Abstract
The incorporation of superhydrophobic properties into metal organic framework (MOF) materials is highly desirable to enhance their hydrolytic stability, gas capture selectivity in the presence of humidity and efficiency in oil–water separations, among others. The existing strategies for inducing superhydrophobicity into MOFs have several weaknesses, such as increased cost, utilization of toxic reagents and solvents, applicability for limited MOFs, etc. Here, we report the simplest, most eco-friendly, and cost-effective process to impart superhydrophobicity to MOFs, involving a rapid (90 min) treatment of MOF materials with solutions of sodium oleate, a main component of soap. The method can be applied to both hydrolytically stable and unstable MOFs, with the porosity of modified MOFs approaching, in most cases, that of the pristine materials. Interestingly, this approach was used to isolate superhydrophobic magnetic MOF composites, and one of these materials formed stable liquid marbles, whose motion could be easily guided using an external magnetic field. We also successfully fabricated superhydrophobic MOF-coated cotton fabric and fiber composites. These composites exhibited exceptional oil sorption properties achieving rapid removal of floating crude oil from water, as well as efficient purification of oil-in-water emulsions. They are also regenerable and reusable for multiple sorption processes. Overall, the results described here pave the way for an unprecedented expansion of the family of MOF-based superhydrophobic materials, as virtually any MOF could be converted into a superhydrophobic compound by applying the new synthetic approach.
Keywords: MOFs, post-synthetic modification, superhydrophobic materials, porous materials, oil-in-water separation
Introduction
Hydrophobic and superhydrophobic materials, i.e., materials with a water contact angles greater than 90 and 150°, respectively, are of interest for a variety of applications, including oil–water separations, moisture-repellent coatings, waterproof clothing, etc.1−6 Incorporating porosity into such materials broadens the range of their applications, enabling the removal of trace lipophilic contaminants from water or air, carbon dioxide capture under humid conditions, and selective recognition of hydrophobic molecules, among other functions.7−9
Metal–organic frameworks (MOFs) represent a class of porous materials that have gained substantial attention over the past two decades due to their remarkable properties and utility in various fields such as gas storage, ion exchange, catalysis, and so on.10−20
Most of these materials are hydrophilic and therefore either hydrolytically unstable or their properties are significantly altered in the presence of moisture. To date, several strategies have been employed to render MOFs hydrophobic, with the two most common approaches involving: (a) the utilization of polytopic organic ligands with fluorinated, polyaromatic, or long-chain alkyl groups, and (b) postsynthetic modification (PSM).21−25
PSM methods appear to be more attractive for applications as they primarily modify the outer surface of the MOF particles rather than the internal structure, thus largely preserving the porosity of the original materials.26−28 However, several limitations are associated with reported PSM approaches such as high cost, the use of toxic reagents, and applicability to only a limited number of MOFs, among others.22,29−35 Moreover, for applications related to the sorption/removal of lipophilic contaminants from aqueous media, the hydrophobic/superhydrophobic MOFs should be prepared in a form that enables easy recovery after use.36,37 Thus, research efforts should also be focused on this direction.
Oleic acid, in its sodium salt form (CH3(CH2)7CH=CH(CH2)7COONa), is a primary component of soap, functioning as an emulsifying agent with significant surface activity and solubility in aqueous media. Sodium oleate is particularly cost-effective since it can be readily prepared by neutralizing free oleic acid or saponifying triglycerides.38 In addition, it exhibits low toxicity and no carcinogenic effects.39,40 Oleate anion is an excellent ligand for metal ions, coordinating to them through its carboxylate group,41,42 and has been widely used as capping agent for metal nanoparticles.43−45 Considering all above features of sodium oleate, it appears to be an ideal reagent for modifying the wetting properties of MOFs through a straightforward and affordable route involving coordination of metal centers with the powerful oleate ligand. It is anticipated that sodium oleate could effectively transform most MOFs into hydrophobic/superhydrophobic materials, regardless of the type of metal ion, ligand, or metal coordination environment. This modification may proceed through the binding of oleate anions to the external surface of MOF particles, replacing the surface-terminated hydroxyl/water ligands.46,47
Here, we demonstrate the facile and rapid transformation of several MOFs, including Zr6O4(OH)4(BDC)6 (UiO-66, with BDC2–=terephthalate anion),48 Zr6O4(OH)4(NH3+–BDC)6Cl6 (MOR-1),49 Zn(mIm)2 (ZIF-8, with mIm=2-methylimidazole),50 Cu3(BTC)2(H2O)3 (HKUST-1, with BTC3–=trimesate),51 and Al(OH)(BDC) (MIL-53(Al))52 into superhydrophobic materials, simply by treating them with solutions of the inexpensive and harmless sodium oleate (purity ≥ 82%). Simultaneously, these materials, except for modified MIL-53(Al), retain a significant percentage (70–100%) of the internal porosity of the original (untreated) MOFs. By applying this newly developed method, we also managed to isolate superhydrophobic MOF-Fe3O4 composites. Interestingly, one of these materials was able to form robust magnetic liquid marbles, whose motion could be controlled with an external magnet. Additionally, we successfully prepared superhydrophobic MOF-coated cotton fabrics and fibers either via in situ modification of MOFs immobilized on the substrate or postsynthetic immobilization of the superhydrophobic material onto the substrate with the aid of poly(methyl methacrylate) (PMMA). These composite materials can quickly sorb floating oil from aqueous media and efficiently purify oil-in-water emulsions. At the same time, these fabric/fiber-based sorbents are also regenerable and reusable for multiple cycles and can be easily recovered after the separation process by simply pulling them out.
Results and Discussion
Post-Synthetic Modification of UiO-66
Our studies on the conversion of MOFs into superhydrophobic materials initiated with water stable MOFs, specifically focusing on UiO-66, one of the most extensively studied MOFs.6,13,16,17,20,21,36,37,48,49 At an early stage, UiO-66 was treated with an aqueous solution of sodium oleate with a concentration of 18.5 mM for 90 min at room temperature. Subsequently, the MOF was isolated by filtration and underwent rinsing with water and then acetone to remove all weakly bound oleate anions. The resultant material, namely, UiO-66-Oleate-1, floated on the water surface (Figure S1), which was the initial evidence of its hydrophobicity. In contrast, UiO-66 formed a fine colloidal suspension in water. Contact angle measurements of UiO-66-Oleate-1 demonstrated its superhydrophobic nature, revealing a water contact angle (WCA) of 167 ± 5° (Figure S2), whereas the WCA of the untreated UiO-66 sample was 25 ± 5° (Figure 1a). PXRD measurements and Le Bail refinement revealed that the UiO-66-Oleate-1 retains the structural features of the original MOF (Figure S3).
Figure 1.
Digital images of water droplets on thin films of (a) UiO-66, (b) UiO-66-green soap, and (c) UiO-66-Oleate-5 along with the determined WCA values. (d) Digital image of UiO-66 and UiO-66-Oleate-5 dispersing in water/chloroform mixtures. (e) Comparative PXRD data for UiO-66 and UiO-66-Oleate-5. (f) High resolution Zr 3d core-level photoelectron spectra of UiO-66 and UiO-66-Oleate-5. The peaks assigned to Zr 3d3/2 and 3d5/2 appear at 182.7/185.1 and 182.4/184.8 eV, respectively. (g) Suggested mechanism for the interaction of oleate anions with the UiO-66 framework.
Considering that oleate is a main component of soap, we attempted to convert UiO-66 into a superhydrophobic material by treating it with an aqueous solution of green soap. To our delight, such treatment resulted in imparting superhydrophobicity to UiO-66, exactly like its treatment with the oleate solution, as revealed by WCA measurements (Figure 1b). The above finding emphasizes the simplicity and effectiveness of the new method.
To find out whether the inserted oleate molecules affect the internal surface area of the MOF, nitrogen gas physisorption studies were performed for the activated UiO-66-Oleate-1. The calculated Brunauer–Emmett–Teller (BET) surface area for UiO-66-Oleate-1 was determined to be 336 m2/g, indicating a significant void reduction compared to pristine UiO-66 (1041 m2/g) (Figure S4). These findings prompted us to investigate whether we could be able to generate superhydrophobic materials while maintaining the inherent porosity of UiO-66. Thus, we altered our experimental procedure by modifying the sodium oleate concentrations (i.e., 12.3, 6.2, 4.3, 2.5, and 1.8 mM and the materials denoted as UiO-66-Oleate-2 up to UiO-66-Oleate-6, respectively). The new MOFs displayed superhydrophobic properties (WCAs > 151 ± 5°), except for UiO-66-Oleate-6, which turned out to be hydrophilic with a WCA of 58 ± 5° (Figure S2). Therefore, the optimum concentration to impart superhydrophobicity to UiO-66 was found to be 2.5 mM. The completely different wetting properties of UiO-66 and UiO-66-Oleate-5 are illustrated in Figure 1a,c,d. Nitrogen gas physisorption data for the activated UiO-66-Oleate-5 showed that a significant percentage of the original porosity is retained (BET surface area of 725 m2/g) proving that the oleate molecules are predominantly located on the outer surface of the MOF particles (Figure S5). The BET surface area for the activated UiO-66-Oleate-3 (683 m2/g) turned out to be somewhat lower compared to UiO-66-Oleate-5 (Figure S6).
The nonlocal density functional theory (NLDFT) pore size distribution analysis showed that both UiO-66 and UiO-66-Oleate-5 materials have pore sizes of ≈5.6 and ≈9 Å (Figures S7 and S8), consistent with those reported in the literature.53,54 The absence of changes in pore widths observed after treating the MOF with sodium oleate further supports the incorporation of oleate anions on the external surface rather than within the internal structure of the material. This restriction on the entry of oleate into the pores of the MOF is presumably due to the significantly larger size of oleate anions (≈2 nm)42 (Figure S9) compared to the pore widths of UiO-66. The reduction in BET surface areas of UiO-66 samples upon treatment with increased oleate concentration likely aligns with the formation of a dense oleate layer at the surface of the MOF, preventing gas molecules from diffusing into the pores.
UiO-66-Oleate-5 and UiO-66-Oleate-3 could additionally be isolated following the same experimental procedure by utilizing an ethanolic solution of sodium oleate. The solvent modification does not appear to significantly affect the porosity and the wetting properties of the materials (see Figures S6 and S10).
Considering that UiO-66-Oleate-5 accomplishes both significant porosity and superhydrophobicity, it was extensively characterized via a variety of techniques. PXRD data and Le Bail refinement revealed that UiO-66-Oleate-5 maintains the structural features of the pristine material (Figure 1e and Figure S11). To further investigate the composition, 1H NMR data were obtained after digesting UiO-66-Oleate-5 in a highly alkaline (2 M NaOH) D2O solution. This process resulted in the decomposition of the framework into insoluble Zr(IV) species and soluble terephthalate/oleate anions. The results revealed the characteristic signals of oleate ions in the aliphatic region of the 1H NMR spectrum (Figure S12). According to the peak integrals, the oleate to MOF molar ratio was determined to be ≈0.08. Similar results were obtained for UiO-66-Oleate-1 (Figure S13). FT-IR data in the spectrum of UiO-66-Oleate-5 revealed the presence of characteristic bands of oleate at 2926 and 2854 cm–1, which correspond to the asymmetric and symmetric stretch of −CH2–, respectively (Figure S14). Field emission-scanning electron microscopy (FE-SEM) images illustrated that the morphology of both UiO-66 and UiO-66-Oleate-5 particles was similar (Figure S15).
