Abstract
Pheochromocytomas (PCCs) and/or paragangliomas (PGLs) are a challenge to diagnose during pregnancy because of elusive signs and testing difficulties. We report a 25-year-old woman with no pertinent medical history who presented to the hospital with hypertension, vision loss, and weakness and was initially diagnosed with preeclampsia. Imaging showed hemangioblastomas in the medulla and thoracic spine, pancreatic cysts, and a renal cyst. The endocrinology service was consulted for possible PCCs associated with von Hippel-Lindau disease (VHL). Serum and urine normetanephrine levels were elevated despite the lack of overt PCCs/PGLs seen on magnetic resonance imaging and magnetic resonance angiography. The patient was medically managed with doxazosin and then labetalol. Despite successful resection of the hemangioblastoma in the medulla, the patient suffered respiratory distress requiring tracheostomy and venous-venous extracorporeal membrane oxygenation (V-V ECMO) and fetal demise. After 3 months, the patient was discharged to rehabilitation. Follow-up genetics were heterozygous for VHL and Lynch syndrome. DOTATATE positron emission tomography/computed tomography scan showed a small hepatic focus of a maximum standard uptake value of 12.1. Altogether, this case illustrates the importance of prompt diagnosis and proper management of PCCs/PGLs during pregnancy and incorporating genetic information during surveillance to lower morbidity and mortality.
Keywords: pheochromocytoma, paraganglioma, pregnancy, von Hippel-Lindau disease, Lynch syndrome, genetics
Introduction
Pheochromocytomas (PCCs) and paragangliomas (PGLs) are rare neuroendocrine tumors that are often missed during pregnancy, especially when the symptoms can mimic more common conditions related to gestation. They are estimated to occur in only 0.007% of all pregnancies but with a high maternal and fetal mortality up to 48% and 55%, respectively, if not managed appropriately (1). PCCs/PGLs associated with both von Hippel-Lindau disease (VHL) and Lynch syndrome (LS) during pregnancy has not been reported to our knowledge.
VHL is inherited in an autosomal-dominant manner, with 80% of individuals having an affected parent and an incidence of approximately 1 in 36 000. Various mutations of this tumor suppressor gene allow for unchecked cellular response to hypoxia, causing tumors such as hemangioblastomas, renal cell carcinoma, PCCs/PGLs, and many others (2). PCCs associated with VHL tend to be seen in younger patients, are often small, multiple, or bilateral, may be extra-adrenal, and are less likely to be associated with symptoms or biochemical evidence of catecholamine production than those without VHL (3, 4). Catecholamine production due to PCCs in VHL almost exclusively produces normetanephrine, and PGLs are more variable with catecholamine production (5, 6). Furthermore, some PCCs/PGLs express the luteinizing hormone/chorionic gonadotropin receptor (LHCGR), which may explain their dramatic manifestation in pregnancy (7). Altogether, the possibility of occult PCCs/PGLs needs to be considered whenever a patient with suspected VHL requires surgery because of the potential risk of anesthetic complications, including sympathetic overactivity and severe hypertension.
LS is an autosomal-dominant genetic disorder with a reported incidence of approximately 1 in 279. It is caused by germline mutations in the mismatch repair genes of MLH1, MSH2, MSH6, PMS2, and rarely EPCAM, increasing risks for malignancies such as gastrointestinal, genitourinary, brain, and skin (8). There are very few reported cases of LS presenting with PCCs in the adrenal gland and a case of PGL of the retroperitoneuml (9-13) (abstract). We present a pregnant patient who has the increased risks of PCCs/PGLs associated with genetic mutations of VHL and LS and discuss the proper management and follow-up plan.
Case Presentation
A 25-year-old woman with gravida 1 para 0 and no pertinent medical history presented to a clinic at 9 weeks’ gestation with dizziness, nausea, and vomiting, which improved temporarily on antiemetics but progressed with time. Around 20 weeks, she began to have tongue swelling, dysphagia, and episodes of systolic blood pressures around 170s to 200s mm Hg with associated vision loss and bilateral lower-extremity weakness. She was diagnosed with preeclampsia and treated with nifedipine, labetalol, and methylprednisolone at an outside hospital. A magnetic resonance imaging (MRI) scan of the brain showed a mass in the dorsal medulla. She was transferred to our hospital for a higher level of care at 22 weeks’ gestation.
