Abstract
Current strategies in human immunodeficiency virus type 1 (HIV-1) vaccine development are often based on the production of different vaccine antigens according to particular genetic clades of HIV-1 variants. To determine if virus virulence or genetic distance had a greater impact on HIV-1 vaccine efficacy, we designed a series of heterologous chimeric simian/human immunodeficiency virus (SHIV) challenge experiments in HIV-1 subunit-vaccinated rhesus macaques. Of a total of 22 animals, 10 nonimmunized animals served as controls; the remainder were vaccinated with the CCR5 binding envelope of HIV-1W6.1D. In the first study, heterologous challenge included two nonpathogenic SHIV chimeras encoding the envelopes of the divergent clade B HIV-1han2 and HIV-1sf13 strains. In the second study, all immunized animals were rechallenged with SHIV89.6p, a virus closely related to the vaccine strain but highly virulent. Protection from either of the divergent SHIVsf13 or SHIVhan2 challenges was demonstrated in the majority of the vaccinated animals. In contrast, upon challenge with the more related but virulent SHIV89.6p, protection was achieved in only one of the previously protected vaccinees. A secondary but beneficial effect of immunization on virus load and CD4+ T-cell counts was observed despite failure to protect from infection. In addition to revealing different levels of protective immunity, these results suggest the importance of developing vaccine strategies capable of protecting from particularly virulent variants of HIV-1.
A safe, effective prophylactic human immunodeficiency virus (HIV) vaccine is urgently needed to curb the current AIDS epidemic (20, 44). Effective HIV type 1 (HIV-1) vaccines must be capable of protecting immunized individuals from infection with a broad array of diverse viral variants. Current strategies in HIV-1 vaccine development are often based on designing immunogens according to genetically defined clades of HIV-1 which may be predominant in a specific country or continent. However, given the genetic diversity of HIV-1, the induction of sterilizing immunity by vaccination may not be an objective that can be readily achieved by the first-generation HIV-1 vaccines likely to be widely used in humans (2). Protection from high virus loads and disease progression is often cited as a more realistic short-term goal. Despite many efforts, an ideal vaccine candidate has not yet emerged. This is in part due to the poor immunogenicity of the envelope glycoprotein, the tremendous variability of the virus (3, 49), its ability to evade and impair the host's immune system, and its ability to persist by integrating into the host cell genome of a number of different cell types (2, 12, 27). It is generally believed that an effective HIV-1 vaccine must be capable of inducing neutralizing antibodies as well as strong cell-mediated immune responses in outbred populations (6, 27). Inclusion of an HIV-1 envelope antigen(s) in candidate vaccine strategies is thought to be a necessary component of a prophylactic HIV-1 vaccine to induce responses capable of blocking infection (6, 12).
To date only live attenuated viruses have been reported to protect against markedly heterologous and pathogenic challenges (17, 18, 28, 36, 38, 58). Safety issues with respect to attenuated AIDS vaccines (4, 5, 66) have raised serious concerns that may preclude the widespread clinical use of this approach. Furthermore, not all live attenuated vaccines have proved to be protective (42). Subunit vaccines, on the other hand, are relatively safe but have not induced broad antiviral responses (16). Despite this criticism, it has been shown that recombinant HIV-1 vaccines can protect against heterologous but nonpathogenic HIV-2 infection (1). New strategies are being developed to expose highly conserved and functionally critical sites of the virus envelope that can be targeted by broad neutralizing antibodies (35), reemphasizing the potential importance of HIV-1 envelope antigens as components of an effective HIV-1 vaccine.
Comparative evaluation of various vaccine candidates requires model systems that permit the practical use of relatively large groups of outbred nonhuman primates. Chimeric simian/human immunodeficiency viruses (SHIV) that express the envelope of HIV-1 and are infectious for various macaque species have been developed (39, 41). The possibility to use SHIV for preclinical HIV-1 vaccine efficacy enables the study of both the immunogenicity and the efficacy of new-generation HIV-1 enveloped-based vaccine candidates in macaques. The availability of certain SHIVs which are pathogenic (31, 51) also provides the possibility to determine vaccine protection from disease if protection from infection fails. We previously used the SHIV model to demonstrate that macaques immunized with recombinant envelope of the clinical isolate HIV-1W6.1D could be protected from infection with homologous SHIVW6.1D (45). As proof of principle, we set out to determine if after protection from initial homologous challenge, protection could be achieved from heterologous and/or highly virulent pathogenic SHIVs in these same animals. For this purpose, we used a series of dual CCR5- and CXCR4-utilizing HIV-1 envelope SHIV chimeras which were selected on the basis of their genetic distance or similarity to the envelope sequence of the vaccine. In the context of our findings of protection and more detailed analysis of the virus strains used, we question the strategy of designing clade-based vaccines without consideration of the antigenic components of particularly virulent variants.
MATERIALS AND METHODS
Animals.
Captive-bred mature (4- to 5-year-old) outbred rhesus macaques were housed at the Biomedical Primate Research Center, Rijswijk, The Netherlands. The animals were negative for antibodies to simian T-cell-tropic virus type 1, simian type D retrovirus, and herpes B virus. During the course of the study, the animals were checked twice daily for appetite and general behavior, and stools were checked for consistency. Body weight and body temperature were measured every time an animal was sedated for blood collection or immunization. Animals which suffered from opportunistic infections that were not responsive to treatment, had a body weight loss of >10%, and developed persistently low CD4 counts and high virus loads were euthanized, and full pathology was performed to confirm the diagnosis of AIDS. The Institutional Animal Care and Use Committee approved study protocols according to international ethical and scientific standards and guidelines.
SHIV strains.
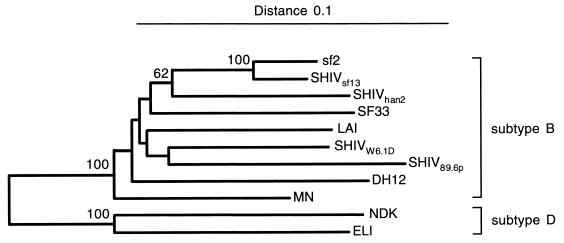
SHIVW6.1D (50) was constructed by replacing a NheI-to-AvrII fragment, encompassing gp120 and gp41 of the chimeric virus SHIV-4 (39), with the equivalent region of the envelope of the W6.1D molecular clone from virus isolate 320.3. The parental HIV-1 isolate 320.3 was derived from a Dutch AIDS patient (24), was dualtropic, and could infect T cells as well as macrophages (23). SHIVW6.1D was propagated on autologous rhesus peripheral blood mononuclear cells (PBMC) to generate a cell-free virus stock. The 50% macaque infectious dose (MID50) was determined by in vivo titration in Macaca mulatta (7). The chimeric viruses SHIVsf13 and SHIVhan2 were generated using SIVmac239 as the background virus (including gag, pol, vif, vpx, and nef) as described by Kuwata et al. (33). SHIVsf13 was constructed using env, vpu, vpr, rev, and tat from HIV-1sf13. SHIVhan2 contains part of env and vpu from HIVhan2 plus tat and vpr from HIV-1NL432 (33, 34). Preparation of the virus stocks in rhesus PBMC and in vivo titration in M. mulatta were done as described by Bogers et al. (7). SHIV89.6P was constructed with SIVmac239 expressing the HIV-1 env of the primary isolate HIV-189.6p (15) and the associated auxiliary genes tat, vpu, and rev as described previously (52). After in vivo passage, this virus became pathogenic (32, 51). This virus stock was titrated in vivo at GTC Mason Laboratories and generously distributed by N. Letvin (Beth Israel Hospital, Boston, Mass.). The SHIVs chosen for heterologous challenge (sf13, han2, and 89.6p) were selected based on their complete env nucleotide sequences. The phylogenetic tree comparing their genetic relatedness is depicted in Fig. 1. For the first challenge study we chose SHIVsf13 and SHIVhan2 because of their relative relatedness (bootstrap value 78%) to the vaccine strain based on this phylogenetic analysis. All three of the heterologous SHIVs used were comparable with respect to use of both CCR5 and CXCR5 coreceptors (8, 30, 51, 52).
FIG. 1.
Phylogenetic tree of Env protein sequences of HIV-1 and SHIV, based on the well-aligned positions in the Env region. The root was placed between the sequences from subtypes B and D. The distance between two sequences is obtained by summing the lengths of the connecting horizontal branches, using the scale at the top. Bootstrap values higher than 50% are given at the internodes in percentages; 2,000 replicates were analyzed.
