Abstract
Background and Aims:
High levels of serum matrix metalloproteinase-7 (MMP-7) have been linked to biliary atresia (BA), with wide variation in concentration cutoffs. We investigated the accuracy of serum MMP-7 as a diagnostic biomarker in a large North American cohort.
Approach and Results:
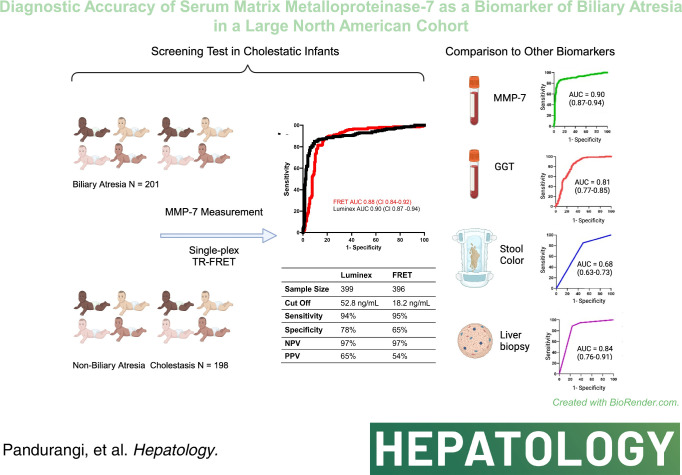
MMP-7 was measured in serum samples of 399 infants with cholestasis in the Prospective Database of Infants with Cholestasis study of the Childhood Liver Disease Research Network, 201 infants with BA and 198 with non-BA cholestasis (age median: 64 and 59 days, p = 0.94). MMP-7 was assayed on antibody-bead fluorescence (single-plex) and time resolved fluorescence energy transfer assays. The discriminative performance of MMP-7 was compared with other clinical markers. On the single-plex assay, MMP-7 generated an AUROC of 0.90 (CI: 0.87–0.94). At cutoff 52.8 ng/mL, it produced sensitivity = 94.03%, specificity = 77.78%, positive predictive value = 64.46%, and negative predictive value = 96.82% for BA. AUROC for gamma-glutamyl transferase = 0.81 (CI: 0.77–0.86), stool color = 0.68 (CI: 0.63–0.73), and pathology = 0.84 (CI: 0.76–0.91). Logistic regression models of MMP-7 with other clinical variables individually or combined showed an increase for MMP-7+gamma-glutamyl transferase AUROC to 0.91 (CI: 0.88–0.95). Serum concentrations produced by time resolved fluorescence energy transfer differed from single-plex, with an optimal cutoff of 18.2 ng/mL. Results were consistent within each assay technology and generated similar AUROCs.
Conclusions:
Serum MMP-7 has high discriminative properties to differentiate BA from other forms of neonatal cholestasis. MMP-7 cutoff values vary according to assay technology. Using MMP-7 in the evaluation of infants with cholestasis may simplify diagnostic algorithms and shorten the time to hepatoportoenterostomy.
INTRODUCTION
Biliary atresia (BA) is an obliterative infantile cholangiopathy, which can rapidly progress to cirrhosis without appropriate and timely surgical management.1,2 Infants with BA present in the neonatal period with acholic stools, jaundice, and variable levels of hepatosplenomegaly, which result from an inflammatory and fibrosing obstruction of bile ducts. Without a greater understanding of the mechanisms of disease, the primary treatment is the removal of diseased/obstructed bile ducts in a hepatoportoenterostomy (HPE) operation.1,3–5 Published studies suggest a critical role of age at diagnosis in achieving successful biliary drainage after HPE and surviving with the native liver.2,6 Making the diagnosis in the young infant, however, remains a challenge due to a major overlap of clinical and biochemical parameters with other causes of neonatal cholestasis. The diagnostic algorithm for BA includes acholic stools, laboratory testing for cholestasis and other confounding conditions, imaging studies, and liver biopsy. Ultimately, patients with suspected BA undergo an intraoperative cholangiogram, followed by HPE when the biliary tree is not visualized. This evaluation is time-consuming, costly, and invasive, underscoring the need for reliable, noninvasive biomarkers for BA.
A study from the Childhood Liver Disease Research Network (ChiLDReN) showed that a logistic regression model of seven clinical and biochemical parameters yielded an 81% predictive rate for BA, which remains below the accuracy provided by liver biopsy alone and is intrinsically time-consuming and costly.7 Another study performed on samples from ChiLDReN applied proteomics to quantify the relative abundance of ~1129 proteins in the serum of a discovery and validation cohorts identified matrix metalloproteinase-7 (MMP-7) as a biomarker with high discriminative properties for BA.8 In a subsequent study in a Chinese cohort, a serum MMP-7 concentration of 52.8 ng/mL differentiated BA from other causes of neonatal cholestasis with 98.67% sensitivity and 95% specificity.9 These findings have been supported by other investigators; however, the reported cutoff values predictive of diagnosis were highly variable in these studies, and none included North American subjects.10–13
In the present study, we measured MMP-7 levels in a cohort of North American infants at the time of diagnosis of BA and non-BA cholestasis. We hypothesized that high serum concentrations of MMP-7 predict BA. Specifically, we aimed to quantify the diagnostic performance of MMP-7, compare it with other clinical markers, and examine the influence of the assay technology on establishing cutoff values.
