Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a profound global impact, with millions of confirmed cases and deaths worldwide. While most cases are mild, a subset progresses to severe respiratory complications and death, with factors such as thromboembolism, age, and underlying health conditions increasing the risk. Vascular endothelial damage has been implicated in severe outcomes, but specific biomarkers remain elusive. This study investigated syndecan-1 (SDC-1), a marker of endothelial damage, as a potential prognostic factor for COVID-19, focusing on the Japanese population, which is known for its aging demographics and high prevalence of comorbidities.
Methods
A multicenter retrospective study of COVID-19 patients in Fukushima Prefecture in Japan who were admitted between February 2020 and August 2021 was conducted. SDC-1 levels were measured along with other clinical and laboratory parameters. Outcomes including thrombosis, 28-day survival, and disease severity were assessed, and disease severity was categorized according to established guidelines.
Results
SDC-1 levels were correlated with disease severity. Patients who died from COVID-19 had greater SDC-1 levels than survivors, and the area under the receiver operating characteristic curve (AUC) analysis suggested the potential of the SDC-1 level as a predictor of mortality (AUC 0.714). K‒M analysis also revealed a significant difference in survival based on an SDC-1 cutoff of 10.65 ng/mL.
Discussion
This study suggested that SDC-1 may serve as a valuable biomarker for assessing COVID-19 severity and predicting mortality within 28 days of hospitalization, particularly in the Japanese population. However, further investigations are required to assess longitudinal changes in SDC-1 levels, validate its predictive value for long-term survival, and consider its applicability to new viral variants.
Conclusions
SDC-1 is emerging as a potential biomarker for assessing the severity and life expectancy of COVID-19 in the Japanese population, offering promise for improved risk stratification and patient management in the ongoing fight against the virus.
Keywords: Syndecan-1, Coronavirus, Coronavirus disease 2019
Background
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had an enormous impact on global health and the economy [1, 2]. More than 767 million cases have been confirmed worldwide, and 6.9 million people have died as of May 2023 [3]. Although the World Health Organization (WHO) declared the end of the COVID-19 state of emergency in May 2023, the threat of infection by mutant strains remains.
Most cases of COVID-19 are mild or asymptomatic, but a small percentage of those who develop pneumonia progress to acute respiratory distress syndrome (ARDS) and require mechanical ventilation. The leading cause of death in COVID-19 patients is respiratory failure due to ARDS [4]. Although respiratory failure is the most common cause of death, mortality has been found to be greater in patients complicated by thromboembolism [5]. Mortality is also known to be greater in elderly individuals and in patients with underlying medical conditions such as hypertension, ischemic heart disease (IHD), and diabetes mellitus (DM) [5]. Numerous studies and meta-analyses have been conducted on biomarkers to determine disease severity and predict prognosis to identify target populations that should be heavily treated to improve survival [6–10].
Vascular endothelial damage is considered a risk factor for thrombosis, but there is no consensus on biomarkers suggestive of vascular endothelial damage in COVID-19 patients. A candidate biomarker for vascular endothelial damage is syndecan-1 (SDC-1), a core protein of heparan sulfate proteoglycans expressed on endothelial cells. SDC-1 forms glycocalyx that plays a role in regulating vascular permeability and preventing thrombus formation and leukocyte adhesion [11]. Previous studies found that circulating SDC-1 is an important marker of glycocalyx degradation associated with severity and prognosis in cardiovascular disease (CVD) [11–14]. Under inflammatory conditions, the accumulation of proteases, including matrix metalloproteinases, thrombin, and plasmin, accelerates the shedding of SDC-1 from the endothelial surface [15, 16]. Several indicators have demonstrated that serum SDC-1 levels are significantly correlated with the Sequential Organ Failure Assessment (SOFA) score in patients with sepsis [17–19], and that non-survivors of sepsis have significantly higher SDC-1 levels than survivors. These findings suggest the potential utility of SDC-1 as a biomarker linking endotheliopathy to organ failure [19–21].
Japan has an aging society with a high proportion of elderly people and a high prevalence of underlying diseases such as hypertension and diabetes. However, during the early stages of the COVID-19 pandemic, the mortality rate in Japan was lower than that in other countries [22].
The purpose of this study was to investigate whether there is an association between SDC-1 levels and disease severity and life expectancy in Japanese COVID-19 patients.
