Abstract
Adequate pain relief is usually achieved with the simultaneous use of two or more different classes of analgesics, often called multimodal analgesia. The purpose of this article is to highlight the use of perioperative multimodal analgesia and the need to individualize the treatment plan based on the presenting condition, and to adjust it based on the response to analgesia for a given patient. This case series presents the alleviation of acute pain in three cats undergoing different major surgical procedures. These cases involved the administration of different classes of analgesic drugs, including opioids, non-steroidal anti-inflammatory drugs, tramadol, ketamine, gabapentin and local anesthetics. The rationale for the administration of analgesic drugs is discussed herein. Each case presented a particular challenge owing to the different cause, severity, duration and location of pain. Pain management is a challenging, but essential, component of feline practice: multimodal analgesia may minimize stress while controlling acute perioperative pain. Individual response to therapy is a key component of pain relief in cats.
Optimal pain relief is generally achieved with the simultaneous use of two or more different classes of analgesics: this is known as multimodal analgesia. In this approach, each analgesic acts at different levels of the nociceptive pathway to produce analgesia. 1 This case series presents the use of multimodal analgesia for controlling perioperative acute pain in three cats undergoing major surgical procedures in a veterinary teaching institution [Ontario Veterinary College (OVC)]. The rationale for the administration of different analgesics is discussed in each case.
Case description
Case 1
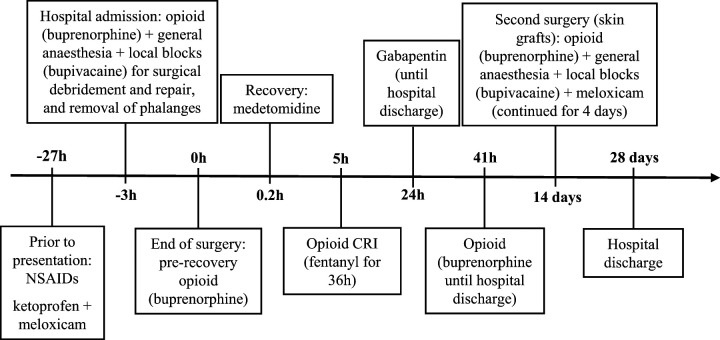
A 5.3 kg 8-year-old male neutered domestic shorthair (DSH) was presented with caustic burns after accidentally stepping on spilled drain-cleaning product 5 days earlier. The lesions affected all feet, including foot pads, and superficial and deep soft tissues. Approximately 24 h before, the referring veterinarian had administered ketoprofen and meloxicam in close temporal association. On admission to OVC, the cat was ambulatory and presented with moderate lameness and minimal response to palpation of the limbs. In the emergency setting, intramuscular (IM) buprenorphine (0.02 mg/kg) was given and used as part of the anesthetic premedication. Prior to, and under, general anesthesia for wound debridement and repair, blockade of the distal branches of the radial, ulnar, median, common peroneal and tibial nerves was performed with bupivacaine 0.5% (2.5 mg per limb), according to the technique described elsewhere. 2 Necrotic skin, tendons and multiple phalanges were removed during surgery. Prior to extubation, a second dose of buprenorphine (0.02 mg/kg) was administered intravenously (IV). During anesthetic recovery, the cat was given medetomidine (1.7 μg/kg IV) owing to emergence delirium. Five hours postoperatively, a constant rate infusion (CRI) of fentanyl was started (1–3 μg/kg/h IV) for the next 36 h. The cat was then administered buprenorphine again (0.01–0.02 mg/kg IV PRN) until hospital discharge (28 days later). Pain assessment was performed by the attending clinician and trained staff, and was based on the cat’s behavior and response to gentle palpation of the limbs. Gabapentin (18.9 mg/kg PO q8h) was started 24 h after surgery and continued during hospitalization. Two weeks after the first intervention, a second surgical procedure was carried out for skin grafts. Premedication, induction agents and local blocks were the same as used in the first anesthetic episode. Meloxicam (0.1 mg/kg SC) was administered intraoperatively and continued for 4 days postoperatively (0.05 mg/kg SC q24h) (Figure 1).
