Recent advances in the treatment of acute ischaemic stroke have focused largely on drug treatments, and yet the number of effective and widely practicable treatments remains limited. After a spate of trials with negative results, no neuroprotective agents have yet been licensed for acute stroke. Although thrombolysis with tissue plasminogen activator is now available in the United States and Canada, most eligible patients are not treated, and thrombolysis remains the subject of considerable debate in the international research community.1,2 Other important interventions for people with acute stroke include organised care in multidisciplinary stroke units and routine use of aspirin in acute ischaemic stroke.3,4 Stroke is the second most common cause of death worldwide, and with no major panacea for acute stroke imminent, we must not ignore stroke prevention.5
Medical and surgical treatments to prevent stroke carry some risk (and some cost). These preventive strategies should be targeted at those who are at the highest absolute risk of stroke, because these individuals are likely to derive the greatest absolute benefit.6 These patients generally have a history of occlusive vascular diseases with symptoms—that is, prior ischaemic stroke or transient ischaemic attack, coronary heart disease, or peripheral vascular disease. Among the 80% of patients who survive an acute stroke, the risk of recurrent stroke is highest within the first few weeks and months; about 10% in the first year and about 5% per year thereafter. These patients are also at a major risk of other vascular disease, including myocardial infarction, emphasising the need for early preventive treatments.7 Individual risk factors such as a history of hypertension, smoking, hyperlipidaemia, increased blood glucose concentration, and obesity are important considerations for all patients, especially those at high risk.
Summary points
Reduction of blood pressure is effective at preventing a first stroke, but it is not clear which patients with stroke should be treated with antihypertensives (and at what blood pressure), what the best drug regimen is, or when treatment should start
Risky or expensive medical interventions for stroke prevention should be targeted at those at high risk because the absolute benefits are greatest in such patients
Lowering cholesterol concentration with drugs may reduce the risk of non-fatal stroke, but the effects on the risk of fatal stroke and haemorrhagic stroke are unclear
The balance of risk and benefits of endarterectomy in most patients with a stenosis without symptoms is unclear
A diet rich in fresh fruit and vegetables and low in salt and fat, regular exercise, and the avoidance of smoking may reduce the lifetime risk of first stroke, but the effects on secondary prevention of stroke are unclear
This article focuses on medical interventions that are most appropriate for individuals at high absolute risk of stroke and other serious vascular events. Dietary and lifestyle interventions needed to reduce the population burden of stroke are not discussed. The modification of other vascular risk factors is generally supported by observational evidence (and common sense) and includes smoking cessation, moderation of alcohol intake, treating diabetes and monitoring glucose concentrations, and weight reduction and exercise.8–12 None of these have been rigorously evaluated in randomised controlled trials of secondary stroke prevention.
Methods
We have attempted to find the best available evidence for the topics we discuss. We searched the Cochrane Library, and we used the search strategy developed by the Cochrane Stroke Group.13 We also assessed information published in Clinical Evidence.14 We outline six common interventions for stroke prevention, discuss for whom they are indicated, and provide the evidence supporting their use.
