Abstract
Background
Understanding changes in diagnostic performance after symptom onset and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure within different populations is crucial to guide the use of diagnostics for SARS-CoV-2.
Methods
The Test Us at Home study was a longitudinal cohort study that enrolled individuals across the United States between October 2021 and February 2022. Participants performed paired antigen-detection rapid diagnostic tests (Ag-RDTs) and reverse-transcriptase polymerase chain reaction (RT-PCR) tests at home every 48 hours for 15 days and self-reported symptoms and known coronavirus disease 2019 exposures immediately before testing. The percent positivity for Ag-RDTs and RT-PCR tests was calculated each day after symptom onset and exposure and stratified by vaccination status, variant, age category, and sex.
Results
The highest percent positivity occurred 2 days after symptom onset (RT-PCR, 91.2%; Ag-RDT, 71.1%) and 6 days after exposure (RT-PCR, 91.8%; Ag-RDT, 86.2%). RT-PCR and Ag-RDT performance did not differ by vaccination status, variant, age category, or sex. The percent positivity for Ag-RDTs was lower among exposed, asymptomatic than among symptomatic individuals (37.5% (95% confidence interval [CI], 13.7%–69.4%) vs 90.3% (75.1%–96.7%). Cumulatively, Ag-RDTs detected 84.9% (95% CI, 78.2%–89.8%) of infections within 4 days of symptom onset. For exposed participants, Ag-RDTs detected 94.0% (95% CI, 86.7%–97.4%) of RT-PCR–confirmed infections within 6 days of exposure.
Conclusions
The percent positivity for Ag-RDTs and RT-PCR tests was highest 2 days after symptom onset and 6 days after exposure, and performance increased with serial testing. The percent positivity of Ag-RDTs was lowest among asymptomatic individuals but did not differ by sex, variant, vaccination status, or age category.
Keywords: SARS-CoV-2, rapid antigen test, SARS-CoV-2 variant, COVID-19, RT-PCR
Rapid antigen tests had highest diagnostic performance 2 days after symptom onset and 6 days after exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Diagnostic performance did not differ by vaccination status, age, sex, or SARS-CoV-2 variant.
Antigen-detection rapid diagnostic tests (Ag-RDTs) are commonly used to diagnose coronavirus disease 2019 (COVID-19) due to their availability for home use, relatively low cost, and ability to return results in 15–20 minutes [1, 2]. Previous US Food and Drug Administration Safety Communications describe methods to minimize the risk of false-negative COVID-19 antigen test results in symptomatic and asymptomatic individuals [3, 4]. However, important questions remain about when to begin testing, particularly among those with symptoms or after close contact with an infected person [5, 6].
Many demographic and viral factors, including age, sex, vaccination status, and severe acute respiratory syndrome coronavirus (SARS-CoV-2) variant, have been associated with changes and differences in symptoms and viral kinetics of SARS-CoV-2 infection [7]. Vaccination, younger age, and infection with the Omicron variant have been associated with fewer symptoms, lower severity of infection, and a higher likelihood of asymptomatic infections [8–10]. The incubation period, or the time from exposure to infection, has been found to differ by variant and age category, which informs testing strategies after exposure to SARS-CoV-2 [11–14]. Furthermore, men have been found to have higher mean and peak viral loads than women throughout SARS-CoV-2 infection [15]. The performance of molecular diagnostics, including reverse-transcriptase polymerase chain reaction (RT-PCR) tests and Ag-RDTs is closely related to detectable viral load; therefore, it is important to determine whether these differences in viral dynamics and symptoms have an impact on diagnostic performance [3].
Using data from the prospective cohort study, Test Us at Home [16], we examined home-collected paired serial Ag-RDTs and RT-PCR tests to compare the percent positivity of these tests and how they differed by time since symptom onset and exposure. We also explored how these findings varied based on vaccination status, variant, sex, and age. The results of this study will inform pragmatic use of at-home Ag-RDTs to detect SARS-CoV-2.
METHODS
Study Population
We used data from the Test Us at Home study, a longitudinal cohort study that evaluated the performance of serial use of Ag-RDTs for detection of COVID-19 [16]. This study enrolled participants aged ≥2 years across the United States between October 2021 and February 2022. The Test Us at Home study aimed to understand the performance of SARS-CoV-2 diagnostics during the onset of infection and in asymptomatic infections. Participants were required to be asymptomatic on enrollment, and recruitment was targeted toward communities across the continental United States with a high incidence of SARS-CoV-2 infection. All participants provided written consent for this study, which was approved by the WIRB-Copernicus Group Institutional Review Board (no. 20214875).
All Test Us at Home participants were asked to conduct Ag-RDT and RT-PCR testing every 48 hours over a 15-day period and record their results within a study app. Every 48 hours, participants received a push notification in the study app, which notified them to begin testing. Additional reminders were sent every 2 hours until testing was complete. During each testing session, 2 anterior nasal swab samples were self-collected at home; one swab was used for performing an Ag-RDT, while the other was sent to a central laboratory for RT-PCR testing. Additional detail about the study design, protocol, and participants are described elsewhere [3, 16].
Only participants who completed ≥1 Ag-RDT or RT-PCR test were included in this analysis. Participants were included in the day past symptom onset (DPSO) analyses if they self-reported any symptoms during the study period and had ≥1 positive RT-PCR result (Supplementary Figure 1). Participants who had an RT-PCR–positive result >14 days before or after symptom onset were excluded, as these symptoms were assumed to be unrelated to the observed infection [17]. Furthermore, participants who reported symptoms on the first day of testing were excluded, to ensure that we most accurately calculated DPSO 0. Participants who reported exposure to SARS-CoV-2 before their RT-PCR–positive result were included in the day past exposure (DPE) analysis. Those with an index RT-PCR–positive result >14 days after the reported exposure were excluded from the DPE analyses. Participants with both symptoms and exposure were included in both analyses if they met both eligibility criteria.
