Abstract
Background:
Video-oculography constitutes a highly-sensitive method of characterizing ocular movements, which could detect subtle premotor changes and contribute to the early diagnosis of Parkinson’s disease (PD).
Objective:
To investigate potential oculomotor differences between idiopathic PD (iPD) and PD associated with the G2019S variant of LRRK2 (L2PD), as well as to evaluate oculomotor function in asymptomatic carriers of the G2019S variant of LRRK2.
Methods:
The study enrolled 129 subjects: 30 PD (16 iPD, 14 L2PD), 23 asymptomatic carriers, 13 non-carrier relatives of L2PD patients, and 63 unrelated HCs. The video-oculographic evaluation included fixation, prosaccade, antisaccade, and memory saccade tests.
Results:
We did not find significant differences between iPD and L2PD. Compared to controls, PD patients displayed widespread oculomotor deficits including larger microsaccades, hypometric vertical prosaccades, increased latencies in all tests, and lower percentages of successful antisaccades and memory saccades. Non-carrier relatives showed oculomotor changes with parkinsonian features, such as fixation instability and hypometric vertical saccades. Asymptomatic carriers shared multiple similarities with PD, including signs of unstable fixation and hypometric vertical prosaccades; however, they were able to reach percentages of successful antisaccade and memory saccades similar to controls, although at the expense of longer latencies. Classification accuracy of significant oculomotor parameters to differentiate asymptomatic carriers from HCs ranged from 0.68 to 0.74, with BCEA, a marker of global fixation instability, being the parameter with the greatest classification accuracy.
Conclusions:
iPD and LRRK2-G2019S PD patients do not seem to display a differential oculomotor profile. Several oculomotor changes in asymptomatic carriers of LRRK2 mutations could be considered premotor biomarkers.
Keywords: Parkinson’s disease, LRRK2, oculomotor, early diagnosis, premotor, microsaccade, antisaccade, eye-tracking
INTRODUCTION
Eye movements are the result of the integrated work of several regions of the cerebral cortex and subcortical structures. Thus, in different pathological processes and depending on the damaged brain structures, oculomotor alterations can be relatively distinct and can be used with varying accuracy for diagnostic or monitoring purposes [1]. In the field of movement disorders, ocular movements are of particular interest because of the direct involvement of the basal ganglia in their generation [2]. Clinical oculomotor examination is limited by its qualitative nature and low sensitivity; however, non-invasive infrared eye-tracking systems with high temporal and spatial resolution can provide accurate data for detecting subtle changes that can be used as biomarkers of the pathological process. To that end, different aspects of oculomotor function can be assessed, such as visually-guided saccades or prosaccades, intentional saccades, and fixation stability. Prosaccades are quick ocular movements triggered by an external visual stimulus that aim to fix the target in the fovea. On the other hand, intentional saccades, such as antisaccades or memory saccades, represent more complex responses, since they are generated according to a given cognitive demand; therefore, their elaboration involves different cognitive processes such as working memory, inhibition, and error monitoring [3]. Thus, both prosaccades and intentional saccades are generated by a fronto-parietal network with projections to the basal ganglia and the superior colliculus, the saccade trigger; however, intentional saccades require the support of additional regions, including the dorsolateral prefrontal cortex or the dorsal striatum [3]. Additionally, fixation can be evaluated by describing spontaneous ocular movements that interrupt fixation, such as microsaccades and square wave jerks (SWJs). Microsaccades are small ocular movements that shift the eye position less than 1° and occur at a typical rate of 1–2 Hz [4]. SWJ is defined as a short amplitude, conjugated ocular movement which moves the eye away from the fixation target, followed by a return saccade that shifts the eye to the initial position. In biphasic SWJs, the return saccade exceeds the initial fixation point, which requires a second return saccade to move the eye to the fixation target. Currently, both microsaccades and SWJ are considered as part of the same physiological continuum and, while they occur in healthy individuals, also increase in several neurological disorders.
In Parkinson’s disease (PD), prosaccades and intentional saccades have been the most studied. Prosaccades are characteristically hypometric with increased latency, more so in the vertical plane, whereas intentional saccades show an increased error rate [2, 5–10]. Visual fixation has been rarely studied in PD, but some works describe more frequent and larger fixational ocular movements [11–14]. In addition, oculomotor performance has been correlated with disease progression and cognitive status [15, 16]. Importantly, some of these oculomotor changes were identified from the earliest stages of PD, in de novo patients and even in at-risk individuals [6–8]. Given that video-oculography is able to evidence these changes in a highly accurate and quantitative manner, oculomotor dysfunction may be considered a promising subclinical biomarker to characterize and monitor the progression of the disease even in preclinical stages.
One of the most accessible settings to study biomarkers of the preclinical stages of PD are unaffected carriers of mutations in the leucine-rich repeat kinase 2 (LRRK2) gene, known to be at increased risk for PD. LRRK2 mutations are the most frequent cause of genetic parkinsonism, being the G2019S mutation the commonest known cause [17]. The G2019S mutation of LRRK2 shows an age-dependent penetrance and is associated to a form of the disease clinically and pathologically indistinguishable from idiopathic PD. However, oculomotor behavior has not been studied in this population.
Our aim here is to demonstrate whether oculomotor abnormalities, in addition to reflecting an established PD state, could also reflect subclinical involvement in asymptomatic carriers of the G2019S variant of LRRK2. We also want to test whether idiopathic PD (iPD) and PD associated with the G2019S variant of LRRK2 (L2PD) have similar oculomotor behavior.