Sequences and analysis of the SHIV constructs.
The nucleotide sequences of the recombinant env gene structures from the SHIV constructs used in this study (32, 34, 39, 50, 51) were reconstructed from the following entries from the EMBL nucleotide sequence database (given by accession number): AF038399 (SHIV-4), U34603 (Ach320/W6.1D), U43141 (han2), M19921 (NL432), L07422 (sf13), and U89134 (SHIV89.6p). These reconstructed sequences were aligned with other HIV-1 group M subtype B and D env sequences available from EMBL or GenBank (data not shown). The phylogenetic tree in Fig. 1 was constructed by amino acid alignment. Only the env region that is in all SHIVs was taken into account, and only positions that could be well aligned were included. This resulted in 514 positions on a total of 914 Env alignment sites. For this amino acid-based tree, no corrections for multiple substitutions were made, as the goal of the tree construction was to assess the actual relationships between the different Env proteins rather than to reconstruct evolution. The neighbor-joining tree was constructed with the software package TREECON (62), disregarding insertions and deletions.
Immunization and challenge schedule.
After five immunizations with recombinant gp120W6.1D antigen derived from HIV-1 clone 320.3 isolated from a Dutch AIDS patient (24), 10 out of 12 animals (three groups of 4 animals each) were found to be protected against homologous challenge with SHIVW6.1D. This antigen (100 μg in a volume of 0.5 ml) was formulated as previously specified (45, 56). To confirm protection against heterologous challenge, all 12 animals were boosted (sixth immunization) 32 weeks after the first challenge with 100 μg of recombinant HIV-1gp120W6.1D antigen in SBAS1 (group A), or SBAS2 (groups B and C) as previously described (45). Four weeks after the boost, half of the animals were challenged intravenously with 50 MID50 of the rhesus PBMC-derived virus stock of either SHIVsf13 or SHIVhan2. Four nonimmunized naive animals, two for each separate challenge virus, served as controls.
To investigate whether vaccination could protect against challenge with highly virulent SHIV, all 12 vaccines were boosted again (seventh immunization) 20 weeks after the second challenge. Four weeks thereafter, the animals were challenged iv with 50 MID50 of pathogenic SHIV89.6p. Two new nonimmunized naive animals served as controls.
Measurement of plasma virus load and detection of proviral DNA.
Plasma virus load was determined with a quantitative competitive RNA reverse transcription (RT)-PCR using plasma from EDTA-treated blood samples (57). The lower detection limit of this RNA PCR is 40 RNA eq/ml. As target sequence, the highly conserved 267-bp region in the SIV gag gene was chosen with primer and probe regions being homologous for SIVmac and chimeric SHIV viruses.
For the detection of proviral DNA in PBMC and lymph node cells, DNA was purified by sodium dodecyl sulfate-proteinase K digestion followed by ethanol precipitation. Nested PCR was performed for two regions of the chimeric SHIV genome, utilizing SIV gag and HIV-1 env primers as described before (9). The detection limit of both the SIV gag and HIV-1 env PCR assay is 1 copy of proviral DNA per 1.5 × 105 cell eq. To enable discrimination between different challenge viruses, DNA PCR products were digested with specific restriction enzymes. Quantitative RNA RT-PCR as well as nested DNA PCR assays for both regions of the proviral genome were performed at 2-week intervals. All assays were performed at multiple time points and with multiple samples, including PBMC, lymph nodes, and autopsy tissues.
Detection of HIV-1 Env- and SIV Gag-specific antibodies.
Anti-HIV-1 Env and SIV Gag antibodies in serum were measured by antigen-specific enzyme-linked immunosorbent assays (ELISA). Microtiter plates (96 wells; Titertex, ICN, Zoetermeer, The Netherlands) were coated with 1 μg of gp120 of HIV-1W6.1D or gag of SIVmac251 (MRC, SIVP27, ARP643) per ml overnight at 4°C. Uncoated sites were blocked with phosphate-buffered saline–0.1% Tween 20–1% bovine serum albumin–4% newborn calf serum for 1 h at 37°C. Serum was incubated for 1.5 h at 37°C. Serum dilutions of 1/50 or 1/500 and serial twofold dilutions were tested. Bound antibody was detected by incubation with sheep anti-human immunoglobulin-biotin antibodies (Amersham International, Amersham, Buckinghamshire, United Kingdom) for 1.5 h at 37°C. Streptavidin-horseradish peroxidase conjugate (Amersham) was added for 0.5 h, followed by o-phenyldiamine dihydrochloride substrate (Sigma Chemical Co., St. Louis, Mo.). Addition of 3 M HCl stopped the reaction. Optical density (OD) was measured at 492 nm. The titers reported in this study represent the reciprocal of the serum dilution giving an OD of more than the mean + 2 standard deviations of the OD of a control serum (of an uninfected rhesus monkey) at the same dilution.
Determination of virus neutralization titers.
Neutralization assays were performed as previously described (11, 65), with minor modifications. Virus was titrated in five replicates on 4 × 104 C8166 cells. The cells were pretreated for 60 min with 5 μg of Polybrene (Sigma) per ml in RPMI 1640 (Gibco BRL, Life Technologies BV, Breda, The Netherlands) with 10% fetal calf serum (Gibco BRL) and antibiotics in 96-well flat-bottomed plates (Falcon Labware, Becton Dickinson, Oxford, United Kingdom). The supernatant was changed on days 1, 2, and 3 and thereafter twice a week. At days 14 and 21, cultures were scored for the presence of syncytia. The viral 50% infective dose 50% (ID50) of the virus stock was calculated as in previous studies, using the Kärber formula (65). Sera from vaccinated and control animals were heat inactivated at 56°C for 30 min and serially diluted in duplicate from 1:10 to 1:320 in a 75-μl volume. Virus was added at 10 to 100 ID50 in a 75-μl volume. The 96-well plates were incubated for 1 h at 37°C; subsequently C8166 cells were added, to make a final volume of 225 μl. As controls, cultures with C8166 cells only, virus only, and cells and virus without serum were used. The neutralizing titer of a particular serum was defined as the reciprocal of the highest dilution giving no syncytia compared with control serum from uninfected animals. To determine if differences between groups were statistically significant, we used either the nonparametric Mann-Whitney rank-sum test or the Student's t test (22).
Measurement of circulating CD4+ T cells.
To monitor the quantitative changes in PBMC subsets in infected macaques (37), fluorescence-activated cell sorting analysis was performed. For single-, double-, or triple-color staining, 50 to 100 μl of heparinized blood was incubated with 10 to 20 μl of monoclonal antibody mix (Becton Dickinson, Lincoln Park, N.Y.) for 15 min at room temperature. Three milliliters of lysing solution (Becton Dickinson, Etten-Leur, The Netherlands) was added, and cells were incubated for 15 min at room temperature. The cells were centrifuged for 10 min at 200 × g. Supernatant was aspirated, and the cells were resuspended in 5 ml of phosphate-buffered saline with 1 to 2% formaldehyde and stored overnight at 4°C.
Flow cytometry was performed on a FACsort using the CellQuest software (Becton Dickinson), and 5,000 events in the lymphocyte gate were analyzed per monoclonal antibody mix. Combinations of CD29 conjugated to fluorescein isothiocyanate, CD4 conjugated to phycoerythrin, and CD8 conjugated to peridinin chlorophyll protein (CD29FITC, CD4PE, and CD8PerCP, respectively) and of CD3FITC, CD4PE, and CD8PerCP were made to measure the percentage of CD4+ and CD4+ CD29+ (memory) T cells. Anti-mouse FITC-conjugated immunoglobulin (IgFITC), -IgPE, and -IgPerCP were used as control antibodies. To allow calculation of the absolute number of circulating CD4 and CD4 memory cells, whole white blood cell counts were performed.
RESULTS
We previously demonstrated that macaques immunized with recombinant gp120 of the clinical isolate HIV-1W6.1D formulated with the adjuvant SBAS1 or SBAS2 were protected from infection with homologous SHIVW6.1D, (Table 1) (45). As proof of principle, we set out to determine if the immunity observed in these animals would be effective in eliciting protection from heterologous versus highly virulent SHIV challenges. Approximately 6 months after challenge, all animals were reboosted with the same vaccine and challenged 1 month later with the respective heterologous SHIV strain. The first heterologous challenge was performed with either the nonpathogenic SHIVhan2 or SHIVsf13 chimera (Fig. 1).
TABLE 1.
