Abstract
Two siblings, a 6-month-old sexually intact male weighing 2.5 kg (cat 1) and a sexually intact female (cat 2) British Shorthair cat weighing 2.3 kg, were examined because of a 3-week history of polyuria, lethargy and laboured breathing. One year previously, another sibling (cat 3) had been presented because of similar, yet more severe, clinical signs at the age of 5 months. Physical examination revealed lethargy, dehydration and polypnoea with slightly increased inspiratory effort. Diagnostic investigation revealed severe hypercalcaemia (cats 1–3), renal azotaemia (cats 1 and 3) and a radiologically generalised miliary interstitial pattern of the lungs (cats 1–3) attributable to hypervitaminosis D caused by ingestion of commercial cat food. Cat 3 was euthanased. Cats 1 and 2 were treated with isotonic saline solution (180 ml/kg IV daily), sucralfate (30 mg/kg PO q12h), terbutaline (only cat 1: 0.1 mg/kg SC q4h), furosemide (1.5 mg/kg IV q8h) and tapering doses of prednisolone. Cat 2 was normal on day 14. Cat 1 had stable renal disease and was followed up to day 672. The radiological generalised military interstitial pattern of the lungs had improved markedly. Excessive cholecalciferol-containing commercially available cat food poses a great hazard to cats. Supportive treatment may result in long-term survival and improvement of radiological pulmonary abnormalities.
Hypervitaminosis D in cats has been reported from ingestion of vitamin D3-containing rodenticide and pet food. Affected animals present with lethargy, chronic weight loss, anorexia, polyuria, episodic vomiting and signs of respiratory disturbance, such as cough and difficulty in breathing. Laboratory changes in cats with hypervitaminosis D included hypercalcaemia, hyperphosphataemia, alkalosis, high urea and creatinine concentrations, hypercalciuria and a decrease in urine concentrating ability.1–4
Case descriptions
A 6-month-old sexually intact male British Shorthair cat (cat 1) weighing 2.5 kg presented to the Veterinary Teaching Hospital of the Ludwig Maximilian University of Munich for a 3-week history of polyuria, lethargy and laboured breathing breathing. The cat’s littermate (2.3 kg sexuallyintact female – cat 2) also living in their household, showed similar, but less severe, signs. One year previously, a sibling of the two cats (cat 3) (same parents, but from a previous litter) had been presented by the same owners because of similar, yet more severe, clinical signs (polyuria, polydipsia, lethargy, anorexia, coughing for 3 months) at the age of 5 months. This cat had been euthanased owing to his overall bad condition, poor prognosis and financial constraints after the initial diagnostic evaluation had revealed renal failure with marked renal azotaemia, metabolic acidosis, hyperphosphataemia, marked anaemia and severe hypercalcaemia, as well as severe radiographic lung changes characterised by a generalised bronchointerstitial pattern and peribronchial cuffing.
On physical examination cat 1 was lethargic and moderately dehydrated. It showed polypnoea with slightly increased inspiratory effort; its rectal temperature was slightly below the reference interval (37.4°C). On palpation, the size of the kidneys seemed to be within the normal range for an adult cat. 5 Other physical examination findings were unremarkable.
Initial diagnostic work-up included a complete blood count, serum biochemistry, venous blood gas analysis, urinalysis (including culture), thoracic radiographs, and abdominal ultrasound.
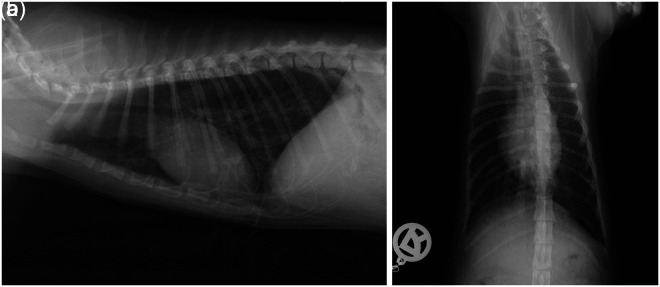
Abnormal serum biochemical findings are summarised (Table 1). Blood gas analysis revealed mixed acidosis (pH 7.18, reference interval 7.34–7.44). On urinalysis, a decreased urine specific gravity of 1.016, 1+ protein, 3+ glucose, 2–8 leukocytes/high power field (hpf) and 2 granular casts/hpf were identified. Urine culture was negative. Thoracic radiographs showed evidence of a generalised miliary interstitial pattern and peribronchial cuffing throughout the lungs, as well as evidence of bronchial mineralisation (Figure 1). Abdominal ultrasound showed borderline large kidneys (length: right kidney, 4.2 cm; left kidney, 4.3 cm) with a hyperechoic cortex and a broad medullary rim sign. The other organs were unremarkable.
