Abstract
PURPOSE
Open-label phase II study (RELATIVITY-060) to investigate the efficacy and safety of first-line nivolumab, a PD-1–blocking antibody, plus relatlimab, a lymphocyte-activation gene 3 (LAG-3)–blocking antibody, plus chemotherapy in patients with previously untreated advanced gastric cancer (GC) or gastroesophageal junction cancer (GEJC).
METHODS
Patients with unresectable, locally advanced or metastatic GC/GEJC were randomly assigned 1:1 to nivolumab + relatlimab (fixed-dose combination) + chemotherapy or nivolumab + chemotherapy. The primary end point was objective response rate (ORR; per RECIST v1.1 by blinded independent central review [BICR]) in patients whose tumors had LAG-3 expression ≥1%.
RESULTS
Of 274 patients, 138 were randomly assigned to nivolumab + relatlimab + chemotherapy and 136 to nivolumab + chemotherapy. Median follow-up was 11.9 months. In patients with LAG-3 expression ≥1%, BICR-assessed ORR (95% CI) was 48% (38 to 59) in the nivolumab + relatlimab + chemotherapy arm and 61% (51 to 71) in the nivolumab + chemotherapy arm; median progression-free survival (95% CI) by BICR was 7.0 months (5.8 to 8.4) versus 8.3 months (6.9 to 12.1; hazard ratio [HR], 1.41 [95% CI, 0.97 to 2.05]), and median overall survival (95% CI) was 13.5 months (11.9 to 19.1) versus 16.0 months (10.9 to not estimable; HR, 1.04 [95% CI, 0.70 to 1.54]), respectively. Grade 3 or 4 treatment-related adverse events (TRAEs) occurred in 69% and 61% of all treated patients, and 42% and 36% of patients discontinued because of any-grade TRAEs in the nivolumab + relatlimab + chemotherapy and nivolumab + chemotherapy arms, respectively.
CONCLUSION
RELATIVITY-060 did not meet its primary end point of improved ORR in patients with LAG-3 expression ≥1% when relatlimab was added to nivolumab + chemotherapy compared with nivolumab + chemotherapy. Further studies are needed to address whether adding anti–LAG-3 to anti–PD-1 plus chemotherapy can benefit specific GC/GEJC patient subgroups.
INTRODUCTION
Gastric cancer (GC), including gastroesophageal junction cancer (GEJC), is the fourth leading cause of cancer-related death worldwide each year, with a 5-year relative survival rate of 6% or less for patients with metastatic disease.1
CONTEXT
Key Objective
Does the combination of relatlimab, a lymphocyte-activation gene 3 (LAG-3)–blocking antibody, and nivolumab, a PD-1–blocking antibody, plus chemotherapy provide clinical benefit in patients with previously untreated advanced gastric or gastroesophageal junction cancer?
Knowledge Generated
RELATIVITY-060 did not meet its primary end point of improved objective response rate with nivolumab + relatlimab + chemotherapy compared with nivolumab + chemotherapy in patients whose tumors had LAG-3 expression ≥1%. Median progression-free survival and median overall survival were also not improved; however, disease control rate was comparable. The safety profile of nivolumab + relatlimab + chemotherapy was acceptable and consistent with the known safety profiles of the immunotherapy and chemotherapy components.
Relevance (E.M. O'Reilly)
The results from the RELATIVITY-060 trial endorse a current standard of care (chemotherapy/anti-PD-1 antibody) as the addition of LAG-3 blockade in untreated upper esophagogastric cancers does not add significant benefit.*
*Relevance section written by JCO Associate Editor Eileen M. O'Reilly, MD.
Nivolumab, a PD-1–blocking antibody, plus oxaliplatin-based chemotherapy demonstrated superior overall survival (OS) and progression-free survival (PFS) benefit and maintained health-related quality of life with an acceptable safety profile compared with chemotherapy in previously untreated patients with non–human epidermal growth factor receptor 2 (HER2)–positive gastroesophageal adenocarcinoma in the CheckMate 649 trial.2,3 On the basis of these results, nivolumab + chemotherapy was approved as a first-line treatment for unresectable advanced or metastatic GC/GEJC/esophageal adenocarcinoma in many countries including the United States.4
Dual immunotherapies, such as nivolumab plus ipilimumab, a cytotoxic T lymphocyte antigen-4–blocking antibody, have resulted in clinical benefit versus chemotherapy for the treatment of multiple tumor types, suggesting that combining nivolumab with other checkpoint inhibitors, such as relatlimab, a lymphocyte-activation gene 3 (LAG-3)–blocking antibody, has the potential for a synergistic effect.5-11 The distinct immune checkpoints PD-1 and LAG-3 are often coexpressed on tumor-infiltrating lymphocytes and contribute to T-cell dysfunction.12 The combination of nivolumab + relatlimab demonstrated significant PFS benefit compared with nivolumab alone with a manageable safety profile in patients with previously untreated metastatic or unresectable melanoma in the RELATIVITY-047 study.13 On the basis of these results, nivolumab + relatlimab as a fixed-dose combination (FDC) was approved for the treatment of unresectable or metastatic melanoma.14
Here, we present the results from the RELATIVITY-060 study of nivolumab + relatlimab + chemotherapy versus nivolumab + chemotherapy as first-line therapy for patients with unresectable, locally advanced or metastatic GC/GEJC.
METHODS
Study Design and Eligibility
RELATIVITY-060 is a randomized, open-label, multicenter, phase II study conducted at 70 centers across 17 countries in Asia, Europe, North America, South America, and Australia. Eligible patients were at least age 18 years with histologically/cytologically confirmed unresectable and either locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma and had not previously received systemic treatment. Adjuvant or neoadjuvant chemotherapy, radiotherapy, and/or chemoradiotherapy were permitted if given at least 6 months before random assignment. Patients had an Eastern Cooperative Oncology Group performance status score of 0 or 1; at least one measurable lesion per RECIST v1.1; and tumor tissue for biomarker analyses.15 Patients with HER2-positive status were excluded. Additional details are provided in the protocol.
This study was conducted in accordance with Good Clinical Practice guidelines as defined by the International Council for Harmonisation. The institutional review board or independent ethics committee approved the study protocol and subsequent amendments at each site. All patients provided written informed consent on the basis of the principles of the Declaration of Helsinki.
Randomization and Blinding
Patients were randomly assigned 1:1 to nivolumab + relatlimab + investigator's (INV's) choice of oxaliplatin-based chemotherapy (oxaliplatin + capecitabine [XELOX], oxaliplatin + leucovorin + fluorouracil [FOLFOX], or oxaliplatin + tegafur/gimeracil/oteracil potassium [SOX] regimen; additional details are provided in the protocol) or nivolumab + INV's choice of oxaliplatin-based chemotherapy, using interactive web response technology (block size of 2). Randomization was stratified according to LAG-3 expression (≥1% v <1%) and PD-L1 combined positive score (CPS; <1 [including indeterminate] v ≥1 to <5 v ≥5). Geographic region (Japan/Taiwan v rest of the world) was planned as a stratification factor but was not used because of lack of patients enrolled from Japan and Taiwan. INVs were not blinded to treatment allocation.
Procedures
Patients assigned to XELOX received nivolumab + relatlimab FDC (nivolumab 360 mg and relatlimab 120 mg) or nivolumab 360 mg administered intravenously (IV) over 60 or 30 minutes, respectively, on days 1 and 22 of each 6-week treatment cycle. Oxaliplatin 130 mg/m2 was administered IV on days 1 and 22 of each cycle, and capecitabine 1,000 mg/m2 was administered orally twice daily on days 1-14 and days 22-35 of each cycle. Patients assigned to FOLFOX received nivolumab + relatlimab FDC (nivolumab 480 mg and relatlimab 160 mg) or nivolumab 480 mg administered IV over 60 or 30 minutes, respectively, on days 1 and 29 of every odd-numbered 6-week treatment cycle and day 15 of every even-numbered 6-week cycle. Oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, and fluorouracil 400 mg/m2 were administered IV on days 1, 15, and 29 of each cycle, and fluorouracil 1,200 mg/m2 IV continuous infusion over 24 hours (or per local standard) on days 1, 2, 15, 16, 29, and 30 of each cycle. SOX treatment details are provided in the Data Supplement (online only). Treatment continued until progressive disease, unacceptable toxicity, withdrawal of consent, or study end. Additional details are in the protocol. Treatment was permitted beyond initial RECIST v1.1–defined progressive disease, on the basis of INV assessment. Dose escalations or reductions of nivolumab + relatlimab or nivolumab were not allowed. Dose modifications, delays, and discontinuation of chemotherapy components could be modified separately and were permitted according to local standards.