Figure 1.
Timeline to demonstrate the analgesic drugs that were administered to cat 1 during hospitalization.
NSAIDs = non-steroidal anti-inflammatory drugs; CRI = constant rate infusion
Case 2
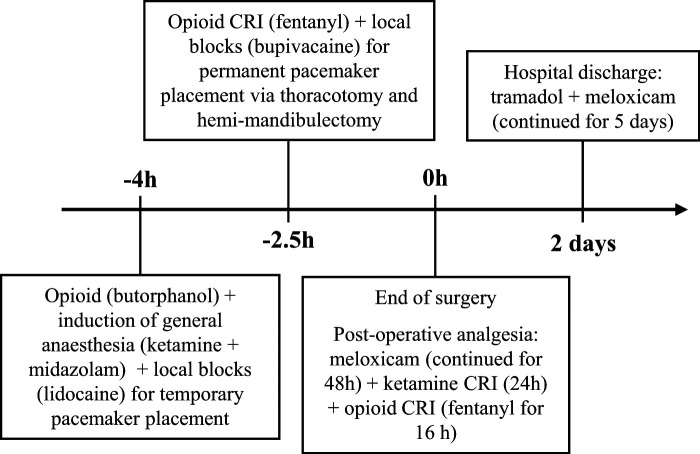
A 4 kg 13-year-old male neutered DSH was presented for permanent epicardial pacemaker placement and hemi-mandibulectomy after previous diagnostic work-up. No signs of pain-related behaviors were observed or noted during physical examination. The cat was premedicated with butorphanol (0.4 mg/kg IV) and induction of anesthesia was performed with a combination of ketamine (5 mg/kg) and midazolam (0.25 mg/kg). Lidocaine 2% (20 mg) was infiltrated in the subcutaneous (SC) tissue for temporary pacemaker placement via the jugular vein. One and a half hours later, fentanyl (2 μg/kg bolus IV; 5–15 μg/kg/h IV CRI) was started for the surgical procedure. An intercostal nerve block was performed by infiltrating 1.25 mg of bupivacaine 0.5% in each of the two adjacent intercostal nerves cranial and caudal to the left fourth intercostal space where the surgical incision was made. 2 The permanent epicardial pacemaker placement was performed via thoracotomy followed by a right hemi-mandibulectomy via a ventral approach. Anesthetic recovery was uneventful. Postoperative analgesic drugs included the administration of meloxicam (0.1 mg/kg SC q24h for 48 h), ketamine (2–16 μg/kg/min IV CRI for 24 h) and fentanyl (2–4 μg/kg/h IV CRI for 16 h). The patient was eating well and seemed to be comfortable at discharge 2 days after surgery. Tramadol (2.5 mg/kg PO q8—12h) and meloxicam (0.04 mg/kg PO q24h) were prescribed for 5 days (Figure 2).
Figure 2.