Table 1.
Results of laboratory analysis of cat 1
| Day 1 | Day 2 | Day 14 | Day 21 | Day 42 | Day 77 | Day 672 | Reference interval | |
|---|---|---|---|---|---|---|---|---|
| Creatinine (µmol/l) | 363 | 388 | 453 | 453 | 295 | 346 | 0–168 | |
| BUN (mmol/l) | 36.1 | 27.7 | 36 | 27.4 | 28.2 | 20.76 | 5.0–11.3 | |
| Ionised calcium (mmol/l) | 2.02 | 2.3 | 1.36 | 1.33 | 1.35 | 1.35 | 1.35 | 1.20–1.35 |
| Phosphate (mmol/l) | 2.7 | 2.7 | 2.63 | 1.76 | 0.97–2.36 | |||
| PTH (pg/ml) | 7 | 3–24 | ||||||
| PTHrp (pmol/l) | 0.5 | <0.8 | ||||||
| 25(OH)D3 (nmol/l) | 240.9 | 63 | 48–192 | |||||
| 1,25(OH)2D3 (pg/ml) | 400 | 125 | <20 (30) |
BUN = blood urea nitrogen; PTH = parathyroid hormone; PTHrp = parathyroid hormone related protein
Figure 1.
Thorax, right lateral (a) and dorsoventral (b) view of cat 1 at presentation. The lungs were well inflated and the diaphragm flattened, which may represent dyspnoea. There was evidence of a generalised miliary interstitial pattern and peribronchial cuffing throughout the lungs, and a curvilinear, interrupted, mineral opacity line superimposed on the cardiac silhouette in the area of the ascending aorta and the left ventricular outflow tract, as well as evidence of bronchial mineralisation. Differential diagnosis for the generalised interstitial pattern included interstitial mineralisation secondary to hypercalcaemia, bronchopneumonia and, less likely, neoplasia secondary to a round cell tumour. Fungal pneumonia, metastatic neoplasia or uraemic pneumonitis were considered unlikely
The cat was hospitalised for further diagnostic work-up and supportive treatment. After 4 days the sibling, cat 2, was presented. Apart from intermittent slightly labored breathing, physical examination findings were unremarkable. Serum biochemistry also revealed hypercalcaemia (Table 2). The rest of the serum parameters, as well as the CBC, showed no abnormalities. The urine had a specific gravity of 1.014 and contained 1+ protein.
Table 2.
Results of laboratory analysis of cat 2
| Day 1 | Day 2 | Day 14 | Day 21 | Day 42 | Day 77 | Day 672 | Reference interval | |
|---|---|---|---|---|---|---|---|---|
| Creatinine (µmol/l) | 112 | 102 | 102 | 0–168 | ||||
| BUN (mmol/l) | 11 | 15.1 | 14.3 | 5.0–11.3 | ||||
| Ionised calcium (mmol/l) | 1.98 | 1.91 | 1.31 | 1.29 | 1.27 | 1.25 | 1.20–1.35 | |
| Phosphate (mmol/l) | 2.16 | 1.98 | 0.97–2.36 | |||||
| PTH (pg/ml) | 3 | 3–24 | ||||||
| PTHrp (pmol/l) | <0.8 | |||||||
| 25(OH)D3 (nmol/l) | 218.9 | 48–192 | ||||||
| 1,25(OH)2D3 (pg/ml) | 266 | <20 (30) |
BUN = blood urea nitrogen; PTH = parathyroid hormone; PTHrp = parathyroid hormone related protein
Results of thoracic radiographs and abdominal ultrasound of cat 2 were similar to the findings for cat 1.
