Abstract
PURPOSE
Hearing loss occurs in 50%-70% of children treated with cisplatin. Scientific efforts have led to the recent approval of a pediatric formula of intravenous sodium thiosulfate (STS) for otoprotection by the US Food and Drug Administration, the European Medicines Agency, and the Medicines and Health Regulatory Authority in the United Kingdom. To inform stakeholders regarding the clinical utility of STS, the current review summarizes available literature on the efficacy, pharmacokinetics (PK), and safety of systemic STS to minimize cisplatin-induced hearing loss (CIHL).
DESIGN
A comprehensive narrative review is presented.
RESULTS
Thirty-one articles were summarized. Overall, systemic STS effectively reduces CIHL in the preclinical and controlled clinical study settings, in both adults and children with cancer. The extent of CIHL reduction depends on the timing and dosing of STS in relation to cisplatin. Both preclinical and clinical data suggest that systemic STS may affect plasma platinum levels, but studies are inconclusive. Delayed systemic administration of STS, at 6 hours after the cisplatin infusion, does not affect cisplatin-induced inhibition of tumor growth or cellular cytotoxicity in the preclinical setting, nor affect cisplatin efficacy and survival in children with localized disease in the clinical setting.
CONCLUSION
Systemic administration of STS effectively reduces the development and degree of CIHL in both the preclinical and clinical settings. More studies are needed on the PK of STS and cisplatin drug combinations, the efficacy and safety of STS in patients with disseminated disease, and the ability of STS to prevent further deterioration of pre-established hearing loss.
STS effectively reduces cisplatin-induced hearing loss. In clinical settings, STS has been demonstrated to be safe for children with localized disease given 6h after cisplatin.
INTRODUCTION
Platinum compounds have contributed significantly to increased survival rates in children with solid tumors including osteosarcoma, germ cell tumors, hepatic tumors, neuroblastoma, nasopharyngeal carcinoma, retinoblastoma, and medulloblastoma. However, ototoxicity in the form of irreversible hearing loss, tinnitus, and vestibular dysfunction is a consequence of this chemotherapy. Estimates suggest that some degree of cisplatin-induced hearing loss (CIHL) develops in 50%-70% of treated children.1-6 The main mechanism whereby cisplatin damages the inner ear is the formation of high levels of reactive oxygen species (ROS), eventually resulting in cochlear hair cell apoptosis.7,8 Hearing loss related to carboplatin treatment is also observed, but the overall prevalence is lower (0%-25%).9-14 Ototoxic effects are thought to be more pronounced in patients who receive both cisplatin and carboplatin, with prevalence rates of 75% reported.13,15 In young children (age 5 years and younger), the cumulative incidence of CIHL is higher compared with that in older children (older than 5 years) and develops early during therapy.16
Other treatment-related risk factors may induce or enhance ototoxic effects, including vincristine administration,17 cranial irradiation,18 brain surgery,19 and supportive care medication.16 Genetic susceptibility may explain why certain patients are more prone to developing CIHL compared with others who receive similar treatments.20
CIHL can negatively affect daily functioning by delayed speech and language development,21 reduced academic performance,22 impaired neurocognitive functioning,23 social isolation, emotional deprivation, and consequent impaired quality of life (QoL)24 compared with peers without hearing loss. In later life, hearing loss may affect cognition25 and has been sighted as a risk factor for dementia.26-28 This may occur directly, through changes in auditory input affecting the brain structures responsible for cognition, or indirectly through factors such as heightened social isolation, depression, impaired self-confidence, reduced physical activity, or decreased engagement in intellectually stimulating activities.29-31 This is particularly concerning as pediatric patients with cancer are already at risk for comorbidities, including accelerated aging32 and impaired QoL.33,34
Given the high prevalence and clinical impact of CIHL, it would be advantageous to reduce or, preferably, prevent this permanent toxicity as much as possible. Reducing the dose of cisplatin, or replacing it with another chemotherapeutic agent, requires randomized clinical trials to prove equal efficacy, otherwise this could negatively affect survival.35 The advent of preventative agents to reduce CIHL is clearly welcome.
The otoprotective effect of sodium thiosulfate (STS) has been explored across multiple studies over several decades, either via systemic or local administration. STS is thought to reduce cisplatin-induced toxicity by two mechanisms. First, STS can bind to cisplatin, leading to the formation of inactive cisplatin compounds. Second, STS enters cochlear cells via cotransporter-2, where it influences antioxidant enzymes. It elevates antioxidant glutathione levels inhibiting intracellular ROS formation induced by cisplatin.36,37 Systemic administration requires a sufficient amount of STS to cross the blood-labyrinth barrier to obtain a preventative effect. Local applications such as intratympanic and intracochlear injections, administering STS directly to the ear, are not the subject of this review.38,39
Scientific evidence, alongside results from two randomized pediatric clinical trials,40,41 have recently led to marketing authorization for intravenously (IV) administered pediatric STS, by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the UK Medicines and Health Regulatory Authority (MHRA). Currently, this compound is licensed as a cisplatin otoprotectant, for children age 1 month or older, who have nonmetastatic solid tumors (neuroblastoma, hepatoblastoma, nasopharyngeal carcinoma, osteosarcoma, germ cell tumors, medulloblastoma, and other rare tumors), at a dose of 10-20 g/m2 (dependent on body weight) administered over 15 minutes, 6 hours after the end of the cisplatin infusion.42
A recent survey showed that North American health care providers consider CIHL to be a concerning toxicity.43 It is therefore important that all stakeholders are well informed on the use of STS. To date, an overview of all studies on STS is lacking. This review summarizes available literature (N = 31) on the efficacy, pharmacokinetics (PK), and safety of systemic STS in the prevention of CIHL. The majority of this article pertains to cisplatin, although mention will be made of STS and carboplatin.
OTOPROTECTIVE EFFECT OF SYSTEMIC STS
Preclinical Studies
In 1988, Otto et al44 first described the otoprotective effect of STS in vivo. They injected 17 guinea pigs with intramuscular (IM) cisplatin for 8 days (1.5 mg/kg total per day), and 11 with cisplatin and STS (16 g/kg total per day). At 10 days after cisplatin, auditory brainstem responses (3-30 kHz) were measured and converted to hearing threshold levels (HTLs). In cisplatin only-treated animals, a HTL shift of >40 dB from baseline was observed at all frequencies, whereas the HTLs in the STS group remained unchanged.44 Four other studies performed between 1995 and 2000 completed similar experiments in hamsters and guinea pigs, which also reported normal HTLs (0-20 dB) at ≥30 days after cisplatin and after carboplatin in animals that received STS.45-48 One other study (N = 14) only found a significant difference in favor of STS in the very high frequency range (30 kHz; P = .07).49
Dickey et al50 specifically assessed HTLs when IV STS (8 g/m2) was administered at different time points after a single infusion of intra-arterial (IA) cisplatin (6 mg/kg). Compared with rats treated with cisplatin only (N = 15), a significant difference in favor of STS was found when it was administered at 4 or 8 hours after cisplatin (n = 7: P < .05), but not after 12 hours.50
Cochlear outer hair cells (OHCs) have also been assessed in multiple studies. Cisplatin-treated rodents showed a reduction in number of OHCs of 32%-65%, whereas only minor losses of 5%-14% were reported in animals receiving STS.45-47 Overall, the studies show that systemic STS effectively reduces the development of hearing loss, and this may vary with the timing of administration (Table 1).
TABLE 1.
Preclinical Studies on the Otoprotective Effect of STS
| Author, Year | Species | No.: STS Group | No.: Comparison Group | Platinum Treatment | STS Specification | Evaluation Methods | FU Time, Post-Tx | Hearing Function Outcomes | P | OHC Count | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| With STS | Without STS | ||||||||||
| Otto et al,44 1988 | Guinea pig | 11: CIS + STS | 17: CIS only 10: normal saline |
CIS IM 1.5 mg/kg total per day 8 days |
STS IP 16 g/kg total per day Concurrent with CIS |
ABR (3-30 kHz) | 10 days | No HTL shift from baseline | >40 dB HTL shift from baseline | NA | — |
| Church et al,45 1995 | Hamster | 10: CIS + STS | 10: CIS only 22: no Tx |
CIS IP 3 mg/kg total per day EOD 5 injections |
STS IP 16 g/kg total per day Concurrent with CIS |
ABR (2-20 kHz) Electron microscopy |
30 days | HTLs <10 dB at all frequencies | HTLs 30-48 dB at 8-20 kHz | NA | Untreated: N = 2,672 CIS only: 32% loss CIS + STS: 14% loss |
| Neuwelt et al,46 1996 | Guinea pig | 6: CARBO + STS | 12: CARBO only 6: saline |
CARBO SC 24 mg/kg 1 injection |
STS IP 1.83 g/kg 2, 4, 8, and 24h after CARBO |
CAP (2-32 kHz) OHC count (unspecified) |
4 weeks | HTLs 0-10 dB at all frequencies | HTLs 40-60 dB at all frequencies | NA | CARBO only: 65% loss CARBO + STS: 5% loss |
| Kaltenbach et al,47 1997 | Hamster | 10: CIS + STS | 10: CIS only 4: no Tx |
CIS IP 3 mg/kg 5 injections |
STS IP 16 g/kg 30 min before CIS |
ABR (2-20 kHz) Electron microscopy |
30-35 days | HTLs 10 dB at all frequencies | HTLs 50 dB at 16 kHz | NA | Untreated: N = 2,670 CIS only: 44% loss CIS + STS: 9% loss |
| Muldoon et al,48 2000 | Guinea pig | 2: CIS + STS | 2: CIS + saline | CIS IV 6 mg/kg 1 infusion |
STS bolus IP or 15-min IV infusion 11.6 g/m2 2h after CIS |
ABR (4-32 kHz) | 8 weeks | HTLs 0-20 dB at all frequencies | HTLs 30-50 dB at all frequencies | NA | — |
| Dickey et al,50 2005 | Rat | 7: CIS + STS | 15: CIS + saline | CIS IA 3 mL/min 6 mg/kg 1 infusion |
STS IV 8 g/m2 4, 8, and 12h after CIS |
ABR (4-20 kHz) | 7 days | STS 4h: HTLs 0-5 dB at all frequencies STS 8h: HTLs 0-10 dB at all frequencies STS 12h: HTLs 10-20 dB at all frequencies |
HTLs 10-25 dB at all frequencies HTLs 10-20 dB at all frequencies HTLs 10-30 dB at all frequencies |
<.05 <.05 >.05 |
— |
| Videhult Pierre et al,49 2017 | Guinea pig | 7: CIS + STS | 7: CIS + saline | CIS IV in 3 min 8 mg/kg 1 infusion |
STS 20 sec IV infusion 1 mL/0.3 kg 30 min before CIS |
ABR (3-30 kHz) | 4 days | HTLs 0-25 dB at all frequencies | HTLs 5-35 dB at all frequencies | .07 (only at 30 kHz) | — |
Abbreviations: ABR, auditory brainstem response; CAP, compound action potential; CARBO, carboplatin; CIS, cisplatin; EOD, every other day; FU, follow-up; HTL, hearing threshold level; IA, intra-arterial; IM, intramuscular; IP intraperitoneal; IV, intravenous; NA, not assessed; OHC, outer hair cell; SC, subcutaneous; STS, sodium thiosulfate; Tx, treatment.