Measures
Participants were prompted to self-report symptoms (fever, body aches, fatigue, rash, nausea, abdominal pain, diarrhea, loss of smell, runny nose, cough, headache, or other) every 48 hours, immediately before testing. Participants also had the ability to report the onset of symptoms at any time if symptoms developed between testing periods. The first day that a participant reported ≥1 symptoms was termed DPSO 0.
Participants self-reported close-contact exposures to COVID-19 at the time of baseline study enrollment and before each testing period. They were asked to report the date of their exposure, the proximity of contact, and the duration of exposure with the infected person. An exposure was defined as being within 6 feet of an infected person without a mask for ≥15 minutes over a 24-hour period. DPE 0 was defined as the first day of the reported exposure.
Vaccination status, sex, and age were self-reported during the enrollment survey. Vaccination status was operationalized into 2 groups: vaccinated (≥1 dose) and unvaccinated (0 doses). Self-reported age was used to assign people by age group, as children (<18 years old) or adults (≥18 years old).
For molecular testing (RT-PCR), 2 high-sensitivity RT-PCR assays (Roche Cobas 6800 SARS-CoV-2 PCR and Quest RC COVID-19 PCR DTC) were performed on each anterior nasal swab sample received at Quest Laboratories, and an additional tiebreaker assay (Hologic Aptima SARS-CoV-2 Transcription Mediated Amplification assay) was performed if assay results were discordant. Samples positive on 2 of 3 assays were counted as a true-positive. Cycle threshold (Ct) values for the E gene from Roche Cobas SARS-CoV-2 test were used to quantify viral load. Participants’ SARS-CoV-2 variants were determined using whole-genome sequencing of SARS-CoV-2 by amplicon-based next-generation sequencing on extracted RNA. Participants without sequencing results were excluded in variant stratified analyses (n = 20).
Participants were assigned to 1 of 3 rapid antigen tests: Quidel QuickVue At-Home OTC COVID-19 Test, Abbott BinaxNOW COVID-19 Antigen Self Test, or BD Veritor At-Home COVID-19 Test. Test assignment was determined using an automated algorithm based on enrollment numbers and geographic location of the participants. Participants were provided with test-specific instructions with images, per respective emergency use authorizations, to mimic real-world testing conditions. During each testing session, participants were asked to provide an interpretation of each Ag-RDT result (positive, negative, or invalid) and upload a picture of the test result to the study app. All self-reported positive test results were confirmed by study coordinators using uploaded images.
For data analysis, demographic factors for eligible participants were tabulated and described. Ct values were averaged on each DPSO and DPE, stratified by variant, sex, age category, and vaccination status, and 95% confidence intervals (CIs) were calculated using Wilson's method [18]. Ct values for symptomatic and asymptomatic participants were also calculated by DPE. The percent positivity of symptomatic and/or exposed participants was calculated for RT-PCR tests and Ag-RDTs by DPSO and DPE with 95% CIs [18]. The denominator for percent positivity was the number of participants who tested positive on the composite RT-PCR result at least once during the observation period (DPSO −14 to 14 and DPE 0–14) and recorded a test on that specific DPSO or DPE. The numerator for percent positivity was the number of participants with a positive test result (Ag-RDT or RT-PCR) on each DPSO or DPE.
Cumulative positivity was calculated at DPSO 4, DPSO 6, DPE 4, and DPE 6. This was defined as the sum of participants with ≥1 positive test result between the beginning of the observation period (ie, DPSO −14 or DPE 0) to the day of calculation, divided by the total number participants who tested positive by RT-PCR comparator at least once during the study. In other words, for cumulative positivity on DPSO 4, it would include the participants who tested positive anytime between DPSO −14 to DPSO 4, divided by the total number of participants who tested positive by molecular comparator during the study period. Analyses for DPSO were stratified by vaccination status, variant, sex, and age category. DPE analyses were stratified by vaccination status and symptom status, due to sample size limitations (comparator group n < 20). All analyses were conducted using R software, version 4.2.1 [19].
RESULTS
Characteristics of Symptomatic and Exposed Participants
Among the 7361 Test Us At Home participants, 146 tested positive for SARS-CoV-2 during the study period, reported symptoms during their infection, and were asymptomatic at the first test, making them eligible for the DPSO analysis. In addition, 96 participants tested positive after a close-contact exposure to SARS-CoV-2 and were eligible for the DPE analysis (Supplementary Figure 1). The majority of exposed participants (69.8%) reported that their close-contact exposure occurred with someone in their household. Among the participants included in DPE analyses, 85 (88.5%) developed symptoms at some point during their infection and were included in both analyses; the mean time from exposure to symptom onset was 4.69 days (interquartile range, 2–6 days) (Table 1). Most participants (88.4%) were previously uninfected with SARS-CoV-2, between the ages of 18 and 44 years, and female (64.4%). Approximately 60% of participants had received ≥2 doses of a SARS-CoV-2 vaccine, and >30% were unvaccinated. The majority of participants included in DPSO analyses (69.2%) were infected with the Omicron variant.
Table 1.
