Abstract
The Lewis acid mediated reaction of allyltributylstannane compounds with β-hydroxy-α-diazo carbonyls gives β-allyl-α-diazo carbonyl products in good yields. This reaction proceeds via a vinyl diazonium ion intermediate which is intercepted by the allylstannane nucleophile. Importantly, the diazo functional group is retained over the course of the reaction to give diazo-containing scaffolds with increased molecular complexity. Methallyltrimethylsilane also serves as a functional allyl transfer reagent in this reaction.
Graphical Abstract
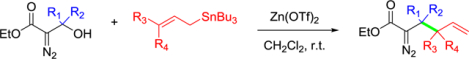
The Lewis acid mediated allylation of β-hydroxy-α-diazo carbonyls gives β-allyl-α-diazo carbonyl products. The diazo functional group is retained over the course of the reaction giving functional group rich products.
Reactions that incorporate allyl groups onto organic scaffolds have proven to be highly useful transformations. Brown’s allylation reaction,1–3 the Hosomi-Sakurai reaction,4–6 the Tsuji–Trost reaction,7–9 and the 1,4-conjugate addition of allyl species to α,β-unsaturated carbonyl compounds10–13 have been widely adopted in synthesis as they deliver versatile building blocks. We recently reported that vinyl diazonium ions generated by treating α-diazo-β-hydroxy carbonyls with Lewis acids (Scheme 1A) readily serve as activated electrophiles in conjugate addition reactions with enoxysilane, indole and alcohol nucleophiles (Scheme 1, equation B and C).14–18 These bond forming reactions proceed in high yield and the diazo functional group is maintained over the course of these reactions giving access to more complex diazo containing scaffolds that can be taken advantage of in subsequent transformations. For example, allyl alcohols add to vinyl diazonium ions to give tetrahydro-3H-furo[3,4-c]pyrazoles via an addition/cycloaddition sequence.18 In view of the importance of allylation reactions, we sought to develop a reaction that would deliver an allyl nucleophile to the β position of a vinyl diazonium ion to give a more functional group rich α-diazo-β-allyl carbonyl product. In this paper we describe the results of these studies.
Scheme 1:

Addition of nucleophiles to vinyl diazonium ions.
Our first objective was to identify a suitable reagent to deliver the allyl group. A variety of allyl metal compounds are known to participate in allylation reactions but concerns about compatibility issues with the Lewis acidic reaction conditions and the sensitive diazo functional group limit the possible nucleophiles in this case. We noted that enoxysilanes and indoles had comparable nucleophilicity on the Mayr reactivity scales and we hypothesized that an allyl metal species with similar nucleophilicity might be competent in these reactions as well. Allylstannanes, and to a lesser degree allylsilanes, fall into the correct nucleophilicity range.19–21 Importantly, Hosomi and Sakurai showed that allylsilanes react with enones via conjugate addition in the presence of Lewis acid.10 Similarly, allylstannanes are well known nucleophiles in carbonyl addition reactions,22 although their use in 1,4-conjugate addition reactions has received less attention.11, 23–25 With this in mind, we hypothesized that these allyl species would be competent nucleophiles toward vinyl diazonium ions resulting in an allyl transfer reaction.
To initiate these studies, we chose benzaldehyde derived β-hydroxy-α-diazoester (1, Table 1) to act as the vinyl diazonium progenitor because it gave high yields of addition products with other nucleophiles. We chose allyltributylstannane (2) as the initial nucleophile due to its simplicity and commercial availability. We were pleased to observe that treating diazo 1 with stannane 2 in the presence of 1 equiv. of Zn(OTf)2 at room temperature gave the desired addition product in quantitative yield. Reducing the Lewis acid loading to sub-stoichiometric levels gave the desired product, but in diminished yields. For the remainder of the study, we chose to use a full equivalent of Lewis acid.
Table 1:
Effect of Lewis acid loading on yield.

|
To assess the scope of the reaction we treated a variety of β-hydroxy-α-diazoesters with allyltributylstannane (2) in the presence of Zn(OTf)2 (Table 2).
Table 2:
Allylation of various diazonium ions
|
Electron rich aryl rings on the diazo component gave good yields of the allylated products. For example, the 4-methoxyphenyl derivative of the diazo ester gave allyl addition product 4 in 88% yield, while the furanyl derivative gave 5 in 70% yield in addition to several unidentified side products. On the other hand, an electron poor phenyl ring bearing a trifluoromethyl substituent gave the addition product 6 in only 5% yield. The difference in these results is likely a consequence of the stability of the vinyl diazonium ion intermediate that is formed upon Lewis acid catalyzed dehydroxylation.17 In an attempt to circumvent this problem, the reaction was tried again using the stronger Lewis acid aluminum (III) triflate. However, this only resulted in creating inseparable byproducts, likely due to degradation of the diazo, without any significant increase to the yield.