Clinical Studies
Historically, STS was administered systemically at the same time as cisplatin, the goal being to increase the dose of cisplatin without increasing toxicity.51-60 This approach changed in more recent clinical studies, where different approaches to the administration of STS were investigated to avoid interference with the antitumor effect of IV cisplatin on the one hand and IA carboplatin on the other: (1) separating IV STS from IV cisplatin by time (eg, 6 hours after the end of cisplatin infusion) with the aim of avoiding STS being in the circulation alongside peak serum cisplatin levels (to avoid interference with the antitumor effect of cisplatin while retaining an otoprotective effect)40,41; and (2) separating IV STS from IA carboplatin by space, specifically via blood-brain barrier disruption (BBBD; Appendix Fig A1, online only). To increase the IA delivery of carboplatin across the BBB, transient osmotic disruption of the barrier via mannitol is used. After 2 hours, the BBB is re-established and IV STS is administered (Appendix Fig A1).61-63 BBBD studies evaluate STS in patients with CNS tumors treated with IA carboplatin. In these studies, STS is used to counteract carboplatin in the circulation and potentially mitigate the effects of carboplatin on organs outside of the CNS.
STS Studies With a Control Group
Randomized Controlled Trials
Brock et al40 designed the SIOPEL 6 trial in which 109 children with standard-risk hepatoblastoma were randomly assigned to receive treatment with six cycles of single-agent IV cisplatin (80 mg/m2) and surgery with (n = 57) or without (n = 52) IV STS (20 g/m2), administered 6 hours after the end of the cisplatin, as a 15-minute infusion. Patients in each arm were matched by tumor type, prognostic group, and treatment received. Pure tone audiometry (PTA) showed that the incidence of hearing loss in those who received cisplatin only was 63%, compared with 33% in the STS group (P = .002; relative risk, 0.52 [95% CI, 0.33 to 0.81]) and that the grade of hearing loss was significantly less in those who received STS.40 A similar observation was reported by Freyer et al41 in the ACCL0431 STS otoprotection trial. Children with any type of tumor treated with cisplatin (290-466 mg/m2) were eligible and randomly assigned to receive IV STS 16 g/m2 over 15 minutes, 6 hours after the end of the cisplatin infusion (n = 49) or not (n = 55). Patients were not matched by tumor type, biology, stage, risk, or treatment. A significant reduction of hearing loss in the STS group (29%) compared with the observation group (56%; P = .00022)41 was observed.
Non-RCTs
In adults who received IA carboplatin after BBBD for CNS tumors (N = 15), Doolittle et al61 showed that the percentage of hearing loss (at 4 and 8 kHz) was 52%, compared with historical controls who received no STS, when IV STS (16-20 g/m2) was administered 2 hours after carboplatin, and improved to only 29% when STS was given after 4 hours. In an earlier phase II study, Neuwelt et al62 reported 33% hearing loss in 15 patients who received IV STS at 16 or 20 g/m2 after IA carboplatin following BBBD, compared with a small number of patients who received STS at a dose of four or 8 g/m2 (n = 4). These BBBD studies show that delayed timing of STS in relation to carboplatin administration as well as a higher dose of STS both increase the extent of the hearing protection.
Adults with head and neck squamous cell carcinoma (HNSCC) who received cisplatin (IV or IA, 66-100 mg/m2) with STS (12-14 g/m2 over 2-4 hours) at the same time revealed minor yet significant mean differences in HTL shifts up to 4 kHz (5.3 dB in the STS group v 8.9 dB in the non-STS group; P < .001)60 and at higher frequencies (20 dB at 10-12 kHz v 15-25 dB at 8-10 kHz, respectively: lowest P = .016).53
The most common mild-to-moderate adverse events related to systemic STS reported include nausea and vomiting, nephrotoxicity, neutropenia, hypernatremia, hypophosphatemia, hypokalemia, and hypermagnesemia,40,41,62 and no late side effects have been reported to date.
In summary, systemic STS has been shown to effectively reduce the occurrence of hearing loss in controlled clinical studies in both adults and children with cancer. In children, RCTs have only been pursued with one specific STS compound. Otoprotection seems to depend on the dosing of STS and timing of administration (Table 2).
TABLE 2.
Clinical Studies on the Otoprotective Effect of STS (with a control group)
| Author, Year | Design | Patient Characteristics | No.: STS Group | No.: Comparison Group | Platinum Treatment | STS Specification | Audiometry | FU Time | Hearing Function Outcomes | P | Adverse Events | Survival | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With STS | Without STS | ||||||||||||
| Studies in adults | |||||||||||||
| Zuur et al,60 2007 | RCT: phase 3 | HNSCC Median age: 55 years |
78: CIS + STS + radiotherapy (70 Gy) | 80: CIS only + radiotherapy (70 Gy) | CIS + STS CIS IA; 4× 150 mg/m2 CIS only CIS IV; 3× 100 mg/m2 |
STS IV 9 g/m2 for 30 minutes + 12 g/m2 for 2 hours Concurrent with CIS |
PTA 0.25-16 kHz |
Median: 8 weeks post-Tx | HTL shifts >10 dB over time Mean HTL shift up to 4 kHz: 5.3 dB, and up to 12.5 kHz: 20.4 dB |
HTL shifts >10 dB over time Mean HTL shift up to 4 kHz: 8.9 dB, and up to 12.5 kHz: 19.0 dB |
<.001 (≤4 kHz) | NA | NA |
| Ishikawa et al,53 2015 | Prospective cohort study | HNSCC Age: 45-82 years |
7: CIS + STS + radiotherapy (60-70 Gy) | 11: CIS only + radiotherapy (60-70 Gy) | CIS + STS CIS IA; 2-5× 100-180 mg/m2 CIS only CIS IV; 1-3× 66-85 mg/m2 |
STS IV 14 g/m2 for 4 hours Concurrent with CIS |
PTA 0.125-12 kHz |
1-3 weeks post-Tx | From baseline HTL shifts of 20 dB at 10 and 12 kHz |
From baseline HTL shifts of 15-25 dB at 8 and 10 kHz |
.028 .039 .016 .027 |
NA | NA |
| Studies in mixed cohorts of children and adults | |||||||||||||
| Neuwelt et al,62 1998 | Cohort study STS group: prospective Comparison group: retrospective |
Brain tumors Age: 2-68 years |
15: CARBO + STS 16 or 20 mg/m2 4: CARBO + STS 4 or 8 mg/m2 |
19: CARBO only | BBBD + CARBO IA over 10-min 400 mg/m2 per month 4-12 courses |
STS IV over 15 minutes 4-20 mg/m2 2 hours after CARBO |
PTA 0.25-8 kHz |
Each month during Tx | HL in 33% with STS 16 or 20 mg/m2a Average loss after first cycle: 3.7 ± 2.0 dB at 8 kHz |
HL in 79% Average loss after first cycle: 20.8 ± 5.9 dB at 8 kHz |
<.05 | Mild nausea, vomiting HN, ↑ blood pressure |
NA |
| Doolittle et al,61 2001 | Cohort study STS group: prospective Comparison group: retrospective |
Brain tumors Age: 4-67 years |
24: CARBO + STS after 2 hours 17: CARBO + STS after 4 hours |
19: CARBO only | BBBD + CARBO IA over 10-min 400 mg/m2 per month 1-7 courses |
STS IV over 15 minutes 16 or 20 mg/m2 2 or 4 hours after CARBO |
PTA 0.25-8 kHz |
Each month during Tx | STS2h: HL in 52% HTL shift at 8 and 4 kHz: 41.7 dB and 35.4 dB, respectively STS4h: HL in 29% HTL shift at 8 and 4 kHz: 34.1 dB and 28.6 dB, resp. |
HL in 84% HTL shift at 8 and 4 kHz: 64.4 dB and 51.6 dB, respectively |
.001 (at 8 kHz) .0075 (at 4 kHz) |
NA | NA |
| Studies in children and adolescents | |||||||||||||
| Freyer et al,41 2017 | RCT: multicenter, open-label, phase 3 | Any tumor Age: 1-18 years |
49: CIS + STS | 55: CIS only | CIS IV 6× CIS + STS: 393 mg/m2 (290-420) CIS only: 387 mg/m2 (305-466) |
STS IV over 15 minutes 16 g/m2 6 hours after CIS |
PTA 0.5-8 kHz |
4 weeks post-Tx EFS + OS: median 3.5 years |
HL in 29% | HL in 56% | .00022 | Nephrotoxicity HP, HK |
EFS + OS in both groups: P = .36 and .07, respectively EFS + OS in LD (N = 77): P = .73 and .88, respectively OS in DD (N = 47): P = .009 |
| Brock et al,40 2018 | RCT | SR HBL Age: 0-8 years |
57: CIS + STS | 52: CIS only | CIS IV 6× 80 mg/m2 |
STS IV over 15 minutes 20 mg/m2 6 hours after CIS |
PTA 1-8 kHz |
During Tx EFS + OS: median 3.0 years |
HL in 33% | HL in 63% | .002 | Neutropenia, HM, HP, HK | 3-yr EFS in CIS + STS group: 82%, 95% CI 69%-90%; in CIS alone group 79%, 95% CI 65%-88% 3-yr OS CIS + STS: 98%, 95% CI 88%-100%; CIS alone 92%, 95% CI 81%-97% |
Abbreviations: BBBD, blood-brain barrier disruption; CARBO, carboplatin; CIS, cisplatin; DD, disseminated disease; EFS, event-free survival; FU, follow-up; HBL, hepatoblastoma; HK, hypokalemia; HL, hearing loss; HM, hypermagnesemia; HN, hypernatremia; HNSCC, head and neck squamous cell carcinoma; HP, hypophosphatemia; HTL, hearing threshold level; IA, intra-arterial; IV, intravenous; LD, localized disease; NA, not assessed; OS, overall survival; RCT, randomized controlled trial; SR, standard risk; STS, sodium thiosulfate; Tx, treatment.