Characteristics of Participants Included in Analyses
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Included in DPSO (n = 146) |
Included in DPE (n = 96) |
|
| Symptomatic | 146 (100.0) | 85 (88.5) |
| No. of previous infections | ||
| 0 | 129 (88.4) | 85 (88.5) |
| 1 | 14 (9.6) | 9 (9.4) |
| ≥2 | 3 (2.1) | 2 (2.1) |
| Age | ||
| <18 y | 22 (15.1) | 17 (17.7) |
| 18–44 y | 91 (62.3) | 52 (54.2) |
| 45–64 y | 29 (19.9) | 24 (25.0) |
| ≥65 y | 4 (2.7) | 3 (3.1) |
| Sex | ||
| Male | 47 (32.2) | 37 (38.5) |
| Female | 94 (64.4) | 58 (60.4) |
| Missing | 5 (3.4) | 1 (1.0) |
| Race | ||
| White | 116 (79.5) | 81 (84.4) |
| Asian | 7 (4.8) | 3 (3.1) |
| Black/African-American | 8 (5.5) | 4 (4.2) |
| Multiracial | 8 (5.5) | 3 (3.1) |
| Other | 4 (2.8) | 5 (5.2) |
| Missing | 3 (2.1) | 0 (0.0) |
| Hispanic | 18 (12.3) | 6 (6.2) |
| Educational level | ||
| Bachelor's degree or higher | 65 (44.5) | 47 (49.0) |
| Some college | 31 (21.2) | 18 (18.8) |
| High school graduate | 23 (15.8) | 15 (15.6) |
| Did not finish high school | 22 (15.1) | 13 (13.5) |
| Don’t know | 0 (0.0) | 3 (3.1) |
| Missing | 5 (3.4) | 0 (0.0) |
| Vaccination status | ||
| Unvaccinated | 47 (32.2) | 32 (33.3) |
| 1 vaccine dose | 6 (4.1) | 3 (3.1) |
| ≥2 vaccine doses | 93 (63.7) | 61 (63.5) |
| SARS-CoV-2 variant | ||
| Delta | 34 (23.3) | 15 (15.6) |
| Omicron | 101 (69.2) | 75 (78.1) |
| Unknown | 11 (7.5) | 6 (6.2) |
Abbreviation: DPE, day past exposure; DPSO, day past symptom onset; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Ct Values by DPSO
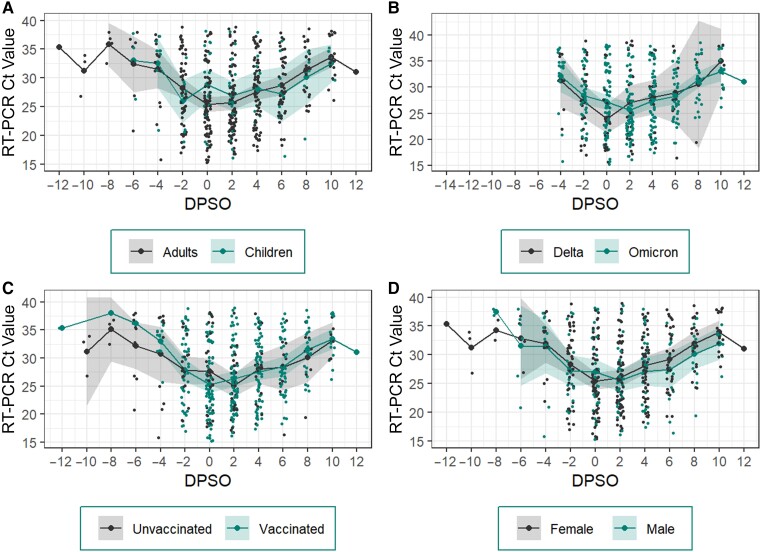
For all symptomatic participants, the mean nadir Ct value was observed on DPSO 2 (Ct, 26.0) (Figure 1). The nadir Ct value for adults occurred on the day of symptom onset (DPSO 0), and Ct values among children were higher than in adults (adults, 25.41 [95% CI, 24.1–26.8]; children, 28.78 [26.0–31.5]) (Figure 1A). Participants infected with the Delta variant appeared to experience the nadir Ct value earlier than those infected with the Omicron variant. With the Delta variant, this peak occurred at DPSO 0, or the first day of symptom onset; however, among participants with the Omicron variant, peak viral load occurred on DPSO 2 (Figure 1B). Vaccinated participants also experienced their peak viral load earlier than unvaccinated participants (DPSO 0 vs 2) (Figure 1C). After DPSO 2, male participants had lower Ct values than female participants, though this difference was not statistically significant (Figure 1D). No significant differences in the magnitude of viral loads were observed by DPSO onset among children compared with adults, or by variant, vaccination status, or sex.
Figure 1.
Reverse-transcriptase polymerase chain reaction (RT-PCR) cycle threshold (Ct) values by day past symptom onset (DPSO). Mean Ct values on DPSO among adults and children (A), Delta and Omicron and severe acute respiratory syndrome coronavirus 2 variants (B), vaccinated and unvaccinated participants (C), and female and male participants (D). Dots represent individual participant values; lines, mean Ct values; and shaded regions, 95% confidence intervals.