Biomarker Analyses
LAG-3 expression was determined using an analytically validated immunohistochemistry assay. LAG-3 expression was reported as the percent of LAG-3–positive cells resembling lymphocytes, relative to all nucleated cells within the tumor region.16 PD-L1 expression was assessed using the PD-L1 immunohistochemistry (IHC) 28-8 pharmDx assay (Dako, Agilent) assay. CPS was defined as the number of PD-L1–positive tumor cells, lymphocytes, and macrophages divided by the total number of viable tumor cells and multiplied by 100.17 Additional details are shown in the Data Supplement.
Assessments
The primary end point was objective response rate (ORR) by blinded independent central review (BICR) in patients with LAG-3 expression ≥1% per RECIST v1.1.15 Secondary end points included safety; ORR (BICR) in patients with LAG-3 expression <1% and all randomly assigned patients; and ORR as assessed per INV, duration of response (DOR; BICR and INV), PFS (BICR and INV), and OS, all in patients with LAG-3 expression ≥1% and <1% and all randomly assigned patients. Key exploratory end points included pharmacokinetic, biomarker, and immunogenicity analyses (Data Supplement). Additional details are described in the Data Supplement.
Safety
Adverse events (AEs) were reported up to 100 days after the last treatment dose according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (INV). Immune-mediated AEs (IMAEs) were consistent with immune-mediated mechanisms or components for which noninflammatory etiologies have been ruled out. Definitions of treatment-related AEs (TRAEs) and additional methods are described in the protocol.
Statistical Analyses
Sample size of the study was determined on the basis of the primary objective, and calculations were based on the methodology of Fleiss et al.18 Assuming a 40% prevalence of LAG-3 positivity, randomization of approximately 250 patients in a 1:1 ratio would ensure that 100 patients with LAG-3 expression ≥1% were enrolled. This would provide approximately 71% power for testing the ORR difference between the two arms, with a 0.15 one-sided (0.30 two-sided) significance level, assuming ORRs of 75% (nivolumab + relatlimab + chemotherapy) and 60% (nivolumab + chemotherapy). The maximum width of the two-sided 70% CI for the ORR difference would be 0.192. ORR and corresponding 95% CIs were determined using the Clopper-Pearson method. ORR difference (and 70% CI) in patients with LAG-3 expression ≥1% and the associated P value was compared between arms using a two-sided Cochran-Mantel-Haenszel test. Two-sided 95% CI for unweighted difference was calculated using Newcombe method. Additional details are described in the Data Supplement. Safety was analyzed by descriptive statistics using NCI CTCAE v5.0 by treatment group.
RESULTS
Patient Baseline Characteristics
From October 16, 2018, to November 4, 2019, 461 patients were assessed for eligibility, and of these, 274 patients were randomly assigned to the nivolumab + relatlimab + chemotherapy arm (n = 138) or the nivolumab + chemotherapy arm (n = 136); 271 patients received one or more doses of the assigned treatment (136 and 135 patients, respectively; Fig 1). At the data cutoff on August 27, 2020, the median follow-up (time from randomization date to the last known date alive or death date) was 11.9 months. The baseline characteristics were balanced across the treatment groups (Table 1). Twenty-two (16%) and 29 (21%) patients had received previous systemic therapy in the nivolumab + relatlimab + chemotherapy and nivolumab + chemotherapy arms, respectively (Table 1).
FIG 1.
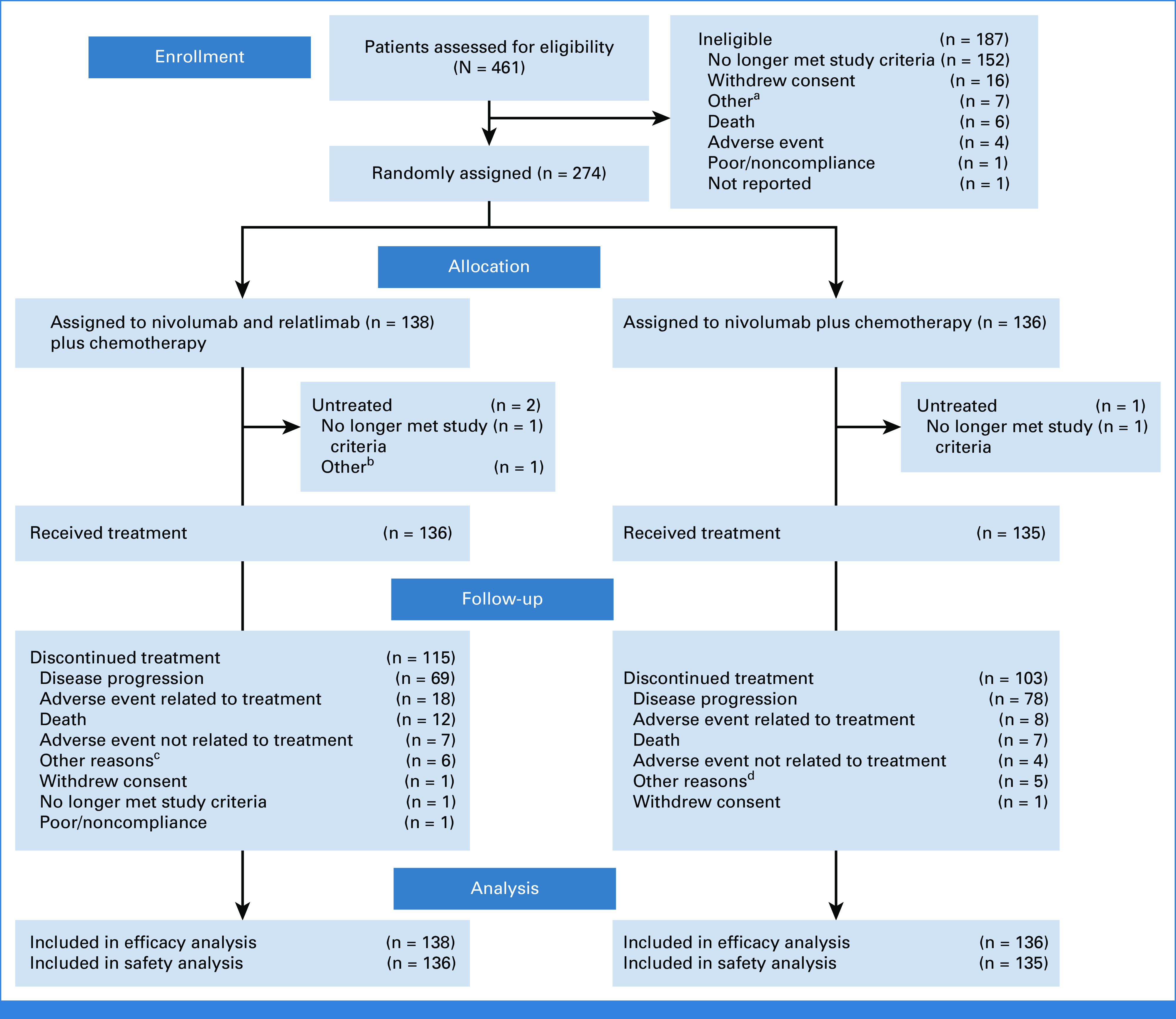
CONSORT diagram. aOther reasons included clinical progression (n = 2), and one each of patient could not wait for centralized review, patient went for laparoscopic surgery, patient did not meet eligibility criteria, rapid progression of malignant neoplasm disease, and not possible to obtain a tumor sample. bOther reason was patient had myocardial infarction (n = 1). cOther reasons included one each of INV decision, patient request, physician decision, total gastric surgery, clinical progression, and additional reasons. dOther reasons included patient request (n = 2), and one each of INV decision, clinical progression, and bedridden patient. AE, adverse event; INV, investigator.
