Conspectus
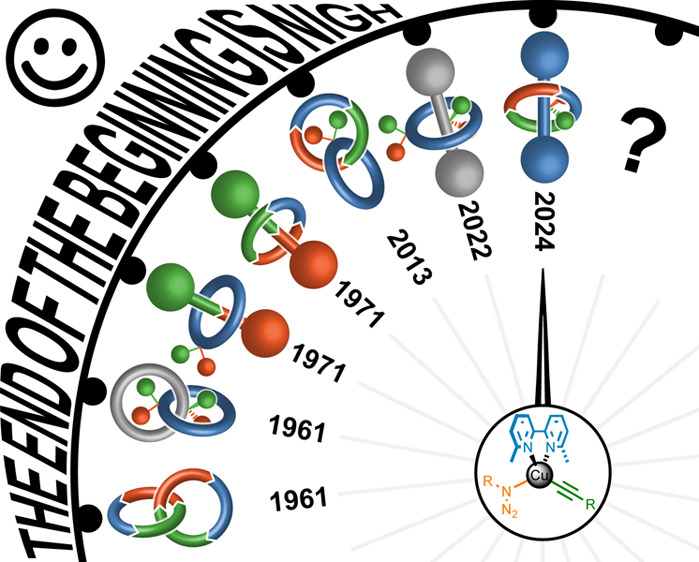
Stereochemistry has played a key role in the development of synthetic chemistry for the simple reason that the function and properties of most molecules, from medicine to materials science, depend on their shape and thus the stereoisomer used. However, despite the potential for rotaxanes and catenanes to display unusual forms of stereochemistry being identified as early as 1961, this aspect of the mechanical bond remained underexplored and underexploited; until 2014 it was only possible to access chiral rotaxanes and catenanes whose stereoisomerism is solely attributable to the mechanical bond using chiral stationary phase high performance liquid chromatography, which limited their production on scale and thus inhibited the investigation of their properties and applications. Furthermore, the stereogenic units of such molecules and analogues were often poorly described, which made it hard to fully articulate both what had been achieved in the field and what problems were left to solve. Relatively recently, methods to access rotaxanes and catenanes that display mechanical stereochemistry selectively have been developed, making these intriguing structures available for study in a range of prototypical applications including catalysis, sensing, and as chiral luminophores.
In this Account, we briefly discuss the history of mechanical stereochemistry, beginning in 1961 when the potential for mechanical stereoisomerism was first identified, before defining how mechanical stereochemistry arises from a structural point of view. Building on this, using simple stereochemical arguments, we confirm that the complete set of unique stereogenic units of two-component rotaxanes and catenanes have finally been identified and categorized unambiguously, with the last being identified only in 2024. After pausing to discuss some of the stereochemical curiosities that arise when molecules contain both covalent and mechanical stereogenic units, and the potential for stereoisomerism to arise due to co-conformational movement, we use our stereochemical framework to summarize our efforts to develop conceptually general approaches to [2]catenanes and [2]rotaxanes containing all of the possible mechanical stereogenic units. In particular, we highlight how the nature of a mechanical stereogenic unit affects the available strategies for their stereoselective synthesis. We finish by highlighting recent prototypical chemical applications of interlocked molecules that rely on their mechanical stereochemistry, before discussing future directions and challenges.
Taken together, we propose that the transition of such molecules from being hard to make and poorly described, to being available in high stereopurity using clearly articulated methodological and stereochemical concepts suggests that the field is finally maturing. Thus, we are now coming to the end of the beginning of mechanical stereochemistry. The stage is now set for such molecules to play a functional role in a range of areas, indeed in any chemical or physical application where control over molecular shape is required.
Key References
de Juan, A.; Lozano, D.; Heard, A. W.; Jinks, M. A.; Suarez, J. M.; Tizzard, G. J.; Goldup, S. M. A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes. Nat. Chem. 2022, 14 ( (2), ), 179. .1 A general approach to mechanically planar chiral rotaxanes that takes advantage of high diastereoselectivity and mechanical motion, demonstrated through the synthesis of 11 highly enantioenriched targets.
Maynard, J. R. J.; Gallagher, P.; Lozano, D.; Butler, P.; Goldup, S. M. Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes. Nat. Chem. 2022, 14 ( (9), ), 1038. .2 The first stereoselective synthesis of mechanically axially chiral catenanes, which was enabled by proper stereochemical analysis that also led to the discovery of the mechanical axial stereogenic unit of rotaxanes.
Pairault, N.; Rizzi, F.; Lozano, D.; Jamieson, E. M. G.; Tizzard, G. J.; Goldup, S. M. A catenane that is topologically achiral despite being composed of oriented rings. Nat. Chem. 2023, 15 ( (6), ), 781. .3 A clear demonstration that the mechanically planar chiral stereogenic unit of catenanes is not necessarily topological in nature.
Heard, A. W.; Goldup, S. M. Synthesis of a Mechanically Planar Chiral Rotaxane Ligand for Enantioselective Catalysis. Chem 2020, 6 ( (4), ), 994. .4 The first demonstration of a mechanically planar chiral ligand in catalysis.
Introduction
The selective synthesis of stereoisomers is a problem that continues to engage the synthetic community, driven both by the intellectual challenge it presents and the technological importance of providing stereopure molecules for applications from medicine to materials science. However, although mechanically interlocked molecules (MIMs)5 have attracted significant interest as components of molecular machines,6,7 the stereochemical properties of the mechanical bond have received less attention. This is despite there being opportunities for stereoisomerism distinct from that of classical covalently bonded molecules. Indeed, optical and geometric isomerism can arise in MIMs even when their covalent subcomponents are stereochemically trivial because of how the underlying symmetry properties of the subcomponents interact in the geometrically restricted environment of the mechanical bond.8
In this Account, we provide an overview of how the study of molecules displaying mechanical stereochemistry has progressed since the first racemic syntheses of mechanically chiral molecules in the 1990s. We also discuss how our understanding of mechanical stereochemistry has evolved during these synthetic efforts. Our focus is on the stereochemistry that arises in molecules composed of two covalent subcomponents (e.g., [2]catenanes and [2]rotaxanes) that contain the minimum number of crossing points for a mechanical bond to exist. We conclude with a discussion of the next frontiers of mechanical stereochemistry, from the possible applications of these molecules to future synthetic challenges.
Describing Mechanical Stereochemistry
The Canonical Mechanical Stereogenic Units
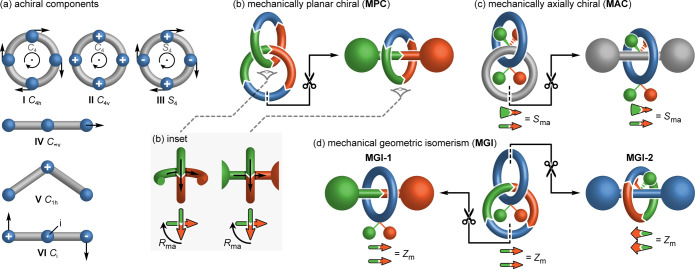
In 1961,9 Wasserman and Frisch highlighted that chirality could arise in catenanes even when the component rings are achiral provided that the two rings are oriented by a sequence of atoms (Figure 1a10), or the two faces of the macrocycles are distinguishable (Figure 1b). Later,11 Schill recognized that chiral rotaxanes arise if the macrocycle is oriented and the two ends of the axle are distinguishable (Figure 1c) and also that geometric isomerism arises if the macrocycle faces and the two ends of the axle are distinguishable (Figure 1d). We later described such molecules as displaying “conditional mechanical stereochemistry” because their stereochemistry is conditional on the symmetry of the subcomponents.8a
Figure 1.
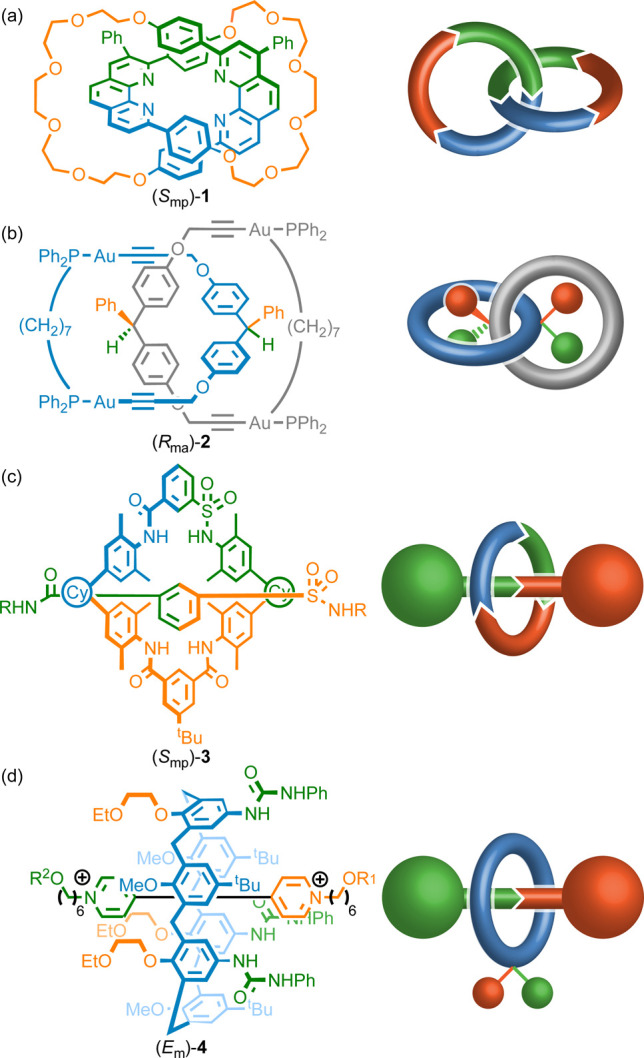
The canonical mechanical stereogenic units identified by Wassermann and Frisch9 (a, b) and Schill11 (c, d) and representative early chemical examples of such structures which were separated by PCSP-HPLC (113 and 3(14) [R = 4-C6H4CPh3; Cy = 1,1-cyclohexyl]), reported as a racemate (218) or synthesized stereoselectively (416 [R1 = C(O)CH(cyclohexyl)2; R2 = C(O)CHPh2]).10
Molecules whose stereochemistry relies solely on these canonical stereogenic units received varying levels of attention between their initial identification9 and 201412 when we made our first contribution. Specifically, the enantiomers of chiral catenanes and rotaxanes (Figure 1) composed of oriented components (e.g., 1(13) and 3(14) respectively) had been separated using preparative chiral stationary phase HPLC (PCSP-HPLC)15 and several stereoselective syntheses of rotaxane geometric isomers had been reported, albeit these relied on the use of macrocycles that adopt a cone-shaped conformation, typically calixarenes (e.g., 4(16)), rather than simple prochiral17 rings as envisaged by Schill.11 However, no report of enantioenriched catenanes composed of facially dissymmetric rings (e.g., 2(18)) had been disclosed.
