Abstract
Tritrichomonas foetus is a protozoan parasite that has been associated with chronic diarrhea in cats. This study aimed to determine (i) the prevalence of T foetus shedding in cats from three different populations in southern Ontario, and (ii) associations between the presence of T foetus and potential cat management, health and demographic risk factors. A cross-sectional study was conducted involving 140 cats from a cat clinic in Guelph, 46 cats from a humane society in Guelph and 55 cats from two cat shows. Risk factor information was assessed through a questionnaire. The InPouch TF (feline) culture method was used to determine the presence of T foetus in all samples. Polymerase chain reaction was conducted on all samples positive by the InPouch TF, as well as 132 negative samples. The assays were interpreted in series and the prevalence of T foetus shedding and 95% confidence intervals (CI) were estimated at 0.7% (95% CI: 0.0–3.9%; n = 140) from the cat clinic, 0% (95% CI: 0.0–7.7%; n = 46) from the humane society and 23.6% (95% CI: 13.2–37.0%; n = 55) from the cat shows. ‘Attendance at cat shows’ was the only variable significant in both the univariable and multivariable analyses (P <0.05). No significant association was found between the presence of T foetus and diarrhea at the time of sampling or having a history of diarrhea in the past 6 months. The prevalence of T foetus was highly variable among populations of cats in southern Ontario, with shedding being most common in show cats.
Introduction
Tritrichomonas foetus is a protozoan parasite that has been isolated from cats with diarrhea. 1 Tritrichomonas foetus does not have a cyst stage and reproduces by binary fission inside the host. 2 The parasite has been identified in cat colonies with a history of chronic diarrhea, especially those containing pedigree cats or young cats from rescue centers.3 –5 The organism has been isolated from healthy cats, but these cats were housed with cats with chronic diarrhea and T foetus-positive feces. 6 To date, ronidazole appears to be the most effective drug for eliminating the organism. Without treatment, the diarrhea may resolve spontaneously; however, cats can remain carriers of the organism. 2 The commercially available InPouch TF (feline) culture system (Biomed Diagnostics) and polymerase chain reaction (PCR) currently appear to be the most reliable assays for detecting T foetus. 3
Tritrichomonas foetus has been detected in fecal samples from cats in the USA, 3 the UK, 5 Canada, 7 Italy, 4 Switzerland, 8 Australia, 9 New Zealand, 10 Korea, 11 Spain 12 and Germany. 13 The prevalence of T foetus among cats in Canada is unknown. By extension, veterinary awareness of this potential pathogen is lacking and practitioners do not routinely test for the organism. The objectives of this study were to determine the prevalence of T foetus in different cat populations in southern Ontario, as well as to determine risk factors for fecal shedding.
Materials and methods
A cross-sectional study was performed involving 241 cats. One hundred and forty cats were sampled through a cat clinic in Guelph (CCG) between June and August 2011. Forty-six cats were sampled from the humane society in Guelph (HSG); 26 cats were sampled in summer 2011 and 20 cats were sampled in winter 2012. Fifty-five cats were sampled at cat shows; 30 cats were sampled from a show in London, Ontario (ON), in November 2011, and 25 cats were recruited from a show in Ottawa, ON, in December 2011.
Cats eligible for enrollment into the study included (i) any cat presented to the CCG regardless of presenting complaint/current treatment, other than if treated with ronidazole within the previous month; (ii) all cats present at the HSG at the time of sampling; and (iii) cats present in the auditorium of the attended cat shows. Exclusion criteria included kittens <16 weeks old unless a voided fecal sample could be obtained, any cat treated for T foetus in the past 6 months, fractious cats where performing a rectal swab would be prohibitively difficult without sedation and moribund/dying cats. The questionnaire was administered to all cat owners to assess demographic and risk factor information, including signalment, the number of cats per house, diet, the source of the cat (breeder, store, etc), travel, attendance at cat shows, water source (municipal, filtered, bottled, well, drains, toilet, other), fecal consistency of other cats in the house and treatment with antibiotics within the past 6 weeks. Owners were asked to score the cat’s current feces using a modified Purina fecal scoring chart (Nestlé-Purina Pet Food) where 1 = formed, hard and dry; 2 = formed, soft or wet; 3 = does not hold form, very moist, occurs as piles; and 4 = no defined shape, puddles or spots. A score of 3 or 4 on this system was considered consistent with diarrhea.