METHODS
Study design and population
This is a case-control study of 399 infants with cholestasis, with and without BA. Serum samples and data on clinical and histological obstructive features were obtained from infants enrolled in the Prospective Database of Infants with Cholestasis study (ClinicalTrials.gov identifier: NCT00061828) of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded ChiLDReN (www.childrennetwork.org), originated from pediatric liver centers in the United States and Canada. ChiLDReN is a collaborative research network dedicated to the study of pediatric cholestatic liver diseases among centers in North America. Prospective Database of Infants with Cholestasis is a collection of clinical information and a repository of blood and tissue samples from children with diagnoses of neonatal cholestatic liver diseases. Infants under the age of 180 days presenting with cholestasis at participating centers are eligible to be enrolled. Exclusion criteria include acute liver failure, previous hepatobiliary surgery, bacterial or fungal sepsis, shock hepatopathy, malignancy, primary hemolytic disease, parenteral nutrition–associated cholestasis, extracorporeal membrane oxygenation–associated cholestasis, or birthweight less than 1500 g. A separate group of 73 infants younger than 6 months of age without cholestasis undergoing a blood draw for medical indications unrelated to liver disease (eg, hernia repair, otolaryngologic evaluation) was enrolled at Cincinnati Children’s Hospital Medical Center to serve as age-matched normal controls.
Serum samples were collected from participants at the time of initial evaluation and enrollment in Prospective Database of Infants with Cholestasis. The diagnosis of BA was confirmed by the finding of luminal obstruction of extrahepatic bile ducts by histopathological examination, which was performed by ChiLDReN pathologists who were unaware of the clinical/surgical diagnosis. All samples were obtained after written informed consent from the participant’s parents/legal guardians. Study protocols were approved by the Institutional Review Boards of all participating institutions (Salus IRB #20044) and conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the Declaration of Istanbul.
Biochemical studies and MMP-7 quantification
Serum levels of ALT, AST, gamma-glutamyl transferase (GGT), total bilirubin, and direct bilirubin were determined in clinical laboratories at the time of initial evaluation of all patients. The concentration of MMP-7 was determined in the same serum sample by 2 assays. The first assay was the Millipore Luminex assay (MilliporeSigma, USA; heretofore referred to as single-plex), and the second assay was the time resolved fluorescence energy transfer (TR-FRET) for MMP-7 (THUNDER MMP-7 Biomarker Assay Kit from BioAuxilium, Montreal, Canada; distributed in the United States by Dx4Liver Inc., Phoenix, AZ). For both assays, samples were run according to the manufacturer’s protocol. Samples were run blinded to sample diagnosis and in duplicate. All samples with a coefficient of variance >5% were run a second time. Controls were included in every assay run.
Statistical analysis
An a priori power calculation for sample size was performed using the data reported by Yang et al, in which 75 of the 135 subjects had BA and 60 had non-BA cholestasis.9 Based on this information, 155 subjects in each group were powered to detect a moderate correlation (r > 0.5) between serum MMP-7 and BA under 0.05 significance level, two-sided, at 80%. However, given the need to determine a broader range of serum MMP-7 in North American infants for BA and the correlative analyses of MMP-7 with other markers of cholestasis, we increased the number to 200 subjects in each group.
In our sample, the data did not follow a normal distribution using the Shapiro-Wilk test, so nonparametric tests (Mann-Whitney U and Fisher) were used in the analysis of numeric and categorical data, respectively. Measurements of MMP-7 are presented with medians and IQR. The AUROC was calculated for MMP-7, and each of the other clinical markers was analyzed. The optimal cutoff points to maximize the sensitivity and specificity of the test were calculated using Youden Index. Multivariable logistic regression with AUROC assessed the performance of MMP-7 and other biomarkers. The likelihood ratio was used to compare the difference in the predictive performance of nested models with MMP-7 alone. Statistical significance was defined as p<0.05. Statistical analyses were performed using the R programming language (R Core Team, 2021), GraphPad Prism (GraphPad Software, San Diego, CA), and MedCalc Software Ltd Diagnostic test evaluation calculator (Version 22.003, https://www.medcalc.org/calc/diagnostic_test.php).
RESULTS
Patient characteristics
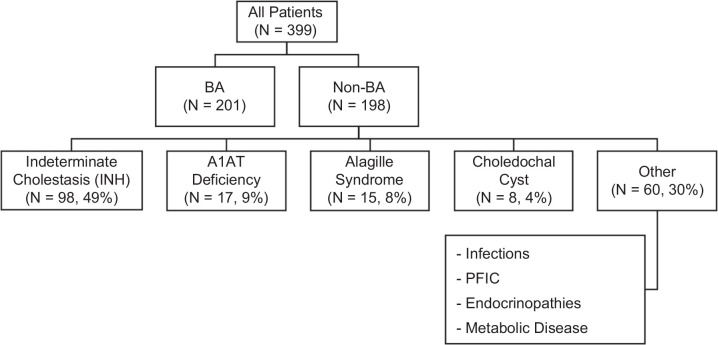
Serum samples and clinical data were retrieved for 399 infants with cholestasis; 201 patients had BA, and 198 had other forms of cholestasis. There were no significant differences between groups in age at presentation (Table 1) or gestational age. A female predominance was seen in BA (Table 1, p = 0.003). Baseline biochemistries showed higher total bilirubin (p = 0.01), direct bilirubin (p = 0.01), and GGT (p<0.001) in the BA group; there were no significant differences in the elevated baseline levels of aminotransferases between groups (Table 1). The racial distribution between groups did not significantly differ (Asian: p = 0.46, Black: p = 0.8, Multiracial: p = 0.43, Other: p = 0.12, White: p = 0.84). In the non-BA cohort, the most common diagnoses were indeterminate cholestasis/idiopathic neonatal hepatitis (49%, N = 98), alpha-1-antitrypsin (A1AT) deficiency (9%, N = 17), Alagille syndrome (8%, N = 15), and choledochal cyst (4%, N = 8). The remaining 30% were composed of more rare diagnoses combined in a category of “Other,” such as infections, genetic forms of intrahepatic cholestasis (collectively known as progressive familial intrahepatic cholestasis), endocrinopathies, and metabolic diseases (Figure 1).