Timeline to demonstrate the analgesic drugs that were administered to cat 2 during hospitalization. CRI = constant rate infusion
Case 3
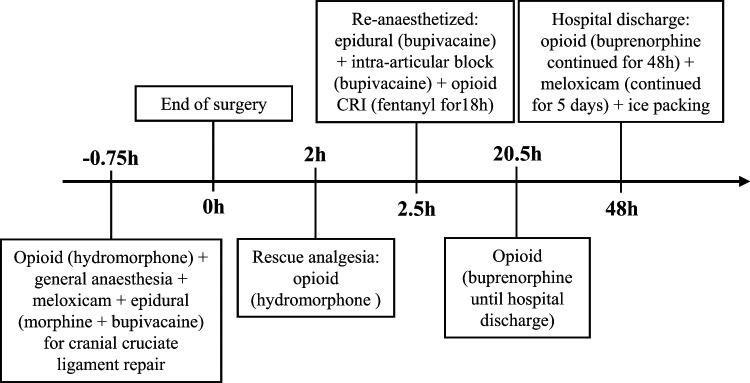
A 9 kg 13-year-old male neutered DSH was presented for evaluation of a 2-week history of right hind limb lameness and stifle joint instability that were consistent with a cranial cruciate ligament rupture. The cat expressed behavioral signs of pain, such as growling with attempts to escape during palpation of the affected pelvic limb. The cat was admitted for stabilization of the right stifle by performing a right lateral extra-capsular suture technique. Hydromorphone (0.04 mg/kg) was administered IM as part of the anesthetic premedication. Under general anesthesia, an epidural injection with morphine (0.1 mg/kg) and bupivacaine (0.8 mg/kg) was performed; meloxicam (0.08 mg/kg SC) was administered pre- emptively. The surgical procedure lasted for 45 min. Two hours after recovery from anesthesia the cat showed signs of discomfort, including restlessness and vocalization. The animal was extremely reactive to touch of the surgical wound area and became extremely aggressive while rolling around the cage. A dose of hydromorphone (0.05 mg/kg IM) was administered, but failed to improve manic reaction of aggression. The clinicians hypothesized that clinical signs were possibly related to central sensitization and wind-up pain. The cat was re-anesthetized and a second epidural injection of bupivacaine, as well as an intra-articular injection of the same local anesthetic (2.5 mg) was performed. A CRI of fentanyl (2–3 μg/kg/h IV) was instituted for the following 18 h. The cat returned to its normal behavior and appeared to remain comfortable until hospital discharge. The fentanyl CRI was stopped and buprenorphine (0.02 mg/kg IV PRN) was administered for the subsequent 18 h. Prescribed treatments included meloxicam (0.05 mg/kg PO q48h for 5 days) and buprenorphine (8 μg/kg buccally q8h for 2 days). The owners were also instructed to perform ice packing by applying a cold compress to the incision three times daily (Figure 3).
Figure 3.
Timeline to demonstrate the analgesic drugs that were administered to cat 3 during hospitalization. CRI = constant rate infusion
Discussion
This case series reports the use of multimodal analgesia for the treatment of surgical, with or without pre- existing pain in three cats. Each case is an example of a particular clinical challenge owing to different cause, severity, duration and location of pain. A lack of consistent underlying approach among clinicians towards pain management was observed, particularly in cases 1 and 2. This is challenging in any veterinary teaching hospital where a single case may be dealt by several different specialists. This report emphasizes the pros and cons of each analgesic therapy, and their combinations employed herein.
Opioid drugs produce analgesia by binding to opioid receptors within the central and peripheral nervous system. They are the cornerstone for the treatment of acute and postoperative pain if one considers their efficacy, safety margins and versatility. 3 Buprenorphine is a partial agonist at µ opioid receptors that binds avidly to, and dissociates slowly from, these receptors. 4 The drug was administered to the cat described as case 1 at presentation owing to its provision of long-term moderate analgesia with few adverse effects.5,6 However, buprenorphine does not elicit a maximal clinical response as a full agonist. Therefore, had the clinicians known that surgery was recommended immediately, a fentanyl CRI would have been a better option because it provides dose-dependent analgesia as a full-agonist, it can be titrated to effect in order to produce inhalant anesthetic-sparing effects and it may be easily administered for postoperative analgesia. The fentanyl infusion was initiated approximately 5 h after buprenorphine administration in an attempt to minimize an interaction with buprenorphine; the mixing of opioid analgesics may produce antagonistic properties and interfere with optimal pain relief as discussed below. The IV route was chosen for administration of buprenorphine after discontinuation of fentanyl CRI because recent studies have shown that the drug provides better anti-nociception after IV administration, and has a faster onset of action, when compared with SC or IM administration. 7
Following induction of anesthesia, ‘declaw’ blocks, as described previously, were performed to provide analgesia and prevent central sensitization in case 1; this technique has been performed in combination with other analgesic modalities in cats undergoing onychectomy. 2 Pre-emptive analgesia (ie, administration of agent before the noxious event) may help to reduce the total amount of anesthetics needed during surgery, as well as anesthetic-induced cardiopulmonary depression and the postoperative analgesics required. Local anesthetics are ideal for providing pre-emptive analgesia as these compounds inhibit nervous impulse generation and conduction by reversibly blocking voltage-dependent sodium channels, thus blocking the transmission of nociceptive input to the spinal cord, and inhibiting central sensitization. 8 These drugs are relatively safe when appropriate doses and techniques are used, albeit central nervous system and cardiovascular toxic effects may be observed when accidentally administered IV or greater than recommended amounts are used. 9 In all the cases described herein, toxic doses were calculated and care was taken to ensure that IV administration did not occur. Indeed, close monitoring of patients is essential when administering local anesthetics at dosages that are close to the ones considered to be toxic for that species.