Reduction of blood pressure
The risk of stroke doubles for every 7.5 mm Hg increase in usual diastolic blood pressure; antihypertensives have been shown to reduce stroke risk by about 38%.15 A recent consensus statement has advocated a patient centred multidisciplinary approach to the evaluation and treatment of hypertension, particularly patients at the highest risk of stroke.16 Hypertension is the most important and treatable risk factor for stroke, but there is surprisingly limited evidence about the effectiveness of modifying blood pressure in secondary prevention of stroke. A meta-analysis of data from nine randomised controlled trials on the effects of drugs for lowering blood pressure in survivors of stroke estimated a reduction in the relative risk of recurrent stroke of 29% (95% confidence interval, 5% to 47%).17 Whether patient characteristics such as baseline blood pressure were important variables were not shown. The authors also identified several limitations of the analyses and concluded that further evidence was needed. Evidence also remains limited about when to begin antihypertensive treatment after stroke and which drugs to use, although there is limited indirect evidence from randomised trials of primary prevention to support using low dose diuretics or low dose β blockers.18 Recently, the Swedish trial in old patients with hypertension (STOP-2) published data on 6614 hypertensive patients randomised to conventional antihypertensives (atenolol, metolprolol, pindolol, or hydrocholorthiazide plus amiloride) or newer antihypertensives (enalapril, lisinopril, felodipine, or isradipine).19 Both groups showed important decreases in blood pressure (about 35/17 mm Hg) but no major differences in primary end points, including fatal and non-fatal stroke, showing that the conventional and newer antihypertensives are similar at preventing major events or death from cardiovascular disease. A large ongoing randomised trial assessing the balance of benefits and risks of treatment among survivors of stroke with an angiotensin converting enzyme inhibitor (perindopril, given singly or with a diuretic, indepamide) should provide additional information.20
Reducing cholesterol concentration
Recent guidelines recommend the use of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (“statins”) to reduce cholesterol concentrations after myocardial infarction, thereby reducing the risk of death from coronary artery disease and fatal or non-fatal stroke16; the effects on fatal stroke and on haemorrhagic stroke are unclear. The strong association between cholesterol concentrations and future coronary heart disease shows that all people with stroke should reduce their cholesterol concentrations by dietary means.21 A systematic review of the evidence supports cholesterol reduction with a statin in people with prior stroke, a history of coronary heart disease, and a cholesterol concentration greater than 5 mmol/l (or low density lipoprotein cholesterol concentration greater than 3 mmol/l).22 The benefits of using drugs to reduce cholesterol concentrations among people with a prior stroke but no history of coronary heart disease remains uncertain. Two ongoing clinical trials should provide further information.23,24 A prospective overview of individual patient data from all randomised controlled trials of reduced cholesterol concentrations is also ongoing and should summarise the overall effects of reduction of cholesterol concentration among different groups of people with a prior stroke.25
Angiotensin converting enzyme inhibitors
The results of the recently published large scale multicentre heart outcomes prevention evaluation (HOPE) trial suggest that activation of the renin-angiotensin system is an independent risk factor in people with cardiovascular disease, and that the use of angiotensin converting enzyme inhibitors may reduce vascular risk in this population.26 Overall, 9297 patients with any evidence of coronary artery disease, stroke, or peripheral vascular disease were randomised to receive either ramipril 10 mg daily or placebo. The trial was terminated early when 13.9% of patients given ramipril had reached the primary end point (myocardial infarction, primary stroke, or death from cardiovascular causes) compared with 17.5% of patients given placebo. These results correspond to a risk reduction of 25% for death from cardiovascular disease, 20% for myocardial infarction, and 32% for stroke. The reduction in vascular events was larger than might have been expected from the size of the reductions in blood pressure, again supporting the hypothesis that angiotensin converting enzyme inhibitors act not only by reducing blood pressure. The implications of this trial for clinical practice are that if 50% of people in developed countries and 25% of people in developing countries with vascular disease were to take angiotensin converting enzyme inhibitors, 400 000 deaths and 600 000 non-fatal cardiovascular events could be prevented every year, but at a substantial cost.26 The cost effectiveness (and appropriate costs) of large scale use of these drugs has not been determined.
Antiplatelet drugs
A systematic review by the Antiplatelet Trialists' Collaboration showed that among high risk patients, antiplatelet drugs reduced the odds of any serious vascular event (non-fatal myocardial infarction, non-fatal stroke, or death from vascular causes) by about 25%.7 The review determined that among people with a prior ischaemic stroke, antiplatelet drugs avoided 38 serious vascular events for every 1000 people treated for about three years. The risk of intracranial bleeding with antiplatelet treatment is small, at most one or two per 1000 people per year in trials of long term treatment. Likewise, the risk of non-fatal major extracranial bleeding was only about 3 per 1000 per year. In general, the benefits of antiplatelet therapy in high risk individuals outweigh any hazards.
