Abstract
Nanotechnology is vital to many current industries, including electronics, energy, textiles, agriculture, and theranostics. Understanding the chemical mechanisms of nanomaterial synthesis has contributed to the tunability of their unique properties, although studies frequently overlook the potential impact of impurities. Impurities can show adverse effects, clouding the interpretation of results or limiting the practical utility of the nanomaterial. On the other hand, as successful doping has demonstrated, the intentional introduction of impurities can be a powerful tool for enhancing the properties of a nanomaterial. This Review examines the complex role of impurities, unintentionally or intentionally added, during nanoscale synthesis and their effects on the performance and usefulness of the most common classes of nanomaterials: nanocarbons, noble metal and metal oxide nanoparticles, semiconductor quantum dots, thermoelectrics, and perovskites.
Keywords: nanocarbon, semiconductor, thermoelectric, perovskite, nanomaterial, nanoparticle, doping, contaminants, purity
1. Introduction
Nanomaterials offer novel, emergent, or amplified phenomena over their bulk counterparts due to their small size, high surface-to-volume ratio, variable morphology, and interesting quantum, optoelectronic, and magnetic properties. These nanomaterial qualities can be tuned by controlling synthesis parameters, such as energy input (e.g., temperature, light, electricity), pH, reactant concentration, reaction time, and the presence of nonreacting additives (impurities); indeed, a true vision of nanomaterials’ origin and function can only be achieved if these parameters are fully controlled and understood.
Reaction impurities can influence the synthesis and resulting properties of nanomaterials to a greater degree than one may initially anticipate. In the context of nanomaterials, “impurity” can be defined as any foreign substance or element that is present in the nanostructured material, either inadvertently from external sources or deliberately added to influence and modulate its size, morphology, and various physical and chemical characteristics. Some impurities behave beneficially and impart desirable effects on nanomaterial syntheses.1−3 These impurities impact the synthetic conditions, compete with the primary reaction, and direct the formation of the resulting nanomaterial.4−10 Conversely, even otherwise inert impurities can compromise nanomaterial synthesis and applicability.11−14
Impurities can originate from chemical reagents, from the environment of the synthesis, or as byproducts (i.e., secondary impurities), and limitations in achieving exceptional and reproducible batch-to-batch or vendor-to-vendor chemical purity (cost, time, and feasibility) make managing impurities an enduring problem. Rather than combat these impurities, there exists a body of research dedicated to examining their role during chemical synthesis to offer a semblance of control over, and understanding of, their impact.15−22 Moreover, theoretical studies are now incorporating impurities as an integral part of their analyses.23,24
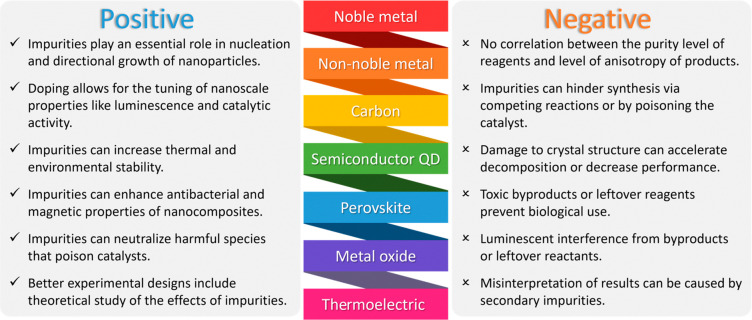
In this Review, we explore the impact of impurities on nanoparticle (NP) synthesis, whether they are intentionally introduced or not. We examine works that investigate the role of purposeful impurities alongside literature reports that encountered evidence of their effects (even if the role of the impurities is not investigated or, in some cases, misattributed). Figure 1 shows some of the positive and negative effects of impurities on the synthesis of nanomaterials. While impurities can play a massive role in the synthesis and application of some nanomaterials, their impact is widely varied material-to-material. Therefore, this Review is divided for ease of reading into sections based on the prevalent nanomaterials being used today, including noble metal, non-noble metal, metal oxide, and carbon NPs, semiconductor quantum dots (QDs), and thermoelectric and perovskite nanomaterials.