Performance of SARS-CoV-2 Diagnostics by DPSO
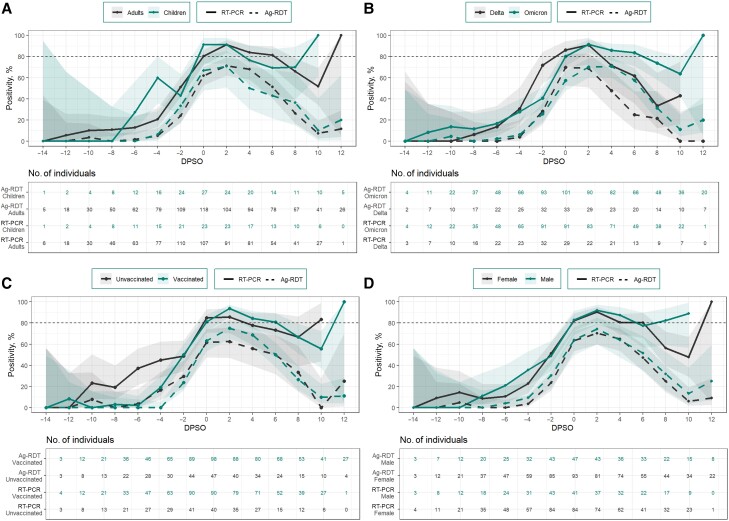
Among all symptomatic individuals, RT-PCR tests and Ag-RDTs performed highest on DPSO 2, or 2 days after symptom onset (RT-PCR, 91.2% [95% CI, 84.6%–95.2%]; Ag-RDT, 71.1% [62.7%–78.2%]) (Supplementary Table 1). On DPSO 2, RT-PCR tests detected 91.2% of infections (95% CI, 84.6%–95.2%), and Ag-RDTs detected 71.1% (62.7%–78.2%). RT-PCR testing and Ag-RDTs performed similarly among children and adults (Figure 2A and Supplementary Table 2), vaccinated and unvaccinated individuals (Figure 2C and Supplementary Table 4), and male and female participants (Figure 2D and Supplementary Table 5). Ag-RDTs detected 84.9% of all infections cumulatively by DPSO 4.
Figure 2.
Reverse-transcriptase polymerase chain reaction (RT-PCR) and antigen-detection rapid diagnostic test (Ag-RDT) positivity by day past symptom onset (DPSO). Filled lines represent RT-PCR results; dashed lines, Ag-RDT results; and shaded regions, 95% confidence intervals. Percent positivity of Ag-RDT and RT-PCR by DPSO is shown among adults (black) and children (green) (A), individuals with the Delta (black) and Omicron (green) variants (B), unvaccinated (black) and vaccinated (green) individuals (C), and female (black) and male (green) participants (D). Percent positivity was defined as the number of participants with a positive result (Ag-RDT or RT-PCR) on each DPSO divided by the number of participants who tested positive on RT-PCR at least once during the observation period (DPSO −14 to 14) and recorded a test on that specific DPSO.
On the day of symptom onset (DPSO 0), RT-PCR had a percent positivity of 86.2% (95% CI, 69.4%–94.5%) and 80.2% (70.9%–87.1%) for Delta and Omicron variants, respectively (Figure 2B and Supplementary Table 3). For both variants, the highest percent positivity for RT-PCR was achieved on DPSO 2 (Delta, 90.9% [95% CI, 72.2%–97.5%]; Omicron, 91.6% [83.6%–95.9%]). There were no significant differences in the performance of Ag-RDTs between participants with Delta and Omicron variants; however, we did observe that participants with the Omicron variant had their highest percent positivity on DPSO 4 (70.7% [95% CI, 60.1%–79.5%), while those with the Delta variant which had their highest percent positivity on DPSO 0 (69.7% [52.7%–82.6%]).
Ct Values by DPE
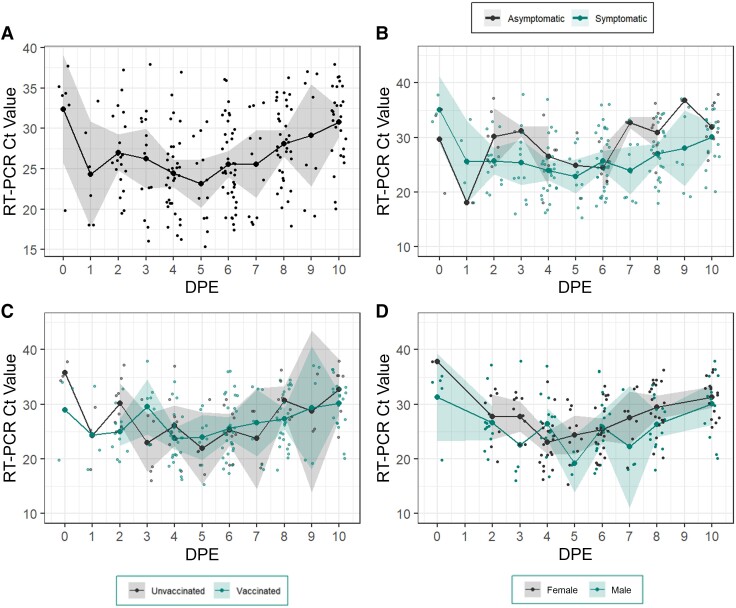
For participants who experienced a close-contact SARS-CoV-2 exposure, the lowest mean Ct value occurred on DPE 5 (Ct, 23.6 [95% CI, 20.6–26.6]) (Figure 3A). Despite wide CIs, symptomatic individuals had lower mean Ct values than asymptomatic individuals throughout the infection period, except on DPE 6, when Ct values were roughly equivalent (Figure 3B). We did not observe any differences in Ct values between vaccinated and unvaccinated participants (Figure 3C). Finally, male participants appeared to have lower Ct values than female participants throughout their infections; however, due to sample size, CIs were wide (Figure 3D).
Figure 3.
Cycle threshold (Ct) values by day past exposure (DPE). Mean Ct values on day past exposure (DPE) among all exposed participants (A), symptomatic and asymptomatic participants (B), vaccinated and unvaccinated participants (C), and female and male participants (D). Dots represent individual participant values; lines, mean Ct values; and shaded regions, 95% confidence intervals.