Samples were collected via a rectal swab moistened with 0.9% saline. Alternatively, if a fecal sample was obtained within 6 h of voiding, approximately 0.03 g of feces was collected on a moistened swab. The sample was inoculated into the InPouch TF (feline) culture system immediately at the cat shows and HSG, and within a maximum of 15 min of collection at the CCG. The pouches were kept in the dark at room temperature and transported vertically to the Ontario Veterinary College where they were incubated at 35oC for up to 6 days and examined daily for motile trophozoites according to the manufacturer’s guidelines. On day 6 of incubation, 1.5 ml aliquots of pouch liquid were frozen at −80oC for T foetus PCR, which was performed at the Animal Health Laboratory, University of Guelph. PCR using specific primers (TFR3 and TFR4), which target parts of the 28S and 18S rRNA genes 14 was performed on the pouch fluid of (i) the initial 90 samples collected from the CCG, (ii) all samples positive for T foetus on the InPouch TF culture method, (iii) all samples collected at cat shows and (iv) a randomly selected negative sample for each positive sample on the InPouch TF from the same population (identified by using a random number generator).
This study was approved by the Animal Care Committee and the Research Ethics Board at the University of Guelph.
Statistical analysis
Stata 11 MP software was used for statistical analysis. An Exact McNemar’s test was conducted to determine if there was a significant difference in the proportion of samples that tested positive with the InPouch TF culture system and T foetus PCR. The kappa statistic and the prevalence-adjusted bias-adjusted kappa (PABAK) were calculated to determine the level of agreement between the two tests. 15
Prevalence estimates and 95% confidence intervals (CIs) for T foetus shedding in the three different populations were calculated by interpreting the InPouch TF and PCR tests in series. 15
Initially, univariable, exact logistic regression models were built to assess the relationship between infection with T foetus (by either diagnostic method) and the presence of diarrhea at the time of sampling, history of diarrhea in the past 6 months, history of diarrhea in another cat in the house in the past 6 months, age, sex, attendance at cat shows, history of travel outside of ON, being fed a raw food diet and being housed with more than five cats. Two variable exact logistic regression models were also created to examine the potential confounding effect of ‘attendance at cat shows’. ‘Attendance at a cat show’ was considered a confounding variable if it caused a >20% change in the log odds ratio of a significant variable. 15 P-values were calculated using the score method. Statistical significance was defined as P <0.05.
Results
Overall, 15/241 cats were positive by either the InPouch TF culture system or PCR. By the InPouch TF, 0.7% (95% CI: 0.0–3.9%; n = 140) cats from the CCG, 0% (95% CI: 0.0–7.7%; n = 46) cats from the HSG and 23.6% (95% CI: 13.2–37.0%; n = 55) cats from cat shows were positive for T foetus. Positive cats represented 12/29 catteries/households from cat shows and 1/128 houses in the CCG population.
Of the samples subjected to T foetus PCR, 0.0% (95% CI: 0.0–4.0%; n = 92) cats from the CCG, and 25.5% (95% CI: 14.7−39.0%; n = 55) cats from cat shows were positive.
The prevalence of T foetus shedding in cats and 95% CI based on the assays interpreted in series were estimated at 0.7% (95% CI: 0.0–3.9%; n = 140) from the cat clinic, 0% (95% CI: 0.0–7.7%; n = 46) from the humane society and 23.6% (95% CI: 13.2–37.0%; n = 55) from cat shows, ie, exactly the same as by InPouch TF culture alone.
Demographic information is displayed in Table 1. Fifty-five cats were sampled at cat shows from 23 catteries and six houses. Cats sampled at cat shows represented approximately 20% of breeders/owners at each show. Four (1.7%) of the samples were collected using voided feces.
Table 1.