Virus status following SHIV challenge
| Group | Animal | Statusa
|
|||||
|---|---|---|---|---|---|---|---|
| 1st challenge (45)
|
2nd challenge
|
3rd challenge
|
|||||
| Homologous SHIV strain | Outcome | Heterologous, nonvirulent SHIV strain | Outcome | Related, highly virulent SHIV strain | Outcome | ||
| A | 9143 | W6.1D | Protected | han2 | Protected | 89.6p | Infected |
| 9157 | W6.1D | Protected | sf13 | Protected | 89.6p | Infected | |
| 9172 | W6.1D | Infected | sf13 | (prot, W6.1D) | 89.6p | (prot, W6.1D) | |
| 9206 | W6.1D | Infected | han2 | Transient | 89.6p | Infected | |
| B | 9150 | W6.1D | Protected | han2 | Infected | 89.6p | (prot, han2) |
| 9175 | W6.1D | Protected | sf13 | Transient | 89.6p | Infected | |
| 9214 | W6.1D | Protected | sf13 | Transient | 89.6p | Infected | |
| 9208 | W6.1D | Protected | han2 | Protected | 89.6p | Infected | |
| C | 9171 | W6.1D | Protected | han2 | Protected | 89.6p | Infected |
| 9203 | W6.1D | Protected | sf13 | Protected | 89.6p | Infected | |
| 9205 | W6.1D | Protected | han2 | Protected | 89.6p | Protected | |
| 9241 | W6.1D | Protected | sf13 | Protected | 89.6p | Infected | |
| D | AA002 | W6.1D | Infected | ||||
| J040 | W6.1D | Infected | |||||
| L146 | W6.1D | Infected | |||||
| Y005 | W6.1D | Infected | |||||
| K7D | han2 | Infected | |||||
| KXO | han2 | Infected | |||||
| KKO | sf13 | Infected | |||||
| VC1 | sf13 | Infected | |||||
| BJC | 89.6p | Infected | |||||
| WT5 | 89.6p | Infected | |||||
For explanation of boldface, see Results.
Heterologous challenge with SHIVhan2.
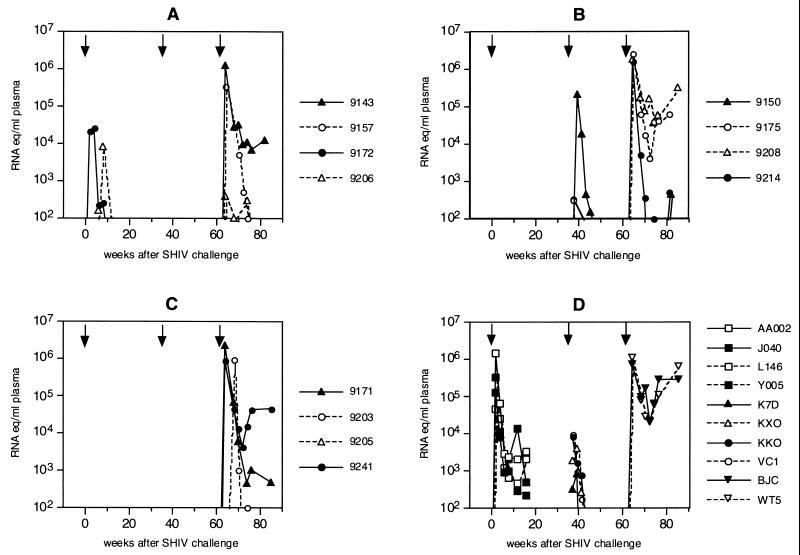
SHIVhan2 contains the envelope of HIV-1han2, a clinical clade B isolate from an HIV-1-infected patient from Hannover, Germany (54). The HIV-1han2 isolate has recently been selected and evaluated for vaccine efficacy studies in chimpanzees (8). The SHIVhan2 envelope nucleotide sequence is related to but distinct from that of the vaccine strain SHIVW6.1D (Fig. 1). Control animals K7D and KXO challenged with SHIVhan2 became readily and persistently infected (Table 1). Virus RNA load peaked at 4 weeks after challenge and declined to undetectable virus loads after 12 weeks (Fig. 2D). At all time points, proviral DNA was detected (data not shown). No evidence of disease progression was observed, despite persistent infection. Half of the twelve vaccinated animals were challenged with SHIVhan2. One of these six (9206) was previously infected with SHIVW6.1D (Table 1) and will therefore be discussed separately (see below, “Live attenuated vaccine effect”). Four of the five previously completely protected animals challenged with SHIVhan2 (9143, 9208, 9171, and 9205) were again completely protected from the heterologous SHIVhan2 infection (Table 1). Neither viral RNA nor proviral DNA was detected in these four animals at any of the multiple time points after challenge or later at autopsy. The one animal that became infected, 9150, was persistently infected. At all time points tested, proviral SHIVhan2 DNA was detected in the PBMC of this animal. In animal 9150, viral RNA levels peaked with a higher virus load (1.5 × 105 RNA copies/ml [Fig. 2B]) than in the control monkeys (K7D and KXO; 3.9 × 102 and 2.1 × 103 RNA copies/ml [Fig. 2D]).
FIG. 2.
Plasma viral RNA levels of rhesus macaques challenged with SHIVW6.1D (first arrow), SHIVhan2 (second arrow, triangles) or SHIVsf13 (second arrow, circles) and SHIV89.6p (third arrow) as determined by quantitative RT-PCR (SIV gag) (57). Group A was immunized with gp120W6.1D-SBAS1; group B was immunized with gp120W6.1D-SBAS2; group C was immunized with gp120W6.1D in an experimental adjuvant and in SBAS2; group D consists of nonimmunized control animals. For details, see Materials and Methods.
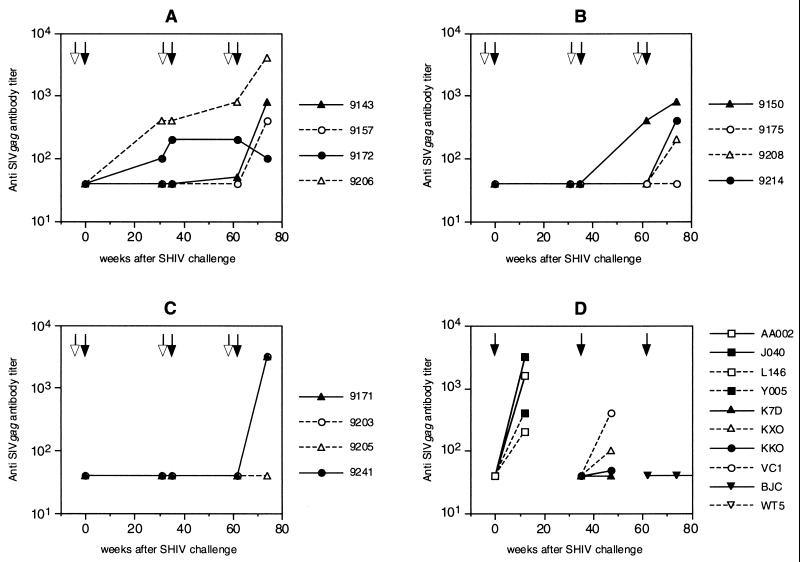
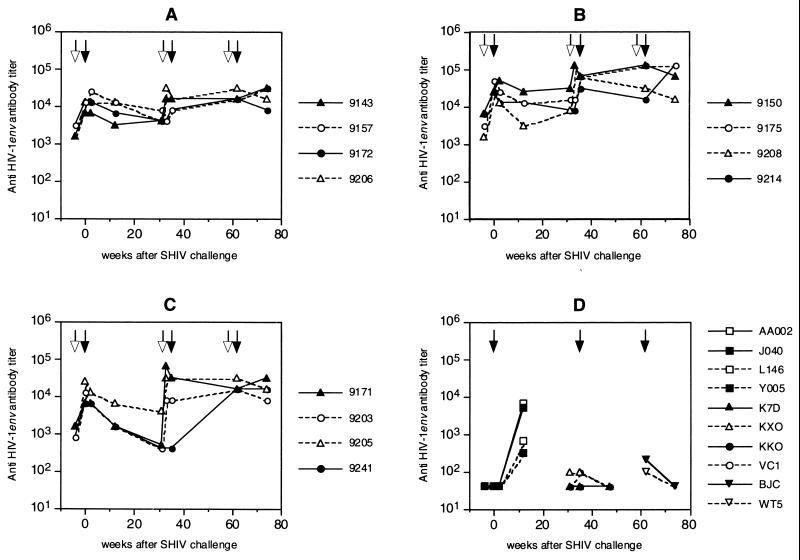
All humoral and cellular immune responses before the first challenge with SHIVW6.1D have been described before (45). No single immune correlate with protection against SHIVW6.1D infection could be attributed to the vaccine preparation at that time. Anti-SIV Gag antibodies proved to be a good marker for virus infection. After the heterologous SHIVhan2 challenge, antibodies against SIV Gag were observed in the control monkeys (Fig. 3D) and in monkey 9150, which became persistently infected with SHIVhan2 (Fig. 3B). In all vaccinated and challenged animals, anti-gp120 antibody titers declined after the first SHIVW6.1D challenge. In the vaccinees titers were boosted by the sixth immunization, 4 weeks prior to the heterologous SHIVhan2 challenge. The highest anti-gp120 ELISA titers were found in group B (Fig. 4B) (P < 0.05, Student's t test) as described before (45).