In the next step, we decided to investigate the influence of pH on the superhydrophobic properties of UiO-66-Oleate-5, as well as its wettability in different types of aqueous media. The experiments were carried out by depositing liquid droplets of extremely alkaline/acidic aqueous solutions alongside samples of sea, lake, and tap water. The measured WCAs demonstrated that UiO-66-Oleate-5 remains highly hydrophobic in a wide pH range from 0 to 13, and similar results were observed in various aqueous media (Figure S16). In addition, it is worth mentioning that the material displays a WCA of 150 ± 5° even after drying at 150 °C for 12 h (Figure S16). Additionally, PXRD studies revealed that the modified UiO-66 MOF retains its structural characteristics after treatment with highly acidic/alkaline solutions and the various aqueous media (Figures S17 and S18).
The above results suggest a strong binding of oleate ions to the MOF. Thermogravimetric (TGA) analysis (see section Calculation of the linker deficiencies in UiO-66 MOF, SI) revealed linker deficiencies (Figure S19) in the as prepared UiO-66 MOF, with the missing linkers replaced by water and hydroxyl terminal ligands. Considering the strong affinity of Zr4+ for carboxylate ligands, oleate anions could easily substitute the terminal OH–/H2O ligands on the Zr4+ centers. X-ray photoelectron spectroscopy (XPS) (Figure 1f and Figure S20) data were used to confirm the possible ligation of oleate ions to the Zr4+ anions. Specifically, the XPS spectrum of UiO-66-Oleate-5 revealed a remarkable negative shift (about −0.3 eV) in the Zr 3d5/2 and 3d3/2 core-level signals (Figure 1f), in comparison to the corresponding signals in the pristine UiO-66. These results suggest an increase of the electron density around Zr4+ nodes after oleate modification, providing direct proof of the coordination of oleate anions to the Zr4+ centers.55,56 Additionally, zeta potential measurements showed that UiO-66 has neutral surface charge, thus excluding probable electrostatic interactions of the MOF’s surface with the oleate anions (Figure S21). Energy dispersive spectroscopy (EDS) and XPS data indicated the absence of Na+ in the modified material (Figures S22 and S20), which implies that the insertion of oleate anions likely results in the release of surface, terminal hydroxyl ligands to achieve charge neutrality, as depicted in Figure 1g.
Post Synthetic Modification of Other Hydrolytically Stable MOFs
The described synthetic method is also applicable to other Zr4+ MOFs, such as MOR-1.49 Treating MOR-1 with an aqueous oleate solution of 0.8 mM resulted in a superhydrophobic material with a WCA of 155 ± 5°, denoted as MOR-1-Oleate (Figure S23). Oleate concentrations below that value generated hydrophilic materials. The activated MOR-1-Oleate shows a BET surface area (846 m2/g), approaching that (1114 m2/g) of original material (Figure S24). PXRD data and Le Bail refinement indicated that the modified ammonium analogue of UiO-66 retained the structural characteristics of the pristine material (Figure S25). FT-IR studies also demonstrated the existence of the characteristic IR bands of oleate at 2926 and 2854 cm–1 (Figure S26). It was also important to investigate whether our approach could be successful in imparting superhydrophobicity to other types of MOFs. For these studies, we selected ZIF-8(50) and MIL-53(Al)(52) as representative water-stable MOFs. Using a similar synthetic protocol to that applied for Zr4+ MOFs, we isolated the superhydrophobic versions of ZIF-8 and MIL-53(Al). The minimum sodium oleate concentrations required to impart superhydrophobicity to these MOFs were found to be 4.5 and 9.8 mM, respectively. The new materials denoted as ZIF-8-Oleate and MIL-53-Oleate exhibited superhydrophobic behavior with WCAs of 154 ± 5 and 153 ± 5°, respectively (Figure S23). Despite the fact that ZIF-8 and MIL-53 contain metal ions with no terminal ligands, which could be promptly exchanged by oleate anions, they are easily transformed to superhydrophobic materials through their treatment with sodium oleate solutions. This can be explained by the presence of surface-terminated hydroxyl/water groups in these materials (and presumably most MOFs), which can be readily replaced with oleate ligands.46,47 The determined BET surface area for ZIF-8-Oleate (1156 m2/g) was found to be identical, within the error limit, to that (1144 m2/g) of pristine material (Figure S27). However, the BET surface area of MIL-53-Oleate (411 m2/g) was significantly decreased compared to that (986 m2/g) of the original MOF (Figure S28). PXRD measurements and Le Bail refinement indicated that the modified MOFs retained the structural features of the pristine materials (Figures S29 and S30). In addition, FT-IR studies confirmed the presence of oleate ions in each of the modified MOF materials (Figures S31 and S32).
Post Synthetic Modification of Hydrolytically Unstable MOFs
It was challenging to test our method for modification of MOFs that are hydrolytically unstable, especially with MOFs like HKUST-1, which undergoes phase transformation upon exposure to water.57 In this case, an aqueous solution of sodium oleate cannot be used because it leads to immediate degradation of the MOF’s structure. Therefore, we chose ethanol, which is considered a green solvent,58 instead of water to impart superhydrophobicity to HKUST-1. Initially, we proceeded as in the case of UiO-66, by experimenting with different oleate concentrations (i.e., 46, 31, and 15 mM), while investigating the wettability and surface area of the modified materials denoted as HKUST-1-Oleate-1, HKUST-1-Oleate-2, and HKUST-1-Oleate-3. WCA data showed that HKUST-1-Oleate-1 and HKUST-1-Oleate-2 are superhydrophobic with WCA values of 167 ± 5 and 166 ± 5°, respectively, demonstrating the effectiveness of our method even for hydrolytically unstable MOFs (Figure S33 and Figure 2a). However, the BET surface areas for these materials varied significantly. HKUST-1-Oleate-2 displayed a BET surface area of 1359 m2/g, which is close to that of pristine HKUST-1 being 1476 m2/g, confirming that the oleate molecules occupy the outer surface of the HKUST-1 particles (Figure 2c). On the contrary, HKUST-1-Oleate-1 exhibits significantly lower BET surface area (839 m2/g, Figure S34). Regarding HKUST-1-Oleate-3, although it fully retains the porosity of the original material (Figure S35), the observed water contact angle was determined at 47 ± 5° (Figure S33). Sodium oleate concentrations lower than 31 mM led to WCA values of <90°, probably due to the insufficient abundance of surface anchored oleate groups.
Figure 2.
Digital images of water droplets on thin films of (a) HKUST-1-Oleate-2 and (b) HKUST-1, along with the determined WCA values. (c) Nitrogen physisorption isotherms at 77 K for HKUST-1 and HKUST-1-Oleate-2. (d) Comparative PXRD data for HKUST-1 and HKUST-1-Oleate-2. (e) FE-SEM image of HKUST-1. (f) FE-SEM image of HKUST-1-Oleate-2. (g) Suggested mechanism for the interaction of oleate ions with the HKUST-1 framework.
Pore size distribution studies indicated no change in the pore sizes of HKUST-1, which were found to be ≈5.5 and ≈9.1 Å (Figures S36 and S37), in accordance with literature values,59,60 upon treatment with oleate anions. Similar to UiO-66, the decrease of the BET surface areas of HKUST-1 samples when treated with concentrated oleate solutions can be attributed to the formation of a dense oleate layer at the MOF’s surface, considering that oleate anions are too large to penetrate the pores of HKUST-1.
As HKUST-Oleate-2 is both superhydrophobic and highly porous (largely retaining the porosity of the pristine material), it was characterized in more detail. PXRD measurements and Le Bail refinement for HKUST-1-Oleate-2 revealed no structural alternations compared with the pristine material (Figure 2d and Figure S38). FT-IR data further confirmed the presence of the characteristic IR bands of oleate such as the alkyl −CH2– vibrational peaks at 2926 and 2854 cm–1 (Figure S39). In addition, 1H NMR spectroscopy measurements also confirmed the grafting of oleate ions (Figure S40). Based on the peak integrals, the Oleate to HKUST-1 molar ratio was determined to be ≈0.07. FE-SEM images demonstrated that the morphology of HKUST-1 and HKUST-1-Oleate-2 particles was similar (Figure 2e,f).
We then investigated the wetting properties of the superhydrophobic HKUST-1-Oleate-2 with various water samples (sea, lake, and tap water) and acidic/alkaline aqueous solutions. The calculated WCAs (Figure S41) showed that HKUST-1-Oleate-2 remains superhydrophobic in a pH range of 3 to 10 and in different types of aqueous media. Furthermore, PXRD indicated that modified HKUST-1 preserves its structural integrity after treatment with various aqueous solutions for 4 or even 24 h (Figures S42 and S43).
Additionally, we aimed to investigate whether the superhydrophobic HKUST-1-Oleate-2 exhibits improved stability to humidity compared to the original framework. Both MOFs were placed in a closed, controlled humid system at room temperature for 3 days. The results revealed that the PXRD pattern of HKUST-1 contained diffraction peaks not existing in the pattern of the pristine MOF, indicating structural alterations upon interaction with water vapors (Figure S44). On the contrary, HKUST-1-Oleate-2 retained its structural features as it was revealed by Le Bail refinement (Figure S45). In conjunction with PXRD data, N2 physisorption studies further confirmed the structural differentiations observed in HKUST-1 by showing a remarkable decrease in its BET surface area down to 178 m2/g (Figure S46), while the oleate modified material retained a substantial portion of its porosity with a BET surface area of 978 m2/g (Figure S47). The above clearly indicates that the post synthetic modification significantly enhances the stability of HKUST-1 in a humid environment.
Finally, similarly to the investigation conducted for the UiO-66 MOF, we explored the mechanism of oleate modification in the case of HKUST-1. Based on N2 physisorption and pore size distribution studies for HKUST-1-Oleate-2, the interaction between oleate ions and HKUST-1 takes place predominantly in the external surface resulting in the preservation of large void space. XPS data for HKUST-1-Oleate-2 (Figures S48 and S49) revealed a negative shift, up to −0.2 eV, in the Cu 2p3/2 and 2p1/2 core-level signals, in comparison to the corresponding signals for the pristine HKUST-1 material. This is consistent with an increase in the electron density around the Cu2+ metal ions, implying coordination and thus charge transfer interactions between the oleate anions and Cu2+ centers.55,56 The EDS spectrum and XPS data of HKUST-1-Oleate-2 (Figures S50 and S48) revealed no Na+ residues from sodium oleate. Taking into consideration the above results, we suggest that the ligation of oleate anions to Cu2+ centers coincides with the release of terminal OH– groups from the external surface of HKUST-1 particles (Figure 2g).