TABLE 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | NIVO + RELA + Chemo (n = 138) | NIVO + Chemo (n = 136) |
|---|---|---|
| Age, years, median (range) | 62 (24-79) | 63 (23-84) |
| Sex, No. (%) | ||
| Male | 94 (68) | 98 (72) |
| Female | 44 (32) | 38 (28) |
| Race, No. (%) | ||
| White | 128 (93) | 122 (90) |
| Asian | 5 (4) | 8 (6) |
| Black | 0 | 1 (<1) |
| Other | 5 (4) | 5 (4) |
| Geographic region, No. (%) | ||
| Europe | 71 (51) | 77 (57) |
| United States and Canada | 22 (16) | 22 (16) |
| Asia | 2 (1) | 3 (2) |
| Rest of world | 43 (31) | 34 (25) |
| ECOG PS,a No. (%) | ||
| 0 | 55 (40) | 61 (45) |
| 1 | 83 (60) | 74 (54) |
| Primary tumor location at initial diagnosis, No. (%) | ||
| GC | 68 (49) | 70 (51) |
| GEJC | 70 (51) | 66 (49) |
| Lauren classification, No. (%) | ||
| Intestinal | 33 (24) | 42 (31) |
| Diffuse | 36 (26) | 31 (23) |
| Mixed | 7 (5) | 8 (6) |
| Unknown | 62 (45) | 55 (40) |
| Signet ring cell carcinoma, No. (%) | 20 (14) | 27 (20) |
| Disease stage, No. (%) | ||
| Metastatic | 128 (93) | 122 (90) |
| Unresectable locally advanced | 6 (4) | 9 (7) |
| Locally recurrent | 4 (3) | 5 (4) |
| Site of metastasis, No. (%) | ||
| Liver | 63 (46) | 51 (38) |
| Peritoneal | 56 (41) | 59 (43) |
| Helicobacter pylori infection,b No. (%) | 15 (11) | 19 (14) |
| LAG-3 expression, No. (%) | ||
| <1% | 41 (30) | 38 (28) |
| ≥1% | 97 (70) | 98 (72) |
| PD-L1 CPS, No. (%) | ||
| <1 or indeterminate | 29 (21) | 33 (24) |
| ≥1 to <5 | 35 (25) | 28 (21) |
| ≥5 | 74 (54) | 75 (55) |
| Chemo received on study,c,d No. (%) | ||
| FOLFOX | 103 (75) | 97 (71) |
| XELOX | 34 (25) | 38 (28) |
| Previous systemic therapy,e No. (%) | 22 (16) | 29 (21) |
| Adjuvant therapy | 4 (3) | 11 (8) |
| Neoadjuvant | 18 (13) | 21 (15) |
| Locally advanced | 1 (<1) | 1 (<1) |
| Previous radiotherapy, No. (%) | 21 (15) | 23 (17) |
| Previous surgery, No. (%) | 45 (33) | 47 (35) |
NOTE. Percentages may not add up to 100 because of rounding.
Abbreviations: chemo, chemotherapy; CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFOX, oxaliplatin + leucovorin + fluorouracil; GC, gastric cancer; GEJC, gastroesophageal junction cancer; LAG-3, lymphocyte-activation gene 3; NIVO, nivolumab; RELA, relatlimab; SOX, oxaliplatin + tegafur/gimeracil/oteracil potassium; XELOX, oxaliplatin + capecitabine.
Not reported, n = 1 (NIVO + chemo).
Unknown, n = 79 (NIVO + RELA + chemo, n = 43; NIVO + chemo, n = 36).
Patients who received at least one dose of assigned treatment.
One patient in each arm received SOX.
Patient may have received more than one type of therapy.
Patient Disposition and Study Drug Exposure
At data cutoff, 15% of patients in the nivolumab + relatlimab + chemotherapy arm and 21% in the nivolumab + chemotherapy arm were still receiving study treatment, with a median (range) duration of therapy of 6.1 months (0.1-19.9) and 7.4 months (0.5-20.6), respectively (Data Supplement, Table S1). Disease progression was the most common reason for discontinuation in both treatment arms (51% v 58%, respectively; Data Supplement, Table S1).
The majority of patients received ≥90% of the planned relative dose intensity for nivolumab + relatlimab FDC (66%) and nivolumab (65%) regardless of the chemotherapy regimens; the cumulative and relative dose intensity of the chemotherapy component was lower in patients treated with nivolumab + relatlimab + chemotherapy relative to patients treated with nivolumab + chemotherapy for both XELOX and FOLFOX treatment regimens. Cumulative dose and dose delays are shown in the Data Supplement (Table S2).
In both arms, 34% of patients received subsequent anticancer therapy, including radiotherapy (6% v 7%), surgery (4% v 4%), and systemic therapy (29% v 26%; Data Supplement, Table S3).
Response
In patients with LAG-3 expression ≥1%, BICR-assessed ORR (95% CI) was 48% (38 to 59) with nivolumab + relatlimab + chemotherapy versus 61% (51 to 71) with nivolumab + chemotherapy, and the difference in ORR (nivolumab + relatlimab + chemotherapy over nivolumab + chemotherapy) was –13 (70% CI, –20 to –6; P = .0711; Table 2). In patients with LAG-3 expression <1%, BICR-assessed ORR (95% CI) was 32% (18 to 48) for nivolumab + relatlimab + chemotherapy versus 55% (38 to 71) for nivolumab + chemotherapy, and in all randomly assigned patients, BICR-assessed ORR (95% CI) was 44% (35 to 52) versus 60% (51 to 68), respectively. Concordance rates between BICR- and INV-assessed ORR were 83% for the nivolumab + relatlimab + chemotherapy arm and 78% for the nivolumab + chemotherapy arm. BICR-assessed ORR favored nivolumab + chemotherapy over nivolumab + relatlimab + chemotherapy across most prespecified subgroups (Fig 2).
TABLE 2.
Response, Disease Control, and PFS
| Outcome | LAG-3 ≥1% | LAG-3 <1% | All Randomly Assigned | |||
|---|---|---|---|---|---|---|
| NIVO + RELA + Chemo (n = 97) | NIVO + Chemo (n = 98) | NIVO + RELA + Chemo (n = 41) | NIVO + Chemo (n = 38) | NIVO + RELA + Chemo (n = 138) | NIVO + Chemo (n = 136) | |
| BICR-assessed | ||||||
| ORRa,b | ||||||
| No. (%) | 47 (48) | 60 (61) | 13 (32) | 21 (55) | 60 (43) | 81 (60) |
| [95% CI] | 38 to 59 | 51 to 71 | 18 to 48 | 38 to 71 | 35 to 52 | 51 to 68 |
| Difference of ORRc (70% CI) | –13 (–20 to –6) | –25 (–36 to –15) | –17 (–23 to –10) | |||
| Pd | .0711 | NA | NA | |||
| Best overall response,a No. (%) | ||||||
| CR | 6 (6) | 10 (10) | 2 (5) | 0 | 8 (6) | 10 (7) |
| PR | 41 (42) | 50 (51) | 11 (27) | 21 (55) | 52 (38) | 71 (52) |
| SD | 34 (35) | 18 (18) | 11 (27) | 7 (18) | 45 (33) | 25 (18) |
| PD | 1 (1) | 10 (10) | 2 (5) | 4 (11) | 3 (2) | 14 (10) |
| Unable to determine | 7 (7) | 5 (5) | 7 (17) | 0 | 14 (10) | 5 (4) |
| DCRa | ||||||
| No. (%) | 89 (92) | 83 (85) | 32 (78) | 34 (89) | 121 (88) | 117 (86) |
| 95% CI | 84 to 96 | 76 to 91 | 62 to 89 | 75 to 97 | 81 to 93 | 79 to 91 |
| TTR, months,e median (range) | 1.5 (1.2-5.3) | 1.5 (1.2-5.6) | 1.6 (1.3-4.0) | 2.8 (1.2-7.2) | 1.5 (1.2-5.3) | 2.0 (1.2-7.2) |
| DOR, months,e median (95% CI) | 5.7 (4.4 to 10.0) | 10.1 (6.4 to 13.3) | 5.4 (3.3 to 7.0) | 8.5 (5.3 to 13.6) | 5.6 (5.1 to 7.0) | 9.7 (6.7 to 12.5) |
| PFS, months, median (95% CI) | 7.0 (5.8 to 8.4) | 8.3 (6.9 to 12.1) | 6.6 (4.7 to 7.1) | 9.7 (6.9 to 13.7) | 6.8 (5.8 to 7.3) | 9.4 (7.0 to 11.5) |
| INV-assessed | ||||||
| ORRa,b | ||||||
| No. (%) | 51 (53) | 55 (56) | 13 (32) | 16 (42) | 64 (46) | 71 (52) |
| 95% CI | 42 to 63 | 46 to 66 | 18 to 48 | 26 to 59 | 38 to 55 | 43.5 to 61 |
| Best overall response,a No. (%) | ||||||
| CR | 3 (3) | 5 (5) | 0 | 0 | 3 (2) | 5 (4) |
| PR | 48 (49) | 50 (51) | 13 (32) | 16 (42) | 61 (44) | 66 (49) |
| SD | 33 (34) | 27 (28) | 19 (46) | 18 (47) | 52 (38) | 45 (33) |
| PD | 6 (6) | 12 (12) | 2 (5) | 4 (11) | 8 (6) | 16 (12) |
| Unable to determine | 7 (7) | 4 (4) | 7 (17) | 0 | 14 (10) | 4 (3) |
| DCRa | ||||||
| No. (%) | 84 (87) | 82 (84) | 32 (78) | 34 (89) | 116 (84) | 116 (85) |
| 95% CI | 78 to 93 | 75 to 90 | 62 to 89 | 75 to 97 | 77 to 90 | 78 to 91 |
| TTR, months,f median (range) | 1.6 (0.9-5.5) | 1.4 (1.2-8.5) | 1.5 (1.2-4.1) | 2.3 (1.0-16.6) | 1.6 (0.9-5.5) | 1.5 (1.0-16.6) |
| DOR, months,f median (95% CI) | 6.9 (4.4 to 8.3) | 8.3 (6.9 to 12.45) | 5.5 (3.0 to 6.9) | 7.5 (5.6 to 13.6) | 5.9 (4.4 to 7.3) | 8.3 (6.9 to 11.1) |
| PFS, months, median (95% CI) | 7.0 (5.8 to 8.4) | 8.3 (5.9 to 9.7) | 5.4 (4.2 to 7.6) | 11.0 (6.9 to 13.7) | 6.6 (5.4 to 7.6) | 8.3 (6.9 to 9.7) |
Abbreviations: BICR, blinded independent central review; chemo, chemotherapy; CR, complete response; DCR, disease control rate; DOR, duration of response; INV, investigator; LAG-3, lymphocyte-activation gene 3; NA, not applicable; NIVO, nivolumab; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; RELA, relatlimab; SD, stable disease; TTR, time to response.