The same cat received medetomidine during anesthetic recovery and shortly after extubation. The α2 adrenergic agonists are sedatives with muscle relaxant and analgesic properties that are widely used in veterinary medicine. 10 In our experience, small doses of (dex) medetomidine can be used to treat emergence delirium and/or dysphoria after general anesthesia in healthy cats. Transient increases in systemic vascular resistance with reflex bradycardia and further decreases in cardiac output are usually observed after α2 adrenergic administration. Therefore, their use should be restricted to animals that can tolerate such cardiovascular effects. 11
Gabapentin was administered to cat 1 as neuropathic pain was suspected owing to damage of nerve endings and because the drug has been shown to decrease postoperative opioid requirements in humans. 12 This drug has been used widely in the treatment of neuropathic pain, although its mechanism of action is not fully elucidated, and doses are commonly based on anecdotal reports. 13 In cats, the pharmacokinetics of gabapentin have been determined; 14 it seems to be effective as an adjuvant analgesic in the treatment of acute pain. 15 Increasing doses of up to 50 mg/kg PO have been recommended. 13 Gabapentin may be used adjunctively in the treatment of hyperalgesia and allodynia in order to potentiate the effect of other analgesic drugs. 15
Fourteen days after presentation, cat 1 required a second surgical intervention, and the anesthetic and analgesic protocols were similar to the first episode. At this time, meloxicam was administered for treatment of postoperative pain for 4 days. Non-steroidal anti-inflammatory drugs (NSAIDs) are popular for their anti-inflammatory, analgesic and antipyretic properties. Their analgesic effects are secondary to the inhibition of cyclooxygenase enzymes in cell membranes. 16 In healthy cats, short-term administration of NSAIDs has not been correlated with adverse effects, 17 although this may not be the case in cats with naturally-occurring disease in the clinical setting. Cats have limited hepatic glucuronidation and consequent NSAID metabolism, 18 which may induce the development of adverse effects, such as gastric irritation, hepatic and renal damage, and inhibition of platelet aggregation. 19 Meloxicam was chosen as part of the pain management protocol as a recent study in cats has shown that its metabolites are products of oxidative metabolism, and excretion occurs primarily via feces. 20 This drug was given to all cases with no detectable clinical side effects. In case 1, previously to the first anesthetic episode, the cat had been administered two different NSAIDs in close temporal association and, for this reason, NSAID treatment was withheld initially. Although a wash-out interval when switching NSAIDs has not been validated, the use of two NSAIDs in close temporal association is contraindicated. 21
In case 2, lidocaine was infiltrated in the SC tissue before temporary pacemaker placement via the jugular vein to minimize surgical stimulation and provide analgesia. Prior to the thoracotomy, an intercostal block was performed as part of the multimodal analgesic regimen. A mandibular block could have been beneficial prior to the hemi-mandibulectomy, but other analgesic techniques were preferred owing to the risk of seeding neoplastic cells. 22
Butorphanol is anκagonist and a µ opioid receptor antagonist that provides mild and short-acting analgesic effects. 23 The drug was administered as part of the anesthetic premedication in case 2 because temporary pacemaker placement was deemed to cause only mild pain. Retrospectively, a fentanyl or remifentanil CRI would have been a better option to start with in order to avoid a possible opioid interaction between butorphanol and fentanyl. The clinicians tried to minimize this interaction by initiating the fentanyl CRI approximately 1.5 h after butorphanol administration, when the effects of butorphanol were likely to be wearing off. 23 In laboratory settings there has been controversy as to whether or not administration of an agonist–antagonist with a pure µ agonist will diminish analgesic efficacy.24,25 However, mixing full, partial and/or agonist-antagonist opioids should be avoided whenever possible as these combinations may provide less than optimal analgesic effects and unpredictable pain relief in the clinical setting.