Comparing Our Method with Other PSM Approaches
At this point, it is useful to compare the new method with known approaches and modifications to generate superhydrophobicity in MOFs. As shown in Table S1, our method represents (a) one of the fastest, being completed in less than 2 h, while other methods require reaction times of at least 12 h, and (b) one of the very few that does not utilize organic solvents, except for the transformation of hydrolytically unstable MOFs requiring the use of ethanol. In contrast to several known PSM methods that are effective only in MOFs bearing linkers with specific functional groups,6,22,23,27 this method is applicable to MOFs with a variety of metal ions and linkers. Moreover, in our approach, superhydrophobicity is conferred by ligation of oleate ions, an inexpensive, widely available, and low-toxicity organic substance.38−45 The estimated cost for the conversion of UiO-66 into a superhydrophobic material, considering the retail price of crude sodium oleate (purity ≥82%, cost ≈58 €/kg) and the negligible solvent and energy costs (as the process takes place in aqueous solutions and ambient temperature for only 90 min), is calculated approximately 4 € per kg of MOF. In contrast, other methods involve the use of costly and/or toxic materials such as perfluorinated agents, phosphate-containing surfactants, polymer coatings etc., or require specialized equipment and synthetic conditions.22,23,27,30−32,35 Therefore, the above comparison demonstrates that the method described here is widely applicable and probably the “greenest” and least expensive method ever described for the conversion of MOFs into superhydrophobic materials. Importantly, this method also results in the isolation of superhydrophobic materials with surface areas comparable to those of the pristine MOFs. Notably, modified HKUST-1 and ZIF-8 retained up to 92 and 100% of the original materials’ porosity, respectively. Such remarkable porosity retention for superhydrophobic HKUST-1 and ZIF-8 has been reported in the literature.31,35 However, the synthetic approaches toward these modified MOFs entail perfluorinated or other hazardous reagents and toxic solvents.
Formation of Magnetic Liquid Marbles
Superhydrophobic/hydrophobic particles enclosing liquid droplets enable the isolation of nonstick droplets, known as liquid marbles. These hold potential applications in a variety of different fields, for example gas sensing, miniaturized synthesis and micro reactors, cultivation of microorganisms, etc.61,62 The development of liquid marbles with strong magnetic properties has significant interest, as the motion of magnetic liquid marbles can be easily controlled using an external magnetic field, allowing them to act as mobile phases to transfer miscellaneous types of liquids to specific locations on various surfaces.63 This feature of magnetic liquid marbles is attractive for microfluidic applications.64 To isolate a magnetic MOF-based liquid marble, we aimed to prepare superhydrophobic, magnetic MOF composites, utilizing the synthetic route employed for the isolation of the superhydrophobic MOF materials. Specifically, a mixture of UiO-66 and Fe3O4 (mass ratio UiO-66:Fe3O4 = 100:10) was treated with an 18.5 mM aqueous oleate solution at room temperature. Remarkably, the resulting composite, i.e., UiO-66-Oleate-1-Fe3O4, displayed a WCA of 156 ± 5° (Figure 3a).
Figure 3.

(a) Digital image of a water droplet on a thin film of UiO-66-Oleate-1-Fe3O4 with the indication of the determined WCA value. (b) Digital stereoscopic image of a liquid marble based on the UiO-66-Oleate-1-Fe3O4 material. (c) A water droplet rolling over UiO-66-Oleate-1-Fe3O4 particles. (d) Controlling the motion of the liquid marble using an external magnet.
PXRD data once again confirmed that this new material retained the structural features of the pristine UiO-66 MOF (Figures S51 and S52). N2 physisorption measurements for the activated UiO-66-Oleate-1-Fe3O4 showed a surface area of 338 m2/g (Figure S53). FT-IR and 1H NMR spectroscopy also confirmed the presence of oleate ions (Figures S54 and S55). FE-SEM images revealed that the particles of the magnetic composite had morphology resembling that of the UiO-66 MOF (Figure S56). EDS data confirmed the presence of Fe in the magnetic composites (Figure S57). Notably, UiO-66-Oleate-1-Fe3O4 effectively stabilized water droplets by forming concrete liquid marbles (Figure 3b). The droplets could easily roll over a pile of magnetic composite while picking up MOF particles until the droplets were entirely covered (Figure 3c and Video S1). The resulting liquid marbles displayed excellent mechanical robustness and their motion can readily be controlled with an external magnetitic field (Figure 3d and Video S2). We should note that additional superhydrophobic MOF-Fe3O4 composites could be isolated, such as UiO-66-Oleate-2-Fe3O4 and HKUST-1-Oleate-1-Fe3O4 (their detailed characterization is provided in SI, Figures S58–S70). However, these composites could not form liquid marbles as stable as those made by UiO-66-Oleate-1-Fe3O4.
The fact that UiO-66-Oleate-2-Fe3O4 is not superhydrophobic, showing WCA= 141 ± 5° (Figure S58), in contrast to UiO-66-Oleate-1-Fe3O4 (Figures 3a), likely justifies the limited capability of the former material to create stable liquid marbles. Regarding HKUST-1-Oleate-1-Fe3O4, despite exhibiting superhydrophobicity with WCA = 164 ± 5° (Figure S58), it forms liquid marbles with lower mechanical robustness compared to those of UiO-66-Oleate-1-Fe3O4. The likely explanation for this aligns with the significantly larger sizes (up to 37 μm) of HKUST-1-Oleate-1-Fe3O4 particles (Figure S68) compared to those (up to 0.2 μm) of UiO-66-Oleate-1-Fe3O4 (Figure S56), which may affect the enclosing properties of the materials.
Immobilization of the Superhydrophobic MOFs onto Substrates
We also desired to investigate the possibility of incorporating the superhydrophobic MOFs into stable substrates. Initially, we successfully immobilized UiO-66-Oleate into a cotton fabric and impart superhydrophobicity through a two-step procedure involving in situ growth of UiO-66 on the cotton fabric and then treatment of UiO-66@Cotton fabric with an aqueous sodium oleate solution at ambient temperature (Figure 4a). The resulting UiO-66-Oleate@Cotton fabric floated on the water surface, in contrast to unmodified UiO-66@Cotton fabric, which easily submerged (Figure 4b). The WCA for the composite was determined to be 150 ± 5° (Figure 4c). PXRD and FTIR data confirmed the presence of UiO-66-Oleate onto the cotton fabric (Figure 4d and Figure S71). Moreover, FE-SEM and EDS studies revealed the presence of MOF particles on the textile fibers (Figure 4e and Figure S72).
Figure 4.
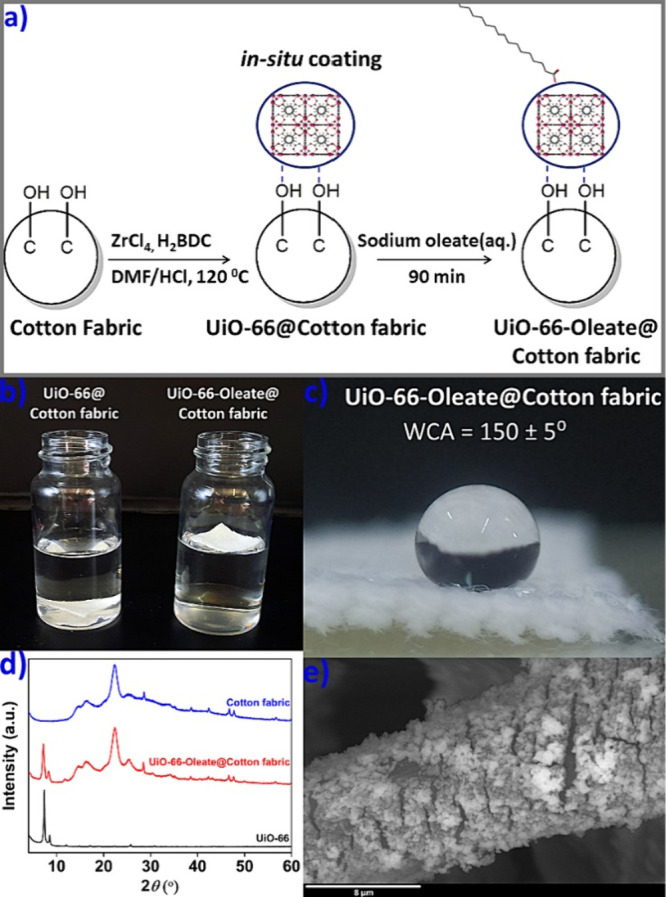
(a) Two-step in situ coating route for the isolation of UiO-66-Oleate@Cotton fabric, (b) Digital image of UiO-66-Oleate@Cotton fabric floating on the water surface in comparison to UiO-66@Cotton fabric. (c) Digital image of a water droplet on UiO-66-Oleate@Cotton fabric, along with the determined WCA value. (d) Comparative PXRD data of UiO-66, UiO-66-Oleate@Cotton fabric, and cotton fabric. (e) FE-SEM image of UiO-66-Oleate@Cotton fabric.
As an alternative method, we employed a postsynthetic approach for the immobilization of the superhydrophobic MOF onto the cotton fabric. Thus, we effectively incorporated the superhydrophobic UiO-66-Oleate-5 onto the cotton fabric using the inexpensive and nontoxic poly(methyl methacrylate) (PMMA) as an adhesive agent. UiO-66-Oleate-5 was suspended in a PMMA solution in acetone (mass ratio MOF:PMMA = 10:1) via ultrasonication. A piece of cotton fabric was submerged into the stirred coating solution for a few seconds. After a dipping and drying procedure at room temperature, a dense UiO-66-Oleate-5-PMMA coating was obtained on the cotton fabric (Figure 5a). The MOF displayed strong adhesion, as the quantity of MOF immobilized onto cotton fabrics remained identical after water treatment of the fabric composites for 24 h (Experimental Section). UiO-66-Oleate-5-PMMA@Cotton fabric floats on the water surface unlike UiO-66-PMMA@Cotton fabric (Figure 5b). The water contact angle for UiO-66-Oleate-5-PMMA@Cotton fabric was estimated to be 144 ± 5° (Figure 5c). PXRD and FTIR studies also revealed the effective incorporation of UiO-66-Oleate-5 onto the cotton substrate (Figure 5d and Figure S73). FE-SEM images and EDS data revealed the coating of the fabric fibers by MOF particles (Figure 5e and Figure S74).
Figure 5.
(a) Postsynthetic immobilization of UiO-66-Oleate-PMMA mixture onto the cotton fabric/fiber. (b) Digital image of UiO-66-Oleate-5-PMMA@Cotton fabric floating on the water surface in comparison to UiO-66-PMMA@Cotton fabric. (c) Digital image of a water droplet on UiO-66-Oleate-5-PMMA@Cotton fabric, along with the determined WCA value. (d) Comparative PXRD data of UiO-66, UiO-66-Oleate-5-PMMA@Cotton fabric, and PMMA@Cotton fabric. (e) FE-SEM image of UiO-66-Oleate-5-PMMA@Cotton fabric. (f) Digital image of UiO-66-Oleate-3-PMMA@Cotton floating on the water surface in comparison to cotton substrate. (g) Digital image of liquid droplets (dye solutions and water) on UiO-66-Oleate-3-PMMA@Cotton, along with the determined WCA value.
By employing the same method, we were able to prepare a cotton fiber-based composite denoted as UiO-66-Oleate-3-PMMA@Cotton. The modified cotton fiber displayed superhydrophobicity in contrast to the original substrate and the WCA was calculated to be 165 ± 5° (Figure 5f,g). FE-SEM, EDS, and PXRD data confirmed the successful immobilization of UiO-66-Oleate-3 onto the cotton fiber (Figures S75–S77).