Demographic data for cats enrolled in the study
| Characteristics of sample populations | Cat clinic | Humane society | Cat shows |
|---|---|---|---|
| Total cats surveyed | 140 | 46 | 55 |
| Age (months): | |||
| Mean | 78.7 | 22.7 | 14.7 |
| Range | 3–221 | 3–96 | 4–192 |
| Not available | 1 (0.7%) | 17 (37.0%) | 2 (3.6%) |
| Breed: | |||
| Purebred | 19 (13.6%) | 0 (0.0%) | 53 (96.0%) |
| Mixed breed | 121 (86.4%) | 46 (100%) | 2 (4.0%) |
| Number of cats in household: | |||
| Mean | 2 | Not available | 16 |
| Range | 1–10 | 2–41 | |
| Diarrhea at sampling: | |||
| Yes | 24 (17.1%) | 25 (54.4%) | 18 (32.7%) |
| No | 112 (80.0%) | 19 (41.3%) | 28 (50.9%) |
| Unknown | 4 (2.9%) | 2 (4.3%) | 9 (16.4%) |
Breeds sampled included Abyssinian (one), American Ringtail (five), American Shorthair (one), Bengal (four), Burmilla (one), Balinese Longhair (one), Balinese Shorthair (one), British Shorthair (three), Cornish Rex (eight), Devon Rex (three), Exotic Shorthair (one), Himalayan (two), Japanese Bobtail (one), Maine Coon (four), Maine Coon–Persian cross (one), Manx (one), Ocicat (two), Oriental Shorthair (five), Persian (five), Ragdoll (five), Russian Blue (one), Scottish Fold (two), Siamese (five), Siamese cross (one), Siberian (four), Sphynx (three), Tonkinese (two) and mixed breed (168). Breeds positive for T foetus included Bengal (two), Balinese Longhair (one), British Shorthair (one), Cornish Rex (two), mixed breed (one), Japanese Bobtail (one), Ocicat (one), Oriental Shorthair (two), Ragdoll (two) and Sphynx (two).
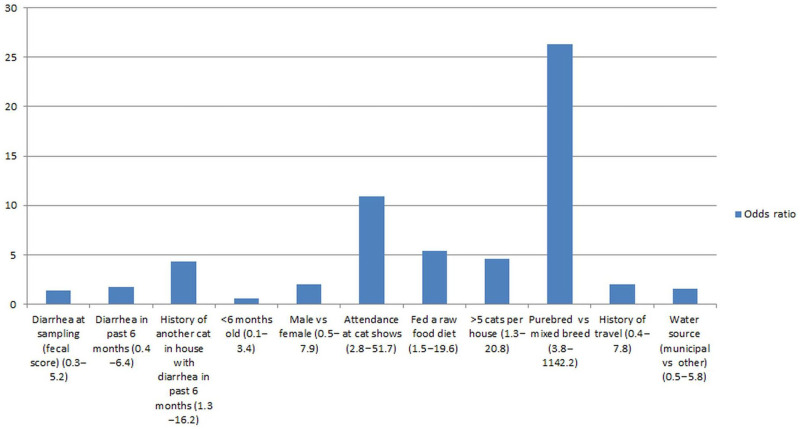
Exact logistic regression revealed that the following variables increased the odds of shedding T foetus: being of a pedigree breed, living with a cat with diarrhea, multi-cat houses (>5 cats), being fed a raw food diet and attendance at cat shows (Figure 1; Table 2). No significant association was found between the presence of T foetus and diarrhea at the time of sampling, having a history of diarrhea in the past 6 months, travel, age, sex and water source. A regression model using T foetus shedding as the risk factor and diarrhea at the time of sampling or in the past 6 months as the outcome also did not reveal a significant association. The inclusion of the variable ‘attendance at cat shows’ in these regression models resulted in all variables becoming non-significant; however, the odds ratios of risk factors identified on univariable analysis remained positive (Table 3).
Figure 1.
Univariable risk factor analysis for the presence of T foetus by the InPouch or T foetus polymerase chain reaction with 95% confidence interval in brackets. Unless otherwise stated the referent category is the absence of the stated exposure
Table 2.
Univariable risk factor analysis for the presence of T foetus by the InPouch TF or T foetus polymerase chain reaction
| Risk factor | Odds ratio | P | 95% CI |
|---|---|---|---|
| Diarrhea at time of sampling (fecal score) | 1.4 | 0.74 | 0.3–5.2 |
| Diarrhea in past 6 months | 1.7 | 0.48 | 0.4–6.4 |
| History of another cat in house with diarrhea in past 6 months | 4.4 | <0.01 | 1.3–16.2 |
| <6 months old | 0.6 | 0.43 | 0.1–3.4 |
| Male vs female | 2.0 | 0.26 | 0.5–7.9 |
| Attendance at cat shows | 10.9 | <0.01 | 2.8–51.7 |
| Fed a raw food diet | 5.4 | <0.01 | 1.5–19.6 |
| >5 cats per house | 4.6 | <0.01 | 1.3–20.8 |
| Purebred vs mixed breed | 26.3 | <0.01 | 3.8–1142.2 |
| History of travel | 2.0 | 0.27 | 0.4–7.8 |
| Water source (municipal vs other) | 1.6 | 0.42 | 0.5–5.8 |
CI = confidence interval
Table 3.