Figure 1.
Positive and negative effects of impurities on the synthesis of nanomaterials.
2. Noble Metal Nanoparticles
Noble metal NPs, particularly those of Au and Ag, have captured significant attention since the groundbreaking research conducted by Michael Faraday,25 though their rich history spans several centuries. Initially employed in ancient Rome to add vibrant colors to glass, noble metal NPs have evolved to serve a wide range of contemporary applications in diverse fields such as optoelectronics,26,27 catalysis,28,29 sensors,30,31 biomedicine,32−34 and imaging.31 Some of the key factors contributing to their popularity are their nontoxic nature and the ability for researchers to precisely tune their optical, electronic, and thermal properties during synthesis. This section starts by presenting studies that encountered evidence of impurities affecting the synthesis of noble metal nanoparticles, followed by works in which impurities are explicitly considered in the experimental design and, finally, reports that highlight the role of impurities on the applicability of the nanomaterial.
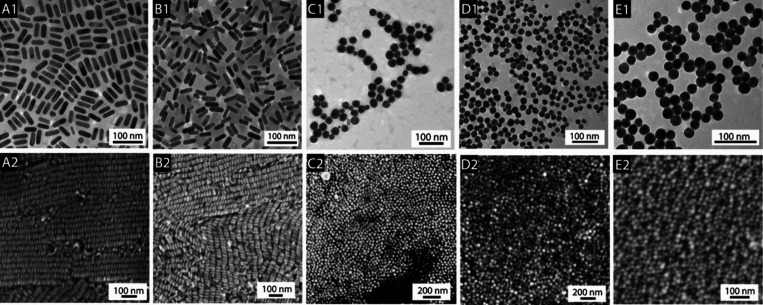
In a 2008 letter to Langmuir, Smith and Korgel demonstrated shape disparity during gold nanoparticle (AuNP) synthesis based solely on the vendor source and purity of commercial cetyltrimethylammonium bromide (CTAB), a common NP surfactant. More specifically, CTAB from Acros (≥99%), Sigma (≥99%), and Aldrich (95%) produced spherical AuNPs, whereas CTAB from Fluka (≥96%) and MPBiomedicals (>98%) resulted in Au nanorods (Figure 2).35 Evidently, there is significant disparity in purity between vendors, though different impurity levels can even be found lot-to-lot within the same manufacturer.5 The authors posit that an impurity present in some CTAB batches slows the colloidal growth rate for AuNPs, resulting in rods. Iodide in CTAB has shown similar shape control4,18,36−38 and can inhibit Au nanorod growth by preventing the adsorption of Au at the (111) facet, limiting elongation. Jessl and co-workers have established the correlation between the amount of iodide present in CTAB (i.e., small impurities of cetyltrimethylammonium iodide, or CTAI) and Ag+ concentration in Ag-assisted Au nanorod synthesis.18 The authors note that the exhaustive conversion of CTAI-Au+ to CTAI-Ag+ is critical to the formation of rods: CTAI-Au+ and CTAI-Ag+ exhibit similar affinities for Au (110) facets, though Ag+ expresses a higher reduction potential. Hence, CTAI-Ag+ causes the initial symmetry breaking of the seeds and drives asymmetric growth in the (100) direction. Ag+ also acts to prevent the unfavorable formation of CTAB-Au+ to increase the yield of Au nanorods, though excess Ag+ can result in the adsorption of CTAB-Ag+ on the (100) plane and reduce the rate of asymmetric growth. In another CTAB study, Liu and co-workers demonstrate the role of Ag+ as a shape-regulating agent to produce Au nanorods and elongated bipyramids.39 The authors propose that Ag+ selectively adsorbs to the (110) facets of Au surfaces by underpotential deposition (UPD), forming a protective monolayer of Ag0 while simultaneously promoting Au0 growth on the (111) and (100) facets. When applying single-crystalline Au seeds, this growth direction creates rods; when applying multicrystalline seeds, the products are similarly multicrystalline (bipyramids therein).