Subsequently, we decided to apply the same protocols to HKUST-1. Unfortunately, the two-step procedure was not efficient, as the MOF particles were released from the fabric during the hydrophobic modification. We also explored different surface immobilization methods, such as enriching the substrate with pendant carboxyl or catechol groups, but we encountered the same difficulty.65,66
Hence, we focused on the MOF-PMMA approach by applying the same experimental procedure described above for UiO-66-Oleate-5. However, efficient adhesion of HKUST-1-Oleate-2 onto the cotton fabrics was achieved using a larger amount of PMMA (MOF to PMMA mass ratio was 1:1). The new composite (denoted as HKUST-1-Oleate-2-PMMA@ Cotton fabric) displayed high hydrophobicity with a WCA of 147 ± 5° (Figure 6a). The efficient immobilization of HKUST-1-Oleate-2 was confirmed by PXRD, FTIR and FE-SEM/EDS measurements (Figure 6b,c and Figures S78 and S79). Importantly, the immobilized HKUST-1-Oleate-2 material retained its structural characteristics upon interaction with water (Figure 6d).
Figure 6.

(a) Digital image of a water droplet on HKUST-1-Oleate-2-PMMA@Cotton fabric, along with the determined WCA value. (b) Comparative PXRD data of HKUST-1, HKUST-1-Oleate-2-PMMA@Cotton fabric, and PMMA@Cotton fabric. (c) FE-SEM image of HKUST-1-Oleate-2-PMMA@Cotton fabric. (d) Comparative PXRD data of HKUST-1-Oleate-2-PMMA@ Cotton fabric before and after water treatment.
Oil/Water Separation
In the past decades, an enormous volume of oily wastewater has been regularly discharged into the ecosystem from various oil-based activities such as petroleum refining, metalworking and machining, food processing, chemicals production, textile manufacturing, etc. Furthermore, frequent oil leakage accidents during transportation and refilling incidents pose considerable environmental issues. Oily wastewater may contain various toxic substances, which severely affect the marine habitat and the seafood resulting to the accumulation of hazardous chemicals into the human body.2,36,37,67,68 Therefore, there is a crucial demand to develop novel, cost-effective and environmentally friendly oil/water separation materials.69
Aiming toward practical oil–water separation applications, the lipophilicity of the superhydrophobic cotton fabric composites (UiO-66-Oleate@Cotton fabric, UiO-66-Oleate-5-PMMA@Cotton fabric, and HKUST-1-Oleate-2-PMMA@Cotton fabric) was also tested. As shown in Figure 7a–c, the MOF@fabric composites can easily sorb crude oil droplets. Hence, they were investigated for the removal of crude oil floating in water. It was found that all three different fabrics successfully sorbed the oil from the water surface (Figures S80 and S81, Figure 7d–g, and Video S3). On the contrary, unmodified cotton fabric is highly hydrophilic. Thus, it is easily submerged upon contact with water (Figure 4b) and its sorption capacity for crude oil under static conditions is negligible. The oil-laden fabrics could be regenerated with n-hexane, and the sorption/regeneration procedure was repeated up to 10 times (Figures S80 and S81 , Figure 7h,i, and Video S3). PXRD data revealed no structural differentiations of the MOF-oleate materials after 10 cycles of sorption/regeneration (Figures S82–S84). Similar crude oil removal efficiency and reusability was observed for UiO-66-Oleate-3-PMMA@Cotton as depicted in Figure S85.
Figure 7.
Digital images demonstrating the oleophilicity and hydrophobicity of (a) UiO-66-Oleate@Cotton fabric, (b) UiO-66-Oleate-5-PMMA@Cotton fabric, and (c) HKUST-1-Oleate-2-PMMA@Cotton fabric. (d–g) Crude oil sorption from the water surface by HKUST-1-Oleate-2-PMMA@Cotton fabric. (h) Desorbed oil from the oil-laden fabric after its treatment with n-hexane and the regenerated HKUST-1-Oleate-2-PMMA@Cotton fabric. (i) Oil-free water solution and the HKUST-1-Oleate-2-PMMA@Cotton fabric after the 10th cycle of sorption/regeneration.
Furthermore, we tested the capability of the MOF@cotton composites for treatment of oil-in-water emulsions. Specifically, we have investigated the efficiency of the UiO-66-Oleate-5-PMMA@Cotton fabric for purification of vacuum pump oil-in-water emulsions (initial oil concentration ≈540 ppm). The fabric was fixed onto a magnetic stirring bar, by applying ordinary adhesive, to remain immersed during the demulsification process. The experiment was conducted under mild stirring, at ambient conditions, for 20 h and the purification efficiency was determined via UV–vis spectroscopy (Experimental Section). As shown in Figure 8a, UiO-66-Oleate-5-PMMA@Cotton fabric sorbed most of the oil, resulting in a transparent mixture (removal efficiency ≈89.6%). As a further step, we decided to investigate more thoroughly the separation kinetics as well as the performance of the composite in more concentrated oil-in-water mixtures. The sorption data revealed that the equilibrium is reached at 5 h with a separation efficiency of 87.2%, while only a minor increase of 2.4% was achieved after 20 h of contact time (Figure S86). On top of that, UiO-66-Oleate-5-PMMA@Cotton fabric maintained its great separation performance even in emulsions of increased concentrations (Figure 8b,c and Figure S87) with the highest sorption capacity determined at 1.53 g of vacuum pump oil/g of UiO-66-Oleate-5 (Figure S88). UiO-66-Oleate-5-PMMA@Cotton fabric also demonstrated high efficiency for the purification of crude oil-in-water emulsion (Figure 8d and Figure S87). Additionally, we tested the unmodified cotton for demulsification of a vacuum pump oil emulsion. Based on the variance in absorbance intensities at 238 nm (Figure S89), the unmodified fabric demonstrated demulsification efficiency of only 14.3%. The fabric composites after demulsification were studied via PXRD and FT-IR spectroscopy. PXRD data indicated no structural alterations, while FT-IR measurements confirmed the sorption of vacuum pump oil, presenting a strong band at ≈2900 cm–1 that is assigned to the −CH2– vibrations of the hydrocarbons (Figure 8e,f). We also investigated the potential reusability of the demulsifier. FT-IR data confirmed the desorption of most sorbed pump oil (Figure 8e,f) by treating the oil-loaded fabric with n-hexane. Importantly, the fabric sorbent could be reused up to four cycles of sorption/regeneration with no significant loss of separation performance (Figure 8g). The reused fabric was further characterized via FTIR and PXRD data (Figures S90 and S91), indicating no structural modifications. In addition, no leaching of MOF particles from the fabric was observed, as the quantity of the MOF immobilized remained unaltered after the emulsion purification processes.
Figure 8.
Vacuum pump oil-in-water emulsions with initial oil concentrations (a) C ≈ 540 ppm, (b) C ≈ 780 ppm, and (c) C ≈ 1040 ppm before and after a 20 h treatment with UiO-66-Oleate-5-PMMA@Cotton fabric. (d) Crude oil-in-water emulsion with initial oil concentration C ≈ 1005 ppm before and after a 20 h treatment with UiO-66-Oleate-5-PMMA@Cotton fabric. (e) Comparative PXRD data of UiO-66-Oleate-5-PMMA@Cotton fabric before and after demulsification of vacuum pump oil-in-water emulsion (with initial oil concentration of ≈1040 ppm) as well as after regeneration with n-hexane. (f) Comparative FTIR spectra of a vacuum pump oil sample, UiO-66-Oleate-5-PMMA@Cotton fabric before and after demulsification of vacuum pump oil-in-water emulsion (with initial oil concentration of ≈1040 ppm) as well as after regeneration with n-hexane. (g) Demulsification performance, evaluated on vacuum pump oil-in-water emulsion with initial oil concentration of ≈1040 ppm, of UiO-66-Oleate-5-PMMA@Cotton fabric during four cycles of sorption/regeneration.
Conclusions
A new synthetic strategy for the conversion of a series of hydrophilic, water-stable MOFs into superhydrophobic materials is reported, in which oleate anions are strongly bound to the outer surface of MOF particles via the formation of metal ion-oleate coordination bonds. This method is particularly cost-effective and environmentally friendly as it does not require organic solvents or perfluorinated agents, proceeds at room temperature, and is completed within 1 to 2 h. Interestingly, even a treatment of MOFs with an aqueous solution of green soap, whose main component is sodium oleate, led to the isolation of superhydrophobic MOF materials. This method can also be effective in converting hydrolytically unstable MOFs into superhydrophobic materials, but an ethanolic solution of the fatty acid anion must be used. Remarkably, the superhydrophobic MOFs produced through the new method preserve, in most cases, a considerable percentage of the internal porosity of the pristine materials. The same approach was extended to the isolation of superhydrophobic MOF-Fe3O4 magnetic composites. One of the isolated composites could form robust magnetic liquid marbles with their motion easily manipulated by an external magnetic field. Such property is of interest for applications in microfluidics. Furthermore, we have succeeded in preparing superhydrophobic MOFs immobilized in cotton fabric or fiber. These composite fabric-based materials can remove crude oil from the water surface and effectively decontaminate oil-in-water emulsions. Simultaneously, they can be easily retrieved after use and are also easily regenerated and reused multiple times.
Overall, the present work provides a facile and quite simple synthetic approach that could potentially be applied for converting any known MOF or newly synthesized MOF compounds to superhydrophobic materials. Therefore, the family of superhydrophobic MOFs can be significantly expanded to include compounds with a variety of structural features, pore sizes, and functional groups. The resulting materials are anticipated to exhibit unprecedented properties arising from the combination of the intrinsic MOF characteristics with superhydrophobicity.
Experimental Section
Materials
Zirconium chloride (ZrCl4), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), aluminum nitrate nonahydrate (Al(NO3)3·9H2O), 2-aminoterephthalic acid (NH2–H2BDC), terephthalic acid (H2BDC), 2-methylimidazole (C4H6N2), and poly(methyl methacrylate) ((C5H8O2)n) were purchased from Alfa Aesar. Iron Chloride Hexahydrate (FeCl3·6H2O), copper nitrate trihydrate (Cu(NO3)2·3H2O), sodium sulfite (Na2SO3), trimesic acid (H3BTC), methylene blue hydrate (C16H18ClN3S·xH2O), methyl orange 85% (C14H14N3NaO3S), and sodium oleate (>82%) were purchased from Aldrich. The solvents were used as received. Green soap, cotton fabric, and cotton fiber were obtained from commercial sources.
MOF Syntheses
The pristine MOFs, i.e., UiO-66, MOR-1, MIL-53(Al), ZIF-8, and HKUST-1 were synthesized following experimental procedures similar to those reported previously in the literature with some minor modifications.70−74
Post Synthetic Modification of Water Stable MOFs with Sodium Oleate
Aqueous-Based Preparation of UiO-66-Oleate-1
A total of 100 mg of UiO-66 was added to an 8 mL aqueous solution of sodium oleate with a concentration of 18.5 mM. The resulting suspension was stirred at ambient temperature for 90 min. The modified UiO-66 was isolated via centrifugation, washed with water and acetone, and dried at 80 °C. The rest of the modified UiO-66 samples (i.e., UiO-66-Oleate-2 up to UiO-66-Oleate-6) were prepared following the same experimental procedure by adjusting the concentration of the sodium oleate solution (i.e., 12.3, 6.2, 4.3, 2.5,and 1.8 mM). Yield: 75–102 mg (depending on the sodium oleate concentration).