Most patients who received 4 or 8 mg/m2 developed hearing loss.
STS Studies Without a Control Group
Since 1982, several clinical studies (mainly phase I or II trials) have investigated the concomitant administration of STS with cisplatin, either to increase the dose of cisplatin to enhance treatment efficacy, or to reduce platinum-related toxicities (including hearing loss).51,52,54-59,63-66 Reichman et al57 performed PTA (up to 20 kHz) in 11 adults with cervical cancer, who received IV cisplatin over 2 hours (200 mg/m2; 2-5 courses) with IV STS (3.3-6.6 g/m2) at the same time. After the first course, 44% developed hearing loss at >8 kHz. Thereafter, 77% developed hearing loss at 8 kHz; 55% at 6 kHz; and 11% at 1-4 kHz.57 Kim et al54 (1993) reported 50% hearing loss during treatment in 18 adults with different tumor types, who received IV cisplatin over 6 hours (180 mg/m2 for 1-6 cycles) with STS (2-4 g/m2). One study observed 10% self-reported moderate-to-severe hearing loss, in adults with HNSCC (N = 79), treated with IA cisplatin (150 mg/m2 for four courses) administered at the same time as IV STS (12 g/m2 for 2 hours).51 Two comparable studies in populations with the same diagnosis and treatment reported 60% CIHL after end of treatment as measured by PTA (N = 70),65 and 23% of evaluated ears to be under consideration for hearing aids at 7.5 weeks after the last cisplatin cycle (N = 146).59
Continuous hearing deterioration was reported after cisplatin + STS in adults with pre-existing hearing loss (ie, present before start of cancer treatment),55,58 and in a child with CIHL who received STS near the end of the cisplatin regimen with the goal to avoid a further decrease of HTLs.64 Neuwelt et al63 found differences in hearing loss occurrence between children who received IV STS at 2 hours versus 4 hours after BBBD + IA carboplatin, with percentages of 60% and 33% (loss of ≥40 dB at 2-8 kHz) reported, respectively. An additional study performed in adolescents and young adults (N = 13)66 reported a hearing loss incidence of 46% after intraperitoneal (IP) treatment with hyperthermic cisplatin (55-100 mg/m2) in parallel with IV STS administered over 12 hours. As the studies described above did not include control groups, a conclusion on the otoprotective effect of STS in these populations is hard to ascertain, although the comparison between 2-hour versus 4-hour delay in administration of STS confirms the advantage of the longer delay (Table 3).
TABLE 3.
Clinical Studies on the Otoprotective Effect of STS (without a control group)
| Author, Year | Design | Patient Characteristics | Platinum Treatment | STS Specification | Audiometry | FU Time | Evaluable for HL | Hearing Function Outcomes | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Studies in adults | |||||||||
| Howell et al,52 1982 | Phase I trial | 17 patients with IP tumors Mean age: 52 years (31-65) |
CIS IP dialysis over 4 hours; 6 courses Start at 90 mg/m2, escalated to 270 mg/m2 |
STS IV, over 12 hours 2.13 g/m2 per hour Concurrent with CIS |
PTA, unspecified | Post-Tx | 5 patients | No HL observed | Increased serum creatinine, vomiting, hematologic toxicity, abdominal pain, decreased serum bicarbonate and potassium |
| Pfeifle et al,56 1985 | Phase I trial | 24 patients with different tumor types Median age: 56 years (15-73) |
CIS IV over 2 hours 180-834 mg/m2 1-3 courses |
STS IV for 3 hours First hour 3.3 g/m2, thereafter 9.9 g/m2 Start 1 hour before CIS |
PTA, unspecified | 1 month post-Tx | 5 complete courses | VIIIth nerve toxicity in 1 course at 225 mg/m2 and 3 courses at 202.5 mg/m2; not in 1 course at 180 mg/m2 Incomplete courses: 8.4% with a change in hearing after Tx |
Proteinuria, increased serum creatinine, hematuria, myelosuppression, nausea, vomiting, decreased serum magnesium levels |
| Markman et al,55 1990 | Phase I trial | 36 patients with solid tumors Median age: 56 years (25-72) |
CIS IV over 2 hours 150-200 mg/m2 1-6 courses |
STS IV for 3 hours First hour 3.3 g/m2, thereafter 6.6 g/m2 Start 1 hour before CIS |
PTA (0.25-20 kHz) | During Tx | 22 | 55%: normal baseline at ≤8 kHz, of whom 58% developed HL in this range 45%: HL at 3-8 kHz at baseline; all showed continuous deterioration 77%: normal hearing at >8 kHz; all completely lost hearing in this range |
Emesis, myelosuppression, increased creatinine, renal insufficiency, peripheral neuropathy |
| Reichman et al,57 1991 | Phase II trial | 11 patients with cervical cancer Median age: 43 years (25-57) |
CIS IV over 2 hours 200 mg/m2 2-5 courses |
STS IV First hour 3.3 g/m2, thereafter 6.6 g/m2 Start 1 hour before CIS |
PTA (1-20 kHz) | During Tx | 9 | 44% HL at >8 kHz after 1 CIS course 77% mild-moderate HL at 8 kHz 55%: mild-moderate HL at 6 kHz 11%: mild-moderate HL at 1-4 kHz |
Low Hb requiring red blood cell transfusion, nausea, vomiting, peripheral neuropathy, increased creatinine |
| Kim et al,54 1993 | Phase I trial | 28 patients with different tumor types Median age: 51 years (31-72) |
CIS IV over 4 hours 180 mg/m2 1 to ≥6 courses |
STS IV over 6 hours First hour 4 g/m2, thereafter 2 g/m2 Concurrent with CIS |
PTA (1-8 kHz) | During Tx | 18 | 50% with HL 88% developed HL after course 1-2 Mostly at 4-8 kHz |
Slight increase in serum creatinine, myelosuppression, nausea, vomiting, peripheral neuropathy |
| Madasu et al,65 1997 | Inception cohort study | 70 patients with HNSCC Mean age: 56 years EBRT (68-70 Gy) |
CIS IA 150 mg/m2 4 courses |
STS IV Unspecified |
PTA (0.25-4 kHz) | During Tx | 49 | 25% HL after 1 course; 60% HL after 4 courses Mostly at 4-8 kHz |
NA |
| Van Rijswijk et al,58 1997 | Phase II trial | 29 patients with ovarian cancer Median age: 54 years (23-72) |
CIS IP over 6 hours 200 mg/m2 1-6 courses |
STS IP over 6 hours 4 g/m2 as a bolus, followed by 12 g/m2 Concurrent with CIS |
PTA (0.25-8 kHz) | During Tx | 23 | 35% with HL 75% had pre-existing HL that deteriorated (>15-30 dB) 25% developed new HL (drop of 15 dB in 1 ear; drop of >10 dB in 2 ears) |
Intra-abdominal adhesions, inflow and outflow obstructions, septic peritonitis, ileus, nausea, vomiting, leukopenia, thrombocytopenia, increased creatinine |
| Balm et al,51 2004 | Phase II trial | 79 patients with HNSCC Mean age: 54 years (29-79) EBRT (70 Gy) |
CIS IA 150 mg/m2 4 courses |
STS IV 9 g/m2 over 30 minutes, followed by 12 g/m2 over 2 hours |
PTA, unspecified CTCAE |
3 months post-Tx | 79 | 10% reported HL grade 3 Results of audiometry not reported |
Hematologic toxicities, mucositis, skin reactions, nausea, toxicity of the upper gastrointestinal tract, cardiotoxicity, treatment-related death, mucosal defect original tumor site, swallowing difficulties |
| Zuur et al,59 2007 | Prospective cohort study | 146 patients with HNSCC Median age: 54 years EBRT (70 Gy) |
CIS IA 150 mg/m2 4 courses |
STS IV 9 g/m2 over 30 minutes, followed by 12 g/m2 over 2 hours |
PTA (0.125-16 kHz) | During Tx and after a median of 7.5 weeks | Variable (range 141 before to 91 after Tx) | Largest HTL shifts after second and third CIS dose: average of 8 dB at 1-4 kHz and 24 dB at 8-12.5 kHz 59 ears (23%) under consideration for hearing aids |
NA |
| Studies in children, adolescents, and young adults | |||||||||
| Neuwelt et al,63 2006 | Phase I trial | 12 patients with brain tumors Age: 17 months—12 years |
CARBO IA 400 mg/m2 in 2 days after BBBD 2-12 courses |
STS IV 1 dose of 10-16 g/m2 at 2 or 4 hours after CARBO; extra dose 4 hours after dose 1 in case of pre-existing HL |
PTA (0.5-8 kHz) | During Tx | 11 | 55% received STS at 4 hours (of whom 67% had pre-existing HL); 33% had HTLs of ≥40 dB at 2-8 kHz 45% received STS at 2 hours (no pre-existing HL), of whom 60% developed HL (≥40 dB at 2-8 kHz) |
Increased sodium levels, myelosuppression, infection, cardiovascular toxicity, metabolic toxicity, gastrointestinal toxicity, neurologic toxicity, pulmonary toxicity, abdominal pain |
| Womack et al,66 2014 | Retrospective data review | 13 patients with IP tumors Mean age: 19 years (10-30) |
Hyperthermic CIS IP 55-100 mg/m2 1 course |
STS IV over 12 hours Dose unknown Before, during, or 12 hours post-CIS |
PTA (0.25-16 kHz) | 2-15 months | 13 | 46% with loss of 10-15 dB at one single frequency | NA |
| Harao et al,64 2020 | Case report | 9-year-old boy with MBL Craniospinal irradiation (23.4 Gy) + PF boost (32.4 Gy) |
CIS IV 75 mg/m2 8 courses |
STS IV 16 g/m2 At CIS course 6 and 7 |
PTA (1-8 kHz) | During Tx and after 12 months | — | At fifth cycle: HL up to ≥40 dB at 2-8 kHz At the end of seventh cycle: no deterioration of hearing After 12 months: ↓ HTLs at 2-8 kHz; 0.125-1 kHz within normal limits |
NA |
Abbreviations: BBBD, blood-brain barrier disruption; CARBO, carboplatin; CIS, cisplatin; CTCAE, Common Terminology Criteria for Adverse Events; EBRT, external-beam radiotherapy; FU, follow-up; HL, hearing loss; HNSCC, head and neck squamous cell carcinoma; HTL, hearing threshold level; IA, intra-arterial; IP, intra-peritoneal; IV, intravenous; MBL, medulloblastoma; NA, not assessed; PF, posterior fossa; PTA, pure tone audiometry; RCT, randomized controlled trial; STS, sodium thiosulfate; Tx, treatment.