MATERIALS AND METHODS
Participants
The study included 5 groups of participants: iPD, L2PD, asymptomatic carriers of LRRK2 G2019S mutation or nonmanifesting carriers (L2NMC), non-carrier relatives of L2PD patients, and unrelated healthy controls (HCs). All individuals were Caucasian and came from Cantabria, a region in northern Spain. Participants with iPD, L2PD, L2NMC, and non-carrier relatives of L2PD were previously enrolled in a local PD research cohort described elsewhere [18], and were consecutively invited to participate in the present study. Participants were evaluated with the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III), in “on” condition in individuals with PD. Global cognitive function was assessed using the Montreal Cognitive Assessment (MoCA). Patients with iPD and L2PD had to meet current criteria of PD diagnosis as inclusion criteria for the study [19]. In patients with iPD, the most frequent mutations in LRRK2 (G2019S, R1441G, and R1441C) and GBA (N370S and L444P) genes were ruled out. LRRK2 G2019S mutation carriers were considered as nonmanifesting carriers (L2NMC) when they did not have a diagnosis nor manifested signs of PD (exclusion criteria when UPDRS-III > 4). In addition, we included a separate group of non-carrier relatives of L2PD based on the rationale of previous works that report a higher prevalence of PD markers in these individuals than in general population, suggesting the presence of genetic or environmental factors different from the LRRK2 mutation that are shared with affected relatives [20]. Non-carrier relatives of L2PD were excluded if they had a diagnosis or manifested signs of PD (UPDRS-III > 4). For all groups, other exclusion criteria were a clinical diagnosis of dementia or any other neurological condition such as cerebrovascular disease or traumatic brain injury, as well as severe visual loss that could interfere with the oculomotor evaluation. Ophthalmologic pathologies (such as cataracts) with preserved visual acuity were not considered exclusion criteria, since the oculographic device has been tested in these conditions with adequateperformance [21].
Unrelated HCs were participants in the Valdecilla Study of Memory and Brain Aging, a prospective cohort that aims to investigate healthy brain aging and the early stages of neurodegenerative diseases. Participants are community-dwelling individuals older than 55 and living in Cantabria. Exclusionary criteria are a previous diagnosis of dementia or any other cognitive disorder, PD, cerebrovascular disease, or any other neurological or medical condition that might interfere with cognition. The study protocol includes a clinical interview and a neurological examination; oculomotor and neuropsychological evaluations; a brain MRI; and collection of plasma and cerebrospinal fluid samples. Cohort participants with normal neuropsychological and brain MRI exams and normal levels of Alzheimer’s disease biomarkers in cerebrospinal fluid (including amyloid-β1–42/1 –40 ratio, P-tau181, and total tau) were selected as a healthy control group to compare their oculomotor evaluation with the groups of PD, L2NMC, and non-carrier relatives.
The same research team examined all participants and all tests were carried out at the Marqués de Valdecilla University Hospital. The study was approved by our local Ethics Committee (Comité Ético de Investigación de Cantabria) and all subjects gave their written informed consent according to the Declaration of Helsinki.
Oculomotor evaluation
All participants underwent a similar oculomotor evaluation with the same device and following the same protocol [22]. Preserved visual acuity was confirmed before the evaluation. Subjects were tested with glasses off. Patients with PD were tested in ON condition. Evaluations were conducted with OSCANN (Aura Innovative Robotics, SL), an eye-tracker device based on video-oculography technology [21]. Visual targets were bright green circles of 2 centimeters in diameter displayed on a dark gray background on a screen 60 centimeters away from the subject. Each oculomotor test was preceded by practice trials that allowed to confirm the understanding of the task, followed by a nine-point calibration. Parameters used to describe the oculomotor performance were calculated according to the published methodology [23]. The evaluation included fourtests:
Fixation test: In this test, the target remained static in the center of the screen for 10 seconds, and subjects were asked to keep their gaze on it as still as possible. This test allows assessing fixation stability through the detection of superimposed ocular movements, such as microsaccades or SWJs. Oculomotor parameters evaluated in this test were the number, amplitude, and peak velocity of microsaccades; the number of biphasic SWJs; and the Bivariate Contour Ellipse Area (BCEA). BCEA represents the area around the target in which 68% of the gaze fixations are framed [24]. This way, the BCEA parameter is a marker of fixation stability, with greater values indicative of more scattered fixations.
Prosaccade test: This test included 12 horizontal trials and 8 vertical trials. Each trial started with a central fixation target, which was then replaced by the random appearance of eccentric targets at 5, 10, or 20 degrees to the right or left in horizontal trials; and targets at 5 or 12 degrees up or down in vertical trials. After a latency period of 3000 ms, each peripheral target was substituted by the reappearance of the central target. Subjects were asked to look at targets as fast as possible. We evaluated saccadic latency (defined as the time delay between the appearance of a peripheral target and the onset of the ocular movement); saccadic peak velocity; and spatial error (defined as the deviation of the final gaze position from the target, measured as positive or negative error).
Antisaccade test: This test was structured exactly as the prosaccade test, but the instructions were: “When the target appears at one side, look at the opposite location, in a mirrored way. If you realize that you have looked at the target, try to correct yourself by looking at the opposite location.” The oculomotor parameters were the percentage of corrective antisaccades (defined as an antisaccade performed after an erroneous saccade towards the target), the percentage of successful antisaccades (representing the sum of correct and corrective antisaccades), and corrective antisaccade latency (defined as the time delay between the appearance of a peripheral target and the onset of a corrective antisaccade).
Memory saccade test: Similarly to the prosaccade test, the target appeared eccentrically and was subsequently replaced by the central target. Then, the central target disappeared and the screen remained blank. The directions were: “Keep your gaze fixed on the target when it appears at one side and when it comes back to the center. When the central target disappears, look at the location where it had previously appeared”. The analyzed parameters were the percentage of correct memory saccades and the correct memory saccade latency.