Further work-up was focused on possible differential diagnosis for hypercalcaemia. Blood samples were submitted for measurement of parathyroid hormone (PTH), parathyroid hormone related hormone PTHrp, calcidiol (25-hydroxyvitamin D3, 25(OH)D3) and calcitriol (1,25-dihydroxyvitamin D3, 1,25(OH)2D3; Vet-Med-Labor, Idexx Laboratories).
Treatment of both cats was initiated with potassium-supplemented (ad 20 mmol/l) isotonic saline solution (180 ml/kg IV daily) (0.9% sodium chloride; B Braun), sucralfate (30 mg/kg PO q12h: Ulcogant; Merck) and terbutaline (only cat 1: 0.1 mg/kg SC q4h; Bricanyl, AstraZeneca). Furosemide (1.5 mg/kg IV q8h) (Dimazon; Intervet) was started after rehydration was achieved. One day after treatment initiation, ionized blood calcium had increased in cat 1 and only slightly decreased in cat 2 (Tables 1 and 2).
Further work-up included in-house snap tests for feline leukaemia virus and feline immunodeficiency virus, as well as immunofluorescence assay for the detection of Toxoplasma gondii antibodies to exclude an underlying infectious cause of the pulmonary changes. Results were negative in both cats. Thereafter, prednisolone (2 mg/kg PO q24h: Prednisolone; CP-Pharma) was added to the cats’ current medication to enhance calciuresis.
Results of the PTH measurement were in the lower reference interval in both cats. PTHrp was low (only measured in cat 1). Assessment of the vitamin D status revealed a markedly elevated 1.25(OH)2D3 in both cats, as well as an elevated 25(OH)D3, consistent with hypervitaminosis D (Tables 1 and 2).
Thorough questioning of the owner about any vitamin D-containing products in their household revealed no potential source for the vitamin D intoxication. A detailed feeding anamnesis was taken, which showed that all three cats had exclusively been fed commercial cat food (the same two different brands). All three cats had mainly received wet food of one single brand (food 1: about 80% of their daily food ration) and, to a lesser extent, dry food (food 2) of another brand. Therefore, samples of both diets were sent to a special laboratory for analysis of the cholecalciferol (vitamin D3) content by high-performance liquid chromatography (LUFA-ITL GmbH, Agrolab Laborgruppe, Kiel, Germany). A second sample of food 1 was submitted for measurement of its calcium and phosphate content (Department of Nutrition and Dietetics, Ludwig Maximilian University Munich, Germany) (Table 3).
Table 3.
Analysis of the different diets that were fed
| Vitamin D3-declared* (IU/kg) | Vitamin D3-measured** (IU/kg) | Calcium-declared* (g/kg) | Calcium-measured** (g/kg) | Phosphate-declared* (g/kg) | Phosphate-measured** (g/kg) | |
|---|---|---|---|---|---|---|
| Food 1: wet food (Almo Nature Kitten with chicken) | OS: 1500 | OS: 46,900 | OS: 10.5 | OS: 0.7 | OS: 7.5 | OS: 1.9 |
| DM: 6466 | DM: 202,155 | DM: 45 | DM: 3.0 | DM: 32 | DM: 8.2 | |
| Food 2: dry food (Royal Canin Feline Kitten 36) | OS: 700 | OS: 978 | OS: 11 | OS: 9.6 | ||
| DM: 753 | DM: 1052 | DM: 11.8 | DM: 10.3 | |||
| Renal diet: wet food (Hill’s Feline k/d) | OS: 235 | OS: 1.4 | OS: 0.9 | |||
| DM: 1046 | DM: 6.3 | DM: 3.8 |
Stated label amount
Actual content that was measured in a certified laboratory
OS = original substance (wet weight, as fed); DM = dry matter
A diagnosis of vitamin D intoxication with soft-tissue calcification of the lungs and nephropathy caused by ingestion of commercial cat food was established for the kittens (cats 1 and 2). The same diagnosis was made presumptively for cat 3 as clinical signs, food brands and feeding habits were identical in all three cats. However, PTH, PTHrp, 25(OH)D3, 1,25(OH)2D3 and vitamin D3 content in the food fed were not measured in cat 3.