Output from bivariable risk factor analysis, including ‘attendance at cat shows’ the model
| Risk factor | Odds ratio | P | 95% CI |
|---|---|---|---|
| Diarrhea at time of sampling (fecal score) | 0.9 | >0.99 | 0.2–3.9 |
| Diarrhea in past 6 months | 2.6 | 0.22 | 0.5–12.2 |
| History of another cat in house with diarrhea in past 6 months | 2.4 | 0.32 | 0.5–11.1 |
| <6 months old | 1.4 | 0.74 | 0.3–9.1 |
| Male vs female | 1.6 | 0.23 | 0.6–9.6 |
| Fed a raw food diet | 3.3 | 0.08 | 0.8–14.2 |
| >5 cats per house | 4.2 | 0.07 | 0.8–26.5 |
| History of travel | 0.4 | 0.28 | 0.1–2.0 |
| Water source (municipal = 1) | 3.7 | 3.66 | 0.9–16.3 |
CI = confidence interval
Being of a pedigree breed was highly correlated with ‘attendance at cat shows’ (Spearman’s rho = 0.61, P = <0.01) and therefore was not included in a model with ‘attendance at cat shows’ owing to issues associated with collinearity. 15
Test comparison
Based on the Exact McNemar’s test, there was no significant difference in the proportions of cats positive from the InPouch TF and PCR conducted on InPouch TF culture fluid (P >0.99). The kappa statistic (92.1%, P <0.01) and the PABAK (97.3%, P <0.01) indicated a high level of agreement between the two tests; PABAK was calculated because the overall prevalence of T foetus was <20% and the kappa statistic is unstable for prevalences <20% or >80%. 15
Discussion
This study sampled cats at a primary healthcare clinic, humane society and two cat shows in ON. There was a marked difference in prevalence of T foetus infection in the cat show population (23.6%) compared with the other populations (0.7% CCG, 0.0% HSG). This may be owing to breed predispositions, group housing, or other demographic and pet management factors. The cat show populations differed from the clinic population with respect to age, housing density, prevalence of diarrhea and the proportion of purebreds. However, a specific breed predisposition seems unlikely, as in this study 72/241 cats were purebred, and T foetus was isolated from 10 different breeds, including one mixed breed cat. Tritrichomonas foetus has also been reported in a variety of breeds in other studies.3,16 With respect to housing density, most cats sampled from the CCG were living in households containing two cats, while most cats sampled from cat shows were living in multi-cat households (mean 16, range 2–41). A plausible explanation for the potential association between housing density and T foetus infection is that T foetus does not have a cyst stage, 2 and close contact is important to facilitate spread of the organism. Other studies on T foetus in cat show populations have reported similarly elevated prevalences, for example, 21.0% in Norway (n = 52), 16 36.0% in the USA (n = 117) 3 and 15.0% in Germany (n = 230). 13 Studies on the prevalence of T foetus in the general population reveal slightly lower estimates, for example, 8.8% (n = 147) in Japan 17 and 10.0% (n = 173) in the USA. 18 However, the majority or all positive cats in the two aforementioned studies were of pedigree breeds.
No significant association was found between the presence of T foetus and diarrhea at the time of sampling (fecal score 3 or 4), or having history of diarrhea in the past 6 months. This is in contrast to previous research;13,19 –21 however, it has been reported that subclinical shedding of T foetus does occur 22 and thus it is possible to isolate T foetus from a clinically normal cat. Intermittent shedding of T foetus in cats’ feces is also likely, and each cat was only sampled once during this study. The possibility remains that the occurrence of diarrhea in a cat infected with T foetus may be multifactorial, and further investigation into the pathogenesis of this organism is warranted.