FIG. 3.
Circulating anti-SIVmac239 Gag antibodies in vaccinated and challenged rhesus monkeys. Animals were immunized at weeks -4, 31, and 58 (open arrows) and challenged (closed arrows) at week 0 with SHIVW6.1D, at week 35 with SHIVhan2 (triangles) or SHIVsf13 (circles), and at week 62 with SHIV89.6p. Groups are as defined in the legend to Fig. 2. For details, see Materials and Methods.
FIG. 4.
Circulating anti-HIVW6.1D Env antibodies in vaccinated and challenged rhesus monkeys. Animals were immunized at weeks -4, 31, and 58 (open arrows) and challenged (closed arrows) at week 0 with SHIVW6.1D, at week 35 with SHIVhan2 (triangles) or SHIVsf13 (circles), and at week 62 with SHIV89.6p. Groups are as defined in the legend to Fig. 2. For details, see Materials and Methods.
Neutralizing antibodies against SHIVhan2 as well as the vaccine strain SHIVW6.1D were measured at the day of the heterologous SHIVhan2 challenge (Table 2). As expected, the naive nonvaccinated control monkeys did not develop neutralizing antibodies. Vaccinated animals, however, had high neutralizing antibody titers to the homologous SHIVW6.1D virus strain (Table 2) and the heterologous challenge virus SHIVhan2. Correlation with protection from infection and a high neutralization titer was not observed. Monkey 9150 became infected with SHIVhan2 despite comparable high neutralizing antibody titers against this virus strain (Table 2).
TABLE 2.
Neutralization titers against SHIV
| Monkey | SHIV challenge (1st, 2nd, 3rd) | Titera
|
||||||
|---|---|---|---|---|---|---|---|---|
| Day of SHIVsf13/han2 challenge
|
Day of SHIV89.6p challenge
|
|||||||
| W6.1D, 11b | han2, 19 | sf13, 19 | W6.1D, 11 | han2, 19 | sf13, 19 | 89.6p, 6.5 | ||
| Infected | ||||||||
| 9143 | W6.1D, han2, 89.6p | 320 | 160 | 80 | 160 | <20 | ||
| 9157 | W6.1D, sf13, 89.6p | >640 | 160 | >640 | 40 | 20 | ||
| 9172 | W6.1D, sf13, 89.6p | 160 | 160 | >640 | 80 | <20 | ||
| 9206 | W6.1D, han2, 89.6p | >640 | >640 | >640 | 320 | <20 | ||
| 9150 | W6.1D, han2, 89.6p | >640 | >640 | >640 | 160 | <20 | ||
| 9175 | W6.1D, sf13, 89.6p | 320 | 160 | >640 | 80 | <20 | ||
| 9214 | W6.1D, sf13, 89.6p | 320 | 80 | >640 | <20 | <20 | ||
| 9208 | W6.1D, han2, 89.6p | >640 | >640 | >640 | >640 | <20 | ||
| 9171 | W6.1D, han2, 89.6p | >640 | >640 | >640 | >640 | <20 | ||
| 9203 | W6.1D, sf13, 89.6p | >640 | 40 | >640 | <20 | <20 | ||
| 9205 | W6.1D, han2, 89.6p | >640 | >640 | >640 | 320 | <20 | ||
| 9241 | W6.1D, sf13, 89.6p | 160 | 40 | <20 | 40 | <20 | ||
| Controls | ||||||||
| K7D | han2 | <20 | <20 | |||||
| KXO | han2 | <20 | <20 | |||||
| KKO | sf13 | <20 | 20 | |||||
| VC1 | sf13 | <20 | <20 | |||||
| BJC | 89.6p | <20 | <20 | |||||
| WT5 | 89.6p | <20 | <20 | |||||
For explanation of boldface, see Results.
TCID50.
Heterologous challenge with SHIVsf13.
SHIVsf13 contains the envelope of the HIV-1sf13, a biological variant from HIVsf2, isolated from an AIDS patient from San Francisco (14). HIV-1sf2 can infect chimpanzees (47) and is highly similar to the North American clade B consensus sequence (48). Control animals KKO and VC1 challenged with SHIVsf13 became readily and persistently infected with this virus (Table 1; Fig. 2D). Plasma viral RNA levels peaked at 2 weeks after challenge and showed slightly higher peak levels (4.2 × 103 and 4.8 × 103 RNA copies/ml) and similar kinetics as in the SHIVhan2-infected control animals (Fig. 2D). At all time points, proviral DNA was detected (data not shown). Like SHIVhan2, SHIVsf13 was nonpathogenic. Of the five previously protected vaccinated animals challenged with SHIVsf13, three (9157, 9203, and 9241) were completely protected from SHIVsf13 infection (Table 1). Two animals (9175 and 9214) became transiently infected. Low viral RNA levels were detected in the circulation of these two animals only at 2 weeks postchallenge; RNA was not detected at later time points (Fig. 2B). Importantly, using highly sensitive nested DNA PCRs, no proviral DNA was detected in circulating PBMC at any time point after challenge in these animals. Evidence to suggest only a transient infection with SHIVsf13 in the two vaccinees 9175 and 9214 was based on the absence of anti-SIV Gag antibodies (Fig. 3B), repeated negative DNA PCR on multiple samples and time points, and detailed PCR analysis at autopsy. In these animals we found no correlation between protection from infection and neutralization antibody titers (Table 2). Monkeys 9175 and 9214 became infected with SHIVsf13 despite good neutralizing titers against SHIVsf13. In some cases, these titers were even higher than in monkeys 9203 and 9241, which were also protected from SHIVsf13 challenge (Table 2).
Challenge with related but highly virulent SHIV89.6p.
Approximately 5 months after the first heterologous challenge with either SHIVhan2 or SHIVsf13, all animals were reboosted and 1 month later challenged with the related (Fig. 1) but highly virulent SHIV89.6p (51). This chimeric virus expresses the envelope derived from a cytopathic, macrophagetropic primary HIV-189.6 isolate (15, 52). Following in vivo passage, this virus proved to be pathogenic in rhesus monkeys (51, 52), causing rapid CD4+ T-cell depletion (32). After challenge with SHIV89.6p, the control animals BJC and WT5 developed persistent and high levels of plasma viral RNA (Fig. 2D). The SHIV89.6p plasma viral RNA levels were approximately 2 logs higher than SHIVhan2 or SHIVsf13 plasma RNA levels in control animals (Fig. 2D) and remained high until the time of euthanasia, 47 weeks after challenge. Proviral DNA was detected at all time points after challenge until euthanasia (data not shown). Of the 12 vaccinated animals, only 3 were completely protected from this vigorous SHIV89.6p challenge. Of the seven previously completely protected animals (9143, 9157, 9208, 9171, 9203, 9205, and 9241 [Table 1]), only one (9205) proved to be completely protected from infection by all three SHIV challenges. At the time of euthanasia of this monkey (20 weeks after SHIV89.6p challenge), neither viral DNA nor RNA could be detected in PBMC or lymph nodes using the highly sensitive nested PCR for two different viral sequences.
Complete protection from the vigorous pathogenic SHIV89.6p challenge was observed in only one (9205) of seven animals previously protected from the more divergent but nonpathogenic challenge viruses. A plasma RNA virus load lower than in the control monkeys was observed in five other vaccinated animals (9143, 9157, 9171, 9203, and 9241) that became infected with SHIV89.6p (Table 1; Fig. 2). This reduced virus load was lower than the pathogenic threshold of 105 RNA eq/ml of plasma that has previously been defined (57). RNA levels in the control monkeys BJC and WT5 were above the threshold of 105 RNA eq/ml of plasma (Fig. 2). One of the seven previously protected animals, monkey 9208, had steady-state virus loads above 105 RNA eq/ml of plasma (Fig. 2B), strongly suggesting that this animal could eventually develop disease.