Per RECIST 1.1.
CI on the basis of the Clopper-Pearson method.
Strata adjusted difference in ORR (NIVO + RELA + chemo over NIVO + chemo) on the basis of the Cochran-Mantel-Haenszel method of weighting.
On the basis of two-sided stratified Cochran-Mantel-Haenszel test.
Number of responders: LAG-3 ≥1%, NIVO + RELA + chemo (n = 47) and NIVO + chemo (n = 60); LAG-3 <1%, NIVO + RELA + chemo (n = 13) and NIVO + chemo (n = 21); all randomly assigned NIVO + RELA + chemo (n = 60) and NIVO + chemo (n = 81).
Number of responders: LAG-3 ≥1%, NIVO + RELA + chemo (n = 51) and NIVO + chemo (n = 55); LAG-3 <1%, NIVO + RELA + chemo (n = 13) and NIVO + chemo (n = 16); all randomly assigned NIVO + RELA + chemo (n = 64) and NIVO + chemo (n = 71).
FIG 2.
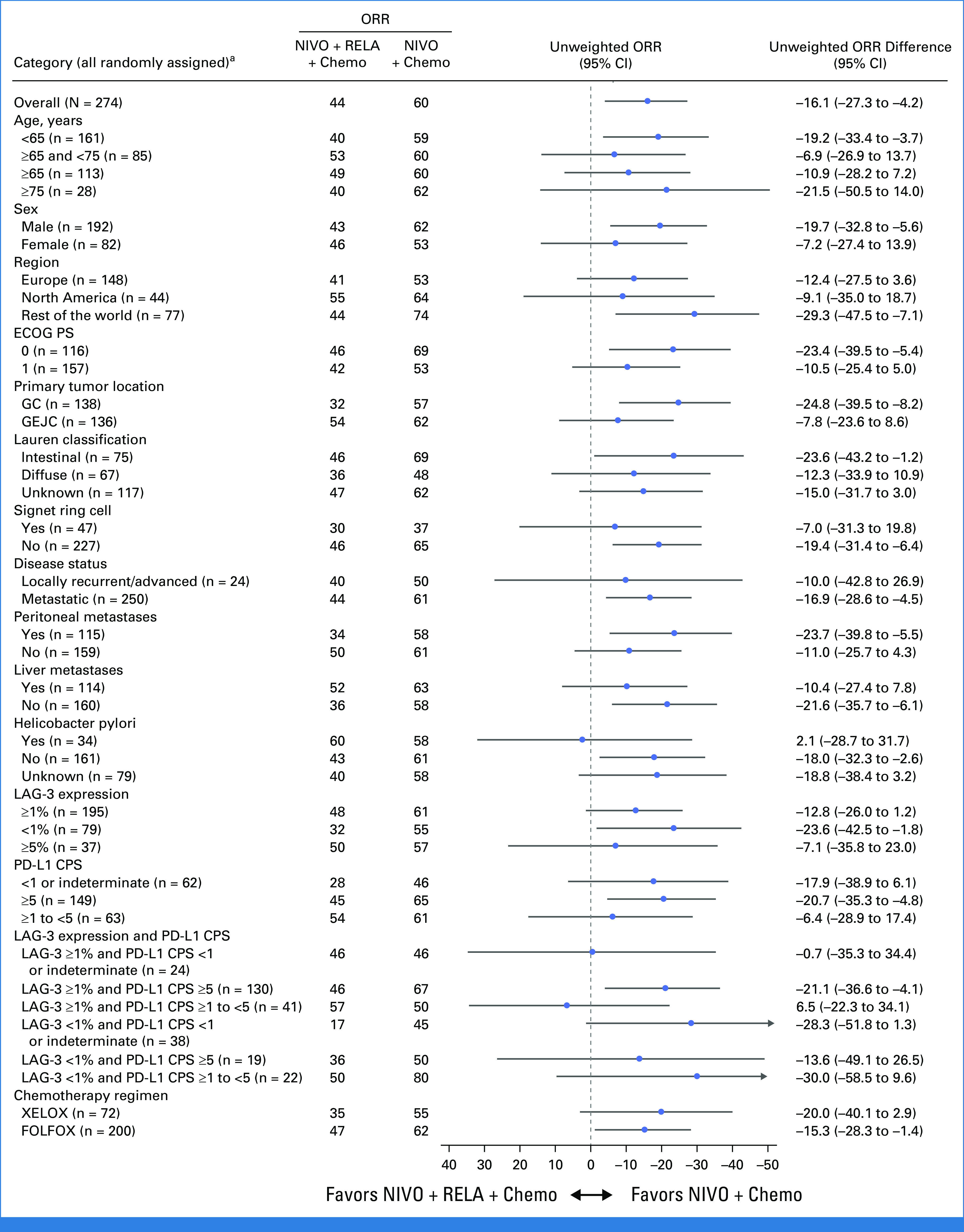
Subgroup analysis of unweighted ORR difference per BICR in all randomly assigned patients. Two-sided 95% CI for unweighted difference was calculated using Newcombe method. ORR difference is not computed for subsets with <10 patients in at least one treatment group. ORR difference: NIVO plus RELA minus NIVO. aORR difference was not computed for subset category with <10 patients in at least one treatment group. BICR, blinded independent central review; chemo, chemotherapy; CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFOX, oxaliplatin + leucovorin + fluorouracil; GC, gastric cancer; GEJC, gastroesophageal junction cancer; LAG-3, lymphocyte-activation gene 3; NIVO, nivolumab; ORR, objective response rate; RELA, relatlimab; XELOX, oxaliplatin + capecitabine.
Median time to response was similar between treatment arms (Table 2). BICR-assessed median (95% CI) DOR in the nivolumab + relatlimab + chemotherapy and nivolumab + chemotherapy arms was 5.7 months (4.4 to 10.0) versus 10.1 months (6.4 to 13.3) in patients with LAG-3 expression ≥1%, 5.4 months (3.3 to 7.0) versus 8.5 months (5.3 to 13.6) in patients with LAG-3 expression <1%, and 5.6 months (5.1 to 7.0) versus 9.7 months (6.7 to 12.5) in all responders. For patients with LAG-3 expression ≥1%, BICR-assessed disease control rate (DCR) was 92% in the nivolumab + relatlimab + chemotherapy arm and 85% in the nivolumab + chemotherapy arm, and patients with LAG-3 expression <1% had DCR of 78% and 89%, respectively (Table 2). Results per INV are shown in Table 2.