In 2011,19 we observed well-expressed diastereomerism in sterically crowded rotaxanes containing covalent stereochemistry. This observation prompted us to develop auxiliary methodologies for the stereoselective synthesis of mechanically stereogenic molecules. Our guiding objective was to be able to synthesize structures where the mechanical bond provides the sole source of stereoisomerism to allow the potential applications of mechanical stereochemistry to be identified unambiguously. In addition to synthetic challenges, our studies revealed problems relating to the description of mechanical stereochemistry, as highlighted by the relatively recent identification of noncanonical chiral2 and geometric20,21 stereogenic units. Thus, we have also worked to systematize the description of mechanical stereochemistry—in order to know if we have achieved our goal, we need to know how many stereogenic units there are to conquer!
Defining the Fundamental Mechanical Stereogenic Units
The stereoisomers of molecules displaying mechanical stereochemistry are related by inversion of the relative orientation of the two interlocked components. We recently highlighted21 that such isomerism can only arise when neither covalent subcomponent contains a C2 rotational axis parallel to the macrocycle plane/perpendicular to the axle long axis as this rotation corresponds to the notional process of inverting the orientation of the MIM components; if this rotation is a symmetry operation of the separated component it precludes mechanical steroisomerism. In hindsight this requirement should have been obvious but, to our knowledge, it had not been stated previously. The only achiral macrocycle point group symmetries that meet this requirement are Cnh, Cnv, S2n, which allowed us to confirm that the complete set of mechanical stereogenic units in catenanes had already been identified; Cnh symmetric macrocycles (I, Figure 2a) are by definition oriented and Cnv symmetric rings (II) are facially dissymmetric and so catenanes composed of pairs of rings of these symmetries correspond to the chiral catenanes identified by Wasserman and Frisch,9 whereas interlocking one Cnh symmetric ring with one Cnv ring produces the geometric stereogenic unit identified more recently by Gaeta and Neri.20 The only surprise arising from this analysis is that S2n rings also give rise to mechanical stereochemistry. However, inspecting a simple representation of an S4 symmetric structure (III) reveals that these rings are also oriented; S2n symmetry arises when the ring is oriented, but the horizontal mirror symmetry is lifted.
Figure 2.
(a) Achiral ring (I–III) and axle (IV–VI) components that give rise to (b) MPC (inset shows the view used to assign the mechanical stereochemistry), (c) MAC, and (d) MGI stereochemistry.10
Thus, we define the three conditional mechanical stereogenic units of catenanes as arising when two oriented (Cnh or S2n, Figure 2b) or facially dissymmetric (Cnv, Figure 2c) rings, or one oriented and one facially dissymmetric ring (Figure 2d) are combined. In the latter case, the structure is achiral as the stereochemistry is characterized by oriented lines (vectors) that can be arranged coplanar in a syn or anti arrangement. We describe such catenanes as displaying mechanical geometric isomerism (MGI). The stereochemistry arising when two facially dissymmetric rings are combined is characterized by vectors perpendicular to each ring that cannot be made coplanar, whereas the equivalent vectors arising from interlocked oriented rings are parallel to the macrocycle plane. The former are usually termed “mechanically axially” chiral (MAC) catenanes5 whereas the latter have historically been simply referred to as “topologically chiral” catenanes because their stereochemistry is a topological property22 of the structure when the orientation arises from a sequence of atoms within the macrocycle. However, we have suggested that “mechanically planar” chiral (MPC) is more appropriate because this stereochemistry can also be Euclidean3 and the catenane and rotaxane stereogenic units, to which this label was originally applied,15,12 are related by a notional ring opening process (Figure 2b).
It is relatively trivial to perform the same first-principals stereochemical analysis for rotaxanes, with the result that mechanical stereochemistry arises in rotaxanes if the axle is oriented (Cnv symmetry, e.g., IV) or facially dissymmetric (Cnh or Ci symmetry, e.g., V or VI respectively).21 However, it is more intuitive to recognize that rotaxanes and catenanes are related by a notional ring-opening-and-stoppering process. Applying this approach, MPC (Figure 2b) and MAC (Figure 2b) catenanes are each related to a chiral rotaxane mechanical stereogenic unit and thus we have proposed that the same nomenclature be used to rotaxane and catenane stereochemistry. The MPC rotaxane stereogenic unit corresponds to that originally proposed by Schill,11 whereas MAC rotaxanes were overlooked until 2022.2
Finally, if the oriented ring of an MGI catenane is chosen for the ring opening process (Figure 2d), the rotaxane product displays geometric isomerism, which corresponds to that first identified by Schill.11 However, if the facially dissymmetric ring is opened, the corresponding rotaxane displays a form of geometric isomerism that we have only recently highlighted.21 We proposed that the labels “type 1 MGI” (MGI-1) and “type 2 MGI” (MGI-2) be applied to disambiguate these forms or rotaxane stereoisomerism, where the numeral simply refers to the order of their identification.
Assigning Mechanical Stereochemistry
Given that the stereoisomers of rotaxanes and catenanes are clearly related by a notional ring opening process, it seems sensible that (i) the method used to assign their absolute stereochemistry should avoid automatic inversion of the stereolabel when this process is considered and (ii) the methods used should require the minimum number of arbitrary rules. We recently fell afoul of these rules; our proposed method for assigning the stereochemistry of axially chiral rotaxanes could not be extended to type 2 rotaxane geometric isomers without causing an inversion of stereolabel compared with the corresponding catenane.23 To overcome this problem, we have revised our methods for assigning the axial stereogenic unit of rotaxanes and catenanes.
Pleasingly, this change resulted in a fully self-consistent approach to assigning the fundamental mechanical stereogenic units. In broad terms (see ESI for full details) one: (i) defines the vector associated with each component using simple rules based on the Cahn-Ingold-Prelog24 priorities of atoms; (ii) views these vectors at the crossing point between the two components (Figure 2b inset); (iii) if the vectors cannot be made coplanar, identifies if the direction of rotation from the head of the front vector to the tail of the rear vector is clockwise (R) or anticlockwise (S); (iv) if the vectors can be made coplanar, identifies if they are parallel (Z) or antiparallel (E). We propose that the suffixes “mp”, “ma” and “m” be applied to the stereolabels for MPC, MAC and MGI structures respectively (e.g., Rmp, Rma and Zm) to indicate the mechanical origin of the stereoisomerism.25
Molecules Containing Covalent and Mechanical Stereogenic Units
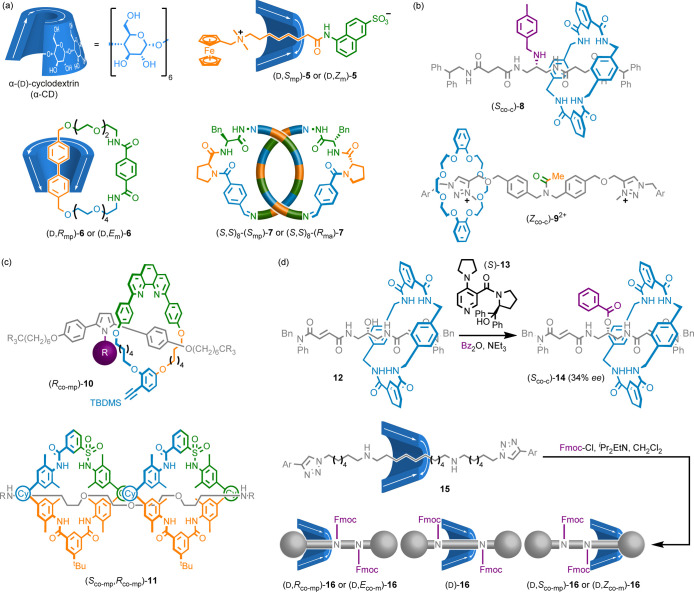
Many mixed covalent/mechanical diastereomers have been reported.8a However, due to difficulties in clearly describing their stereochemistry, the importance of these early results was often obscured. Most commonly, these molecules contain cyclodextrin (CD) rings (Scheme 1a), which are both oriented and facially dissymmetric, as well as containing covalent stereogenic units and so rotaxane 5(26) and catenane 6(27) exist as mechanical diastereomers. Such molecules were often described as “orientational” isomers. However, all mechanical stereochemistry depends on the relative orientation of the subcomponents.
Scheme 1. (a) MIMs Containing Covalent Stereocenters Whose Stereochemistry can be Described Using One of Two Mechanical Stereodescriptors. (b) MIMs that Display Co-conformational Covalent Stereochemistry (Ar = 3,4-di-tBu-C6H3). (c) MIMs that Display Co-conformational MPC Stereochemistry (R1 = 4′-cyclohexyl-1,1′-biphenyl, R2 = C(O)-(4-C6H4)–CH2O-(4-C6H4)-CPh3). (d) Operation of Information Ratchets 12 and 16 (Ar = 3,4-di-tBu-C6H3)10.
MIMs containing mechanical and covalent stereochemistry can typically be fully specified using one of two possible mechanical stereodescriptors combined with the relevant covalent stereolabels. For example, the mechanical stereochemistry of 5 and 6 can be specified as MGI or MPC but using both labels is redundant because one automatically specifies the other. We hesitate to arbitrarily privilege one description over the other. However, in the case of catenanes 6 the MPC label seems more appropriate as this captures a key feature of the stereochemistry; although MGI stereochemistry is not a topological property of the structure, the MPC stereochemistry is. For this reason, we prefer the covalent-MPC description of 6 and, for consistency, apply the same approach to rotaxanes 5. Similarly, the configuration of peptidic catenane 7(28) can be fully specified using a MAC or MPC stereolabel alongside the covalent configuration, of which we prefer the MPC description for the same reason as above.
Co-conformational Stereochemistry
We have so far focused on conditional mechanical stereochemistry that is invariant with mechanical motion. However, co-conformational movement can result in new stereogenic units. In the simplest case, the position of one subcomponent desymmetrizes the other such that covalent co-conformational stereochemistry arises (Scheme 1b), as in enantioselective catalyst 8,29 which is chiral because the ring desymmetrizes an axle covalent prochiral center that is bulky enough to prevent racemization (c.f., atropisomerism). Similarly, rotaxane 9 displays dynamic co-conformational covalent geometric isomerism as the amide geometric isomers can exchange by shuttling of the macrocycle either side of the amide bond as well as by single bond rotation.30
Co-conformational mechanical stereochemistry arises when one component desymmetrizes the other (Scheme 1c). Rotaxane 10 displays co-conformational MPC stereochemistry as the position of the oriented ring desymmetrizes the axle component.31 Similarly, rotaxane 11 exists as two non-interchanging32 co-conformational diastereomers, one of which is chiral and the other is meso (shown) because the oriented rings desymmetrize the bilaterally symmetrical axle.33
Co-conformational stereochemistry has been harnessed to generate directed mechanical motion (Scheme 1d). Rotaxane 12 behaves as a molecular information ratchet under enantioselective acylation of the OH unit mediated by (S)-13.34 Similarly, information ratchet 15 relies on the fact that shuttling of the ring results in mixed covalent/co-conformational epimeric structures that react at different rates with FmoCl,35 resulting in kinetic asymmetry36 and thus a mixture of rotaxanes 16 in ratios that do not accord with their relative stability.