After the challenge with SHIV89.6p, all vaccinated animals that became infected developed anti-SIV Gag antibodies except 9175 (Fig. 3B), which had been previously infected with SHIVsf13. In the completely protected animal 9205, no SIV Gag antibodies were detected. In the SHIV89.6p-challenged control animals, plasma virus levels were so high that anti-SIV Gag antibodies were likely complexed with plasma antigen and could not be detected. Indeed, it has been reported that in this pathogenic SHIV89.6p model rapid progression to disease is often associated with high level viremia and antigenemia, making seroconversion difficult to detect (40). Alternatively, this may be due to profound immunosuppression by this virus.
The final immunization prior to SHIV89.6p challenge did not boost the anti-gp120 antibody response further but did maintain antibody titers. In the one animal that was protected from SHIV89.6p infection (9205), anti-gp120 titers declined after challenge, confirming protection from infection (Fig. 4). In control animals challenged with SHIVW6.1D, anti-gp120 antibodies were detected, but only low titers in animals challenged with SHIVhan2, SHIVsf13 or SHIV89.6p were observed early postinfection. On the day of the SHIV89.6p challenge, 4 weeks after the last immunization, neutralizing antibody titers against the SHIVW6.1D vaccine strain were maintained. However, neutralizing antibody titers to SHIVhan2 and SHIVsf13 were generally lower than at the time of the second challenge, despite the prechallenge boost. These antibodies were not able to neutralize the highly virulent SHIV89.6p (Table 2), and again no correlation with protection from infection and neutralizing antibody titers was found.
Live attenuated vaccine effect.
During the course of this study, a number of immunized animals became infected but appeared to control or limit the infection. We chose to keep these animals in the study (boldface regions in Tables 1 and 2) even though they had failed the criteria for the first level of protection, which we defined as protection from infection. We reasoned that if these vaccinated animals had truly controlled their infection, that this might be of benefit for generating better immunity to subsequent heterologous or virulent challenges. Importantly, the data were not consistent with this hypothesis.
Animal 9206 was transiently infected with SHIVhan2. Only at one time point (2 weeks postchallenge) were very low levels of viral RNA detected in the circulation (36 RNA eq/ml of plasma; around the detection limit [data not shown]). However, it could not be determined whether the viral RNA originated from the SHIVhan2 challenge or whether this was reactivation of the previous SHIVW6.1D infection with from the earlier challenge in this animal (Table 1). No proviral DNA specific for SHIVhan2 could be detected in circulating PBMC at any time point after SHIVhan2 challenge in monkey 9206, confirming control of infection. The relative protection from heterologous SHIVhan2 infection in animal 9206 could have been attributed to a live attenuated vaccine effect induced by the SHIVW6.1D virus that this animal was carrying (Table 1). Only a minor peak and decline in SIV Gag antibodies was observed (Fig. 3A) after SHIVhan2 infection, further confirming control of infection.
Similarly to animal 9206 previously infected with SHIVW6.1D, protection from heterologous SHIVsf13 infection in animal 9172 could be attributed to a live attenuated vaccine effect associated with the SHIVW6.1D virus that it was infected with earlier (Table 1). Additionally, monkey 9172 had only a slight peak and decline of SIV Gag antibodies after the heterologous SHIVsf13 challenge, indicating that this monkey most probably did not support a secondary infection with the heterologous challenge virus.
Two of five animals that were previously infected with either SHIVW6.1D (monkey 9172) or with SHIVhan2 (monkey 9150) were protected from SHIV89.6p infection, in contrast to those animals that were previously transiently infected (9206, 9175, and 9214). At the time of euthanasia of monkeys 9172 and 9150 (24 and 18 weeks after SHIV89.6p challenge), neither viral DNA nor RNA of SHIV89.6p origin could be found in PBMC or lymph nodes of these monkeys, and virus appeared to have been cleared. However, in the PBMC of monkey 9150, SHIVhan2 DNA, but no circulating viral RNA, could be detected at almost all time points measured after SHIV89.6p challenge (also at the time of euthanasia). Thus, we attributed protection from SHIV89.6p infection in these two animals to a persistent low level, rather than short-lived transient, infection. In the two partially protected animals 9172 and 9150, the anti-Gag antibody titer did not increase after challenge, further confirming protection from SHIV89.6p infection.
Of importance was the observation that not all animals that were previously infected with a nonpathogenic virus were protected from SHIV89.6p challenge, particularly monkeys 9206 (infected with SHIVW6.1D and transiently infected with SHIVhan2) and 9175 and 9214 (transiently infected with SHIVsf13) (Table 1). These animals, therefore, did not benefit from a live attenuated vaccine effect after SHIV89.6p challenge, again suggesting that a persistent rather than a transient presence of virus was necessary to sustain this type of protection.
Analysis of protection from disease.
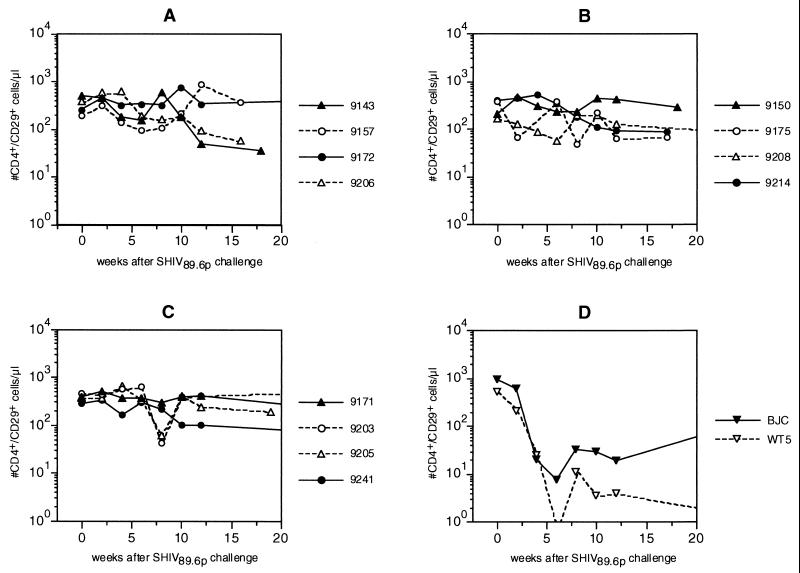
Circulating CD4+ and CD4+ CD29+ (memory) T-cell counts were monitored as a follow-up to the pathogenic SHIV89.6p challenge (Fig. 5). Infection with this SHIV strain causes marked CD4+ T-cell decline (32, 51). Indeed, in this study in the two infected control animals, numbers of circulating CD4+ (data not shown) and CD4+ CD29+ (Fig. 5D) T cells declined very rapidly after infection with SHIV89.6p. By 6 weeks postchallenge, the number of circulating CD4+ CD29+ T cells was at its lowest point and remained low thereafter. One of the two control monkeys (WT5) developed clinically advanced AIDS, which was confirmed by autopsy and histopathology 41 weeks after challenge. The other control animal (BJC) also developed very low numbers of circulating CD4+ CD29+ T cells. Pathology of this animal euthanized at 47 weeks postinfection revealed lymphadenopathy, gastritis with lymphoid follicle formation, and hepatitis.
FIG. 5.
Absolute circulating CD4+ CD29+ T-cell counts in rhesus monkeys after challenge with SHIV89.6p. Groups are as defined in the legend to Fig. 2. For details, see Materials and Methods.
In immunized animals the dramatic pattern of CD4+ (data not shown) and/or a CD4+ CD29+ T-cell loss (Fig. 5A to C) seen in the control animals was not observed. However, in three of the four animals that developed a persistently high virus load (9143, 9175, and 9208), an initial but transient decline of CD4+ CD29+ T-cell levels was observed. Subsequently, a very slow decline was observed over time in some of these animals. Only animals immunized with gp120 HIVW6.1D-SBAS2 and infected with SHIV89.6p but previously completely protected from SHIVW6.1D and SHIVsf13 or SHIVhan2 (9208, 9171, 9203, 9205, and 9241) were followed for an extended period of time (47 weeks) for possible disease progression. Evidence of disease progression was not observed in these immunized animals, nor did the extensive pathology performed on them reveal lesions suggestive of AIDS or disease progression. Furthermore, there was no apparent benefit from the live attenuated vaccine effect in immunized animals previously infected with nonpathogenic challenge viruses. The CD4+ T cells in these animals were comparable to those in other vaccines that were previously protected from all other challenges.