Medium dose aspirin (75-325 mg daily) is the agent that has been most thoroughly evaluated, but direct randomised comparisons provide no clear evidence that any one dose of aspirin is more effective than another.7 Gastrointestinal side effects (dyspepsia, constipation) are clearly dose related. One recent trial assessing different doses of aspirin in patients undergoing carotid endarterectomy confirmed previous trial evidence that adverse events are less common in patients receiving lower doses of aspirin.27
A recent systematic review comparing thienopyridines (ticlopidine and clopidogrel) with aspirin showed a 12% absolute reduction in the odds of recurrent stroke, corresponding to seven strokes avoided per 1000 patients treated with a thienopyridine (instead of aspirin) for two years.28 The combination of aspirin and dipyridamole in the second European secondary prevention study (ESPS-2) showed a small advantage over aspirin alone, but with wide confidence intervals including the possibility of almost no extra benefit.29 A systematic review suggested that, compared with aspirin, the combination reduces the risk of stroke but has no effect on myocardial infarction and little or no overall effect on “serious vascular events.”30 The European and Australian stroke prevention in reversible ischemia trial (ESPRIT) should provide further information about the benefits of adding dipyridamole to aspirin.31 Both the thienopyridines and dipyridamole plus aspirin are more expensive than aspirin, and, given the modest benefits when compared with aspirin alone, such regimens should probably only be considered in patients with an allergy to aspirin or those with further vascular events while receiving aspirin alone. In the latter case, drugs should only be switched after reconsidering the suspected mechanism of the stroke, and further investigations should be undertaken so as to rule out other treatable causes such as severe carotid stenosis or paroxysmal atrial fibrillation.
Anticoagulants for patients in atrial fibrillation
Anticoagulants are the drugs of choice for preventing stroke in high risk patients with atrial fibrillation. A systematic review evaluated six trials comparing anticoagulants (target international normalised ratio about 2.0-3.0) with placebo in 2900 patients with atrial fibrillation.32 Anticoagulants reduced the relative risk of stroke by 62% (48% to 72%), corresponding to a reduction in the absolute risk of stroke of 2.7% per year for primary prevention and 8.4% per year for secondary prevention. The rate of intracranial haemorrhage averaged 0.3% per year in the group receiving anticoagulants and 0.1% in the placebo group.
Warfarin (target international normalised ratio 2.2 to 3.1) has been compared with aspirin for stroke prevention in 2837 patients with atrial fibrillation in five trials.32 Both agents were effective but warfarin especially. Overall, warfarin reduced the relative risk of stroke by 36% (14% to 52%) compared with aspirin. One trial was subsequently excluded from the meta-analysis owing to important differences in the patient population. The relative risk of reduction of stroke with warfarin was re-estimated at 49% (26% to 65%), corresponding to an absolute reduction in risk of stroke per year of 0.6% for primary prevention and 7.0% for secondary prevention. One additional clinical trial compared warfarin (target international normalised ratio 2.0 to 3.5) with indobufen (a reversible inhibitor of cyclo-oxygenase) but did not find any major difference in the rate of recurrent stroke between the two groups (absolute risk reduction 1.0%, −1.7% to 3.7%).33
A recent consensus statement based on the available evidence recommends warfarin both for patients of any age who have atrial fibrillation and specific risk factors for stroke (previous transient ischaemic attack, stroke, other systemic embolism, hypertension, left ventricular dysfunction) and for patients older than 75 years with atrial fibrillation and no risk factors.16 Either warfarin or antiplatelet therapy is suggested for patients aged 65-75 with atrial fibrillation and no risk factors, depending on the status of the patient. Anticoagulation increases the risk of serious bleeding for patients in normal sinus rhythm. Warfarin (target international normalised ratio 2.0-3.0) is also recommended for patients after myocardial infarction who also have other risk factors, including non-valvular atrial fibrillation, a decreased left ventricular ejection fraction, or left ventricular thrombus.
Aspirin is a reasonable option for patients with atrial fibrillation who cannot tolerate anticoagulants, although it is not as effective as anticoagulation. In a systematic review of six trials comparing antiplatelet therapy with placebo (3337 high risk patients with atrial fibrillation; 40% with prior stroke), aspirin reduced the overall incidence of stroke by 22% (2% to 38%), with a reduction in the absolute risk of stroke per year of 1.5% for primary prevention and 2.5% for secondary prevention.32
In general, moderate intensity anticoagulation (target international normalised ratio 2.0-3.0) is recommended. Therapy should be tailored to the individual, depending not only on the risk of recurrent stroke but also on bleeding risks (for example, a tendency to fall, recent gastrointestinal bleeding, liver disease, dementia, uncontrolled hypertension) and the potential to benefit from treatment. The best time to start anticoagulation after an ischaemic stroke is unclear. Aspirin does reduce the risk of recurrent ischaemic stroke and may be the best initial treatment immediately after stroke.34 When anticoagulants are being considered for long term use, the treatment preferences of the patient should also be considered, because the benefits of warfarin in the trials may not reflect clinical practice owing to probable differences in anticoagulant monitoring and patient compliance. Indeed, evidence from several observational studies shows that warfarin is generally underused in people with atrial fibrillation at risk of stroke, and that the risk of haemorrhage may be lower than the risks associated with not prescribing warfarin when warranted.