PK OF PLATINUM AND SYSTEMIC STS
Preclinical Studies
Saito et al67 investigated the effect of systemic STS on the PK of cisplatin. Guinea pigs received three injections of IM cisplatin (7.5 mg/kg) with (n = 24) or without (n = 15) IP STS (1,000 mg/kg), administered concurrently with cisplatin, and 1-6 hours thereafter. Free cisplatin (FP) and total cisplatin (TP) were analyzed by inductively coupled mass spectrometry (ICP-MS). In terms of elimination, lower FP and TP concentrations were found in plasma of guinea pigs who received STS at 6 and 24 hours (P < .05).67 By contrast, Harned et al68 did not observe differences in cisplatin concentrations in plasma at 6 hours after administration in eight mice treated with IP cisplatin (4 mg/kg total per day for 4 days) and IP STS (3.5 g/kg total per day) compared with two mice that received cisplatin only, as measured by atomic absorption spectrometry (AAS).
To date, to our knowledge, only one study has investigated the effect of systemic STS on the PK of carboplatin by using AAS. In guinea pigs, Cmax in plasma (ie, the peak plasma concentration) was approximately 23 μg/mL in both groups that received carboplatin (24 mg/kg) with STS (11.6 g/m2 administered 2 hours after carboplatin), and carboplatin area under the plasma concentration-time curve (AUC) values were also comparable (61 μg/mL/h in the group with STS and 69 μg/mL/h in those without STS). Carboplatin clearance was reported to be similar in groups treated with and without STS (208 and 184 mL/h/kg, respectively: P = .33).48
In 10 guinea pigs that received IV STS (103 mg/kg) without cisplatin or carboplatin, the maximum concentration of STS was observed 10 minutes after administration (Cmax mean: 300 μM), with very low STS concentrations observed at 200 minutes after administration (mean: 1.5 μM), assessed in plasma by high-performance liquid chromatography (HPLC) analysis.69 Because of faster elimination from the bloodstream, the concentration of STS measured in the perilymph of the inner ear exceeded that of blood at the later time point, with mean perilymph concentrations of 55 μM and 7.0 μM observed at 10 and 200 minutes, respectively.
Because of different study end points and reported PK outcomes, a conclusion on the effect of systemic STS on cisplatin and carboplatin levels cannot be drawn (Table 4).
TABLE 4.
Preclinical Studies on the Pharmacokinetics of STS and Platinum
| Author, Year | Study End Point | Species | Treatment Groups | Platinum Treatment | STS Specification | Samples | Evaluation Method | PK Results | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax, µg/mL | AUC, µg-h/mL | T1/2, Minutes | Elimination/Clearance | ||||||||
| Saito et al,67 1997 | Effect of STS on the PK of CIS | Guinea pig | 10: CIS + STS concurrent + after 1 hour 14: CIS + STS at 3 and 6 hours 15: CIS only 8: STS only |
CIS IM 7.5 mg/kg 3 injections 5-day interval |
STS IP, 1,000 mg/kg; concurrent with CIS, and after 1, 3, and 6 hours | Blood (2-3 mL) perilymph (3-4 µL) at 1, 3, 6, and 24 hours | ICP-MS (FP and TP) | Plasma CIS + STS: 3.5 ± 1.0 CIS only: 1.9 ± 0.7 Perilymph CIS + STS: 0.4 ± 0.1 CIS only: 0.4 ± 0.1 |
NA | NA | Plasma Lower FP and TP concentrations in the CIS + STS group at 6 hours and 24 hours (P < .05) Perilymph Lower PT concentrations in the CIS + STS group at 24 hours (P < .05) |
| Muldoon et al,48 2000 | Effect of STS on the PK of CARBO | Guinea pig | 3: CARBO + STS 3: CARBO + saline |
CARBO 24 mg/kg |
STS 11.6 g/m2 for 2 hours | Blood (0.5 mL each) at 5 minutes, 30 minutes, and 1-6 hours | AAS | Both groups: approximately 23.0 (range 15-29) | With STS: 60.7 ± 19.6 Without STS: 68.5 ± 21.5 |
NA | CARBO clearance With STS: 208 ± 51 mL/h/kg Without STS: 184 ± 44 mL/h/kg (P = .33) |
| Harned et al,68 2008 | PK of STS | Mouse | 6: STS only | — | STS IP 3.5 g/kg | Blood at 1 and 15 minutes after injection | Methylene blue test | 1 minute: 1,717 ± 345 5 minutes: 8,598 ± 493 |
NA | NA | NA |
| Effect of STS on the PK of CIS | Mouse | 8: CIS + STS 2: CIS only |
CIS IP 4 mg/kg total per day for 4 days |
STS IP 3.5 g/k total per day concurrently with CIS | Blood at 15 minutes, 45 minutes, 1 hour, and 6 hours | AAS | NA | NA | CIS concentrations after 6 hours did not differ between groups (no data) | ||
| Pierre et al,69 2009 | PK of STS | Guinea pig | 10: STS only 2: saline |
— | STS IV 103 mg/kg as a bolus injection | Perilymph (1 μL) and blood (0.35 mL) after 10 and 30 minutes; 1, 2, and 3 hours | HPLC | Perilymph: approximately 60 μM Plasma: approximately 300 μM |
Perilymph: 51.7 Plasma: 105 |
Perilymph: 50 Plasma: 20 |
Perilymph mean STS concentrations at 200 min: approximately 6 μg/ml Plasma mean STS concentrations at 200 minutes: approximately 1.5 μg/mL |
Abbreviations: AAS, atomic absorption spectrometry; AUC, area under the plasma drug concentration-time curve; CARBO, carboplatin; CIS, cisplatin; Cmax, peak plasma concentration of the drug after administration; FP, free platinum; HPLC, high-performance liquid chromatography; ICP-MS, inductively coupled mass spectrometry; IM, intra-muscular; IP, intraperitoneal; IV, intravenous; NA, not assessed; PK, pharmacokinetics; PT, platinum; STS, sodium thiosulfate; TP, total platinum; Tx, treatment; T1/2, time required for the concentration of the drug to reach half of its original value.
Clinical Studies
Howell et al52 studied the effect of STS on the PK of cisplatin in 17 adults with IP tumors, who received IP cisplatin administered by dialysis over 4 hours (90-270 mg/m2) with IV STS over 12 hours (2.13 g/m2). Blood was obtained every 60 minutes after cisplatin and analyzed using HPLC. Cisplatin Cmax in plasma was 7.5 μg/mL and no effect of STS administration was observed (P > .05). This was also the case for t1/2 (half-life), which remained around 50-60 minutes in plasma when STS was added (P > .05).52 Similar observations have been reported by Pfeifle et al.56 In adults with different tumor types who received IV cisplatin (100 mg/m2) without STS (n = 5) or IV cisplatin (200 mg/m2) with IV STS (3.3-9.9 g/m2; n = 6) for 3 hours, no significant differences were observed in t1/2 (36.5 v 38.5 minutes, respectively) or plasma clearance (222 v 234 mL/min/m2, respectively). Similarly, plasma Cmax and AUC were approximately twice as high for the group of patients receiving the higher dose of cisplatin, suggesting that STS did not affect cisplatin PK.56 In a study by Goel et al70 in 14 adults treated concurrently with IP cisplatin (90 mg/m2) and STS (12 g/m2 over 6 hours), a nonsignificant reduction in the mean total plasma AUC from 8.8 μg/mL/h without STS to 6.7 μg/mL/h with STS was reported up to 21 hours (25%; P > .05). This difference became significant for the last 3 hours of exposure (up to 24 hours) with a reduction of 54% (P < .05).70
On the basis of the studies described above, it seems unlikely that STS has a major impact on systemic cisplatin PK levels even when the drugs are administered concurrently, but a firm conclusion on this is not possible to draw and requires further research. This is currently being assessed in the ongoing Paediatric Hepatic International Tumour Trial (PHITT; ClinicalTrials.gov identifier: NCT03017362). In all studies, platinum was measured (not specifically cisplatin or carboplatin), consisting of intact drug, aqua complexes, and platinum-bound species (including STS-bound platinum) being measured simultaneously. It is therefore unknown whether systemic STS administration reduces the fraction of active platinum species (Table 5). Further research studies using sensitive ICP-MS approaches are needed to measure cisplatin levels in patients treated with and without STS.71
TABLE 5.