Levels of protective immunity.
Several levels of protective immunity were observed during this study. First, complete (so-called sterilizing immunity) protection from infection was observed with no evidence (by any of the stringent criteria) of the presence of challenge virus. Second, evidence of transient infection was observed in which at only one time point was viral RNA, but not proviral DNA, detected. In these animals, virus could not be detected in autopsy material with any of the assays available. Third, a persistent but contained viral infection, in which plasma viral RNA was reduced and proviral DNA persisted, was also documented. Fourth, protection from disease was observed in animals with an active viral infection (persistent low plasma virus load) but which maintained CD4+ T cells within the normal range.
DISCUSSION
This study was designed to evaluate the vaccine efficacy of HIV-1W6.1D-vaccinated macaques challenged with heterologous low-virulence nonpathogenic SHIVs compared to challenge with the related but highly virulent SHIV89.6p chimera. For comparative purposes, all three heterologous SHIVs were chosen for the ability to utilize CCR5 as well as CXCR4 coreceptors. It was observed that the number of protected animals per group began to decline from 10 of 12 with the homologous SHIVW6.1D challenge (45), to 4 of 5 with the SHIVhan2, and 3 of 5 with the SHIVsf13 heterologous low-virulence challenges. This difference is, however, not statistically significant (P = 0.79, χ2 test). However, when animals found to be protected were rechallenged with the more homologous (Fig. 1) but highly virulent SHIV89.6p, very few were protected from infection (P < 0.025, χ2 test with Yates' correction [Table 1]). This suggested that virulence rather than genetic distance may be a predominant factor in cases of HIV-1 vaccine failure. Retrospectively, these observations are supported by comparison of virus loads between naive SHIVsf13-infected and SHIVhan2-infected animals, revealing that SHIVsf13 infection causes higher virus loads than SHIVhan2 (57), correlating with fewer animals protected from SHIVsf13 challenge. Despite the greater genetic distance from the vaccine strain, less virulent challenge strains were easier to protect from. Alternatively, a challenge which was more closely related to the vaccine strain but more virulent was more difficult to protect from infection.
In the absence of complete protection from infection, some immunized animals appeared to be capable of clearing infection (Table 1, transient infection). This observation was similar to what we have previously described in a separate vaccine study (63) which we further confirmed by rigorous PCR analysis of these animal's tissues following necropsy. Second, in cases where infection was not cleared and persisted at low levels, protection from infection by subsequent exposure was observed, as previously reported (19–21, 59, 64). This type of protection is analogous to the protection afforded by live attenuated vaccines (2, 9, 17, 19, 28, 36, 58, 59, 64). Importantly, however, not all animals that were previously infected with a particular SHIV strain were protected from the virulent SHIV89.6p challenge, in particular animals 9206, 9175, and 9214 (Table 1, boldface region). This indicates that immunity induced by a live attenuated vaccine approach is not always sufficient to protect from virulent virus infection. These findings, together with the safety concerns of live attenuated vaccines (4, 5, 66), are important issues to consider with regard to live attenuated HIV-1 vaccine development. Finally, although immunization with recombinant HIV-1W6.1D gp120 did not protect the majority of the monkeys from infection with the highly virulent SHIV89.6p, clear evidence of a beneficial effect on maintenance of the number of circulating CD4+ T cells and suppression of virus load was observed (Fig. 2 and 5). In immunized SHIV89.6p-infected monkeys, viral RNA load peaked 2 weeks after infection but declined thereafter (Fig. 2) below the critical pathogenic threshold of 105 RNA copies/ml of plasma (57). This is suggestive of prolonged survival for these animals. None of the immunized animals revealed clinical or pathological evidence of disease progression to AIDS during the study. One of the two control animals developed terminal AIDS and was euthanized 47 weeks following infection. The other control animal had developed pathological evidence of AIDS at the time of euthanasia. It is possible that differences in disease progression between vaccinated animals and controls would have become even more dramatic if the animals could have been monitored indefinitely for survival.
Although this study was undertaken to investigate whether vaccine protection could be induced against heterologous versus virulent challenges, we cannot discard the possible effect of repeated exposure (challenge) on boosting and broadening immunity. However, the concern of boosting by challenge was not supported by assays of the humoral immune responses. Antibody titers to envelope did not increase, nor did antibodies to Gag develop in animals which remained virus negative. A possible role of cytotoxic T lymphocytes cannot be ruled out. At multiple time points, PBMC and lymph node cells were negative for the presence of virus, with highly sensitive nested PCRs confirming protection from infection. Subsequent studies with naive animals immunized and challenged with the same heterologous viruses will be required to completely unravel the vaccine- versus possible virus challenge-induced effects on boosting immunity and facilitating protection from disease. In a separate study using the same antigen but a different adjuvant formulation and immunization schedule, cynomolgous monkeys were not protected from infection with a divergent and relatively virulent SHIVsf13 challenge (55). However, in that study viral RNA in plasma was not measured and thus the effect of protection from disease could not be assessed (55).
The clinical relevance of the envelope subunit used in this study is emphasized by the CCR5 coreceptor usage (23) of this particular HIV-1W6.1D isolate from which the gp120 was derived (60). However, the use of current subunit preparations as components of HIV vaccines has the limitation of not inducing broad neutralizing immune responses against primary isolates (16, 43, 46, 61). With these envelope antigen preparations, we were able to neither induce broad neutralizing antibodies nor achieve broad protection from infection with vigorous pathogenic challenge. Although we achieved only a narrow spectrum of protection from infection, we did observe a beneficial effect on protection from disease. From our initial studies with this vaccine (45) and other recent studies (30, 53), it is clear that effective protection cannot be correlated with neutralizing antibodies alone or other immune effector mechanisms separately. Indeed, our findings compiled from the evaluation of over 10 different HIV-1 vaccine candidates in preclinical efficacy studies in macaques have revealed that effective protection will require the coordination of multiple effector mechanisms (25, 29). Potent and balanced T-helper responses appear to be critical for protection from infection (25, 30). Furthermore, preservation of the T-helper compartment appears to be essential at the level of protection from disease in cases where infection overcomes vaccine-induced immunity (26).
One of the key issues in the development of an HIV vaccine remains the problem of heterologous vaccine protection across genetically diverse and defined clades of HIV-1. Indeed, current clinical trial strategies are based on the development of different vaccines for each clade. Although the study described here focused on clade B, a number of observations were made which should be taken into consideration before clade-based vaccine strategies are undertaken. Indeed, consideration of antigenic relatedness or virulence properties appears to be of critical importance. Failure of the vaccine to protect was related to the virulence of the challenge virus rather than the genetic distance from the vaccine strain. These findings have implications for and question the relatively arbitrary development of clade-based HIV-1 vaccines. It will likely be important to take the antigenic properties of more virulent variants into consideration in the selection of vaccine strains and the development of specific HIV-1 vaccine immunogens.
In the absence of protection from highly virulent viruses, our observations reveal a possible effect on protection from disease. From a public health point of view, however, the first vaccine priority must remain protection from infection. New strategies must be explored which are able to present critical conserved structures of the HIV-1 envelope to the immune system (10) to elicit host responses capable of inhibiting infection from a wide variety of primary isolates (35). Furthermore, the addition of nonenvelope antigens to target possible virulence factors such as Nef or Tat may be of additional benefit (13). Such observations reveal the importance and will likely revitalize efforts to use HIV-1 envelope immunogens in combination with other structural or regulatory antigens. Indeed, the combination of different immune mechanisms directed to a number of conserved viral targets may be necessary to broaden vaccine protection from infection by a diverse array of highly virulent HIV-1 variants.
ACKNOWLEDGMENTS
We thank J. Schouw for kind and efficient administrative assistance. We are grateful to P. Frost and E. M. Kuhn for excellent veterinary care and pathology, respectively. C. Bruck and G. Voss (SmithKline Beecham Biologicals, Rixensart, Belgium) kindly provided the vaccine formulations for this study. N. Letvin (Beth Israel Hospital, Harvard Medical Center, Boston, Mass.) kindly provided the SHIV9.6p challenge stock used in this study. Program EVA and the MRC supplied many of the reagents for immunological analysis.
