Quantitative sensory testing in 3 different body areas implied a potential involvement of spinal sensitization in patients with chronic low back pain.
Keywords: Quantitative sensory testing, Chronic pain, Central sensitization, Secondary hyperalgesia
Abstract
Introduction:
In 85% of patients with chronic low back pain (CLBP), no specific pathoanatomical cause can be identified. Besides primary peripheral drivers within the lower back, spinal or supraspinal sensitization processes might contribute to the patients' pain.
Objectives:
The present study conceptualized the most painful area (MP) of patients with nonspecific CLBP as primarily affected area and assessed signs of peripheral, spinal, and supraspinal sensitization using quantitative sensory testing (QST) in MP, a pain-free area adjacent to MP (AD), and a remote, pain-free control area (CON).
Methods:
Fifty-nine patients with CLBP (51 years, SD = 16.6, 22 female patients) and 35 pain-free control participants individually matched for age, sex, and testing areas (49 years, SD = 17.5, 19 female participants) underwent a full QST protocol in MP and a reduced QST protocol assessing sensory gain in AD and CON. Quantitative sensory testing measures, except paradoxical heat sensations and dynamic mechanical allodynia (DMA), were Z-transformed to the matched control participants and tested for significance using Z-tests (α = 0.001). Paradoxical heat sensations and DMA occurrence were compared between cohorts using Fisher's exact tests (α = 0.05). The same analyses were performed with a high-pain and a low-pain CLBP subsample (50% quantile).
Results:
Patients showed cold and vibration hypoesthesia in MP (all Ps < 0.001) and mechanical hyperalgesia (P < 0.001) and more frequent DMA (P = 0.044) in AD. The results were mainly driven by the high-pain CLBP subsample. In CON, no sensory alterations were observed.
Conclusion:
Mechanical hyperalgesia and DMA adjacent to but not within MP, the supposedly primarily affected area, might reflect secondary hyperalgesia originating from spinal sensitization in patients with CLBP.
1. Introduction
Chronic low back pain (CLBP) is a particularly challenging chronic pain condition because in approximately 85% of cases, no specific pathoanatomical cause can be identified,35 which hinders mechanism-based treatment approaches. Various lumbar structures such as facet joints57 or intervertebral disks29 might contribute to CLBP, as well as central processes, ie, central sensitization.3,22,49 Different definitions of central sensitization exist,13 including the originally described activity-dependent central sensitization at the spinal dorsal horn neuron65 and the broader definition of the International Association for the Study of Pain, that is “increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input.”28 Although it is not possible to directly measure neuronal hyperexcitability, and thus central sensitization, in humans, certain sensory signs serve as proxies.3 Quantitative sensory testing (QST)44 allows the standardized assessment of such sensory signs, for example, allodynia, hyperalgesia, or increased temporal summation of pain.3 Of note, to infer a central—and not peripheral—origin of the respective sensory signs, QST has to be performed in more than one body area.3 For instance, dynamic mechanical allodynia (DMA) and mechanical hyperalgesia are hallmarks of spinal sensitization as demonstrated in experimentally induced activity-dependent central sensitization,31,48,56 but only if present in a secondary area, ie, a region surrounding the primarily affected area. If detected in the primarily affected area, DMA and mechanical hyperalgesia could be due to peripheral or spinal sensitization.3 In addition, supraspinal sensitization may contribute to DMA, mechanical hyperalgesia, and other signs of pain hypersensitivity in any body area given that alterations in supraspinal neuronal circuits can have widespread effects, for example in the case of dysfunctional descending pain inhibition.5 One option to differentiate supraspinal from spinal or peripheral sensitization is to assess pain hypersensitivity at body areas remote from the primarily affected or the secondary area.3
The present study aimed to apply these concepts in patients with nonspecific CLBP and pain-free control participants. The most painful area (MP) within the lower back of patients with CLBP was considered a proxy for the primarily affected area. A pain-free area adjacent to MP (AD) was conceptualized as secondary area surrounding the primarily affected area. The pain-free nondominant hand served as remote, pain-free control area (CON). In these 3 body areas, sensory alterations were examined using the QST battery provided by the German Research Network on Neuropathic Pain (DFNS)44 to infer a putative presence of peripheral, spinal, and supraspinal sensitization in the patient cohort. In addition, it was explored whether QST measures indicative of spinal or supraspinal sensitization were related to patient-reported outcome measures supposedly associated with central pain processes, namely pain catastrophizing,7,54,59 sleep quality,7 and widespread pain.42
2. Methods
2.1. Participants
Patients with nonspecific CLBP and individually age- and sex-matched pain-free control participants between 18 and 80 years of age were consecutively recruited through the Balgrist University Hospital, online advertisement, and oral communication. Patients with CLBP needed to present with CLBP as primary pain complaint without signs of serious underlying pathology (eg, infection, fractures, or inflammation) or radiculopathy (ie, motor and sensory deficits) and of a duration longer than 3 months. Control participants could not have experienced low back pain lasting longer than 3 consecutive days during the last year. Exclusion criteria comprised any self-reported major medical (eg, severe heart disease) or psychiatric (eg, major depressive disorder) condition other than CLBP, pregnancy, or inability to follow study instructions. Ethical approval was obtained from the local ethics committee “Kantonale Ethikkommission Zürich” (Nr.: PB_2019-00136, PB_2016-02051, and EK-04/2006). The study was registered on ClinicalTrials.gov (NCT04433299 and NCT02138344) and performed in accordance with the Declaration of Helsinki (2013). All participants provided written informed consent before the start of the experiment.
2.2. Quantitative sensory testing
The QST battery was part of a larger study protocol (Clinical Research Priority Program “Pain”, https://www.crpp-pain.uzh.ch/en.html) that comprised 3 sessions of approximately 3 hours and electronic questionnaires, including the Hospital Anxiety and Depression Scale (HADS; anxiety and depression subscales, each scored from 0 to 21, with higher scores meaning greater anxiety or depression),66 the Pain Catastrophizing Scale (PCS; scored from 0 to 52, with higher scores meaning more pronounced pain catastrophizing),52 the Pain and Sleep Questionnaire 3-item index (PSQ-3; scored from 0 to 300, with higher scores meaning more severe pain-related sleep disturbances),4 the painDETECT (scored from 0 to 38, with higher scores indicating a more likely neuropathic pain component),17 and the Widespread Pain Index (WPI; scored from 0 to 19, with higher scores indicating a larger number of painful body regions).64 The electronic questionnaires also included a question about regular pain-relevant medication intake that was classified into M01A (anti-inflammatory and anti-rheumatic drugs and nonsteroids), N02 (analgesics), N03 (antiepileptics), N05 (psycholeptics), and N06 (psychoanaleptics) based on the ATC/DDD classification by the World Health Organization (http://www.whocc.no/atc_ddd_index/). Quantitative sensory testing was performed in the first session after a clinical examination (for details see section 2.3) and a neurophysiological assessment.
During the clinical examination, the location of the patients' MP within the lower back was identified. In addition, the patients were asked to indicate the area closest to MP (rostrally), which they perceived as pain-free (AD). If the patients' MP was lateralized, AD was assessed at the contralateral body side to avoid potential confounding of QST measures by tension in the erector spinae muscle. If the patients' MP was in the midline of the back, the body side to assess AD was randomly chosen. The nondominant hand served as the remote, pain-free control area (CON) (Fig. 1). For CON, normal sensory integrity was tested before the QST session for all participants by bedside sensory testing of vibration, thermal, pinprick, and light touch sensation. Control participants were assessed at the identical testing sites as the patient with CLBP they had been matched to.
Figure 1.