TABLE 1.
Demographic information and baseline laboratory data for BA and non-BA cohorts. p-values comparing cohorts were obtained using the Mann-Whitney U/Wilcoxon rank sum test. Sex comparison was performed with Fisher exact test.
| BA (N=201) | Non-BA (N=198) | p | |
|---|---|---|---|
| Age median (IQR), days | 64 (45–75) | 59 (42–83.3) | 0.94 |
| Gestational age median (IQR), weeks | 39 (37–40) | 39 (37–40) | 0.85 |
| Sex, n %) | |||
| Male | 96 (47.8) | 124 (62.9) | 0.003 |
| Female | 105 (52.2) | 73 (37.1) | — |
| TB, mg/dL | 8 (6.4–9.8) | 6.8 (5.2–10.3) | 0.01 |
| DB, mg/dL | 4.8 (3.4–5.9) | 4.1 (2.9–5.9) | 0.01 |
| ALT, U/L | 132 (77.8–213) | 121 (71–218.5) | 0.52 |
| AST, U/L | 187.5 (124.8–285) | 164 (102–266) | 0.11 |
| GGT, U/L | 600.5 (323–882.5) | 144 (91.3–377.3) | <0.001 |
Abbreviations: BA, biliary atresia; DB, direct bilirubin; GGT, gamma-glutamyl transferase; TB, total bilirubin.
FIGURE 1.
Patient distribution and etiological diagnoses of patients without BA. Flowchart of subject enrollment. A total of 399 infants were enrolled in this study, among whom 201 infants had BA and 198 had other cholestatic diseases (non-BA). The most common diagnoses seen in the non-BA group are outlined. Abbreviations: A1AT, alpha-1-antitrypsin; BA, biliary atresia; INH, idiopathic neonatal hepatitis; PFIC, progressive familial intrahepatic cholestasis.
Discriminative performance of MMP-7
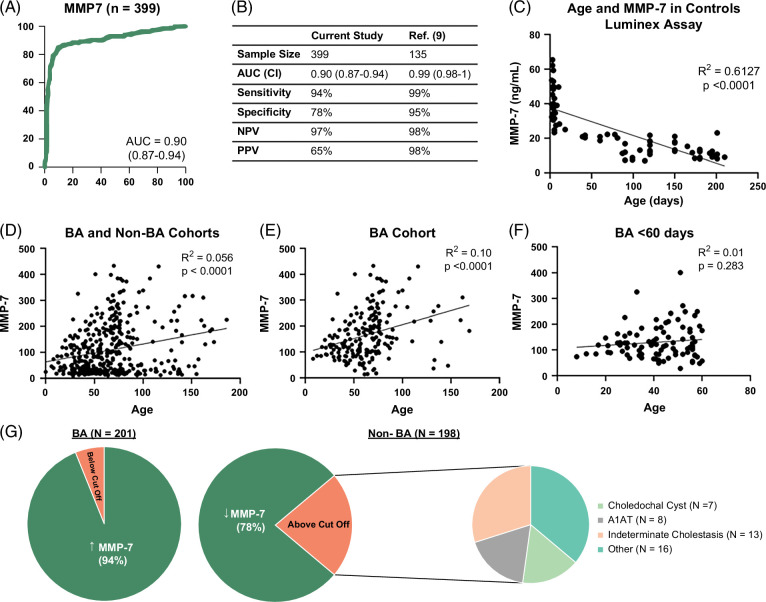
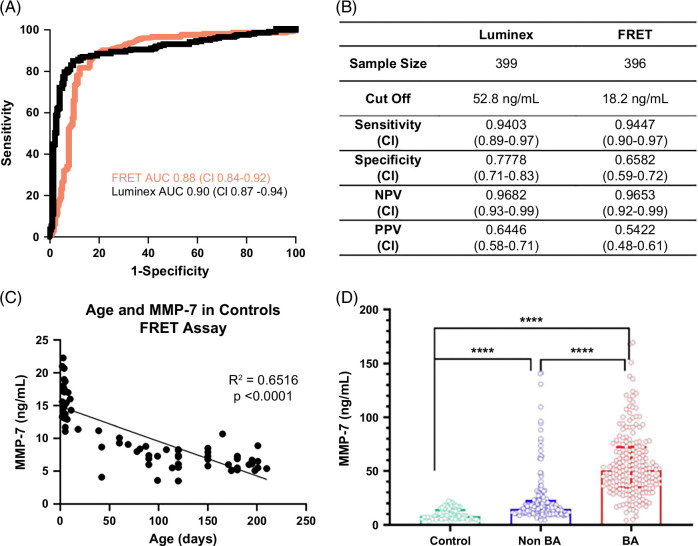
MMP-7 in this North American cohort produced an AUROC of 0.90 (CI: 0.87–0.94; Figure 2A) for the diagnosis of BA. Next, we sought to directly test the performance of MMP-7 using the previously reported cutoff level of MMP-7 at 52.8 ng/mL to distinguish BA from non-BA groups. In our North American cohort, this cutoff produced a sensitivity of 94.03% (CI: 0.89–0.97), specificity of 77.78% (CI: 0.71–0.83), positive predictive value (PPV) of 64.46% (CI: 0.58–0.71), and negative predictive value (NPV) of 96.82% (CI: 0.93–0.99). The overall MMP-7 performance in the North American cohort was lower when compared with the Chinese cohort described (Figure 2B).9 To examine if the North American cohort has an alternative cutoff point, we applied the Youden Index-generated cutoff of 67.4 ng/mL, which yielded a sensitivity of 90.55% (CI: 0.85–0.94), specificity of 84.34% (CI: 0.78–0.89), PPV of 71.25% (CI: 0.65–0.77), and NPV of 95.42% (CI: 0.91–0.98). This overall performance at a cutoff of 67.4 ng/mL had similar discriminative properties when compared with the previously reported cutoff of 52.8 ng/mL.