Preparation of UiO-66-Oleate-5 and UiO-66-Oleate-3 in Ethanol
These materials were isolated through the procedure described above via the utilization of an ethanolic instead of an aqueous sodium oleate solution. Yield: 80 mg.
Preparation of MOR-1-Oleate
A total of 100 mg of MOR-1 was added to an 8 mL aqueous solution of sodium oleate with a concentration of 0.8 mM. The resulting suspension was stirred at ambient temperature for 90 min. The modified MOF was isolated via centrifugation, washed with water and acetone, and dried at 80 °C. Yield: 80 mg.
Preparation of MIL-53(Al)-Oleate
A total of 100 mg of MIL-53(Al) was added to an 8 mL aqueous solution of sodium oleate with a concentration of 9.8 mM. The resulting suspension was stirred at ambient temperature for 90 min. The modified MOF was isolated via centrifugation, washed with water and acetone, and dried at 80 °C. Yield: 75 mg.
Preparation of ZIF-8-Oleate
A total of 100 mg of ZIF-8 was added to an 8 mL aqueous solution of sodium oleate with a concentration of 4.5 mM. The resulting suspension was stirred at ambient temperature for 90 min. The modified MOF was isolated via centrifugation, washed with water and acetone, and dried at 80 °C. Yield: 85 mg.
Post Synthetic Modification of HKUST-1 with Sodium Oleate
Preparation of HKUST-1-Oleate-1
A total of 100 mg of HKUST-1 was added to an 8 mL ethanolic solution of sodium oleate with a concentration of 46 mM. The resulting suspension was stirred at ambient temperature for 90 min. The modified MOF was isolated via centrifugation, washed several times with EtOH, and dried at 80 °C. The rest of the modified HKUST-1 samples (i.e., HKUST-1-Oleate-2 and HKUST-1-Oleate-3) were prepared following the same experimental procedure by adjusting the concentration of the sodium oleate solution (i.e., 31 and 15 mM). Yield: 80–90 mg (depending on the sodium oleate concentration).
Post Synthetic Modification of UiO-66 with Green Soap
Preparation of UiO-66-green Soap
A total of 100 mg of UiO-66 was added to an aqueous solution of green soap (55 mg in 8 mL). The resulting suspension was stirred at room temperature for 90 min. The modified UiO-66 was isolated via centrifugation, washed with water and acetone, and dried at 80 °C. Yield: 99 mg.
Synthesis of Fe3O4
A total of 61.5 mg (0.49 mmol) of Na2SO3 was dissolved in 5 mL of distilled water. The Na2SO3 solution were gradually added to a 30 mL aqueous solution of FeCl3·6H2O (810.9 mg, 3 mmol) under vigorous stirring. After the addition, the color of the solution changed from light yellow to red. Thereafter, a NH3 aqueous solution (1.5 M) of 15 mL is gradually added to the red solution leading to the precipitation of a black solid while the color of the solution changed back to yellow. The resulting oxide (its PXRD is shown in Figure S92) was isolated via filtration, washed several times with water, and dried in air. Yield: 240 mg.
Isolation of the Magnetic Superhydrophobic/Hydrophobic Composites
Preparation of UiO-66-Oleate-1-Fe3O4
A total of 100 mg of UiO-66 and 10 mg of Fe3O4 were added to an 8 mL aqueous solution of sodium oleate with a concentration of 18.5 mM. The resulting mixture was stirred at room temperature for 90 min. The magnetic composite was isolated via centrifugation, washed with water and acetone, and dried at 80 °C. UiO-66-Oleate-2-Fe3O4 was isolated following the same experimental procedure by adjusting the sodium oleate solution concentration to 2.5 mM. Yield: 80–90 mg (depending on the sodium oleate concentration).
Preparation of HKUST-1-Oleate-1-Fe3O4
A total of 100 mg of HKUST-1 and 10 mg of Fe3O4 were added to an 8 mL ethanolic solution of sodium oleate with a concentration of 31 mM. The resulting mixture was stirred at room temperature for 90 min. The magnetic composite was isolated via centrifugation, washed several times with EtOH and dried at 80 °C. Yield: 75 mg.
Immobilization of the Superhydrophobic MOFs onto Substrates
Preparation of UiO-66-Oleate@Cotton Fabric
UiO-66-Oleate@Cotton fabric was prepared in two separate steps. The first step consists of the in situ immobilization of UiO-66 onto the fabric substrate via a solvothermal reaction. The second step relates to the hydrophobic modification of UiO-66@Cotton fabric using a sodium oleate solution at room temperature.
A typical procedure is the following:
First Step
One piece of circular shaped cotton fabric (diameter 1.5 cm) weighing a total of 35 mg was washed three consecutive times with 5 mL of MeOH and then dried at 80 °C for 2 h. Afterward, the cotton fabric, ZrCl4 (62.5 mg, 0.268 mmol), and H2BDC (62.3 mg, 0.375 mmol) was placed in 20 mL glass vial containing a mixture of DMF/HCl (7.5 mL/0.5 mL). The reaction mixture was ultrasonicated for 10 min and the container was then sealed and allowed to react in an oven operated at 120 °C for 24 h. The next day the mixture was cooled at room temperature and the modified fabric was primarily washed multiple times with deionized H2O to remove the UiO-66 that has not been incorporated into the substrate and additionally with acetone. The final product was dried at 80 °C. The immobilization process was repeated twice to increase the amount of the MOF particles onto the substrate. The UiO-66 powder not immobilized on the substrate was isolated and further used in the preparation of UiO-66-Oleate-5.
2nd step
UiO-66@Cotton fabric was added to a 10 mL aqueous solution of sodium oleate (2.7 mM) in a 20 mL glass vial. The mixture was kept under magnetic stirring for 90 min at room temperature. Thereafter the modified fabric was primarily washed with deionized H2O and acetone. The final product was dried at 80 °C.
Preparation of UiO-66-Oleate-5-PMMA@Cotton fabric
5 mg of PMMA were dissolved in 25 mL acetone under ultrasonication for 15 min. Then 10 mg of UiO-66-Oleate-5 were added to 5 mL of the PMMA solution, and the resulting mixture was stirred for 12 h at ambient temperature in order to get a uniform suspension. A piece of circular shaped cotton fabric (diameter 1.5 cm) weighing a total of 35 mg was immersed into the suspension for a few seconds while stirring, and then dried in air. The dipping and drying procedure was repeated numerous times until all the suspension was used up. The MOF-PMMA composite was left to dry at 80 °C overnight to achieve better adhesion of the MOF into the fabric.
Preparation of UiO-66-Oleate-3-PMMA@Cotton
5 mg of PMMA were dissolved in 25 mL acetone under ultrasonication for 15 min. Then 10 mg of UiO-66-Oleate-3 were added to 5 mL of the PMMA solution, and the resulting mixture was stirred for 12 h at ambient temperature in order to get a uniform suspension. A piece of cotton fiber (≈100 mg) was immersed into the suspension for a few seconds while stirring, and then dried in air. The dipping and drying procedure was repeated numerous times until all the suspension was used up. The MOF-PMMA composite was left to dry at 80 °C overnight to achieve better adhesion of the MOF into the cotton fiber.
Preparation of HKUST-1-Oleate-2-PMMA@Cotton fabric
10 mg of PMMA were dissolved in 5 mL acetone under ultrasonication for 15 min. Then 10 mg of HKUST-1-Oleate-2 were added to the PMMA solution, and the resulting mixture was stirred for 12 h at ambient temperature to get a uniform suspension. A piece of circular shaped cotton fabric (diameter 1.5 cm) weighing a total of 35 mg was immersed into the suspension for a few seconds while stirring, and then dried in air. The dipping and drying procedure was repeated numerous times until all the suspension was used up. The MOF-PMMA composite was left to dry at 80 °C overnight to achieve better adhesion of the MOF into the fabric.
Determination of the Zr content (mg/cm2) of the UiO-66-Oleate@Cotton fabric
As the MOF was chemically bound to the fabric, releasing it without causing its decomposition was not feasible. Thus, direct determination of the MOF content was not possible in this case. Only the Zr content of the MOF immobilized into the fabric could be thus determined, after decomposing the MOF to ZrO2. Initially we attempted to determine the Zr content of the superhydrophobic fabric via its thermal treatment at 800 °C. However, we observed that a portion of the fabric did not burn after the heating process and thus, it was not possible to determine the Zr content (based on the ZrO2 mass) accurately. Consequently, we decided to detach the MOF-oleate from the fabric via treatment with an alkaline solution. The superhydrophobic composite was stirred for 60 min in a 20 mL NaOH solution (2 M). Extremely alkaline conditions led to the decomposition of the MOF and formation of an insoluble Zr(IV) phase. The oleate and terephthalate ions were soluble under these conditions, while the cotton fabric remained intact. The white solid was isolated via centrifugation, washed with water and dried at 80 °C. PXRD for the solid revealed a mixture of several phases. Thus, the solid underwent an 1 h thermal treatment at 800 °C to transform it to pure ZrO2. Based on the final mass of the oxide, the Zr content of the fabric was determined to be 0.17 mg/cm2 of the fabric.
Determination of the MOF content into the MOF-PMMA composites
By taking advantage of the high solubility of PMMA in acetone, the release of the MOF particles from MOF-PMMA@Cotton fabric or fiber composites could be easily achieved. As a result, the MOF content of the composites could be readily determined. Thus, the composites were immersed in acetone and sonicated for several minutes. The substrate was then removed, and the mixture was centrifuged to isolate the MOF particles that were released. Acetone dissolves PMMA and as a result, MOF particles cannot remain fixed into the fabric. The released MOF solid was dried in air. In the case of HKUST-1-Oleate-2-PMMA@Cotton fabric, the treatment with acetone was conducted multiple times to ensure the dissolution of PMMA, which was present in a much higher (10-fold) content compared to those in UiO-66-Oleate-5-PMMA@Cotton fabric and UiO-66-Oleate-3@Cotton. The MOF content for the three different composites was determined at 6.2 mg of UiO-66-Oleate-5 per 1.77 cm2 of cotton fabric, 6 mg of UiO-66-Oleate-3 per 100 mg of cotton fiber and 6.5 mg of HKUST-1-Oleate-2 per 1.77 cm2 of cotton fabric.
Evaluation of the Adhesion of the UiO-66-Oleate-5-PMMA Coating on Cotton Fabric After Water Treatment
A piece of UiO-66-Oleate-5-PMMA@Cotton fabric was placed in a glass vial containing 10 mL of distilled water and was kept under mild stirring for 24 h at room temperature. The weight of the fabric composite remained identical following the water treatment proving the strong adhesion of the MOF-PMMA coating.
Preparation of Thin MOF Films for Water Contact Angle Studies
Thirty mg of the oleate modified MOF was dispersed in 1.5 mL of CH2Cl2 in a glass vial. The mixture was subjected to ultrasonication for 10 min and kept under stirring. A small portion of the resulting suspension was spread on a microscope coverslip using a Pasteur pipet and dried in air. This step was repeated several times until the slip was covered with a thin film of the MOF. In advance of water contact angle determination, the MOF films remained at room temperature for a minimum of 1 h.
Activation of UiO-66, MOR-1, MIL-53, and ZIF-8 Samples Prior Gas Physisorption Studies
A total of 100 mg of the MOF was placed in a glass vial containing 4 mL of EtOH. The mixture was then stirred at ambient temperature for 24 h. The solid was isolated via centrifugation, washed with acetone, and dried at 80 °C overnight. The solvent exchange procedure was repeated two additional times.