Performance of SARS-CoV-2 Diagnostics by DPE
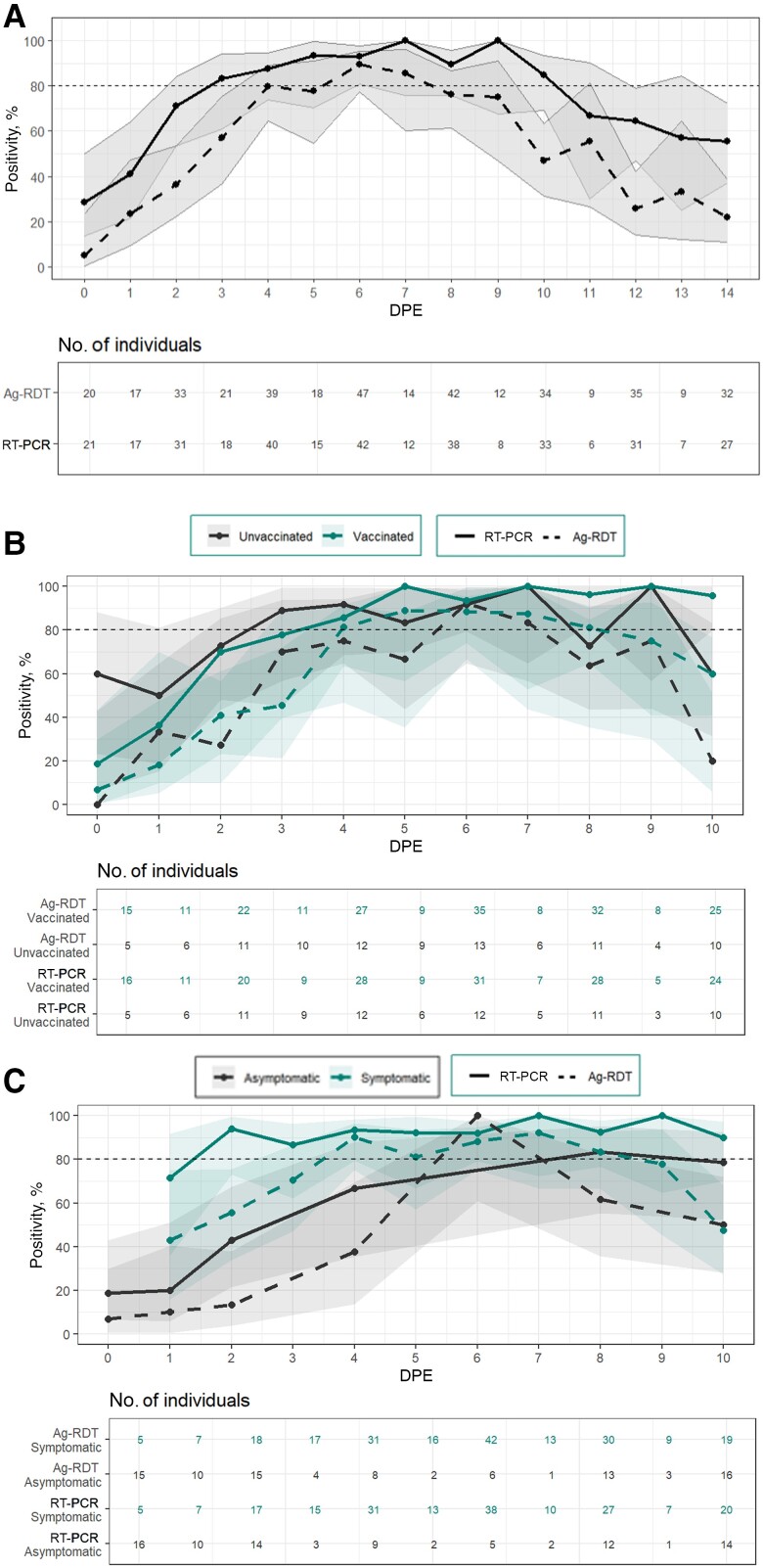
Among exposed individuals, the percent positivity for RT-PCR tests and Ag-RDTs was highest on DPE 6, or 6 days after close-contact exposure to SARS-CoV-2 (Figure 4A and Supplementary Table 6). On DPE 6, RT-PCR detected 91.8% of infections (95% CI, 80.8%–96.8%), and Ag-RDTs detected 86.2% (75.1%–92.8%). Among vaccinated individuals, the highest percent positivity of RT-PCR was on DPE 8, with percent positivity of 96.6% (95% CI, 82.8%–99.8%) (Figure 4B). For unvaccinated participants, the highest percent positivity of RT-PCR occurred on DPE 6 (93.8% [95% CI, 71.7%–99.7%]). The highest percent positivity for Ag-RDTs occurred on DPE 6 among both vaccinated and unvaccinated individuals. We observed no differences between percent positivity for RT-PCR and Ag-RDTs between male and female participants.
Figure 4.
Reverse-transcriptase polymerase chain reaction (RT-PCR) and antigen-detection rapid diagnostic test (Ag-RDT) positivity by day past exposure (DPE). Filled lines represent RT-PCR results; dashed lines, Ag-RDT results; shaded regions, 95% confidence intervals. Exposure was defined as being within 6 feet of an infected person without a mask for ≥15 minutes over a 24-hour period. DPE 0 was defined as the first day of the reported exposure. The percent positivity for Ag-RDTs and RT-PCR tests by day past exposure is shown among all exposed participants (A), unvaccinated (black) and vaccinated individuals (green) (B), and asymptomatic (black) and symptomatic (green) participants (C). Percent positivity was defined as the number of participants with a positive test result (Ag-RDT or RT-PCR) on each DPE divided by the number who tested positive with RT-PCR at least once during the observation period (DPE 0 to 10) and recorded a test on that specific DPE.