FIGURE 2.
(A) Shows the AUROC of MMP-7 in the North American cohort. (B) Diagnostic performance of MMP-7 at a cutoff of 52.8 ng/mL and its comparison to a Yang et al9 study in the Chinese population. (C–F) Correlation of MMP-7 with age in healthy infants and in the entire cohort (BA and non-BA cohorts), BA cohort alone, and BA cohort of infants younger than 60 days of age. (G) Pie charts show the percentage of patients above the cutoff of 52.8 ng/mL for patients with BA (left) and the percentage of patients below the cutoff of 52.8 ng/mL for patients without BA (right). The distribution of diagnoses of patients without BA with MMP-7 levels above 52.8 ng/mL is displayed in the far right. Abbreviations: A1AT, alpha-1-antitrypsin; BA, biliary atresia; MMP-7, matrix metalloproteinase-7.
Influence of age and characteristics of false-positive and false-negative results
To better understand potential factors that influence the discriminative properties of the test, we examined the influence of postnatal age on the concentration of MMP-7. First, we measured serum MMP-7 in 73 healthy infants without cholestasis (median age 90 days, IQR 5–150 days). Simple regression analysis of MMP-7 in relation to age (days after birth) in controls showed a negative linear relationship, with a decrease in MMP-7 values as age increases, with an overall moderate effect size of R2>0.60 (p<0.0001, Figure 2C). In the cholestatic cohort, simple regression analysis of MMP-7 in relation to age (days after birth) showed an overall positive linear relationship in the total cohort (BA and non-BA cohorts, R2 = 0.06, p<0.0001), BA alone (R2 = 0.12, p<0.0001), or BA younger than 60 days at diagnosis (R2 = 0.01, p = 0.28) (Figure 2D–F). However, the low R2 values implied low numerical variation according to age.
We next examined how clinical parameters contribute to false-positive and false-negative results. Ninety-four percent of the patients with BA had an MMP-7 level at or >52.8 ng/mL (Figure 2G), with only 12 patients (6%) with values below this cutoff. There were no significant differences seen in age, gestational age, presence of syndromic BA, or biochemistries (aminotransferases, total bilirubin, direct bilirubin, and GGT) between the patients with BA below the cutoff and those above the cutoff. Analyzing the cohort of patients without BA, 78% of them had an MMP-7 level below the cutoff of 52.8 ng/mL. Among the 22% of patients without BA who had levels above the cutoff, the most common diagnoses were indeterminate cholestasis (30%, N = 13), A1AT deficiency (18%, N = 8), choledochal cyst (16%, N = 7), and individual diseases grouped as “other” (36%, N = 16). Patients without BA who had MMP-7 levels above the cutoff were older (median [IQR] for age in days: above cutoff 78 [57–134] days vs. below cutoff 54 [40–77] days, p<0.0001) and had higher ALT (ALT in units per liter above 150.5 [90.5–291] vs. below 107 [67.5–196.5], p = 0.015), AST (AST in units per liter above 217 [123.3–410] vs. below 137 [99–247], p = 0.013), and GGT levels (GGT in units per liter above 378 [123.5–945.5] vs. below 132 [84–265], p = 0.0001). Based on these differences, we examined the performance of MMP-7 in diagnosing BA when compared with GGT and other clinical variables.
Performance of MMP-7 in comparison with other clinical markers
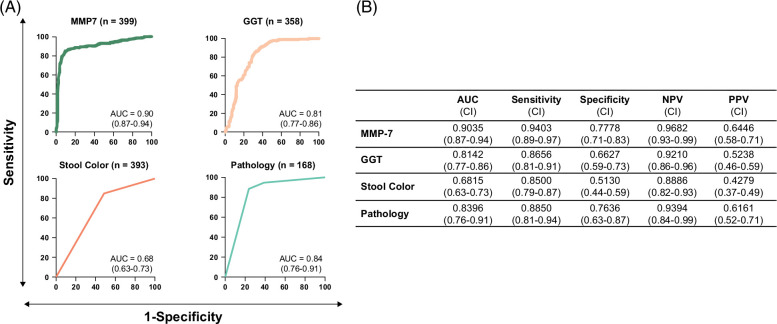
Serum GGT levels (N = 358), the presence of acholic stool (N = 393), and obstructive features on histology (N = 168) have been reported to have discriminative properties for BA. To examine how they perform in relation to MMP-7, we generated AUROCs for each variable, along with sensitivity, specificity, NPV, and PPV at respective optimal cutoff points. MMP-7 performed best among the clinical predictors assessed (Figure 3), with GGT generating an AUROC of 0.81 (CI: 0.77–0.86). Acholic stools, as defined by caregiver or physician observation, were an overall poor predictor of BA, with an AUROC of 0.68 (CI: 0.63–0.73). Obstructive features on liver histology were the second-best predictor of BA, with an AUROC of 0.84 (CI: 0.76–0.91). Despite the numerically lower performance when compared with MMP-7, the findings raised the possibility that the combination of MMP-7 with these markers may produce a composite AUROC with greater discriminative properties for BA.
FIGURE 3.
Comparison of MMP-7 performance with other clinical variables. (A) AUROCS for serum MMP-7, GGT, stool color, and pathology in differentiating BA from non-BA cholestasis in logistic regression analysis. The AUROC value is shown with a 95% CI. (B) Overall AUC values for each variable, along with sensitivity, specificity, NPV, and PPV calculated at each respective optimal cutoff point. Abbreviations: GGT, gamma-glutamyl transferase; MMP-7, matrix metalloproteinase-7.