Figure 2.
Electron microscopy images (top, TEM; bottom, SEM) of CTAB-stabilized AuNPs. The letters designate the CTAB supplier: (A) Fluka (52370, ≥96%), (B) MP Biomedicals (194004, >98%), (C) Acros (22716V, ≥99%), (D) Sigma (H5882, ≥99%), and (E) Aldrich (855820, 95%). Interestingly, sourcing CTAB from different vendors results in either rods or spheres, though the label purity does not predict the shape direction (e.g., the Aldrich CTAB is only 95% yet produced spheres). Reproduced from ref (35). Copyright 2008 American Chemical Society.
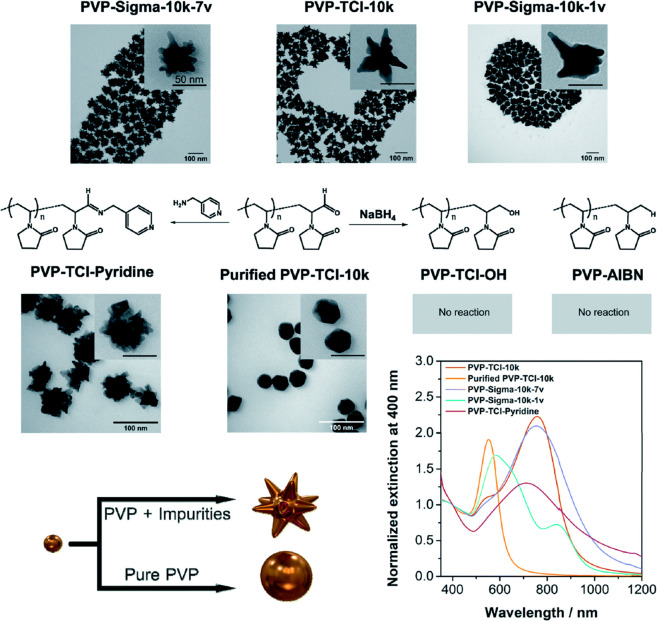
Polyvinylpyrrolidone (PVP) is a popular additive during NP synthesis for metastability and to direct anisotropic growth. Silver nanoparticles (AgNPs) synthesized in the presence of purified PVP via continuous flow synthesis experience enhanced plasmonic properties independently of an added reductant, resulting in smaller particles than using unpurified PVP.8 However, in batch synthesis, AgNPs made using unpurified PVP exhibited much higher scattering intensity across the small angle scattering q-range because of the partial reduction of Ag in purified PVP. Similarly, the impurities in PVP can enhance the degree of anisotropy for Au nanostars (Figure 3).40 Taladriz-Blanco and co-workers showed another instance where disparities between supplier PVP batches resulted in wildly different Au nanostructures: in-house purified PVP resulted in Au spheres, whereas “dirty” PVP from certain vendors contained precursor impurities that shape-direct AuNPs to create nanostars. Importantly, they expanded on this discovery by recreating the nanostar formation with purified PVP by adding hydrazine. Despite the popularity of PVP as a surfactant within the research community, the identity of its impurities and their effects still needs to be deciphered.
Figure 3.
TEM images and extinction spectra of Au-NSTs synthesized using unpurified PVP, with different end groups, from two suppliers vs purified PVP. Reprinted with permission from ref (40). Copyright 2022 Royal Society of Chemistry.
