Abstract
PURPOSE
National estimates of cancer clinical trial participation are nearly two decades old and have focused solely on enrollment to treatment trials, which does not reflect the willingness of patients to contribute to other elements of clinical research. We determined inclusive, contemporary estimates of clinical trial participation for adults with cancer using a national sample of data from the Commission on Cancer (CoC).
METHODS
The data were obtained from accreditation information submitted by the 1,200 CoC programs, which represent more than 70% of all cancer cases diagnosed in the United States each year. Deidentified, institution-level aggregate counts of annual enrollment to treatment, biorepository, diagnostic, economic, genetic, prevention, quality-of-life (QOL), and registry studies were examined. Overall, study-type estimates for the period 2013-2017 were estimated. Multiple imputation by chained equations was used to account for missing data, with summary estimates calculated separately by type of program (eg, National Cancer Institute [NCI]–designated cancer centers) and pooled.
RESULTS
The overall estimated patient participation rate to cancer treatment trials was 7.1%. Patients with cancer participated in a wide variety of other studies, including biorepository (12.9%), registry (7.3%), genetic (3.6%), QOL (2.8%), diagnostic (2.5%), and economic (2.4%) studies. Treatment trial enrollment was 21.6% at NCI-designated comprehensive cancer centers, 5.4% at academic (non–NCI-designated) comprehensive cancer programs, 5.7% at integrated network cancer programs, and 4.1% at community programs. One in five patients (21.9%) participated in one or more cancer clinical research studies.
CONCLUSION
In a first-time use of national accreditation information from the CoC, enrollment to cancer treatment trials was 7.1%, higher than historical estimates of <5%. Patients participated in a diverse set of other study types. Contributions of adult patients with cancer to clinical research is more common than previously understood.
INTRODUCTION
Patient participation in clinical trials is critical for developing new treatments for cancer. An accurate understanding of how commonly patients participate in trials is necessary for trialists and policymakers seeking to devise strategies to conduct the most relevant trials.1 In the 1990s and early 2000s, the rate of adult cancer treatment trial participation was estimated to be 2%-3%.2-4 These studies relied on enrollments to government-sponsored trials only and did not include enrollments to industry-sponsored studies. No new evidence on the basis of original data has been examined for many years to determine an inclusive estimate of trial participation for all adults with cancer. Moreover, the participation of patients in cancer studies has focused solely on their enrollment to treatment trials.2-4 This is clearly important for the development of new treatments but does not reflect patient contributions to other important elements of clinical research, such as biorepository or quality-of-life (QOL) studies.
CONTEXT
Key Objective
What is a contemporary estimate of enrollment to cancer treatment trials and other categories of cancer clinical research studies across a diverse set of clinical care facilities in the United States?
Knowledge Generated
In a first-time use of national accreditation and enrollment data submitted to the Commission on Cancer, we estimated that the overall participation rate to cancer treatment trials was 7.1% from 2013 to 2017. Patients participated in a diverse set of other study types, including biorepository (12.9%), registry (7.3%), genetic (3.6%), quality-of-life (2.8%), diagnostic (2.5%), and economic (2.4%) studies.
Relevance (S.B. Wheeler)
These contemporary data provide encouraging insights about the increasing numbers of people with cancer participating in cancer clinical research studies, including but not limited to cancer treatment studies; however, more efforts are needed to expand study access and to anticipate and mitigate barriers to clinical research participation, especially among underrepresented groups.*
*Relevance section written by JCO Associate Editor Stephanie B. Wheeler, PhD, MPH.
In a first-time collaboration, the American Cancer Society Cancer Action Network collaborated with the Commission on Cancer (CoC) to rigorously identify a more contemporary rate of adult clinical trial participation. The CoC is a consortium of cancer programs and member organizations who develop and implement standards, quality measures, and quality improvement projects in support of accrediting cancer care facilities across the United States.5 We used CoC repository data to identify a contemporary estimate of enrollment to cancer treatment trials across a diverse set of clinical care facilities in the United States. This repository also provided first-time estimates of enrollment to other types of cancer studies that inform patient care, including patient QOL, genetic and diagnostic studies, and biorepository studies.
METHODS
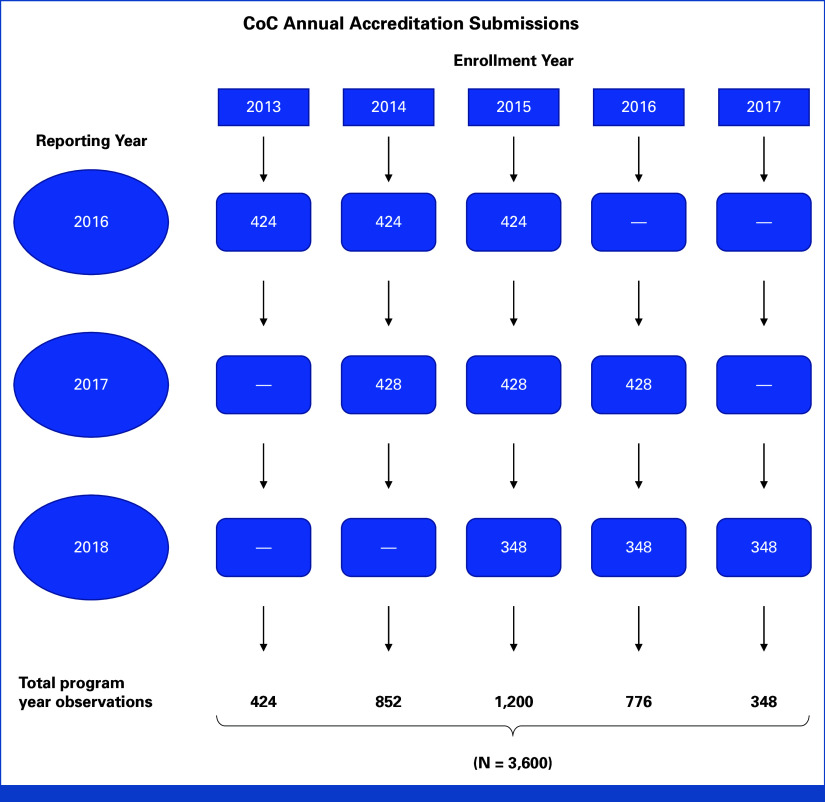
Data were from the CoC accreditation repository.5 Institutions participating in the CoC accreditation program represent more than 70% of all cases of cancer diagnosed in the United States each year.6-10 Programs are accredited every 3 years, and each year, the CoC receives clinical trial enrollment data for accreditation purposes from approximately one third of all CoC member programs (Fig 1). Thus, there are three reporting program panels, each reporting every 3 years, with each program reporting enrollment data for each of the previous 3 years. We received complete data over the 3-year period from 2016 through 2018. The data are self-reported in aggregate format for each accredited program (or site); thus, individual patient-level attributes were not available.