Methods
Study location and patients
This was a multicenter retrospective study conducted at Fukushima Medical University Hospital and Iwaki City Medical Center. The participants were all COVID-19 patients who were admitted to Fukushima Medical University Hospital and Iwaki City Medical Center between February 2020 and August 2021.
Severity classification of COVID-19
COVID-19 cases are generally divided into four severity categories based on the guidelines of the Ministry of Health, Labor and Welfare in Japan [23]. Mild cases were defined as cases with mild clinical symptoms but no evidence of pneumonia on CT scan. Moderate cases 1 were defined as cases with mild respiratory symptoms and mild hypoxemia with pneumonia but no evidence of oxygen requirements. Moderate cases 2 were defined as cases with SpO2 less than 93% with oxygen required. Severe cases were defined as cases with intensive care unit management and those requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO) support. The WHO classification offers more detailed criteria for each severity level, especially concerning respiratory symptoms and oxygen saturation. The WHO classification of mild, moderate, severe, and critical roughly corresponds to the MHLW’s classification of disease severity as mild, moderate-1, moderate-2, and severe.
Blood samples
Blood samples were collected on the day of admission or the day after admission. White blood cell count, neutrophil count, lymphocyte count, hemoglobin, platelet count, LD, PT-INR, APTT, D-dimer, CRP, total bilirubin, ALT, AST, and creatinine were measured. SDC-1 was measured by an enzyme-linked immunosorbent assay established by the R&D Center, Shino-Test Corporation, using residual serum from blood tests [24]. Serum was separated from blood samples collected using blood collection tubes containing a serum separator.
Data collection
Other data included age, sex, body mass index (BMI), smoking history, underlying medical conditions, laboratory data, intensive care unit admission, use of mechanical ventilation or ECMO, and outcome. The associations of these outcomes with thrombosis incidence, disease severity, and survival at 28 days after admission were analyzed.
Statistical analysis
The data are presented as the median, 25th-75th percentile or number (%). Statistical analysis was performed with EZR. Wilcoxon’s rank-sum test, chi-square test, Student’s t test, and Kruskal-Wallis test were used for between-group comparisons, as appropriate. Multivariate analysis was also performed using logistic regression analysis. The usefulness of each parameter in predicting outcome was assessed by area under the receiver operating characteristic (ROC) curve (AUC) analysis; a P value < 0.05 was considered to indicate statistical significance.
Study approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the General Ethics Committee of Fukushima Medical University (Approval Review Number: General 2021-056). This retrospective observational study was conducted, and all patients were clearly informed that the study was a clinical study and were given the opportunity to opt out, as well as consent to participate in the study.
Results
Patient characteristics
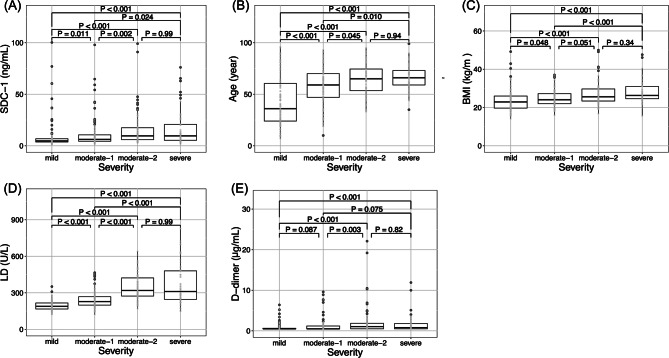
A total of 381 COVID-19 patients were included in this study. Table 1 shows the clinical characteristics of these patients. A total of 118, 108, 90, or 65 patients were classified as having mild, moderate 1, moderate 2, or severe disease, respectively. As in previous reports, age and BMI tended to increase with disease severity [25, 26]. In addition, males had higher disease severity on average than females. A greater percentage of patients with more severe disease had a history of smoking, hypertension, IHD, COPD, or DM. The more severely ill patients were admitted to the ICU and required mechanical ventilation and ECMO support. Two patients (2.2%) in the moderately ill group were also critically ill on admission and received ECMO support. Twenty-two patients died while hospitalized, 15 of whom died within 28 days of admission. White blood cell count, serum LD, CRP, and serum creatinine levels increased with disease severity. Pulmonary function tests were not performed during hospitalization for COVID-19 patients because of efforts to prevent infection to avoid the spread of pathogens in the hospital.
Table 1.