123I-ioflupane SPECT (DaT-SPECT)
DaT-SPECT imaging was performed in the L2NMC group, with image acquisition as described elsewhere [25]. Scans were reviewed by expert faculty from the Department of Nuclear Medicine and rated as normal or reduced uptake based on visual inspection.
Statistical analysis
Demographic characteristics were compared among study groups with ANOVA for quantitative variables, and χ2 for qualitative variables. To compare oculomotor performance between groups, we used a T-test and a multivariate analysis with General Linear Models, with the oculomotor parameter as the dependent variable and age and sex as covariates. The false discovery rate (FDR) method was applied to correct for multiple comparisons. In order to investigate potential correlations between oculomotor parameters and other parameters associated to the disease, such as UPDRS-III and MoCA, we used Pearson’s r and a linear regression analysis with age and sex as covariates. Finally, we explored the diagnostic utility of oculomotor parameters to differentiate L2NMC from HCs with ROC curves. Differences between groups were considered statistically significant when p < 0.05. Analyses were carried out with The Statistical Packages for Social Sciences (SPSS 26.0.0.0).
RESULTS
Demographics
The study population consisted of 129 participants, composed of 30 PD (16 iPD and 14 L2PD), 23 asymptomatic carriers of LRRK2 G2019S mutation or nonmanifesting carriers (L2NMC), 13 non-carrier relatives of L2PD patients, and 63 unrelated HCs. L2PD and L2NMC belonged to 19 different pedigrees. Baseline characteristics of each group are displayed in Table 1. We did not find significant differences in age or sex among groups. In the post-hoc analysis, both PD groups showed higher UPDRS-III scores than the other groups, as expected, and L2PD patients obtained lower MoCA scores than L2NMC (mean difference –4.00, p = 0.043). When comparing both PD groups, L2PD patients tended to show longer disease duration and higher levodopa-equivalent daily dose than the iPD group, but the differences were not statistically significant. Also, their UPDRS-III scores were almost equal.
Table 1.
Descriptives
| iPD | L2PD | L2NMC | Non-carrier relatives | HC | p | |
| (n = 16) | (n = 14) | (n = 23) | (n = 13) | (n = 63) | ||
| Age, y | 66.25 (11.12) | 69.29 (13.56) | 61.48 (8.37) | 62.38 (8.65) | 63.29 (5.94) | 0.06 |
| Sex, % female | 56.3% | 57.1% | 73.9% | 46.2% | 77.8% | 0.10 |
| UPDRS-III | 28.63 (13.97) | 28.07 (17.08) | 2.26 (2.31) | 2.60 (2.18) | – | <0.0001 |
| MoCA | 24.27 (5.19) | 22.21 (5.54) | 26.22 (3.53) | 24.46 (3.12) | – | 0.07 |
| Disease duration, y | 5.13 (2.13) | 7.21 (4.78) | – | – | – | 0.14 |
| Levodopa daily dose, mg | 391.33 (219.04) | 634.29 (211.18) | – | – | – | 0.07 |
Demographic characteristics of participants, with standard deviations in brackets. P-values represent the group level comparison calculated with ANOVA. iPD, idiopathic Parkinson’s disease; HC, healthy controls; L2NMC, Non Manifesting Carrier of the LRRK2 G2019 S mutation; L2PD, Parkinson’s disease with LRRK2 G2019 S mutation.
Oculomotor performance
Idiopathic PD and L2PD groups
We did not find significant differences between iPD and L2PD patients. In the fixation test, biphasic SWJs tended to be more frequent in the iPD group than in L2PD (Table 2), but it did not reach significance (p = 0.12). To test the potential influence of other variables in the comparison between iPD and L2PD, we explored additional multivariate models including disease duration, levodopa-equivalent daily dose, and MoCA scores as covariates. As in the initial analysis, we did not observe significant differences in the oculomotor performance between PD groups.
Table 2.
Comparative oculomotor performance between groups
| iPD (n = 16) | L2PD (n = 14) | L2NMC (n = 23) | Non-carrier relatives (n = 13) | HC (n = 63) | p PD vs. HC | p L2NMC vs. HC | p Non-carriers vs. HC | |
| Fixation test | ||||||||
| Microsaccade number | 5.00 (3.18) | 5.50 (2.95) | 7.05 (5.81) | 5.29 (3.73) | 5.85 (5.63) | 0.75 (0.83) | 0.43 (0.53) | 0.93 (0.93) |
| Microsaccade amplitude, ° | 0.45 (0.17) | 0.49 (0.14) | 0.50 (0.25) | 0.52 (0.35) | 0.35 (0.13) | <0.001 (<0.01) | <0.001 (<0.01) | 0.05 (0.14) |
| Microsaccade peak velocity, °/s | 28.47 (9.59) | 28.89 (6.02) | 30.01 (11.28) | 32.60 (17.57) | 23.43 (7.39) | <0.01 (<0.01) | <0.01 (0.02) | 0.1 (0.19) |
| SWJ, number | 1.19 (1.52) | 0.43 (0.65) | 1.25 (2.12) | 0.43 (0.79) | 0.46 (0.94) | 0.11 (0.15) | 0.02 (0.04) | 0.58 (0.68) |
| BCEA, °2 | 1.12 (1.25) | 0.81 (0.94) | 1.42 (1.50) | 1.82 (2.12) | 0.64 (0.96) | 0.26 (0.31) | <0.01 (0.02) | 0.02 (0.07) |
| Prosaccade test | ||||||||