The percent positivity of RT-PCR for exposed, symptomatic participants was consistently high (>90%) starting at DPE 2 (Figure 4C and Supplementary Table 7). The percent positivity for Ag-RDTs among symptomatic, exposed participants peaked at DPE 4 (90.3% [95% CI, 75.1%–96.7%). Though CIs were wide, the performances of Ag-RDTs and RT-PCR were consistently lower among asymptomatic individuals, and the percent positivity on DPE 4 was 66.7% (95% CI, 35.4%–87.9%) for RT-PCR and 37.5% (13.7%–69.4%) for Ag-RDTs.
DISCUSSION
We report the performance of nasal-swab Ag-RDTs and RT-PCR in home-based settings by time since symptom onset and exposure by sex, age category, vaccination status, and variant. We identified 3 important findings: (1) the performance of Ag-RDTs and RT-PCR peaked on DPSO 2 for symptomatic individuals and on DPE 6 for exposed individuals; (2) the timing of viral peak did not differ by sex, vaccination status, or age; (3) the performance of SARS-CoV-2 diagnostic tests is similar among vaccinated and unvaccinated participants, adults and children, and male and female participants and for Delta and Omicron variants. Taken together, these findings reinforce the importance of Ag-RDTs for detection of SARS-CoV-2 virus.
As the pandemic enters its fourth year, use of COVID-19 diagnostics has shifted away from general screening and mandated testing toward personal risk assessment, with most people using Ag-RDTs in response to acute symptoms or COVID-19 exposure [20]. It is increasingly important to advise individuals on the timing of Ag-RDT use, to facilitate accurate test interpretation and minimize false-negative results. The present results reinforce the importance of serial testing when individuals are either symptomatic or exposed to SARS-CoV-2, in line with previous recommendations [4]. For symptomatic individuals in our study, >85% of PCR-confirmed infections were detected with Ag-RDTs by DPSO 4, indicating that serial testing on DPSO 2 and DPSO 4 offers an effective strategy for detection of SARS-CoV-2 with Ag-RDTs. Among exposed participants, Ag-RDTs detected nearly 80% of PCR-confirmed infections cumulatively by DPE 6, and performance was highest on DPE 6. We observed lower performance of Ag-RDTs among asymptomatic, exposed individuals compared with symptomatic, exposed individuals, also indicating the need for serial testing within this group.
Several prior studies have examined Ag-RDT performance when tests are used serially, but these studies predate the arrival of the Omicron variants in the United States and widespread vaccination coverage [21, 22]. We observed that viral load peaked on DPSO 4 for participants with the Omicron variant, compared with DPSO 0 for the Delta variant, consistent with other recent studies [23]. However, we did not observe that Ag-RDT performance differed significantly by variant, despite the differences in viral peaks.
A previous study of 225 individuals with PCR-confirmed SARS-CoV-2 infections in the spring of 2021 similarly found that RT-PCR had a positivity rate of approximately 60% on the day of illness onset (defined as symptom onset among symptomatic individuals and first RT-PCR–positive result among asymptomatic individuals) [22]. These investigators also found that Ag-RDTs had lower sensitivity among participants with ≥1 dose of the SARS-CoV-2 vaccine, which differs from our own findings. The observed difference may be explained by the vaccine's declining effect on immunity over time, as many individuals in our study had received the SARS-CoV-2 vaccine >6 months before enrollment, as well as the difference in SARS-CoV-2 variants.
The SARS-CoV-2 vaccine has been found to have lower efficacy in preventing infection from the Delta and Omicron variants compared with previous variants, and studies have found no effect of the SARS-CoV-2 vaccine on symptomatic disease 20 weeks after vaccination [24, 25]. We also observed that peak viral load did not differ between vaccinated and unvaccinated individuals, consistent with previous reports [26]. This further emphasizes the value of reevaluating SARS-CoV-2 diagnostic performance as new variants continue to arise.
Children have consistently demonstrated milder clinical presentations than adults when infected with SARS-CoV-2; however, the mechanism behind this difference between children and adults remains unknown [27, 28]. Pediatric patients often present with fewer or atypical symptoms than adults, which can complicate diagnosis [29]. Furthermore, most previous research on pediatric SARS-CoV-2 infections has occurred in hospitalized patients, even though the majority of pediatric infections are mild and self-limited. Despite the differences in severity of infections among children and adults, our results match previous findings, which showed no differences in viral loads between children and adults [30, 31]. Although viral load did not differ significantly between children and adults, it has been suggested that the difference in severity among children and adults may be associated with a faster rate of viral clearance, leading to milder infections [32]. Also notably, diagnostic performance did not differ significantly between the 2 groups.
Finally, previous studies have found that males have higher peak viral loads than females during infection after adjusting for symptoms, as well as higher rates of severe infection and mortality rates [15, 33]. We did not observe differences in diagnostic performance by sex, nor did we see substantial differences in viral load by DPSO. This may be due to sociological differences in how men and women report their symptoms and perceived risk [34–36], as well as the potential immunological differences in the timing of symptom onset within an infection among men and women, which were not addressed in the design of the current study [37, 38]. Future studies examining objective symptom measures, including temperature, may provide additional information in assessing the comparison of diagnostic performance between men and women by DPSO.
Among the strengths of the current study, it is one of the first to analyze the longitudinal diagnostic performance of RT-PCR tests and Ag-RDTs for COVID-19 based on days past acute symptom onset or exposure to SARS-CoV-2. It assessed serial paired longitudinal data to evaluate the performance of Ag-RDTs and RT-PCR over the duration of infection, using a large nationwide sample of children and adults. It is also, to the best of our knowledge, the first study to quantify time from exposure to Ag-RDT positivity.