Auxiliary Syntheses of MPC Structures
The properties of the underlying stereogenic units in rotaxanes and catenanes feeds directly into methods for their synthesis.37 For example, if the axle or macrocycle components of an MPC rotaxane are disconnected the resulting components are always achiral (Scheme 2a). Thus, additional chiral information needs to be included in the forward synthesis if we are to avoid producing a racemic mixture of enantiomeric products.
Scheme 2. (a) Retrosynthesis Demonstrating that Dividing the Axle or Macrocycle Component of an MPC Rotaxane Results in Achiral Components. (b) A Chiral Derivatization Approach to MPC Rotaxanes 22. (c) A Stereoselective Chiral Auxiliary Approach to Rotaxanes 24. Ar = 3,4-di-tBu-C6H310.
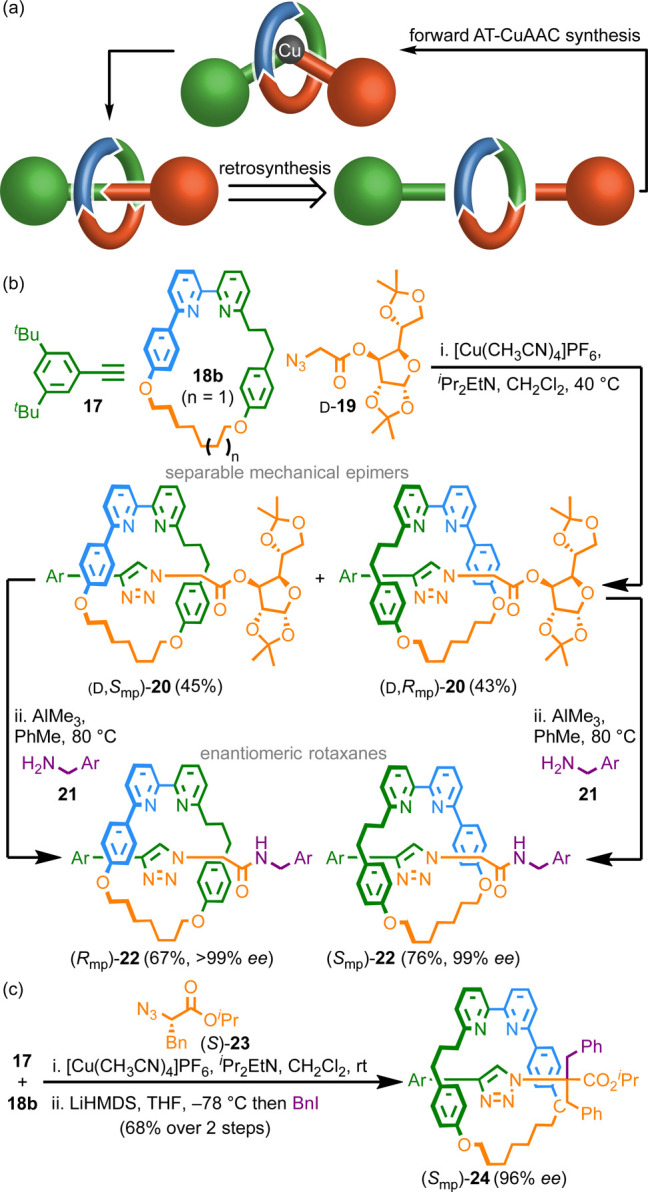
In 2014,12 we demonstrated one solution to this problem through an active template38 Cu-mediated alkyne–azide cycloaddition39 (AT-CuAAC40) coupling between alkyne 17 and glucose-derived azide (d)-19 mediated by readily available41 oriented macrocycle 18b to produce rotaxanes 20 (Scheme 2b). Diastereomeric rotaxanes 20 were separated by flash chromatography and the covalent stereochemistry removed by aminolysis to give rotaxanes 22 in high stereopurity. Luckily, both (d-Rmp)-20 and (d-Smp)-20 could be analyzed by single-crystal X-ray diffraction (SCXRD), which allowed their absolute stereochemistry to be assigned unambiguously, the first time this had been achieved for an MPC rotaxane. Indeed, throughout the discussions below, the absolute configuration of the mechanical stereogenic unit, where provided, was assigned using SCXRD. However, we have shown that it is possible to computationally model, and thus predict, the vibrational circular dichroism spectra of MPC rotaxanes.62 Although computationally expensive at present, in future, this may provide a method to assign the mechanical configuration of molecules without the need to generate crystals suitable for SCXRD.
The AT-CuAAC reaction is particularly beneficial for the synthesis of separable mechanical epimers as it is efficient even when forming highly sterically crowded products.19 This crowding ensures that the covalent and mechanical stereogenic units interact strongly, leading to well-expressed diastereoisomerism. The same steric hindrance is also potentially beneficial when considering stereoselective mechanical bond formation. Indeed, in 2018 we extended our approach to the first true chiral auxiliary synthesis of an MPC rotaxane by using α-chiral azide (S)-23 as the auxiliary (Scheme 2c).42 High stereoselectivity (96% de) was observed when aryl acetylene 17 was the coupling partner, although selectivities fell when less hindered alkynes were used. Subsequent alkylation of the stereocenter removed the covalent stereochemistry, giving rotaxane (Smp)-24 in 96% ee without separation of the intermediate diastereomers.
The same stereochemical considerations apply to MPC catenanes; disconnection of either ring gives rise to achiral starting materials, requiring the inclusion of a temporary source of stereochemical information in the forward synthesis. In our first report, an exocyclic pendant auxiliary was used to direct the formation of the mechanical bond in low (50% de) stereoselectivity (Scheme 3a).43 After separation of diastereomers 26, the pendant auxiliary could be removed by oxidation-tautomerization-hydrolysis sequence to give enantiopure catenane 27. More recently, we extended our α-chiral azide-type auxiliary to the synthesis of MPC catenane 29 in up to 82% ee (Scheme 2b).44 In this case we removed the covalent stereocenter by Rh-mediated decarbonylation. Although this sequence proceeded in low isolated yield, in part due to several challenging purifications, we were able to use the same method to synthesize co-conformationally mechanically chiral catenane (Sco-mp)-30 in high stereopurity by installing the mechanical bond selectively over one side of a C2v ring (Scheme 2c).
Scheme 3. (a) Synthesis of MPC Catenane 27 (Ar = 4-OMe-C6H4). (b) Synthesis of MPC Catenane 29. (c) Co-conformationally MPC Catenane 30(10).
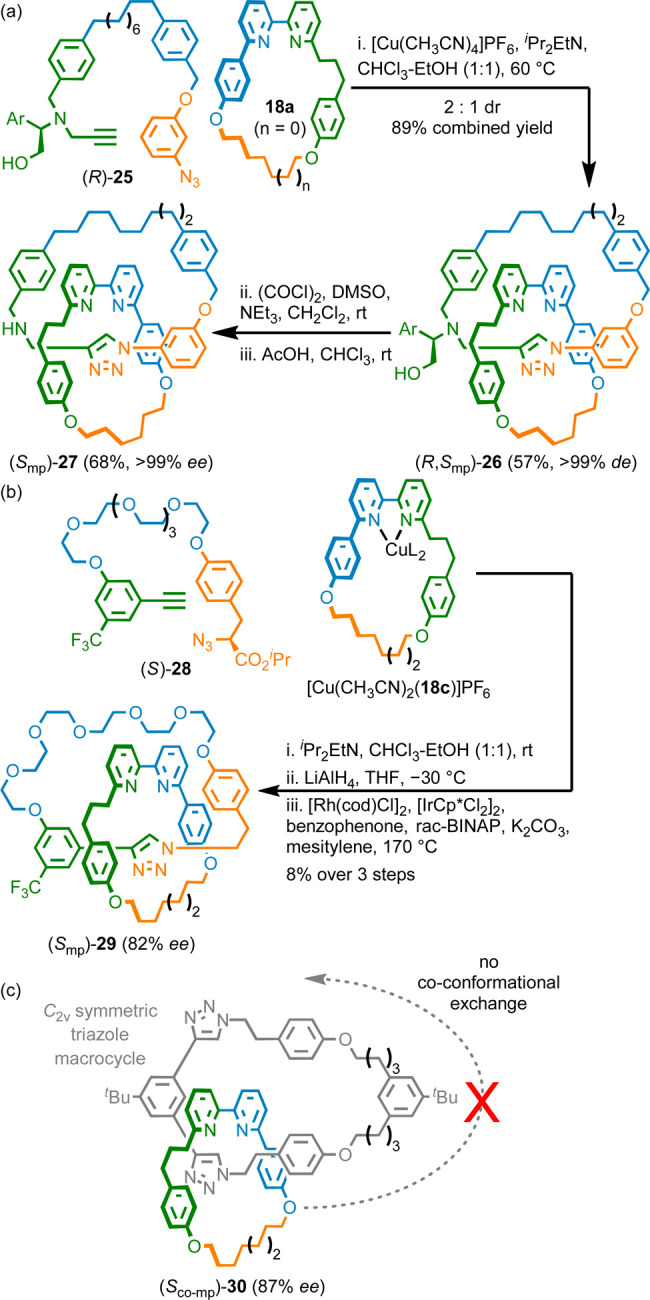
Our AT-CuAAC approach to MPC rotaxanes and catenanes all make use of a bipyridine macrocycle motif, which led us to investigate whether it was possible to direct stereoselective formation of MPC rotaxanes and catenanes using a single chiral macrocycle.45 Pleasingly, macrocycle (S)-31 was found to produce both catenanes (e.g., 33) and rotaxanes (e.g., 35) in very high stereoselectivity (Scheme 4a). The auxiliary moiety was removed by reduction of the carboxylic acid followed by an oxidation-tautomerization-hydrolysis sequence (c.f. 29) in the case of the reported rotaxanes whereas the auxiliary cleaved spontaneously during the mechanical bond forming step in the synthesis of 33. The origin of the latter serendipitous reactivity is as yet unknown. This flexible methodology also allowed the synthesis of all three diastereomers (meso and both enantiomers) of co-conformationally MPC [3]rotaxanes 38 (Scheme 4b) in which a centrosymmetric axle is encircled by two identical oriented macrocycles, simply by varying the enantiomer of macrocycle 31 used during an iterative46 AT-CuAAC strategy.45
Scheme 4. (a) MPC Catenane 33 and Rotaxane 35 Synthesized from Macrocycle (S)-31 (Ar = 3,4-di-tBu-C6H3). (b) Synthesis of Co-conformational MPC [3]Rotaxanes 38 (Ar = 3,4-di-tBu-C6H3). (c) MPC Catenane 39 Whose Stereochemistry is Euclidean (R = CH2OH)10.