Occasionally, impurities act as key components during NP formation. Polyvinyl acetate (PVAc) is commonly used as a NP ligand (e.g., in the radiolytic formation of AgNPs) and plays an essential role in the nucleation and growth process. However, Shin and co-workers demonstrated that purified PVAc does not produce AgNPs during radiolytic synthesis (γ-ray irradiation using 60Co source).4 Instead, AgNP synthesis only occurred when sodium acetate (NaOAc), a common impurity in PVAc, was present in sufficient quantities. Before irradiation, the acetate ion interacts with AgNO3, forming an Ag+-OAc– complex which is more amenable to reduction through irradiation than the Ag+-NO3– complex. Additionally, the Ag nuclei can also create an acetate complex, playing a role in promoting the growth of the nanoparticle.
The monounsaturated 18-C amine-bearing surfactant oleylamine (OAm) is frequently used for nonaqueous NP synthesis. However, the technical grade (i.e., ca. 70%) reagent contains impurities of varying chain length which can strongly influence as-synthesized NP size and shape.41,42 Two common impurities, elaidylamine (trans-9-octadeceneamine, ELAm, >15%) and octadecylamine (ODAm, <1–15%), impart opposing roles in the growth of ultranarrow Au nanowires (AuNWs).9 More specifically, organic syntheses of Au nanowires using specialty OAm blends result in shorter wires when doped with ODAm than with ELAm (in blends doped with both impurities, ODAm overpowers the effect of ELAm). Apart from these, other impurities in the form of octadecadienamine (5%–36%), hexadecylamine (1%–7%), and hexadecenamine are also present in substantial amounts; indeed, these impurities impact the physical properties of technical OAm and can thereby indirectly affect NP growth and stability.43
Ionic liquids (ILs) are increasingly common reaction media for NP synthesis and surface functionalization.44 They are also widely known for the controversies surrounding the impact of water, halide, acid, or parent material impurities on their physical properties. Expectedly, AgNPs produced within 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) expressed variable dispersion and agglomeration between different lots of ionic liquid solvent.6 Lazarus and co-workers spiked purified [BMIM][BF4] with water, chloride, or 1-methylimidazole and reported that contaminant levels as low as 250 ppm result in broadening and red-shifting of the plasmon band. They hypothesized that water contamination in the bulk IL interferes with the double-layer stabilization of the colloid, whereas the halide and 1-methylimidazole act as competing ligands. Accordingly, similar levels of chloride or 1-methylimidazole lead to anisotropic AgNP growth.
Impurities can be intentionally incorporated to affect the synthesis of noble metal nanoparticles. For example, controlled addition of a magnetite impurity shows that its presence during AgNPs synthesis can enhance their structural and antibacterial properties, or even integrate with the NPs to impart a magnetic response.45,46 Further, saturation magnetization of Ag-magnetite NPs increased by 29-fold after introducing a small concentration of cobalt nano ferrite, which makes the nanocomposite an alluring material toward various applications which can utilize these properties. Density functional theory (DFT) and atomistic models have shown that 1–2% of small impurities in the matrix of the larger atom in a binary metallic system cause a transition from a crystalline bulk-like structure to a nanocrystalline icosahedral (Ih) structure.47 These are especially interesting because of their enhanced catalytic and magnetic properties. But for single component clusters, face-centered cubic (fcc) truncated octahedron (TO) is more stable than Ih (i.e., for Au, Ag, and Cu). Panizon and co-workers showed that adding a small impurity, such as Cu, Co, or Ni, with these noble materials can relieve strain and induce drastic morphological changes. They studied the critical impurity percentage (I%) required for transforming Ih to fcc lattice and vice versa and the energy difference between the two-lattice structures at a particular I%.