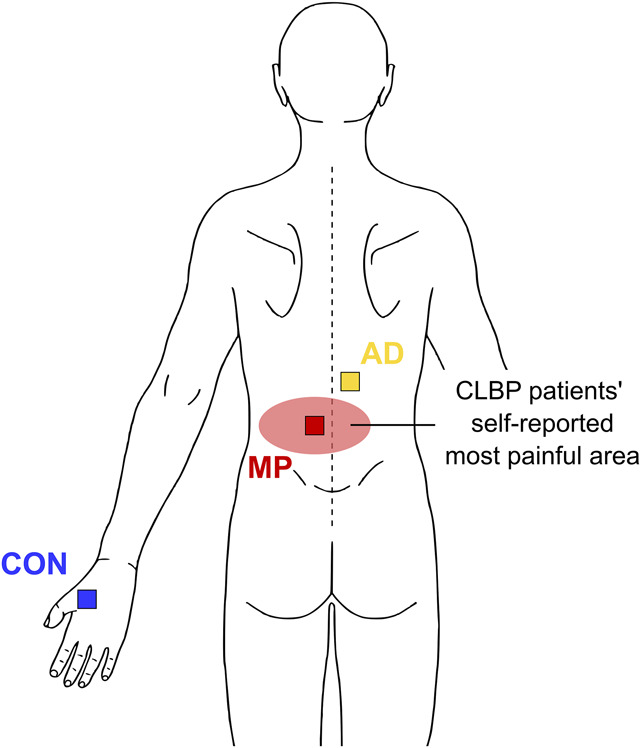
Schematic illustration of QST testing areas. For the most painful area (MP), superficial QST measures were assessed in the centre of MP, PPT was assessed over the erector spinae muscle at the segmental level of MP and VDT was assessed over the processus spinosus at the segmental level of MP. For the pain-free area adjacent to MP (AD), all QST measures were assessed over the erector spinae muscle (contralaterally to MP if MP was lateralized). For the remote, pain-free control area (CON), the standard DFNS locations were used, ie, dorsum of the hand for superficial QST measures and the thenar eminence for PPT. DFNS, German Research Network on Neuropathic Pain; PPT, pressure pain thresholds; QST, quantitative sensory testing; VDT, vibration detection thresholds.
In MP, the full DFNS QST protocol44 was performed to assess sensory loss and gain of function. In AD and CON, a reduced QST protocol focusing on the sensory gain of interest was performed. The reduced protocol comprised the following: cold pain thresholds (CPT), heat pain thresholds (HPT), mechanical pain thresholds (MPT), mechanical pain sensitivity (MPS), DMA, wind-up ratio (WUR), and pressure pain thresholds (PPT). Because of time constraints within the larger study protocol, MPS and DMA were evaluated with 2 (instead of 5) stimulus-response-function blocks. Pilot measurements in our laboratory showed that MPS and DMA values depend on the number of included blocks, and therefore, MPS and DMA were calculated based on the first 2 stimulus-response-function blocks also in MP to ensure comparability across all areas. Based on DFNS recommendations, CON was always assessed first. Given that the applied QST protocol deviated from the full DFNS QST protocol, a comparison of the CLBP patients' QST measures to the DFNS reference database would not have been valid. Therefore, the control participants who underwent the identical QST protocol as the patients with CLBP were used as reference group.
All QST measures except paradoxical heat sensations (PHS) and DMA were Z-transformed to the control participants. For that, control participants were divided into age-based tertiles and values of patients with CLBP were Z-transformed to the control participant tertile of their age. This allowed for calculation of Z-scores referenced to identical body areas, which would not have been possible with the DFNS reference database. To avoid the influence of single extreme outliers on the analysis, the maximum and minimum Z-score value was set to ±4.11
Quantitative sensory testing changes in patients with CLBP were tested by comparing the Z-values of patients with CLBP to an assumed ideal healthy population (ie, an ideal Z-value distribution with mean = 0 and SD = 1) using Z-tests with a conservative α = 0.001 to reduce the risk for false-positive results.53 Fisher's exact tests with α = 0.05 were used to compare the presence or absence of PHS and DMA between the cohorts. False discovery rate (FDR) multiple comparison correction for 3 tests was performed for those QST measures that were assessed on all 3 body areas. As an additional exploratory analysis, the same approach was performed with the patients with CLBP divided into a high-pain and a low-pain subsample (based on 50% quantile).
Associations between QST measures indicative of spinal (mechanical sensory gain of function in AD, ie, MPT, PPT, MPS, and WUR) or supraspinal sensitization (thermal and mechanical sensory gain of function in CON, ie, CPT, HPT, MPT, PPT, MPS, and WUR) and patient-reported outcome measures supposedly associated with central pain processes (ie, PCS, PSQ-3, and WPI) were investigated using Spearman correlations. Because of the exploratory nature of these correlation analyses, no multiple comparison correction was performed.
2.3. Clinical examination
The clinical examination was based on an evidence-based diagnostic classification system for low back pain60,61 and comprised diagnostic tests to investigate the most likely underlying nociceptive source of the patients' nonspecific CLBP, for example, provocation tests for discogenic/facetogenic/sacroiliac (ie, nociceptive) or radicular (ie, neuropathic) pain. Together with the painDETECT and the WPI, this information was used to characterize the patient cohort in potential underlying nociceptive, neuropathic, or nociplastic pain mechanisms.51 Based on the Delphi consensus study, the following features were considered to be indicative of nociceptive pain mechanisms: clear discogenic/facetogenic/sacroiliac symptom provocation pattern, painDETECT scores ≤12, and localized pain extent. Localized pain extent was inferred if patients with CLBP did not meet the criteria of widespread (contralateral limb) pain assessed using the WPI.23,64
3. Results
3.1. Participants
From the recruited 64 patients with CLBP and 48 control participants, 8 participants were excluded because of discontinuation (3 patients), abnormal sensory findings (2 control participants), suspected neurological (1 patient) or psychiatric (1 patient) conditions, or development of low back pain between the time of inclusion and the experimental session (1 control participant). Thus, the final sample comprised 59 patients with CLBP and 45 control participants. Participant characteristics are described in Table 1. Ten of the 45 control participants had been matched to another chronic pain cohort of the Clinical Research Priority Program “Pain” for MP and AD and were therefore only included in analyses related to CON.
Table 1.
Participant characteristics.