After a hospitalisation period of 5 and 7 days, respectively, the two kittens (cats 1 and 2) improved markedly and were discharged on isotonic saline solution (50 ml/kg SC q12h), furosemide (1.5 mg/kg PO q8h), sucralfate (30 mg/kg PO q8h) and prednisolone (2 mg/kg PO q24h). In the following weeks, those medications were slowly tapered down, taking the clinical and laboratory findings into account. The owners were advised to feed cooked minced meat, owing to its low calcium content, in combination with a phosphate binder (sucralfate). As soon as the blood calcium had decreased, both cats were switched to a commercial renal diet (food 3).
Follow-up examinations were performed 2, 3, 6, 11 and 96 weeks after discharge. After a gradual, but slow, decline of the blood ionised calcium during hospitalisation (Tables 1 and 2), it had normalised in both cats after 2 weeks. At that time cat 2 was clinically normal (The condition of cat 1 was improving slowly; after 11 weeks, it still exhibited mild dyspnoea following exercise. Urine specific gravity remained low in cat 1 (after discontinuation of the diuretic medication), but glucosuria and cylinduria resolved completely. Urine protein/creatinine ratio was 0.13 (reference interval 0–0.5). After 96 weeks cat 1 still had polyuria and polydipsia due to chronic renal disease with serum urea and creatinine elevated approximately twofold. Mild non-regenerative anaemia was present that was attributed to chronic renal disease.
Measurement of serum vitamin D3 metabolites was repeated in cat 1 after 11 weeks. The level of 25(OH)D3 had returned to within the reference interval, whereas 1.25(OH)2D3, despite a distinct decrease, was still markedly elevated (Tables 1 and 2).
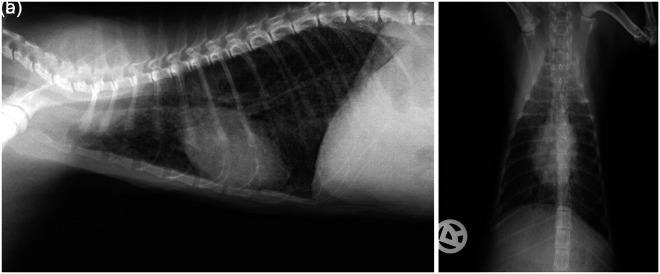
The radiographic pulmonary changes were re-evaluated after 11 weeks in cat 1 and an improved generalised unstructured bronchointerstitial pattern was observed. The bronchial mineralisation appeared less conspicuous than in the first set of radiographs (Figure 2).
Figure 2.
Thorax, right lateral (a) and dorsoventral (b) view of cat 1 after (84 days) 12 weeks. An improved generalised unstructured bronchointerstitial pattern was observed. The bronchial mineralisation appeared less conspicuous and the mineral opacity line superimposed on the cardiac silhouette showed larger gaps than in the first set of radiographs
Discussion
Diet and skin are sources of vitamin D3. In dogs and cats the synthesis of vitamin D3 in the skin is inadequate and much lower than in other mammals. Therefore, vitamin D supply depends exclusively on dietary vitamin D3.6,7 Vitamin D3 is converted in the liver to 25(OH)D3, then hydroxylated, primarily in the kidney, to 1,25(OH)2D3 by the enzyme 1α-hydroxylase and, finally, to 24,25-dihydroxycholecalciferol [24,25(OH)2D3] with the aid of 24-hydroxylase.8–11 One study in the USA demonstrated that 21/49 canned cat foods exceeded 7500 IU/kg of cholecalciferol, which corresponds to 30 times the vitamin D requirement in kittens.12,13 Mostly, the high vitamin D levels were considered to be caused by the use of raw food materials rich in vitamin D, for example oily marine fish, rather than the over-zealous supplementation of vitamins. 12 The minimum requirement of 250 IU/kg (dry matter) of cholecalciferol is considered safe if the calcium and phosphate concentrations are 12 and 8 g/kg respectively. 8 However, this might not necessarily reflect the optimum vitamin D allowance and may be different for other types of cat foods with different calcium and phosphate levels. 14
In addition, higher levels do not necessarily represent toxic levels. The National Research Council (NRC) 2006 recommendations allow a minimum of 224 IU/kg (5.6 µg/kg) and a maximum of 30,000 IU/kg (750 µg/kg) in the dry matter of cholecalciferol for kittens after weaning. 15 The levels of vitamin D in cats foods should be between the minimum allowance and the safe upper limit, which was established by Sih et al. 4
While the dry food (food 2) in this case series contained 978 IU vitamin D3/kg (original substance), the wet food that was predominantly given (food 1) had a vitamin D3 content of 46,900 IU/kg (original substance). Both were measured in a certified laboratory (LUFA-ITL GmbH, Agrolab Laborgruppe, Kiel, Germany). Relating to the dry matter, this food (food 1: 23.2% dry matter) contained 202,155 IU vitamin D3/kg, whereas the second food (food 2: 93% dry matter) contained 1052 IU vitamin D3/kg. The calcium and phosphate contents of food 1 were measured in a certified laboratory (Department of Nutrition and Dietetics, Ludwig Maximilian University Munich, Germany). The calcium content was 0.7 g/kg (original substance) respectively 3.0 g/kg dry matter. Phosphorus content was 1.9 g/kg (original substance) respectively 8.2 g/kg dry matter (Table 3). The calcium:phosphorus (Ca:P) ratio was 0.4.