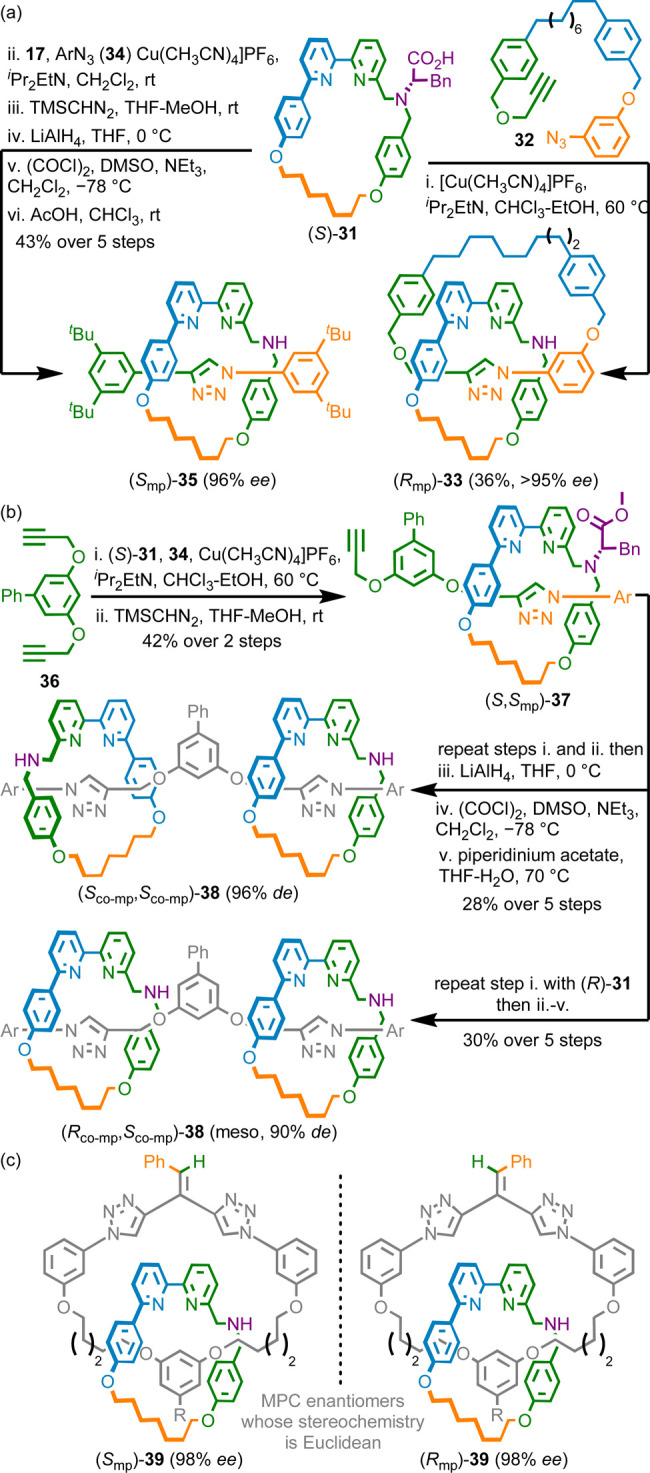
We used the same methodology to synthesize MPC catenane 39 whose stereochemistry is Euclidean in nature, the first example of such a structure, in high stereopurity (Scheme 4c).3 The triazole-containing ring of 39 is oriented by the exocyclic double bond and so catenane 39 contains an MPC stereogenic unit. However, the stereochemistry of 39 is not a topological property of the structure because double bond geometry is not defined in the corresponding molecular graph.22
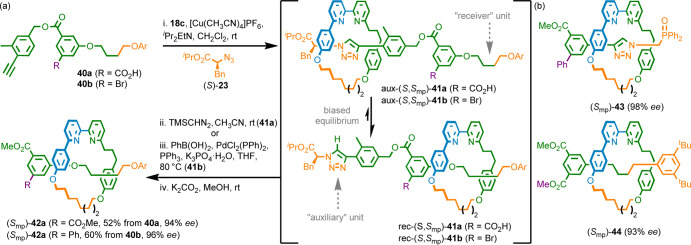
Finally, as commented above, the selectivity with α-chiral azide-based auxiliaries (e.g., 23) depends strongly on the steric demand of the alkyne coupling partner.42 During investigations of this effect, we serendipitously identified that an o-Me aryl acetylene motif delivered both high diastereoselectivity in combination with azide 23, and resulted in products where the bipyridine macrocycle is displaced from the triazole formed, presumably due to the same steric hindrance that ensures high de in the mechanical bond formation.1 We took advantage of this observation to develop a chiral interlocking auxiliary strategy, as exemplified in the synthesis of rotaxanes 42 (Scheme 5a). Coupling of (S)-23, 18c and alkynes 40 gave rotaxanes 41 that exist as a mixture of co-conformations in which the macrocycle preferentially encircles the alkyl ether receiver unit where it presumably engages in CH···N H-bonds with the polarized axle ether protons. Subsequent esterification (rotaxane 41a) or cross coupling (rotaxane 41b) traps the macrocycle over the ether receiver unit after which transesterification with basic MeOH removes the auxiliary unit to give rotaxanes 42 in excellent stereopurity (94% and 98% ee respectively). To demonstrate the power of this approach we synthesized 11 highly enantioenriched (93–98% ee) MPC rotaxanes, including (Scheme 5b) functionalized (e.g., 43) and extremely challenging examples where the receiver unit presents almost no attractive interactions for the macrocycle (e.g., 44).
Scheme 5. (a) Interlocking Chiral Auxiliary Synthesis of MPC Rotaxanes 42 (Ar = 3,4-di-tBu-C6H3). (b) Rotaxanes 43 and 44 Made Using the Chiral Interlocking Auxiliary Approach10.
Syntheses of MAC Rotaxanes and Catenanes
A key difference between MPC and MAC stereochemistry is that in the latter case, disconnecting one of the prochiral subunits results in a chiral fragment that, in the forward reaction, is symmetrized (Scheme 6a).47 We combined this observation with the recognition that MAC molecules display co-conformational covalent stereochemistry. If the relative movement of the rings is sterically hindered this results in separable diastereomers. The challenge then becomes controlling which face of the incoming prochiral macrocycle interacts with which component of the half-axle/pre-macrocycle.
Scheme 6. (a) Schematic Retrosynthesis of a MAC Catenane via a Chiral Synthon that is Symmetrized in the Forward Reaction Highlighting the Potential for Co-conformational Diastereoisomerism. (b) Synthesis of MAC Catenane 48 (R = CO2Me). (c) Synthesis of MAC Rotaxane 51 (Ar = 3,4-di-tBu-C6H3). (d) Direct Enantioselective Synthesis of MAC Rotaxane 53 (Ar = 3,4-di-tBu-C6H3)10.
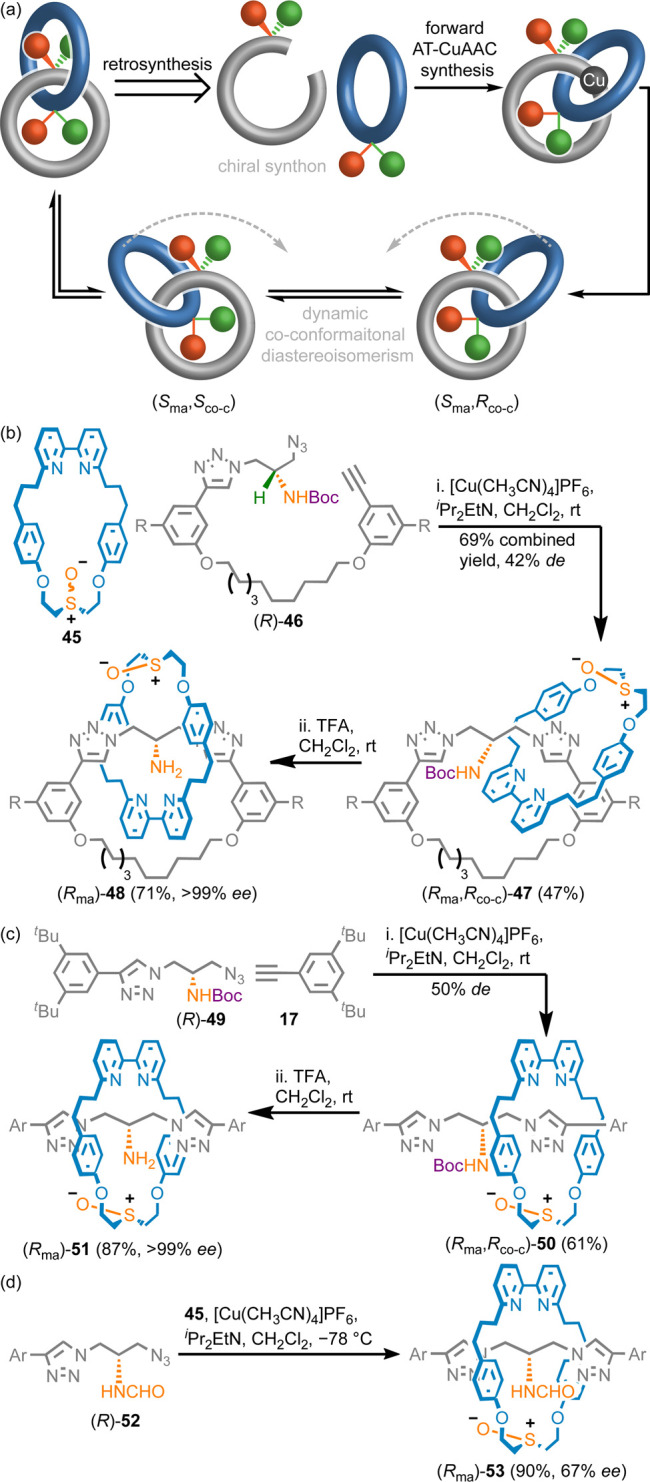
Coupling of prochiral macrocycle 45 with chiral pre-macrocycle (R)-46 gave diastereomeric catenanes 47 (42% de) that differ in the relative orientation of the rings–the SO unit can point toward or away from the NHBoc substituent–but whose co-conformational stereochemistry is fixed by the method of synthesis (Scheme 6b).2 Separation of stereoisomers 47 followed by removal of the Boc group from the major (Rma,Rco-c)-47 isomer gave MAC catenane (Rma)-48 in excellent stereopurity (>99% ee). Similarly, half-axle (R)-49 was coupled with macrocycle 45 to give diastereomers 50 in 50% de (Scheme 6c). These were separated and the Boc group removed to give MAC rotaxane (Rma)-51 in excellent stereopurity (>99% ee).
Recently,23 we revisited the synthesis of rotaxanes 50. The results obtained when the N protecting group and reaction conditions were varied led us to propose that the observed selectivity arises due to an NH···O interaction between the NH unit of the axle and the sulfoxide moiety of the macrocycle during the mechanical bond forming step. Under optimized conditions (CH2Cl2, −78 °C) we were able to increase the stereoselective formation (Rma)-50 (80% de). This understanding allowed us to develop a direct enantioselective synthesis of MAC rotaxane 53 (Scheme 6d) in which the NCHO substituent is too small to prevent exchange of the macrocycle between prochiral compartments. Thus, the MAC stereogenic unit is the only fixed source of stereoisomerism in 53. Coupling of formamide half-axle (R)-52 with 45 and 17 at low temperature resulted in rotaxane (Rma)-53 in up to 67% ee.