| Horizontal latency, ms | 265.31 (39.92) | 300.56 (115.49) | 271.13 (38.34) | 259.14 (41.55) | 246.26 (43.80) | 0.05 (0.09) | <0.01 (0.02) | 0.26 (0.37) |
| Vertical latency, ms | 329.69 (99.36) | 288.16 (59.15) | 291.50 (49.36) | 324.97 (141.46) | 263.01 (32.78) | <0.001 (<0.01) | <0.01 (0.02) | <0.01 (0.02) |
| Horizontal peak velocity, °/s | 304.58 (73.27) | 312.61 (57.97) | 326.41 (75.43) | 320.63 (87.77) | 314.17 (54.48) | 0.91 (0.92) | 0.57 (0.63) | 0.85 (0.89) |
| Vertical peak velocity, °/s | 221.56 (54.54) | 221.54 (35.78) | 240.87 (45.23) | 232.46 (63.97) | 253.27 (48.67) | 0.02 (0.03) | 0.17 (0.24) | 0.14 (0.25) |
| Horizontal positive error, ° | 0.51 (0.28) | 1.08 (1.39) | 0.60 (0.33) | 0.63 (0.54) | 0.55 (0.31) | 0.21 (0.26) | 0.80 (0.84) | 0.50 (0.62) |
| Horizontal negative error, ° | –0.66 (0.53) | –0.63 (0.67) | –0.45 (0.51) | –0.45 (0.36) | –0.42 (0.61) | 0.19 (0.25) | 0.90 (0.90) | 0.76 (0.84) |
| Vertical positive error, ° | 0.59 (0.59) | 0.50 (0.25) | 0.62 (0.61) | 0.48 (0.30) | 0.51 (0.35) | 0.92 (0.92) | 0.30 (0.40) | 0.46 (0.61) |
| Vertical negative error, ° | –1.04 (1.22) | –0.50 (0.22) | –0.57 (0.46) | –0.70 (0.41) | –0.29 (0.31) | <0.01 (<0.01) | <0.01 (0.02) | <0.001 (<0.01) |
| Antisaccade test | ||||||||
| % Corrective antisaccades | 84.71 (25.88) | 75.19 (41.53) | 93.18 (17.87) | 93.76 (10.83) | 97.21 (6.16) | <0.01 (<0.01) | 0.10 (0.16) | 0.15 (0.25) |
| % Successful antisaccades | 72.19 (28.81) | 67.69 (39.40) | 86.96 (18.51) | 80.77 (17.89) | 91.83 (13.45) | <0.001 (<0.01) | 0.13 (0.20) | 0.02 (0.07) |
| Horizontal corrective antisaccade latency, ms | 713.84 (172.38) | 729.18 (392.37) | 599.14 (147.14) | 632.78 (207.22) | 546.61 (95.16) | <0.001 (<0.01) | 0.05 (0.09) | 0.08 (0.17) |
| Vertical corrective antisaccade latency, ms | 722.35 (223.15) | 845.87 (559.78) | 637.83 (181.08) | 625.74 (209.99) | 552.70 (123.13) | <0.001 (<0.01) | <0.01 (0.02) | 0.07 (0.17) |
| Memory saccades | ||||||||
| % Correct memory saccades | 80.95 (21.48) | 85.00 (26.10) | 92.27 (12.12) | 78.46 (27.26) | 93.44 (12.16) | 0.01 (0.02) | 0.48 (0.56) | <0.001 (<0.01) |
| Horizontal latency, ms | 610.15 (534.27) | 447.67 (269.71) | 467.06 (279.50) | 420.59 (194.69) | 351.04 (155.24) | <0.01 (0.02) | 0.01 (0.02) | 0.16(0.25) |
| Vertical latency, ms | 534.56 (271.97) | 614.54 (600.04) | 639.10 (553.13) | 458.30 (249.66) | 359.36 (113.29) | <0.01 (<0.01) | <0.001 (<0.01) | <0.01 (0.03) |
Mean values of oculomotor parameters, with standard deviations in brackets. P-values are the result of general linear models with the oculomotor variable as the dependent variable and age and sex as covariates. In brackets, p-values corrected for multiple comparisons (FDR). °, degrees; BCEA, Bivariate Contour Ellipse Area; iPD, idiopathic Parkinson’s disease; HC, healthy controls; L2NMC, Non Manifesting Carrier of the LRRK2 G2019S mutation; L2PD, Parkinson’s disease with LRRK2 G2019S mutation; SWJ, square wave jerk.
Therefore, to increase the sample size and to facilitate the interpretation of the results, we decided to merge both PD samples into a common PD group to look for differences from HCs. When compared with HCs, PD patients showed fixation microsaccades in a similar number but with different features, including significantly higher amplitude and peak velocity. Their prosaccades were also different from HCs, especially in the vertical plane, with significantly greater saccade latency, lower peak velocity, and larger negative error, indicating a tendency to make hypometric saccades. The antisaccades and memory saccades revealed widespread abnormalities, including increased saccade latencies and lower percentages of corrective antisaccades, successful antisaccades, and correct memory saccades (Table 2). We did not observe changes in these results after correcting for multiple comparisons.
Asymptomatic carriers (L2NMC)
L2NMC demonstrated several similar findings to PD patients (Fig. 1). In the fixation test, L2NMC showed microsaccades with greater amplitude and peak velocity than HCs, as well as more frequent SWJs (Table 2). These results led to a significantly larger BCEA, indicating a more scattered fixation. Additionally, L2NMC performed slightly hypometric vertical prosaccades, with greater values of negative error. Saccade latencies were increased in all horizontal and vertical prosaccades, antisaccades, and memory saccades. However, in spite of this excess of time needed to perform antisaccades and memory saccades, L2NMC reached a similar percentage of correct responses in these tests as HCs. Similarly to the PD group, no changes were observed after correcting for multiple comparisons.