The clinical characteristics of the COVID-19 patients
| Mild (n = 119) |
Moderate-1 (n = 105) |
Moderate-2 (n = 90) |
Severe (n = 65) |
P values | |
|---|---|---|---|---|---|
| Age (median [IQR], year) | 36 [24.0, 60.5] | 59[47.0, 70.0] | 65 [53.5, 74.5] | 66 [59.0, 73.0] | < 0.001 |
| Sex (male, %) | 55 (46.2) | 55 (52.4) | 61 (67.8) | 46 (70.8) | 0.001 |
|
BMI (median [IQR], kg/m2) |
22.9 [19.7, 26.0] |
24.0 [22.0, 27.2] |
25.6 [23.4, 29.7] |
26.3 [24.6, 30.9] |
< 0.001 |
| Smoking (%) | 41 (36.3) | 44 (44.4) | 55 (63.2) | 28 (50.0) | 0.002 |
| Comorbidity | |||||
| Hypertension (%) | 26 (21.8) | 41 (39.0) | 50 (55.6) | 41 (63.1) | < 0.001 |
| DM (%) | 14 (11.8) | 21 (20.0) | 24 (26.7) | 24 (36.9) | 0.001 |
| COPD (%) | 0 (0.0) | 0 (0.0) | 7 (7.8) | 5 (7.7) | < 0.001 |
| IHD (%) | 2 (1.7) | 3 (2.9) | 8 (8.9) | 3 (4.6) | 0.064 |
| Asthma (%) | 13 (10.9) | 4 (3.8) | 5 (5.6) | 4 (6.2) | 0.18 |
| Blood biomarkers | |||||
|
White blood cell (median [IQR], 103/µL) |
4.80 [3.65, 6.05] |
4.70 [3.90, 6.10] |
7.15 [5.12, 9.65] |
7.00 [5.40, 9.80] |
< 0.001 |
|
Neutrophils (median [IQR], 103/µL) |
2.80 [1.90, 3.60] |
3.20 [2.40, 4.30] |
5.55 [3.70, 7.70] |
5.40 [3.80, 8.60] |
< 0.001 |
|
Lymphocytes (median [IQR], 103/µL) |
1.40 [1.10, 1.80] |
1.00 [0.80, 1.50] |
0.80 [0.50, 1.08] |
0.90 [0.60, 1.20] |
< 0.001 |
|
Hemoglobin (median [IQR], g/dL) |
14.1 [12.6, 15.7] |
13.9 [12.2, 14.9] |
13.5 [11.9, 14.8] |
14.4 [12.7, 15.4] |
0.027 |
|
Platelet (median [IQR], 103/µL) |
219.0 [182.5, 267.5] |
193.0 [159.0, 239.0] |
200.5 [156.2, 249.5] |
189.0 [161.0, 231.0] |
0.002 |
|
Total Bilirubin (median [IQR], mg/dL) |
0.50 [0.40, 0.67] |
0.50 [0.40, 0.60] |
0.60 [0.40, 0.70] |
0.60 [0.40, 0.80] |
0.018 |
|
LD (median [IQR], U/L) |
189.0 [167.0, 216.5] |
228.0 [199.0, 268.0] |
319.0 [273.2, 422.2] |
322.0 [247.0, 480.0] |
< 0.001 |
|
Creatinine (median [IQR], mg/dL) |
0.71 [0.58, 0.86] |
0.76 [0.63, 0.96] |
0.84 [0.68, 0.97] |
0.84 [0.71, 1.02] |
< 0.001 |
|
CRP (median [IQR], mg/dL) |
0.14 [0.05, 0.40] |
1.26 [0.40, 4.18] |
5.14 [2.86, 9.05] |
4.02 [1.67, 9.17] |
< 0.001 |
|
D-dimer (median [IQR], µg/mL) |
0.50 [0.50, 0.65] |
0.50 [0.50, 1.20] |
1.05 [0.50, 1.87] |
0.80 [0.50, 1.80] |
< 0.001 |
|
SDC-1 (median [IQR], ng/mL) |
4.90 [3.70, 7.00] |
6.30 [4.40, 11.90] |
10.15 [6.03, 18.25] |
10.30 [5.40, 25.40] |
< 0.001 |
| ICU (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 54 (96.4) | < 0.001 |
| ECMO (%) | 0 (0.0) | 0 (0.0) | 2 (2.2) | 5 (7.7) | 0.001 |
| Hospital mortality within 28days (%) | 0 (0.0) | 1 (1.0) | 4 (4.4) | 10 (15.4) | < 0.001 |
IQR: interquartile range, BMI: body mass index, DM: diabetes mellitus, COPD: chronic obstructive pulmonary disease, IHD: ischemic heart disease, LD: lactate dehydrogenase, CRP: C-reactive protein, SDC-1: syndecan-1, ICU: intensive care unit, ECMO: extracorporeal membrane oxygenation
Prediction of disease severity and hospital mortality using serum SDC-1 levels