PFS and OS
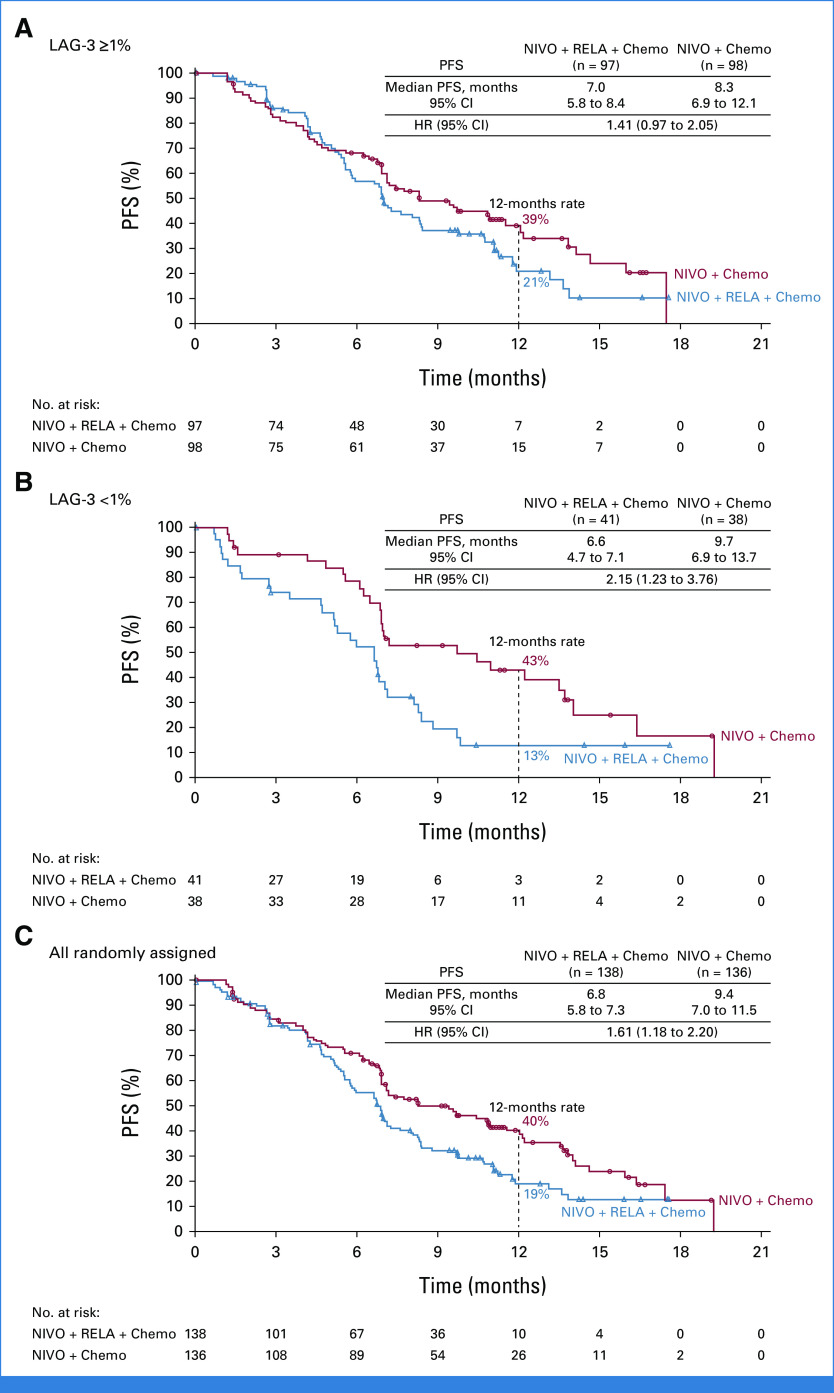
Median PFS (mPFS; 95% CI) by BICR in the nivolumab + relatlimab + chemotherapy arm versus the nivolumab + chemotherapy arm, respectively, was 7.0 months (5.8 to 8.4) versus 8.3 months (6.9 to 12.1) for patients with LAG-3 expression ≥1% (hazard ratio [HR], 1.41 [95% CI, 0.97 to 2.05]; Fig 3A), 6.6 months (4.7 to 7.1) versus 9.7 months (6.9 to 13.7) for patients with LAG-3 expression <1% (HR, 2.15 [95% CI, 1.23 to 3.76]; Fig 3B), and 6.8 months (5.8 to 7.3) versus 9.4 months (7.0 to 11.5) for all randomly assigned patients (HR, 1.61 [95% CI, 1.18 to 2.20]; Fig 3C). Similar results were observed for PFS by INV (Table 2). BICR-assessed PFS favored nivolumab + chemotherapy over nivolumab + relatlimab + chemotherapy across the majority of prespecified subgroups (Data Supplement, Fig S1A).
FIG 3.
Kaplan-Meier estimates of PFS per BICR. (A) Patients with LAG-3 expression ≥1%; (B) patients with LAG-3 expression <1%; and (C) all randomly assigned patients. Symbols represent censored observations. BICR, blinded independent central review; chemo, chemotherapy; HR, hazard ratio; LAG-3, lymphocyte-activation gene 3; NIVO, nivolumab; PFS, progression-free survival; RELA, relatlimab.
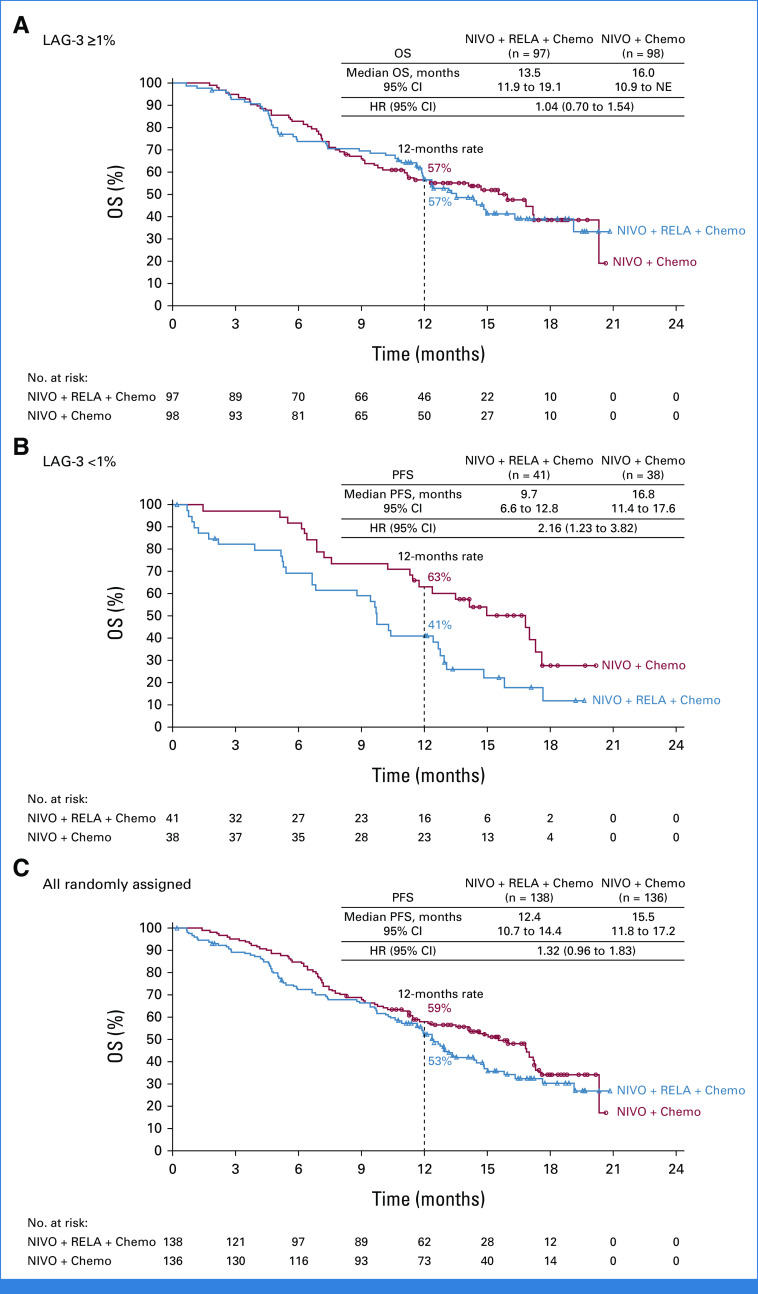
Median OS (mOS; 95% CI) in the nivolumab + relatlimab + chemotherapy arm versus the nivolumab + chemotherapy arm, respectively, was 13.5 months (11.9 to 19.1) versus 16.0 months (10.9 to not estimable) for patients with LAG-3 expression ≥1% (HR, 1.04 [95% CI, 0.70 to 1.54]; Fig 4A), 9.7 months (6.6 to 12.8) versus 16.8 months (11.4 to 17.6) for patients with LAG-3 expression <1% (HR, 2.16 [95% CI, 1.23 to 3.82]; Fig 4B), and 12.4 months (10.7 to 14.4) versus 15.5 months (11.8 to 17.2) for all randomly assigned patients (HR, 1.32 [95% CI, 0.96 to 1.83]; Fig 4C). OS favored nivolumab + chemotherapy over nivolumab + relatlimab + chemotherapy across the majority of prespecified subgroups (Data Supplement, Fig S1B).
FIG 4.
Kaplan-Meier estimates of OS. (A) Patients with LAG-3 expression ≥1%; (B) patients with LAG-3 expression <1%; and (C) all randomly assigned patients. Symbols represent censored observations. Chemo, chemotherapy; HR, hazard ratio; LAG-3, lymphocyte-activation gene 3; NE, not estimable; NIVO, nivolumab; OS, overall survival; RELA, relatlimab.
Safety
Any-grade TRAEs were reported for 124 (91%) patients (grade 3/4, n = 94 [69%]; grade 5, n = 0) in the nivolumab + relatlimab + chemotherapy arm and 129 (96%) patients (grade 3/4, n = 82 [61%]; grade 5, n = 1 [<1%]) in the nivolumab + chemotherapy arm (Table 3). The most frequently reported grade 3/4 TRAEs were neutropenia (21%), fatigue (7%), diarrhea (4%), peripheral neuropathy (4%), and anemia (4%) in the nivolumab + relatlimab + chemotherapy arm and neutropenia (17%), fatigue (7%), peripheral neuropathy (7%), and diarrhea (6%) in the nivolumab + chemotherapy arm. Serious any-grade TRAEs were reported in 38% (grade 3/4, 34%) of patients in the nivolumab + relatlimab + chemotherapy arm and 28% (grade 3/4, 26%; grade 5, <1%) of patients in the nivolumab + chemotherapy arm. Any-grade TRAEs leading to discontinuation were reported in 42% (grade 3/4, 21%) of patients in the nivolumab + relatlimab + chemotherapy arm and 36% (grade 3/4, 19%) of patients in the nivolumab + chemotherapy arm (Table 3). The most common any-grade TRAEs leading to discontinuation were peripheral neuropathy (7% v 10%) and peripheral sensory neuropathy (5% v 4%) in the nivolumab + relatlimab + chemotherapy arm and in the nivolumab + chemotherapy arm, respectively.