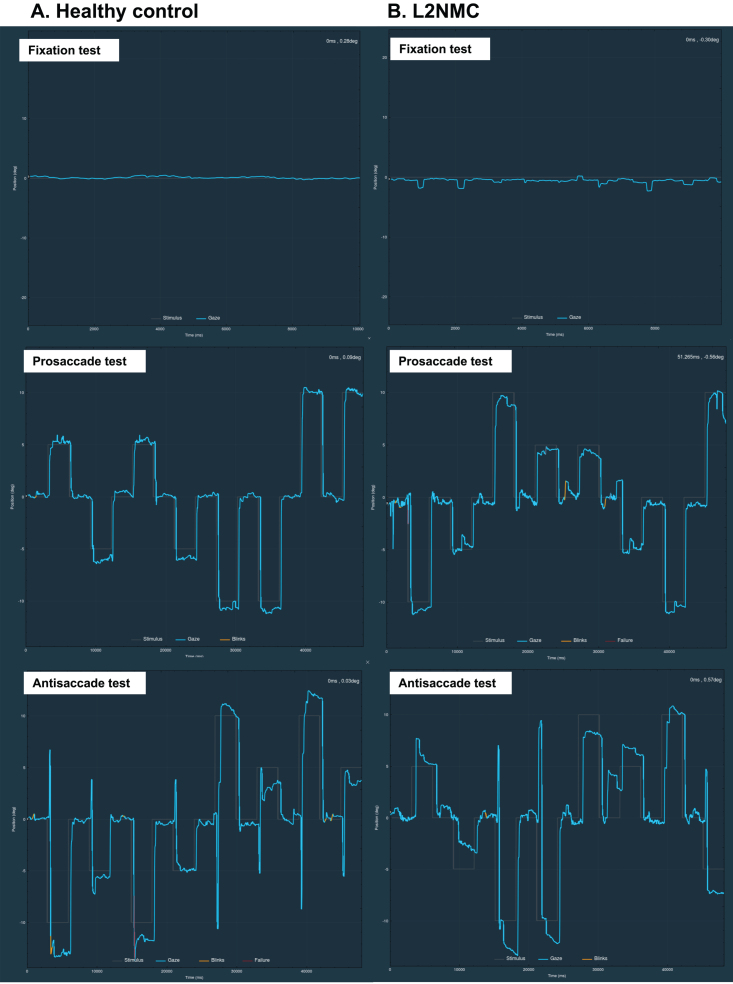
Fig. 1.
Clinical examples of fixation and vertical prosaccade and antisaccade tests in a healthy control (A) and in a LRRK2 nonmanifesting carrier (L2NMC) (B). In the L2NMC, frequent SWJs during fixation and slow corrections of antisaccade errors in the antisaccade test can be appreciated. In the ordinate axis, 0 indicates the center of the screen, positive values the superior part, and negative values the inferior part of the screen. Blue lines represent the participant’s ocular movement. Gray lines indicate the expected gaze location, which corresponds to the target position in the prosaccade test, and to the opposite position of the target in the antisaccade test. Blinks are marked in yellow and pupil detection failure (usually also due to blinks) in red. deg, degrees; ms, milliseconds.
To further explore whether these oculomotor abnormalities were associated with a nearer clinical onset, we split the L2NMC sample into two groups, a group with normal DaT-SPECT uptake and a group with reduced DaT-SPECT uptake. Although the sample size was small, with only 8 subjects in the normal and 13 in the abnormal DaT-SPECT group, we observed that fixation parameters such as microsaccade amplitude, number of SWJ, and BCEA tended to be greater in subjects with abnormal than in those with normal DaT-SPECT, with a borderline significantly higher number of SWJ (p = 0.07). Horizontal prosaccade peak velocity was significantly lower in the group with reduced DaT-SPECT uptake (p = 0.04). Interestingly, although it did not reach statistical significance, prosaccades, antisaccades, and memory saccade latencies also tended to be greater in subjects with abnormal DaT-SPECT (Supplementary Table 1).
Non-carrier relatives
As previous groups, non-carrier relatives tended to make microsaccades with greater amplitude and peak velocity, which led to a significantly larger BCEA, although these results were no longer significant after correcting for multiple comparisons. Also, they showed vertical prosaccades with increased latency and negative error. Their performance in the antisaccade and memory saccade tests tended to be poorer than in controls, with lower percentages of correct responses and increased latencies, especially in the memory saccade test (Table 2).
Oculomotor correlates
We were also interested in investigating potential correlations between oculomotor parameters and UPDRS-III and MoCA scales in order to explore more deeply the association of oculomotor changes with disease progression. Firstly, we observed that UPDRS-III and MoCA were strongly correlated (r –0.48, p < 0.0001), indicating that both scales are related to disease severity.
UPDRS-III scores showed significant correlations with multiple saccade parameters (Supplementary Table 2). Higher scores were associated with longer latencies in the three tests, larger spatial error in the prosaccade test and lower percentages of corrective and successful antisaccades. On the other hand, we did not find any association between UPDRS-III scores and fixation parameters.
MoCA scores were not correlated with fixation or prosaccade parameters, but we observed moderate correlations between lower MoCA scores and poorer performance in the antisaccade and memory saccade tests, including longer latencies and lower success rates. We then analyzed the correlations between oculomotor parameters and MoCA scores only in the asymptomatic carrier group. In this case, we found a borderline negative correlation between MoCA scores and antisaccade latencies (horizontal antisaccades: r –0.40, p = 0.083; vertical antisaccades: r –0.51, p = 0.050) (Supplementary Table 2).
Classification accuracy of oculomotor parameters in asymptomatic carriers
We calculated the classification utility of the studied variables to distinguish the group of L2NMC from HCs. All oculomotor parameters that showed differences with respect to HCs showed an AUC ranging from 0.68 to 0.74, with BCEA being the parameter that showed the greatest classification accuracy (AUC 0.74, p < 0.01) (Supplementary Table 3).