Removing impurities has been the norm in experimental design, but purification is not always practical. Karadaghi and co-workers48 demonstrated the feasibility of recycling ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [BMIM][NTf2] for the synthesis of platinum nanoparticles (PtNPs). Using the recycled solvent reduces the cost of synthesis lower than when using the 1-octadecene. In the process of synthesizing single-crystalline magnetite nanocrystals within the ionic liquid trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide ([P6,6,6,14][NTf2]) through an iono-polyol approach, the authors hypothesized that the enhanced uniformity observed when employing twice-recycled [P6,6,6,14][NTf2] could be attributed to the existence of residual “FeO” seeds. These seeds are believed to act as nucleation sites in subsequent reactions, leading to improved consistency in the resulting nanocrystals.49 Halides are common impurities in OAm and they negatively impact the controlled synthesis of AgNPs (by forming Ag halide precipitants), thus necessitating halide removal from the solvent before synthesis.50 In some situations, impurities can be removed successfully,51 but separating all the ions in other cases will compromise the quality of the nanomaterial. In another example, halides and elemental impurities in a solution of AuNPs can poison the Au catalyst. A purification approach is impractical since there are several sources for these impurities (solvent, reagents, experimental setup, drying agents, etc.). Considering the possible nature of the impurities and their affinity toward Au, Kumar and co-workers introduced a sacrificial acid into the media to neutralize the impurities and free the catalyst.52 Even seldomly considered wet chemistry practices like clean glassware and the quality of Milli-Q water (the trace elements and pH) used for synthesis can dramatically influence the aspect ratio of Au nanorods.53
Remnant reagent impurities following NP synthesis can impede their target application. For example, excess sodium citrate in AuNPs induces cytotoxicity in human alveolar cells,54 and free CTAB present at or above 10 μM is known to be generally toxic to humans.55 Schmidt and co-workers found that the robust absorption of chloride anions, originating from the reactant, in the membrane of carbon-supported Pt fuel cells during oxygen reduction reactions (ORR) can impose a kinetic constraint in the formation of H2O2.56 Chloride can be introduced in the preparation of the cell or as a contaminant in humidified feed streams and acts as a site-blocking species, reducing the available active sites for the ORR. Enhanced formation of peroxides reduces the stability of the membranes and causes degradation. Therefore, purified and chloride-free electrolytes and feed streams avoid performance losses.
Conversely, remnant impurities can instead enhance the properties of the nanomaterial. Formic acid oxidation (FAO) is enhanced when using a glassy carbon (GC) electrode modified with PtNPs synthesized in the presence of hydrocarbon impurities.12 The authors hypothesize that parts per million quantities of organic impurities (acetonitrile being prominent) disrupt CO poisoning of the Pt surface while simultaneously increasing the current peak of FAO to CO2. Indeed, susceptibility to CO poisoning is a known limitation of PtNPs in FAO fuel cells.57 These hydrocarbon impurities are common in fuel cell manufacture and extend the life of the Pt surface.12 Osawa and co-workers found that formic acid adsorbed on a Pt catalyst in a FAO reaction acts as a reaction intermediate instead of a site-blocking agent.57 Adsorbed formic acid undergoes a more rapid adsorption–desorption equilibrium than bulk formic acid and can decompose to CO2 and H+ ions. In contrast, the direct bulk oxidation pathway of formic acid to carbon dioxide is negligible,58 suggesting that adsorbed formic acid acts as an essential intermediate in the methanol electro-oxidation reaction.
3. Non-Noble Metal Nanoparticles
Transition metals are useful chemical catalysts because of their vacant d-orbitals and their ability to have variable valence states. At the nanoscale, the catalytic59 and electrocatalytic60 potential of transition metals are enhanced, and much research is focused on transition metal NPs from cheap and abundant materials like aluminum, iron, copper, and nickel.61,62 Because impurities cause strain in the nanocrystal, some impurities are intentionally introduced in the synthesis in order to control the catalytic power of non-noble metal NPs; however, other unintended impurities can have negative or positive effects. This section presents studies that encountered evidence of impurities affecting the synthesis, in liquid media or the gas phase, of non-noble metal nanoparticles.