| Patients with CLBP (n = 59) | Control participants (n = 45) | Test statistic | P | Effect size | |
|---|---|---|---|---|---|
| Age [y] | 50.8 (16.64) | 48.1 (16.85) | t = 0.8 | 0.412 | d = 0.16 |
| Sex (female:male) [n] | 37:22 | 26:19 | 0.687* | ||
| BMI [kg/m2] | 23.9 (3.47) | 23.4 (2.87) | t = 0.8 | 0.422 | d = 0.16 |
| HADS anxiety | 4 (2.5–7.0) | 3 (2.0–5.0) | W = 789.0 | 0.036 | r = 0.22 |
| HADS depression | 3 (1.0–6.0) | 1 (0–2.0) | W = 594.5 | <0.001 | r = 0.37 |
| PCS | 10 (4.0–21.0) | 2 (0–8.0) | W = 484.0 | <0.001 | r = 0.46 |
| CLBP characteristics | |||||
| Pain characteristics | |||||
| Clinical pain intensity [NRS]† | 4 (3.0–5.0) | ||||
| Pain duration [mo] | 79 (15.5–202.3)‡ | ||||
| Spatial pain extent [%]§ | 1.3 (0.55–2.30) | ||||
| PSQ-3 | 84 (25.0–127.5) | ||||
| WPI | 4 (2.0–6.0) | ||||
| Nociceptive pain features | |||||
| Clear symptom provocation pattern (n/%) | 37/62.7 | ||||
| painDETECT ≤ 12 (n/%) | 47/82.5‖ | ||||
| Localized pain (n/%) | 56/94.9 | ||||
| Myofascial features¶ | |||||
| Myofascial component (n/%) | 50/84.7 |
| High-/low-pain CLBP subsamples | |||||
|---|---|---|---|---|---|
| High-pain (n = 32) | Low-pain (n = 27) | ||||
| Age [y] | 48.0 (16.90) | 54.2 (16.00) | t = 1.4 | 0.157 | d = −0.37 |
| Sex (female:male) [n] | 19:13 | 18:9 | 0.600 | ||
| BMI [kg/m2] | 24.0 (3.24) | 23.8 (3.78) | t = 0.2 | 0.811 | d = 0.06 |
| HADS anxiety | 5 (2.8–7.3) | 4 (2.5–6.5) | W = 463.0 | 0.641 | r = 0.06 |
| HADS depression | 3 (1.0–6.0) | 2 (2.0–6.0) | W = 421.5 | 0.878 | r = 0.02 |
| PCS | 11 (4.8–23.3) | 10 (3.5–15.0) | W = 515.0 | 0.209 | r = 0.16 |
| Clinical pain intensity [NRS]† | 5 (4.0–6.0) | 3 (2.0–3.0) | W = 864.0 | <0.001 | r = 0.87 |
| Pain duration [mo] | 39 (12.0–142.5) | 135 (22.5–260.0) | W = 301.5 | 0.069 | r = 0.24 |
| Spatial pain extent [%]§ | 1.4 (0.68–2.25) | 1.1 (0.40–2.00) | W = 501.0 | 0.297 | r = 0.14 |
| PSQ-3 | 99 (74.5–148.8) | 35 (11.0–92.0) | W = 635.5 | 0.002 | r = 0.40 |
| WPI | 4 (3.0–6.0) | 4 (1.0–6.0) | W = 461.0 | 0.662 | r = 0.06 |
High-pain (NRS ≥ 4/10) and low-pain (NRS < 4/10) CLBP subsamples were formed based on the 50% quantile of clinical pain intensities. Values are presented as mean (SD) for continuous variables and as median (interquartile range) for ordinal or nonnormally distributed variables. T-statistics refer to unpaired t-tests and W-statistics to Wilcoxon rank sum tests. Effect sizes are reported as Cohen's d (small: < 0.5, medium: 0.5–0.8, large: > 0.8)9 for t-tests and r (small: 0.1–< 0.3, medium: 0.3–< 0.5, large: ≥ 0.5)10 for Wilcoxon rank sum tests.
Fisher's exact test.
Average clinical pain intensity over the past 4 weeks, self-reported through electronic questionnaires completed before the QST session.
N = 40 because of 1 missing value (participant did not indicate month of pain onset).
Extent of typically painful LBP-associated body areas (in % of total body area), ie, the lower back, the buttocks and the legs, were assessed using pain drawings.45
Proportion relative to 57 patients with CLBP because of 2 missing values in the painDETECT.
Based on the clinical examination.
BMI, body mass index; CLBP, chronic low back pain; HADS, Hospital Anxiety and Depression Scale; NRS, numeric rating scale; PCS, Pain Catastrophizing Scale; PSQ-3, Pain and Sleep Questionnaire three-item index; WPI, Widespread Pain Index. Bold entries: P < 0.05.
3.2. Quantitative sensory testing
In MP, patients with CLBP presented with increased cold detection thresholds (CDT) in combination with decreased cold pain thresholds (CPT) and vibration detection thresholds (VDT), indicating sensory loss of function (Table 2, Fig. 2). The pain-free area adjacent to MP was located contralaterally to MP in 44 of 59 patients with lateralized MP and randomly assigned to a body side in 15 of 59 patients with MP in the midline of the back. In AD, patients with CLBP showed increased MPS and more frequent DMA compared with control participants, reflecting sensory gain of function (Table 2, Fig. 2). In all remaining QST measures, as well as in CON, patients with CLBP showed neither sensory gain nor loss of function (Table 2, Fig. 2). Fifteen extreme Z-scores (ie, >4 or < −4) were identified and adjusted to 4 or −4, respectively (MP > 4: 1 warm detection threshold (WDT), 1 DMA; MP < −4: 2 CDT, 2 WDT, 1 mechanical detection threshold (MDT), 4 VDT; AD > 4: 1 MPS, 1 DMA; AD < −4: 1 MPT; CON > 4: 1 WUR).
Table 2.
Quantitative sensory testing measures in patients with chronic low back pain and control participants.
| Patients with CLBP* (n = 59) | Control participants* (n = 45) | Patients with CLBP Z-score |
Z statistic | P | |
|---|---|---|---|---|---|
| MP† | |||||
| CDT [°C] | 3.0 (2.14) | 2.3 (1.39) | −0.5 (1.30) | −4.2 | <0.001 |
| WDT [°C] | 3.3 (1.49) | 3.0 (0.84) | −0.1 (1.78) | −0.9 | 0.380 |
| TSL [°C] | 6.8 (3.02) | 6.5 (2.13) | −0.1 (1.14) | −0.5 | 0.629 |
| CPT [°C] | 11.9 (10.35) | 15.9 (10.22) | −0.5 (1.25) | −3.9 | <0.001‡ |
| HPT [°C] | 43.1 (3.66) | 42.7 (3.46) | −0.1 (1.10) | −1.0 | 0.447‡ |
| PPT [kg/cm2] | 5.3 (2.66) | 6.0 (2.45) | 0.3 (1.20) | 2.6 | 0.027‡ |
| MPT [mN] | 33.4 (36.80) | 29.4 (30.56) | −0.1 (1.20) | −1.0 | 0.495‡ |
| MPS [NRS] | 4.8 (5.46) | 3.8 (4.33) | 0.2 (1.08) | 1.6 | 0.107‡ |
| WUR [NRS ratio] | 3.0 (1.98) | 3.6 (2.25) | −0.3 (0.93) | −2.1 | 0.112‡ |
| MDT [mN] | 16.9 (31.68) | 7.6 (6.01) | −0.4 (1.44) | −2.9 | 0.004 |
| VDT [V.U.] | 5.5 (2.62) | 6.5 (1.35) | −0.6 (1.55) | −4.8 | <0.001 |
| PHS [count] | 2 | 3 | 0.357§ | ||
| DMA [count] | 13 | 6 | 0.608ठ| ||
| AD† | |||||
| CPT [°C] | 10.4 (10.67) | 11.6 (11.59) | −0.1 (0.95) | −0.6 | 0.572‡ |
| HPT [°C] | 43.3 (3.79) | 43.4 (3.12) | 0.1 (1.22) | 0.6 | 0.557‡ |
| PPT [kg/cm2] | 5.8 (2.40) | 5.9 (2.46) | 0.1 (0.88) | 0.4 | 0.699‡ |
| MPT [mN] | 45.2 (67.95) | 55.2 (83.74) | 0.2 (1.22) | 1.5 | 0.425‡ |
| MPS [NRS] | 4.0 (3.96) | 1.6 (1.29) | 0.8 (1.54) | 6.0 | <0.001‡ |
| WUR [NRS ratio]‖ | 3.1 (2.04) | 3.2 (1.82) | −0.2 (1.07) | −1.4 | 0.167‡ |
| DMA [count] | 13 | 1 | 0.044 ‡ § | ||
| CON | |||||
| CPT [°C] | 8.9 (9.32) | 10.6 (7.75) | −0.2 (1.15) | −1.4 | 0.258‡ |
| HPT [°C] | 45.1 (3.64) | 44.4 (3.34) | −0.2 (1.09) | −1.9 | 0.167‡ |
| PPT [kg/cm2] | 4.3 (2.06) | 4.2 (1.64) | 0.1 (1.34) | 0.8 | 0.626‡ |
| MPT [mN] | 54.5 (69.08) | 40.6 (33.2) | −0.1 (1.41) | −0.6 | 0.561‡ |
| MPS [NRS] | 4.7 (5.64) | 2.9 (3.31) | 0.4 (1.06) | 2.9 | 0.006‡ |
| WUR [NRS ratio] | 3.4 (8.38) | 2.8 (1.72) | −0.2 (1.05) | −1.4 | 0.167‡ |
| DMA [count] | 10 | 3 | 0.215ठ|
All QST measures except for CPT, HPT, and VDT were log-transformed before Z-score conversion. Values are presented as mean (SD). Minimum and maximum Z-score value for each QST measure was set to ±4 to reduce influences of single extreme outliers. Effect sizes are not reported because for Z-tests against mean = 0 and SD = 1, Cohen's d is identical to the mean of the Z-scores.