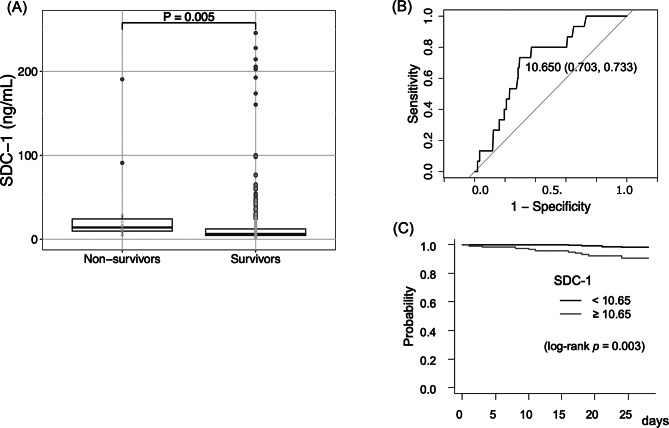
SDC-1 values significantly increased with disease severity (Kruskal-Wallis test, p < 0.001) (Fig. 1). The median level of SDC-1 in healthy volunteers (n = 8, median age 32 years) was 2.6 ng/mL (range: 1.8–3.8 ng/mL), which was significantly lower than the mild case of the COVID-19 patients (p = 0.002, data not shown). The patients who died within 28 days of hospitalization and those who survived were divided into two groups, and a comparison between the two groups using SDC-1 values revealed a significant difference (Mann-Whitney U test, p = 0.005) (Fig. 2). ROC curve analysis revealed that the AUC for predicting mortality in COVID-19 patients within 28 days of hospitalization for serum SDC-1 was 0.714 (95% CI, sensitivity 73.3%, specificity 70.3%), with an optimal cutoff value of 10.65 ng/mL (Fig. 2).
Fig. 1.
Factors associated with COVID-19 severity. (A) SDC-1; (B) age; (C) BMI; (D) LD; (E) D-dimer. The center bold line is the median value; the bottoms and tops of the boxes represent the 25th and 75th percentiles, respectively; and the whiskers are 95% confidence intervals. SDC-1, syndecan-1; BMI, body mass index; LD, lactate dehydrogenase
Fig. 2.
Prediction of in-hospital mortality using serum SDC-1 levels. (A) Serum SDC-1 levels in survivors and nonsurvivors. The lines in the graph indicate the medians with interquartile ranges. The differences between the survivors and nonsurvivors were analyzed using the Mann-Whitney U test (P = 0.005). (B) Receiver operating characteristic (ROC) curve analysis of the ability of serum SDC-1 levels to predict in-hospital mortality. The area under the ROC curve (AUC) for predicting hospital mortality was 0.714. (C) Survival rate according to the Kaplan-Meier method in which patients with SDC-1 were divided into two groups according to the cutoff point. Survival analysis by the Kaplan-Meier method revealed a significant difference in the SDC-1 value (p = 0.003). SDC-1, syndecan-1
Age and SDC-1 are predictive of poor prognosis in COVID-19 patients
COVID-19 patients were divided into two groups using an SDC-1 cutoff of 10.65 ng/mL. The Kaplan-Meier survival test showed a significant difference between the two groups (log-rank p = 0.003; Fig. 2). The group with SDC-1 levels ≥ 10.65 ng/mL had a significantly greater mortality rate within 28 days of hospitalization (HR 6.2; 95% CI 2.0-19.7; p = 0.001; Table 2). Cox proportional hazards regression analysis was performed with older age, BMI > 30 kg/m2, diabetes status, CVD status, and SDC-1 status as independent variables. Age and SDC-1 were found to be statistically significant. An SDC-1 concentration ≥ 10.65 ng/mL had a hazard ratio of 7.3 (95% confidence interval of 1.50–34.8, p = 0.012). According to the multivariate analysis, BMI > 30 kg/m2, diabetes status, and heart disease status were not significantly different between the two groups (Table 2).