Choice of antithrombotic agent for patients in sinus rhythm
One systematic review evaluated nine trials (1214 patients) comparing oral anticoagulants (warfarin) with placebo or no treatment in patients with prior stroke in normal sinus rhythm. No clear benefit of anticoagulation on death or dependency, overall mortality, or recurrent stroke was found.35 Additionally, anticoagulants significantly increased the absolute risk of fatal intracranial haemorrhage by 2.0% (0.4% to 3.6%) and the absolute risk of fatal and non-fatal extracranial haemorrhage by 5.0% (3.0% to 7.2%). One randomised trial compared aspirin with oral anticoagulant (target international normalised ratio 3.0-4.5) in 1316 people with prior transient ischaemic attack or non-disabling stroke in normal sinus rhythm, but the trial was stopped early because of an excess of cerebral haemorrhages in the anticoagulant group.36 At least two further randomised trials (including the European and Australian stroke prevention in reversible ischemia trial30 and the warfarin-antiplatelet recurrent stroke study37) are in progress, comparing low intensity anticoagulation (target international normalised ratio 1.4-3.0) with aspirin.
Carotid endarterectomy
Symptomatic stenosis—One recent systematic review of three trials including 6143 patients with angiographically confirmed mild, moderate, or severe carotid stenosis, compared carotid surgery with best medical therapy within four and six months of the onset of symptoms.38 The benefit from surgery was related to the degree of stenosis. For people with severe stenosis (greater than 70% by angiography), surgery almost completely abolished the risk of ipsilateral stroke over several years. People with moderate stenosis (50%-70% by angiography) also benefited, although to a lesser extent, and it is generally thought that the risk of stroke is not great enough to make endarterectomy worthwhile in this group. Importantly, not all patients with operable lesions benefit from surgery; further research is ongoing to determine who might benefit most. Important considerations include risk factors for surgery (for example, age, being female, peripheral vascular disease, occlusion of the contralateral internal carotid artery, hypertension) and the complication rates for angiography, anaesthesia, and endarterectomy, which must be assessed for individual centres. People with mild stenosis (less than 50%) do not benefit from carotid endarterectomy. Trials comparing endarterectomy with stent placement and endarterectomy under local anaesthesia with general anaesthesia are under way to identify lower risk methods of treating carotid stenosis.39–41
Stenosis without symptoms—A systematic review of all of the available randomised data shows that the efficacy of surgery for carotid stenosis without symptoms remains unproved and that further randomised trial evidence is needed; trials are ongoing.42
Conclusions
Considerable evidence in the literature supports an active approach towards both the primary and secondary prevention of stroke. It is reassuring that much of this evidence comes from randomised trials and systematic reviews that have considered risk factors and have identified clinically reasonable treatments and alternatives. Furthermore, many of these treatments (appropriate use of anticoagulants, aspirin, antihypertensives, and statins) are also cost effective.43 Further trials are under way to assess the value both of reducing blood pressure after acute stroke and of drugs to lower cholesterol concentrations in stroke prevention. Measures to promote smoking cessation, moderate alcohol intake, improved diet, and regular exercise may be associated with health gain but not necessarily a reduction in the risk of recurrent stroke.
Figure.