Evaluation of the Structural Robustness of UiO-66-Oleate-5 and HKUST-1-Oleate-2 in Various Aqueous Media and pH Values
Prior to the stability studies, the materials were dispersed in CH2Cl2, centrifuged, and dried in air for 1 h. Thereafter, 15 mg of the MOF were placed in a glass vial containing 8 mL of the corresponding aqueous solution and the sample was left undisturbed for 4 to 24 h at room temperature. Upon completion of the treatment, the solid was isolated via centrifugation, washed with water and acetone, and dried in air. The structural stability of the treated samples was investigated by PXRD studies.
Oil/Water Separation
Static Crude Oil Removal
Two drops of crude oil (≈25 mg) were added to 100 mL of distilled water. Thereafter a piece of superhydrophobic UiO-66-Oleate@Cotton fabric (dimensions 4 × 4 cm2) was placed on the surface on each side, for a few minutes, until the floating oil was sorbed. The oil laden fabric composite was then treated twice with a 40 mL solution of n-hexane and dried in air for 30 min. The regenerated material was then reused for the following sorption cycle. The same process was conducted for a total of 10 cycles. The same experimental procedure was applied to the rest of hydrophobic/superhydrophobic PMMA-cotton fabric and PMMA-cotton fiber composites. The crude oil removal efficiency of UiO-66-Oleate-3-PMMA@Cotton was evaluated for two sorption/regeneration cycles.
Preparation of oil in water emulsions
The o/w emulsions of different concentrations were prepared by mixing the oil and water samples and ultrasonicating the resulting mixture for at least 30 min. The ultrasonication process was repeated, if necessary, until the oil particles were completely suspended.
Evaluation of the o/w emulsion separation efficiency of UiO-66-Oleate-5-PMMA@ Cotton fabric
A typical experiment is the following: A circular shaped UiO-66-Oleate-5-PMMA@Cotton fabric (1.5 cm diameter) was fixed onto the magnetic stirring using ordinary adhesive. Subsequently the fabric was placed in a glass vial containing 10 mL of oil emulsion and stirred at 300 rpm, at room temperature, for a certain time interval. When the demulsification process was completed, the fabric was retrieved and the demulsification efficiency was determined. The regeneration of the composite was accomplished by treatment with n-hexane and the fabric was dried in air for 30 min. Finally, the regenerated UiO-66-Oleate-5-PMMA@Cotton fabric was fixed again onto the magnetic stirring bar prior to the next sorption/regeneration cycle. The weight of the fabric composite remained identical following the demulsification process owning to the strong adhesion of the MOF-PMMA coating.
Estimation of the o/w emulsion separation efficiency
The % demulsification efficiency was estimated via UV–vis spectroscopy. A typical o/w emulsion spectrum displays an absorbance band peaking at 238 nm (Figure S93). Hence, the absorbance intensity at this specific wavelength was selected and the % separation efficiency was estimated by the difference of the absorbance intensities at 238 nm for the initial emulsion and the treated sample. Similar method has been previously reported in literature.75
Characterization Techniques
Powder X-ray diffraction measurements were performed on a Bruker D2-Phaser X-ray diffractometer (CuKa radiation source, wavelength = 1.54184 Å). High quality diffraction data, suitable to be used for Le Bail refinement, were obtained using a step of 0.01° and a scan rate of 1.8 s per 0.01° (overall measurement time was approximately 172 min). The Le Bail refinement was performed using TOPAS.761H NMR spectra were measured with a Bruker 500 MHz spectrometer. ATR-IR spectra were recorded in the range of 4000–400 cm–1 range using an Agilent Cary 630 ATR. Thermogravimetric analyses (TGA) were performed on a STA 449C Jupiter in air atmosphere with a heating rate of 10 °C min–1. N2 physisorption isotherms were measured at 77 K on a Quantachrome Novatouch LX2 sorption analyzer. Before analysis, UiO-66, MOR-1, MIL-53 and ZIF-8 samples were EtOH exchanged and degassed at 150 °C under vacuum (<10–5 Torr) for 12 h. Regarding the HKUST-1 materials, no solvent exchange was applied, and the degassing was performed at 120 °C under vacuum (<10–5 Torr) for 12 h. The specific surface areas were calculated by applying the Brumauer-Emmett-Teller (BET) method to the adsorption branch of isotherms in the 0.05–0.25 relative pressure (P/Po) range. CO2 physisorption measurements were performed on a Quantachrome NOVA 3200e sorption analyzer at 0 °C. Prior measurement, all samples were degassed (<10–5 Torr) at 120–150 °C for 12 h. The corresponding pore-size distribution plots were obtained by fitting the CO2 adsorption data of the isotherms to nonlocal density functional theory (NLDFT) model.77 Field Emission-Scanning Electron Microscopy (FE-SEM)/Energy Dispersive Spectroscopy (EDS) measurements were performed with a Phenom Pharos G2 Desktop FEG-SEM (Thermo Fisher Scientific) on Cr sputtered specimens (Q150T ES Plus automatic sputter coater, Quorum Technologies Ltd.), as well as with a JEOL JSM-6390LV scanning electron microscope (SEM) equipped with an Oxford INCA PentaFET-x3 energy dispersive X-ray spectroscopy (EDS) detector. XPS data were collected on a SPECS spectrometer using a Phoibos 100 1D-DLD electron analyzer and an A1 Kα radiation as the energy source (1486.6 eV). Binding energy values were corrected for charging by assigning a binding energy of 284.8 eV to the C 1s signal of adventitious carbon. Water contact angles (WCA) were initially determined from digital images obtained with the use of smartphone equipped with macro-lens, by using the drop shape analysis utility of the ImageJ software, particularly the Low-Bond Axisymmetric Drop Shape Analysis (LBADSA) method.78,79 The WCA values were further confirmed utilizing an Attension Theta Flex (Biolin Scientific) contact angle meter. Digital images of the magnetic liquid marbles were obtained using a KERN stereoscope equipped with an ODC 87/88 microscope camera. Zeta potential was measured on a suspension of the MOF in water (pH ≈ 7) using a Malvern Zetasizer Nano ZS (Malvern Panalytical, Worcestershire, UK) in a two-electrode capillary cell.
Acknowledgments
The work was implemented within the framework of the Action “Research and Innovation Synergies in the Attica Region” and was cofinanced by the European Regional Development Fund (ERDF) of the European Union and national resources through the E.P. Attica 2014-2020 (project code: ATTP4-0359579). We also thank the Thermal Analysis unit of the Network of Research Supporting Laboratories at the University of Ioannina.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.3c19536.
Miscellaneous Characterization data; Contact angle data in various aqueous media and pH solutions; Pore size distribution results; Characterization data for modified MOR-1, ZIF-8, MIL-53 and the hydrophobic magnetic composites; Calculation of the linker deficiencies in the UiO-66 MOF; Details of oil in water separation properties for the modified cotton fabrics and cotton fiber (PDF)
Video S1 showing the formation of magnetic liquid marbles (MP4)
Video S2 showing the movement of magnetic liquid marbles using an external magnet (MP4)
Video S3 showing the crude oil-in-water separation process (MP4)
Author Present Address
∥ Department of Chemistry, Northwestern University, Evanston, 60208, IL, United States (A.D.P.)
Author Present Address
Dimitrios A. Evangelou – Department of Chemistry, University of Ioannina, GR-45110 Ioannina, Greece
Author Present Address
Anastasia D. Pournara – Department of Chemistry, Northwestern University, Evanston, 60208, IL, United States
Author Present Address
Vasiliki I. Karagianni – Department of Chemistry, University of Ioannina, GR-45110 Ioannina, Greece
Author Present Address
Christos Dimitriou – Department of Physics, University of Ioannina, GR-45110 Ioannina, Greece
Author Present Address
Evangelos K. Andreou – Department of Materials Science and Technology, University of Crete, GR-70013 Heraklion, Greece
Author Present Address
Yiannis Deligiannakis – Department of Physics, University of Ioannina, GR-45110 Ioannina, Greece
Author Present Address
Gerasimos S. Armatas – Department of Materials Science and Technology, University of Crete, GR-70013 Heraklion, Greece
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Falde E. J.; Yohe S. T.; Colson Y. L.; Grinstaff M. W. Superhydrophobic Materials for Biomedical Applications. Biomaterials 2016, 104, 87–103. 10.1016/j.biomaterials.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.; Tai N. H. Carbon Materials as Oil Sorbents: A Review on the Synthesis and Performance. J. Mater. Chem. A 2016, 4 (5), 1550–1565. 10.1039/C5TA08321D. [DOI] [Google Scholar]
- Zhang P.; Lv F. Y. A Review of the Recent Advances in Superhydrophobic Surfaces and the Emerging Energy-Related Applications. Energy 2015, 82, 1068–1087. 10.1016/j.energy.2015.01.061. [DOI] [Google Scholar]
- Zimmermann J.; Reifler F. A.; Fortunato G.; Gerhardt L. C.; Seeger S. A Simple, One-Step Approach to Durable and Robust Superhydrophobic Textiles. Adv. Funct. Mater. 2008, 18 (22), 3662–3669. 10.1002/adfm.200800755. [DOI] [Google Scholar]