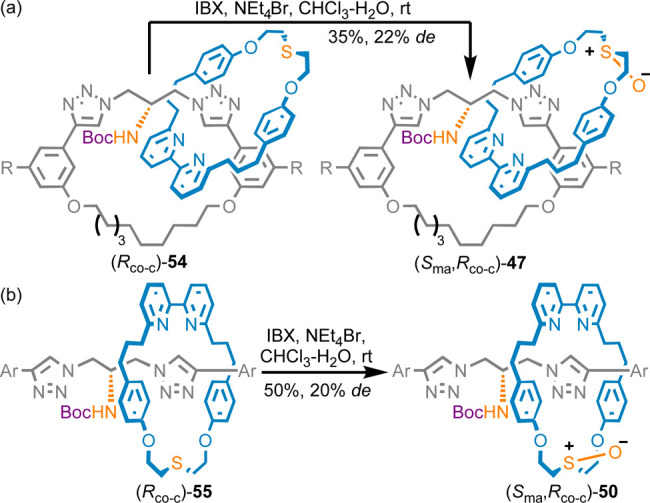
Finally, it is also possible to form the MAC stereogenic unit by taking advantage of co-conformational stereochemistry to control a stereoselective desymmetrization of the faces of one of the components (Scheme 7).2 Catenane (Rco-c)-54, whose co-conformational configuration is fixed, could be oxidized stereoselectively to give diastereomeric catenanes 47. Interestingly, the major stereoisomer, (Sma,Rco-c)-47, was the opposite of that obtained in the direct AT-CuAAC synthesis of the same molecule (Scheme 6b). Similarly, rotaxanes 50 could be accessed stereoselectively by oxidation of rotaxane (Rco-c)-55, again with opposite stereoselectivity to the direct AT-CuAAC approach (Scheme 6c).
Scheme 7. Diastereoselective Oxidation of Co-conformationally Chiral (a) Catenane 54 (R = CO2Me) and (b) Rotaxane 55 (Ar = 3,4-di-tBu-C6H3) with 2-Iodoxybenzoic Acid (IBX)10.
MGI Catenanes and Rotaxanes (Type 1)
Most MGI-1 rotaxanes48 and all reported MGI catenanes49 are derived from calixarene or similar50 macrocycles, where the facial dissymmetry is provided by a fixed cone-shaped conformation. Systems where the facial dissymmetry is provided by a single prochiral center, as proposed by Schill,11 have been largely51 overlooked.
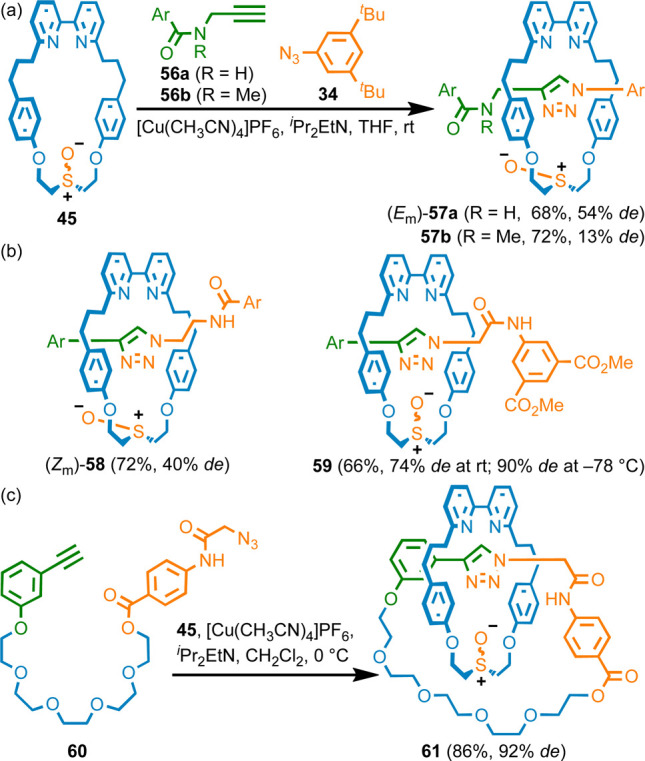
The facial control required for the synthesis of MAC rotaxanes is identical to that required for the synthesis of MGI structures. Thus, we explored the formation of type I MGI rotaxanes using sulfoxide macrocycle 45. As expected, if one of the coupling partners contained an H-bond donor, a much higher stereoselectivity was obtained; coupling of secondary amide-containing alkyne 56a gave rotaxane Em-57a (Scheme 8a) in reasonable selectivity (54% de). When analogous tertiary amide half-axle 56b was used, corresponding rotaxane 57b was produced in low stereoselectivity (13% de). Interestingly, rotaxane Zm-58, which is synthesized from a secondary amide containing azide half-axle, was produced stereoselectively (40% de) but the macrocycle orientation with respect to the triazole is identical to that of 57a (Scheme 8b); although the NH···O interaction appears to be important in directing the reaction, the flexibility of macrocycle 45 means that it is hard to predict a priori the major isomer formed. Rotaxane 59, which was designed to contain a more polarized NH unit was formed in high stereoselectivity at rt (72% de), which increased to 90% de at −40 °C. Using the same approach (Scheme 8c), catenane 61 could be synthesized from pre-macrocycle 60, which also contains an electron deficient amide, in good stereoselectivity (92% de).
Scheme 8. (a) Diastereoselective Synthesis of MGI-1 Rotaxanes 57. (b) MGI-1 Rotaxanes 58 and 59 Synthesized Stereoselectively from 45. (c) Diastereoselective Synthesis of MGI Catenane 61. Ar = 3,4-di-tBu-C6H310.
Synthesis of Type 2 MGI Rotaxanes
Type 2 MGI rotaxanes present an unusual synthetic challenge. First, as with MAC systems (Scheme 6a), retrosynthetic analysis using a direct AT-CuAAC approach (Scheme 9a) highlights that chiral half-axle synthons are almost inevitable, even though the stereogenic unit itself is nonchirotopic. In the forward synthesis, stereoselectivity could arise through interactions between the substituents of the nascent prochiral unit and the bilaterally dissymmetric macrocycle, which is similar to how selectivity arises in the synthesis of MPC rotaxanes. This analysis also highlights that the same diastereomeric mixture will arise whether the starting materials are racemic or enantiopure.
Scheme 9. (a) Schematic Retrosynthesis of the MGI-2 Stereogenic Unit. (b) Attempted Direct Diastereoselective Synthesis of Rotaxanes 62 (Ar = 3,4-di-tBu-C6H3). (c) Chiral Interlocking Auxiliary Synthesis of Rotaxanes 65 (Ar = 3-CO2Me-5-Ph–C6H3)10.
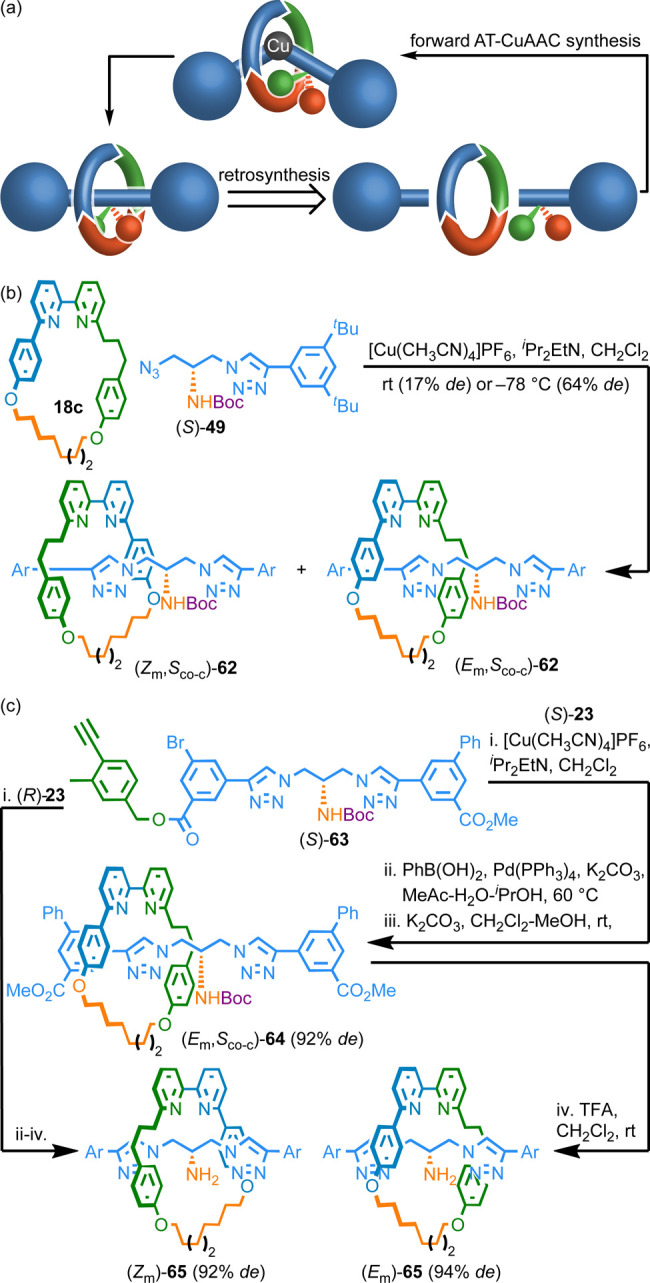
Unfortunately, the direct AT-CuAAC reaction between macrocycle 18c and half-axle (S)-49 to give diastereomeric rotaxanes 62, which contain an MGI-2 stereogenic unit and a co-conformational stereogenic center gave poor selectivity (17% de) at rt (Scheme 9b).21 This is perhaps unsurprising; the desired process resembles the synthesis of MPC rotaxanes, which we have established requires hindered α-chiral azide auxiliaries,42 a problem here as it would necessitate a 1,1 bis-azide building block. Although the selectivity could be improved at the expense of conversion by reducing the reaction temperature, the diastereomers produced were not separable and so we were unable to produce an MGI-2 rotaxane in high stereopurity using this approach.
Thus, we applied our chiral interlocking auxiliary strategy for the synthesis of MPC rotaxanes (Scheme 5), which controls the relative orientation of the axle and macrocycle, to the synthesis of the MGI-2 stereogenic unit (Scheme 9c). Coupling half-axle (S)-63 with α-chiral azide (S)-23 followed by Suzuki coupling and transesterification gave type II MGI rotaxane (Em,Sco-c)-64 in 92% de.52 Removal of the Boc group gave (Em)-65 (94% de) whose only stereochemistry arises from the MGI-2 stereogenic unit. Repeating the same sequence replacing azide (S)-23 with (R)-23 gave (Zm)-65 (92% de). It should be noted that stereoselectivity in this synthesis depends on the stereochemical relationship between the azide and the alkyne half-axle components; unlike in the direct synthesis, using racemic coupling partners would result in an equimolar mixture of the product diastereomers.