FIG 1.
Flow diagram illustrating the repository design using CoC accreditation enrollment data. Each year, the CoC receives clinical trial enrollment data for accreditation purposes from approximately one third of all CoC member programs. Thus, there are three reporting program panels, each of which reports its accreditation data every 3 years. At each reporting time, each program is required to report enrollment data for each of the previous 3 years. CoC, Commission on Cancer.
Institutions were not identifiable, and no geospatial identifiers were provided to the research team. Given the absence of identifiable patient-level data, institutional review board approval of this study was not required.
CoC Data
Under CoC program requirements, any patient diagnosed and/or treated at the site is included in the program analytic caseload, the site-level denominator of patients with cancer, representing a surrogate for the size of the cancer program. Enrollment rates were based on this analytic caseload denominator.
The CoC collects data on multiple categories of studies (Table 1). Patients may have participated in more than one type of cancer study; thus, category-specific indications of study participation were not mutually exclusive.
TABLE 1.
Definitions of Types of Cancer Studies
| Type of Trial or Study | CoC Terminology | Description |
|---|---|---|
| Treatment | Treatment trials | Participants receive specific interventions according to the research plan or protocol. These interventions may be medical products, such as drugs or devices; procedures; or changes to participants' behavior, such as diet. |
| Biorepository | Cancer-specific biorepositories or tissue banks | Cancer specific-biobanks that collect cancer tissue or blood samples specifically for cancer research purposes |
| Diagnostic | Diagnostic trials | Examining tests or procedures used to identify or diagnose cancer |
| Economics | Economics of care related to cancer care | Assesses the costs and effectiveness of cancer interventions and/or analyzes the financial impact of oncology care on patients |
| Quality-of-life | Quality-of-life or supportive care trials | A broad concept or term used to define observational studies (usually questionnaires or longitudinal studies) that include subjective evaluations of both positive and negative aspects of the patient's life that are affected by the diagnosis and/or treatment of cancer |
| Genetics | Genetic studies | Studies that examine contributing genes or how different exposures could modify the effect of a gene mutation that may be a risk for cancer development OR genetic assessments that examine genetic polymorphisms and mutations for early risk assessment |
| Registries | Patient registries | Patient registries with an underlying cancer research focus—epidemiologic studies. Must have underlying cancer research focus, such as National Oncologic PET Registry |
Aggregate data were submitted for individual cancer programs defined according to their type of facility, program structure, services provided, and the number of cases accessioned each year (Table 2).12 Categories of programs included in the analysis were Academic (non–National Cancer Institute [NCI]–designated) Comprehensive Cancer Programs (ACADs), Community Cancer Programs (CCPs), Comprehensive Community Cancer Programs (CCCPs), Integrated Network Cancer Programs (INCPs), and NCI-Designated Comprehensive Cancer Center Programs (NCIPs).13 Given our focus on adult patients with cancer to clinical trials, enrollments from Pediatric Cancer Programs were not obtained. Additionally, data from Freestanding Cancer Center Programs, Hospital Associate Cancer Programs, and Veterans Affairs Cancer Programs were not available. Given the limited distinction between CCPs and CCCPs, and the likelihood of referrals across programs as specified in the definition, these programs were combined under CCPs.
TABLE 2.
Definitions of Institutional Programs
| Program Name | Acronym | Description |
|---|---|---|
| Academic Comprehensive Cancer Program | ACAD | The facility participates in postgraduate medical education in at least four program areas. The facility accessions more than 500 newly diagnosed cancer cases each year. The facility offers the full range of diagnostic and treatment services either on-site or by referral |
| Integrated Network Cancer Program | INCP | The organization owns, operates, leases, or is part of a joint venture with multiple facilities providing integrated cancer care and offers comprehensive services. At least one facility in the category is a hospital and must be a CoC-accredited cancer program. Generally, INCPs are characterized by a unified cancer committee, standardized registry operations with a uniform data repository, and coordinated service locations and practitioners. Each entity of the INCP meets performance expectations for the quality measures under the umbrella of the integrated program. The INCP participates in cancer-related clinical research either by enrolling patients in cancer-related clinical trials or by referring patients for enrollment at another facility or through a physician's office. Participation in the training of resident physicians is optional, and there is no minimum caseload requirement for this category |
| NCI-Designated Comprehensive Cancer Center Program | NCIP | The facility secures an NCI peer-reviewed Cancer Center Support Grant and is designated Comprehensive Cancer Center by the NCI. A full range of diagnostic and treatment services and staff physicians are available. Participation in the training of resident physicians is optional, and there is no minimum caseload requirement |
| Veterans Affairs Cancer Program | VACP | The facility provides care to military veterans and offers the full range of diagnostic and treatment services either on-site or by referral, preferably to CoC-accredited cancer program(s). There is no minimum caseload required |
| Comprehensive Community Cancer Program | CCCP | The facility accessions 500 or more newly diagnosed cancer cases each year. The facility provides a full range of diagnostic and treatment services either on-site or by referral |
| Community Cancer Program | CCP | The facility accessions more than 100 but fewer than 500 newly diagnosed cancer cases each year and provides a full range of diagnostic and treatment services, but referral for a portion of diagnosis or treatment may occur |
| Hospital Associate Cancer Program | HACP | The facility accessions 100 or fewer newly diagnosed cancer cases each year and has a limited range of diagnostic and treatment services available on-site. Other services are available by referral. Clinical research is not required. Participation in the training of resident physicians is optional |
| Pediatric Cancer Program | PCP | The facility provides care only to children, or the pediatric oncology program is a component within a larger CoC-accredited facility. The facility may be associated with a medical school and participate in training pediatric residents. The facility or pediatric oncology program offers the full range of diagnostic and treatment services for pediatric patients either on-site or by referral. The facility is required to participate in cancer-related clinical research focused on pediatric patients. There is no minimum caseload requirement for this category |
| Freestanding Cancer Center Program | FCCP | The facility is a nonhospital-based program and offers at least one cancer-related treatment modality. The full range of diagnostic and treatment services are available by referral. Referral to CoC-accredited cancer program(s) is preferred. There is no minimum caseload requirement for this category |
NOTE. Adapted from American College of Surgeons.11
Abbreviations: CoC, Commission on Cancer; NCI, National Cancer Institute.