There was a high level of agreement between the InPouch TF and T foetus PCR on InPouch TF culture fluid in this study (PABAK = 97.3%, P <0.01). Two discordant results were obtained. One sample was positive by the InPouch TF, but negative by PCR. This pouch was inoculated with a greater than usual amount of feces; very low numbers of motile trophozoites and an overgrowth of fecal microflora were observed in this pouch. It is therefore possible that the presence of inhibitors in the feces of this cat led to a false-negative PCR. 23 The cat that was positive on PCR, but not on the InPouch TF, was from a cattery where T foetus was diagnosed in another cat, and the entire cattery had been treated with ronidazole 1 year previously. The cat was being treated with metronidazole at the time of sampling; treatment with antibiotics such as metronidazole has been shown to temporarily decrease the numbers of T foetus in feces and can produce false-negative culture results.19,24 The fact that PCR was not conducted on all samples may have led to a lower prevalence estimation in the CCG and HSG populations, as PCR is generally considered a more sensitive assay than the InPouch TF; however, this was not thought to be a major limitation of the study as PCR on (i) 91 negative samples collected from the CCG did not yield any positive results, and only one out of 41 negative samples collected from cat shows was positive on PCR of culture fluid. While PCR on feces appears to be more sensitive than the InPouch TF in some studies, 3 an evaluation of single tube nested PCR when compared with the InPouch TF did not find PCR alone to be superior to culture; however, utilizing the two tests in combination is reported to increase the chance of attaining a positive test.14,25
The 15 min lag time between sample collection and InPouch TF inoculation at the CCG was not thought to have compromised the validity of the test because survival of T foetus for at least 30 mins has been reported in water, cat food and urine. 26 Survival of T foetus in the environment for short periods is thought to be via the formation of pseudocysts. 27
Limitations of this study included the relatively small sample sizes in the cat show and humane society populations in terms of animals and households/catteries. The small number of positive cats overall limited our ability to conduct multivariable statistical analyses and explore interaction effects. Participation rates were variable among the study populations, with cat show owners having the highest non-response rate. Cattery owners may have been more inclined to participate in the study if they suspected that T foetus was present in their cats, leading to an overestimation of the prevalence in this population. Conversely, this may have been a deterrent from participation in the study.
Conclusions
Based on our results, we conclude that the prevalence of T foetus appears to be highly variable among cat populations in ON, with infection being more common in cats at cat shows. A larger study involving a cat show population at the cattery level should be conducted in order to increase statistical power for a risk factor analysis and investigate factors specific to the management of these cats.
Acknowledgments
We thank the staff at the cat clinic and humane society, facilitators of the cat shows, and Drs Sabrina Thomas and Erin Harrison for assistance with sample collection. We also thank Drs Hugh Cai, Krishna Shakya and Jacob Avula, and Ms Mary Lake for diagnostic and technical support.
Footnotes
Funding: Funding for this project was provided by the Ontario Veterinary College (OVC) Pet Trust fund. A summer student position was funded by the OVC Dean’s Office. The infrastructure for statistical analyses was supported through a grant to David L. Pearl from the Canada Foundation for Innovation and the Ontario Research Fund.
The authors do not have any potential conflicts of interest to declare.
Accepted: 29 December 2012
References
- 1. Levy MG, Gookin JL, Poore M, et al. Tritrichomonas foetus and not Pentatrichomonas hominis is the etiologic agent of feline trichomonal diarrhea. J Parasitol 2003; 89: 99–104. [DOI] [PubMed] [Google Scholar]
- 2. Payne PA, Artzer M. The biology and control of Giardia spp and Tritrichomonas foetus. Vet Clin N Am Small Anim Pract 2009; 39: 993–1007. [DOI] [PubMed] [Google Scholar]