The company that produced food 1 states that no vitamin D is added and that the entire vitamin D content derives from natural ingredients (Table 4). However, it is unrealistic that the declared vitamin D content would be achieved solely by those ingredients. According to the European Union regulations the amount of additives that is declared is the added amount, not the total amount present in the food. If vitamin D is added as an additive the total amount of vitamin D in the product should not be higher than 2275.7 IU/kg dry matter.16–18 Obviously, food 1 had been enriched with excessive amounts of vitamin D. The product therefore contained an illegal level of vitamin D.
Table 4.
Composition of food 1 (as indicated by the manufacturer)
| Ingredients | Percentage (%) |
|---|---|
| Chicken meat | 75 |
| Rice | 9 |
| Cheese | 7.5 |
| Sunflower oil | 4.5 |
| Chicken liver | 1.5 |
| Egg | 1.5 |
Calculation of diet revealed that cat 1 received 10,332 IU cholecalciferol, 374 mg calcium and 610 mg phosphate per day. According to the NRC, the safe upper limit in the diet for cholcalciferol is 30,000 IU/kg (dry matter); 7520 IU/1000 kcal metabolisable energy (ME) for kittens after weaning. 15 However, cat 1 received 29,436 IU cholecalciferol/1000 kcal ME. The recommended calcium allowance by the NRC is 8.0 g calcium/kg dry matter. Though calcium requirements were not met owing to the low calcium content of food 1 and could not be balanced by the small amounts of food 2, and the Ca:P ratio of the diet was inverse, vitamin D intoxication was possible. 14 A Ca:P ratio in the range of 1.31:2.61 is reported to be adequate for growing kittens.15,19
In a case series of food-induced hypervitaminosis D from Japan, the vitamin D3 contents of these diets were reported to be 63,700 IU/kg (original substance). One more recent report from the UK describes two dogs with hypervitaminosis D that were fed the same food which was over supplemented with vitamin D.3,20
Concentration of plasma 25(OH)D3 is generally used as an index of the vitamin D status, and elevated concentrations indicate hypervitaminosis D. Plasma concentration of 25(OH)D3 is linearly related to dietary intake of vitamin D3. Therefore, circulating levels of 25(OH)D3 could be used as a bioassay for the vitamin D content in the diet. 8
Calcitriol is regarded to be the most active vitamin D3 metabolite with regard to calcium metabolism. Calcitriol has a high affinity for the vitamin D receptor (VDR) and a low affinity for the transport protein Vitamin D binding protein (DBP). Binding to the VDR in the nucleus of target cells causes exaggerated gene expression. Binding to DBP increases functional half-life and protects against vitamin D intoxication. Free vitamin D metabolites are functional in vivo. High 25(OH)D3 concentrations will cause excessive synthesis of 1,25(OH)2D3, but also, together with vitamin D and its other metabolites, exceed the binding capacity of DBP, thereby increasing the amount of free 1,25(OH)2D3 that is accessible to target cells.10,11 This may result in increased biological activity of 1,25(OH)2D3 without a concomitant increase in the total plasma.9,21 Vitamin D intoxication will develop eventually if the input of vitamin D or its metabolites exceed the adaptive capacity of the body to eliminate them.9–11
Cats 1 and 2 had severely increased plasma 25(OH)D3 and 1,25(OH)2D3 levels, supporting the diagnosis of hypervitaminosis D. Although the increased 1,25(OH)2D3 concentration is always observed, as mentioned earlier, a decreased clearance rate could explain this finding. It is also possible that the food was also over-fortified with 1,25(OH)2D3. Increased plasma 25(OH)D3 and 1,25(OH)2D3 levels were also found in two dogs with hypervitaminosis D caused by consumption of commercial dog food. 20