Clinical Studies on the Pharmacokinetics of STS and Platinum
| Author, Year | Study End Point | Patient Characteristics | STS Group | Comparison Group | Platinum Treatment | STS Specification | Samples | Evaluation Method | PK Results | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax, µg/mL | AUC, µg-h/mL | T1/2, Minutes | Elimination/Clearance | |||||||||
| Howell et al,52 1982 | Effect of STS on the PK of CIS | IP tumors Mean age: 52 years (31-65) |
17: CIS + STS | — | CIS IP dialysis over 4 hours 6 courses 90-270 mg/m2 |
STS IV, over 12 hours 2.13 g/m2 per hour Concurrent with CIS |
Blood + peritoneal dialysate (every 60 minutes after CIS) | HPLC (FP) | Plasma: 7.5 PC: 85 No effect of STS (P > .05) |
Plasma: 7.2 ± 5.5 PC: 97.1 ± 64.9 Addition of STS: 2.9- and 1.9-fold increase (P < .01)a |
Plasma: 50-66 PC: 51-53 No effect of STS (P > .05) |
Plasma + PC Exponential decrease in CIS concentrations over time |
| Pfeifle et al,56 1985 | Effect of STS on the PK of CIS | Different tumor types Median age: 56 years (15-73) |
6: CIS + STS | 5: CIS only | CIS + STS: IV 202.5 mg/m2 7 courses CIS only: IV 100 mg/m2 8 courses |
STS IV for 3 hours First hour 3.3 g/m2, thereafter 9.9 g/m2 Start 1 hour before CIS |
Blood (22 time points up to 7 hours after CIS) | HPLC | With STS 6.9 Without STS: 3.2 |
With STS: 17.1 Without STS: 8.3 |
With STS: 36.5 Without STS: 38.5 |
Elimination rate With STS: 0.019 min/L Without STS: 0.018 min/L Clearance level With STS: 222 mL/min/m2 Without STS 234 mL/min/m2 |
| Goel et al,70 1989 | Effect of STS on the PK of CIS | IP tumors Mean age: 59 years (29-57) |
14: CIS + STS | — | CIS IP 90 mg/m2 5 courses |
STS IV in a bolus of 4 g/m2 before CIS, followed by 12 g/m2 over 6 hours | Blood + peritoneal dialysate (20 time points up to 24 hours after CIS) | HPLC | NA | Plasma With STS: 6.7 ± 2.2 Without STS: 8.8 ± 3.8: P < .05b PC With STS: 96.5 ± 54.4 Without STS: 149 ± 38.0: P < .05 |
Plasma: 80 ± 65 PC: 72 ± 59 |
Plasma clearance level: 59 ± 52 mL/min |
| Neuwelt et al,62 1998 | PK of STS in the presence of CARBO | Brain tumors Age: 2-68 years |
25: CARBO + STS 4-16 g/m2 8: CARBO + STS 20 g/m2 |
— | CARBO IA over 10 minutes 400 mg/m2 per month 4-12 courses |
STS IV over 15 minutes 4-20 g/m2 2 hours after CARBO |
Blood + urine (directly after STS injection, after 15 minutes, and after 24 hours) | Methylene blue test | Plasma (20 mg/m2) End bolus: 33.1 Urine (20 mg/m2) 15 minutes after bolus: 198.1 |
NA | NA | STS levels not detectable at 24 hours post-Tx |
Abbreviations: AUC, area under the plasma drug concentration-time curve; BL, bilateral; CARBO, carboplatin; CIS, cisplatin; Cmax, peak plasma concentration of a drug after administration; FP, free platinum; HPLC, high-performance liquid chromatography; IA, intra-arterial; IP, intraperitoneal; IV, intravenous; NA, not assessed; PC, peritoneal cavity; PK, pharmacokinetics; PT, platinum; STS, sodium thiosulfate; TP, total platinum; Tx, treatment; T1/2, time required for the concentration of the drug to reach half of its original value; UL, unilateral.
When the peritoneum to plasma AUC ratio was calculated separately for each patient, no variation in this ratio with dose was observed (P > .05: mean ratio for all cisplatin courses 12.4 [range 2.9-37.4]).
Significant difference only for the last 3 hours of exposure where a reduction of 54% was observed (P < .05).
ONCOLOGIC SAFETY OF SYSTEMIC STS
Preclinical Studies
In small cell lung carcinoma (SCLC) cell lines, Muldoon et al72 reported chemoprotection of cisplatin (15 μg/mL) and carboplatin (200 μg/mL) when STS (2,000 μg/mL) was administered concurrently, or 2-4 hours after administration (85%-95% live cells), compared with cell lines treated with cisplatin or carboplatin only (10%-20% live cells). However, when a broader time interval was studied, Dickey et al50 found only very minimal protection (0%-10%) in cell lines treated with cisplatin (30-50 µM) and STS (8 g/m2) after 6-8 hours, which was similar compared with the cisplatin-only group. Another study also reported no difference in cell survival between neuroblastoma cell lines that received cisplatin with or without STS after 6 hours (0%-60%: P > .05).68
Furthermore, Neuwelt et al73 studied tumor volume (TV) in rats with LX-1 human SCLC xenografts. TV of untreated rats was approximately 29 mm3 (n = 8), approximately 4.3 mm3 for rats treated with carboplatin (200 mg/m2: n = 8), and approximately 3.7 mm3 for rats that received systemic IV STS (8 g/m2: n = 8) at 4-8 hours (P < .0001; all groups).73 In another study, mice were treated with IP cisplatin only (4 mg/kg total per day for 4 days: n = 6) or with IP cisplatin and STS (3.5 g/kg total per day: n = 6). For mice treated with STS after 6 hours, TV was max 800 mm3 (P > .05); for the mice treated with STS and cisplatin at the same time (n = 6), this was 1,400 mm3 (P < .05). In addition, within these mice, the time to tumor progression was also similar between the cisplatin only and STS-at-6-hours group (210 days, P = .9), but shorter for the group that received cisplatin and STS concomitantly (20 days, P = .03).68 In a similar designed study with carboplatin (200 mg/m2) and STS, comparable findings were reported.48
The studies described above indicate that the delayed administration of STS at 6 hours after cisplatin does not reduce the impact of cisplatin on tumor growth and cell survival. A similar conclusion from delayed STS and carboplatin is difficult to draw from these data (Table 6).
TABLE 6.
Preclinical Studies on the Effect of STS on Platinum Antitumor Efficacy
| Author, Year | Species | Treatment Groups | Platinum Treatment | STS Specification | Evaluation Method | Results | P | |
|---|---|---|---|---|---|---|---|---|
| With STS | Without STS | |||||||
| Muldoon et al,48 2000 | Rat with LX-1 human SCLC xenograft | 8: CARBO + STS at 2 and 6 hours 8: CARBO + STS at 8 hours 20: CARBO only 20: no Tx |
CARBO 200 mg/m2 | STS 8 g/m2 at 2, 6 or 8 hours after CARBO | Time to tumor progression | STS 2 hours and 6 hours: 6.4 ± 0.8 days STS 8 hours group: 8.1 ± 0.7 days |
CARBO only: 8.9 ± 0.6 days | .012 .188 |
| Muldoon et al,72 2001 | SCLC cell line + human fibroblast cell strain | CARBO + STS CIS + STS CIS or CARBO only |
CARBO 200 μg/mL CIS 15 μg/mL |
STS 2,000 μg/mL: immediately, 2 hours, or 4 hours after PT | Live cell number by using CPA kit | CIS + STS immediately, at 2 hours, or 4 hours: 90%-95% live cells CARBO + STS immediately, at 2 hours, or 4 hours: 85%-90% live cells |
CIS only: 10% live cells CARBO only: 20% live cells |
NA |
| Neuwelt et al,73 2004 | Rat with LX-1 human SCLC xenograft | 8: CARBO + STS 8: CARBO only 8: no Tx |
CARBO 200 mg/m2 | STS IV 8 g/m2 at 4 or 8 hours after CARBO | Tumor volume | CARBO + STS: 3.7 ± 0.6 mm3 | CARBO only: 4.3 ± 1.0 mm3 Untreated: 29.1 ± 4.1 mm3 |
<.0001 (all groups) |
| Dickey et al,50 2005 | Human GBL, OC, MBL and SCLC cell lines | CIS + or - STS | CIS 30-50 µM | STS IV 8 g/m2 0, 2, 4, 6 or 8 hours after CIS |
Cell viability and immunoblotting assays | CIS + STS: 70%-100% protection of cells up to 2 hours after CIS; 30%-45% at 4 hours; 0%-10% at 6-8 hours post-CIS | CIS only: reduction in cell viability of 58% (GBL cells), 81% (OC cells), and 100% (MBDL and SCLC cells) | NA |
| Harned et al,68 2008 | Human NBL cell lines | 6: CIS + or - STS | CIS 0-2 μg/mL | STS 0.5-1.0 mg/mL at 0 or 6 hours after CIS | Cytotoxicity by using FDIMA | CIS + STS concurrently (5/6): survival fraction of cells 70%-100% CIS + STS after 6h (5/6): survival fraction of cells 0%-60% |
CIS only (5/6): survival fraction of cells 0%-60% | <.05 >.05 |
| Mouse with NBL xenografts | 6: CIS + STS concurrently 6: CIS + STS after 6 hours 6: CIS only 6: no Tx |
CIS IP 4 mg/kg total per day 4 days |
STS IP 3.5 g/k total per day concurrently with CIS or after 6 hours 4 days |
Tumor volume + time to tumor progression | Tumor volume CIS + STS concurrently: 1,400 mm3 CIS + STS after 6 hours: max. 800 mm3 Tumor progression CIS + STS concurrently: 20 days CIS + STS after 6 hours: 210 days |
Tumor volume CIS only: max. 800 mm3 Tumor progression CIS only: 210 days |
<.05 >.05 .03 .90 |
|
Abbreviations: CIS, cisplatin; CPA, cell proliferation assay; FDIMA, fluorescence/digital imaging microscopy assay; GBL, glioblastoma; IP, intraperitoneal; IV, intravenous; MBL, medulloblastoma; NA, not assessed; NBL, neuroblastoma; OC, ovarian carcinoma; PT, platinum; SCLC, small cell lung carcinoma; STS, sodium thiosulfate; Tx, treatment.
Clinical Studies
In children with standard-risk hepatoblastoma, Brock et al40 reported that event-free survival (EFS) and overall survival (OS) were similar between the IV STS group (EFS, 82% [95% CI, 69 to 90]; OS, 98% [95% CI, 88 to 100]) and the non-STS group (EFS, 79% [95% CI, 65 to 88]; OS, 92% [95% CI, 81 to 97])40 at a median follow-up time of 3 years. In children with any tumor type at a similar follow-up time, Freyer et al41 reported no difference between the IV STS group and controls regarding EFS and OS (P = .36 and .07, respectively). Although there was no evidence of a tumor-protective effect from STS in the whole cohort, a post hoc analysis confirmed no effect in 77 patients with localized disease (EFS and OS: P = .73 and .88, respectively) but revealed a survival difference in OS in 47 patients with disseminated disease in the STS-treated group (P = .009; relative hazard ratio, 4.10 [95% CI, 1.30 to 12.97]).41 In a subsequent follow-up paper, the same authors (2022) noted that the children with disseminated disease who did not receive STS had a better than originally predicted survival (compared with the literature) and that the difference between the two arms was most probably related to an imbalance in prognostic groups.74
In conclusion, systemic STS is safe to administer in children with localized disease (Table 2). For now, STS is not approved for use in those with metastatic disease, although a biologically plausible rationale is lacking for why a 6-hour delay in STS administration after cisplatin infusion would lead to reduced antitumor efficacy.75
FUTURE PERSPECTIVES
The STS compound used in the pediatric RCTs has recently been licensed by the FDA (Pedmark) and EMA and MHRA (Pedmarqsi), and is ready for implementation into current practice in children with cancers requiring cisplatin therapy. These include mainly neuroblastoma, hepatoblastoma, nasopharyngeal carcinoma, osteosarcoma, medulloblastoma, germ cell tumors, and rarely other cancers. A guideline published prelicensing, however, recommends it for use in children with standard-risk hepatoblastoma only.76 This guideline therefore requires a postlicensing update. To study the efficacy and safety of STS in metastatic disease, specific tumor diagnoses RCTs or single-arm trials for which adequately available historic outcomes are needed. Preferably, these studies should be supported by biologic and imaging studies to assess both otoprotection and treatment response in the presence of properly administered (delayed) STS.