Combinative and composite models of MMP-7
To directly test this possibility, we evaluated the performance of MMP-7 with other parameters individually or in combination using logistic regression. The addition of an individual clinical parameter to MMP-7 produced AUROCs of 0.907 (MMP-7+GGT, CI: 0.88–0.95), 0.902 (MMP-7+stool color, CI: 0.88–0.94), and 0.876 (MMP-7+pathology, CI: 0.82–0.95) (Table 2). Likelihood ratio analysis showed a significant increase in model prediction, with each of these clinical variables combined with MMP-7 compared to MMP-7 alone (Table 2). A composite model of MMP-7, GGT, and stool color increased AUROC to 0.906 (CI: 0.89–0.95). The numerical increase in AUROC is minimally different in each of the logistic regression models compared with MMP-7 alone. In each of the models, MMP-7 has the largest odds ratio of regression coefficients, demonstrating its degree of impact on the prediction of BA (Table 2).
TABLE 2.
Logistic regression models of each of the clinical markers studied in combination with MMP-7 were created to assess differences between these combined models versus MMP-7 alone. The second column shows the respective coefficients for each variable in the model. The fourth column shows the likelihood ratio comparison of each logistic regression model compared with MMP-7 alone
| Model | Odds ratio | AUROC (CI) | Likelihood ratio, p |
|---|---|---|---|
| MMP-7 + GGT | MMP-7=107.892 GGT=6.018 |
0.907 (0.88–0.95) | <0.0001 |
| MMP-7 + stool color | MMP-7=153.946 Pale stool=3.414 |
0.902 (0.88–0.94) | <0.0001 |
| MMP-7 + pathology | MMP-7=26.962 Pathology (score 2)=2.674 Pathology (score 3)=10.956 |
0.876 (0.82–0.95) | <0.0001 |
| MMP-7 + GGT + stool color | MMP-7=91.076 GGT=4.836 Stool color=3.169 |
0.906 (0.89–0.95) | <0.0001 |
| MMP-7 + GGT + stool color + pathology | MMP-7=19.891 GGT=2.894 Stool color=3.781 Pathology (score 2)=1.948 Pathology (score 3)=8.142 |
0.897 (0.86–0.97) | <0.0001 |
Abbreviations: GGT, gamma-glutamyl transferase; MMP-7, matrix metalloproteinase-7.
Influence of assay technology on MMP-7 quantification
Clinical studies uniformly report highly distinguishing properties of MMP-7 for BA but differ in serum concentrations and cutoff levels.9–13 To investigate if the variability relates to assay types, we quantified MMP-7 in the same serum samples of BA and non-BA cohorts utilizing the TR-FRET assay. Using the remaining volume of serum samples from 396 infants (199 BA, 197 non-BA), the TR-FRET assay generated an AUROC of 0.88 (CI: 0.84–0.92), which largely overlapped with that from the Luminex assay (Figure 4A). Of note, concentrations of MMP-7 for all patients shifted to lower values, as reflected by the cutoff by TR-FRET at 18.2 ng/mL. Despite the lower concentrations, the TR-FRET assay yielded similar sensitivity (94.47%, CI: 0.90–0.97), specificity (65.82%, CI: 0.59–0.72), PPV (54.22%, CI: 0.48–0.61), and NPV (96.53%, CI: 0.92–0.99) when compared with concentrations produced by the Luminex assay (Figure 4B). A simple regression analysis of MMP-7 in relation to age (days after birth) in controls quantified by the TR-FRET assay showed a negative linear relationship, with a decrease in MMP-7 values as age increases, with an overall moderate effect size of R2>0.65 (p<0.0001, Figure 4C), which differed from the levels in subjects with and without BA (Figure 4D). Last, using data from the AUROCs for both assays, we generated minimal and maximal cutoffs that increase sensitivity and specificity to 95% and range with lower accuracy (Figure 5).
FIGURE 4.
ROC curves for TR-FRET and Luminex assays. (A) Superimposed ROC curves for the Luminex assay (black line) and TR-FRET assay (red line). (B) The table on the right displays the AUROCs of Luminex and TR-FRET assays, along with sensitivity, specificity, NPV, and PPV calculated at each respective cutoff point. (C) Scatter plots show simple logistic regression analysis of MMP-7 concentration and postnatal age in days in healthy controls using the TR-FRET assay. (D) Graphs display the median and IQR of MMP-7 concentrations in healthy controls, non-BA cholestasis, and BA using TR-FRET assays. Abbreviations: BA, biliary atresia; FRET, fluorescence energy transfer; MMP-7, matrix metalloproteinase-7.
FIGURE 5.
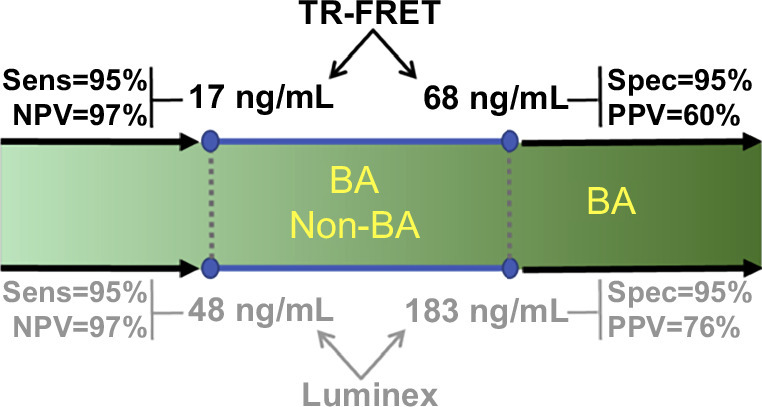
Diagram depicting the cutoffs for serum MMP-7 that increase sensitivity and specificity to 95%, while maintaining high NPV for the TR-FRET (upper panel) and bead-based Luminex assays (lower panel). Abbreviations: BA, biliary atresia; FRET, fluorescence energy transfer.