The EC Centralized Facility program for HIV-1 vaccine development (BMH4-CT95-0206 and BMH4-CT97-2067) supported this study.
REFERENCES
- 1.Abimiku A G, Franchini G, Tartaglia J, Aldrich K, Myagkikh M, Markham P D, Chong P, Klein M, Kieny M P, Paoletti E, et al. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med. 1995;1:321–329. doi: 10.1038/nm0495-321. [DOI] [PubMed] [Google Scholar]
- 2.Almond N M, Heeney J L. AIDS vaccine development in primate models. AIDS. 1998;12:S133–S140. [PubMed] [Google Scholar]
- 3.Anonymous. HIV type 1 variation in World Health Organization-sponsored vaccine evaluation sites: genetic screening, sequence analysis, and preliminary biological characterization of selected viral strains. WHO Network for HIV Isolation and Characterization. AIDS Res Hum Retroviruses. 1994;10:1327–1343. doi: 10.1089/aid.1994.10.1327. [DOI] [PubMed] [Google Scholar]
- 4.Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 5.Baba T W, Liska V, Khimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Green M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiple deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 6.Bloom B R. A perspective on AIDS vaccines. Science. 1996;272:1888–1890. doi: 10.1126/science.272.5270.1888. [DOI] [PubMed] [Google Scholar]
- 7.Bogers W M, Dubbes R, ten Haaft P, Niphuis H, Cheng-Mayer C, Stahl-Hennig C, Hunsmann G, Kuwata T, Hayami M, Jones S, Ranjbar S, Almond N, Stott J, Rosenwirth B, Heeney J L. Comparison of in vitro and in vivo infectivity of different clade B HIV-1 envelope chimeric simian/human immunodeficiency viruses in Macaca mulatta. Virology. 1997;236:110–117. doi: 10.1006/viro.1997.8744. [DOI] [PubMed] [Google Scholar]
- 8.Bogers W M, Koornstra W H, Dubbes R H, ten Haaft P J, Verstrepen B E, Jhagjhoorsingh S S, Haaksma A G, Niphuis H, Laman J D, Norley S, Schuitemaker H, Goudsmit J, Hunsmann G, Heeney J L, Wigzell H. Characteristics of primary infection of a European human immunodeficiency virus type 1 clade B isolate in chimpanzees. J Gen Virol. 1998;79:2895–2903. doi: 10.1099/0022-1317-79-12-2895. [DOI] [PubMed] [Google Scholar]
- 9.Bogers W M, Niphuis H, ten-Haaft P, Laman J D, Koornstra W, Heeney J L. Protection from HIV-1 envelope-bearing chimeric simian immunodeficiency virus (SHIV) in rhesus macaques infected with attenuated SIV: consequences of challenge. AIDS. 1995;9:F13–F18. [PubMed] [Google Scholar]
- 10.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Van-Opstal O, Culp J, Rosenberg M, De-Wilde M, Heidt P, Heeney J L. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine. 1994;12:1141–1148. doi: 10.1016/0264-410x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 12.Burton D R, Moore J P. Why do we not have an HIV vaccine and how can we make one? Nat Med. 1998;4:495–498. doi: 10.1038/nm0598supp-495. [DOI] [PubMed] [Google Scholar]
- 13.Cafaro A, Caputo A, Fracasso C, Maggiorella M T, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo M L, Betti M, Borsetti A, Belli R, Akerblom L, Corrias F, Butto S, Heeney J, Verani P, Titti F, Ensoli B. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat Med. 1999;5:643–650. doi: 10.1038/9488. [DOI] [PubMed] [Google Scholar]
- 14.Cheng-Mayer C, Shioda T, Levy J A. Host range, replicative, and cytopathic properties of human immunodeficiency virus type 1 are determined by very few amino acid changes in tat and gp120. J Virol. 1991;65:6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor R I, Korber B T, Graham B S, Hahn B H, Ho D D, Walker B D, Neumann A U, Vermund S H, Mestecky J, Jackson S, Fenamore E, Cao Y, Gao F, Kalams S, Kunstman K J, McDonald D, McWilliams N, Trkola A, Moore J P, Wolinsky S M. Immunological and virological analyses of persons infected by human immunodeficiency virus type 1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 18.Dittmer U, Brooks D M, Hasenkrug K J. Requirement for multiple lymphocyte subsets in protection by a live attenuated vaccine against retroviral infection. Nat Med. 1999;5:189–193. doi: 10.1038/5550. [DOI] [PubMed] [Google Scholar]
- 19.Dunn C S, Hurtrel B, Beyer C, Gloeckler L, Ledger T N, Moog C, Kieny M P, Mehtali M, Schmitt D, Gut J P, Kirn A, Aubertin A M. Protection of SIVmac-infected macaque monkeys against superinfection by a simian immunodeficiency virus expressing envelope glycoproteins of HIV type 1. AIDS Res Hum Retroviruses. 1997;13:913–922. doi: 10.1089/aid.1997.13.913. [DOI] [PubMed] [Google Scholar]
- 20.Esparza J, Heyward W L, Osmanov S. HIV vaccine development: from basic research to human trials. AIDS. 1996;10:S123–S132. [PubMed] [Google Scholar]
- 21.Gibbs C J, Jr, Peters R, Gravell M, Johnson B K, Jensen F C, Carlo D J, Salk J. Observations after human immunodeficiency virus immunization and challenge of human immunodeficiency virus seropositive and seronegative chimpanzees. Proc Natl Acad Sci USA. 1991;88:3348–3352. doi: 10.1073/pnas.88.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glantz S A. Primer of biostatistics. In: Kaufman B, White B, White J, editors. Medical statistics series. 2nd ed. Vol. 1. Singapore, Republic of Singapore: McGraw-Hill International Editions; 1989. pp. 64–325. [Google Scholar]
- 23.Groenink M, Andeweg A C, Fouchier R A, Broersen S, van-der-Jagt R C, Schuitemaker H, de-Goede R E, Bosch M L, Huisman H G, Tersmette M. Phenotype-associated env gene variation among eight related human immunodeficiency virus type 1 clones: evidence for in vivo recombination and determinants of cytotropism outside the V3 domain. J Virol. 1992;66:6175–6180. doi: 10.1128/jvi.66.10.6175-6180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groenink M, Fouchier R A, de-Goede R E, de-Wolf F, Gruters R A, Cuypers H T, Huisman H G, Tersmette M. Phenotypic heterogeneity in a panel of infectious molecular human immunodeficiency virus type 1 clones derived from a single individual. J Virol. 1991;65:1968–1975. doi: 10.1128/jvi.65.4.1968-1975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeney J, Akerblom L, Barnett S, Bogers W, Davis D, Fuller D, Koopman G, Lehner T, Mooij P, Morein B, de Giuli Morghen C, Rosenwirth B, Verschoor E, Wagner R, Wolf H. HIV-1 vaccine-induced immune responses which correlate with protection from SHIV infection: compiled preclinical efficacy data from trials with ten different HIV-1 vaccine candidates. Immunol Lett. 1999;66:189–195. doi: 10.1016/s0165-2478(98)00157-6. [DOI] [PubMed] [Google Scholar]
- 26.Heeney J L, Beverley P, McMichael A, Shearer G, Strominger J, Wahren B, Weber J, Gotch F. Immune correlates of protection from HIV and AIDS—more answers but yet more questions. Immunol Today. 1999;20:247–251. doi: 10.1016/s0167-5699(98)01437-6. [DOI] [PubMed] [Google Scholar]
- 27.Heeney J L, Bruck C, Goudsmit J, Montagnier L, Schultz A, Tyrrell D, Zolla-Pazner S. Immune correlates of protection from HIV infection and AIDS. Immunol Today. 1997;18:4–8. doi: 10.1016/s0167-5699(97)80005-9. [DOI] [PubMed] [Google Scholar]
- 28.Heeney J L, Holterman L, ten Haaft P, Dubbes R, Koornstra W, Teeuwsen V, Bourquin P, Norley S, Niphuis H. Vaccine protection and reduced virus load from heterologous macaque propagated SIV challenge. AIDS Res Hum Retroviruses. 1994;10:S117–S121. [PubMed] [Google Scholar]
- 29.Heeney J L, Mooij P, Bogers W, Davis D, Morein B, de Giuli Morghen C, Lehner T, Voss G, Bruck C, Koopman G, Rosenwirth B. Multiple immune effector mechanisms as correlates of HIV-1 vaccine protection. In: Girard M, Dodet B, editors. Retroviruses of human AIDS and related animal diseases. 11th ed. Paris, France: Elsevier; 1998. pp. 281–285. [Google Scholar]