Trace impurities in IL media used for transition metal NP syntheses affect the resulting NP morphology and play a crucial role in shape directing. Zhang and co-workers demonstrated that HCl leached from IL and the type of polar protic solvent used in synthesizing tellurium (Te) NPs affected the size and morphology of Te nano- and microstructures.63 The authors found that trace amounts of HCl impurities are present in trihexyltetradecylphosphonium chloride ([P6,6,6,14]Cl) IL and usually exist as HCl2– anions due to a strong interaction with chloride ions. However, polar protic solvents (alcohols, water, and amines) used in the synthesis could liberate HCl from the HCl2– anion by accelerating the cleavage of the P–Te bond in trialkylphosphanetelluride reactants, leading to the formation of highly homogeneous 3D Te fusiform assemblies and 3D aloe-like Te microarchitectures. SEM images in Figure 4 show the construction of fusiform Te structures in the presence of different polar protic solvents that are homogeneous. It has also been reported that a Brønsted acid impurity in the [P6,6,6,14]Cl IL leads to the surface activation of elemental copper. This is vital to obtain a quantitative product yield, leading to the formation of micrometer-sized Cu3–xP particles. According to these findings, trace impurities in ILs affect the quality of NPs synthesized therein and, with careful analysis and control, can be used as a synthetic tool.64
Figure 4.
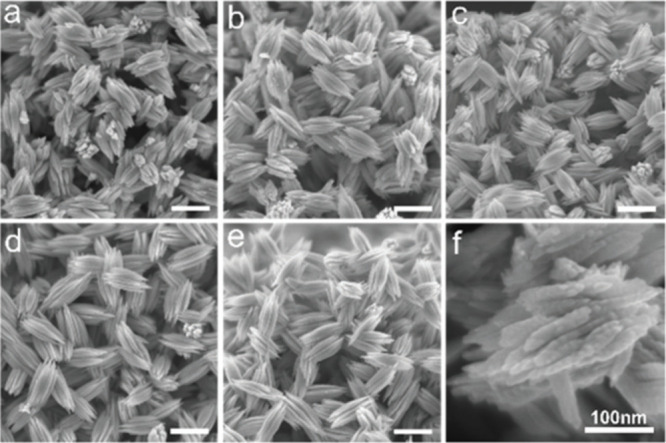
SEM images of Te particles that were obtained by mixing Te with [P66614]Cl IL in different polar protic alcohol solvents (a = ethanol, b = n-butanol, c = n-hexanol, d = n-octanol, e = n-decanol; scale bar for a–e is 300 nm). Panel (f) represents SEM imaging of a particle prepared in n-butanol (scale bar is 100 nm). Reprinted with permission under a Creative Commons Attribution 3.0 Unported License from ref (63). Copyright 2020 Royal Society of Chemistry.
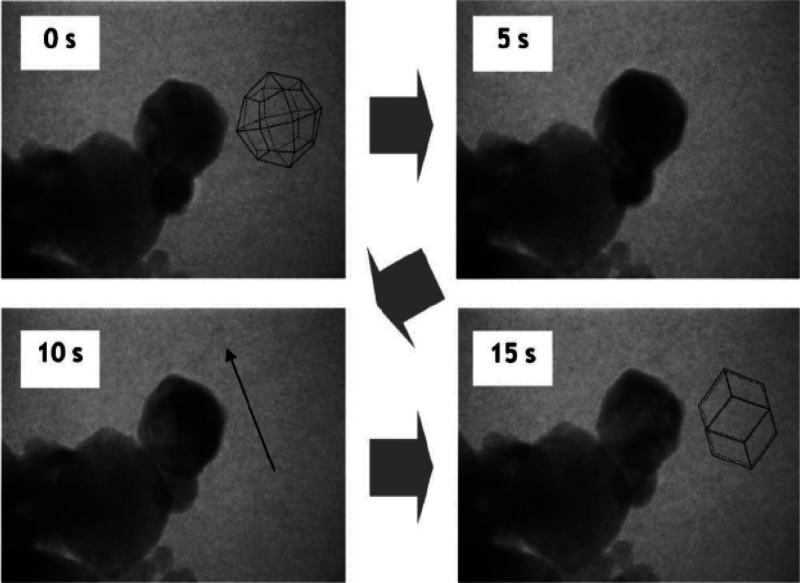
Kido and co-workers reported that incorporating oxygen impurities into the structure of chromium nanoparticles (CrNPs) leads to a phase transition from δ-CrNPs to metastable cubic α-CrNPs (Figure 5).65 The authors estimated the oxygen volume to be 20–30% of the volume of the Cr2O3 surface layer. Kimoto and Nishida also showed that oxygen content in introduced gas is essential for the growth of cubic particles. However, interstitial oxygen in the cubic α-Cr particles can also act as an impurity and alter the growth.66 Trace amount of reactive gas is essential for nucleation for Ti, Co, and W NPs67,68 when homogeneous nucleation is challenging to initiate. Similarly, exposure to oxygen