DISCUSSION
In this large North American cohort, we report that MMP-7 has superior discriminative properties in identifying children with BA as compared with other clinical markers frequently used in clinical practice. At a concentration ≥52.8 ng/mL by the single-plex assay or 18.2 ng/mL by the TR-FRET assay, serum MMP-7 produced high sensitivity and NPV for BA. The addition of GGT, stool color, and pathology to MMP-7 concentrations improved model fit but minimally improved AUROC numerically compared with MMP-7 alone. Comparisons of 2 technologies to quantify serum concentrations produced different cutoff values that were internally consistent within each technology, generated comparable AUROCs, and maintained high discriminative properties as individual assays.
The high sensitivity and NPV of MMP-7 underscore the ability of the assay to identify infants unlikely to have BA, minimizing the likelihood of infants with missed diagnosis when serum MMP-7 values are below the cutoff values. A review of the demographic and laboratory characteristics of the 6% of infants with BA whose serum MMP-7 values were below the cutoff did not show statistical significance when compared to those with high MMP-7 and correctly diagnosed with BA. Among infants in the non-BA cohort, 22% had serum MMP-7 levels above 52.8 ng/mL; in this group of “false positives,” the most common diagnoses were indeterminate cholestasis, A1AT deficiency, and choledochal cyst. As a group, these infants were older and with worse serum biochemistries than those with lower MMP-7 and correctly diagnosed with non-BA cholestasis.
Plotting the concentration of MMP-7 as a function of postnatal age in healthy controls showed a decline in MMP-7 concentrations with an increase in age, which suggested an influence of postnatal development in the suppression of protein expression. In contrast, the assessment of MMP-7 concentrations in infants with cholestasis produced an upward trajectory, suggesting a potential influence of the ongoing liver disease in inducing the expression of MMP-7. These observations have implications for the interpretation of MMP-7, which will tend to be higher in the first 2 postnatal weeks of life, followed by a gradual decrease over time in healthy infants. A limitation of our study is the lack of serum samples from infants born prematurely, which comprise an important patient population that may develop physiologic and pathologic cholestasis, such as cholestasis associated with parenteral nutrition.14 Notwithstanding the potential influence of prenatal and postnatal development on MMP-7 concentration, it is also possible that the higher MMP-7 levels may relate to a longer duration of liver and/or biliary injury. This possibility is supported by the finding that infants with non-BA cholestasis and levels above the cutoff were older and had higher ALT, AST, and GGT. In this context, MMP-7 levels above the cutoff values also occurred in infants with A1AT deficiency, a finding that may relate to the accumulation of the mutant alpha-1-antitrypsin Z (α1-ATZ).15–17
The difference in discriminative cutoff values among previous publications likely relates to differences in the different assay technologies. This is supported by the two different cutoffs produced in the same serum samples by the bead-based Luminex assay and the TR-FRET technology. In our assays, both technologies displayed internal reproducibility and generated comparable AUROCs. Thus, the choice of the assay for potential clinical use may be based on a shorter time-to-result, which may allow for a timely result without compromising assay accuracy.
Despite the high discriminatory performance of serum MMP-7 for BA in this North American population, the AUROC is slightly lower than in Asian populations reported previously, and is similar to the test characteristics reported recently in a European population. Future studies focused on the detailed quantification of MMP-7 in infants born prematurely, and the first 3 postnatal months may be particularly useful for its potential use in identifying BA earlier in the neonatal period.18,19 Recently published data suggest that MMP-7 can be identified on dried blood spots from newborn screening as early as 3 days of life.20 A two-stage screening model utilizing fractionated bilirubin is also used in some parts of the United States to identify potential newborns with BA in nurseries.18,21,22 Future study of MMP-7 concentrations in a much larger cohort of neonates from birth could provide insight into whether incorporation of MMP-7 into a universal fractionated bilirubin screening model could allow for earlier identification and expedite the referral to liver care centers. Ultrasound is another noninvasive method of diagnosis that shows promise in expediting BA diagnosis. The predictive ability of ultrasound in conjunction with MMP-7 is an exciting subject of inquiry for noninvasive screening for BA in cholestatic neonates.23 Beyond diagnosis, MMP-7 could additionally serve a role in prognosticating and monitoring patients with BA after HPE. Evidence from published studies supports that continued elevations in MMP-7 after successful HPE are associated with hepatic fibrosis.15–17,24–26 Collectively, our findings add strong support to the mounting evidence that MMP-7 may be an important tool in the diagnostic algorithm for BA by potentially simplifying the evaluation and facilitating timely access to intraoperative cholangiograms and palliative surgery.
DATA AVAILABILITY STATEMENT
Data sets are deposited in the NIDDK Central Data Repository (https://repository.niddk.nih.gov/studies/probe/).
AUTHOR CONTRIBUTIONS
Sindhu Pandurangi carried out data collection, statistical analysis and interpretation of results, and manuscript writing. Reena Mourya and Shreya Nalluri ran all assays and collected MMP-7 measurements; Lin Fei and Shun Dong assisted with statistical analysis. Sanjiv Harpavat, Stephen L. Guthery, Jean P. Molleston, Philip Rosenthal, Ronald J. Sokol, Kasper S. Wang, Vicky Ng, Estella M. Alonso, Estella M. Alonso, Saul J. Karpen, Kathleen M. Loomes, John C. Magee, Benjamin L. Shneider, Simon P. Horslen, and Jeffrey H. Teckman reviewed the manuscript critically for important intellectual content. Jorge A. Bezerra was responsible for study conception and design, analysis of results, and manuscript preparation and revision. Heather Van Doren, Senior Medical Editor with Arbor Research Collaborative for Health, provided editorial assistance on this manuscript.