Raw values might not reflect Z-scores because (1) most QST measures were log-transformed before Z-score conversion, (2) outlier influences are larger for raw values than for Z-scores, and (3) raw values are not age and sex matched.
N = 35 for control participants because 10 control participants had been matched to another chronic pain cohort of the Clinical Research Priority Program “Pain.”
FDR-corrected for 3 tests.
Fisher's exact test.
N = 58 for patients with CLBP because 1 patient rated the highest possible single stimulus intensity as not painful.
AD, pain-free area adjacent to most painful area; CDT, cold detection threshold; CLBP, chronic low back pain; CON, remote, pain-free control area; CPT, cold pain threshold; DMA, dynamic mechanical allodynia; FDR, false discovery rate; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; NRS, numeric rating scale; PHS, paradoxical heat sensation; PPT, pressure pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; VDT, vibration detection thresholds; V.U., vibration units in X/8; WUR, wind-up ratio. Bold entries: P < 0.05 for PHS and DMA and P < 0.001 for other QST measures.
Figure 2.
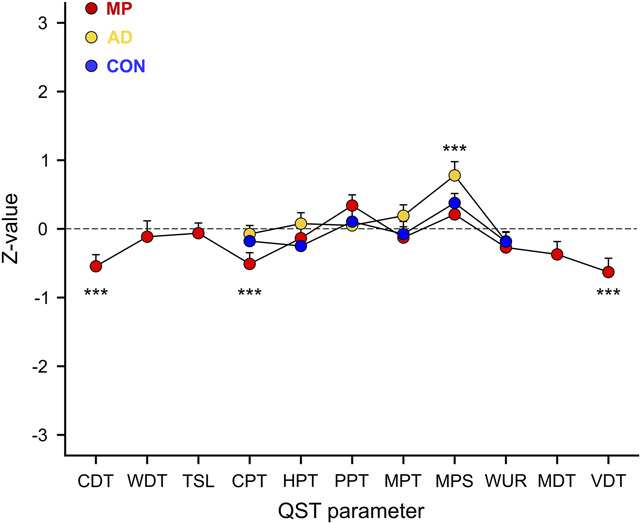
Patients with CLBP show sensory loss of function in MP and sensory gain of function in AD. Quantitative sensory testing (QST) profiles of the 3 tested areas, ie, most painful area (MP), pain-free area adjacent to MP (AD), and remote, pain-free control area (CON). Data are presented as mean Z-values and SEs. CDT, cold detection threshold; CLBP, chronic low back pain; CPT, cold pain threshold; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PPT, pressure pain threshold; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio. ***P < 0.001.
The results in MP and AD were mainly driven by the high-pain (NRS ≥ 4/10) CLBP subsample (Table 3, Fig. 3).
Table 3.
Quantitative sensory testing measures in high-pain and low-pain chronic low back pain subsamples.
| High-pain CLBP subsample Clinical pain intensity ≥ NRS 4/10 (n = 32) |
Low-pain CLBP subsample Clinical pain intensity < NRS 4/10 (n = 27) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Raw values* | Z-scores | Z statistic | P † | Raw values* | Z-scores | Z statistic | P † | |
| MP‡ | ||||||||
| CDT [°C] | 2.9 (2.25) | −0.6 (1.35) | −3.5 | <0.001 | 3.0 (2.04) | −0.5 (1.26) | −2.4 | 0.016 |
| WDT [°C] | 3.4 (1.73) | −0.2 (1.71) | −1.3 | 0.190 | 3.1 (1.15) | 0.0 (1.88) | 0.1 | 0.898 |
| TSL [°C] | 6.9 (3.39) | −0.2 (1.19) | −0.9 | 0.345 | 6.6 (2.56) | 0.1 (1.08) | 0.3 | 0.754 |
| CPT [°C] | 11.6 (10.73) | −0.6 (1.32) | −3.4 | 0.002§ | 12.2 (10.1) | −0.4 (1.19) | −2.1 | 0.099§ |
| HPT [°C] | 43.2 (4.10) | −0.2 (1.23) | −1.1 | 0.428§ | 42.9 (3.11) | −0.1 (0.94) | −0.4 | 0.708§ |
| PPT [kg/cm2] | 5.3 (2.77) | 0.3 (1.18) | 1.8 | 0.226§ | 5.3 (2.57) | 0.4 (1.24) | 1.9 | 0.081§ |
| MPT [mN] | 31.0 (34.12) | −0.0 (1.08) | −0.1 | 0.917§ | 36.3 (40.22) | −0.3 (1.35) | −1.3 | 0.278§ |
| MPS [NRS] | 4.9 (4.59) | 0.3 (1.02) | 1.7 | 0.097§ | 4.6 (6.43) | 0.1 (1.15) | 0.6 | 0.563§ |
| WUR [NRS ratio] | 3.0 (2.00) | −0.3 (0.91) | −1.9 | 0.186§ | 3.1 (1.98) | −0.2 (0.96) | −1.0 | 0.452§ |
| MDT [mN] | 20.3 (37.18) | −0.5 (1.48) | −3.0 | 0.002 | 12.9 (23.66) | −0.2 (1.39) | −0.9 | 0.361 |
| VDT [V.U.] | 5.7 (2.33) | −0.6 (1.39) | −3.3 | <0.001 | 5.2 (2.95) | −0.7 (1.75) | −3.5 | <0.001 |
| PHS [count] | 0 | 0.381 | 2 | 1¶ | ||||
| DMA [count] | 9 | 0.033 § | 4 | 1§¶ | ||||
| AD‡ | ||||||||
| CPT [°C] | 11.7 (10.56) | 0.0 (0.96) | 0.1 | 0.906§ | 8.9 (10.80) | −0.2 (0.93) | −1.0 | 0.373§ |
| HPT [°C] | 43.7 (3.98) | −0.1 (1.32) | −0.4 | 0.726§ | 42.7 (3.54) | 0.2 (1.10) | 1.3 | 0.634§ |
| PPT [kg/cm2] | 5.6 (2.41) | 0.1 (0.91) | 0.5 | 0.647§ | 5.9 (2.43) | 0.0 (0.86) | 0.1 | 0.942§ |
| MPT [mN] | 38.9 (41.8) | 0.3 (1.02) | 1.9 | 0.163§ | 52.6 (90.06) | 0.0 (1.42) | 0.1 | 0.938§ |
| MPS [NRS] | 4.2 (4.12) | 1.0 (1.34) | 5.5 | <0.001 § | 3.7 (3.83) | 0.6 (1.74) | 2.9 | 0.011§ |
| WUR [NRS ratio]‖ | 3.0 (1.66) | −0.2 (1.01) | −1.2 | 0.252§ | 3.2 (2.44) | −0.1 (1.15) | −0.8 | 0.452§ |
| DMA [count] | 8 | 0.381§ | 5 | 0.333§¶ | ||||
| CON | ||||||||
| CPT [°C] | 9.0 (9.94) | −0.2 (1.22) | −1.0 | 0.450§ | 8.8 (8.72) | −0.2 (1.09) | −0.9 | 0.373§ |
| HPT [°C] | 45.5 (3.80) | −0.4 (1.16) | −2.0 | 0.135§ | 44.7 (3.46) | −0.1 (1.00) | −0.6 | 0.708§ |
| PPT [kg/cm2] | 4.5 (2.15) | −0.1 (1.14) | −0.8 | 0.647§ | 4.0 (1.94) | 0.4 (1.52) | 2.0 | 0.081§ |
| MPT [mN] | 52.7 (69.39) | 0.1 (1.48) | 0.4 | 0.917§ | 56.6 (69.97) | −0.3 (1.33) | −1.3 | 0.278§ |
| MPS [NRS] | 4.9 (5.01) | 0.5 (1.04) | 2.7 | 0.009§ | 4.5 (6.41) | 0.2 (1.09) | 1.3 | 0.293§ |
| WUR [NRS ratio] | 4.2 (11.33) | −0.2 (1.14) | −1.1 | 0.252§ | 2.4 (1.36) | −0.2 (0.95) | −0.9 | 0.452§ |
| DMA [count] | 7 | 0.125§ | 3 | 0.667§¶ | ||||
All QST measures except for CPT, HPT, and VDT were log-transformed before Z-score conversion. Values are presented as mean (SD). Minimum and maximum Z-score value for each QST measure was set to ±4 to reduce influences of single extreme outliers. Effect sizes are not reported because for Z-tests against mean = 0 and SD = 1, Cohen's d is identical to the mean of the Z-scores.