- 30.Heeney J L, Teeuwsen V J, van Gils M, Bogers W M, De Giuli Morghen C, Radaelli A, Barnett S, Morein B, Akerblom L, Wang Y, Lehner T, Davis D. Beta-chemokines and neutralizing antibody titers correlate with sterilizing immunity generated in HIV-1 vaccinated macaques. Proc Natl Acad Sci USA. 1998;95:10803–10808. doi: 10.1073/pnas.95.18.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlsson G B, Halloran M, Li J, Park I W, Gomila R, Reimann K A, Axthelm M K, Iliff S A, Letvin N L, Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuwata T, Igarashi T, Ido E, Jin M, Mizuno A, Chen J, Hayami M. Construction of human immunodeficiency virus 1/simian immunodeficiency virus strain mac chimeric viruses having vpr and/or nef of different parental origins and their in vitro and in vivo replication. J Gen Virol. 1995;76:2181–2191. doi: 10.1099/0022-1317-76-9-2181. [DOI] [PubMed] [Google Scholar]
- 34.Kuwata T, Shioda T, Igarashi T, Ido E, Ibuki K, Enose Y, Stahl-Hennig C, Hunsmann G, Miura T, Hayami M. Chimeric viruses between SIVmac and various HIV-1 isolates have biological properties that are similar to those of the parental HIV-1. AIDS. 1996;10:1331–1337. doi: 10.1097/00002030-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 35.LaCasse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 36.Langlois A J, Desrosiers R C, Lewis M G, KewalRamani V N, Littman D R, Zhou J Y, Manson K, Wyand M S, Bolognesi D P, Montefiori D C. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J Virol. 1998;72:6950–6955. doi: 10.1128/jvi.72.8.6950-6955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letvin N L, King N W. Immunologic and pathologic manifestations of infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquir Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 38.Lewis M G, Yalley-Ogunro J, Greenhouse J J, Brennan T P, Jiang J B, VanCott T C, Lu Y, Eddy G A, Birx D L. Limited protection from a pathogenic chimeric simian-human immunodeficiency virus challenge following immunization with attenuated simian immunodeficiency virus. J Virol. 1999;73:1262–1270. doi: 10.1128/jvi.73.2.1262-1270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Lord C I, Haseltine W, Letvin N L, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 40.Lu Y, Pauza C D, Lu X, Montefiori D C, Miller C J. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J Acquir Immune Defic Syndr Hum Retroviral. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Luciw P A, Pratt-Lowe E, Shaw K E, Levy J A, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proc Natl Acad Sci USA. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marthas M L, Sutjipto S, Higgins J, Lohman B, Torten J, Luciw P A, Marx P A, Pedersen N C. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990;64:3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 44.Merson M H. Slowing the spread of HIV: agenda for the 1990s. Science. 1993;260:1266–1268. doi: 10.1126/science.8493570. [DOI] [PubMed] [Google Scholar]
- 45.Mooij P, van der Kolk M, Bogers W M, ten Haaft P J, Van Der Meide P, Almond N, Stott J, Deschamps M, Labbe D, Momin P, Voss G, Von Hoegen P, Bruck C, Heeney J L. A clinically relevant HIV-1 subunit vaccine protects rhesus macaques from in vivo passaged simian-human immunodeficiency virus infection. AIDS. 1998;12:F15–F22. doi: 10.1097/00002030-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murthy K K, Cobb E K, el-Amad Z, Ortega H, Hsueh F C, Satterfield W, Lee D R, Kalish M L, Haigwood N L, Kennedy R C, Steimer K S, Schultz A, Levy J A. Titration of a vaccine stock preparation of human immunodeficiency virus type 1SF2 in cultured lymphocytes and in chimpanzees. AIDS Res Hum Retroviruses. 1996;12:1341–1348. doi: 10.1089/aid.1996.12.1341. [DOI] [PubMed] [Google Scholar]
- 48.Myers G, Korber B, Wain-Hobson S, Jeang K-T, Henderson L E, Pavlakis G N. Human retroviruses and AIDS database. Los Alamos, N.Mex: Los Alamos National Laboratory; 1994. [Google Scholar]
- 49.Osmanov S, Heyward W L, Esparza J. The World Health Organization Network for HIV Isolation and Characterization: summary of a pilot study. AIDS Res Hum Retroviruses. 1994;10:1325–1326. doi: 10.1089/aid.1994.10.1325. [DOI] [PubMed] [Google Scholar]
- 50.Ranjbar S, Jones S, Stott E J, Almond N. The construction and evaluation of SIV/HIV chimeras that express the envelope of European HIV type 1 isolates. AIDS Res Hum Retroviruses. 1997;13:797–800. doi: 10.1089/aid.1997.13.797. [DOI] [PubMed] [Google Scholar]
- 51.Reimann K, Li J T, Veazey R, Halloran M, Park I, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 54.Sauermann U, Schneider J, Mous J, Brunckhorst U, Schedel I, Jentsch K D, Hunsmann G. Molecular cloning and characterization of a German HIV-1 isolate. AIDS Res Hum Retroviruses. 1990;6:813–823. doi: 10.1089/aid.1990.6.813. [DOI] [PubMed] [Google Scholar]
- 55.Stott E J, Almond N, Kent K, Walker B, Hull R, Rose J, Silvera P, Sangster R, Corcoran T, Lines J, Silvera K, Luciw P, Murphy-Corb M, Momin P, Bruck C. Evaluation of a candidate human immunodeficiency virus type 1 (HIV-1) vaccine in macaques: effect of vaccination with HIV-1 gp120 on subsequent challenge with heterologous simian immunodeficiency virus–HIV-1 chimeric virus. J Gen Virol. 1998;79:423–432. doi: 10.1099/0022-1317-79-3-423. [DOI] [PubMed] [Google Scholar]
- 56.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Kryzch U, Marchand M, Ballou W R, Cohen J D. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 57.Ten Haaft P, Verstrepen B, Uberla K, Rosenwirth B, Heeney J. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J Virol. 1998;72:10281–10285. doi: 10.1128/jvi.72.12.10281-10285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Titti F, Sernicola L, Geraci A, Panzini G, Di Fabio S, Belli R, Monardo F, Borsetti A, Maggiorella M T, Koanga-Mogtomo M, Corrias F, Zamarchi R, Amadori A, Chieco-Bianchi L, Verani P. Live attenuated simian immunodeficiency virus prevents super-infection by cloned SIVmac251 in cynomolgus monkeys. J Gen Virol. 1997;78:2529–2539. doi: 10.1099/0022-1317-78-10-2529. [DOI] [PubMed] [Google Scholar]
- 59.Travers K, Mboup S, Marlink R, Gueye-Nidaye A, Siby T, Thior I, Traore I, Dieng-Sarr A, Sankale J L, Mullins C, et al. Natural protection against HIV-1 infection provided by HIV-2. Science. 1995;268:1612–1615. doi: 10.1126/science.7539936. [DOI] [PubMed] [Google Scholar]
- 60.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 61.Vancott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 62.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 63.Verschoor E J, Mooij P, Oostermeijer H, van der Kolk M, ten Haaft P, Verstrepen B, Sun Y, Morein B, Akerblom L, Fuller D H, Barnett S W, Heeney J L. Comparison of immunity generated by nucleic acid-, MF59-, and ISCOM-formulated human immunodeficiency virus type 1 vaccines in rhesus macaques: evidence for viral clearance. J Virol. 1999;73:3292–3300. doi: 10.1128/jvi.73.4.3292-3300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Dalnok G K, Kleinschmidt A, Neumann M, Leib-Moesch C, Erfle V, Brack-Werner R. Productive expression state confers resistance of human immunodeficiency virus (HIV)-2-infected lymphoma cells against superinfection by HIV-1. Arch Virol. 1993;131:419–429. doi: 10.1007/BF01378642. [DOI] [PubMed] [Google Scholar]
- 65.Weber J, Fenyo E, Beddows S, Kaleebu P, Bjorndal A. Neutralization serotypes of human immunodeficiency virus type 1 field isolates are not predicted by genetic subtype. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whatmore A M, Cook N, Hall G A, Sharpe S, Rud E W, Cranage M P. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J Virol. 1995;69:5117–5123. doi: 10.1128/jvi.69.8.5117-5123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]