ACKNOWLEDGMENTS
The authors thank the Scientific and Data Coordinating Center for managing all studies and providing data and specimens, and the Clinical Research Coordinators of individual ChiLDReN Centers for patient recruitment and acquisition of tissue and data.
FUNDING INFORMATION
Supported by the National Institute of Diabetes, Digestive, and Kidney Diseases U01 grants DK62436 (Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL), DK62497 (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH), DK62453 (Children’s Hospital Colorado and University of Colorado School of Medicine, Aurora, CO), DK62481 (Children’s Hospital of Philadelphia, Philadelphia, PA), DK62466 (Children’s Hospital of Pittsburgh, Pittsburgh, PA), DK62500 (UCSF Children’s Hospital, San Francisco, CA), DK62453 (Saint Louis University School of Medicine, St. Louis, MO), DK84536 (Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN), DK84575 (Seattle Children’s Hospital, Seattle WA), DK103135 (Hospital for Sick Children, Toronto, Ontario, Canada), DK103140 (University of Utah, Salt Lake City, UT), DK84538 (Children’s Hospital Los Angeles, Los Angeles, CA), DK062470 (Children’s Healthcare of Atlanta, Atlanta, GA), DK103149 (Texas Children’s Hospital, Houston, TX), and DK62456 (Scientific Data Coordinating Center, Ann Arbor, MI). Also supported by the National Institute of Health National Center for Advancing Translational Sciences, Clinical and Translational Sciences Awards UL1TR001422 (Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL), UL1TR000077 (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH), UL1TR002535 (Children’s Hospital Colorado and the University of Colorado School of Medicine, Aurora, CO), UL1TR000005 (Children’s Hospital of Pittsburgh, Pittsburgh, PA), UL1TR000004 (UCSF Children’s Hospital, San Francisco, CA), UL1TR001108 (Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN), UL1TR000423 (Seattle Children’s Hospital, Seattle WA), UL1TR000130 (Children’s Hospital Los Angeles, Los Angeles, CA), UL1TR000454 (Children’s Healthcare of Atlanta, Atlanta, GA), and UL1TR001878 (The Children’s Hospital of Philadelphia, Philadelphia, PA).
CONFLICTS OF INTEREST
Sanjiv Harpavat has other interests with Syneos Health. Jean P. Molleston received grants from AbbVie, Albireo, CF Foundation, Gilead, and Mirum. Philip Rosenthal consults, is on the speakers’ bureau, and received grants from Mirum. He consults and received grants from Albireo, Dicerna, Takeda, and Traverse. He consults for Audentes, Encoded, and Taysha. He received grants from Arrowhead and Gilead. Ronald J. Sokol advises Albireo and Mirum. He is on the speakers’ bureau for Astellas. Evelyn K. Hsu received grants from Albireo, Gi, and Mirum. Saul J. Karpen consults for Albireo, HemoShear, Intercept, and Mirum. Kathleen M. Loomes consults and received grants from Albireo and Mirum. She consults for Travere. Simon P. Horslen consults for Albireo, Alexion, and iECURE. He received grants from Mirum. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: BA, biliary atresia; ChiLDReN, Childhood Liver Disease Research Network; GGT, gamma-glutamyl transferase; HPE, hepatoportoenterosomy; MMP-7, matrix metalloproteinase-7; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; TR-FRET, time resolved fluorescence energy transfer.
Contributor Information
Sindhu Pandurangi, Email: Sindhu.Pandurangi@utsouthwestern.edu.
Reena Mourya, Email: Reena.Mourya@UTSouthwestern.edu.
Shreya Nalluri, Email: nalluris@bu.edu.
Lin Fei, Email: lin.fei@cchmc.org.
Shun Dong, Email: shun.dong@ku.edu.
Sanjiv Harpavat, Email: harpavat@bcm.edu.
Stephen L. Guthery, Email: stephen.guthery@hsc.utah.edu.
Jean P. Molleston, Email: jpmolles@iu.edu.
Philip Rosenthal, Email: prosenth@ucsf.edu.
Ronald J. Sokol, Email: ronald.sokol@childrenscolorado.org.
Kasper S. Wang, Email: kasper.wang@sickkids.ca.
Vicky Ng, Email: vicky.ng@sickkids.ca.
Estella M. Alonso, Email: ealonso@luriechildrens.org.
Evelyn K. Hsu, Email: evelyn.hsu@seattlechildrens.org.
Saul J. Karpen, Email: saul.karpen@emory.edu.
Kathleen M. Loomes, Email: loomes@email.chop.edu.
John C. Magee, Email: mageej@umich.edu.
Benjamin L. Shneider, Email: Benjamin.Shneider@bcm.edu.
Simon P. Horslen, Email: horslensp@upmc.edu.
Jeffrey H. Teckman, Email: Jeff.Teckman@health.slu.edu.
Jorge A. Bezerra, Email: jorge.bezerra@utsouthwestern.edu.