Raw values might not reflect Z-scores because (1) most QST measures were log-transformed before Z-score conversion, (2) outlier influences are larger for raw values than for Z-scores, and (3) raw values are not age and sex matched.
Statistical comparison to an ideal Z-value distribution (mean = 0, SD = 1) for Z-tests and to control participants for Fisher's exact tests.
N = 35 for control participants because 10 control participants had been matched to another chronic pain cohort of the Clinical Research Priority Program “Pain.”
FDR-corrected for three tests.
N = 31 for high-pain CLBP subsample because 1 patient rated the highest possible single stimulus intensity as not painful.
Fisher's exact test.
AD, pain-free area adjacent to most painful area; CDT, cold detection threshold; CLBP, chronic low back pain; CON, remote, pain-free control area; CPT, cold pain threshold; DMA, dynamic mechanical allodynia; FDR, false discovery rate; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; NRS, numeric rating scale; PHS, paradoxical heat sensation; PPT, pressure pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; VDT, vibration detection thresholds; V.U., vibration units in X/8; WUR, wind-up ratio. Bold entries: P < 0.05 for PHS and DMA and P < 0.001 for other QST measures.
Figure 3.
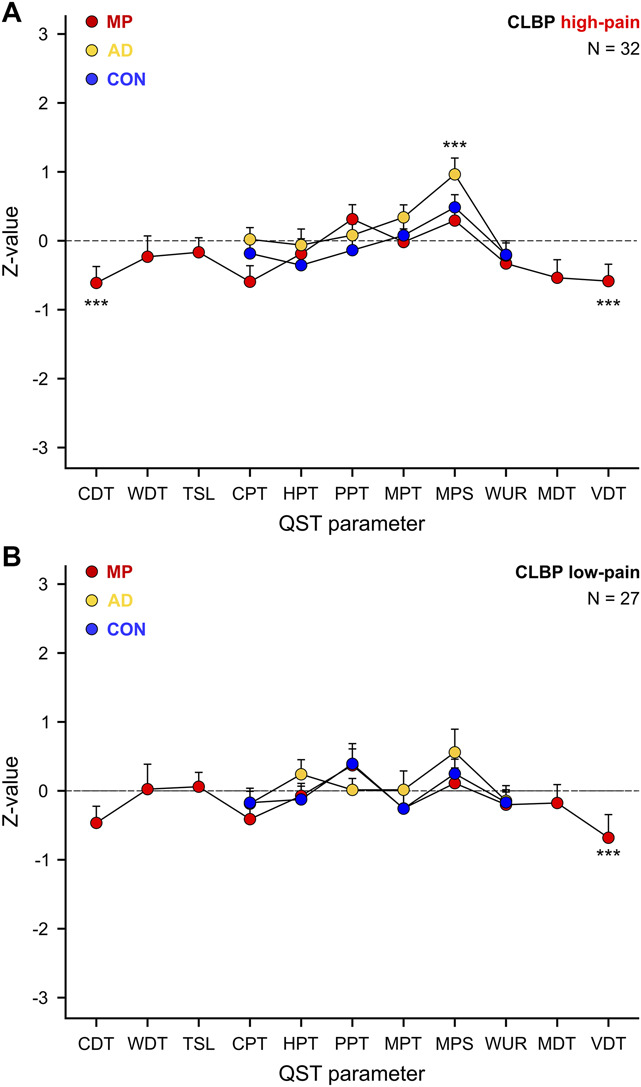
Sensory loss of function in MP and sensory gain of function in AD depends on clinical pain severity. Quantitative sensory testing (QST) profiles of the 3 tested areas, ie, most painful area (MP), pain-free area adjacent to MP (AD), and remote pain-free control area (CON) in a high-pain (clinical pain intensity ≥ NRS 4/10) (A) and a low-pain (clinical pain intensity < NRS 4/10) CLBP subsample (B). Data are presented as mean Z-values and SEs. CDT, cold detection threshold; CLBP, chronic low back pain; CPT, cold pain threshold; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PPT, pressure pain threshold; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio. ***P < 0.001.
Two significant associations (out of 30 tested) between QST measures indicative of spinal or supraspinal sensitization and patient-reported outcome measures supposedly associated with central pain processes were identified. First, patients with lower PPTs in AD, reflecting more pronounced pressure pain sensory gain of function, reported more widespread pain (rho = 0.31, P = 0.017). Second, patients with lower CPTs in CON, reflecting more pronounced cold pain sensory loss of function, had higher PSQ-3 scores, meaning worse sleep quality (rho = −0.30, P = 0.022). The remaining associations were not significant (PCS: all rhos < |0.18|, all Ps > 0.171; PSQ-3: all rhos < |0.18|, all Ps > 0.182; WPI: all rhos < |0.19|, all Ps > 0.150).
Influences of pain-relevant medication intake on QST measures were not analyzed because regular intake was reported by less than a quarter (ie, 14/59) of the patients with CLBP, resulting in low statistical power for a respective subgroup analysis.
3.3. Clinical examination
The patients with CLBP predominantly presented with features of nociceptive pain mechanisms51 (Table 1).
4. Discussion
Using QST in 3 different body areas, this study demonstrated area-specific sensory alterations in patients with nonspecific CLBP compared with pain-free control participants, namely: (1) hypoesthesia in the painful MP, (2) mechanical hyperalgesia and allodynia in the MP-surrounding pain-free AD, and (3) no sensory alterations in the remote, pain-free CON. The sensory alterations were more pronounced in patients with clinically more severe CLBP and indicate an involvement of spinal sensitization in CLBP without evidence for peripheral and supraspinal sensitization. No compelling evidence was found for an association between QST measures indicative of spinal or supraspinal sensitization and pain catastrophizing, sleep quality, or widespread pain.
Tactile hypoesthesia in painful body areas has been previously observed in chronic myofascial pain,18,38 muscle pain,24 and over trigger points,2 which supports a muscular/myofascial (Table 1) component in the present CLBP cohort. The findings further align with reports of decreased tactile acuity in the lower back of patients with CLBP.33,37,40 Also, despite predominant nociceptive pain features in the investigated CLBP cohort, the observed hypoesthesia in MP could indicate an additional neuropathic component because hypoesthesia is a hallmark of nerve damage.32 Neuropathic pain mechanisms have been suggested to play a role in CLBP.6 Peripheral fiber loss could also preclude the detection of hypersensitivity in the affected area50 and thus explain the findings in MP, particularly the absence of deep tissue hypersensitivity. Alternatively, sensory attenuation could result from neglect-like tactile dysfunction39 or enhanced descending pain inhibition, which has been shown to reduce primary hyperalgesia.58 Interestingly, 2 and 4 extreme Z-score values below −4 (and none above 4) were identified for CDT and VDT in MP, respectively, and adjusted to a value of −4 to minimize outlier influences on the analysis. This means that the sensory loss in MP was even more pronounced in the CLBP cohort and that the reported effect size is a conservative estimate of the true group difference. The lack of a statistically significant deep tissue hypersensitivity in form of decreased PPT values was unexpected based on previous studies in patients with low back pain.12 Nevertheless, before multiple comparison correction, a trend (P = 0.009) for decreased PPTs had been observed in MP for patients with CLBP. Besides the mechanisms outlined above, the nonsignificant result for PPTs in MP could also be due to the included CLBP patient cohort. The patients were psychologically relatively mildly affected, as evident in lower HADS and PCS scores compared with other CLBP cohorts19,21,55,62,63 and a small proportion reporting regular pain-relevant medication intake.