Data on enrollment to studies about economics of cancer care and QOL were available separately for the 2016 panel but were combined for panels 2017 and 2018. To derive estimates for these study types, site-specific estimates for enrollment to economics of care and QOL studies for panels 2017 and 2018 were prorated on the basis of the 2016 panel estimates.
Statistical Methods
The primary aim was to derive a national estimate of clinical treatment trial participation during the years 2013-2017. The unit of analysis was each individual set of site-level aggregate data defined by reporting panel (2016, 2017, and 2018 data collection panels) and year of reporting (2013-2017). We also estimated participation rates for other study types, including biorepository or biobank, diagnostic, economic, genetic, QOL, and registry studies.
To account for missing data, multiple imputation by chained equations (MICE) was used.14 This approach relies on the assumption that missing data are missing at random and that randomness can be modeled using available variables. MICE is valuable for imputing a data set with missing values across multiple variables, and for its capacity to manage variables of different types (eg, continuous v categorical), including data with complex missing data patterns.15,16 Thus—along with fields for each of the study categories—the input matrix for the multiple imputation also included program (institution) type, reporting panel, and year. Analyses were conducted in R using the mice package.17 We specified a multiple imputation model with n = 100 imputations and 50 iterations, using a predictive mean model imputation method.18 Estimates of participation rates in each kind of study were calculated on the basis of the imputed matrix as the number of reported enrollments divided by the number of cases, separately by program type, and pooled.
In some instances, programs reported that a portion of patients participated in studies categorized as other. These enrollments were distributed to the known study categories according to observed enrollment distributions as follows. For each study category, a weight was derived. For the treatment and diagnostic trial categories, the weights were using ClinicalTrials.gov data as the proportion of interventional trials over the 10-year period from 2013 through 2022 (inclusive) classified as other intervention type that were coded as having been conducted for the purpose of treatment (33.3%) or diagnosis (2.8%), respectively.19 For the remaining study categories, ClinicalTrials.gov categorizations did not align with our study categories. Thus, remaining weights were calculated as the proportion of the study category enrollment to the sum of total enrollments for all studies. For example, if there were four enrollments to repository studies, and 20 total enrollments, the repository study weight would be 0.2 (4/20). Because treatment and diagnostic trial weights were determined separately, weights for other categories were prorated so the total of weights = 1.0. Enrollments to other studies were then reassigned to a given study category as the number of other study enrollments multiplied by category-specific estimated weight. Under full assignment, 100% of the calculated weight was used. In our example, if five enrollments to other studies were reported, and the repository study enrollment was four and its corresponding weight was 0.20 (as noted above), then 5 × 0.20 = 1 of the other enrollments would be assigned to the repository study total, and the estimated total repository study enrollment would increase to 5. We also calculated estimates using 25%, 50%, and 75% of the estimated weight (ie, partial assignment). Given uncertainty about the extent to which studies coded as other were attributable to known categories, we used 50% partial assignment (rather than 100% full assignment) for our base case estimates.
Finally, we estimated overall participation in any of the seven study categories. This estimate was limited because data on overall participation in any study at each site were not available. Estimates were derived by assuming that study type enrollment categories within sites were strictly nested, so site-level total enrollment was best represented by enrollment to the type of study with maximum participation. For instance, if, among 100 cancer cases, a site reported 10 enrollments to biorepository studies, four enrollments to treatment trials, and five enrollments to registry studies, total study enrollment for the site would be designated as 10% (10/100). This estimate of total study participation is conservative (representing a lower bound) if study type enrollment categories are nonoverlapping (eg, non-nested) to any degree.
RESULTS
Data from 1,200 programs were available, each reporting 3 years of data, representing N = 3,600 site-years of data (Fig 1). The most common program types included CCPs (908, 75.7%), followed by ACCPs (180, 15.0%), INCPs (69, 5.8%), and NCIPs (43, 3.6%). Given the nature of the reporting across the three panel cohorts, the plurality of site-years of data (N = 1,200, 33.3%) for the 2013-2017 period were from year 2015. For treatment trials, data were missing for 16.1% of site-years. Missing data were more common for other study types, including biorepository (55.8%), diagnostic (79.1%), economic (71.1%), genetic (77.3%), QOL (61.5%), and registry (59.0%) studies. Overall, 8.9% of enrollments were to other studies.