Endocrinology was consulted for possible PCCs due to suspicion of VHL with numerous pancreatic cysts, a renal cyst, and hemangioblastomas in the brainstem and thoracic spine on images. Serum and urine normetanephrines were significantly elevated, but metanephrines were normal. MRI from the head to the pelvis was negative for PCCs, and repeated fractionated serum metanephrines 1 day later showed similar results. Doxazosin 2 mg daily was initiated for about a week, then labetalol 200 mg twice daily was added followed by volume expansion. She underwent a successful resection of brainstem hemangioblastoma at 24 weeks’ gestation. Unfortunately, her hospital course was further complicated by acute hypoxic and hypercapnic respiratory failure from aspiration, viral pneumonia, and acute respiratory distress syndrome, which required intubation, tracheostomy, venous-venous extracorporeal membrane oxygenation (V-V ECMO) and subsequent fetal demise at 27 weeks. V-V ECMO was removed after 1 month and tracheostomy was decannulated a month after that. After 3 months of hospitalization, she was discharged to an inpatient rehabilitation.
Diagnostic Assessment
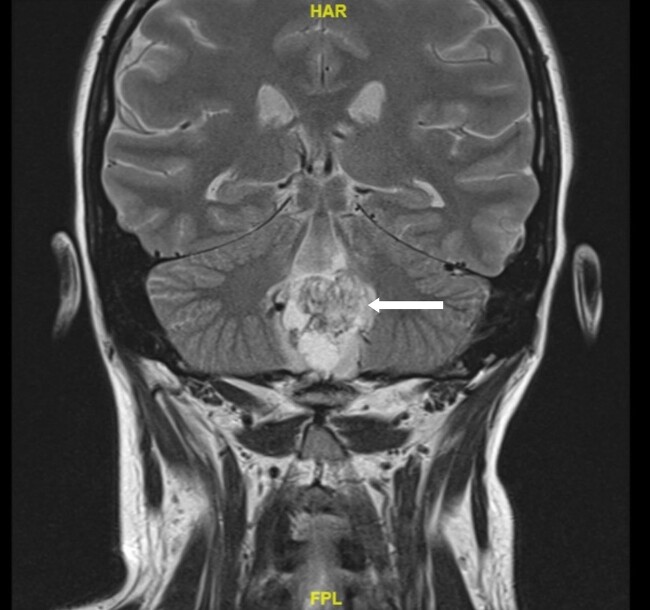
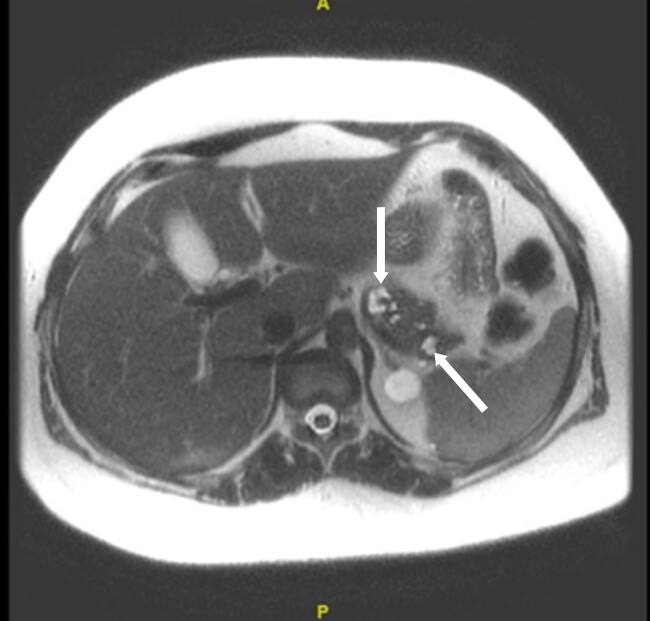
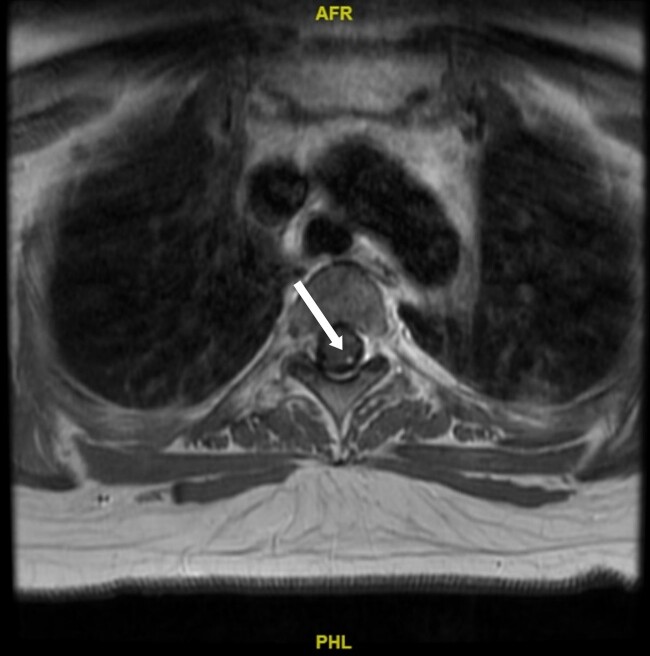
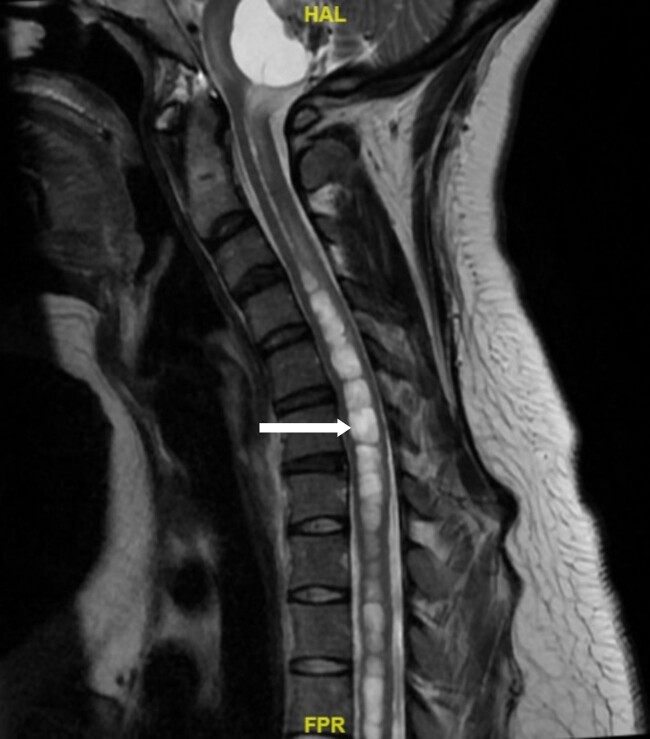
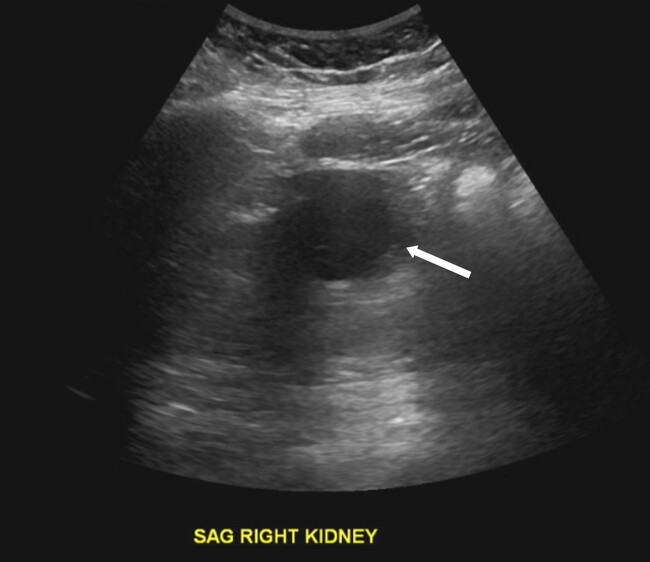
MRI imaging during initial admission showed a 2.6 × 2.1 × 2.7 cm mass centered in the dorsal medulla with cystic and solid components extending across the floor of the fourth ventricle and flow voids posteriorly. The mass extended inferiorly with fullness in the foramen magnum with associated extensive syrinx from C3 to T4 and a punctate enhancing lesion along the left posterior thoracic spinal cord. The ultrasound of the kidneys showed mild hydronephrosis and a 4.5-cm minimally complex cyst in the right kidney. MRI of the abdomen and pelvis showed no definite PCCs/PGLs, but numerous pancreatic cysts with the largest measuring up to 1.6 cm and a single intrauterine gestation in a cephalic position. These phenotypes are demonstrated in Figs. 1 to 5. After medulla mass resection, pathology confirmed a 3.0 × 2.7 × 1.3 cm hemangioblastoma, central nervous system World Health Organization grade 1.