DISCUSSION
Here we show that visual fixation, prosaccades, and intentional saccades are similarly impaired in iPD and L2PD, and that these oculomotor changes occur early in the disease course and can be detected in asymptomatic carriers of LRRK2 mutations. The changes we found in our PD cohorts are consistent with what has been reported in other studies. They describe fixation microsaccades with higher amplitude and peak velocity, a tendency to make hypometric prosaccades, especially in the vertical plane, and lower percentages of corrective antisaccades, successful antisaccades, and correct memory saccades [2, 6, 7, 10–16, 26]. Most oculomotor changes in PD are attributed to the increased inhibitory output of the substantia nigra pars reticulata on the saccade initiating structure, the superior colliculus, affecting the latency and amplitude of saccades [3]. Regarding fixation abnormalities, it has been proposed that the superior colliculus might be triggered during fixation by frontal eye field activity, which is increased in an attempt to compensate for the superior colliculus tonic inhibition [27].
Except for the non-significant higher frequency of SWJ in the iPD group, the oculomotor behavior in iPD and L2PD was identical, both showing multiple changes that differentiated them from HCs. There are no previous studies analyzing oculomotor function in LRRK2-PD, a condition which, although almost identical to iPD, shows group-level differences in several clinical or pathological features. Interestingly, L2NMCs also showed several oculomotor changes similar to PD, such as an unstable fixation, hypometric vertical prosaccades, and increased saccadic latencies in all tests. Several studies suggest that oculomotor changes can constitute an early marker of PD, already detectable at diagnosis and even in presymptomatic stages. The studies conducted in de novo PD have shown an increased prosaccade latency and an increased error rate in the antisaccade task, albeit not consistently [6, 7, 8, 16]. A single study carried out in subjects with iRBD also showed a higher error rate in the antisaccade task, which correlated with UPDRS-III and MoCA scores [26]. Only two previous studies have assessed oculomotor function in asymptomatic carriers of PD mutations. While a sample of 13 Parkin mutation carriers performed similarly to healthy controls [28], a group of 11 PINK1 mutation carriers showed an increased prosaccade latency in comparison with controls, with similar antisaccade and memory saccade latencies [29]. It is important to note that none of the aforementioned studies analyzed visual fixation. In our study we did, and L2NMC showed clear signs of fixation instability, such as larger microsaccades and a higher number of SWJs, which altogether led to a significantly larger BCEA, a parameter representing the global fixational area during the test. This was precisely the variable that showed the greatest accuracy in differentiating L2NMC from HCs, with an AUC of 0.74. Also, and based on our DaT-SPECT data, we consider that fixation instability is an early event in the course of the disease and could be a marker of individuals that are entering the prodromal stage. Although not statistically significant, subjects with a visually abnormal DaT-SPECT showed larger microsaccade amplitude and higher number of SWJs than subjects with normal DaT uptake, suggesting that these parameters become impaired with the progression of the disease. In this sense, we found that UPDRS-III scores correlated with multiple oculomotor parameters, but intriguingly not with fixation parameters. One possible explanation would be that fixation abnormalities reach a ceiling effect in the premotor stage, time before UPDRS-III scores start to increase, which would make it difficult to find a correlation between both parameters. This is specially the case of microsaccade amplitude, which ranges from 0.03 to 1 degree, since saccades larger than 1 degree are not considered microsaccades by definition.
Furthermore, L2NMCs obtained percentages of successful antisaccades and memory saccades similar to controls, however with increased latencies. This pattern contrasts with what was observed in PD patients, who showed an overall poor performance with low rates of success and increased latencies, similar to previous reports [6, 11]. This finding indicates that L2NMC were able to reach a high percentage of success, but, in comparison to HCs, they required more time to do so. Saccade latency is defined by the time lapse between the appearance of the visual target and the start of the ocular movement, so it is heavily impacted by the time needed to process and generate the oculomotor response. In this context, the observed increase of saccade latencies in L2NMC can be interpreted as a decrease of processing speed, an indicator of early premotor cognitive change. The antisaccade and memory saccade paradigms have been traditionally considered sensitive tools of cognitive functioning, with multiple studies showing a correlation between executive function and antisaccade or memory saccade performance both in cognitively normal individuals and patients with cognitive disorders [1, 6, 22, 30]. When considering all groups and as reported by others, we observed a significant correlation between MoCA score and the antisaccade and memory saccade parameters, but not with less cognitively demanding tasks as the fixation and prosaccade tests [6, 8, 11]. Interestingly, MoCA scores in L2NMC were only marginally associated with antisaccade latency, suggesting that saccade latencies would be an earliest and more sensitive marker of processing speed impairment than a global cognitive scale as MoCA.
Contrary to expectations, the group of non-carrier relatives also displayed oculomotor changes compared to HCs. Some of these changes were similar to those observed in PD and L2NMC, with fixation instability resulting in an increased BCEA, hypometric vertical saccades with increased latency and a lower rate of correct antisaccades and memory saccades, although the BCEA increase was not longer significant after correcting for multiple comparisons. The small sample size and the fact that, contrary to HCs, this was not a population selected for being cognitively intact (in fact average MoCA score was 24) may explain the alterations observed in the antisaccade and memory saccade tasks. However, the alterations in fixation and prosaccades had the characteristics of those observed in PD and could be indicative of changes in the PD continuum or a risk state. In this sense, several studies have shown that relatives of PD patients would have an increased risk of PD compared to the general population [31]. In line with this, relatives have been found to show a higher frequency of some recognized PD risk biomarkers, such as hyposmia, reduced striatal DaT binding, or an increased area of substantia nigra hyperechogenicity [18, 32, 33]. Only one previous study has analyzed saccades in unaffected relatives of PD patients. Interestingly, as in our study, they found that a proportion of the relatives had saccadic changes of the type observed in PD. The authors suggest that this marker could identify individuals among relatives at increased risk for PD [34]. We should keep in mind that the G2019S variant of LRRK2 is neither necessary nor sufficient for the development of PD and that additional genetic or environmental factors are required for carriers to develop the phenotype. Accordingly, members of a given family would share some of these additional factors, and therefore a number of non-carrier relatives would also be at increased risk for the disease and would show the associated biomarker changes.