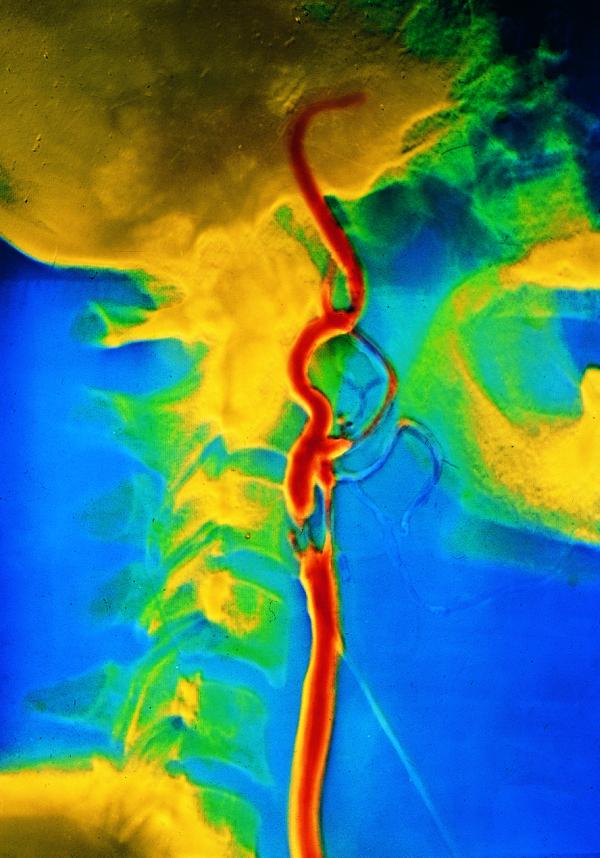
False colour angiogram showing major stenosis in carotid artery
Figure.
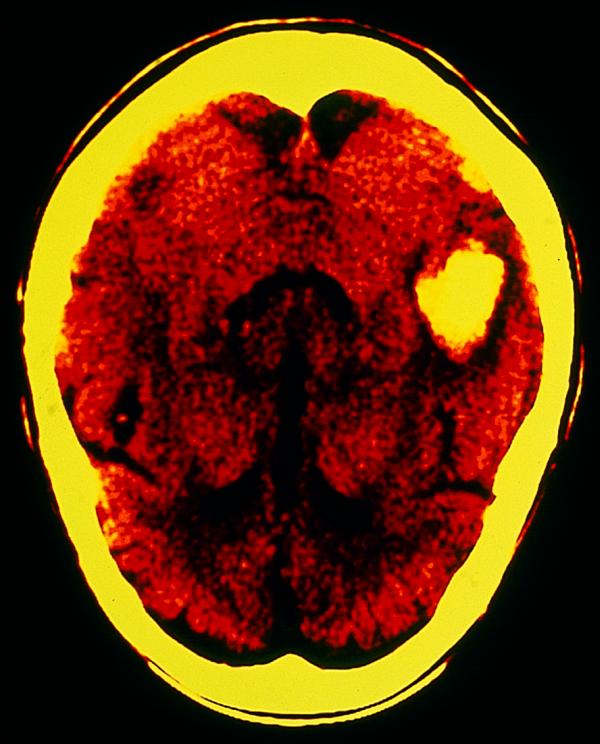
Intracerebral haemorrhage in post-temporal region
Footnotes
Competing interests: PS has received honoraria and expenses from Glaxo Wellcome, Boehringer Ingelheim, and Sanofi to give lectures at symposia. He has also received a research grant from Glaxo Wellcome.
References
- 1.Alberts M. tPA in acute ischemic stroke. United States experience and issues for the future. Neurology. 1988;51(suppl 3):53–5S. doi: 10.1212/wnl.51.3_suppl_3.s53. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Yamaguchi T, del Zoppo G Cochrane Collaboration, editors. Cochrane Library. Issue 4. Oxford: Update Software; 1999. Thrombolytic therapy versus control in acute ischaemic stroke. [Google Scholar]
- 3.Stroke Unit Trialists' Collaboration. Collaborative systematic review of the randomised trials of organised inpatient (stroke unit) care after stroke. BMJ. 1997;314:1151–1159. [PMC free article] [PubMed] [Google Scholar]
- 4.Sandercock P, Gubitz G, Counsell C Cochrane Collaboration, editors. Cochrane Library. Issue 1. Oxford: Update Software; 2000. Antiplatelet therapy for acute ischaemic stroke. [DOI] [PubMed] [Google Scholar]
- 5.Murray DJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 6.Sandercock P. Preventing recurrent stroke and other serious vascular events. In: Stroke. A practical guide to management. 2 ed. Oxford: Blackwell Science Management (in press).