Second, more PK studies are needed to understand the effect of STS on cisplatin kinetics, including measurement of unbound cisplatin and carboplatin. This contrasts to the approach taken in the majority of published studies in this area, in which total plasma platinum is most commonly measured (including aqua complexes and STS-bound platinum). Evaluating platinum in ultrafiltrate samples would provide a measure of free drug levels, which are more likely to correlate with clinical response and toxicity.77,78 Using this method would also be beneficial to confirm the safety of STS when administered 6 hours after the end of cisplatin infusion, as previously suggested.48,50,68 An effort is currently ongoing to study relationships between cisplatin PK, pharmacogenomics, and biomarkers of toxicity, as well as clinical efficacy and toxicity in the PHITT Trial (ISRCTN17869351).79
Third, to administer STS in the appropriate window to obtain otoprotection, cisplatin infusion durations require reduction to a maximum of 6 hours. In Europe, cisplatin is sometimes administered as a 24- to 96-hour infusion, historically implemented to reduce emesis.80,81 However, there is no evidence that longer infusion durations are more effective in terms of antitumor effect compared with shorter durations. Specifically, in the SIOPEL 6 trial, the infusion of cisplatin was reduced from 48 to 6 hours, with no difference in survival outcome when compared with SIOPEL 3.82 In addition, there is no evidence that a single dose of cisplatin is more effective compared with split, daily doses. The latter has been used for patients with germ cell tumors for several decades (ie, 20 mg/m2 for 5 days per cycle), and has shown to result in excellent survival rates.83,84 For future studies, it would therefore be important to design them, with the reduced cisplatin infusion duration and the use of split doses in specific tumor groups, thereby powering the study appropriately, to allow outcome analyses, similar to the previous RCTs in children.
Fourth, there is preliminary evidence from early-phase adult studies that hearing loss deterioration already starts after the first dose of cisplatin and may worsen thereafter with time, independent of other treatments.55,58,64 This implies that it is important to administer STS alongside the first cisplatin cycle of treatment, as it may be detrimental to wait to give STS after hearing loss has already developed. Whether STS may prevent subsequent additional hearing loss, after the onset of CIHL, upon cisplatin rechallenge, is not well understood. A current trial is under way to assess this phenomenon in children with relapsed or refractory hepatoblastoma (ClinicalTrials.gov identifier: NCT05756660).85
Fifth, historically, STS was often administered concomitantly with cisplatin, either to allow the infusion of higher doses of cisplatin or to reduce the development of side effects, including nephrotoxicity.51-60,66 It should be noted that when STS is given for otoprotection at 6 hours after the end of cisplatin, it is unlikely to reduce nephrotoxicity that occurs earlier than ototoxicity. It is therefore important that the currently approved form of high-dose STS in children is not provided with a view to protecting renal function, but only for otoprotection.42
Sixth, future investigation on the potential for STS to prevent CIHL from carboplatin is justified. This may be challenging to achieve in the short term. The PK of the activation reaction distinguishes the two drugs from each other. Cisplatin will already be deactivated systemically after 6 hours, but carboplatin is more chemically stable.77,86 Thus, administering STS after 6 hours would generate a risk of deactivating the carboplatin and negatively affecting its antitumor efficacy. Carefully planned PK and xenograft studies are therefore necessary to determine an appropriate time window for STS administration after carboplatin infusion.
Seventh, it is important to highlight the variations in audiologic testing and end point definitions among the clinical studies reviewed, taking into consideration differences in test frequency range, ototoxicity definitions, and consistency in hearing endpoints. For example, Brock et al40 used the Brock scale, whereas Freyer et al41 used the ASHA criteria. Clemens et al2 concluded that there is good concordance between ototoxicity grading scales; however, severity definitions and intermediate grades diverge. Acknowledging these differences, a recent reevaluation of ACCL0431 with as end point the SIOP ototoxicity criteria revealed a lower incidence of grade ≥2 CIHL in the STS arm compared with the observation arm (3/58 [5.2%] and 18/63 [28.6%], respectively).87 This underscores the need for careful consideration of the type of hearing assessment and ototoxicity grading scale used when interpreting the prevalence of hearing loss in studies. Consequently, we suggest incorporating age-dependent audiologic testing, as recommended by Meijer et al,88 and the SIOP ototoxicity grading scale, to facilitate uniform outcomes regarding platinum-induced hearing loss.89
In conclusion, systemic administration of STS effectively reduces the development of CIHL in both the preclinical and clinical settings. It has been shown to be safe, in children with localized disease, when a window of 6 hours is respected. Even if hearing loss develops, its severity is reduced. More well-executed studies on the PK and safety of STS and cisplatin are needed, especially in patients with metastatic disease. In the future, this will hopefully lead to STS otoprotection becoming standard of care for all cisplatin-treated patients with cancer, thereby decreasing the debilitating impact of CIHL, on speech development, social isolation, neurocognitive development, and consequent QoL, as well as reducing the risk of late sequelae, such as early dementia later in life.
APPENDIX
FIG A1.
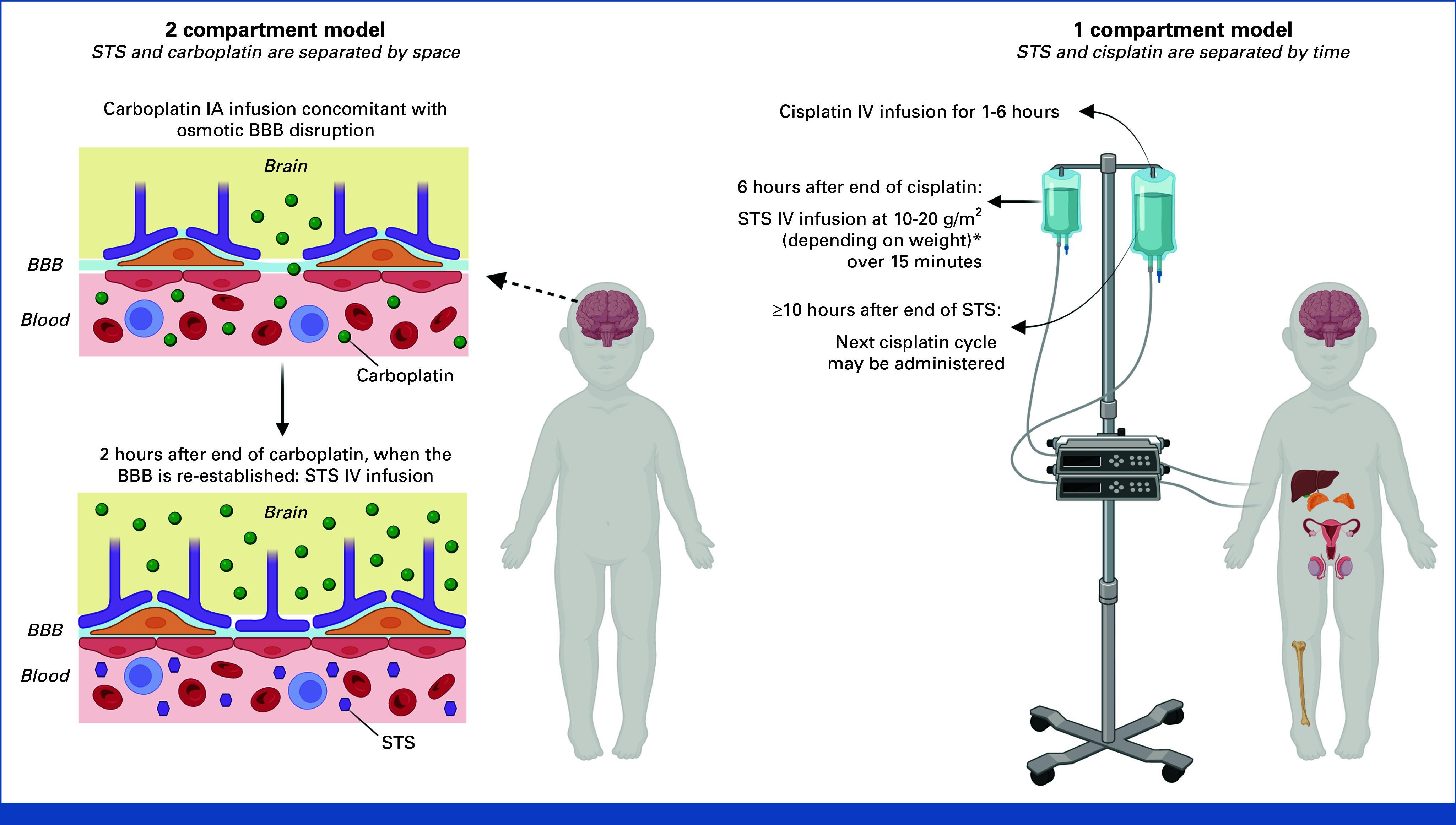
Differences between (A) 2-compartment and (B) 1-compartment models for platinum and STS administration. a<5 kg: 10 mg/m2; 5-10 kg: 15 mg/m2; >10 kg: 20 mg/m2. BBB, blood-brain barrier; IA, intra-arterial; IV intravenous; STS, sodium thiosulfate. Created via BioRender.com.