Intraoperative fentanyl reduces inhalant anesthetic requirements potentially resulting in less cardiopulmonary depression while also providing analgesia. 26 Doses of fentanyl were titrated in case 2 depending on requirement with lower doses being used to provide the conscious cat with analgesia while higher doses were given during surgery in response to painful manipulations. An NSAID and ketamine CRI were combined with fentanyl for postoperative analgesia. Ketamine is a dissociative anesthetic used commonly in cats. This drug is a non-competitive antagonist of N-methyl D-aspartate receptors involved in the transmission and modulation of nociceptive stimuli in the dorsal horn of the spinal cord, a key player in neuropathic and chronic pain. 27 As part of a multimodal approach, ketamine has shown to improve postoperative analgesia in dogs undergoing forelimb amputation. 28 In this case, ketamine CRI was used as an adjunctive analgesic agent in order to minimize the risk of central sensitization, potentially decreasing opioid requirements.
Two days after surgery the same cat was discharged from hospital and prescribed tramadol and meloxicam for 5 days. Tramadol has weak binding affinity at µ receptors, and also inhibits the reuptake of serotonin and norepinephrine. 3 Subcutaneous administration of tramadol has limited effect on nociceptive thresholds 29 while providing adequate analgesia in cats undergoing ovariohysterectomy. 30 However, oral administration of tramadol provides high oral bioavailability while peak concentrations were reached within 45 mins; doses of 2–4 mg/kg induced significant thermal anti-nociception in cats, possibly making it useful for feline postoperative pain control following hospital discharge.31,32 In cats, dysphoria has been observed after injectable tramadol; 29 single administration of the drug does not alter hematologic or biochemical parameters or gastrointestinal function. 19 In this patient, tramadol did not appear to cause any side effects. The drug was combined with meloxicam because a combination of tramadol-NSAID has been shown to produce superior analgesic efficacy than either treatment alone. 19
The cat described in case 3 was premedicated with hydromorphone, which is a full agonist at µ opioid receptors. This drug may be a good option in the clinical setting owing to its low cost, lack of inducing histamine release after IV administration (as may be caused by morphine), high efficacy and long duration of anti- nociceptive effects. 33 Hydromorphone has, however, been observed to cause post-anesthetic hyperthermia in cats 34 and vomiting, 5 similar to other opioids. None of these side effects were observed in this patient. Nonetheless, we recommend close temperature monitoring in cats receiving hydromorphone. In our experience, the administration of this opioid as supplemental analgesia is common in cats undergoing orthopedic surgery; in this patient, however, it failed to provide adequate pain relief. It was hypothesized that the cat had possibly developed central sensitization, which has been reported to occur after intensive nociceptive input and subsequent release of excitatory neurotransmitters. Also known as windup, this phenomenon may be manifested clinically as an exaggerated pain response to low- intensity stimuli or so-called allodynia. 35
Epidural administration of local anesthetics and/or opioids can be used to provide sensory and motor nerve blockade caudal to the diaphragm, such as in abdominal and orthopedic surgeries. 2 This technique has been used in combination with general anesthesia to reduce anesthetic requirements, and improve analgesia and muscle relaxation, 36 and was considered to be adequate in case 3. The use of local anesthetics was also performed post-operatively when the cat showed severe signs of pain, and an intra-articular injection of bupivacaine was performed. The latter procedure has been shown to provide superior analgesia when compared with intra-articular morphine in dogs following stifle surgery. 37 In our experience, the analgesic plan used in case 3 has been successful and efficacious in the treatment of perioperative pain after orthopedic surgery in cats. The lack of analgesic efficacy may have been owing to individual variability in response to a set dose of a specific analgesic treatment.