Given that AD was located adjacent to MP and thus, innervated by adjacent spinal segments, sensory gain of function in AD indicates sensitization at the spinal level. Particularly, the sensory gain of function was restricted to superficial mechanical stimuli, aligning with sensory signs observed in areas of secondary hyperalgesia.31,34,48 Of note, different spinal sensitization mechanisms have to be considered depending on the location of AD. The originally described activity-dependent central sensitization at the spinal dorsal horn neuron results in ipsilateral hypersensitivity within the receptive field of the same spinal dorsal horn neuron.65 By contrast, glia-mediated spinal sensitization has been shown to spread along the spinal cord8,30 and can induce widespread sensitization and hypersensitivity. For patients with MP in the midline and thus, an unclear attribution of AD to the ipsilateral or contralateral body side, activity-dependent and glia-mediated sensitization are possible. For patients with lateralized MP, AD was always assessed contralaterally, and therefore, glia-mediated spinal sensitization is more plausible. In the present study, most patients presented with a lateralized MP. However, according to the obtained pain drawings, their pain frequently (52.3%) extended beyond the midline, making it impossible to identify the side of the spinal cord that innervated MP and thus might have been affected by activity-dependent central sensitization. In addition, MP was used as best available proxy for the primarily affected area, but it does not necessarily reflect the location of a primary nociceptive driver. Finally, similarly to MP, the Z-score adjustment of extreme outlier values in AD led to a slight underestimation of the sensory gain in patients with CLBP, given that 1 MPS and 1 DMA value were adjusted from values above 4 to 4.
Evidence regarding widespread hyperalgesia in CLBP is inconclusive,46 with various studies not reporting sensory alterations in remote body areas of patients with CLBP.15,27,36,43 The absence of widespread hyperalgesia in the present cohort might be because of most patients showing localized pain20 (Table 1). This feature is considered an indicator of nociceptive pain mechanisms that are not expected to be associated with generalized pain hypersensitivity.51 The notion of more widespread clinical pain being associated with experimental pain hypersensitivity beyond the primarily affected area may be reflected in the correlation between higher WPI scores and higher pressure pain sensitivity in AD observed in the present study. However, this correlation might represent a spurious finding because of its small effect size and because it was one of 2 significant correlations out of 30 exploratory correlation analyses, a proportion of positive findings expected by pure chance. The predominance of nociceptive pain mechanisms in the investigated patients with CLBP is further supported by the lower HADS and PCS scores compared with other CLBP studies.21,62,63 According to expert consensus, the absence of significant psychological features supports the dominance of nociceptive or neuropathic pain mechanisms.51 Interestingly, PCS scores were not associated with QST measures indicative of spinal or supraspinal sensitization. This means that the here observed signs of spinal sensitization may be independent of psychological features and might mainly represent a biological phenomenon. To the best of our knowledge, the present study is the first to allow the differentiation between spinal and supraspinal sensitization in nonspecific CLBP based on area-specific sensory alterations.
The observed hypoesthesia in MP and the mechanical hypersensitivity in AD were driven by the high-pain CLBP subsample, adding to previous work in chronic pain cohorts, which showed that clinical pain severity varied across detected QST profiles.16,25,41 Thus, in combination with other factors, clinical pain intensity can be an indicator of pathophysiological mechanism in CLBP. Alternatively, central sensitization might cause more severe CLBP.47 Furthermore, the high-pain CLBP subsample showed higher PSQ-3 scores compared with the low-pain CLBP subsample. This suggests that poorer sleep quality could play a role in the sensory alterations observed in the high-pain CLBP subsample. Yet, the sleep impairment (median PSQ-3 score: 99) was less pronounced compared with previous reports (mean PSQ-3 score: 187.2)4 and the bidirectional relationship between pain intensity and sleep quality1 makes it challenging to determine which of the 2 is more relevant in the context of the present results. A generic association of poor sleep quality with signs of spinal or supraspinal sensitization is not supported by the present study because PSQ-3 scores were not associated with any sensory gain of function in AD or CON, respectively. The correlation between PSQ-3 and more pronounced sensory loss of function, ie, lower CPTs in CON, might, as mentioned above for the correlation between WPI and PPTs in AD, be a spurious finding as the effect size was low and the exploratory correlation analyses were not corrected for multiple comparisons.
One limitation of the present study is that a reduced QST protocol was used in AD and CON, including the consequence that MPS and DMA were calculated based on only 2 stimulus-response-function blocks instead of 5. The reduced QST protocol precluded the detection of potential sensory loss in AD and CON. For CON, sensory loss was unlikely given that the hand was sensory intact in all participants (as assessed by bedside sensory testing). However, sensory loss in AD cannot be ruled out. In addition, the differences between the applied QST protocol and the full DFNS QST protocol hamper the comparability of the absolute QST values between this study and studies that used the full DFNS QST protocol. Nevertheless, the presented relative comparison of patients with CLBP and control participants is valid because the identical QST protocol had been used in both cohorts. Furthermore, the low proportion of patients with CLBP reporting regular pain-relevant medication intake prevented a meaningful analysis of whether medication had an influence on QST measures. Yet, the small number of patients with CLBP relying on regular medication intake also represents a strength of the present study. First, there are fewer confounding medication effects. Second, it might indicate that the included CLBP cohort was relatively mildly affected by their pain with a lesser role of psychosocial factors,26 highlighting the potential relevance of the study's findings from a biological perspective. Finally, it should be kept in mind that QST only allows an indirect assessment of central sensitization as defined by the International Association for the Study of Pain28 by sensory proxies. Alternative methods might more closely reflect changes in neuronal hyperexcitability, for example, the N13 component of somatosensory evoked potentials.14 However, by considering known manifestations of peripheral, spinal, and supraspinal sensitization, QST can be used to infer the presence of central sensitization, if assessed in a primarily affected, secondary, and remote body area.
In conclusion, the combination of QST in 3 different body areas allowed to investigate contributions of peripheral, spinal, and supraspinal sensitization in patients with CLBP. Signs of spinal sensitization were observed in patients with CLBP, predominantly in patients with more severe clinical pain who also displayed poorer sleep quality compared with less severely affected patients. Of particular interest, the included patients with CLBP presented with predominant nociceptive features, namely localized pain and a low degree of psychological interference, and the observed signs of spinal sensitization were independent of pain catastrophizing levels. The present study might thus offer insights into central pain processes involved in nonspecific, nociceptive CLBP phenotypes without pronounced influence of psychological factors.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgments
This study was funded by the Clinical Research Priority Program “Pain” of the University of Zurich. L.S. was supported by the Theodor und Ida Herzog-Egli Stiftung. The authors thank all participants who took part in the study. In addition, the authors thank Lucas Tauschek, Simon Carisch, David Costa Marques, and Anna Mollo for their support during data acquisition and László Demkó for setting up the custom-made software for spatial pain extent calculation. The authors further thank Balgrist Campus for their support and providing the infrastructure to perform the present study.
Data availability statement: Data of participants who gave informed consent for further use of their anonymized data will be made available upon request.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Iara De Schoenmacker, Email: Iara.DeSchoenmacker@balgrist.ch.
Paulina Simonne Scheuren, Email: paulina.scheuren@balgrist.ch.