β-hydroxy-α-diazoesters derived from alkyl aldehydes were also competent reaction partners, but gave lower product yields than the electron rich or electron neutral aryl analogs (e.g. 3, 4, and 5). For example, the substrate derived from n-pentanal gave the allylated product 7 in 62% yield. In this case, the diazo alcohol starting material itself acted as a competitive nucleophile giving 16% yield of the bis-diazo ether product (7b), which we have observed before in other systems.18 A similar alkyl system that has a silyl ether protecting group distal to the reaction center gave allylation product 8 in moderate yield. In this case the lower yield was at least partially due to loss of material during a difficult separation from small amounts of unidentified byproducts. As would be expected, increased steric hindrance around the electrophilic site prevented the reaction from proceeding, and the tert-butyl derivative gave only trace quantities of allylation product 9. However, β-hydroxy-α-diazoesters derived from ketones, which necessarily have a tertiary alcohol at the β-position, were competent reactants. For example, the β-hydroxy-α-diazoester derived from cyclohexanone gave allylation product 10 which contains a new all carbon quaternary center in 74% yield. Similarly, allylation product 11, which also contains a new all carbon quaternary center, was isolated in 52% yield. These two examples are noteworthy because other tertiary β-hydroxy-α-diazoesters did not react productively with enoxy silane nucleophiles under similar reaction conditions. Lastly, we attempted the allylation reaction on a diazo ketone and a diazo amide substrate. The diazo ketone gave the desired product (12) in only 29% yield. This low yield for product 12 can be attributed to a noticeable increase in byproducts during the reaction, which is likely due to the greater instability of diazo ketones. The diazo amide failed to react under these conditions and returned only the diazo alcohol starting material.
With these results in hand, we next looked to determine if other allylstannanes would add to vinyl diazoniums. We prepared allenyltributylstannane (13, Figure 1) as described by Tagliavini and co-workers,26 and we synthesized (E)-cinnamyl tributylstannane (14), (E)-geranyl tributylstannane (15), and (E)-crotyl tributylstannane (16) from their respective allyl acetates using the methodology developed by Takaki and co-workers.27
Figure 1:

Allylstannane nucleophiles
The allenylstannane reacted with β-hydroxy-α-diazoester 1 to deliver a propargyl group giving alkyne 17 in 58% yield. (E)-cinnamyl tributylstannane reacted with 1 to give diaryl compound 18 in 67% yield as a 2:1 mixture of diastereomers. This stannane also reacted with the β-hydroxy-α-diazoester derived from cyclohexanone to give allylation product 19 which contains a new all carbon quaternary center in 69% yield. (E)-geranyl tributylstannane reacted with 1 to give 20, which also contains a new all carbon quaternary center, in 42% yield and 2 to 1 diastereoselectivity. (E)-crotyl tributylstannane (16) was reacted with the tertiary β-hydroxy-α-diazoester derived from 4-heptanone to give allylation product 21 with a new all carbon quaternary center, albeit in a lower yield of 9%. Finally, (E)-crotyl tributylstannane (16) reacted with ethyl 2-diazo-3-hydroxy-3-(3-furyl)propanoate to give allylation product 22 in 77% yield with 2 to 1 diastereoselectivity.
In addition to the allylstannane nucleophiles, we wondered if allylsilane compounds, which are known to be weaker nucleophiles, would function in these reactions. Unfortunately, most of the allylsilanes we tried failed to give any addition products. However, the more nucleophilic 2-methallyltrimethylsilane (23) did function moderately well as an allyl donor and gave conjugate addition product 24 in 38% yield. It seems likely that other allylsilanes with electron donating groups in the 2 position may be productive reaction partners, but unfortunately, these compounds are not trivial to prepare.
In conclusion, we have demonstrated a new allylation reaction whereby allylstannanes react with vinyl diazonium ions to give β-allyl-α-diazoesters. This allylation reaction is operationally simple and occurs under mild conditions at room temperature. 2-Methallyltrimethylsilane was also a competent allyl transfer reagent in this reaction, but gave the product in only moderate yield. Less nucleophilic allylsilanes failed to give any desired products and coupled with Mayr’s reactivity parameters, these results provide a qualitative indication of how nucleophilic a species must be to react with the vinyl diazonium electrophile. The reaction described here gives functional group rich products that contain an alkene and diazo group and is another example of the synthetic utility of vinyl diazonium ions for the formation of quaternary or tertiary carbon centers.
Supplementary Material
Scheme 2:
Addition of 2-methylallyl trimethylsilane
Table 3:
Addition of various allylstannane nucleophiles to vinyl diazonium ions.