- 7.Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 8.Peto R. Smoking and death: the past 40 years and the next 40. BMJ. 1994;309:937–939. doi: 10.1136/bmj.309.6959.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillbom M, Juvela S, Numminen H. Alcohol intake and risk of stroke. J Cardiovasc Risk. 1999;6:223–228. doi: 10.1177/204748739900600406. [DOI] [PubMed] [Google Scholar]
- 10.Sigal R. Cardiovascular disease in diabetes mellitus. In: Godlee F, Goldmann D, Donald A, Barton S, editors. Clinical Evidence, issue 2. London: BMJ Publishing Group; 1999. pp. 32–44. [Google Scholar]
- 11.Brand MB, Mulrow CD, Chiquette E, Angel L, Cornell J, Summerbell C, et al. Cochrane Library, 1999, Issue 3. Oxford: Update Software; 1999. Dieting to reduce body weight for controlling hypertension in adults. Cochrane Collaboration. [Google Scholar]
- 12.Wannamethee SG, Shaper A. Physical activity and the prevention of stroke. J Cardiovasc Risk. 1999;6:213–216. doi: 10.1177/204748739900600404. [DOI] [PubMed] [Google Scholar]
- 13.Search strategy of the Cochrane Stroke Group. Cochrane Library. Issue 1. Oxford: Update Software; 2000. In: Cochrane Collaboration. [Google Scholar]
- 14.Godlee F, Goldmann D, Donald A, Barton S, editors. Clinical Evidence, issue 2. London: BMJ Publishing Group; 1999. [Google Scholar]
- 15.MacMahon S, Rodgers A. The epidemiological association between blood pressure and stroke: implications for primary and secondary prevention. Hypertens Res. 1994;17(suppl I):23–32S. [Google Scholar]
- 16.Gorelick PB, Sacco RL, Smith DB, Alberts M, Mustone-Alexander L, Rader D, et al. Prevention of a first stroke. A review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. JAMA. 1999;281:1112–1120. doi: 10.1001/jama.281.12.1112. [DOI] [PubMed] [Google Scholar]
- 17.The INDANA (Individual Data Analysis of Antihypertensive intervention trials) Project Collaborators. Effect of antihypertensive treatment in patients having already suffered a stroke. Stroke. 1997;28:2557–2562. doi: 10.1161/01.str.28.12.2557. [DOI] [PubMed] [Google Scholar]
- 18.Mulrow C, Jackson R. Treating primary hypertension. In: Godlee F, Goldmann D, Donald A, Barton S, editors. Clinical Evidence, issue 2. London: BMJ Publishing Group; 1999. pp. 524–531. [Google Scholar]
- 19.Hansson L, Lindholm L, Ekbom T, Dahlof B, Lanke J, Schersten B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity. The Swedish trial in old patients with hypertension-2 study. Lancet. 1999;354:1751–1756. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 20.PROGRESS Management Committee. Blood pressure lowering for the secondary prevention of stroke: rationale and design of PROGRESS. J Hypertens. 1996;14(suppl 2):41–6S. [PubMed] [Google Scholar]
- 21.Pekkanen J, Linn S, Heiss G, Suchindron CM, Leon A, Ruskind BM, et al. Ten year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 22.Hebert PR, Gaziano JM, Chan KS, Hennekens CH. Cholesterol lowering with statin drugs, risk of stroke, and total mortality: an overview of randomized trials. JAMA. 1997;278:313–321. [PubMed] [Google Scholar]
- 23.Collins R, Peto R. MRC/BHF heart protection study. Stroke. 1999;30:1304. . (Abstract.) [Google Scholar]
- 24.Goldstein L, for the SPARCL Investigators. Stroke prevention by aggressive reduction in cholesterol levels—the SPARCL study [abstract]. 25th international stroke conference, New Orleans, Feb 2000.