The long-term administration of most of NSAIDs is still considered an off-label treatment in the cat. In some countries (European Union), meloxicam is licensed for long-term use at 0.05 mg/kg/day, and several studies38,39 have supported its safe use when used at low doses (0.01–0.03 mg/kg/day) in the long term. In case 3, the administration of meloxicam at lower than labeled doses was an attempt to use the lowest effective dosage while ensuring adequate analgesia. 40 In all cases, close monitoring was recommended to owners, who were fully informed about off-label administration of NSAIDs and the possible side effects.
The use of multimodal therapy for the treatment of pain in cats is of utmost importance; opioids are considered to be the mainstay of acute pain management used in combination with local anesthetic blocks and NSAIDs. Other classes of analgesics (eg, gabapentin, tramadol, ketamine CRI) may be administered for specific conditions as alternative adjuvants. Critical pain recognition and assessment is a key component to tailor analgesic treatment to the individual needs on a case-by-case basis. The literature suggests that there are likely to be genetic differences between individuals and response to therapy in cats. 41 Multimodal analgesia did not produce undesirable therapeutic effects in the present case series; however, awareness that drug interactions may lead to side effects is warranted. For example, opioids may produce bradycardia and dose-dependent respiratory depression in cats that becomes more pronounced with co-administration of anesthetic agents and/or sedatives in patients undergoing general anesthesia. 3
Conclusions
This case series reported the successful treatment of acute pain in three cats using a multimodal approach. Pain management is a challenging, but essential, component of feline clinical practice, and multimodal analgesia may minimize stress while controlling perioperative acute pain. Rational administration of analgesic drugs may vary according to the cause, severity, duration and location of pain, as well as differences between individual patients. Future investigations may provide a clear understanding of the potential side effects after the combined administration of analgesic drugs.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors for the preparation of this case series.
The authors do not have any potential conflicts of interest to declare.
Accepted: 6 January 2013
References
- 1. Kehlet H, Dahl JB. The value of ‘multimodal’ or ‘balanced analgesia’ in postoperative pain treatment. Anaesth Analg 1993; 77: 1048–1056. [DOI] [PubMed] [Google Scholar]
- 2. Skarda RT, Tranquilli WJ. Local and regional anesthetic and analgesic techniques: cats. In: Tranquilli WJ, Thurmon JC, Grimm KA. (eds). Lumb Jones veterinary anesthesia and analgesia. 4th ed. Ames, MA: Blackwell, 2007, pp 595–604. [Google Scholar]
- 3. Robertson SA. Managing pain in feline patients. Vet Clin Small Anim 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]
- 4. Cowan A, Doxey JC, Harry EJR. The animal pharmacology of buprenorphine, an oripavine agent. Br J Pharmacol 1977; 60: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robertson SA, Wegner K, Lascelles BD. Antinociceptive and side-effects of hydromorphone after subcutaneous administration in cats. J Feline Med Surg 2009; 11: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giordano T, Steagall PV, Ferreira TH, et al. Postoperative analgesic effects of intravenous, intramuscular, subcutaneous or oral transmucosal buprenorphine administered to cats undergoing ovariohysterectomy. Vet Anaesth Analg 2010; 4: 357–366. [DOI] [PubMed] [Google Scholar]