Figure 1.
A magnetic resonance image of the brain. Arrow indicates a 2.6 × 2.1 × 2.7 cm hemangioblastoma centered in the dorsal medulla extending across the floor of the fourth ventricle with cystic and solid components and flow voids posteriorly.
Figure 5.
A magnetic resonance image of the abdomen and pelvis. Arrows indicate numerous pancreatic cysts, the largest measuring about 1.6 cm in the distal body, without definite ductal communication.
Figure 2.
A magnetic resonance image of the spine. Arrow indicates a 2 × 4 mm hemangioblastoma seen along the left posterior thoracic spinal cord at T3 to T4.
Figure 3.
A magnetic resonance image of the spine. Arrow indicates an associated syrinx formation of the spinal cord starting at the level of C3 to C4 and extending to the level of T3 to T4 measuring up to 9 mm in diameter.
Figure 4.
An ultrasound of the kidneys. Arrow indicates a minimally complex cyst seen along the right kidney measuring 4.5 cm.
Her initial fractionated serum metanephrines showed normetanephrine at 313 pg/mL (1709 pmol/L) (reference range ≤ 148 pg/mL [808 pmol/L]), and 24-hour normetanephrine was 1723 mcg/24 hours (9408 nmol/day) (reference range 40-412 mcg/24 hours [218-2250 nmol/day]) (Table 1). Repeat fractioned serum metanephrines a day after had similar results. Initial VHL gene testing with next-generation sequencing was negative.
Table 1.
Biochemical parameters for pheochromocytomas/ paragangliomas during and 3 months after hospitalization
| Laboratory | During hospitalization | After hospitalization | Normal range |
|---|---|---|---|
| Serum, metanephrine | <25 pg/mL (≤127 pmol/L) |
<25 pg/mL (≤127 pmol/L) |
≤ 57 pg/mL (289 pmol/L) |
| Serum, normetanephrine |
313 pg/mL
(1709 pmol/L) |
201 pg/mL
(1097 pmol/L) |
≤148 pg/mL (808 pmol/L) |
| Urine, metanephrine | 199 mcg/24 h (1009 nmol/d) |
— | 25-222 mcg/24 h (127-1126 nmol/d) |
| Urine, normetanephrine |
1723mcg/24 h
(9408 nmol/d) |
— | 40-412 mcg/24 h (218-2250 nmol/d) |
Abnormal values are shown in bold. Values in parenthesis are International System of Units (SI).
Treatment
After endocrinology was consulted, doxazosin was started and titrated to 2 mg daily while other antihypertensives were stopped. After 1 week, labetalol was added and titrated to 200 mg twice a day followed by normal saline in preparation for surgery. Post operation, doxazosin and labetalol were continued.
Outcome and Follow-up
The patient was discharged to inpatient rehabilitation and followed by a multidisciplinary team after being released home. A follow-up genetic testing with targeted sequencing of 85 genes including SDH subtypes was performed by DNA sequencing with deletion/duplication RNA analysis that revealed a heterozygous c.1831dupA (p.I611Nfs*2) pathogenic mutation in the PMS2 gene for LS and a heterozygous p.N78S (c.233A>G) pathogenic mutation in the VHL gene per American College of Medical Genetics and Genomics criteria.