Table 2.
Cox’s proportional hazards regression
| Characteristics | n = 379, (%) | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age, year | ||||||||
| ≧ 65 | 145 (38) | 23.7 | 3.1-180.2 | 0.002 | 12.9 | 1.5–110.0 | 0.018 | |
| < 65 | 234 (62) | 1 | ||||||
| BMI, kg/m2 | ||||||||
| ≧ 30 | 60 (16) | 2.1 | 0.5–8.1 | 0.28 | 3.1 | 0.7–12.7 | 0.11 | |
| < 30 | 291 (77) | 1 | ||||||
| Diabetes mellitus | ||||||||
| yes | 83 (22) | 1.3 | 0.4–4.1 | 0.63 | 0.6 | 0.1–2.8 | 0.52 | |
| no | 296 (78) | 1 | ||||||
| Hypertension | ||||||||
| yes | 158 (42) | 3.9 | 1.2–12.3 | 0.018 | 2.2 | 0.4–11.1 | 0.3 | |
| no | 221 (58) | 1 | ||||||
| Ischemic heart disease | ||||||||
| yes | 16 (4) | 1.5 | 0.2–12.1 | 0.65 | 1.3 | 0.1–11.0 | 0.79 | |
| no | 363 (96) | 1 | ||||||
| SDC-1, ng/mL | ||||||||
| ≧ 10.7 | 119 (31) | 6.2 | 2.0-19.7 | 0.001 | 7.3 | 1.5–34.8 | 0.012 | |
| < 10.7 | 260 (69) | 1 | ||||||
BMI: body mass index, SDC-1: syndecan-1, HR: hazard ratio, CI: confidence interval
Discussion
In this study, we focused on SDC-1, a component of the glycan chain expressed on the surface of the vascular endothelium, as a potential biomarker for assessing the severity of COVID-19 since endothelialitis is thought to be involved in the pathogenesis of severe COVID-19. We investigated the relationships between SDC-1 levels, COVID-19 disease severity, and mortality within 28 days of hospitalization in Japanese COVID-19 patients. Our ROC analysis revealed that SDC-1 levels increase with disease severity, suggesting that SDC-1 may serve as a valuable prognostic marker. These results are consistent with previous studies that compared SDC-1 levels between surviving and deceased COVID-19 patients in the Middle East and China and found that SDC-1 levels were significantly greater in patients who succumbed to COVID-19 than in survivors [27, 28]. In addition, compared with those in non-ICU patients, SDC-1 levels on the first day of admission were significantly elevated in ICU patients [27]. ROC analysis revealed that the AUC for the ability of the SDC-1 concentration to predict ICU admission for COVID-19 patients at admission was 0.705. Furthermore, in predicting COVID-19 mortality, ROC analysis revealed that the optimal cutoff value of SDC-1 for distinguishing nonsurvivors from survivors (813.8 ng/mL) had a sensitivity of 68.6%, a specificity of 78.6%, and an AUC of 0.783 (95% confidence interval [CI] 0.647–0.918, P = 0.002) [28]. Even if the disease is classified as hypothetically mild in terms of severity, a high SDC-1 value might prompt consideration for more intensive treatment to prevent progression to severe disease. Elevated SDC-1 levels may also indicate vascular endothelial damage, and addressing such damage through treatment could potentially improve prognosis.
Other reports and meta-analyses have been published on other biomarkers of disease severity [6, 7], showing a significant association between lymphocyte count, CRP, procalcitonin, LD, and D-dimer and COVID-19 severity [6].
Elevated serum levels of SDC-1 indicate the loss of endothelial glycans, the glycocalyx, which play a role in maintaining vascular integrity and coagulation homeostasis [29]. The exact mechanism underlying the elevated SDC-1 levels in COVID-19 patients remains unclear. The shedding and degradation of the glycocalyx are observed in other disease states, including sepsis and ARDS [30]. Inflammation-induced activation of metalloproteinases, heparanases, and hyaluronidases is thought to play a role in glycocalyx shedding and/or degradation [30].
As a result of endothelial damage, a hypercoagulable state is induced. We assumed that SDC-1 levels would correlate with D-dimer levels. Contrary to our expectation, no correlation was observed between these levels (data not shown), suggesting that COVID-19-associated coagulopathy is not simply a result of endothelialitis. The exaggerated production of cytokine-induced tissue factors on inflammatory cells and endothelial cells, as well as neutrophil extracellular traps released by activated neutrophils, may collaboratively play a role in this condition.