Estimates of Study Participation Overall and by Institution Type
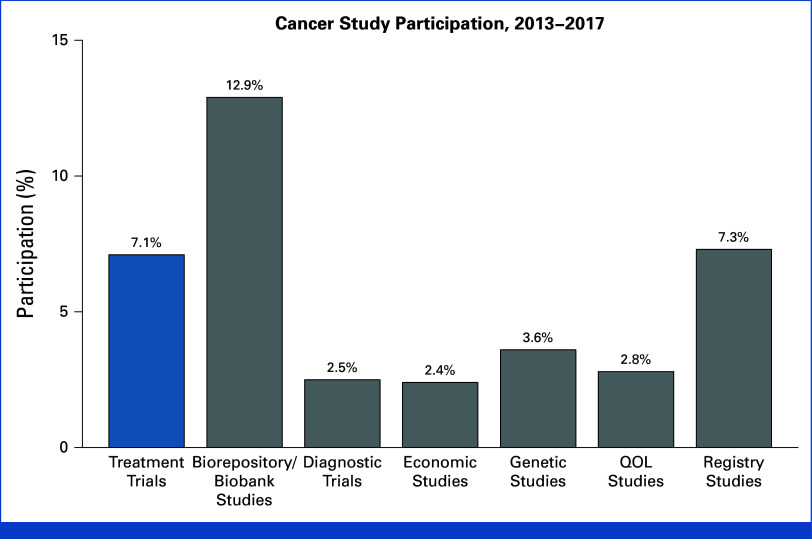
The overall estimate of treatment trial enrollment was 7.1% (Fig 2). Patients participated in a wide variety of other study types, including biorepository (12.9%), registry (7.3%), genetic (3.6%), QOL (2.8%), diagnostic (2.5%), and economic (2.4%) studies.
FIG 2.
Estimated cancer study participation by type of study, 2013-2017. The estimate for treatment trial enrollment is highlighted in blue. QOL, quality-of-life.
Enrollment for different study types varied by type of institution (Appendix Table A1, online only). Estimated treatment trial participation was greatest at NCIPs (21.6%) and lowest at CCPs (4.1%; Fig 3). Nearly two of five patients contributed to biorepository studies at NCIPs (39.1%). For all study categories, participation was highest at NCIPs.
FIG 3.
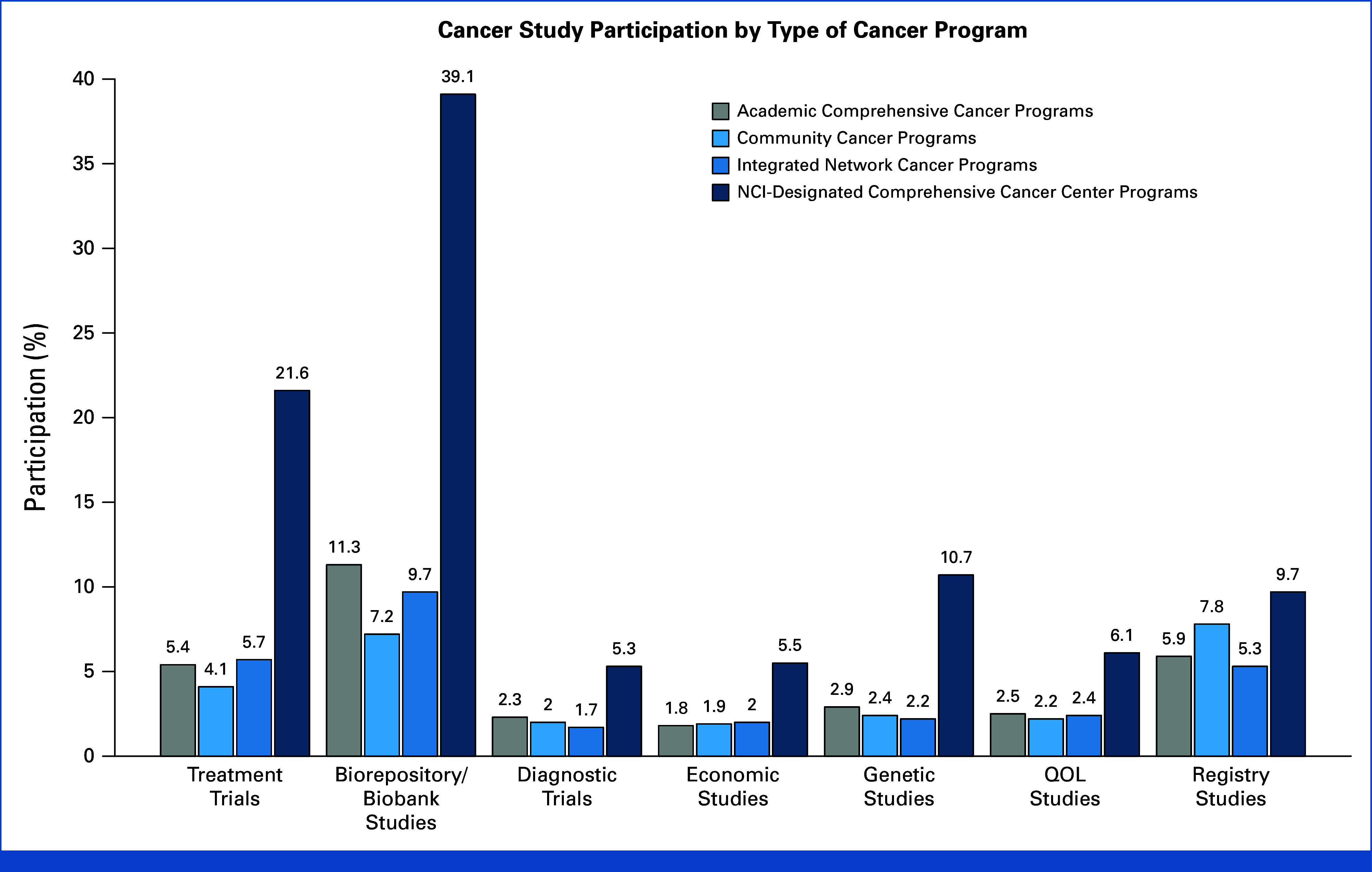
Study participation estimates by category of cancer program. Numerators and denominators are provided in Appendix Table A1. Given large numbers, all pairwise comparisons were strongly (P < .01) statistically significantly different by chi-square tests. NCI, National Cancer Institute; QOL, quality-of-life.