- Rius-Ayra O.; Biserova-Tahchieva A.; Llorca-Isern N. Removal of Dyes, Oils, Alcohols, Heavy Metals and Microplastics from Water with Superhydrophobic Materials. Chemosphere 2023, 311, 137148 10.1016/j.chemosphere.2022.137148. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lin Z.; Luo Y.; Wu R.; Fang R.; Umar A.; Zhang Z.; Zhao Z.; Yao J.; Zhao S. Superhydrophobic MOF Based Materials and Their Applications for Oil-Water Separation. J. Clean. Prod. 2023, 420, 138347 10.1016/j.jclepro.2023.138347. [DOI] [Google Scholar]
- Hou Y.; Wang Z.; Guo J.; Shen H.; Zhang H.; Zhao N.; Zhao Y.; Chen L.; Liang S.; Jin Y.; Xu J. Facile Fabrication of Robust Superhydrophobic Porous Materials and Their Application in Oil/Water Separation. J. Mater. Chem. A 2015, 3 (46), 23252–23260. 10.1039/C5TA05612H. [DOI] [Google Scholar]
- Levkin P. A.; Svec F.; Fréchet J. M. J. Porous Polymer Coatings: A Versatile Approach to Superhydrophobic Surfaces. Adv. Funct. Mater. 2009, 19 (12), 1993–1998. 10.1002/adfm.200801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam P. Z.; Ivy J. F.; Arvapally R. K.; Dos Santos A. M.; Pearson J. C.; Zhang L.; Tylianakis E.; Ghosh P.; Oswald I. W. H.; Kaipa U.; Wang X.; Wilson A. K.; Snurr R. Q.; Omary M. A. Adsorption and Molecular Siting of CO2, Water, and Other Gases in the Superhydrophobic, Flexible Pores of FMOF-1 from Experiment and Simulation. Chem. Sci. 2017, 8 (5), 3989–4000. 10.1039/C7SC00278E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin G.; Serre C.; Cooper A.; Férey G. The New Age of MOFs and of Their Porous-Related Solids. Chem. Soc. Rev. 2017, 46 (11), 3104–3107. 10.1039/C7CS90049J. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Ma K.; Mahle J. J.; Wang H.; Syed Z. H.; Atilgan A.; Chen Y.; Xin J. H.; Islamoglu T.; Peterson G. W.; Farha O. K. Integration of Metal-Organic Frameworks on Protective Layers for Destruction of Nerve Agents under Relevant Conditions. J. Am. Chem. Soc. 2019, 141 (51), 20016–20021. 10.1021/jacs.9b11172. [DOI] [PubMed] [Google Scholar]
- Alezi D.; Belmabkhout Y.; Suyetin M.; Bhatt P. M.; Weseliński L. J.; Solovyeva V.; Adil K.; Spanopoulos I.; Trikalitis P. N.; Emwas A. H.; Eddaoudi M. MOF Crystal Chemistry Paving the Way to Gas Storage Needs: Aluminum-Based Soc-MOF for CH4, O2, and CO2 Storage. J. Am. Chem. Soc. 2015, 137 (41), 13308–13318. 10.1021/jacs.5b07053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341 (6149), 1230444 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Schneemann A.; Bon V.; Schwedler I.; Senkovska I.; Kaskel S.; Fischer R. A. Flexible Metal-Organic Frameworks. Chem. Soc. Rev. 2014, 43 (16), 6062–6096. 10.1039/C4CS00101J. [DOI] [PubMed] [Google Scholar]
- Eddaoudi M.; Li H.; Yaghi O. M. Highly Porous and Stable Metal-Organic Frameworks: Structure Design and Sorption Properties. J. Am. Chem. Soc. 2000, 122 (7), 1391–1397. 10.1021/ja9933386. [DOI] [Google Scholar]
- Kumar P.; Pournara A.; Kim K. H.; Bansal V.; Rapti S.; Manos M. J. Metal-Organic Frameworks: Challenges and Opportunities for Ion-Exchange/Sorption Applications. Prog. Mater. Sci. 2017, 86, 25–74. 10.1016/j.pmatsci.2017.01.002. [DOI] [Google Scholar]
- Zhang Z.; Zhao Y.; Gong Q.; Li Z.; Li J. MOFs for CO2 Capture and Separation from Flue Gas Mixtures: The Effect of Multifunctional Sites on Their Adsorption Capacity and Selectivity. Chem. Commun. 2013, 49 (7), 653–661. 10.1039/C2CC35561B. [DOI] [PubMed] [Google Scholar]
- Rosseinsky M. J. Recent Developments in Metal-Organic Framework Chemistry: Design, Discovery, Permanent Porosity and Flexibility. Microporous Mesoporous Mater. 2004, 73 (1–2), 15–30. 10.1016/j.micromeso.2003.05.001. [DOI] [Google Scholar]
- Kitagawa S.; Kitaura R.; Noro S. I. Functional Porous Coordination Polymers. Angew. Chem., Int. Ed. 2004, 43 (18), 2334–2375. 10.1002/anie.200300610. [DOI] [PubMed] [Google Scholar]
- Shearer G. C.; Chavan S.; Bordiga S.; Svelle S.; Olsbye U.; Lillerud K. P. Defect Engineering: Tuning the Porosity and Composition of the Metal-Organic Framework UiO-66 via Modulated Synthesis. Chem. Mater. 2016, 28 (11), 3749–3761. 10.1021/acs.chemmater.6b00602. [DOI] [Google Scholar]
- Lu W.; Wei Z.; Gu Z. Y.; Liu T. F.; Park J.; Park J.; Tian J.; Zhang M.; Zhang Q.; Gentle T.; Bosch M.; Zhou H. C. Tuning the Structure and Function of Metal-Organic Frameworks via Linker Design. Chem. Soc. Rev. 2014, 43 (16), 5561–5593. 10.1039/C4CS00003J. [DOI] [PubMed] [Google Scholar]
- Shi M.; Huang R.; Qi W.; Su R.; He Z. Synthesis of Superhydrophobic and High Stable Zr-MOFs for Oil-Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125102 10.1016/j.colsurfa.2020.125102. [DOI] [Google Scholar]
- Nguyen J. G.; Cohen S. M. Moisture-Resistant and Superhydrophobic Metal-Organic Frameworks Obtained via Postsynthetic Modification. J. Am. Chem. Soc. 2010, 132 (13), 4560–4561. 10.1021/ja100900c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournara A. D.; Rizogianni S.; Evangelou D. A.; Andreou E. K.; Armatas G. S.; Manos M. J. Zr4+-Terephthalate MOFs with 6-Connected Structures, Highly Efficient As(III/V) Sorption and Superhydrophobic Properties. Chem. Commun. 2022, 58 (63), 8862–8865. 10.1039/D2CC03090J. [DOI] [PubMed] [Google Scholar]
- Rao K. P.; Higuchi M.; Sumida K.; Furukawa S.; Duan J.; Kitagawa S. Design of Superhydrophobic Porous Coordination Polymers through the Introduction of External Surface Corrugation by the Use of an Aromatic Hydrocarbon Building Unit. Angew. Chem., Int. Ed. 2014, 53 (31), 8225–8230. 10.1002/anie.201404306. [DOI] [PubMed] [Google Scholar]
- Wang S.; Morris W.; Liu Y.; McGuirk C. M.; Zhou Y.; Hupp J. T.; Farha O. K.; Mirkin C. A. Surface-Specific Functionalization of Nanoscale Metal–Organic Frameworks. Angew. Chem., Int. Ed. 2015, 54 (49), 14738–14742. 10.1002/anie.201506888. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Sun Q.; Huang H.; Aguila B.; Niu Z.; Perman J. A.; Ma S. A Molecular-Level Superhydrophobic External Surface to Improve the Stability of Metal-Organic Frameworks. J. Mater. Chem. A 2017, 5 (35), 18770–18776. 10.1039/C7TA05800D. [DOI] [Google Scholar]
- Cohen S. M. Postsynthetic Methods for the Functionalization of Metal-Organic Frameworks. Chem. Rev. 2012, 112 (2), 970–1000. 10.1021/cr200179u. [DOI] [PubMed] [Google Scholar]
- Du J.; Zhang C.; Pu H.; Li Y.; Jin S.; Tan L.; Zhou C.; Dong L. HKUST-1 MOFs Decorated 3D Copper Foam with Superhydrophobicity/Superoleophilicity for Durable Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 222–229. 10.1016/j.colsurfa.2019.04.064. [DOI] [Google Scholar]
- Feng L.; Lo S. H.; Tan K.; Li B. H.; Yuan S.; Lin Y. F.; Lin C. H.; Wang S. L.; Lu K. L.; Zhou H. C. An Encapsulation-Rearrangement Strategy to Integrate Superhydrophobicity into Mesoporous Metal-Organic Frameworks. Matter 2020, 2 (4), 988–999. 10.1016/j.matt.2020.01.015. [DOI] [Google Scholar]
- Gao M. L.; Zhao S. Y.; Chen Z. Y.; Liu L.; Han Z. B. Superhydrophobic/Superoleophilic MOF Composites for Oil-Water Separation. Inorg. Chem. 2019, 58 (4), 2261–2264. 10.1021/acs.inorgchem.8b03293. [DOI] [PubMed] [Google Scholar]
- Du J.; Chen L.; Zhou C.; Zhou W.; Shen H.; Zeng X.; Zhou P.; Tan L.; Dong L. Stable Zr-UiO-67 Constructed through Polymeric Network Assisted Post-Synthetic Modification and Its Wettability Modulation. Chem. Commun. 2021, 57 (84), 11021–11024. 10.1039/D1CC04063D. [DOI] [PubMed] [Google Scholar]
- Liu C.; Liu Q.; Huang A. A Superhydrophobic Zeolitic Imidazolate Framework (ZIF-90) with High Steam Stability for Efficient Recovery of Bioalcohols. Chem. Commun. 2016, 52 (16), 3400–3402. 10.1039/C5CC10171A. [DOI] [PubMed] [Google Scholar]
- Yang S.; Peng L.; Sun D. T.; Asgari M.; Oveisi E.; Trukhina O.; Bulut S.; Jamali A.; Queen W. L. A New Post-Synthetic Polymerization Strategy Makes Metal-Organic Frameworks More Stable. Chem. Sci. 2019, 10 (17), 4542–4549. 10.1039/C9SC00135B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q.; He H.; Gao W. Y.; Aguila B.; Wojtas L.; Dai Z.; Li J.; Chen Y. S.; Xiao F. S.; Ma S. Imparting Amphiphobicity on Single-Crystalline Porous Materials. Nat. Commun. 2016, 7, 13300. 10.1038/ncomms13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S.; Sharma S.; Ghosh S. K. Hydrophobic Metal-Organic Frameworks: Potential toward Emerging Applications. APL Mater. 2019, 7 (5), 050701 10.1063/1.5091783. [DOI] [Google Scholar]
- Jayaramulu K.; Geyer F.; Schneemann A.; Kment Š.; Otyepka M.; Zboril R.; Vollmer D.; Fischer R. A. Hydrophobic Metal–Organic Frameworks. Adv. Mater. 2019, 31 (32), 1900820 10.1002/adma.201900820. [DOI] [PubMed] [Google Scholar]
- Rosen M. J.; Kunjappu J. T.. Surfactants and Interfacial Phenomena; John Wiley & Sons, 2001. [Google Scholar]
- Hiasa Y.; Konishi N.; Kitahori Y.; Shimoyama T. Carcinogenicity Study of a Commercial Sodium oleate in Fischer rats. Food Chem. Toxicol. 1985, 23 (6), 619–623. 10.1016/0278-6915(85)90189-9. [DOI] [PubMed] [Google Scholar]
- Kadono T.; Uezu K.; Kosaka T.; Kawano T. Altered Toxicities of Fatty Acid Salts in Green Paramecia Cultured in Different Waters. Z. Naturforsch., C, J. Biosci. 2006, 61 (7–8), 541–547. 10.1515/znc-2006-7-812. [DOI] [PubMed] [Google Scholar]
- Bronstein L. M.; Huang X.; Retrum J.; Schmucker A.; Pink M.; Stein B. D.; Dragnea B. Influence of Iron Oleate Complex Structure on Iron Oxide Nanoparticle Formation. Chem. Mater. 2007, 19 (15), 3624–3632. 10.1021/cm062948j. [DOI] [Google Scholar]