Robin Lütolf, Email: robin.luetolf@bluewin.ch.
Lindsay Mary Gorrell, Email: lindsay.gorrell@balgrist.ch.
Anke Langenfeld, Email: anke.langenfeld@balgrist.ch.
Mirjam Baechler, Email: mirjam.baechler@balgrist.ch.
Jan Rosner, Email: jan.rosner@balgrist.ch.
Brigitte Wirth, Email: brigitte.wirth@balgrist.ch.
Michèle Hubli, Email: michele.hubli@balgrist.ch.
Petra Schweinhardt, Email: petra.schweinhardt@balgrist.ch.
References
- [1].Alsaadi SM, McAuley JH, Hush JM, Lo S, Bartlett DJ, Grunstein RR, Maher CG. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin J Pain 2014;30:755–65. [DOI] [PubMed] [Google Scholar]
- [2].Ambite-Quesada S, Arias-Buria JL, Courtney CA, Arendt-Nielsen L, Fernandez-de-las-Penas C. Exploration of quantitative sensory testing in latent trigger points and referred pain areas. Clin J Pain 2018;34:409–14. [DOI] [PubMed] [Google Scholar]
- [3].Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Drewes AM. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018;22:216–41. [DOI] [PubMed] [Google Scholar]
- [4].Ayearst L, Harsanyi Z, Michalko KJ. The Pain and Sleep Questionnaire three-item index (PSQ-3): a reliable and valid measure of the impact of pain on sleep in chronic nonmalignant pain of various etiologies. Pain Res Manag 2012;17:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bannister K, Dickenson AH. What the brain tells the spinal cord. PAIN 2016;157:2148–51. [DOI] [PubMed] [Google Scholar]
- [6].Baron R, Binder A, Attal N, Casale R, Dickenson AH, Treede RD. Neuropathic low back pain in clinical practice. Eur J Pain 2016;20:861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, Robinson M, Edwards RR, Smith MT. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res 2015;67:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. PAIN 2009;147:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers, 1988. [Google Scholar]
- [10].Cohen J. A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- [11].De Schoenmacker I, Sirucek L, Scheuren PS, Lutolf R, Gorrell LM, Brunner F, Curt A, Rosner J, Schweinhardt P, Hubli M. Sensory phenotypes in complex regional pain syndrome and chronic low back pain-indication of common underlying pathomechanisms. Pain Rep 2023;8:e1110. doi: 10.1097/PR9.0000000000001110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].den Bandt HL, Paulis WD, Beckwee D, Ickmans K, Nijs J, Voogt L. Pain mechanisms in low back pain: a systematic review and meta-analysis of mechanical quantitative sensory testing outcomes in people with non-specific low back pain. J Orthop Sports Phys Ther 2019;49:698–715. [DOI] [PubMed] [Google Scholar]
- [13].den Boer C, Dries L, Terluin B, van der Wouden JC, Blankenstein AH, van Wilgen CP, Lucassen P, van der Horst HE. Central sensitization in chronic pain and medically unexplained symptom research: a systematic review of definitions, operationalizations and measurement instruments. J Psychosom Res 2019;117:32–40. [DOI] [PubMed] [Google Scholar]
- [14].Di Lionardo A, Di Stefano G, Leone C, Di Pietro G, Sgro E, Malara E, Cosentino C, Mollica C, Blockeel AJ, Caspani O, Garcia-Larrea L, Mouraux A, Treede RD, Phillips KG, Valeriani M, Truini A. Modulation of the N13 component of the somatosensory evoked potentials in an experimental model of central sensitization in humans. Scientific Rep 2021;11:20838. doi: 10.1038/s41598-021-00313-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diers M, Koeppe C, Diesch E, Stolle AM, Holzl R, Schiltenwolf M, van Ackern K, Flor H. Central processing of acute muscle pain in chronic low back pain patients: an EEG mapping study. J Clin Neurophysiol 2007;24:76–83. [DOI] [PubMed] [Google Scholar]
- [16].Frey-Law LA, Bohr NL, Sluka KA, Herr K, Clark CR, Noiseux NO, Callaghan JJ, Zimmerman MB, Rakel BA. Pain sensitivity profiles in patients with advanced knee osteoarthritis. PAIN 2016;157:1988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [18].Geber C, Magerl W, Fondel R, Fechir M, Rolke R, Vogt T, Treede RD, Birklein F. Numbness in clinical and experimental pain—a cross-sectional study exploring the mechanisms of reduced tactile function. PAIN 2008;139:73–81. [DOI] [PubMed] [Google Scholar]
- [19].Georgopoulos V, Akin-Akinyosoye K, Smith S, McWilliams DF, Hendrick P, Walsh DA. An observational study of centrally facilitated pain in individuals with chronic low back pain. Pain Rep 2022;7:e1003. doi: 10.1097/PR9.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gerhardt A, Eich W, Janke S, Leisner S, Treede RD, Tesarz J. Chronic widespread back pain is distinct from chronic local back pain: evidence from quantitative sensory testing, pain drawings, and psychometrics. Clin J Pain 2016;32:568–79. [DOI] [PubMed] [Google Scholar]
- [21].Gevers-Montoro C, Romero-Santiago B, Medina-Garcia I, Larranaga-Arzamendi B, Alvarez-Galovich L, Ortega-De Mues A, Piche M. Reduction of chronic primary low back pain by spinal manipulative therapy is accompanied by decreases in segmental mechanical hyperalgesia and pain catastrophizing: a randomized placebo-controlled dual-blind mixed experimental trial. J Pain 2024. doi: 10.1016/j.jpain.2024.02.014 [DOI] [PubMed] [Google Scholar]
- [22].Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010;6:599–606. [DOI] [PubMed] [Google Scholar]
- [23].Harkness EF, Macfarlane GJ, Silman AJ, McBeth J. Is musculoskeletal pain more common now than 40 years ago? two population-based cross-sectional studies. Rheumatology 2005;44:890–95. [DOI] [PubMed] [Google Scholar]
- [24].Hollins M, Sigurdsson A, Fillingim L, Goble AK. Vibrotactile threshold is elevated in temporomandibular disorders. PAIN 1996;67:89–96. [DOI] [PubMed] [Google Scholar]
- [25].Hurtig IM, Raak RI, Kendall SA, Gerdle B, Wahren LK. Quantitative sensory testing in fibromyalgia patients and in healthy subjects: identification of subgroups. Clin J Pain 2001;17:316–22. [DOI] [PubMed] [Google Scholar]
- [26].Huysmans E, Leemans L, Beckwée D, Nijs J, Ickmans K, Moens M, Goudman L, Buyl R, Putman K, Coppieters I. The relationship between cognitive and emotional factors and healthcare and medication use in people experiencing pain: a systematic review. J Clin Med 2020;9:2486. doi: 10.3390/jcm9082486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Imamura M, Alfieri FM, Filippo TR, Battistella LR. Pressure pain thresholds in patients with chronic nonspecific low back pain. J Back Musculoskelet Rehabil 2016;29:327–36. [DOI] [PubMed] [Google Scholar]
- [28].International Association for the Study of Pain. Task force on taxonomy. IASP terminology updated from Part III: pain terms, A current list with definitions and notes on usage. In: Classification of Chronic Pain. 2nd ed. Seattle: IASP, 2017. Available at: https://www.iasp-pain.org/resources/terminology/. Accessed July 4, 2023. [Google Scholar]
- [29].Kallewaard JW, Terheggen MA, Groen GJ, Sluijter ME, Derby R, Kapural L, Mekhail N, van Kleef M. 15. Discogenic low back pain. Pain Pract 2010;10:560–79. [DOI] [PubMed] [Google Scholar]
- [30].Kronschlager MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkuhler J. Gliogenic LTP spreads widely in nociceptive pathways. Science 2016;354:1144–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lamotte RH, Shain CN, Simone DA, Tsai EFP. Neurogenic hyperalgesia—psychophysical studies of underlying mechanisms. J Neurophysiol 1991;66:190–211. [DOI] [PubMed] [Google Scholar]