REFERENCES
- 1. Lakshminarayanan B, Davenport M. Biliary atresia: A comprehensive review. J Autoimmun. 2016;73:1–9. [DOI] [PubMed] [Google Scholar]
- 2. Feldman AG, Mack CL. Biliary atresia: Clinical lessons learned. J Pediatr Gastroenterol Nutr. 2015;61:167–75. [DOI] [PubMed] [Google Scholar]
- 3. Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, et al. Biliary atresia: Current concepts and research directions. Summary of a symposium Hepatology. 1996;23:1682–92. [DOI] [PubMed] [Google Scholar]
- 4. Perlmutter DH, Shepherd RW. Extrahepatic biliary atresia: A disease or a phenotype? Hepatology. 2002;35:1297–304. [DOI] [PubMed] [Google Scholar]
- 5. Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: Current concepts. J Pediatr Gastroenterol Nutr. 2003;37:4–21. [DOI] [PubMed] [Google Scholar]
- 6. Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: Defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol. 2015;12:342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shneider BL, Moore J, Kerkar N, Magee JC, Ye W, Karpen SJ, et al. Initial assessment of the infant with neonatal cholestasis—Is this biliary atresia? PLoS One. 2017;12:e0176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lertudomphonwanit C, Mourya R, Fei L, Zhang Y, Gutta S, Yang L, et al. Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia. Sci Transl Med. 2017;9:eaan8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang L, Zhou Y, Xu PP, Mourya R, Lei HY, Cao GQ, et al. Diagnostic accuracy of serum matrix metalloproteinase-7 for biliary atresia. Hepatology. 2018;68:2069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu JF, Jeng YM, Chen HL, Ni YH, Hsu HY, Chang MH. Quantification of serum matrix metallopeptide 7 levels may assist in the diagnosis and predict the outcome for patients with biliary atresia. J Pediatr. 2019;208:30–7. [DOI] [PubMed] [Google Scholar]
- 11. Jiang J, Wang J, Shen Z, Lu X, Chen G, Huang Y, et al. Serum MMP-7 in the diagnosis of biliary atresia. Pediatrics. 2019;144:e20190902. [DOI] [PubMed] [Google Scholar]
- 12. Sakaguchi H, Konishi KI, Yasuda R, Sasaki H, Yoshimaru K, Tainaka T, et al. Serum matrix metalloproteinase-7 in biliary atresia: A Japanese multicenter study. Hepatol Res. 2022;52:479–87. [DOI] [PubMed] [Google Scholar]
- 13. Aldeiri B, Si T, Huang Z, Torner N, Ma Y, Davenport M, et al. Matrix metalloproteinase-7 and osteopontin serum levels as biomarkers for biliary atresia. J Pediatr Gastroenterol Nutr. 2023;77:97–102. [DOI] [PubMed] [Google Scholar]
- 14. Salvi PS, Fawaz R, Cowles RA. Comparing serum matrix metalloproteinase-7 in parenteral nutrition associated liver disease and biliary atresia. J Pediatr. 2022;249:97–100. [DOI] [PubMed] [Google Scholar]
- 15. Kerola A, Lampela H, Lohi J, Heikkila P, Mutanen A, Hagstrom J, et al. Increased MMP-7 expression in biliary epithelium and serum underpins native liver fibrosis after successful portoenterostomy in biliary atresia. J Pathol Clin Res. 2016;2:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iordanskaia T, Hubal MJ, Koeck E, Rossi C, Schwarz K, Nadler EP. Dysregulation of upstream and downstream transforming growth factor-beta transcripts in livers of children with biliary atresia and fibrogenic gene signatures. J Pediatr Surg. 2013;48:2047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang CC, Chuang JH, Chou MH, Wu CL, Chen CM, Wang CC, et al. Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod Pathol. 2005;18:941–50. [DOI] [PubMed] [Google Scholar]
- 18. Rabbani T, Guthery SL, Himes R, Shneider BL, Harpavat S. Newborn screening for biliary atresia: A review of current methods. Curr Gastroenterol Rep. 2021;23:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bezerra JA, Wells RG, Mack C, Karpen S, Hoofnagle JH, Doo E, et al. Biliary atresia: Clinical and research challenges for the twenty-first century. Hepatology. 2018;68:1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee CS, Ni YH, Chen HL, Wu JF, Hsu HY, Chien YH, et al. A pilot study of biliary atresia newborn screening using dried blood spot matrix metalloproteinase-7. J Pediatr Gastroenterol Nutr. 2023;76:418–23. [DOI] [PubMed] [Google Scholar]
- 21. Harpavat S, Garcia-Prats JA, Anaya C, Brandt ML, Lupo PJ, Finegold MJ, et al. Diagnostic yield of newborn screening for biliary atresia using direct or conjugated bilirubin measurements. JAMA. 2020;323:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kastenberg ZJ, Deneau MR, O’Brien EA, Huynh K, Book LS, Srivastava R, et al. Fractionated bilirubin among 252,892 Utah newborns with and without biliary atresia: A 15-year historical birth cohort study. J Pediatr. 2023;257:113339. [DOI] [PubMed] [Google Scholar]
- 23. Dai SY, Sun YQ, Wu Y, Chen G, Sun S, Dong R, et al. Development and assessment of screening nomogram for biliary atresia based on hepatobiliary ultrasonographic features. Front Pediatr. 2021;9:625451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramachandran P, Balamurali D, Peter JJ, Kumar MM, Safwan M, Vij M, et al. RNA-seq reveals outcome-specific gene expression of MMP7 and PCK1 in biliary atresia. Mol Biol Rep. 2019;46:5123–30. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi H, Li ZX, Yamataka A, Lane GJ, Miyano T. Clinical evaluation of serum levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases as predictors of progressive fibrosis in postoperative biliary atresia patients. J Pediatr Surg. 2002;37:1030–3. [DOI] [PubMed] [Google Scholar]
- 26. He L, Ip DKM, Tam G, Lui VCH, Tam PKH, Chung PHY. Biomarkers for the diagnosis and post-Kasai portoenterostomy prognosis of biliary atresia: A systematic review and meta-analysis. Sci Rep. 2021;11:11692. [DOI] [PMC free article] [PubMed] [Google Scholar]