Additional Analyses
In a sensitivity analysis, we allowed the strategy of distributing enrollments from studies categorized as other to the defined study categories to vary according to the approaches described in the methods. Additionally, we assessed the sensitivity of the findings to data completeness. With seven study domains, observations may have been missing for zero studies up to all seven studies. Thus, we iteratively excluded observations depending on the number of missing values for study-level estimates (from 0 up to seven missing values). In total, with six strategies for distributing other enrollments to specified studies, and eight levels of data completeness, 48 total analyses were conducted for each study category. The base case analysis was similar to, or modestly lower than, the mean of the sensitivity analyses in all instances except registry studies, improving confidence about the internal validity of base case estimates in relation to analysis parameters (Fig 4).
FIG 4.
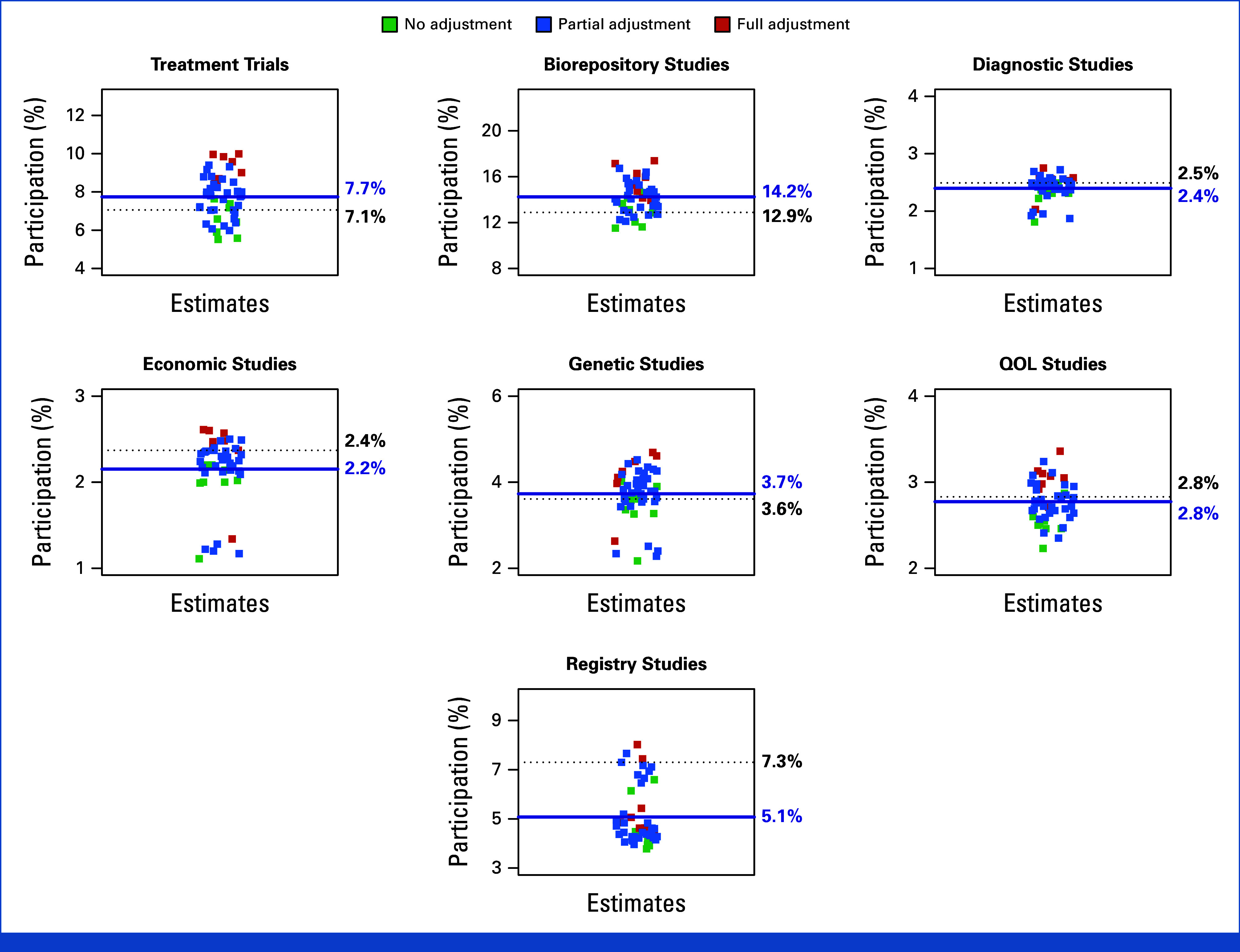
Sensitivity analyses. Estimates were allowed to vary depending on the proportion of enrollments from studies categorized as other and according to data completeness. With seven study domains, observations may have been missing for zero studies up to all seven studies. Forty-eight total analyses were conducted for each study type. The base case finding is indicated by the horizontal dotted line in black and the mean of the sensitivity analyses is indicated by the horizontal line in blue. The base case versus mean (range) sensitivity analysis estimates were 7.1% versus 7.7% (5.5%-10.0%) for treatment trials, 12.9% versus 14.3% (11.5%-17.4%) for biorepository studies, 2.6% versus 2.4% (1.8%-2.8%) for diagnostic studies, 2.4% versus 2.2% (1.1%-2.6%) for economic studies, 3.7% versus 3.6% (2.2%-4.7%) for genetic studies, 2.8% versus 2.8% (2.2%-3.4%) for QOL studies, and 7.3% versus 5.1% (3.8%-8.0%) for registry studies. QOL, quality-of-life.
Overall participation in any category of study was estimated to be at least 21.9%. If biorepository studies were excluded, the estimate was 17.6%, and if both biorepository studies and registry studies were excluded, the estimate was 14.3%.