TABLE 3.
Summary of AEs in All Treated Patients
| Patient | NIVO + RELA + Chemo (n = 136), No. (%) | NIVO + Chemo (n = 135), No. (%) | ||
|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| Any AEa | 135 (99) | 118 (87) | 135 (100) | 115 (85) |
| Serious AEs | 95 (70) | 84 (62) | 82 (61) | 71 (53) |
| AEs leading to discontinuationb | 72 (53) | 44 (32) | 56 (41) | 28 (21) |
| Any TRAEa | 124 (91) | 94 (69) | 129 (96) | 82 (61) |
| Serious TRAEsa | 52 (38) | 46 (34) | 38 (28) | 35 (26) |
| TRAEs leading to discontinuationb | 57 (42) | 29 (21) | 48 (36) | 25 (19) |
| TRAEs reported in ≥15% of patients in either arm | ||||
| Nausea | 53 (39) | 2 (1) | 63 (47) | 5 (4) |
| Fatigue | 52 (38) | 10 (7) | 58 (43) | 9 (7) |
| Diarrhea | 41 (30) | 5 (4) | 45 (33) | 8 (6) |
| Neutropenia | 36 (26) | 28 (21) | 37 (27) | 23 (17) |
| Neuropathy peripheral | 30 (22) | 5 (4) | 43 (32) | 10 (7) |
| Vomiting | 28 (21) | 0 | 34 (25) | 4 (3) |
| Anemia | 26 (19) | 5 (4) | 16 (12) | 5 (4) |
| Peripheral sensory neuropathy | 24 (18) | 3 (2) | 29 (21) | 1 (<1) |
| Decreased appetite | 23 (17) | 3 (2) | 23 (17) | 0 |
| Hypothyroidism | 21 (15) | 0 | 16 (12) | 1 (<1) |
| Thrombocytopenia | 18 (13) | 1 (<1) | 24 (18) | 3 (2) |
| Platelet count decreased | 12 (9) | 1 (<1) | 22 (16) | 2 (1) |
| Immune-mediated AEc | ||||
| Hypothyroidism | 19 (14) | 0 | 15 (11) | 1 (<1) |
| Rash | 13 (10) | 3 (2) | 6 (4) | 1 (<1) |
| Hyperthyroidism | 8 (6) | 1 (<1) | 3 (2) | 0 |
| Diarrhea/colitis | 5 (4) | 4 (3) | 5 (4) | 3 (2) |
| Hepatitis | 5 (4) | 3 (2) | 3 (2) | 3 (2) |
| Adrenal insufficiency | 4 (3) | 2 (1) | 1 (<1) | 0 |
| Pneumonitis | 4 (3) | 0 | 9 (7) | 5 (4) |
| Hypophysitis | 3 (2) | 2 (1) | 0 | 0 |
| Diabetes mellitus | 3 (2) | 2 (1) | 2 (1) | 1 (<1) |
| Thyroiditis | 2 (1) | 1 (<1) | 0 | 0 |
NOTE. Patients who received at least one dose of the assigned treatment. Includes events reported between first dose and 30 days after last dose of trial therapy. Treatment-relatedness was attributed to either nivolumab, relatlimab, or any of the chemotherapies, or all. Common Terminology Criteria for Adverse Events, version 5.0, and Medical Dictionary for Regulatory Activities, version 23.0.
Abbreviations: AE, adverse event; chemo, chemotherapy; NIVO, nivolumab; RELA, relatlimab; TRAE, treatment-related AE.
Grade 5, n = 1 (NIVO + chemo).
Discontinuation of any or all treatment components.
Includes AEs of any grade occurring in ≥1% of patients considered by investigators to be potentially immune-mediated that met the following criteria: occurred within 100 days of the last dose, regardless of causality, treated with immune-modulating medication with no clear alternate etiology, or had an immune-mediated component.
Most IMAEs were grade 1/2 in either treatment arm (Table 3). The most frequent grade 3/4 IMAEs were diarrhea/colitis (3%) for the nivolumab + relatlimab + chemotherapy arm and pneumonitis (4%) for the nivolumab + chemotherapy arm.
Three deaths (autoimmune encephalitis, lung infection, and acute renal failure) in the nivolumab + relatlimab + chemotherapy arm and one death (acute renal insufficiency) in the nivolumab + chemotherapy arm were considered treatment-related.
Exploratory Analyses
Exploratory analysis by LAG-3 and PD-L1 CPS status was performed. Results were consistent with the overall population, except for limited subgroups. Patients with LAG-3 expression ≥5% (n = 37) had mPFS per BICR of 13.1 months in the nivolumab + relatlimab + chemotherapy arm versus 6.9 months in the nivolumab + chemotherapy arm (unstratified HR, 0.75 [95% CI, 0.32 to 1.77]); mOS was not reached in both arms (Data Supplement, Fig S1). Patients with LAG-3 expression ≥1% combined with PD-L1 CPS ≥1 to <5 (n = 41) had mPFS of 8.3 months in the nivolumab + relatlimab + chemotherapy arm versus 8.3 months in the nivolumab + chemotherapy arm (unstratified HR, 0.69 [95% CI, 0.30 to 1.56]; Data Supplement, Fig S1A) and mOS of 15.0 months in the nivolumab + relatlimab + chemotherapy arm versus 11.2 months in the nivolumab + chemotherapy arm (unstratified HR, 0.66 [95% CI, 0.28 to 1.53]; Data Supplement, Fig S1B).
Previous reports demonstrated that LAG-3 can be present in soluble form; therefore, the pharmacodynamic (PD) effect of relatlimab on free soluble LAG-3 (sLAG-3) was investigated.19,20 Free and total sLAG-3 were identified as relatlimab-specific PD biomarkers (Data Supplement, Figs S2A and S2B). Additionally, regardless of response, there was a trend of higher increase in interferon gamma, C-X-C motif chemokine ligand 9, and C-X-C motif chemokine ligand 10 after treatment with nivolumab + relatlimab + chemotherapy relative to nivolumab + chemotherapy (Data Supplement, Figs S2C-S2E).
There were no relatlimab treatment-emergent antidrug antibodies, and the incidence of nivolumab treatment-emergent antidrug antibodies was low.
Nivolumab Cmax and Ctrough were similar between nivolumab + relatlimab + chemotherapy and nivolumab + chemotherapy arms. Additional details are shown in the Data Supplement.
DISCUSSION
To our knowledge, this is the first report of a dual versus single immunotherapy in combination with chemotherapy in patients with GC/GEJC. RELATIVITY-060 did not meet its primary end point of improved ORR per BICR in patients with LAG-3 expression ≥1%, and ORR was numerically higher in the nivolumab + chemotherapy arm. Secondary end points were also not met, with no benefit for nivolumab + relatlimab + chemotherapy relative to nivolumab + chemotherapy for ORR, PFS, and OS in patients with LAG-3 expression ≥1% and <1% and in the all randomly assigned population. The lower cumulative dose intensity of the chemotherapy component in patients treated with nivolumab + relatlimab + chemotherapy relative to patients treated with nivolumab + chemotherapy may have influenced the efficacy results.
The safety profile of nivolumab + relatlimab + chemotherapy was manageable and consistent with the known safety profiles of immunotherapy and chemotherapy, and no new safety signals were identified. There was a small increase in AEs and IMAEs with the addition of a second immunotherapeutic agent.