Our study showed that disease severity was significantly greater in patients with DM, hypertension, and IHD than in those without these comorbidities (Table 1). These comorbidities are strongly associated with vascular endothelial damage [31, 32]. Therefore, we hypothesized that patients with these comorbidities would also have higher SDC-1 levels than those without these comorbidities. However, contrary to our expectation, we did not find significant group differences in the SDC-1 values (data not shown), suggesting that vascular endothelial damage in COVID-19 patients may be different from that in patients with DM, hypertension, and IHD, which are often complicated by atherosclerosis. Although DM and CVD are risk factors for disease severity [33, 34], they may not serve as prognostic predictors (Table 2).
This study has several limitations. First, SDC-1 levels were measured only at admission, and changes over time are unknown. Therefore, it is possible that SDC-1 levels may have increased over time in deceased patients. Second, further validation is necessary to confirm the validity of the SDC-1 as a predictor of long-term survival, as some patients died after 28 days of hospitalization. Third, the subjects in this study were cases from the period when alpha and delta variants were prevalent, and it remains uncertain whether similar results can be obtained for cases involving other variants.
Conclusions
SDC-1 may be a promising biomarker for the prediction of disease severity and life expectancy in Japanese COVID-19 patients.
Acknowledgements
We thank all patients involved in the study.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus
- SDC-1
Syndecan-1
- ELISA
Enzyme-linked immunosorbent assay
- ROC
Receiver operating characteristic
- AUC
Area under curve
- FDP
Fibrinogen/fibrin degradation products
- PT-INR
Prothrombin time international normalized ratio
- CRP
C-reactive protein
- SpO2
saturation of percutaneous oxygen
- ECMO
Extracorporeal membrane oxygenation
Author contributions
KH analyzed the data, interpreted the results, and wrote the first draft. YH, TK, YT, and YS cared for the patients, collected blood samples, and provided clinical data. SY measured SDC-1 using ELISA. DK and MF interpreted the results. TI conceived the clinical questions, designed the experiments, analyzed the data, interpreted the results, and edited the manuscript. All authors read and approved the final manuscript. TI had all the responsibilities for this research.
Funding
This study was supported by JAPS KAKENHI, grant number 22K08510 (To MF and TI).
Data availability
The datasets obtained and analyzed in the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the General Ethics Committee of Fukushima Medical University (Approval Review Number: General 2021-056). This retrospective observational study was conducted, and all patients were clearly informed that the study was a clinical study and were given the opportunity to opt out, as well as consent to participate in the study.
Consent for publication
Informed consent to participate in this study was provided by the enrolled patients or their families.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–9. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 3.Weekly epidemiological update on COVID-. 19–1 June 2023. [cited 2024 May 6]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-june-2023
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26(3):107–8. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayanian S, Reyes J, Lynn L, Teufel K. The association between biomarkers and clinical outcomes in novel coronavirus pneumonia in a US cohort. Biomark Med. 2020;14(12):1091–7. doi: 10.2217/bmm-2020-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar N, Ahmad S, Mahto M, Kumar A, Singh PK. Prognostic value of elevated cardiac and inflammatory biomarkers in patients with severe COVID-19: a single-center, retrospective study. Emerg Crit Care Med. 2022;2(3):122–7. doi: 10.1097/EC9.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampart M, Zellweger N, Bassetti S, Tschudin-Sutter S, Rentsch KM, Siegemund M, et al. Clinical utility of inflammatory biomarkers in COVID-19 in direct comparison to other respiratory infections-A prospective cohort study. PLoS ONE. 2022;17(5):e0269005. doi: 10.1371/journal.pone.0269005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miftode RS, Costache II, Constantinescu D, Mitu O, Timpau AS, Hancianu M, et al. Syndecan-1: from a Promising Novel Cardiac Biomarker to a surrogate early predictor of kidney and Liver Injury in patients with Acute Heart failure. Life (Basel) 2023;13(4):898. doi: 10.3390/life13040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wernly B, Fuernau G, Masyuk M, Muessig JM, Pfeiler S, Bruno RR, et al. Syndecan-1 predicts Outcome