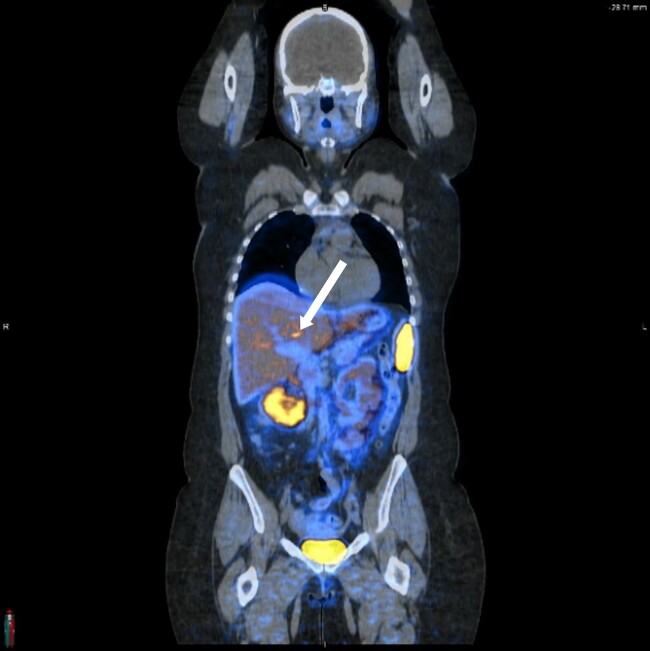
During follow-up, her blood pressure has been well controlled with doxazosin alone. Laboratory tests showed normetanephrine at 201 pg/mL (1097 pmol/L) and metanephrine ≤ 25 pg/mL (≤127 pmol/L) (see Table 1). A Ga-68 DOTATATE scan showed a small hepatic focus of increased tracer activity (maximum standard uptake value of 12.1) as illustrated in Fig. 6.
Figure 6.
A DOTATATE positron emission tomography/computed tomography scan. Arrow indicates a small hepatic focus of increased tracer activity with a maximum standard uptake value of 12.1.
Discussion
Patients with PCCs/PGLs may present with classic symptoms such as headache, hypertension, palpitations, sweating, and others (14). Conditions that can trigger these symptoms and precipitate adrenergic crises include increases in abdominal pressures such as straining or fetal growth, certain foods like cheese and caffeine, drugs, stress, and anesthesia (15). Our patient developed hypertension since 9 weeks of gestation with increasing intensity over time with new onset of vision loss and extremity weakness. It is difficult to distinguish these from common diagnoses during pregnancy such as hyperemesis, gestational hypertension, preeclampsia, eclampsia, and gestational diabetes mellitus (16). Due to the physiological changes of pregnancy, severe hypertension prior to 20 weeks’ gestation is rare and a secondary cause should be investigated (17). During pregnancy in a patient with PCCs/PGLs, the fetus is usually protected from maternal catecholamines due to the degrading monoamine oxidase and catechol O-methyl transferase from the placenta. However, uteroplacental circulation can still be negatively affected, and uteroplacental insufficiency from vasoconstriction can lead to growth restriction or demise (1, 18).
For diagnosis of PCCs/PGLs, plasma-free metanephrines and urinary fractionated metanephrines have the best sensitivities but suboptimal specificities (14, 19, 20). If those are 4 times or more of the upper limit, then PCCs/PGLs are highly probable and provide confidence for further imaging and treatment. If they are below 4 times the upper limit, it could be falsely elevated due to being in a stimulated sympathetic state and/or interfering medications. A clonidine suppression test can further help guide diagnosis (21). However, pregnancy limits other testing options due to the toxicity of certain testing agents (14). Since the patient’s laboratory values were about 2 times the upper limit for plasma-free normetanephrines and greater than 4 times the upper limit for urinary normetanephrines, further imaging and management were pursued. Most sporadic or inherited PCCs will produce both metanephrines and normetanephrines, but VHL-associated PCCs more often exclusively produce normetanephrine due to the lack of phenylethanolamine N-methyltransferase from the upregulated hypoxia-driven angiogenic genes (22).
While our patient did not have any remarkable findings on CT and MRIs, functional imaging or scintigraphy such as meta-iodobenzylguanidine (MIBG) with radioactive iodine or PET/CT with radiotracer 18F-FDG (18F-fluorodeoxyglucose) or Ga-68 DOTATATE is more adept at detecting PGLs with its variable location. These radioactive agents, however, are not safe for the fetus (14, 23).