- 7. Steagall PVM, Pelligand L, Giordano T, et al. Pharmacokinetic and pharmacodynamic modeling after intravenous, intramuscular or subcutaneous administration of buprenorphine in cats. Vet Anaesth Analg 2013; 40: 83–95. [DOI] [PubMed] [Google Scholar]
- 8. Butterworth JFIV, Strichartz GR. Molecular mechanisms of local anesthesia: A review. Anesthesiology 1990; 72: 711–734. [DOI] [PubMed] [Google Scholar]
- 9. Feldman HS, Arthur GR, Covino BG. Comparative systemic toxicity of convulsant and supraconvulsant doses of intravenous ropivacaine, bupivacaine, and lidocaine in conscious dogs. Anesth Analg 1989; 69: 794–801. [PubMed] [Google Scholar]
- 10. Cullen LK. Medetomidine sedation in dogs and cats: a review of its pharmacology, antagonism and dose. Br Vet J 1996; 152: 519–535. [DOI] [PubMed] [Google Scholar]
- 11. Pypendop BH, Verstegen JP. Hemodinamic effects of medetomidine in the dog: a dose titration study. Vet Surg 1998; 27: 612–622. [DOI] [PubMed] [Google Scholar]
- 12. Al-Mujadi H, A-Refai AR, Katzarov MG, et al. Preemptive gabapentin reduces postoperative pain and opioid demand following thyroid surgery. Can J Anaesth 2006; 53: 268–273. [DOI] [PubMed] [Google Scholar]
- 13. Gaynor JS. Other drugs used to treat pain. In: Gaynor JS, Muir WW. (eds). Handbook of veterinary pain management. 2nd ed. Mosby: Elsevier, 2009, pp 260–276. [Google Scholar]
- 14. Siao KT, Pypendop BH, Ilkiw JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res 2010; 71: 817–821. [DOI] [PubMed] [Google Scholar]
- 15. Vettorato E, Corletto F. Gabapentin as part of multi-modal analgesia in two cats suffering multiple injuries. Vet Anaesth Analg 2011; 38: 518–520. [DOI] [PubMed] [Google Scholar]
- 16. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 23: 232–235. [DOI] [PubMed] [Google Scholar]
- 17. Steagall PVM, Moutinho FQ, Mantovani FB, et al. Evaluation of the adverse effects of subcutaneous carprofen over six days in healthy cats. Res Vet Sci 2009; 86: 115–120. [DOI] [PubMed] [Google Scholar]
- 18. Court M, Greenblatt D. Molecular genetic basis for deficient acetominophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms. Pharmacogenetics 2000; 10: 355–369. [DOI] [PubMed] [Google Scholar]
- 19. Brondani JT, Luna SP, Marcello GC, et al. Perioperative administration of vedaprofen, tramadol or their combination does not interfere with platelet aggregation, bleeding time and biochemical variables in cats. J Feline Med Surg 2009; 11: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grudé P, Guittard J, Garcia C, et al. Excretion mass balance evaluation, metabolite profile analysis and metabolite identification in plasma and excreta after oral administration of [14C]-meloxicam to the male cat: preliminary study. Vet Pharmacol Ther 2010; 33: 396–407. [DOI] [PubMed] [Google Scholar]
- 21. Lascelles BDX, McFarland JM, Swann H. Guidelines for safe and effective use of NSAIDs in dogs. Vet Ther 2005; 6: 237–251. [PubMed] [Google Scholar]
- 22. Anderson WI, Dunham BM, King JM, et al. Presumptive subcutaneous surgical transplantation of a urinary bladder transitional cell carcinoma in a dog. Cornell Vet 1989; 79: 263–266. [PubMed] [Google Scholar]
- 23. Carroll GL, Howe LB, Peterson KD. Analgesic efficacy of preoperative administration of meloxicam or butorphanol in onychectomized cats. J Am Vet Med Assoc 2005; 226: 913–919. [DOI] [PubMed] [Google Scholar]