However, our study also has limitations. Paired Ag-RDT and RT-PCR testing, as well as symptom trackers, were completed by participants every 48 hours, but participants were able to report symptoms outside these windows. Assessing diagnostic performance at a finer temporal resolution may be useful in future studies. Symptoms, exposures, and Ag-RDT results were based on participant self-report. In this analysis, we grouped anyone with ≥1 vaccine for SARS-CoV-2 as vaccinated due to sample size limitations; however, there may be heterogeneity in the vaccine responses and immunity within this group. In addition, data collection for this study occurred between October 2021 and February 2022, which captured the transition from the predominance of the Delta to the Omicron variant; however, SARS-CoV-2 has continued to evolve, and current circulating Omicron subvariants may have significant differences in transmission and viral dynamics compared to previous strains [39, 40]. Additional research is indicated to examine diagnostic differences in current and future circulating strains. Finally, due to sample size limitations, we were unable to analyze performance by DPE by sex, age, and variant.
In conclusion, the percent positivity for Ag-RDTs and RT-PCR tests was highest on DPSO 2 and DPE 6, and performance increased with serial testing. The performance of Ag-RDTs was lowest among asymptomatic individuals but did not differ by sex, variant, vaccination status, or age category. This confirms that Ag-RDTs remain a useful tool across populations for detection of SARS-CoV-2 in the setting of symptoms or exposures.
Supplementary Material
Acknowledgments
We are grateful to our study participants and to our collaborators from the National Institutes of Health (National Institute of Biomedical Imaging and Bioengineering and National Heart, Lung, and Blood Institute), who provided scientific input into the design of this study and interpretation of our results but could not formally join as coauthors because of institutional policies, and to the Food and Drug Administration (Office of In Vitro Diagnostics and Radiological Health) for their involvement in the primary Test Us at Home study. We received meaningful contributions from Bruce Tromberg Ph.D., Jill Heemskerk Ph.D., Felicia Qashu Ph.D., Dennis Buxton Ph.D., Erin Iturriaga DNP, MSN, RN, Jue Chen Ph.D., Andrew Weitz Ph.D., and Krishna Juluru MD. We are also thankful to county health departments across the country who helped with recruitment for this siteless study by spreading the word in their networks.
Author contributions. Conceptualization of the research question: N. H., L. G., S. G., C. A., R. M., W. H., Y. C. M., K. R., and A. S. Design of methodology: C. H., B. W., H. L., Y. Y., C. P., P. S., C. W., T. S., E. H., S. S., C. N., V. K., S. C. L., L. O., and J. B. Data collection and curation: C. H., C. W., E. O., S. W., A. Z., B. B., A. F., A. C., and A. S. Formal analysis and writing of the original draft: C. H., B. W., H. L., and A. S. Supervision: B. S. G., Y. C. M., N. F., K. L., J. B., and D. D. M. Administrative support: C. P., P. S., and C. W. Funding acquisition: N. H., K. L., J. B., D. D. M., and A. S. Reviewing and editing of the manuscript: All authors.
Disclaimer. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institute of Biomedical Imaging and Bioengineering, the National Heart, Lung, and Blood Institute, the National Institutes of Health, the Food and Drug Administration, or the US Department of Health and Human Services.
Financial support. This work was supported by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute through the RADx Tech program (grant 3U54HL143541-02S2) and the NIH National Center for Advancing Translational Sciences (Clinical and Translational Science Award grant UL1TR001453).
Contributor Information
Carly Herbert, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; University of Massachusetts Center for Clinical and Translational Science, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Biqi Wang, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Division of Health System Science, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Honghuang Lin, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Division of Health System Science, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Yi Yan, Division of Microbiology, OHT7 Office of Product Evaluation and Quality, Center for Devices and Radiological Health, US Food and Drug Administration, Silver Spring, Maryland, USA.
Nathaniel Hafer, University of Massachusetts Center for Clinical and Translational Science, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Program in Molecular Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Caitlin Pretz, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Pamela Stamegna, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Colton Wright, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Thejas Suvarna, CareEvolution, Ann Arbor, Michigan, USA.
Emma Harman, CareEvolution, Ann Arbor, Michigan, USA.
Summer Schrader, CareEvolution, Ann Arbor, Michigan, USA.
Chris Nowak, CareEvolution, Ann Arbor, Michigan, USA.
Vik Kheterpal, CareEvolution, Ann Arbor, Michigan, USA.
Elizabeth Orvek, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Steven Wong, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Adrian Zai, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Bruce Barton, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Ben S Gerber, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Stephenie C Lemon, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Andreas Filippaios, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Laura Gibson, Division of Infectious Disease, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Sharone Greene, Division of Infectious Disease, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Andres Colubri, Department of Microbiology and Physiological Systems, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Chad Achenbach, Division of Infectious Disease, Department of Medicine, Havey Institute for Global Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
Robert Murphy, Division of Infectious Disease, Department of Medicine, Havey Institute for Global Health, Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
William Heetderks, National Institute of Biomedical Imaging and Bioengineering, NIH, via contract with Kelly Services, Bethesda, Maryland, USA.
Yukari C Manabe, Division of Infectious Disease, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Laurel O’Connor, Department of Emergency Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Nisha Fahey, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Department of Pediatrics, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Katherine Luzuriaga, University of Massachusetts Center for Clinical and Translational Science, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Program in Molecular Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
John Broach, University of Massachusetts Center for Clinical and Translational Science, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Department of Emergency Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Kristian Roth, Division of Microbiology, OHT7 Office of Product Evaluation and Quality, Center for Devices and Radiological Health, US Food and Drug Administration, Silver Spring, Maryland, USA.