The optimum management of PCCs/PGLs during pregnancy is proper medical management to prevent potential adrenergic crises and then surgically remove them after delivery. It is important to perform the sequential treatment with α-adrenergic blockers, then β-adrenergic blockers and followed by volume expansion. Phenoxybenzamine used to be the preferred α-adrenergic blocker, but most hospitals do not have it in stock, and other selective α-adrenergic blockers such as prazosin and doxazosin can be used safely in pregnancy (1, 14, 24). If antepartum surgery is needed, it is usually not recommended during the first trimester due to a higher incidence of miscarriages. It can be conducted during the second trimester and, if after 24 weeks, usually it is performed during or after the cesarean delivery (1, 16, 24).
With a high clinical suspicion for VHL, genetic testing with RNA analysis was performed to improve sensitivity, where it was positive for both VHL and LS. PCCs/PGLs are more common in some subtypes of VHL. Type 1 is associated with missense and truncating mutations that grossly disrupt the folding of the VHL protein, has the lowest risk of less than 5% for PCCs, and is associated with retinal hemangioblastoma, central nervous system hemangioblastoma, renal cell carcinoma, pancreatic cysts, and neuroendocrine tumors such as PGLs (2, 4, 14, 25). Our patient with a genetic mutation of p.N78S (c.233A>G) is most likely to have VHL type 1.
Similarly, each mismatch repair gene for LS has different associated risks. Our patient has the genetic mutation c.1831dupA (p.I611Nfs*2) of the PMS2 gene. Unlike the more common and higher-risk genetic mutations in MLH1 and MSH2, PMS2 has a 3 to 5 times increase in colon and endometrial cancers in people older than 60 years compared to the general population, respectively (26). In terms of PCCs/PGLs, there have been few reported cases in LS (9-13). While these mutations of mismatch repair genes can increase the frequency of PCCs/PGLs, it is hard to say whether they have directly contributed or are a coincidence. For further surveillance, our patient will need close follow-up by a multidisciplinary team.
Learning Points
PCCs/PGLs are rare neuroendocrine disorders that can mimic other conditions and cause high mortality and morbidity during pregnancy if not recognized early.
Accurate diagnosis of PCCs/PGLs is more difficult in pregnancy due to increased confounding factors, and many tests are contraindicated.
Some PCCs/PGLs have rapid development and symptoms during pregnancy due to LHCGR stimulation and increased abdominal pressures of the fetus, uterine contractions, and stress.
Medical management for PCCs/PGLs with right sequential of an α-adrenergic blocker and then a β-adrenergic blocker is crucial.
Many genetic conditions are associated with PCCs/PGLs, and knowing their association can help with earlier diagnosis and close follow-up.
Contributors
Both authors made individual contributions to authorship. S.M. and M.T. were involved in the diagnosis and management of this patient. M.T. and S.M. were involved in the manuscript preparation and submission. Both authors reviewed and approved the final draft.
Abbreviations
- CT
computed tomography
- LHCGR
luteinizing hormone/chorionic gonadotropin receptor
- LS
Lynch syndrome
- MRI
magnetic resonance imaging
- PCC
pheochromocytoma
- PET
positron emission tomography
- PGL
paraganglioma
- VHL
von Hippel-Lindau disease
- V-V ECMO
venous-venous extracorporeal membrane oxygenation
Contributor Information
Michael Tang, Department of Medicine, Texas A&M School of Medicine, Bryan, TX 77807, USA.