Our study has several limitations. First, the sample size was limited and may have affected the power of the study to detect differences between groups. On the other hand, the HC group was selected for the absence of cognitive impairment and negative Alzheimer’s disease biomarkers, using different cognitive assessment procedures than those used in the other groups. Also, HCs were not tested for LRRK2 mutations, since this cohort is not restricted to the study of PD, so we cannot exclude the possible inclusion of asymptomatic LRRK2 mutation carriers. However, it is important to note that none of the HCs had a diagnosis of PD or other neurological disorders, and they did not refer motor complaints nor showed signs of parkinsonism in the neurological examination performed at the inclusion in the cohort. Finally, we did not analyze the possible influence of medication on oculomotor performance.
In conclusion, our study shows that oculomotor changes in LRRK2-PD are similar to those observed in iPD, and that some of these changes can be detected already in early premotor stages. Currently it is believed that many other genetic and/or environmental factors, in addition to the LRRK2 mutation, are implicated and modulate the phenoconversion of asymptomatic carriers. Our findings suggest that oculomotor changes in these individuals constitute a marker of risk, but their precise utility to predict conversion to PD still has to be elucidated. Further studies, ideally longitudinal, will be needed to validate them as useful premotor biomarkers in PD.
Supplementary Material
ACKNOWLEDGMENTS
We are deeply grateful to all participants who generously took part in this study.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD230416.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author.
REFERENCES
- [1]. Anderson TJ, Macaskill MR (2013) Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol 9, 74–85. [DOI] [PubMed] [Google Scholar]
- [2]. White OB, Saint-Cyr JA, Tomlinson RD, Sharpe JA (1983) Ocular motor deficits in Parkinson’s disease. II. Control of the saccadic and smooth pursuit systems. Brain 106(Pt 3), 571–587. [DOI] [PubMed] [Google Scholar]
- [3]. McDowell JE, Dyckman KA, Austin BP, Clementz BA (2008) Neurophysiology and neuroanatomy of reflexive and volitional saccades: Evidence from studies of humans. Brain Cogn 68, 255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Otero-Millan J, Optican LM, Macknik SL, Martinez-Conde S (2018) Modeling the triggering of saccades, microsaccades, and saccadic intrusions. Front Neurol 9, 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Terao Y, Fukuda H, Ugawa Y, Hikosaka O (2013) New perspectives on the pathophysiology of Parkinson’s disease as assessed by saccade performance: A clinical review. Clin Neurophysiol 124, 1491–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Antoniades CA, Demeyere N, Kennard C, Humphreys GW, Hu MT (2015) Antisaccades and executive dysfunction in early drug-naive Parkinson’s disease: The discovery study. Mov Disord 30, 843–847. [DOI] [PubMed] [Google Scholar]
- [7]. Zhou M-X, Wang Q, Lin Y, Xu Q, Wu L, Chen Y-J, Jiang Y-H, He Q, Zhao L, Dong Y-R, Liu J-R, Chen W (2022) Oculomotor impairments in de novo Parkinson’s disease. Front Aging Neurosci 14, 985679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Sun YR, Beylergil SB, Gupta P, Ghasia FF, Shaikh AG (2023) Monitoring eye movement in patients with Parkinson’s disease: What can it tell us? Eye Brain 15, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Ma W, Li M, Wu J, Zhang Z, Jia F, Zhang M, Bergman H, Li X, Ling Z, Xu X (2022) Multiple step saccades in simply reactive saccades could serve as a complementary biomarker for the early diagnosis of Parkinson’s disease. Front Aging Neurosci 14, 912967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Waldthaler J, Stock L, Student J, Sommerkorn J, Dowiasch S, Timmermann L (2021) Antisaccades in Parkinson’s disease: A meta-analysis. Neuropsychol Rev 31, 628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Beylergil SB, Murray J, Noecker AM, Gupta P, Kilbane C, McIntyre CC, Ghasia FF, Shaikh AG (2022) Temporal patterns of spontaneous fixational eye movements: The influence of basal ganglia. J Neuroophthalmol 42, 45–55. [DOI] [PubMed] [Google Scholar]
- [12]. Pinnock RA, McGivern RC, Forbes R, Gibson JM (2010) An exploration of ocular fixation in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. J Neurol 257, 533–539. [DOI] [PubMed] [Google Scholar]
- [13]. Otero-Millan J, Schneider R, Leigh RJ, Macknik SL, Martinez-Conde S (2013) Saccades during attempted fixation in parkinsonian disorders and recessive ataxia: From microsaccades to square-wave jerks. PLoS One 8, e58535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Zhang JY, Zhang B, Ren QG, Zhong Q, Li Y, Liu GT, Ma XT, Zhao CP (2021) Eye movement especially vertical oculomotor impairment as an aid to assess Parkinson’s disease. Neurol Sci 42, 2337–2345. [DOI] [PubMed] [Google Scholar]