David D McManus, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Division of Health System Science, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Division of Cardiology, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Apurv Soni, Program in Digital Medicine, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Division of Health System Science, Department of Medicine, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA; Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Crozier A, Rajan S, Buchan I, McKee M. Put to the test: use of rapid testing technologies for COVID-19. BMJ 2021; 372:n208. [DOI] [PubMed] [Google Scholar]
- 2. Peeling RW, Olliaro PL, Boeras DI, Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis 2021; 21:e290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soni A, Herbert C, Lin H, et al. Performance of rapid antigen tests to detect symptomatic and asymptomatic SARS-CoV-2 infection. Ann Intern Med 2023; 176:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Center for Devices and Radiological Health . At-home COVID-19 antigen tests-take steps to reduce your risk of false negative results: FDA safety communication. US Food and Drug Administration, 2022. Available at: https://www.fda.gov/medical-devices/safety-communications/home-covid-19-antigen-tests-take-steps-reduce-your-risk-false-negative-results-fda-safety. Accessed 24 August 2023.
- 5. Robinson ML, Mirza A, Gallagher N, et al. Limitations of molecular and antigen test performance for SARS-CoV-2 in symptomatic and asymptomatic COVID-19 contacts. J Clin Microbiol 2022; 60:e0018722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brümmer LE, Katzenschlager S, Gaeddert M, et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: a living systematic review and meta-analysis. PloS Med 2021; 18:e1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol 2023; 21:147–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 2022; 22:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet 2022; 399:1618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Du W, Yu J, Wang H, et al. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection 2020; 48:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation period of COVID-19 caused by unique SARS-CoV-2 strains: a systematic review and meta-analysis. JAMA Netw Open 2022; 5:e2228008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dai J, Yang L, Zhao J. Probable longer incubation period for elderly COVID-19 cases: analysis of 180 contact tracing data in Hubei Province, China. Risk Manag Healthc Policy 2020; 13:1111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quesada JA, López-Pineda A, Gil-Guillén VF, Arriero-Marín JM, Gutiérrez F, Carratala-Munuera C. Incubation period of COVID-19: a systematic review and meta-analysis. Rev Clínica Esp Engl Ed 2021; 221:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogata T, Tanaka H, Irie F, Hirayama A, Takahashi Y. Shorter incubation period among unvaccinated Delta variant coronavirus disease 2019 patients in Japan. Int J Environ Res Public Health 2022; 19:1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herbert C, Manabe YC, Filippaios A, et al. Differential viral dynamics by sex and body mass Index during acute SARS-CoV-2 infection: results from a longitudinal cohort study. Clin Infect Dis. 2024;78:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soni A, Herbert C, Pretz C, et al. Design and implementation of a digital site-less clinical study of serial rapid antigen testing to identify asymptomatic SARS-CoV-2 infection. J Clin Transl Sci 2023; 7:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; 172:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927; 22:209–12. [Google Scholar]
- 19. R Core Team . R: A language and environment for statistical computing. Published 2020. Available at: https://www.R-project.org/. Accessed.
- 20. Rader B. Use of at-home COVID-19 tests—United States, August 23, 2021–March 12, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith RL, Gibson LL, Martinez PP, et al. Longitudinal assessment of diagnostic test performance over the course of acute SARS-CoV-2 infection. J Infect Dis 2021; 224:976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chu VT, Schwartz NG, Donnelly MAP, et al. Comparison of home antigen testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection. JAMA Intern Med 2022; 182:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frediani JK, Parsons R, McLendon KB, et al. The new normal: delayed peak SARS-CoV-2 viral loads relative to symptom onset and implications for COVID-19 testing programs. Clin Infect Dis 2024; 78:301–7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gram MA, Emborg HD, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: a nationwide Danish cohort study. PloS Med 2022; 19:e1003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022; 386:1532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boucau J, Marino C, Regan J, et al. Duration of shedding of culturable virus in SARS-CoV-2 Omicron (BA.1) infection. N Engl J Med 2022; 387:275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr 2021; 175:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020; 145:e20200702. [DOI] [PubMed] [Google Scholar]
- 29. Laws RL, Chancey RJ, Rabold EM, et al. Symptoms and transmission of SARS-CoV-2 among children—Utah and Wisconsin, March–May 2020. Pediatrics 2021; 147:e2020027268. [DOI] [PubMed] [Google Scholar]
- 30. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 31. Chung E, Chow EJ, Wilcox NC, et al. Comparison of symptoms and RNA levels in children and adults with SARS-CoV-2 infection in the community setting. JAMA Pediatr 2021; 175:e212025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ke R, Martinez PP, Smith RL, et al. Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness. Nat Microbiol 2022; 7:640–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open 2020; 10:e040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med 1998; 60:150–5. [DOI] [PubMed] [Google Scholar]
- 35. Galasso V, Pons V, Profeta P, Becher M, Brouard S, Foucault M. Gender differences in COVID-19 attitudes and behavior: panel evidence from eight countries. Proc Natl Acad Sci U S A 2020; 117:27285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Umamaheswar J, Tan C. “Dad, wash your hands”: gender, care work, and attitudes toward risk during the COVID-19 pandemic. Socius 2020; 6:2378023120964376. [Google Scholar]
- 37. Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020; 588:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med 2001; 16:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan SA, Bhuiyan MA, Dewan SMR. JN.1: the present public health concern pertains to the emergence of a novel variant of COVID-19. Environ Health Insights 2024; 18:11786302241228958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeworowski LM, Mühlemann B, Walper F, et al. Humoral immune escape by current SARS-CoV-2 variants BA.2.86 and JN.1, December 2023. Euro Surveill 2024; 29:2300740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.