- 24. Briggs SL, Sneed K, Sawyer DC. Antinociceptive effects of oxymorphone-butorphanol-acepromazine combination in cats. Vet Surg 1998; 27: 466–472. [DOI] [PubMed] [Google Scholar]
- 25. Lascelles BDX, Robertson SA. Antinociceptive effects of hydromorphone, butorphanol, or the combination in cats. J Vet Intern Med 2004; 18: 190–195. [DOI] [PubMed] [Google Scholar]
- 26. Ferreira TH, Aguiar AJ, Valverde A, et al. Effect of remifentanil hydrochloride administered via constant rate infusion on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 2009; 70: 581–588. [DOI] [PubMed] [Google Scholar]
- 27. Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain 1999; 82: 111–125. [DOI] [PubMed] [Google Scholar]
- 28. Wagner AE, Walton JA, Hellyer PW, et al. Use of low doses of ketamine administered by constant rate infusion as an adjunct for postoperative analgesia in dogs. J Am Vet Med Assoc 2002; 221: 72–75. [DOI] [PubMed] [Google Scholar]
- 29. Steagall PV, Taylor PM, Brondani JT, et al. Antinociceptive effects of tramadol and acepromazine in cats. J Feline Med Surg 2008; 10: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brondani JT, Luna SPL, Beier SL, et al. Analgesic efficacy of perioperative use of vedaprofen, tramadol or their combination in cats undergoing ovariohysterectomy. J Feline Med Surg 2009; 11: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pypendop BH, Ilkiw JE. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J Vet Pharmacol Ther 2008; 31: 52–59. [DOI] [PubMed] [Google Scholar]
- 32. Pypendop BH, Siao KT, Ilkiw JE. Effects of tramadol hydrochloride on the thermal threshold in cats. Am J Vet Res 2009; 70: 1465–1470. [DOI] [PubMed] [Google Scholar]
- 33. Wegner K, Robertson SA. Dose-related thermal antinociceptive effects of intravenous hydromorphone in cats. Vet Anaesth Analg 2007; 34:132–138. [DOI] [PubMed] [Google Scholar]
- 34. Niedfeldt RL, Robertson SA. Postanesthetic hyperthermia in cats: a retrospective comparison between hydromorphone and buprenorphine. Vet Anaesth Analg 2006; 33: 381–389. [DOI] [PubMed] [Google Scholar]
- 35. Muir WW, Woolf CJ. Mechanisms of pain and their therapeutic implications. J Am Vet Med Assoc 2001; 219: 1346–1356. [DOI] [PubMed] [Google Scholar]
- 36. Kona-Boun JJ, Cuvelliez S, Troncy E. Evaluation of epidural administration of morphine or morphine and bupivacaine for postoperative analgesia after premedication with an opioid analgesic and orthopedic surgery in dogs. J Am Vet Med Assoc 2006; 229: 1103–1112. [DOI] [PubMed] [Google Scholar]
- 37. Sammarco JL, Conzemius MG, Perkowski SZ, et al. Postoperative analgesia for stifle surgery: a comparison of intra-articular bupivacaine, morphine, or saline. Vet Surg 1996; 25: 59–69. [DOI] [PubMed] [Google Scholar]
- 38. Gowan RA, Lingard AE, Johnston L, et al. Retrospective case-control study of the effects of long-term dosing with meloxicam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011; 13: 752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg 2008; 10: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carroll GL, Narbe R, Kerwin SC, et al. Dose range finding study for the efficacy of meloxicam administered prior to sodium urate-induced synovitis in cats. Vet Anaesth Analg 2011; 38: 394–406. [DOI] [PubMed] [Google Scholar]
- 41. Taylor PM, Slingsby LS, Pypendop BH, et al. Variable response to opioid analgesia in cats. Vet Anaesth Analg 2007; 34: 1–16.17238956 [Google Scholar]