Marc Ansari
Travel, Accommodations, Expenses: Novonordisk (travel to ASH meeting), Jazz Pharmaceutical (EBMT congress)
Eric Bouffet
Consulting or Advisory Role: Novartis, Alexion Pharmaceuticals, Gilead Sciences
Research Funding: Roche (Inst)
Archie Bleyer
Patents, Royalties, Other Intellectual Property: Springer Nature Book royalty <$500 paid annually until book's next edition
Brice Fresneau
Consulting or Advisory Role: iqone healthcare (Inst)
James I. Geller
Consulting or Advisory Role: Fennec Pharma
Allison F. O'Neill
Employment: Dana-Farber Cancer Institute
Consulting or Advisory Role: CorMedix
Vassilios Papadakis
Honoraria: EUSA Pharma
Consulting or Advisory Role: MSD, Integris Pharma
Travel, Accommodations, Expenses: Servier, EUSA Pharma
Penelope R. Brock
Consulting or Advisory Role: Fennec Pharma
Travel, Accommodations, Expenses: Fennec Pharma
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by the Princess Máxima Center for pediatric oncology.
AUTHOR CONTRIBUTIONS
Conception and design: Annelot J.M. Meijer, Franciscus A. Diepstraten, Marc Ansari, Eric Bouffet, Archie Bleyer, James I. Geller, Alwin D.R. Huitema, Per Kogner, Allison F. O'Neill, Vassilios Papadakis, Kaukab M. Rajput, Michael Sullivan, Marry M. van den Heuvel-Eibrink, Penelope R. Brock
Provision of study materials or patients: Marc Ansari, Kaukab M. Rajput, Penelope R. Brock
Collection and assembly of data: Annelot J.M. Meijer, Marc Ansari, Per Kogner, Marry M. van den Heuvel-Eibrink, Penelope R. Brock
Data analysis and interpretation: Annelot J.M. Meijer, Marc Ansari, Eric Bouffet, Archie Bleyer, Brice Fresneau, James I. Geller, Alwin D.R. Huitema, Per Kogner, Rudolf Maibach, Allison F. O'Neill, Gareth J. Veal, Michael Sullivan, Marry M. van den Heuvel-Eibrink, Penelope R. Brock
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Use of Sodium Thiosulfate as an Otoprotectant in Patients With Cancer Treated With Platinum Compounds: A Review of the Literature
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Marc Ansari
Travel, Accommodations, Expenses: Novonordisk (travel to ASH meeting), Jazz Pharmaceutical (EBMT congress)
Eric Bouffet
Consulting or Advisory Role: Novartis, Alexion Pharmaceuticals, Gilead Sciences
Research Funding: Roche (Inst)
Archie Bleyer
Patents, Royalties, Other Intellectual Property: Springer Nature Book royalty <$500 paid annually until book's next edition
Brice Fresneau
Consulting or Advisory Role: iqone healthcare (Inst)
James I. Geller
Consulting or Advisory Role: Fennec Pharma
Allison F. O'Neill
Employment: Dana-Farber Cancer Institute
Consulting or Advisory Role: CorMedix
Vassilios Papadakis
Honoraria: EUSA Pharma
Consulting or Advisory Role: MSD, Integris Pharma
Travel, Accommodations, Expenses: Servier, EUSA Pharma
Penelope R. Brock
Consulting or Advisory Role: Fennec Pharma
Travel, Accommodations, Expenses: Fennec Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Al-Khatib T, Cohen N, Carret AS, et al. : Cisplatinum ototoxicity in children, long-term follow up. Int J Pediatr Otorhinolaryngol 74:913-919, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Clemens E, Brooks B, de Vries ACH, et al. : A comparison of the Muenster, SIOP Boston, Brock, Chang and CTCAEv4.03 ototoxicity grading scales applied to 3,799 audiograms of childhood cancer patients treated with platinum-based chemotherapy. PLoS One 14:e0210646, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight KR, Chen L, Freyer D, et al. : Group-wide, prospective study of ototoxicity assessment in children receiving cisplatin chemotherapy (ACCL05C1): A report from the Children's Oncology Group. J Clin Oncol 35:440-445, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kushner BH, Budnick A, Kramer K, et al. : Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer 107:417-422, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Nitz A, Kontopantelis E, Bielack S, et al. : Prospective evaluation of cisplatin- and carboplatin-mediated ototoxicity in paediatric and adult soft tissue and osteosarcoma patients. Oncol Lett 5:311-315, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peleva E, Emami N, Alzahrani M, et al. : Incidence of platinum-induced ototoxicity in pediatric patients in Quebec. Pediatr Blood Cancer 61:2012-2017, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Gentilin E, Simoni E, Candito M, et al. : Cisplatin-induced ototoxicity: Updates on molecular targets. Trends Mol Med 25:1123-1132, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Sheth S, Mukherjea D, Rybak LP, et al. : Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front Cell Neurosci 11:338, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert MP, Shields C, Meadows AT: A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy. Pediatr Blood Cancer 50:223-226, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Smits C, Swen SJ, Theo Goverts S, et al. : Assessment of hearing in very young children receiving carboplatin for retinoblastoma. Eur J Cancer 42:492-500, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Musial-Bright L, Fengler R, Henze G, et al. : Carboplatin and ototoxicity: Hearing loss rates among survivors of childhood medulloblastoma. Childs Nerv Syst 27:407-413, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Qaddoumi I, Bass JK, Wu J, et al. : Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol 30:1034-1041, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens E, de Vries AC, Pluijm SF, et al. : Determinants of ototoxicity in 451 platinum-treated Dutch survivors of childhood cancer: A DCOG late-effects study. Eur J Cancer 69:77-85, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Soliman SE, D'Silva CN, Dimaras H, et al. : Clinical and genetic associations for carboplatin-related ototoxicity in children treated for retinoblastoma: A retrospective noncomparative single-institute experience. Pediatr Blood Cancer 65:e26931, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Landier W, Knight K, Wong FL, et al. : Ototoxicity in children with high-risk neuroblastoma: Prevalence, risk factors, and concordance of grading scales—A report from the Children's Oncology Group. J Clin Oncol 32:527-534, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer AJM, Li KH, Brooks B, et al. : The cumulative incidence of cisplatin-induced hearing loss in young children is higher and develops at an early stage during therapy compared with older children based on 2052 audiological assessments. Cancer 128:169-179, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Moke DJ, Luo C, Millstein J, et al. : Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: A multi-institutional North American cohort study. Lancet Child Adolesc Health 5:274-283, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bass JK, Hua CH, Huang J, et al. : Hearing loss in patients who received cranial radiation therapy for childhood cancer. J Clin Oncol 34:1248-1255, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghazwani Y, Qaddoumi I, Bass JK, et al. : Profound hearing loss following surgery in pediatric patients with posterior fossa low-grade glioma. Neurooncol Pract 5:96-103, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemens E, van der Kooi ALF, Broer L, et al. : The influence of genetic variation on late toxicities in childhood cancer survivors: A review. Crit Rev Oncol Hematol 126:154-167, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Einarsson EJ, Petersen H, Wiebe T, et al. : Severe difficulties with word recognition in noise after platinum chemotherapy in childhood, and improvements with open-fitting hearing-aids. Int J Audiol 50:642-651, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Gurney JG, Tersak JM, Ness KK, et al. : Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the Children's Oncology Group. Pediatrics 120:e1229-e1236, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bass JK, Liu W, Banerjee P, et al. : Association of hearing impairment with neurocognition in survivors of childhood cancer. JAMA Oncol 6:1363-1371, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajput K, Edwards L, Brock P, et al. : Ototoxicity-induced hearing loss and quality of life in survivors of paediatric cancer. Int J Pediatr Otorhinolaryngol 138:110401, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Golub JS, Brickman AM, Ciarleglio AJ, et al. : Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol Head Neck Surg 146:57-67, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang F, Mishra SR, Shrestha N, et al. : Association between hearing aid use and all-cause and cause-specific dementia: An analysis of the UK Biobank cohort. Lancet Public Health 8:e329-e338, 2023 [DOI] [PubMed] [Google Scholar]
- 27.Chern A, Sharma RK, Golub JS: Hearing loss and incident dementia: Claims data from the New York SPARCS Database. Otol Neurotol 43:36-41, 2022 [DOI] [PubMed] [Google Scholar]
- 28.Hendriks S, Ranson JM, Peetoom K, et al. : Risk factors for young-onset dementia in the UK Biobank. JAMA Neurol 81:134-142, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawes P, Völter C: Do hearing loss interventions prevent dementia? Z Gerontol Geriatr 56:261-268, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wayne RV, Johnsrude IS: A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev 23:154-166, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Dawes P, Emsley R, Cruickshanks KJ, et al. : Hearing loss and cognition: The role of hearing AIDS, social isolation and depression. PLoS One 10:e0119616, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Atteveld JE, de Winter DTC, Pluimakers VG, et al. : Frailty and sarcopenia within the earliest national Dutch childhood cancer survivor cohort (DCCSS-LATER): A cross-sectional study. Lancet Healthy Longev 4:e155-e165, 2023 [DOI] [PubMed] [Google Scholar]
- 33.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. : Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297:2705-2715, 2007 [DOI] [PubMed] [Google Scholar]
- 34.van Erp LME, Maurice-Stam H, Kremer LCM, et al. : Health-related quality of life in Dutch adult survivors of childhood cancer: A nation-wide cohort study. Eur J Cancer 152:204-214, 2021 [DOI] [PubMed] [Google Scholar]
- 35.Lee JW, Pussegoda K, Rassekh SR, et al. : Clinical practice recommendations for the management and prevention of cisplatin-induced hearing loss using pharmacogenetic markers. Ther Drug Monit 38:423-431, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Sooriyaarachchi M, George GN, Pickering IJ, et al. : Tuning the metabolism of the anticancer drug cisplatin with chemoprotective agents to improve its safety and efficacy. Metallomics 8:1170-1176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bijarnia RK, Bachtler M, Chandak PG, et al. : Sodium thiosulfate ameliorates oxidative stress and preserves renal function in hyperoxaluric rats. PLoS One 10:e0124881, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu D, Gu J, Chen Y, et al. : Current strategies to combat cisplatin-induced ototoxicity. Front Pharmacol 11:999, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hazlitt RA, Min J, Zuo J: Progress in the development of preventative drugs for cisplatin-induced hearing loss. J Med Chem 61:5512-5524, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brock PR, Maibach R, Childs M, et al. : Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 378:2376-2385, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freyer DR, Chen L, Krailo MD, et al. : Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 18:63-74, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhillon S: Sodium thiosulfate: Pediatric first approval. Paediatr Drugs 25:239-244, 2022 [DOI] [PubMed] [Google Scholar]