in patients with ST-Segment Elevation Infarction Independent from Infarct-related myocardial Injury. Sci Rep. 2019;9(1):18367. doi: 10.1038/s41598-019-54937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki K, Okada H, Sumi K, Tomita H, Kobayashi R, Ishihara T, et al. Serum syndecan-1 reflects organ dysfunction in critically ill patients. Sci Rep. 2021;11(1):8864. doi: 10.1038/s41598-021-88303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanino Y. Roles of extracellular matrix in lung diseases. Fukushima J Med Sci. 2024;70(1):1–9. doi: 10.5387/fms.2023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei S, Gonzalez Rodriguez E, Chang R, Holcomb JB, Kao LS, Wade CE, et al. Elevated Syndecan-1 after trauma and risk of Sepsis: a secondary analysis of patients from the pragmatic, randomized optimal platelet and plasma ratios (PROPPR) trial. J Am Coll Surg. 2018;227(6):587–95. doi: 10.1016/j.jamcollsurg.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176(1):67–81. doi: 10.1111/bph.14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallisalmi M, Tenhunen J, Yang R, Oksala N, Pettilä V. Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta Anaesthesiol Scand. 2012;56(3):316–22. doi: 10.1111/j.1399-6576.2011.02578.x. [DOI] [PubMed] [Google Scholar]
- 18.Johansson PI, Haase N, Perner A, Ostrowski SR. Association between sympathoadrenal activation, fibrinolysis, and endothelial damage in septic patients: a prospective study. J Crit Care. 2014;29(3):327–33. doi: 10.1016/j.jcrc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Anand D, Ray S, Srivastava LM, Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin Biochem. 2016;49(10–11):768–76. doi: 10.1016/j.clinbiochem.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Puskarich MA, Cornelius DC, Tharp J, Nandi U, Jones AE. Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at high risk for intubation after large-volume intravenous fluid resuscitation. J Crit Care. 2016;36:125–9. doi: 10.1016/j.jcrc.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrowski SR, Gaïni S, Pedersen C, Johansson PI. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: an observational study. J Crit Care. 2015;30(1):90–6. doi: 10.1016/j.jcrc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Idogawa M, Tange S, Nakase H, Tokino T. Interactive web-based Graphs of Coronavirus Disease 2019 cases and deaths per Population by Country. Clin Infect Dis. 2020;71(15):902–3. doi: 10.1093/cid/ciaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.001248424.pdf. [cited 2024 May 7]. https://www.mhlw.go.jp/content/001248424.pdf
- 24.Hatanaka K, Ito T, Madokoro Y, Kamikokuryo C, Niiyama S, Yamada S, et al. Circulating Syndecan-1 as a predictor of persistent Thrombocytopenia and Lethal Outcome: a Population study of patients with suspected Sepsis requiring Intensive Care. Front Cardiovasc Med. 2021;8:730553. doi: 10.3389/fcvm.2021.730553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, Kandula S, Huynh M, Greene SK, Van Wye G, Li W, et al. Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis. Lancet Infect Dis. 2021;21(2):203–12. doi: 10.1016/S1473-3099(20)30769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, Aveyard P, et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–9. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karampoor S, Zahednasab H, Farahmand M, Mirzaei R, Zamani F, Tabibzadeh A, et al. A possible pathogenic role of Syndecan-1 in the pathogenesis of coronavirus disease 2019 (COVID-19) Int Immunopharmacol. 2021;97:107684. doi: 10.1016/j.intimp.2021.107684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D, Li L, Chen Y, Ma J, Yang Y, Aodeng S, et al. Syndecan-1, an indicator of endothelial glycocalyx degradation, predicts outcome of patients admitted to an ICU with COVID-19. Mol Med. 2021;27(1):151. doi: 10.1186/s10020-021-00412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission Syndecan-1 level, a marker of endothelial glycocalyx degradation, is Associated with inflammation, protein C depletion, Fibrinolysis, and increased mortality in Trauma patients. Ann Surg. 2011;254(2):194. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 30.Yamaoka-Tojo M. Vascular endothelial glycocalyx damage in COVID-19. Int J Mol Sci. 2020;21(24):9712. doi: 10.3390/ijms21249712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis — an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Zou MH. Molecular insights and therapeutic targets for Diabetic Endothelial Dysfunction. Circulation. 2009;120(13):1266–86. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–45. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543–58. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained and analyzed in the current study are available from the corresponding author on reasonable request.