- [32].Leffler AS, Hansson P. Painful traumatic peripheral partial nerve injury-sensory dysfunction profiles comparing outcomes of bedside examination and quantitative sensory testing. Eur J Pain 2008;12:397–402. [DOI] [PubMed] [Google Scholar]
- [33].Luomajoki H, Moseley GL. Tactile acuity and lumbopelvic motor control in patients with back pain and healthy controls. Br J Sports Med 2011;45:437–40. [DOI] [PubMed] [Google Scholar]
- [34].Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. PAIN 1998;74:257–68. [DOI] [PubMed] [Google Scholar]
- [35].Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017;389:736–47. [DOI] [PubMed] [Google Scholar]
- [36].Meeus M, Roussel NA, Truijen S, Nijs J. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehabil Med 2010;42:884–90. [DOI] [PubMed] [Google Scholar]
- [37].Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan ST, Wasan AD, Kaptchuk TJ, McDonnell C, Carriere J, Rosen B, Gollub RL, Edwards RR. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. PAIN 2019;160:833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moriwaki K, Shiroyama K, Yasuda M, Uesugi F. Reversible tactile hypoesthesia associated with myofascial trigger points: a pilot study on prevalence and clinical implications. Pain Rep 2019;4:e772. doi: 10.1097/PR9.0000000000000772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moseley GL, Gallagher L, Gallace A. Neglect-like tactile dysfunction in chronic back pain. Neurology 2012;79:327–32. [DOI] [PubMed] [Google Scholar]
- [40].Moseley LG. I can't find it! Distorted body image and tactile dysfunction in patients with chronic back pain. PAIN 2008;140:239–43. [DOI] [PubMed] [Google Scholar]
- [41].Mustonen L, Vollert J, Rice ASC, Kalso E, Harno H. Sensory profiles in women with neuropathic pain after breast cancer surgery. Breast Cancer Res Treat 2020;182:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nijs J, Torres-Cueco R, van Wilgen CP, Girbes EL, Struyf F, Roussel N, van Oosterwijck J, Daenen L, Kuppens K, Vanwerweeen L, Hermans L, Beckwee D, Voogt L, Clark J, Moloney N, Meeus M. Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Physician 2014;17:447–57. [PubMed] [Google Scholar]
- [43].Peters ML, Schmidt AJM, Vandenhout MA. Chronic low-back pain and the reaction to repeated acute pain stimulation. PAIN 1989;39:69–76. [DOI] [PubMed] [Google Scholar]
- [44].Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 2006;10:77–88. [DOI] [PubMed] [Google Scholar]
- [45].Rosner J, Lütolf R, Hostettler P, Villiger M, Clijsen R, Hohenauer E, Barbero M, Curt A & Hubli M. Assessment of neuropathic pain after spinal cord injury using quantitative pain drawings. Spinal Cord 2021;59:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Roussel NA, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R. Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clin J Pain 2013;29:625–38. [DOI] [PubMed] [Google Scholar]
- [47].Sachau J, Bruckmueller H, Gierthmuhlen J, Magerl W, May D, Binder A, Forstenpointner J, Koetting J, Maier C, Tolle TR, Treede RD, Berthele A, Caliebe A, Diesch C, Flor H, Huge V, Maihofner C, Rehm S, Kersebaum D, Fabig SC, Vollert J, Rolke R, Stemmler S, Sommer C, Westermann A, Cascorbi I, Baron R. The serotonin receptor 2A (HTR2A) rs6313 variant is associated with higher ongoing pain and signs of central sensitization in neuropathic pain patients. Eur J Pain 2021;25:595–611. [DOI] [PubMed] [Google Scholar]
- [48].Sang CN, Gracely RH, Max MB, Bennett GJ. Capsaicin-evoked mechanical allodynia and hyperalgesia cross nerve territories - evidence for a central mechanism. Anesthesiology 1996;85:491–6. [DOI] [PubMed] [Google Scholar]
- [49].Sanzarello I, Merlini L, Rosa MA, Perrone M, Frugiuele J, Borghi R, Faldini C. Central sensitization in chronic low back pain: a narrative review. J Back Musculoskelet Rehabil 2016;29:625–33. [DOI] [PubMed] [Google Scholar]
- [50].Schmelz M. Lessons learned—moving on from QST sensory profiles. Scand J Pain 2022;22:670–72. [DOI] [PubMed] [Google Scholar]
- [51].Shraim MA, Sluka KA, Sterling M, Arendt-Nielsen L, Argoff C, Bagraith KS, Baron R, Brisby H, Carr DB, Chimenti RL, Courtney CA, Curatolo M, Darnall BD, Ford JJ, Graven-Nielsen T, Kolski MC, Kosek E, Liebano RE, Merkle SL, Parker R, Reis FJJ, Smart K, Smeets RJEM, Svensson P, Thompson BL, Treede RD, Ushida T, Williamson OD, Hodges PW. Features and methods to discriminate between mechanism-based categories of pain experienced in the musculoskeletal system: a Delphi expert consensus study. PAIN 2022;163:1812–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sullivan MJL, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [53].Tampin B, Vollert J, Schmid AB. Sensory profiles are comparable in patients with distal and proximal entrapment neuropathies, while the pain experience differs. Curr Med Res Opin 2018;34:1899–906. [DOI] [PubMed] [Google Scholar]
- [54].Taub CJ, Sturgeon JA, Johnson KA, Mackey SC, Darnall BD. Effects of a pain catastrophizing induction on sensory testing in women with chronic low back pain: a pilot study. Pain Res Manag 2017;2017:7892494. doi: 10.1155/2017/7892494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tesarz J, Gerhardt A, Leisner S, Janke S, Treede RD, Eich W. Distinct quantitative sensory testing profiles in nonspecific chronic back pain subjects with and without psychological trauma. PAIN 2015;156:577–86. [DOI] [PubMed] [Google Scholar]
- [56].Torebjork HE, Lundberg LER, Lamotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol 1992;448:765–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].van Kleef M, Vanelderen P, Cohen SP, Lataster A, Van Zundert J, Mekhail N. 12. Pain originating from the lumbar facet joints. Pain Pract 2010;10:459–69. [DOI] [PubMed] [Google Scholar]
- [58].Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Research Brain Res Rev 2004;46:295–309. [DOI] [PubMed] [Google Scholar]
- [59].Vase L, Nikolajsen L, Christensen B, Egsgaard LL, Arendt-Nielsen L, Svensson P, Jensen TS. Cognitive-emotional sensitization contributes to wind-up-like pain in phantom limb pain patients. PAIN 2011;152:157–62. [DOI] [PubMed] [Google Scholar]
- [60].Vining R, Potocki E, Seidman M, Morgenthal AP. An evidence-based diagnostic classification system for low back pain. J Can Chiropr Assoc 2013;57:189–204. [PMC free article] [PubMed] [Google Scholar]
- [61].Vining RD, Minkalis AL, Shannon ZK, Twist EJ. Development of an evidence-based practical diagnostic checklist and corresponding clinical exam for low back pain. J Manipulative Physiol Ther 2019;42:665–76. [DOI] [PubMed] [Google Scholar]
- [62].Wang LY, Fu TS, Tsia MC, Hung CI. The associations of depression, anxiety, and insomnia at baseline with disability at a five-year follow-up point among outpatients with chronic low back pain: a prospective cohort study. BMC Musculoskelet Disord 2023;24:565. doi: 10.1186/s12891-023-06682-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U. Response to the Letter to the Editor: Re: Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain. A systematic review. Spine 2014;39: 263-273. Spine 2014;39:263–73. [DOI] [PubMed] [Google Scholar]
- [64].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- [65].Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686–8. [DOI] [PubMed] [Google Scholar]
- [66].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]