Exploratory biomarker analyses identified some subgroups that showed numerical improvement in efficacy in the nivolumab + relatlimab + chemotherapy arm relative to the nivolumab + chemotherapy arm, such as mPFS in patients with LAG-3 expression ≥5% and mPFS and mOS in patients with LAG-3 expression ≥1% combined with PD-L1 CPS ≥1 to <5. In RELATIVITY-047, LAG-3 and PD-L1 expression did not appear to be predictive for clinical benefit with nivolumab + relatlimab over nivolumab monotherapy in patients with advanced melanoma; whether LAG-3 or PD-L1 expression is associated with clinical benefit from relatlimab in patients with gastroesophageal cancer will require further investigation. Melanoma is generally characterized by a high inflammatory tumor microenvironment, and tumors are frequently infiltrated with lymphocytes.21 By contrast, GC is delineated by heterogeneous levels of inflammation and tumor-infiltrating lymphocytes.22,23 Additionally, lymphocyte activation status and LAG-3 expression patterns vary among different types of tumor-infiltrating lymphocytes within the tumor microenvironment.24 Interestingly, approximately 71% to 75% of the patients had LAG-3 expression ≥1% in the RELATIVITY-060 and RELATIVITY-047 studies; however, 14% and 36% of the patients had LAG-3 expression ≥5%, respectively, suggesting potentially different biology. In addition, tumor cell PD-L1 was used in RELATIVITY-047, whereas PD-L1 CPS was used in this study. Therefore, comparisons between the two studies should be made with caution.25
Separation of the PFS and OS curves in favor of nivolumab + chemotherapy was more pronounced in the population with LAG-3 expression <1% versus the population with LAG-3 expression ≥1%, possibly indicating a negative interaction when relatlimab was added to nivolumab + chemotherapy in patients with LAG-3 expression <1%. To some extent, this was overcome with higher levels of LAG-3 expression, with mPFS increasing from 6.6 months for patients with LAG-3 expression <1% to 13.1 months for patients with LAG-3 expression ≥5% in the nivolumab + relatlimab + chemotherapy arm.
Several preclinical studies and clinical trials have shown the efficacy of LAG-3–targeting agents on antitumor activity. Most of these studies combined anti–LAG-3 antibodies with additional immune checkpoint inhibitor targets, and studies combining anti–LAG-3 antibodies with chemotherapy are scarce.24,26
Our study has limitations that should be considered when interpreting these results. In this open-label phase II study, sample size was relatively small for correlation between biomarkers and efficacy analyses, and because of the exploratory nature, formal statistical comparisons between the arms were not performed.
In conclusion, adding relatlimab to nivolumab + chemotherapy, the current standard of care, did not show improved response in patients with GC/GEJC; however, a trend toward improved PFS in the small subgroup of patients with LAG-3 expression ≥5% was observed and may warrant further investigation. The safety profile of nivolumab + relatlimab + chemotherapy was manageable and consistent with the known safety profiles of dual immunotherapy and chemotherapy; no new safety signals were identified. Additional studies may identify subgroups of patients that could benefit from relatlimab-based therapies to treat unresectable, locally advanced or metastatic GC/GEJC.
ACKNOWLEDGMENT
The authors thank the patients and their families who made this study possible, and the investigators and the clinical study teams at Bristol Myers Squibb (Princeton, NJ) for study support. The authors thank Alessia Sheriff, global trial manager; Charlie Garnett-Benson, Christopher Harbison, Ruslan Novosiadly, Huidong Gu, Jenn Liu, Jennifer Postelnek, Jia Xin Yu, Jianing Zeng, Mary Giancarli, Naiyu Zheng, Sagar Kawle, and Sheen Wang from Bristol Myers Squibb for contributions to biomarker analyses; Lloye Dillon and John Wojcik for input and contributions to biomarker and diagnostics analyses; Dako (an Agilent Technologies, Inc company, Santa Clara, CA) for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; and LabCorp for collaborative development of the LAG-3 IHC assay, including analytic and clinical assay validations. Professional medical writing and editorial assistance were provided by Christiane Dresch, PhD, of Parexel, funded by Bristol Myers Squibb.
Guillermo Mendez
Consulting or Advisory Role: Merck Serono, Amgen, Lilly, MSD Oncology, Bristol Myers Squibb Argentina, Servier
Speakers' Bureau: Roche, Merck Serono, MSD Oncology, Bristol Myers Squibb Argentina, Servier, Lilly
Travel, Accommodations, Expenses: Merck Serono, Amgen, Servier, Pfizer
Joseph Chao
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Astellas Pharma, Roche, Guardant Health
Speakers' Bureau: Merck, Bristol Myers Squibb
Research Funding: Merck (Inst), Novonco Therapeutics (Inst), Brooklyn Immunotherapeutics (Inst)
Other Relationship: Yiviva, Daiichi Sankyo
Radim Nemecek
Honoraria: Merck Serono, Servier, GlaxoSmithKline
Consulting or Advisory Role: Merck Serono
Travel, Accommodations, Expenses: Merck Serono, Amgen, Servier
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Roche, Servier, Bristol Myers Squibb, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Daiichi Sankyo, Pierre Fabre, Taiho Pharmaceutical, Incyte, Astellas Pharma, GlaxoSmithKline, Nordic Group, Pfizer, Takeda, ALX Oncology, AbbVie, BeiGene, Boehringer Ingelheim, Mirati Therapeutics, Seagen, Terumo, Ipsen, Agenus, Amgen, Arcus Biosciences, BioNTech SE, Debiopharm Group, ElmediX, Eisai, Hookipa Biotech, Simcere
Salah-Eddin Al-Batran
Stock and Other Ownership Interests: Immutep, Frankfurter Institut für Klinische Krebsforschung IKF GmbH
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca/Daiichi Sankyo, Eli Lilly Germany
Speakers' Bureau: Lilly, AIO gGmbH, Bristol Myers Squibb, MCI Group
Research Funding: Celgene, Lilly, Sanofi, German Cancer Aid, German Research Foundation, Federal Ministry of Education and Research, Roche, Vifor Pharma, Eurozyto, Immutep, Ipsen, Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca
Wasat Mansoor
Honoraria: Bristol Myers Squibb, Servier, Amgen, Astellas Pharma
Consulting or Advisory Role: Bristol Myers Squibb, Servier
Speakers' Bureau: Lilly
Travel, Accommodations, Expenses: Ipsen, Servier
Nicholas Maisey
Consulting or Advisory Role: Servier/Pfizer
Roberto Pazo Cid
Consulting or Advisory Role: Roche, Bristol Myers Squibb/Celgene, Eisai Europe, Astellas Pharma, AstraZeneca Spain, Servier, Ipsen
Speakers' Bureau: Bristol Myers Squibb GmbH & Co KG, Servier, AstraZeneca Spain, Astellas Pharma
Travel, Accommodations, Expenses: Lilly, Bristol Myers Squibb GmbH & Co KG, Roche/Genentech
Matthew Burge
Stock and Other Ownership Interests: Pfizer, CSL Limited, Coclear
Honoraria: Servier, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca
Consulting or Advisory Role: Merck Sharp & Dohme, Servier, AstraZeneca, Bristol Myers Squibb/Medarex
Speakers' Bureau: Merck Sharp & Dohme
Research Funding: Amgen (Inst)
Patents, Royalties, Other Intellectual Property: Funding for an investigator-initiated prospective trial (Inst)
David Perez-Callejo
Employment: Roche, Bristol Myers Squibb
Stock and Other Ownership Interests: Roche, Bristol Myers Squibb
R. William Hipkin
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Sourav Mukherjee
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Ming Lei
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Inventor of pending BMS patents
Hao Tang
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Satyendra Suryawanshi
Employment: Bristol Myers Squibb
Leadership: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Ronan J. Kelly
Honoraria: Cardinal Health
Consulting or Advisory Role: Novartis, Lilly, AstraZeneca, Bristol Myers Squibb, Merck, Medscape, Takeda, Onc Live, Daiichi Sanyo, EMD Serono, Eisai, Peerview, Astellas Pharma, Ipsen, Philips Healthcare, Toray Industries, Novocure, Grail, Steris, Exact Sciences
Speakers' Bureau: Bristol Myers Squibb
Research Funding: Bristol Myers Squibb (Inst), Lilly (Inst)
Expert Testimony: Merck
Travel, Accommodations, Expenses: Bristol Myers Squibb
Niall C. Tebbutt
Honoraria: Bristol Myers Squibb, AstraZeneca, Merck, BeiGene, Takeda
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Merck, BeiGene, Takeda
No other potential conflicts of interest were reported.
SUPPORT
Supported by Bristol Myers Squibb.
CLINICAL TRIAL INFORMATION
R.J.K. and N.C.T. are cosenior authors and contributed equally to this article.
DATA SHARING STATEMENT
The Bristol Myers Squibb data sharing policy (https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html) is compliant with ICMJE guidelines. Bristol Myers Squibb will honor legitimate requests for clinical trial data from qualified researchers. Data will be shared with external researchers whose proposed use of the data has been approved. Complete deidentified patient data sets will be eligible for sharing 2 years after completion of the RELATIVITY-060 study. Before data are released, the researcher(s) must sign a Data Sharing Agreement, after which the deidentified and anonymized data sets can be accessed within a secured portal.