- [15]. Zhang Y, Yan A, Liu B, Wan Y, Zhao Y, Liu Y, Tan J, Song L, Gu Y, Liu Z (2018) Oculomotor performances are associated with motor and non-motor symptoms in Parkinson’s disease. Front Neurol 9, 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Stuart S, Lawson RA, Yarnall AJ, Nell J, Alcock L, Duncan GW, Khoo TK, Barker RA, Rochester L, Burn DJ, on behalf of the ICICLE-PD study group (2019) Pro-saccades predict cognitive decline in Parkinson’s disease: ICICLE-PD. Mov Disord 34, 1690–1698. [DOI] [PubMed] [Google Scholar]
- [17]. Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW (2008) Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol 7, 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Sierra M, Sánchez-Juan P, Martínez-Rodríguez MI, González-Aramburu I, García-Gorostiaga I, Quirce MR, Palacio E, Carril JM, Berciano J, Combarros O, Infante J (2013) Olfaction and imaging biomarkers in premotorG2019S-associated Parkinson disease. Neurology 80, 621–626. [DOI] [PubMed] [Google Scholar]
- [19]. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease: MDS-PD Clinical Diagnostic Criteria. Mov Disord 30, 1591–1601. [DOI] [PubMed] [Google Scholar]
- [20]. Schweitzer KJ, Behnke S, Liepelt I, Wolf B, Grosser C, Godau J, Gaenslen A, Bruessel T, Wendt A, Abel F, Müller A, Gasser T, Berg D (2007) Cross-sectional study discloses a positive family history for Parkinson’s disease and male gender as epidemiological risk factors for substantia nigra hyperechogenicity. J Neural Transm 114, 1167–1171. [DOI] [PubMed] [Google Scholar]
- [21]. Hernández E, Hernández S, Molina D, Acebrón R, Cena CEG (2018) OSCANN: Technical characterization of a novel gaze tracking analyzer. Sensors (Basel) 18, 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Lage C, López-García S, Bejanin A, Kazimierczak M, Aracil-Bolaños I, Calvo-Córdoba A, Pozueta A, García-Martínez M, Fernández-Rodríguez A, Bravo-González M, Jiménez-Bonilla J, Banzo I, Irure-Ventura J, Pegueroles J, Illán-Gala I, Fortea J, Rodríguez-Rodríguez E, Lleó-Bisa A, García-Cena CE, Sánchez-Juan P (2021) Distinctive oculomotor behaviors in Alzheimer’s disease and frontotemporal dementia. Front Aging Neurosci 12, 603790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Garcia Cena C, Gómez D, Valdeoliva I (2020) Measurement and analysis of eye movements performance to predict healthy brain aging. IEEE Access 8, 87201–87213. [Google Scholar]
- [24]. Steinman RM (1965) Effect of target size, luminance, and color on monocular fixation. J Opt Soc Am 55, 1158. [Google Scholar]
- [25]. Sánchez-Rodríguez A, Tirnauca C, Salas-Gómez D, Fernández-Gorgojo M, Martínez-Rodríguez I, Sierra M, González-Aramburu I, Stan D, Gutierrez-González A, Meissner JM, Andrés-Pacheco J, Rivera-Sánchez M, Sánchez-Peláez MV, Sánchez-Juan P, Infante J (2022) Sensor-based gait analysis in the premotor stage of LRRK2 G2019S-associated Parkinson’s disease. Parkinsonism Relat Disord 98, 21–26. [DOI] [PubMed] [Google Scholar]
- [26]. Hanuška J, Rusz J, Bezdicek O, Ulmanová O, Bonnet C, Dušek P, Ibarburu V, Nikolai T, Sieger T, Šonka K, Růžička E (2019) Eye movements in idiopathic rapid eye movement sleep behaviour disorder: High antisaccade error rate reflects prefrontal cortex dysfunction. J Sleep Res 28, e12742. [DOI] [PubMed] [Google Scholar]
- [27]. Gorges M, Pinkhardt EH, Kassubek J (2014) Alterations of eye movement control in neurodegenerative movement disorders. J Ophthalmol 2014, 658243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Machner B, Klein C, Sprenger A, Baumbach P, Pramstaller PP, Helmchen C, Heide W (2010) Eye movement disorders are different in Parkin-linked and idiopathic early-onset PD. Neurology 75, 125–128. [DOI] [PubMed] [Google Scholar]
- [29]. Hertel S, Sprenger A, Klein C, Kömpf D, Helmchen C, Kimmig H (2009) Different saccadic abnormalities in PINK1 mutation carriers and in patients with non-genetic Parkinson’s disease. J Neurol 256, 1192–1194. [DOI] [PubMed] [Google Scholar]
- [30]. Mirsky JB, Heuer HW, Jafari A, Kramer JH, Schenk AK, Viskontas IV, Miller BL, Boxer AL (2011) Anti-saccade performance predicts executive function and brain structure in normal elders. Cogn Behav Neurol 24, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Payami H, Larsen K, Bernard S, Nutt J (1994) Increased risk of Parkinson’s disease in parents and siblings of patients. Ann Neurol 36, 659–661. [DOI] [PubMed] [Google Scholar]
- [32]. Montgomery EB, Baker KB, Lyons K, Koller WC (1999) Abnormal performance on the PD test battery by asymptomatic first-degree relatives. Neurology 52, 757–762. [DOI] [PubMed] [Google Scholar]
- [33]. Piccini P, Burn DJ, Ceravolo R, Maraganore D, Brooks DJ (1999) The role of inheritance in sporadic Parkinson’s disease: Evidence from a longitudinal study of dopaminergic function in twins. Ann Neurol 45, 577–582. [DOI] [PubMed] [Google Scholar]
- [34]. Blekher T, Weaver M, Rupp J, Nichols WC, Hui SL, Gray J, Yee RD, Wojcieszek J, Foroud T (2009) Multiple step pattern as a biomarker in Parkinson disease. Parkinsonism Relat Disord 15, 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author.