|
Acknowledgements
Financial support from the National Science Foundation (CHE-2102229) is gratefully acknowledged. Mass spectrometry data was acquired in the Agilent Laboratory for Chemical Analysis at The University of Vermont by Bruce O’Rourke, and in the The Vermont Biomedical Research Network Proteomics Facility (RRID: SCR_018667) which is supported through NIH grant P20GM103449 from the INBRE Program of the National Institute of General Medical Sciences
Footnotes
Conflicts of interest
There are no conflicts to declare.
Footnotes relating to the title and/or authors should appear here.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- (1).Kramer GW; Brown HC J. Org. Chem 1977, 42 (13), 2292–2299. [Google Scholar]
- (2).Boiarska Z; Braga T; Silvani A; Passarella D Eur. J. Org. Chem 2021, 2021 (22), 3214–3222. [Google Scholar]
- (3).Boiarska Z; Braga T; Silvani A; Passarella D European Journal of Organic Chemistry 2021, 2021 (22), 3214–3222. [Google Scholar]
- (4).Hosomi A; Sakurai H Tetrahedron Lett. 1976, 17 (16), 1295–1298. [Google Scholar]
- (5).Akira H; Masahiko E; Hideki S Chem. Lett 1976, 5 (9), 941–942. [Google Scholar]
- (6).Lade JJ; Pardeshi SD; Vadagaonkar KS; Murugan K; Chaskar AC RSC Advances 2017, 7 (13), 8011–8033, 10.1039/C6RA27813B. [DOI] [Google Scholar]
- (7).Tsuji J; Takahashi H; Morikawa M Tetrahedron Lett. 1965, 6 (49), 4387–4388. [Google Scholar]
- (8).Trost BM; Fullerton TJ J. Am. Chem. Soc 1973, 95 (1), 292–294. [Google Scholar]
- (9).Trost BM Tetrahedron 2015, 71 (35), 5708–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hosomi A; Sakurai H J. Am. Chem. Soc 1977, 99 (5), 1673–1675. [Google Scholar]
- (11).Hosomi A; Iguchi H; Endo M; Sakurai H Chemistry Letters 1979, 8 (8), 977–980. [Google Scholar]
- (12).House HO; Fischer WF Jr. J. Org. Chem 1969, 34 (11), 3615–3618. [Google Scholar]
- (13).Yanagisawa A; Habaue S; Yasue K; Yamamoto HJ Am. Chem. Soc 1994, 116 (14), 6130–6141. [Google Scholar]
- (14).Pellicciari R; Natalini B; Sadeghpour BM; Marinozzi M; Snyder JP; Williamson BL; Kuethe JT; Padwa A J. Am. Chem. Soc 1996, 118, 1–12. [Google Scholar]
- (15).Cleary SE; Li X; Yang LC; Houk KN; Hong X; Brewer MJ Am. Chem. Soc 2019, 141 (8), 3558–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Fang J; Howard EM; Brewer M Angew Chem Int Ed Engl 2020, 59 (31), 12827–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Howard EM; Brewer M Acs Catal 2021, 11 (19), 12203–12207. [Google Scholar]
- (18).Peck AM; Brewer M Org. Lett 2023, 25 (15), 2647–2651. [DOI] [PubMed] [Google Scholar]
- (19).Mayr H; Kempf B; Ofial AR Acc. Chem. Res 2003, 36 (1), 66–77. [DOI] [PubMed] [Google Scholar]
- (20).Lakhdar S; Westermaier M; Terrier F; Goumont R; Boubaker T; Ofial AR; Mayr H J. Org. Chem 2006, 71, 9088–9095. [DOI] [PubMed] [Google Scholar]
- (21).Laub HA; Yamamoto H; Mayr H Org. Lett 2010, 12 (22), 5206–5209. [DOI] [PubMed] [Google Scholar]
- (22).Gung BW Additions of Allyl, Allenyl, and Propargylstannanes to Aldehydes and Imines. In Organic Reactions, pp 1–113. [Google Scholar]
- (23).Williams DR; Mullins RJ; Miller NA Chem. Commun 2003, (17), 2220–2221. [DOI] [PubMed] [Google Scholar]
- (24).Beaulieu ED; Voss L; Trauner D Org. Lett 2008, 10 (5), 869–872. [DOI] [PubMed] [Google Scholar]
- (25).Nakamura H; Iwama H; Yamamoto Y J. Am. Chem. Soc 1996, 118 (28), 6641–6647. [Google Scholar]
- (26).Boaretto A; Marton D; Tagliavini GJ Organomet. Chem 1985, 297 (2), 149–153. [Google Scholar]
- (27).Komeyama K; Itai Y; Takaki K Chem.-Eur. J 2016, 22 (27), 9130–9134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