AUTHOR CONTRIBUTIONS
Conception and design: Ronan J. Kelly
Provision of study materials or patients: Susanna Hegewisch-Becker, Guillermo Mendez, Joseph Chao, Radim Nemecek, Kynan Feeney, Eric Van Cutsem, Salah-Eddin Al-Batran, Wasat Mansoor, Nicholas Maisey, Roberto Pazo Cid, Matthew Burge, Niall C. Tebbutt
Collection and assembly of data: Susanna Hegewisch-Becker, Guillermo Mendez, Joseph Chao, Radim Nemecek, Kynan Feeney, Eric Van Cutsem, Salah-Eddin Al-Batran, Wasat Mansoor, Nicholas Maisey, Roberto Pazo Cid, Matthew Burge, Niall C. Tebbutt
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
First-Line Nivolumab and Relatlimab Plus Chemotherapy for Gastric or Gastroesophageal Junction Adenocarcinoma: The Phase II RELATIVITY-060 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Guillermo Mendez
Consulting or Advisory Role: Merck Serono, Amgen, Lilly, MSD Oncology, Bristol Myers Squibb Argentina, Servier
Speakers' Bureau: Roche, Merck Serono, MSD Oncology, Bristol Myers Squibb Argentina, Servier, Lilly
Travel, Accommodations, Expenses: Merck Serono, Amgen, Servier, Pfizer
Joseph Chao
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Astellas Pharma, Roche, Guardant Health
Speakers' Bureau: Merck, Bristol Myers Squibb
Research Funding: Merck (Inst), Novonco Therapeutics (Inst), Brooklyn Immunotherapeutics (Inst)
Other Relationship: Yiviva, Daiichi Sankyo
Radim Nemecek
Honoraria: Merck Serono, Servier, GlaxoSmithKline
Consulting or Advisory Role: Merck Serono
Travel, Accommodations, Expenses: Merck Serono, Amgen, Servier
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Roche, Servier, Bristol Myers Squibb, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Daiichi Sankyo, Pierre Fabre, Taiho Pharmaceutical, Incyte, Astellas Pharma, GlaxoSmithKline, Nordic Group, Pfizer, Takeda, ALX Oncology, AbbVie, BeiGene, Boehringer Ingelheim, Mirati Therapeutics, Seagen, Terumo, Ipsen, Agenus, Amgen, Arcus Biosciences, BioNTech SE, Debiopharm Group, ElmediX, Eisai, Hookipa Biotech, Simcere
Salah-Eddin Al-Batran
Stock and Other Ownership Interests: Immutep, Frankfurter Institut für Klinische Krebsforschung IKF GmbH
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca/Daiichi Sankyo, Eli Lilly Germany
Speakers' Bureau: Lilly, AIO gGmbH, Bristol Myers Squibb, MCI Group
Research Funding: Celgene, Lilly, Sanofi, German Cancer Aid, German Research Foundation, Federal Ministry of Education and Research, Roche, Vifor Pharma, Eurozyto, Immutep, Ipsen, Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca
Wasat Mansoor
Honoraria: Bristol Myers Squibb, Servier, Amgen, Astellas Pharma
Consulting or Advisory Role: Bristol Myers Squibb, Servier
Speakers' Bureau: Lilly
Travel, Accommodations, Expenses: Ipsen, Servier
Nicholas Maisey
Consulting or Advisory Role: Servier/Pfizer
Roberto Pazo Cid
Consulting or Advisory Role: Roche, Bristol Myers Squibb/Celgene, Eisai Europe, Astellas Pharma, AstraZeneca Spain, Servier, Ipsen
Speakers' Bureau: Bristol Myers Squibb GmbH & Co KG, Servier, AstraZeneca Spain, Astellas Pharma
Travel, Accommodations, Expenses: Lilly, Bristol Myers Squibb GmbH & Co KG, Roche/Genentech
Matthew Burge
Stock and Other Ownership Interests: Pfizer, CSL Limited, Coclear
Honoraria: Servier, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca
Consulting or Advisory Role: Merck Sharp & Dohme, Servier, AstraZeneca, Bristol Myers Squibb/Medarex
Speakers' Bureau: Merck Sharp & Dohme
Research Funding: Amgen (Inst)
Patents, Royalties, Other Intellectual Property: Funding for an investigator-initiated prospective trial (Inst)
David Perez-Callejo
Employment: Roche, Bristol Myers Squibb
Stock and Other Ownership Interests: Roche, Bristol Myers Squibb
R. William Hipkin
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Sourav Mukherjee
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Ming Lei
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Inventor of pending BMS patents
Hao Tang
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Satyendra Suryawanshi
Employment: Bristol Myers Squibb
Leadership: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Ronan J. Kelly
Honoraria: Cardinal Health
Consulting or Advisory Role: Novartis, Lilly, AstraZeneca, Bristol Myers Squibb, Merck, Medscape, Takeda, Onc Live, Daiichi Sanyo, EMD Serono, Eisai, Peerview, Astellas Pharma, Ipsen, Philips Healthcare, Toray Industries, Novocure, Grail, Steris, Exact Sciences
Speakers' Bureau: Bristol Myers Squibb
Research Funding: Bristol Myers Squibb (Inst), Lilly (Inst)
Expert Testimony: Merck
Travel, Accommodations, Expenses: Bristol Myers Squibb
Niall C. Tebbutt
Honoraria: Bristol Myers Squibb, AstraZeneca, Merck, BeiGene, Takeda
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Merck, BeiGene, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Janjigian YY, Shitara K, Moehler M, et al. : First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shitara K, Ajani JA, Moehler M, et al. : Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 603:942-948, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bristol Myers Squibb: OPDIVO (nivolumab) injection, for intravenous use [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125554s106lbl.pdf.
- 5.Doki Y, Ajani JA, Kato K, et al. : Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med 386:449-462, 2022 [DOI] [PubMed] [Google Scholar]
- 6.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345-1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz H-J, Van Cutsem E, Luisa Limon M, et al. : First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The phase II CheckMate 142 study. J Clin Oncol 40:161-170, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Yau T, Kang Y-K, Kim T-Y, et al. : Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. JAMA Oncol 6:e204564, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. : Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med 381:2020-2031, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Baas P, Scherpereel A, Nowak AK, et al. : First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 397:375-386, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Park Y, Seo AN, Koh J, et al. : Expression of the immune checkpoint receptors PD-1, LAG3, and TIM3 in the immune context of stage II and III gastric cancer by using single and chromogenic multiplex immunohistochemistry. Oncoimmunology 10:1954761, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tawbi HA, Schadendorf D, Lipson EJ, et al. : Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 386:24-34, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bristol Myers Squibb: OPDUALAG (nivolumab and relatlimab-rmbw) injection, for intravenous use [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761234s000lbl.pdf.
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Dillon LM, Wojcik J, Desai K, et al. : Abstract 1625: Distribution and prevalence of LAG-3 expression in samples of melanoma and gastric/gastroesophageal junction cancer. Cancer Res 81, 2021. (suppl 13; abstr 1625) [Google Scholar]
- 17.Chan T, Neill B, Murga A, et al. : 1235P Analytical performance of PD-L1 IHC 28-8 pharmDx in gastric, gastroesophageal junction (GEJ), and esophageal carcinoma evaluated using combined positive score (CPS). Ann Oncol 33:S1113-S1114, 2022 [Google Scholar]
- 18.Fleiss JL, Tytun A, Ury HK: A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics 36:343-346, 1980 [PubMed] [Google Scholar]
- 19.Ascierto PA, Lipson EJ, Dummer R, et al. : Nivolumab and relatlimab in patients with advanced melanoma that had progressed on anti–programmed death-1/programmed death ligand 1 therapy: Results from the phase I/IIa RELATIVITY-020 trial. J Clin Oncol 41:2724-2735, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long L, Zhang X, Chen F, et al. : The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 9:176-189, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake CG, Lipson EJ, Brahmer JR: Breathing new life into immunotherapy: Review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 11:24-37, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng D, Li M, Zhou R, et al. : Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res 7:737-750, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Yu W, Wang S, Rong Q, et al. : Profiling the tumor-infiltrating lymphocytes in gastric cancer reveals its implication in the prognosis. Genes 13:1017, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao L, Wang H, Xu K, et al. : Update on lymphocyte-activation gene 3 (LAG-3) in cancers: From biological properties to clinical applications. Chin Med J (Engl) 135:1203-1212, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolfi S, Tang T, Ao GL, et al. : 606 Biomarker analyses of baseline tumor specimens and on-treatment changes in sera samples of patients enrolled in the RELATIVITY-047 trial to characterize LAG-3 biology. J Immunother Cancer 10:A635-A636, 2022. (suppl 2) [Google Scholar]
- 26.Chocarro L, Blanco E, Arasanz H, et al. : Clinical landscape of LAG-3-targeted therapy. Immunooncol Technol 14:100079, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Bristol Myers Squibb data sharing policy (https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html) is compliant with ICMJE guidelines. Bristol Myers Squibb will honor legitimate requests for clinical trial data from qualified researchers. Data will be shared with external researchers whose proposed use of the data has been approved. Complete deidentified patient data sets will be eligible for sharing 2 years after completion of the RELATIVITY-060 study. Before data are released, the researcher(s) must sign a Data Sharing Agreement, after which the deidentified and anonymized data sets can be accessed within a secured portal.