Conclusions and Future Directions
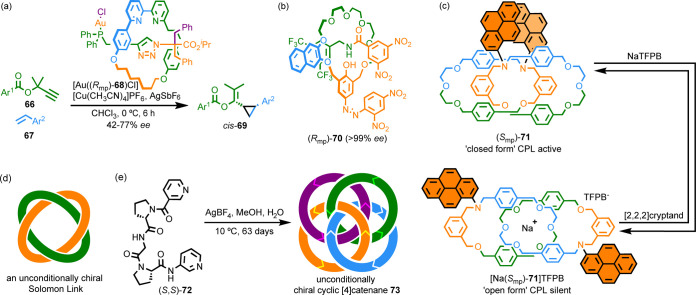
Over the past decade, we have demonstrated that the fundamental mechanical stereogenic units of simple rotaxanes and catenanes can yield to stereoselective synthesis using simple chiral auxiliaries and related approaches. Along the way, we have also identified new mechanical stereogenic units and attempted to systematize the description of mechanically stereogenic molecules, as well as developing self-consistent methods for their assignment. An obvious question of interest to the wider community is what benefits mechanical stereochemistry can bring in chemical applications. We have demonstrated enantioselective catalysis using an MPC rotaxane-based ligand synthesized using our chiral auxiliary strategy (Scheme 10a).4 Others have used CSP-HPLC resolution to produce MPC rotaxanes for the sensing of small organic molecules (Hirose; Scheme 10b),53 chiroptical switching (Schalley)54 and to control the stereochemistry of helical polymers (Takata).55 More recently, Yang, Wang, He, and co-workers reported an MPC catenane that exhibits switchable CPL (Scheme 10c).56 Based on these prototypical examples, there are clearly opportunities to harness mechanical stereochemistry to solve chemical problems.
Scheme 10. (a) MPC Precatalyst [Au(68)Cl]. (b) MPC Rotaxane 70 for the Sensing of Small Chiral Molecules. (c) MPC Catenane 71 that Displays Multistate CPL Switching. (d) Schematic of an Unconditionally Topologically Chiral Solomon Link. (e) Fujita’s Synthesis of Unconditionally Topologically Chiral [4]Catenane 73(10).
If these opportunities are to be realized, further synthetic developments are required. For example, although we have demonstrated general concepts that allow the synthesis of all the fundamental mechanical stereogenic units, we have focused our efforts on the AT-CuAAC reaction because of the synthetic flexibility it brings.39 If we are to make functional molecules, we must broaden the structures that are available for study by applying these concepts to other mechanical bond forming reactions. We note that others have developed complementary stereoselective approaches to MPC rotaxanes; Leigh reported a direct enantioselective synthesis in up to 50% ee using substrate control,57 Kawabata reported a kinetic resolution process that produces an enantiopure product in up to 30% yield58 and Tian and Zhu reported a catalytic enantioselective desymmetrization reaction that proceeds in up to 93% ee.59 The latter examples demonstrate that methods developed for the stereoselective synthesis of covalent structures can be extended to mechanical stereogenic units. Thus, there is clearly an opportunity for those developing stereoselective catalysts to make a significant contribution.
Finally, in tandem with a push toward understanding the benefits mechanical stereochemistry may bring and broadening the generality of the approaches demonstrated so far, it is worth considering where the next stereochemical horizons lie. It is hopefully now clear that synthetic progress goes hand in hand with a proper characterization of stereogenic units; until recently, the structural complexity of even two component interlocked molecules obscured the underlying nature of the stereogenic units that can arise. As the number of crossing points increases, as exemplified by Solomon links (Scheme 10d),60 or the number of interlocked components increases, as in the case of cyclic [n]catenanes (Scheme 10e),61 other opportunities for mechanical stereochemistry arise. Although examples of such systems have been synthesized stereoselectively through the assembly of covalent chiral components, no examples of these structures where the mechanical bond provides the sole source of stereoisomerism have been reported, a similar situation to that which pertained in simple systems when we began our work. Also similarly, the fundamental nature of these stereogenic units is often unclear.
The understanding gained during the studies described in this Account put us in a strong position to attack these next challenges, as well as demonstrating the value in systematizing the discussion of mechanical stereochemistry beyond simple [2]catenanes and rotaxanes. It is our contention that, having established clear definitions of the fundamental mechanical stereogenic units and demonstrated that they can yield to simple synthetic concepts, we have finally reached the end of the beginning, 63 years after Wasserman and Frisch highlighted the potential for mechanical stereochemistry to arise in catenanes—the golden age of mechanical stereochemistry lies ahead!
Acknowledgments
S.M.G thanks the Royal Society (UF130319; RSWF\FT\180010; IE160000; NIF\R1\181686; IEC\R3\193163; NIF\R1\202356), European Union (CoG:724987) and Leverhulme Trust (ORPG-2733) for supporting this research program and the Goldup Group members past and present whose hard work and dedication led to the results described in this Account. Peter R. Gallagher (University of Southampton) is thanked for useful discussions and invaluable assistance in preparing the graphics and electronic Supporting Information.
Biography
Professor Steve Goldup began his independent career in 2009 as a Royal Society University Research Fellow at Queen Mary University of London. In October 2014 the group moved to the University of Southampton where Steve took up the position of Associate Professor before being promoted to Professor of Chemistry in 2017 and appointed as a Royal Society Wolfson Research Fellow in 2019. In 2023 the Goldup Group moved to the University of Birmingham where Steve is Professor of Supramolecular Chemistry leading a team of researchers investigating the synthesis, properties and potential application of mechanically interlocked molecules, including those that display mechanical stereochemistry.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.accounts.4c00195.
Instructions for the assignment of mechanical stereogenic units (PDF)
The author declares no competing financial interest.
Dedication
Dedicated to Professor David Leigh in the year of his 60th birthday.
Supplementary Material
References
- de Juan A.; Lozano D.; Heard A. W.; Jinks M. A.; Suarez J. M.; Tizzard G. J.; Goldup S. M. A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes. Nat. Chem. 2022, 14 (2), 179. 10.1038/s41557-021-00825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. R. J.; Gallagher P.; Lozano D.; Butler P.; Goldup S. M. Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes. Nat. Chem. 2022, 14 (9), 1038. 10.1038/s41557-022-00973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairault N.; Rizzi F.; Lozano D.; Jamieson E. M. G.; Tizzard G. J.; Goldup S. M. A catenane that is topologically achiral despite being composed of oriented rings. Nat. Chem. 2023, 15 (6), 781. 10.1038/s41557-023-01194-1. [DOI] [PubMed] [Google Scholar]
- Heard A. W.; Goldup S. M. Synthesis of a Mechanically Planar Chiral Rotaxane Ligand for Enantioselective Catalysis. Chem 2020, 6 (4), 994. 10.1016/j.chempr.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns C. J.; Stoddart J. F.. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley: 2016. [Google Scholar]
- a Sauvage J. P. From Chemical Topology to Molecular Machines (Nobel Lecture). Angew. Chem., Int. Ed. 2017, 56 (37), 11080. 10.1002/anie.201702992. [DOI] [PubMed] [Google Scholar]; b Stoddart J. F. Mechanically Interlocked Molecules (MIMs)-Molecular Shuttles, Switches, and Machines (Nobel Lecture). Angew. Chem., Int. Ed. 2017, 56 (37), 11094. 10.1002/anie.201703216. [DOI] [PubMed] [Google Scholar]
- Erbas-Cakmak S.; Leigh D. A.; McTernan C. T.; Nussbaumer A. L. Artificial Molecular Machines. Chem. Rev. 2015, 115 (18), 10081. 10.1021/acs.chemrev.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent reviews, see:; a Jamieson E. M. G.; Modicom F.; Goldup S. M. Chirality in rotaxanes and catenanes. Chem. Soc. Rev. 2018, 47 (14), 5266. 10.1039/C8CS00097B. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Evans N. H. Chiral Catenanes and Rotaxanes: Fundamentals and Emerging Applications. Chem.—Eur. J. 2018, 24 (13), 3101. 10.1002/chem.201704149. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pairault N.; Niemeyer J. Chiral Mechanically Interlocked Molecules - Applications of Rotaxanes, Catenanes and Molecular Knots in Stereoselective Chemosensing and Catalysis. Synlett 2018, 29 (6), 689. 10.1055/s-0036-1591934. [DOI] [Google Scholar]; d Nakazono K.; Takata T. Mechanical Chirality of Rotaxanes: Synthesis and Function. Symmetry 2020, 12 (1), 144. 10.3390/sym12010144. [DOI] [Google Scholar]
- Frisch H. L.; Wasserman E. Chemical Topology. J. Am. Chem. Soc. 1961, 83, 3789. 10.1021/ja01479a015. [DOI] [Google Scholar]
- We note that although we have applied a consistent color scheme throughout for aesthetic reasons, only in the schematic diagrams are the colors of different fragments directly related to the method of stereochemical assignment (vectors run from green to orange). In chemical structures, colors are used to aid the reading of the structure and to highlight the underlying symmetry of the components. Although we considered using color to indicate priorities/vectors in the chemical structures, this is challenging or even unhelpful in cases where priorities change over the course of a synthesis. In chemical structures, it should be assumed that the colors used are arbitrary.
- Schill G.Catenanes, Rotaxanes and Knots; Academic Press: New York, 1971. [Google Scholar]
- Bordoli R. J.; Goldup S. M. An efficient approach to mechanically planar chiral rotaxanes. J. Am. Chem. Soc. 2014, 136 (13), 4817. 10.1021/ja412715m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida Y.; Okamoto Y.; Chambron J. C.; Mitchell D. K.; Sauvage J. P. The Separation of Optically-Active Copper (I) Catenates. Tetrahedron Lett. 1993, 34 (6), 1019. 10.1016/S0040-4039(00)77481-8. [DOI] [Google Scholar]
- Yamamoto C.; Okamoto Y.; Schmidt T.; Jager R.; Vogtle F. Enantiomeric resolution of cycloenantiomeric rotaxane, topologically chiral catenane, and pretzel-shaped molecules: Observation of pronounced circular dichroism. J. Am. Chem. Soc. 1997, 119 (43), 10547. 10.1021/ja971764q. [DOI] [Google Scholar]
- Takata and Okamoto reported an attempted stereoselective synthesis of an MPC rotaxane that proceeded in 4% ee. This represents the first stereoselective synthesis of such molecules but is obviously not synthetically useful:; Makita Y.; Kihara N.; Nakakoji N.; Takata T.; Inagaki S.; Yamamoto C.; Okamoto Y. Catalytic Asymmetric Synthesis and Optical Resolution of Planar Chiral Rotaxane. Chem. Lett. 2007, 36, 162. 10.1246/cl.2007.162. [DOI] [Google Scholar]
- Arduini A.; Ciesa F.; Fragassi M.; Pochini A.; Secchi A. Selective synthesis of two constitutionally isomeric oriented calix[6]arene-based rotaxanes. Angew. Chem., Int. Ed. 2004, 44 (2), 278. 10.1002/anie.200461336. [DOI] [PubMed] [Google Scholar]
- IUPAC have noted that several definitions of prochirality are in common use (IUPAC Gold Book: https://goldbook.iupac.org/terms/view/P04859, accessed 25/3/2024). Here, and throughout, we use the term prochiral to mean an achiral structure that becomes chiral if an existing atom/group is replaced by different one.