- 25.Cholesterol Treatment Trialists' Collaboration. Protocol for a prospective collaborative overview of all current and planned randomized trials of cholesterol treatment regimens. Am J Cardiol. 1995;75:1130–1134. doi: 10.1016/s0002-9149(99)80744-9. [DOI] [PubMed] [Google Scholar]
- 26.The Heart Outcomes Prevention Evaluation [HOPE] Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 27.Taylor DW, Barnett HJM, Haynes RB, Ferguson GG, Sackett DL, Thorpe KE, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. Lancet. 1999;353:2179–2184. doi: 10.1016/s0140-6736(99)05388-x. [DOI] [PubMed] [Google Scholar]
- 28.Hankey GJ, Sudlow C, Dunbabin D Cochrane Collaboration, editors. Cochrane Library. Issue 4. Oxford: Update Software; 1999. Thienopyridine derivatives (ticlopidine, clopidogrel) versus aspirin for prevention of stroke and other serious vascular events in high vascular risk patients. [DOI] [PubMed] [Google Scholar]
- 29.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European secondary prevention study 2: dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 30.Wilterdink JL, Easton JD. Dipyridamole plus aspirin in cerebrovascular disease. Arch Neurol. 1999;56:1087–1092. doi: 10.1001/archneur.56.9.1087. [DOI] [PubMed] [Google Scholar]
- 31.De Schryver E.for the ESPRIT Study Group. ESPRIT: mild anticoagulation, acetylsalicylic acid plus dipyridamole or acetylsalicylic acid alone after cerebral ischaemia of arterial origin [abstract] Cerebrovasc Dis 19988(suppl 4)83 [Google Scholar]
- 32.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 33.Morocutti C, Amabile G, Fattapposta F, Nicolosi A, Matteoli S, Trappolini M, et al. Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. Stroke. 1997;28:1015–1021. doi: 10.1161/01.str.28.5.1015. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Sandercock P, Counsell C, Dan HC, Collins R, Liu L, et al. Indications for early aspirin use in acute ischaemic stroke: a combined analysis of 40,000 randomised patients from the Chinese acute stroke trials and the international stroke trial. Stroke. 2000;31:1240–1249. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- 35.Liu M, Counsell C, Sandercock P Cochrane Collaboration, editors. Cochrane Library. Issue 3. Oxford: Update Software; 1999. Anticoagulants for preventing recurrence following ischaemic stroke or transient ischaemic attack. [DOI] [PubMed] [Google Scholar]
- 36.The Stroke Prevention in Reversible Ischaemia Trial (SPIRIT) Study Group. A randomised trial of anticoagulant versus aspirin after cerebral ischemia of presumed arterial origin. Ann Neurol. 1997;42:857–865. doi: 10.1002/ana.410420606. [DOI] [PubMed] [Google Scholar]
- 37.Mohr J.for the WARSS Group. Design considerations for the warfarin-antiplatelet recurrent stroke study Cerebrovasc Dis 19955156–157. [Google Scholar]
- 38.Cina C, Clase C, Haynes R Cochrane Collaboration, editors. Cochrane Library. Issue 3. Oxford: Update Software; 1999. Carotid endarterectomy for symptomatic stenosis. [Google Scholar]
- 39.Brown M.for the CAVATAS Investigators. Results of the carotid and vertebral artery transluminal angioplasty study (CAVATAS) [abstract] Cerebrovasc Dis 19999(suppl 1)21 [Google Scholar]
- 40.Al-Mubarak N, Roubin G, Hobson R, Ferguson R, Brott T, Moore W. Credentialling of stent operators for the carotid revascularization endarterectomy vs stenting trial (CREST) [abstract] Stroke. 2000;31:292. [Google Scholar]
- 41.Gough M, for the GALA Investigators. General versus local anaesthesia for carotid endarterectomy (GALA). www.dcn.ed.ac.uk/gala/
- 42.Benavente O, Moher D, Pham B. Carotid endarterectomy for asymptomatic carotid stenosis: a meta-analysis. BMJ. 1998;317:1477–1480. doi: 10.1136/bmj.317.7171.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hankey GJ, Warlow CP. Treatment and secondary prevention of stroke: evidence, cost, and effects on individuals and populations. Lancet. 1999;34:1457–1463. doi: 10.1016/S0140-6736(99)04407-4. [DOI] [PubMed] [Google Scholar]