- 3. Gookin JL, Stebbins ME, Hunt E, et al. Prevalence of and risk factors for feline Tritrichomonas foetus and Giardia infection. J Clin Microbiol 2004; 42: 2707–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holliday M, Deni D, Gunn-Moore DA. Tritrichomonas foetus infection in cats with diarrhoea in a rescue colony in Italy. J Feline Med Surg 2009; 11: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunn-Moore DA, McCann TM, Reed N, et al. Prevalence of Tritrichomonas foetus infection in cats with diarrhoea in the UK. J Feline Med Surg 2007; 9: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xenoulis PG, Saridomichelakis MN, Read SA, et al. Detection of Tritrichomonas foetus in cats in Greece. J Feline Med Surg 2010; 12: 831–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pham D. Chronic intermittent diarrhea in a 14-month-old Abyssinian cat. Can Vet J 2009; 50: 85–87. [PMC free article] [PubMed] [Google Scholar]
- 8. Frey C, Schild M, Hemphill A, et al. Intestinal Tritrichomonas foetus infection in cats in Switzerland detected by in vitro cultivation and PCR. Parasitol Res 2009; 104: 783–788. [DOI] [PubMed] [Google Scholar]
- 9. Bell ET, Gowan RA, Lingard AE, et al. Naturally occurring Tritrichomonas foetus infections in Australian cats: 38 cases. J Feline Med Surg 2010; 12: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kingsbury DD, Marks SL, Cave NJ, Grahn RA. Identification of Tritrichomonas foetus and Giardia spp infection in pedigree show cats in New Zealand. New Zeal Vet J 2010; 58: 6–10. [DOI] [PubMed] [Google Scholar]
- 11. Lim S, Park SI, Ahn KS, et al. First report of feline intestinal trichomoniasis caused by Tritrichomonas foetus in Korea. Korean J Parasitol 2010; 48: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miro G, Hernandez L, Montoya A, et al. First description of naturally acquired Tritrichomonas foetus infection in a Persian cattery in Spain. Parasitol Res 2011; 109: 1151–1154. [DOI] [PubMed] [Google Scholar]
- 13. Kuehner KA, Marks SL, Kass PH, et al. Tritrichomonas foetus infection in purebred cats in Germany: prevalence of clinical signs and the role of co-infection with other enteroparasites. J Feline Med Surg 2011; 13: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cobo ER, Favetto PH, Lane VM, et al. Sensitivity and specificity of culture and PCR of smegma samples of bulls experimentally infected with Tritrichomonas foetus. Theriogenology 2007; 68: 853–860. [DOI] [PubMed] [Google Scholar]
- 15. Dohoo IR, Martin SW, Stryhn H. Veterinary epidemiologic research. 2nd ed. Charlottetown, Prince Edward Island: VER Incorporated, 2009. [Google Scholar]
- 16. Tysnes K, Gjerde B, Nodtvedt A, Skancke E. A cross-sectional study of Tritrichomonas foetus infection among healthy cats at shows in Norway. Acta Vet Scand 2011; 53: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doi J, Hirota J, Morita A, et al. Intestinal Tritrichomonas suis (=T. foetus) infection in Japanese cats. J Vet Med Sci 2012; 74: 413–417. [DOI] [PubMed] [Google Scholar]
- 18. Stockdale HD, Givens MD, Dykstra CC, Blagburn BL. Tritrichomonas foetus infections in surveyed pet cats. Vet Parasitol 2009; 160: 13–17. [DOI] [PubMed] [Google Scholar]
- 19. Gookin JL, Levy MG, Law JM, et al. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res 2001; 62: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 20. Gookin JL, Copple CN, Papich MG, et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Int Med 2006; 20: 536–543. [DOI] [PubMed] [Google Scholar]
- 21. Bissett SA, Gowan RA, O’Brien CR, et al. Feline diarrhoea associated with Tritrichomonas foetus and Giardia co-infection in an Australian cattery. Aust Vet J 2008; 86: 440–443. [DOI] [PubMed] [Google Scholar]
- 22. Foster DM, Gookin JL, Poore MF, et al. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc 2004; 225: 888–892. [DOI] [PubMed] [Google Scholar]
- 23. Stauffer SH, Birkenheuer AJ, Levy MG, et al. Evaluation of four DNA extraction methods for the detection of Tritrichomonas foetus in feline stool specimens by polymerase chain reaction. J Vet Diagn Invest 2008; 20: 639–641. [DOI] [PubMed] [Google Scholar]
- 24. Kather EJ, Marks SL, Kass PH. Determination of the in vitro susceptibility of feline Tritrichomonas foetus to five antimicrobial agents. J Vet Intern Med 2007; 21: 966–970. [DOI] [PubMed] [Google Scholar]
- 25. Gookin JL, Birkenheuer AJ, Breitschwerdt EB, et al. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol 2002; 40: 4126–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosypal AC, Ripley A, Stockdale-Walden HD. Survival of a feline isolate of Tritrichomonas foetus in water, cat urine, cat food and cat litter. Vet Parasitol 2012; 185: 279–281. [DOI] [PubMed] [Google Scholar]
- 27. Pereira-Neves A, Benchimol M. Tritrichomonas foetus: budding from multinucleated pseudocysts. Protist 2009; 160: 536–551. [DOI] [PubMed] [Google Scholar]