Clinical signs, results of bloodwork and thoracic radiographs of cats in this cases series match those of previously documented cases of hypervitaminosis D.1–4 Although alkalosis is documented frequently in affected cats owing to volume depletion and an increase in bicarbonate reabsorption by the kidneys, cat 1 had a mixed acidosis and cat 2 had no acid–base disturbance. Changes in cat 1 most likely reflect net retention of hydrogen ions due to renal disease and possible restrictive ventilator defects due to calcifications of the lung leading to metabolic and respiratory acidosis. Although pulmonary changes consistent with mineralisation were present radiographically in two cases (cats 1 and 2), clinical and radiographic improvement was observed. To our knowledge this has not been reported before. The very low calcium content of food 1 might have prevented more severe and irreversible calcification of soft tissues, and may have reduced the toxicity of vitamin D. 14
There is little published data on how cats affected by hypervitaminosis D should be treated. Cats 1 and 2 were treated initially with isotonic saline to increase calciuresis, and oral sucralfate to reduce intestinal calcium and phosphorus absorption. Terbutaline was added to achieve bronchodilation in cat 1, which showed dyspnoea. Furosemide was added to the treatment after rehydration was achieved. After all diagnostics had been performed, prednisolone was given in tapering doses for a total of 10 days as isotonic saline and furosemide were insufficient to resolve hypercalcaemia. It resolved on day 14 in both cats. Therefore, it can be concluded that isotonic saline, sucralfate, furosemide and prednisolone successfully treated hypercalcaemia in cats 1 and 2, and can be recommended to treat vitamin D intoxication in cats. Use of bisphosphonates22,23 was discussed with the owner; however, oral alendronate (Fosamax; MSD) was not given because it was impossible to pill cats 1 and 2 and side effects, such as oesophageal ulceration, were feared.
Conclusions
Hypercalcaemia can persist for several weeks despite the withdrawal of the over-fortified diet. Its main transported metabolite, 25(OH)D3, shows a half-life of approximately 15 days in studies of experimental hypervitaminosis in dogs, whereas the hormone 1,25(OH)2D3 has a half-life of approximately 15 h. 11 In cat 1, calcidiol had returned to a concentration within the reference interval 11 weeks after treatment was started. Also, 1,25(OH)2D3 decreased but was still elevated after 11 weeks. The presence of renal failure in cat 1 might have impaired 24-hydroxylation and renal clearance of metabolites. In the two reported dogs with hypervitaminosis D caused by ingestion of commercial dog food 1,25(OH)2D3 normalised in one dog on day 14 and in the other after 37 days, which also indicates long half-lives. 20 After accumulation in fat tissue, 25(OH)D3 is further metabolised slowly.
The reported cases indicate that excessive vitamin D3-containing commercially available cat food poses a great hazard to cats.
This is the first report in which treatment and long-term outcome of hypervitaminosis D, including improvement of radiologic pulmonary abnormalities, could be documented.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case series.
The authors do not have any potential conflicts of interest to declare.