- Wagner M.; Pigliapochi R.; Di Tullio V.; Catalano J.; Zumbulyadis N.; Centeno S. A.; Wang X.; Chen K.; Hung I.; Gan Z.; Dworzak M. R.; Yap G. P. A.; Dybowski C. Multi-Technique Structural Analysis of Zinc Carboxylates (Soaps). Dalton Trans. 2023, 52 (18), 6152–6165. 10.1039/D3DT00184A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkari A.; Wen X.; Orrego-Hernández J.; Da Silva R. R.; Kondo S.; Olsson E.; Härelind H.; Moth-Poulsen K. Synthesis of Highly Monodisperse Pd Nanoparticles Using a Binary Surfactant Combination and Sodium Oleate as a Reductant. Nanoscale Adv. 2021, 3 (9), 2481–2487. 10.1039/D1NA00052G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. H.; Lee D. K.; Jo B. G.; Jeong J. H.; Kang Y. S. Synthesis of Oleate Capped Cu Nanoparticles by Thermal Decomposition. Colloids Surf. A Physicochem. Eng. Asp. 2006, 284–285, 364–368. 10.1016/j.colsurfa.2005.10.067. [DOI] [Google Scholar]
- Wen X.; Lerch S.; Wang Z.; Aboudiab B.; Tehrani-Bagha A. R.; Olsson E.; Moth-Poulsen K. Synthesis of Palladium Nanodendrites Using a Mixture of Cationic and Anionic Surfactants. Langmuir 2020, 36 (7), 1745–1753. 10.1021/acs.langmuir.9b03804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T.; Schmidt J. R. Structure and Thermodynamic Stability of Zeolitic Imidazolate Framework Surfaces. J. Phys. Chem. C 2020, 124 (2), 1458–1468. 10.1021/acs.jpcc.9b10124. [DOI] [Google Scholar]
- Tian F.; Cerro A. M.; Mosier A. M.; Wayment-Steele H. K.; Shine R. S.; Park A.; Webster E. R.; Johnson L. E.; Johal M. S.; Benz L. Surface and Stability Characterization of a Nanoporous ZIF-8 Thin Film. J. Phys. Chem. C 2014, 118 (26), 14449–14456. 10.1021/jp5041053. [DOI] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130 (42), 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- Rapti S.; Pournara A.; Sarma D.; Papadas I. T.; Armatas G. S.; Tsipis A. C.; Lazarides T.; Kanatzidis M. G.; Manos M. J. Selective Capture of Hexavalent Chromium from an Anion-Exchange Column of Metal Organic Resin-Alginic Acid Composite. Chem. Sci. 2016, 7 (3), 2427–2436. 10.1039/C5SC03732H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. S.; Ni Z.; Côte A. P.; Choi J. Y.; Huang R.; Uribe-Romo F. J.; Chae H. K.; O’Keeffe M.; Yaghi O. M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. U.S.A. 2006, 103 (27), 10186–10191. 10.1073/pnas.0602439103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui S. S. Y.; Lo S. M. F.; Charmant J. P. H.; Orpen A. G.; Williams I. D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283 (5405), 1148–1150. 10.1126/science.283.5405.1148. [DOI] [PubMed] [Google Scholar]
- Loiseau T.; Serre C.; Huguenard C.; Fink G.; Taulelle F.; Henry M.; Bataille T.; Férey G. A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. Chem.—Eur. J. 2004, 10 (6), 1373–1382. 10.1002/chem.200305413. [DOI] [PubMed] [Google Scholar]
- Hon Lau C.; Babarao R.; Hill M. R. A Route to Drastic Increase of CO2 Uptake in Zr Metal Organic Framework UiO-66. Chem. Commun. 2013, 49 (35), 3634–3636. 10.1039/c3cc40470f. [DOI] [PubMed] [Google Scholar]
- Han Y.; Liu M.; Li K.; Zuo Y.; Wei Y.; Xu S.; Zhang G.; Song C.; Zhang Z.; Guo X. Facile Synthesis of Morphology and Size-Controlled Zirconium Metal-Organic Framework UiO-66: The Role of Hydrofluoric Acid in Crystallization. CrystEngComm 2015, 17 (33), 6434–6440. 10.1039/C5CE00729A. [DOI] [Google Scholar]
- Yang W.; Yu T.; Sun L.; Liu Q.; Fei Z.; Chen X.; Zhang Z.; Tang J.; Cui M.; Qiao X. Pore-Expanded UiO-66 Pellets for Efficient Bisphenol A Adsorption. Chem. Eng. J. 2023, 455, 140843 10.1016/j.cej.2022.140843. [DOI] [Google Scholar]
- Wang G.; Sharp C.; Plonka A. M.; Wang Q.; Frenkel A. I.; Guo W.; Hill C.; Smith C.; Kollar J.; Troya D.; Morris J. R. Mechanism and Kinetics for Reaction of the Chemical Warfare Agent Simulant, DMMP(g), with Zirconium(IV) MOFs: An Ultrahigh-Vacuum and DFT Study. J. Phys. Chem. C 2017, 121 (21), 11261–11272. 10.1021/acs.jpcc.7b00070. [DOI] [Google Scholar]
- Álvarez J. R.; Sánchez-González E.; Pérez E.; Schneider-Revueltas E.; Martínez A.; Tejeda-Cruz A.; Islas-Jácome A.; González-Zamora E.; Ibarra I. A. Structure Stability of HKUST-1 towards Water and Ethanol and Their Effect on Its CO2 Capture Properties. Dalton Trans. 2017, 46 (28), 9192–9200. 10.1039/C7DT01845B. [DOI] [PubMed] [Google Scholar]
- Capello C.; Fischer U.; Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9 (9), 927–934. 10.1039/b617536h. [DOI] [Google Scholar]
- Cortés-Súarez J.; Celis-Arias V.; Beltrán H. I.; Tejeda-Cruz A.; Ibarra I. A.; Romero-Ibarra J. E.; Sánchez-González E.; Loera-Serna S. Synthesis and Characterization of an SWCNT@HKUST-1 Composite: Enhancing the CO2 Adsorption Properties of HKUST-1. ACS Omega 2019, 4 (3), 5275–5282. 10.1021/acsomega.9b00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng D.; Yuan L.; Zhang P.; Wang L.; Li Z.; Wang Y.; Liu Y.; Shi W. Hydrolytically Stable Foamed HKUST-1@CMC Composites Realize High-Efficient Separation of U(VI). iScience 2021, 24 (9), 102982 10.1016/j.isci.2021.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale G.; Newton M. I. Liquid Marbles: Principles and Applications. Soft Matter 2011, 7 (12), 5473–5481. 10.1039/c1sm05066d. [DOI] [Google Scholar]
- Bormashenko E. Liquid Marbles: Properties and Applications. Curr. Opin. Colloid Interface Sci. 2011, 16 (4), 266–271. 10.1016/j.cocis.2010.12.002. [DOI] [Google Scholar]
- Alp G.; Alp E.; Aydogan N. Magnetic Liquid Marbles to Facilitate Rapid Manipulation of the Oil Phase: Synergistic Effect of Semifluorinated Ligand and Catanionic Surfactant Mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124051 10.1016/j.colsurfa.2019.124051. [DOI] [Google Scholar]
- Draper T. C.; Fullarton C.; Phillips N.; de Lacy Costello B. P. J.; Adamatzky A. Liquid Marble Actuator for Microfluidic Logic Systems. Sci. Rep. 2018, 8 (1), 14153. 10.1038/s41598-018-32540-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld M. J.; Harding J. L.; Reynolds M. M. Immobilization of Metal-Organic Framework Copper(II) Benzene-1,3,5-Tricarboxylate (CuBTC) onto Cotton Fabric as a Nitric Oxide Release Catalyst. ACS Appl. Mater. Interfaces 2015, 7 (48), 26742–26750. 10.1021/acsami.5b08773. [DOI] [PubMed] [Google Scholar]
- Lee H.; Dellatore S. M.; Miller W. M.; Messersmith P. B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318 (5849), 426–430. 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z.; Feng Y.; Seeger S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chem., Int. Ed. 2015, 54 (8), 2328–2338. 10.1002/anie.201405785. [DOI] [PubMed] [Google Scholar]
- Gupta R. K.; Dunderdale G. J.; England M. W.; Hozumi A. Oil/Water Separation Techniques: A Review of Recent Progresses and Future Directions. J. Mater. Chem. A 2017, 5 (31), 16025–16058. 10.1039/C7TA02070H. [DOI] [Google Scholar]
- Obaid M.; Alsadun N.; Shekhah O.; Almahfoodh S.; Zhou S.; Ghaffour N.; Eddaoudi M. Deployment of Superhydrophilic and Super-Antifouling Cr-Soc-MOF-1-Based Membrane for Ultrafast Separation of Stabilized Oil-in-Water Emulsions. ACS Appl. Mater. Interfaces 2023, 15 (25), 31067–31076. 10.1021/acsami.3c05285. [DOI] [PubMed] [Google Scholar]
- Katz M. J.; Brown Z. J.; Colón Y. J.; Siu P. W.; Scheidt K. A.; Snurr R. Q.; Hupp J. T.; Farha O. K. A Facile Synthesis of UiO-66, UiO-67 and Their Derivatives. Chem. Commun. 2013, 49 (82), 9449–9451. 10.1039/c3cc46105j. [DOI] [PubMed] [Google Scholar]
- Rapti S.; Pournara A.; Sarma D.; Papadas I. T.; Armatas G. S.; Hassan Y. S.; Alkordi M. H.; Kanatzidis M. G.; Manos M. J. Rapid, Green and Inexpensive Synthesis of High Quality UiO-66 Amino-Functionalized Materials with Exceptional Capability for Removal of Hexavalent Chromium from Industrial Waste. Inorg. Chem. Front. 2016, 3 (5), 635–644. 10.1039/C5QI00303B. [DOI] [Google Scholar]
- Li J.; Wu Y. N.; Li Z.; Zhu M.; Li F. Characteristics of Arsenate Removal from Water by Metal-Organic Frameworks (MOFs). Water Sci. Technol. 2014, 70 (8), 1391–1397. 10.2166/wst.2014.390. [DOI] [PubMed] [Google Scholar]
- Cravillon J.; Münzer S.; Lohmeier S. J.; Feldhoff A.; Huber K.; Wiebcke M. Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework. Chem. Mater. 2009, 21 (8), 1410–1412. 10.1021/cm900166h. [DOI] [Google Scholar]
- Peng Y.; Krungleviciute V.; Eryazici I.; Hupp J. T.; Farha O. K.; Yildirim T. Methane Storage in Metal-Organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 2013, 135 (32), 11887–11894. 10.1021/ja4045289. [DOI] [PubMed] [Google Scholar]
- Khorshid Z. B.; Doroodmand M. M.; Abdollahi S. UV-Vis. Spectrophotometric Method for Oil and Grease Determination in Water, Soil and Different Mediates Based on Emulsion. Microchem. J. 2021, 160, 105620 10.1016/j.microc.2020.105620. [DOI] [Google Scholar]
- Coelho A. A. TOPAS and TOPAS-Academic: An Optimization Program Integrating Computer Algebra and Crystallographic Objects Written in C++. J. Appl. Crystallogr. 2018, 51 (1), 210–218. 10.1107/S1600576718000183. [DOI] [Google Scholar]
- Ravikovitch P. I.; Wei D.; Chueh W. T.; Haller G. L.; Neimark A. V. Evaluation of Pore Structure Parameters of MCM-41 Catalyst Supports and Catalysts by Means of Nitrogen and Argon Adsorption. J. Phys. Chem. B 1997, 101 (19), 3671–3679. 10.1021/jp9625321. [DOI] [Google Scholar]
- Stalder A. F.; Kulik G.; Sage D.; Barbieri L.; Hoffmann P. A Snake-Based Approach to Accurate Determination of Both Contact Points and Contact Angles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 286 (1–3), 92–103. 10.1016/j.colsurfa.2006.03.008. [DOI] [Google Scholar]
- Stalder A. F.; Melchior T.; Müller M.; Sage D.; Blu T.; Unser M. Low-Bond Axisymmetric Drop Shape Analysis for Surface Tension and Contact Angle Measurements of Sessile Drops. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364 (1–3), 72–81. 10.1016/j.colsurfa.2010.04.040. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