DISCUSSION
In a first-time use of CoC national accreditation and enrollment data, the estimated participation rate to cancer treatment trials was 7.1% from 2013 to 2017. All (100%) sensitivity estimates were at least 5.5%, providing confidence that this contemporary estimate of treatment trial participation is higher than historical estimates of 2%-3%.2-4,20 Patients also participated in a diverse set of other types of studies, including biorepository, genetic, and QOL studies. Moreover, at least one in five patients (21.9%) contribute to any kind of clinical research study. Participation in cancer clinical research studies still has room for improvement. Nonetheless, these results suggest that contributions to clinical research for adults with cancer is more common than is typically realized.
The conventional understanding of participation in adult cancer treatment trials was informed by studies about enrollment to NCI-sponsored cooperative group trials conducted in the 1990s-2000s. Tejeda et al4 reported that 4.0% of patients age 20-49 years and 1.5% of patients age 50 years or older participate in clinical trials. Sateren et al3 reported that among adult patients with cancer between April 1998 and April 1999, 2.5% enrolled in clinical trials. Murthy et al2 reported that trial participants represented 1.7% of the total number of incident cancer cases diagnosed from 2000 to 2002. These studies informed landmark policy documents, including a 2010 Institute of Medicine report, which indicated that approximately 3 percent of adult patients with cancer participate in clinical trials.20 But patients also routinely participate in trials sponsored by pharmaceutical companies that enroll at least as many patients as NCI-sponsored trials.21-23 Our estimate that 7.1% of patients participate in treatment trials is consistent with this premise and reflects recent studies suggesting that overall treatment trial participation is between 6% and 8%.24-27 Notably, these estimates are also similar to estimates from other industrialized countries such as the United Kingdom and France.28,29
Among CoC sites, fully 96.4% represented ACAD, CCP, and INCP sites enrolling 4.1%-5.7% of patients to therapeutic trials; by contrast, NCIP enrollment substantially exceeds these estimates (21.6%). NCIPs, by definition, receive dedicated federal funding to conduct clinical trials, the kind of support not typically available for other programs, especially community-based sites where most patients in the United States receive their care. Therefore, efforts to increase clinical trial enrollment may depend, critically, on providing the kinds of infrastructure and staff support necessary to offer clinical trials at non-NCIP sites, an implication reinforced by evidence that patients at community centers are less likely to have access to locally available clinical trials.24 One model for outreach to community centers to provide locally available trials is the NCI's Community Oncology Research Program, a national network bringing studies to individuals in their own communities.30
Studies that examine participation in clinical research studies have almost entirely focused on clinical treatment trials.2-4 This study demonstrates that patients contribute to a wide variety of other clinical research studies. QOL studies—once rare—are now commonly included within treatment trials to provide self-reporting from patients about their treatment symptoms, functional impairment, and overall well-being, and are important for informing treatment decision making in many disease settings.31,32 The blood, serum, and tissue samples that patients contribute to biorepository studies are essential for basic research to identify mechanisms of new potential agents, especially for contemporary molecular-targeted therapies.33-35 Diagnostic studies aim to improve cancer diagnosis and assessment.36 Genetic studies examine patterns of genetic signatures to guide therapy toward a patient's specific disease.37 Economic studies examine how the financial impact of a cancer diagnosis may drive decision making by patients, clinicians, and payers, and are increasingly relevant, given the recognition of the devastating impact of a cancer diagnosis and treatment on individual financial well-being.38-41
The results of this study represent the first national contemporary estimates of the participation of patients with cancer in all types of clinical research studies. Although the participation of patients in clinical treatment trials has been widely examined, to our knowledge, little to no research has characterized national patient participation patterns in biorepository, diagnostic, economic, genetic, QOL, or registry studies, all of which are important avenues of cancer clinical research.
However, our study has limitations. CoC-approved institutions are larger, more frequently located in urban centers, and have more cancer-related services available to patients than nonapproved institutions.7 This could limit the generalizability of the estimates, although notably, nearly three of four patients with cancer receive care in CoC-approved institutions, suggesting any bias would be modest.42 Since data were deidentified and aggregated by study type at the institution level, it was not possible to examine representation by important factors, such as sex, race, ethnicity, or geography, nor was it possible to characterize institutions, in general, by these variables. Moreover, because the data represented a single set of reporting for all CoC programs, it was not possible to evaluate trends over time. The data were originally obtained for accreditation purposes and were not verified. A verification mechanism would better serve research purposes and would be especially advantageous for examining nontreatment studies, for which missing data were common. Additionally, the strategy of partially distributing enrollments assigned to an other study category may have biased the results high, because of misclassification, or low, if assumptions about how to redistribute the enrollments were too conservative. Also, some sites may have entered zeroes when in fact information was missing, which could bias the results low. Furthermore, patients may have been diagnosed at one center and received care, and possibly clinical trial treatment, at another center, which could create unknown biases in recording enrollment totals, including the possibility that some individual patients were counted twice in enrollment totals. Conversely, since data were aggregated at the institutional level, we were unable to account for the possibility that individual patients may have participated in multiple trials. Finally, the extent to which patients participated in more than a single category of study was unknown. Thus, only minimum estimates of total enrollment participation across all types of studies could be estimated. Efforts to reduce the analytical limitations of the CoC data source and to enable its routine analysis could prove invaluable for evaluating and tracking enrollment to a diverse set of oncology studies over time.