Accepted: 29 November 2012
References
- 1. Moore FM, Kudisch M, Richter K, Faggella A. Hypercalcemia associtated with rodenticide poisoning in three cats. J Am Vet Med Assoc 1988; 193: 1099–1100. [PubMed] [Google Scholar]
- 2. Peterson EN, Kirby R, Sommer M, Bovee KC. Cholecalciferol rodenticide intoxication in a cat. J Am Vet Med Assoc 1991; 199: 904–906. [PubMed] [Google Scholar]
- 3. Morita T, Awakura T, Shimada A, Umemura T, Nagai T, Haruna A. Vitamin D toxicosis in cats: natural outbreak and experimental study. J Vet Med Sci 1995; 57: 831–837. [DOI] [PubMed] [Google Scholar]
- 4. Sih TR, Morris JG, Hickman MA. Chronic ingestion of high concentrations of cholecalciferol in cats. Am J Vet Res 2001; 62: 1500–1506. [DOI] [PubMed] [Google Scholar]
- 5. Park IC, Lee HS, Kim JT, Nam SJ, Choi R, Oh KS, et al. Ultrasonographic evaluation of renal dimension and resistive index in clinically healthy Korean domestic short-hair cats. J Vet Sci 2008; 9: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. How KL, Hazewinkel HA, Mol JA. Photosynthesis of vitamin D3 in cats. Vet Rec 1994; 134: 384. [DOI] [PubMed] [Google Scholar]
- 7. How KL, Hazewinkel HA, Mol JA. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol 1994; 96: 12–18. [DOI] [PubMed] [Google Scholar]
- 8. Morris JG, Earle KE, Anderson PA. Plasma 25-hydroxyvitamin D in growing kittens is related to dietary intake of cholecalciferol. J Nutr 1999; 129: 909–912. [DOI] [PubMed] [Google Scholar]
- 9. Tryfonidou MA, Oosterlaken-Dijksterhuis MA, Mol JA, van den Ingh TS, van den Brom WE, Hazewinkel HA. 24-Hydroxylase: potential key regulator in hypervitaminosis D3 in growing dogs. Am J Physiol Endocrinol Metab 2003; 284: 505–513. [DOI] [PubMed] [Google Scholar]
- 10. Vieth R. The mechanisms of vitamin D toxicity. Bone Miner 1990; 11: 267–272. [DOI] [PubMed] [Google Scholar]
- 11. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008; 88: 582–586. [DOI] [PubMed] [Google Scholar]
- 12. Morris JG. Vitamin synthesis by kittens. Vet Clin Nutr 1996; 3: 88–92. [Google Scholar]
- 13. Morris JG, Earle KE. Vitamin D and calcium requirements of kittens. Vet Clin Nutr 1996; 3: 93–96. [Google Scholar]
- 14. Vieth R, Fraser D, Kooh SW. Low dietary calcium reduces 25-hydroxycholecalciferol in plasma of rats. J Nutr 1987; 117: 914–918. [DOI] [PubMed] [Google Scholar]
- 15.National Research Council. Nutrient requirement of dogs and cats. Washington, DC: National Academy Press, 2006, pp 364–365. [Google Scholar]
- 16.Commission Regulation (EC) No 767/2009 of the European Parliament and of the Council of 13 July 2009 on the placing on the market and use of feed OJEC 1.9.2009 L229/1–28.
- 17.European Union. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Official Journal of the European Union 18.10.2003 L268/29–43. [Google Scholar]
- 18.European Union. 2004/C 50/01 List of the authorised additives in feedingstuffs (1) published in application of Articla 9t (b) of Council Directive 70/524/EEC concerning additives in feedingstuffs. Official Journal of the European Union 25.2.2004 50/1–144. [Google Scholar]
- 19. Morris JG, Earle KE. Growing kittens require less dietary calcium than current allowances. J Nutr 1999; 129: 1698–1704. [DOI] [PubMed] [Google Scholar]
- 20. Mellanby RJ, Mee AP, Berry JL, Herrtage ME. Hypercalcaemia in two dogs caused by excessive dietary supplementation of vitamin D. J Small Anim Pract 2005; 46: 334–338. [DOI] [PubMed] [Google Scholar]
- 21. Deluca HF, Prahl JM, Plum LA. 1,25-Dihydroxyvitamin D is not responsible for toxicity caused by vitamin D or 25-hydroxyvitamin D. Arch Biochem Biophys 2011; 505: 226–230. [DOI] [PubMed] [Google Scholar]
- 22. Price PA, Buckley JR, Williamson MK. The amino bisphosphonate ibandronate prevents vitamin D toxicity and inhibits vitamin D-induced calcification of arteries, cartilage, lungs and kidneys in rats. J Nutr 2001; 131: 2910–2915. [DOI] [PubMed] [Google Scholar]
- 23. Rumbeiha WK, Kruger JM, Fitzgerald SF, Nachreiner RF, Kaneene JB, Braselton WE, et al. Use of pamidronate to reverse vitamin D3-induced toxicosis in dogs. Am J Vet Res 1999; 60: 1092–1097. [PubMed] [Google Scholar]