- McArdle C. P.; Van S.; Jennings M. C.; Puddephatt R. J. Gold(I) macrocycles and topologically chiral [2]catenanes. J. Am. Chem. Soc. 2002, 124 (15), 3959. 10.1021/ja012006+. [DOI] [PubMed] [Google Scholar]
- Lahlali H.; Jobe K.; Watkinson M.; Goldup S. M. Macrocycle size matters: ″small″ functionalized rotaxanes in excellent yield using the CuAAC active template approach. Angew. Chem., Int. Ed. 2011, 50 (18), 4151. 10.1002/anie.201100415. [DOI] [PubMed] [Google Scholar]
- Gaeta C.; Talotta C.; Mirra S.; Margarucci L.; Casapullo A.; Neri P. Catenation of calixarene annulus. Org. Lett. 2013, 15 (1), 116. 10.1021/ol303142c. [DOI] [PubMed] [Google Scholar]
- Savoini A.; Gallagher P. R.; Saady A.; Goldup S. M. The Final Stereogenic Unit of [2]Rotaxanes: Type 2 Geometric Isomers. J. Am. Chem. Soc. 2024, 146 (12), 8472. 10.1021/jacs.3c14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flapan E.When Topology Meets Chemistry; Cambridge University Press: 2012. [Google Scholar]
- Gallagher P. R.; Savoini A.; Saady A.; Maynard J. R. J.; Butler P. W. V.; Tizzard G. J.; Goldup S. M. Facial Selectivity in Mechanical Bond Formation: Axially Chiral Enantiomers and Geometric Isomers from a Simple Prochiral Macrocycle. J. Am. Chem. Soc. 2024, 146 (13), 9134. 10.1021/jacs.3c14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn R. S.; Ingold C.; Prelog V. Specification of Molecular Chirality. Angew. Chem., Int. Ed. 1966, 5 (4), 385. 10.1002/anie.196603851. [DOI] [Google Scholar]
- The MPC stereogenic unit can also be assigned by viewing the vector of one component passing through the oriented ring away from the observer.8a,12,43 This “steering wheel” approach gives the same result as the skew line formalism, and so these methods are equivalent (see SI for an example).
- Isnin R.; Kaifer A. E. A New Approach to Cyclodextrin-Based Rotaxanes. Pure Appl. Chem. 1993, 65 (3), 495. 10.1351/pac199365030495. [DOI] [Google Scholar]
- Armspach D.; Ashton P. R.; Ballardini R.; Balzani V.; Godi A.; Moore C. P.; Prodi L.; Spencer N.; Stoddart J. F.; Tolley M. S.; Wear T. J.; Williams D. J.; Stoddart J. F. Catenated Cyclodextrins. Chem.—Eur. J. 1995, 1 (1), 33. 10.1002/chem.19950010109. [DOI] [Google Scholar]
- Schroder H. V.; Zhang Y.; Link A. J. Dynamic covalent self-assembly of mechanically interlocked molecules solely made from peptides. Nat. Chem. 2021, 13 (9), 850. 10.1038/s41557-021-00770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak Y.; Erbas-Cakmak S.; Leigh D. A. Asymmetric Catalysis with a Mechanically Point-Chiral Rotaxane. J. Am. Chem. Soc. 2016, 138 (6), 1749. 10.1021/jacs.6b00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corra S.; de Vet C.; Baroncini M.; Credi A.; Silvi S. Stereodynamics of E/Z isomerization in rotaxanes through mechanical shuttling and covalent bond rotation. Chem 2021, 7 (8), 2137. 10.1016/j.chempr.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki Y.; Ikeyatsu K.; Mutoh Y.; Hosoya S.; Saito S. Synthesis of Mechanically Planar Chiral rac-[2]Rotaxanes by Partitioning of an Achiral [2]Rotaxane: Stereoinversion Induced by Shuttling. Org. Lett. 2017, 19 (16), 4347. 10.1021/acs.orglett.7b02043. [DOI] [PubMed] [Google Scholar]
- The diastereomers of 11 cannot interchange because the rings cannot shuttle past one another. Although this is typically the case, Loeb and co-workers reported a rotaxane in which the rings can shuttle past one another:; Zhu K.; Baggi G.; Loeb S. J. Ring-through-ring molecular shuttling in a saturated [3]rotaxane. Nat. Chem. 2018, 10 (6), 625. 10.1038/s41557-018-0040-9. [DOI] [PubMed] [Google Scholar]
- Schmieder R.; Hubner G.; Seel C.; Vogtle F. The first cyclodiasteromeric [3]rotaxane. Angew. Chem., Int. Ed. 1999, 38 (23), 3528.. [DOI] [PubMed] [Google Scholar]
- Alvarez-Perez M.; Goldup S. M.; Leigh D. A.; Slawin A. M. A chemically-driven molecular information ratchet. J. Am. Chem. Soc. 2008, 130 (6), 1836. 10.1021/ja7102394. [DOI] [PubMed] [Google Scholar]
- Liu E.; Cherraben S.; Boulo L.; Troufflard C.; Hasenknopf B.; Vives G.; Sollogoub M. A molecular information ratchet using a cone-shaped macrocycle. Chem 2023, 9 (5), 1147. 10.1016/j.chempr.2022.12.017. [DOI] [Google Scholar]
- Aprahamian I.; Goldup S. M. Non-equilibrium Steady States in Catalysis, Molecular Motors, and Supramolecular Materials: Why Networks and Language Matter. J. Am. Chem. Soc. 2023, 145 (26), 14169. 10.1021/jacs.2c12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. R. J.; Goldup S. M. Strategies for the Synthesis of Enantiopure Mechanically Chiral Molecules. Chem 2020, 6 (8), 1914. 10.1016/j.chempr.2020.07.012. [DOI] [Google Scholar]
- Denis M.; Goldup S. M. The active template approach to interlocked molecules. Nat. Rev. Chem. 2017, 1 (8), 0061. 10.1038/s41570-017-0061. [DOI] [Google Scholar]
- Saady A.; Goldup S. M. Triazole formation and the click concept in the synthesis of interlocked molecules. Chem 2023, 9 (8), 2110. 10.1016/j.chempr.2023.07.001. [DOI] [Google Scholar]
- Aucagne V.; Hanni K. D.; Leigh D. A.; Lusby P. J.; Walker D. B. Catalytic ″click″ rotaxanes: a substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 2006, 128 (7), 2186. 10.1021/ja056903f. [DOI] [PubMed] [Google Scholar]
- Lewis J. E. M.; Bordoli R. J.; Denis M.; Fletcher C. J.; Galli M.; Neal E. A.; Rochette E. M.; Goldup S. M. High yielding synthesis of 2,2′-bipyridine macrocycles, versatile intermediates in the synthesis of rotaxanes. Chem. Sci. 2016, 7, 3154. 10.1039/C6SC00011H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenis M. A. J.; Chibueze C. S.; Jinks M. A.; Nicu V. P.; Visscher L.; Goldup S. M.; Buma W. J. Vibrational circular dichroism spectroscopy for probing the expression of chirality in mechanically planar chiral rotaxanes. Chemical Science 2020, 11 (32), 8469. 10.1039/D0SC02485F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks M. A.; de Juan A.; Denis M.; Fletcher C. J.; Galli M.; Jamieson E. M. G.; Modicom F.; Zhang Z.; Goldup S. M. Stereoselective Synthesis of Mechanically Planar Chiral Rotaxanes. Angew. Chem., Int. Ed. 2018, 57 (45), 14806. 10.1002/anie.201808990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M.; Lewis J. E. M.; Modicom F.; Goldup S. M. An Auxiliary Approach for the Stereoselective Synthesis of Topologically Chiral Catenanes. Chem 2019, 5 (6), 1512. 10.1016/j.chempr.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rubio A.; Savoini A.; Modicom F.; Butler P.; Goldup S. M. A Co-conformationally ″Topologically″ Chiral Catenane. J. Am. Chem. Soc. 2022, 144 (27), 11927. 10.1021/jacs.2c02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Rodríguez-Rubio A.; Saady A.; Tizzard G. J.; Goldup S. M. A chiral macrocycle for the stereoselective synthesis of mechanically planar chiral rotaxanes and catenanes. Chem 2023, 9 (5), 1195. 10.1016/j.chempr.2023.01.009. [DOI] [Google Scholar]
- Lewis J. E.; Winn J.; Cera L.; Goldup S. M. Iterative Synthesis of Oligo[n]rotaxanes in Excellent Yield. J. Am. Chem. Soc. 2016, 138 (50), 16329. 10.1021/jacs.6b08958. [DOI] [PubMed] [Google Scholar]
- The exception to this general statement is if the axle is divided at the prochiral center itself such that the reaction centre is converted from sp2 to sp3 in the forward synthesis. Frustratingly, the half-axle that contains the sp2 hybridized center can also be described as prochiral but, confusingly, this is an alternative definition of the same word!17
- Selected examples:; a Arduini A.; Ciesa F.; Fragassi M.; Pochini A.; Secchi A. Selective synthesis of two constitutionally isomeric oriented calix[6]arene-based rotaxanes. Angew. Chem., Int. Ed. 2005, 44 (2), 278. 10.1002/anie.200461336. [DOI] [PubMed] [Google Scholar]; b Arduini A.; Bussolati R.; Credi A.; Faimani G.; Garaudee S.; Pochini A.; Secchi A.; Semeraro M.; Silvi S.; Venturi M. Towards controlling the threading direction of a calix[6]arene wheel by using nonsymmetric axles. Chem.—Eur. J. 2009, 15 (13), 3230. 10.1002/chem.200801926. [DOI] [PubMed] [Google Scholar]; c Pierro T.; Gaeta C.; Talotta C.; Casapullo A.; Neri P. Fixed or invertible calixarene-based directional shuttles. Org. Lett. 2011, 13 (10), 2650. 10.1021/ol200753c. [DOI] [PubMed] [Google Scholar]; d Arduini A.; Bussolati R.; Credi A.; Secchi A.; Silvi S.; Semeraro M.; Venturi M. Toward directionally controlled molecular motions and kinetic intra- and intermolecular self-sorting: threading processes of nonsymmetric wheel and axle components. J. Am. Chem. Soc. 2013, 135 (26), 9924. 10.1021/ja404270c. [DOI] [PubMed] [Google Scholar]; e Ciao R.; Talotta C.; Gaeta C.; Margarucci L.; Casapullo A.; Neri P. An oriented handcuff rotaxane. Org. Lett. 2013, 15 (22), 5694. 10.1021/ol4026974. [DOI] [PubMed] [Google Scholar]; f Zanichelli V.; Ragazzon G.; Arduini A.; Credi A.; Franchi P.; Orlandini G.; Venturi M.; Lucarini M.; Secchi A.; Silvi S. Synthesis and Characterization of Constitutionally Isomeric Oriented Calix[6]arene-Based Rotaxanes. Eur. J. Org. Chem. 2016, 2016 (5), 1033. 10.1002/ejoc.201501522. [DOI] [Google Scholar]; g La Manna P.; Talotta C.; Gaeta C.; Soriente A.; De Rosa M.; Neri P. Threading of an Inherently Directional Calixarene Wheel with Oriented Ammonium Axles. J. Org. Chem. 2017, 82 (17), 8973. 10.1021/acs.joc.7b01388. [DOI] [PubMed] [Google Scholar]; h Bazzoni M.; Andreoni L.; Silvi S.; Credi A.; Cera G.; Secchi A.; Arduini A. Selective access to constitutionally identical, orientationally isomeric calix[6]arene-based [3]rotaxanes by an active template approach. Chem. Sci. 2021, 12 (18), 6419. 10.1039/D1SC00279A. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Cera G.; Arduini A.; Secchi A.; Credi A.; Silvi S. Heteroditopic Calix[6]arene Based Intervowen and Interlocked Molecular Devices. Chem. Rec. 2021, 21 (5), 1161. 10.1002/tcr.202100012. [DOI] [PubMed] [Google Scholar]; j Andreoni L.; Bonati F. C.; Groppi J.; Balestri D.; Cera G.; Credi A.; Secchi A.; Silvi S. Selective enhancement of organic dye properties through encapsulation in rotaxane orientational isomers. Chem. Commun. 2023, 59 (33), 4970. 10.1039/D3CC01135F. [DOI] [PubMed] [Google Scholar]