An accurate understanding of the contributions of patients to clinical research is necessary for understanding how to target strategies for improving participation. This is vital since the rapid enrollment of patients to clinical research studies is necessary to quickly advance new therapies for patients with cancer. This study shows that enrollment to clinical treatment trials is about twice as high as usually realized; this may reflect increased trial participation over time, although further research is needed. Although the overall rate remains low, this study reinforces previous research showing that many system-level structural and clinical barriers limit patients from even having the opportunity to consider trial participation for their care.24 Furthermore, the likelihood of a patient enrolling in a clinical trial is highly influenced by the type of institution at which they seek treatment. Thus, in addition to continuing to study why patients decline trial participation, priority should be given to better characterizing and developing strategies to mitigate the structural and clinical barriers to participation. A key strategy for policymakers aiming to increase trial enrollment would be to improve clinical research infrastructure investments for community-based sites.43-45 Moreover, clinical research is informed by a broad array of studies, not just treatment trials; our study presents first-time overall national estimates for participation in biorepository, diagnostic, economic, genetic, QOL, and registry studies. These estimates may set benchmarks for future studies about access, barriers, and disparities to participation in these critical elements of the clinical research process.
This study demonstrates the substantial contributions of patients to clinical research, which should be appropriately recognized by researchers and policymakers, since without these contributions, clinical research as currently conducted would not be feasible.
ACKNOWLEDGMENT
The authors thank the Commission on Cancer and all the patients and their providers who contribute to cancer clinical research studies throughout the United States.
APPENDIX
TABLE A1.
Study Participation Counts by Type of Cancer Program
| Study Type | Institution Type (numerator, annual) | |||
|---|---|---|---|---|
| ACAD | CCP | INCP | NCIP | |
| Treatment | 17,030 | 28,460 | 10,683 | 41,465 |
| Biorepository | 35,333 | 49,748 | 18,105 | 75,005 |
| Diagnostic | 7,343 | 13,935 | 3,109 | 10,115 |
| Economic | 5,630 | 12,918 | 3,721 | 10,559 |
| Genetic | 8,968 | 16,287 | 4,108 | 20,590 |
| QOL | 7,965 | 14,895 | 4,528 | 11,727 |
| Registry | 18,644 | 53,761 | 9,945 | 18,663 |
| Denominator annual | 314,088 | 690,067 | 187,300 | 191,926 |
NOTE. Given large numbers, all pairwise comparisons were strongly (P < .01) statistically significantly different by chi-square tests.
Abbreviations: ACAD, Academic Comprehensive Cancer Program; CCP, Community Cancer Program; INCP, Integrated Network Cancer Program; NCIP, National Cancer Institute–Designated Comprehensive Cancer Center Program; QOL, quality-of-life.
Joseph M. Unger
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: AstraZeneca
Lawrence N. Shulman
Consulting or Advisory Role: Genentech
Research Funding: Celgene (Inst), Independence Blue Cross (Inst)
Mark E. Fleury
Research Funding: Merck (Inst), Genentech (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Sanofi (Inst), Amgen (Inst), Seagen (Inst), Parexel (Inst), Bayer (Inst), EMD Serono (Inst), Foundation Medicine (Inst), NeoGenomics Laboratories (Inst)
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 2117
DISCLAIMER
The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
PRIOR PRESENTATION
Presented in part at the ASCO Quality Care Symposium, Boston, MA, September 23-25, 2021.
SUPPORT
Supported by the Public Health Sciences Division of the Fred Hutchinson Cancer Center.
AUTHOR CONTRIBUTIONS
Conception and design: Joseph M. Unger, Lawrence N. Shulman, Mark E. Fleury
Financial support: Joseph M. Unger
Administrative support: Heidi Nelson, Mark E. Fleury
Provision of study materials or patients: Joseph M. Unger, Lawrence N. Shulman, Mark E. Fleury
Collection and assembly of data: Joseph M. Unger, Mark E. Fleury
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
National Estimates of the Participation of Patients With Cancer in Clinical Research Studies Based on Commission on Cancer Accreditation Data
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Joseph M. Unger
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: AstraZeneca
Lawrence N. Shulman
Consulting or Advisory Role: Genentech
Research Funding: Celgene (Inst), Independence Blue Cross (Inst)
Mark E. Fleury
Research Funding: Merck (Inst), Genentech (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Sanofi (Inst), Amgen (Inst), Seagen (Inst), Parexel (Inst), Bayer (Inst), EMD Serono (Inst), Foundation Medicine (Inst), NeoGenomics Laboratories (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Tran G, Harker M, Chiswell K, et al. : Feasibility of cancer clinical trial enrollment goals based on cancer incidence. JCO Clin Cancer Inform 10.1200/CCI.19.00088 [DOI] [PubMed] [Google Scholar]
- 2.Murthy VH, Krumholz HM, Gross CP: Participation in cancer clinical trials: Race-sex-and age-based disparities. JAMA 291:2720-2726, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Sateren WB, Trimble EL, Abrams J, et al. : How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 20:2109-2117, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Tejeda HA, Green SB, Trimble EL, et al. : Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst 88:812-816, 1996 [DOI] [PubMed] [Google Scholar]
- 5.American College of Surgeons : Commission on Cancer; Improving Outcomes for Patients with Cancer. https://www.facs.org/Quality-Programs/Cancer/CoC [Google Scholar]
- 6.American College of Surgeons : National Cancer Database. https://www.facs.org/Quality-Programs/Cancer/NCDB [Google Scholar]
- 7.Bilimoria KY, Bentrem DJ, Stewart AK, et al. : Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: Implications for studies that use the National Cancer Data Base. J Clin Oncol 27:4177-4181, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Boffa DJ, Rosen JE, Mallin K, et al. : Using the National Cancer Database for outcomes research: A review. JAMA Oncol 3:1722-1728, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Mohanty S, Bilimoria KY: Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol 109:629-630, 2014 [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Database : American College of Surgeons. National Cancer Database Tools. https://www.facs.org/media/buvfsm0p/22_ca_ncdbtools_8-5x11_v01.pdf [Google Scholar]