Shumei Meng, Department of Medicine, Texas A&M School of Medicine, Bryan, TX 77807, USA; Division of Endocrinology, Baylor University Medical Center, Dallas, TX 75246, USA.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
- 1. Gruber L, Young WF, Bancos I. Pheochromocytoma and paraganglioma in pregnancy: a new era. Curr Cardiol Rep. 2021;23(6):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nielsen SM, Rhodes L, Blanco I, et al. Von Hippel-Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34(18):2172‐2181. [DOI] [PubMed] [Google Scholar]
- 3. Eisenhofer G, Walther MM, Huynh TT, et al. Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab. 2001;86(5):1999‐2008. [DOI] [PubMed] [Google Scholar]
- 4. Li SR, Nicholson KJ, Mccoy KL, Carty SE, Yip L. Clinical and biochemical features of pheochromocytoma characteristic of Von Hippel–Lindau syndrome. World J Surg. 2019;44(2):570‐577. [DOI] [PubMed] [Google Scholar]
- 5. Eisenhofer G, Lenders JWM, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel–Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med. 1999;340(24):1872‐1879. [DOI] [PubMed] [Google Scholar]
- 6. Rednam SP, Erez A, Druker H, et al. Von Hippel–Lindau and hereditary pheochromocytoma/paraganglioma syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23(12):e68‐e75. [DOI] [PubMed] [Google Scholar]
- 7. Lopez AG, Duparc C, Renouf S, et al. Expression of LHCGR in pheochromocytomas unveils an endocrine mechanism connecting pregnancy and epinephrine overproduction. Hypertension. 2022;79(5):1006‐1016. [DOI] [PubMed] [Google Scholar]
- 8. Idos G, Valle L. Lynch syndrome. In: Adam MP Feldman J Mirzaa GM, et al., ed. GeneReviews®—NCBI Bookshelf. University of Washington; 1993-2024:1‐43. [Google Scholar]
- 9. Rodríguez K, Harris KT, Singla N. Adrenal pheochromocytoma in a patient with Lynch syndrome. Urol Case Rep. 2022;42:102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riff BP, Katona BW, Wilkerson ML, Nathanson KL, Metz DC. HNPCC-associated pheochromocytoma. Pancreas. 2015;44(4):676‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colton MD, Tompkins K, O’Donnell E, et al. Case of metastatic pheochromocytoma and meningiomas in a patient with Lynch syndrome. JCO Precis Oncol. 2022:6: e2100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perrier R, Van Galen P, Pasieka JL, Magliocco T, Innes AM. An unusual tumor spectrum in Lynch syndrome caused by MSH6 mutation. Hered Cancer Clin Pract. 2010;8(Suppl 1):P17. [Google Scholar]
- 13. Ares BJ, Pujante P, Rodriguez R, Lanes S, Delgado E, Menéndez-Torre E. Presented at 22nd European Congress of Endocrinology, online, September 5–9, 2020, OC7.7 (Abstract OC7.1 – OC7.7). Endocrine Abstracts is Published by Bioscientifica; 2020. [Google Scholar]
- 14. Neumánn HPH, Young WF, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552‐565. [DOI] [PubMed] [Google Scholar]
- 15. Därr R, Lenders JWM, Hofbauer LC, Naumann B, Bornstein SR, Eisenhofer G. Pheochromocytoma—update on disease management. Ther Adv Endocrinol Metab. 2012;3(1):11‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yulia A. Pheochromocytoma in pregnancy: a review of the literature. Obstet Gynaecol Cases Rev. 2016;3(5):96. [Google Scholar]
- 17. Wiles K, Damodaram M, Frise C. Severe hypertension in pregnancy. Clin Med (Lond). 2021;21(5):e451‐e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bancos I, Atkinson EJ, Eng C, et al. Maternal and fetal outcomes in phaeochromocytoma and pregnancy: a multicentre retrospective cohort study and systematic review of literature. Lancet Diabetes Endocrinol. 2021;9(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lenders JWM, Pacák K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma. JAMA. 2002;287(11):1427‐1434. [DOI] [PubMed] [Google Scholar]
- 20. Sawka AM, Jaeschke R, Singh RJ, Young WF. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab. 2003;88(2):553‐558. [DOI] [PubMed] [Google Scholar]
- 21. Pappachan JM, Raskauskiene D, Sriraman R, Edavalath M, Hanna F. Diagnosis and management of pheochromocytoma: a practical guide to clinicians. Curr Hypertens Rep. 2014;16(7):442. [DOI] [PubMed] [Google Scholar]
- 22. Eisenhofer G, Huynh T, Pacák K, et al. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel–Lindau syndrome. Endocr Relat Cancer. 2004;11(4):897‐911. [DOI] [PubMed] [Google Scholar]
- 23. Itani M, Mhlanga J. Imaging of pheochromocytoma and paraganglioma. In: Codon Publications eBooks; 2019:41‐61. [PubMed] [Google Scholar]
- 24. Oliva R, Angelos P, Kaplan EL, Bakris GL. Pheochromocytoma in pregnancy. Hypertension. 2010;55(3):600‐606. [DOI] [PubMed] [Google Scholar]
- 25. Huang Y, Hu WW, Huang X. Retinal hemangioblastoma in a patient with Von Hippel-Lindau disease: a case report and literature review. Front Oncol. 2022:12:963469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dominguez-Valentin M, Sampson JR, Seppälä TT, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the prospective Lynch syndrome database. Genet Med. 2020;22(1):15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.