- 43.Orgel E, Freyer DR, Ullrich NJ, et al. : Assessment of provider perspectives on otoprotection research for children and adolescents: A Children's Oncology Group Cancer Control and Supportive Care Committee survey. Pediatr Blood Cancer 67:e28647, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otto WC, Brown RD, Gage-White L, et al. : Effects of cisplatin and thiosulfate upon auditory brainstem responses of Guinea pigs. Hear Res 35:79-85, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Church MW, Kaltenbach JA, Blakley BW, et al. : The comparative effects of sodium thiosulfate, diethyldithiocarbamate, fosfomycin and WR-2721 on ameliorating cisplatin-induced ototoxicity. Hear Res 86:195-203, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Neuwelt EA, Brummett RE, Remsen LG, et al. : In vitro and animal studies of sodium thiosulfate as a potential chemoprotectant against carboplatin-induced ototoxicity. Cancer Res 56:706-709, 1996 [PubMed] [Google Scholar]
- 47.Kaltenbach JA, Church MW, Blakley BW, et al. : Comparison of five agents in protecting the cochlea against the ototoxic effects of cisplatin in the hamster. Otolaryngol Head Neck Surg 117:493-500, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Muldoon LL, Pagel MA, Kroll RA, et al. : Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res 6:309-315, 2000 [PubMed] [Google Scholar]
- 49.Videhult Pierre P, Haglöf J, Linder B, et al. : Cisplatin-induced metabolome changes in serum: An experimental approach to identify markers for ototoxicity. Acta Otolaryngol 137:1024-1030, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Dickey DT, Wu YJ, Muldoon LL, et al. : Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther 314:1052-1058, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Balm AJ, Rasch CR, Schornagel JH, et al. : High-dose superselective intra-arterial cisplatin and concomitant radiation (RADPLAT) for advanced head and neck cancer. Head Neck 26:485-493, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Howell SB, Pfeifle CL, Wung WE, et al. : Intraperitoneal cisplatin with systemic thiosulfate protection. Ann Intern Med 97:845-851, 1982 [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa E, Sugimoto H, Hatano M, et al. : Protective effects of sodium thiosulfate for cisplatin-mediated ototoxicity in patients with head and neck cancer. Acta Otolaryngol 135:919-924, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Kim S, Howell SB, McClay E, et al. : Dose intensification of cisplatin chemotherapy through biweekly administration. Ann Oncol 4:221-227, 1993 [DOI] [PubMed] [Google Scholar]
- 55.Markman M, D'Acquisto R, Iannotti N, et al. : Phase-1 trial of high-dose intravenous cisplatin with simultaneous intravenous sodium thiosulfate. J Cancer Res Clin Oncol 117:151-155, 1991 [DOI] [PubMed] [Google Scholar]
- 56.Pfeifle CE, Howell SB, Felthouse RD, et al. : High-dose cisplatin with sodium thiosulfate protection. J Clin Oncol 3:237-244, 1985 [DOI] [PubMed] [Google Scholar]
- 57.Reichman B, Markman M, Hakes T, et al. : Phase II trial of high-dose cisplatin with sodium thiosulfate nephroprotection in patients with advanced carcinoma of the uterine cervix previously untreated with chemotherapy. Gynecol Oncol 43:159-163, 1991 [DOI] [PubMed] [Google Scholar]
- 58.van Rijswijk RE, Hoekman K, Burger CW, et al. : Experience with intraperitoneal cisplatin and etoposide and i.v. sodium thiosulphate protection in ovarian cancer patients with either pathologically complete response or minimal residual disease. Ann Oncol 8:1235-1241, 1997 [DOI] [PubMed] [Google Scholar]
- 59.Zuur CL, Simis YJ, Lansdaal PE, et al. : Risk factors of ototoxicity after cisplatin-based chemo-irradiation in patients with locally advanced head-and-neck cancer: A multivariate analysis. Int J Radiat Oncol Biol Phys 68:1320-1325, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Zuur CL, Simis YJ, Lansdaal PE, et al. : Ototoxicity in a randomized phase III trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J Clin Oncol 25:3759-3765, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Doolittle ND, Muldoon LL, Brummett RE, et al. : Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin Cancer Res 7:493-500, 2001 [PubMed] [Google Scholar]
- 62.Neuwelt EA, Brummett RE, Doolittle ND, et al. : First evidence of otoprotection against carboplatin-induced hearing loss with a two-compartment system in patients with central nervous system malignancy using sodium thiosulfate. J Pharmacol Exp Ther 286:77-84, 1998 [PubMed] [Google Scholar]
- 63.Neuwelt EA, Gilmer-Knight K, Lacy C, et al. : Toxicity profile of delayed high dose sodium thiosulfate in children treated with carboplatin in conjunction with blood-brain-barrier disruption. Pediatr Blood Cancer 47:174-182, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Harao T, Yamada A, Kinoshita M, et al. : Prevention of cisplatin-induced hearing-loss by sodium thiosulfate in medulloblastoma. Pediatr Int 62:1204-1206, 2020 [DOI] [PubMed] [Google Scholar]
- 65.Madasu R, Ruckenstein MJ, Leake F, et al. : Ototoxic effects of supradose cisplatin with sodium thiosulfate neutralization in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 123:978-981, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Womack AM, Hayes-Jordan A, Pratihar R, et al. : Evaluation of ototoxicity in patients treated with hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin and sodium thiosulfate. Ear Hear 35:e243-e247, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saito T, Zhang ZJ, Manabe Y, et al. : The effect of sodium thiosulfate on ototoxicity and pharmacokinetics after cisplatin treatment in Guinea pigs. Eur Arch Otorhinolaryngol 254:281-286, 1997 [DOI] [PubMed] [Google Scholar]
- 68.Harned TM, Kalous O, Neuwelt A, et al. : Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin Cancer Res 14:533-540, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Pierre PV, Engmér C, Wallin I, et al. : High concentrations of thiosulfate in scala tympani perilymph after systemic administration in the Guinea pig. Acta Otolaryngol 129:132-137, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Goel R, Cleary SM, Horton C, et al. : Effect of sodium thiosulfate on the pharmacokinetics and toxicity of cisplatin. J Natl Cancer Inst 81:1552-1560, 1989 [DOI] [PubMed] [Google Scholar]
- 71.Brouwers EE, Tibben MM, Rosing H, et al. : Sensitive inductively coupled plasma mass spectrometry assay for the determination of platinum originating from cisplatin, carboplatin, and oxaliplatin in human plasma ultrafiltrate. J Mass Spectrom 41:1186-1194, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Muldoon LL, Walker-Rosenfeld SL, Hale C, et al. : Rescue from enhanced alkylator-induced cell death with low molecular weight sulfur-containing chemoprotectants. J Pharmacol Exp Ther 296:797-805, 2001 [PubMed] [Google Scholar]
- 73.Neuwelt EA, Pagel MA, Kraemer DF, et al. : Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther 309:594-599, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Orgel E, Villaluna D, Krailo MD, et al. : Sodium thiosulfate for prevention of cisplatin-induced hearing loss: Updated survival from ACCL0431. Lancet Oncol 23:570-572, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brock P, Meijer A, Kogner P, et al. : Sodium thiosulfate as cisplatin otoprotectant in children: The challenge of when to use it. Pediatr Blood Cancer 70:e30248, 2023 [DOI] [PubMed] [Google Scholar]
- 76.Freyer DR, Brock PR, Chang KW, et al. : Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: A clinical practice guideline. Lancet Child Adolesc Health 4:141-150, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qin Z, Ren G, Yuan J, et al. : Systemic evaluation on the pharmacokinetics of platinum-based anticancer drugs from animal to cell level: Based on total platinum and intact drugs. Front Pharmacol 10:1485, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brouwers EE, Tibben M, Rosing H, et al. : The application of inductively coupled plasma mass spectrometry in clinical pharmacological oncology research. Mass Spectrom Rev 27:67-100, 2008 [DOI] [PubMed] [Google Scholar]
- 79.University of Birmingham : Paediatric Hepatic International Tumour Trial. PHITT, 2017. https://www.birmingham.ac.uk/Documents/college-mds/trials/crctu/phitt/Protocol/Current/PHITT-Protocol-version-3-0-17Oct2018.pdf [Google Scholar]
- 80.Jordan NS, Schauer PK, Schauer A, et al. : The effect of administration rate on cisplatin-induced emesis. J Clin Oncol 3:559-561, 1985 [DOI] [PubMed] [Google Scholar]
- 81.Jacobs C, Bertino JR, Goffinet DR, et al. : 24-hour infusion of cis-platinum in head and neck cancers. Cancer 42:2135-2140, 1978 [DOI] [PubMed] [Google Scholar]
- 82.Maibach R, Childs M, Rajput K, et al. : SIOPEL 6: A multicenter open-label randomized phase III trial of the efficacy of sodium thiosulphate (STS) in reducing ototoxicity in patients receiving cisplatin (Cis) monotherapy for standard-risk hepatoblastoma (SR-HB). J Clin Oncol 32, 2014. (suppl; abstr TPS10094) [Google Scholar]
- 83.Grimison PS, Stockler MR, Thomson DB, et al. : Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: Updated analysis of a randomized trial. J Natl Cancer Inst 102:1253-1262, 2010 [DOI] [PubMed] [Google Scholar]
- 84.Funt SA, McHugh DJ, Tsai S, et al. : Four cycles of etoposide plus cisplatin for patients with good-risk advanced germ cell tumors. Oncologist 26:483-491, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Children's Hospital Medical Center Cincinnati : Sodium Thiosulfate Otoprotection During Salvage Cisplatin Therapy, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05756660 [Google Scholar]
- 86.Terheggen PM, Begg AC, Emondt JY, et al. : Formation of interaction products of carboplatin with DNA in vitro and in cancer patients. Br J Cancer 63:195-200, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orgel E, Knight KR, Villaluna D, et al. : Reevaluation of sodium thiosulfate otoprotection using the consensus International Society of Paediatric Oncology Ototoxicity Scale: A report from the Children's Oncology Group study ACCL0431. Pediatr Blood Cancer 70:e30550, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meijer AJM, van den Heuvel-Eibrink MM, Brooks B, et al. : Recommendations for age-appropriate testing, timing, and frequency of audiologic Monitoring during childhood cancer treatment: An International Society of Paediatric Oncology Supportive Care Consensus Report. JAMA Oncol 7:1550-1558, 2021 [DOI] [PubMed] [Google Scholar]
- 89.Brock PR, Knight KR, Freyer DR, et al. : Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol 30:2408-2417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