- 11.American College of Surgeons : Commission on Cancer. Cancer Program Standards: Ensuring Patient-Centered Care. https://www.facs.org/media/t5spw4jo/2016-coc-standards-manual_interactive-pdf.pdf [Google Scholar]
- 12.American College of Surgeons : Commission on Cancer. Cancer Program Standards: Ensuring Patient-Centered Care. https://www.facs.org/Quality-Programs/Cancer/CoC/standards/2016 [Google Scholar]
- 13.American College of Surgeons : 2020 Standards and Resources. https://www.facs.org/quality-programs/cancer-programs/commission-on-cancer/standards-and-resources/2020/ [Google Scholar]
- 14.van Buuren S, Oudshoorn CGM: Multivariate Imputation by Chained Equations: MICE V1.0 User’s Manual. TNO Report PG/VGZ/00.038. Leiden, the Netherlands, TNO Preventie en Gezondheid, 2000. https://stefvanbuuren.name/publication/2000-01-01_vanbuuren2000/ [Google Scholar]
- 15.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30:377-399, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Azur MJ, Stuart EA, Frangakis C, et al. : Multiple imputation by chained equations: What is it and how does it work? Int J Methods Psychiatr Res 20:40-49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Buuren S, Groothuis-Oudshoorn K: MICE: Multivariate imputation by chained equations in R. J Stat Softw 45:1-67, 2011 [Google Scholar]
- 18.Little RJA: Missing data adjustments in large surveys. J Business Econ Stat 6:287-301, 1988 [Google Scholar]
- 19.Zarin DA, Tse T, Williams RJ, et al. : The ClinicalTrials.gov results database--update and key issues. N Engl J Med 364:852-860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IOM (Institute of Medicine) : Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington, DC, The National Academies Press, 2010 [PubMed] [Google Scholar]
- 21.Anderson ML, Chiswell K, Peterson ED, et al. : Compliance with results reporting at ClinicalTrials.gov. N Engl J Med 372:1031-1039, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrhardt S, Appel LJ, Meinert CL: Trends in National Institutes of Health funding for clinical trials registered in ClinicalTrials.gov. JAMA 314:2566-2567, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch BR, Califf RM, Cheng SK, et al. : Characteristics of oncology clinical trials: Insights from a systematic analysis of ClinicalTrials.gov. JAMA Intern Med 173:972-979, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Unger JM, Vaidya R, Hershman DL, et al. : Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst 111:245-255, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green AK, Tabatabai SM, Aghajanian C, et al. : Clinical trial participation among older adult Medicare Fee-for-Service beneficiaries with cancer. JAMA Oncol 8:1786-1792, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittell H, Calip GS, Pierre A, et al. : Racial and ethnic inequities in US oncology clinical trial participation from 2017 to 2022. JAMA Netw Open 6:e2322515, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unger JM: A ground's-eye view on racial and ethnic disparities in cancer clinical trial participation. JAMA Netw Open 6:e2322436, 2023 [DOI] [PubMed] [Google Scholar]
- 28.Mc Daid C, Hodges Z, Fayter D, et al. : Increasing participation of cancer patients in randomised controlled trials: A systematic review. Trials 7:16, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ousseine YM, Bouhnik AD, Mancini J: Health literacy and clinical trial participation in French cancer patients: A national survey. Curr Oncol 29:3118-3129, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute : Community Oncology Research Program (NCORP). https://ncorp.cancer.gov/ [Google Scholar]
- 31.Bottomley A, Pe M, Sloan J, et al. : Moving forward toward standardizing analysis of quality of life data in randomized cancer clinical trials. Clin Trials 15:624-630, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Bansal D, Bhagat A, Schifano F, et al. : Role of patient-reported outcomes and other efficacy endpoints in the drug approval process in Europe (2008-2012). J Epidemiol Glob Health 5:385-395, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambrosone CB, Nesline MK, Davis W: Establishing a cancer center data bank and biorepository for multidisciplinary research. Cancer Epidemiol Biomarkers Prev 15:1575-1577, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Sergi CM: Biorepository—A key component of research studies. Contemp Clin Trials 112:106655, 2022 [DOI] [PubMed] [Google Scholar]
- 35.National Cancer Institute : Division of Cancer Treatment and Diagnosis. Cancer Diagnosis Program—Biorepositories and Biospecimen Research Branch. https://biospecimens.cancer.gov/default.asp [Google Scholar]
- 36.National Cancer Institute : Division of Cancer Treatment and Diagnosis. Cancer Diagnosis Program. https://cdp.cancer.gov/ [Google Scholar]
- 37.American Cancer Society : Understanding Genetic Testing for Cancer Risk. https://www.cancer.org/healthy/cancer-causes/genetics/genetic-testing-for-cancer-risk/understanding-genetic-testing-for-cancer.html [Google Scholar]
- 38.Halpern MT, Shih YT, Yabroff KR, et al. : A framework for cancer health economics research. Cancer 127:994-996, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris S, Devlin N, Parkin D, et al. : Economic Analysis in Healthcare (ed 2). Chichester, UK, John Wiley & Sons, 2012 [Google Scholar]
- 40.Ramsey S, Blough D, Kirchhoff A, et al. : Washington state cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 32:1143-1152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankaran V, Unger JM, Darke AK, et al. : S1417CD: A prospective multicenter cooperative group-led study of financial hardship in metastatic colorectal cancer patients. J Natl Cancer Inst 114:372-380, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fong ZV, Chang DC, Hur C, et al. : Variation in long-term oncologic outcomes by type of cancer center accreditation: An analysis of a SEER-Medicare population with pancreatic cancer. Am J Surg 220:29-34, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minasian LM, Unger JM: What keeps patients out of clinical trials? JCO Oncol Pract 16:125-127, 2020 [DOI] [PubMed] [Google Scholar]
- 44.Unger JM, Hershman DL, Osarogiagbon RU, et al. : Representativeness of black patients in cancer clinical trials sponsored by the National Cancer Institute compared with pharmaceutical companies. JNCI Cancer Spectr 4:pkaa034, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodcock J, Araojo R, Thompson T, et al. : Integrating research into community practice—Toward increased diversity in clinical trials. N Engl J Med 385:1351-1353, 2021 [DOI] [PubMed] [Google Scholar]