- a Gaeta C.; Talotta C.; Mirra S.; Margarucci L.; Casapullo A.; Neri P. Catenation of calixarene annulus. Org. Lett. 2013, 15 (1), 116. 10.1021/ol303142c. [DOI] [PubMed] [Google Scholar]; b Zanichelli V.; Dallacasagrande L.; Arduini A.; Secchi A.; Ragazzon G.; Silvi S.; Credi A. Electrochemically Triggered Co-Conformational Switching in a [2]catenane Comprising a Non-Symmetric Calix[6]arene Wheel and a Two-Station Oriented Macrocycle. Molecules 2018, 23 (5), 1156. 10.3390/molecules23051156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected examples:; a Xue M.; Su Y. S.; Chen C. F. Isomeric squaraine-based [2]pseudorotaxanes and [2]rotaxanes: synthesis, optical properties, and their tubular structures in the solid state. Chem.—Eur. J. 2010, 16 (28), 8537. 10.1002/chem.201000773. [DOI] [PubMed] [Google Scholar]; b Xia Y.-X.; Xie T.; Han Y.; Chen C.-F. Triptycene-derived calix[6]arene analogues: synthesis, structure and complexation with paraquat derivatives. Org. Chem. Front. 2014, 1 (2), 140. 10.1039/c3qo00055a. [DOI] [Google Scholar]; c Wang H. X.; Meng Z.; Xiang J. F.; Xia Y. X.; Sun Y.; Hu S. Z.; Chen H.; Yao J.; Chen C. F. Guest-dependent directional complexation based on triptycene derived oxacalixarene: formation of oriented rotaxanes. Chem. Sci. 2016, 7 (1), 469. 10.1039/C5SC03511B. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Cui J. S.; Ba Q. K.; Ke H.; Valkonen A.; Rissanen K.; Jiang W. Directional Shuttling of a Stimuli-Responsive Cone-Like Macrocycle on a Single-State Symmetric Dumbbell Axle. Angew. Chem., Int. Ed. 2018, 57 (26), 7809. 10.1002/anie.201803349. [DOI] [PubMed] [Google Scholar]; e Li K. A.; Wang Z.; Xie C. D.; Chen T.; Qiang H.; Liu Y. A.; Jia X. S.; Hu W. B.; Wen K. Unidirectional complexation of pillar[4]arene[1]benzoquinoneoxime with alkyl alcohols. Org. Biomol. Chem. 2019, 17 (20), 4975. 10.1039/C9OB00665F. [DOI] [PubMed] [Google Scholar]
- a Saito F.; Bode J. W. Synthesis and stabilities of peptide-based [1]rotaxanes: molecular grafting onto lasso peptide scaffolds. Chem. Sci. 2017, 8 (4), 2878. 10.1039/C7SC00021A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b McCarthy D. R.; Xu K.; Schenkelberg M. E.; Balegamire N. A. N.; Liang H.; Bellino S. A.; Li J.; Schneebeli S. T. Kinetically controlled synthesis of rotaxane geometric isomers. Chem. Sci. 2024, 15, 4860. 10.1039/D3SC04412B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- We note that the stereochemistry of 65 can specified with two of three possible stereolabels: MGI-2/co-conformational covalent, MGI-2, co-conformational MPC or co-conformational covalent/co-conformational MPC. Of these we prefer the MGI-2/co-conformational covalent description as it highlights the fixed MGI-2 stereochemistry of the molecule.
- Hirose K.; Ukimi M.; Ueda S.; Onoda C.; Kano R.; Tsuda K.; Hinohara Y.; Tobe Y. The Asymmetry is Derived from Mechanical Interlocking of Achiral Axle and Achiral Ring Components -Syntheses and Properties of Optically Pure [2]Rotaxanes. Symmetry 2018, 10 (1), 20. 10.3390/sym10010020. [DOI] [Google Scholar]
- Gaedke M.; Witte F.; Anhauser J.; Hupatz H.; Schroder H. V.; Valkonen A.; Rissanen K.; Lutzen A.; Paulus B.; Schalley C. A. Chiroptical inversion of a planar chiral redox-switchable rotaxane. Chem. Sci. 2019, 10 (43), 10003. 10.1039/C9SC03694F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwari F.; Nakazono K.; Koyama Y.; Takata T. Induction of Single-Handed Helicity of Polyacetylenes Using Mechanically Chiral Rotaxanes as Chiral Sources. Angew. Chem., Int. Ed. 2017, 56 (47), 14858. 10.1002/anie.201707926. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gong J.; Wang X.; Li W. J.; Wang X. Q.; He X.; Wang W.; Yang H. B. Multistate Circularly Polarized Luminescence Switching through Stimuli-Induced Co-Conformation Regulations of Pyrene-Functionalized Topologically Chiral [2]Catenane. Angew. Chem., Int. Ed. 2022, 61, e202210542 10.1002/anie.202210542. [DOI] [PubMed] [Google Scholar]
- Tian C.; Fielden S. D. P.; Perez-Saavedra B.; Vitorica-Yrezabal I. J.; Leigh D. A. Single-Step Enantioselective Synthesis of Mechanically Planar Chiral [2]Rotaxanes Using a Chiral Leaving Group Strategy. J. Am. Chem. Soc. 2020, 142 (21), 9803. 10.1021/jacs.0c03447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi A.; Lakshmi B. V.; Ueda Y.; Yoshimura T.; Matayoshi A.; Furuta T.; Kawabata T. Enantioselective preparation of mechanically planar chiral rotaxanes by kinetic resolution strategy. Nat. Commun. 2021, 12 (1), 404. 10.1038/s41467-020-20372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. F.; Chia X. L.; Tian C.; Zhu Y. Mechanically planar chiral rotaxanes through catalytic desymmetrization. Chem 2022, 8 (10), 2843. 10.1016/j.chempr.2022.08.009. [DOI] [Google Scholar]
- Selected examples:; a Lincheneau C.; Jean-Denis B.; Gunnlaugsson T. Self-assembly formation of mechanically interlocked [2]- and [3]catenanes using lanthanide ion [Eu(III)] templation and ring closing metathesis reactions. Chem. Commun. 2014, 50 (22), 2857. 10.1039/c3cc49640f. [DOI] [PubMed] [Google Scholar]; b Zhu R.; Lubben J.; Dittrich B.; Clever G. H. Stepwise halide-triggered double and triple catenation of self-assembled coordination cages. Angew. Chem., Int. Ed. 2015, 54 (9), 2796. 10.1002/anie.201408068. [DOI] [PubMed] [Google Scholar]; c Wood C. S.; Ronson T. K.; Belenguer A. M.; Holstein J. J.; Nitschke J. R. Two-stage directed self-assembly of a cyclic [3]catenane. Nat. Chem. 2015, 7 (4), 354. 10.1038/nchem.2205. [DOI] [PubMed] [Google Scholar]; d Sawada T.; Yamagami M.; Ohara K.; Yamaguchi K.; Fujita M. Peptide [4]Catenane by Folding and Assembly. Angew. Chem., Int. Ed. 2016, 55 (14), 4519. 10.1002/anie.201600480. [DOI] [PubMed] [Google Scholar]; e Feng T.; Li X.; An Y. Y.; Bai S.; Sun L. Y.; Li Y.; Wang Y. Y.; Han Y. F. Backbone-Directed Self-Assembly of Interlocked Molecular Cyclic Metalla[3]Catenanes. Angew. Chem., Int. Ed. 2020, 59 (32), 13516. 10.1002/anie.202004112. [DOI] [PubMed] [Google Scholar]; f Cui Z.; Gao X.; Lin Y. J.; Jin G. X. Stereoselective Self-Assembly of Complex Chiral Radial [5]Catenanes Using Half-Sandwich Rhodium/Iridium Building Blocks. J. Am. Chem. Soc. 2022, 144 (5), 2379. 10.1021/jacs.1c13168. [DOI] [PubMed] [Google Scholar]
- Selected examples:; a Nierengarten J. F.; Dietrich-Buchecker C. O.; Sauvage J. P. Synthesis of a doubly interlocked [2]-catenane. J. Am. Chem. Soc. 1994, 116 (1), 375. 10.1021/ja00080a045. [DOI] [PubMed] [Google Scholar]; b Pentecost C. D.; Chichak K. S.; Peters A. J.; Cave G. W.; Cantrill S. J.; Stoddart J. F. A molecular solomon link. Angew. Chem., Int. Ed. 2007, 46 (1–2), 218. 10.1002/anie.200603521. [DOI] [PubMed] [Google Scholar]; c Schouwey C.; Holstein J. J.; Scopelliti R.; Zhurov K. O.; Nagornov K. O.; Tsybin Y. O.; Smart O. S.; Bricogne G.; Severin K. Self-assembly of a giant molecular Solomon link from 30 subcomponents. Angew. Chem., Int. Ed. 2014, 53 (42), 11261. 10.1002/anie.201407144. [DOI] [PubMed] [Google Scholar]; d Beves J. E.; Danon J. J.; Leigh D. A.; Lemonnier J. F.; Vitorica-Yrezabal I. J. A Solomon link through an interwoven molecular grid. Angew. Chem., Int. Ed. 2015, 54 (26), 7555. 10.1002/anie.201502095. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Cui Z.; Lu Y.; Gao X.; Feng H. J.; Jin G. X. Stereoselective Synthesis of a Topologically Chiral Solomon Link. J. Am. Chem. Soc. 2020, 142 (32), 13667. 10.1021/jacs.0c05366. [DOI] [PubMed] [Google Scholar]; f August D. P.; Jaramillo-Garcia J.; Leigh D. A.; Valero A.; Vitorica-Yrezabal I. J. A Chiral Cyclometalated Iridium Star of David [2]Catenane. J. Am. Chem. Soc. 2021, 143 (2), 1154. 10.1021/jacs.0c12038. [DOI] [PubMed] [Google Scholar]; g Feng H.-N.; Sun Z.; Chen S.; Zhang Z.-H.; Li Z.; Zhong Z.; Sun T.; Ma Y.; Zhang L. A Star of David [2]catenane of single handedness. Chem 2023, 9 (4), 859. 10.1016/j.chempr.2022.11.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.