can alter (and often widen) the shape and size distributions of some metal NPs. For example, magnesium nanoparticles (MgNPs) form a MgO shell and exhibit a broader size distribution than Cu, Co, Fe, Nb, and Mo.69 The MgO formed at the Mg/MgO interface can also lead to hollow, sharp facet voids due to the different diffusion rates of Mg and oxygen. The presence of oxygen or water can be problematic for the homogeneous nucleation of these alkaline earth metals due to their lower reduction potentials. For the bimetallic gas-phase synthesis of MgTi, the introduction of trace amounts of H2 or CH4 to the reaction directs the growth of resulting NPs.70,71 Indeed, the presence of H2 directs MgTi NPs toward hexagonal platelets whereas CH4 directs the growth toward trigonal platelets. The authors posit that a strong Ti–C affinity results in a TiC core in the presence of CH4, leading to the formation of TiC/Mg core–shell NPs. On the other hand, TiHx forms in the presence of trace H2, leading to the development of a MgTi solid solution (Figure 6).
Figure 5.
Images of the morphology transition of α-CrNPs from a rhombic dodecahedron to a cubic one upon incorporating an oxygen impurity. The shape was elongated along the direction shown by the arrow (bottom left panel). Adapted with permission from ref (65). Copyright 2005 Elsevier.
Figure 6.
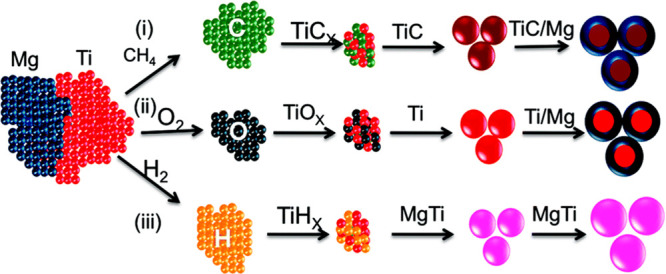
Schematic representation of the nucleation and growth of MgTiNPs. Gas impurities of CH4, O2, or H2 result in core/shell TiC/Mg NPs, core/shell TiO2 NPs, or MgTiNPs as depicted by routes (i), (ii), or (iii), respectively. Adapted with permission from ref (70). Copyright 2017 Royal Society of Chemistry.
4. Carbon Nanomaterials
Carbon is an indispensable element of organic chemistry and life, and the invention of Buckminsterfullerene72 opened the door of carbon nanomaterials to the world. Graphene and carbon nanotubes (CNTs)73 have since become the most critical structures discovered, while fluorescent carbon dots (FCDs)74,75 are the newest addition to this family. Carbon-based nanomaterials have gained significant attention because of their extraordinary electrical and thermal conductivities, mechanical strength, high thermal stability, low toxicity, good biocompatibility, and synthetic tunability.76−78 They have already been successfully used for drug and gene delivery,79 bioimaging,80 energy storage,81 electrochemical sensors,82 and even dye-sensitized solar cells.83 Recent studies have revealed the role of impurities in the synthesis and performance of these carbon nanomaterials. This section starts by presenting studies that encountered evidence of impurities affecting the synthesis of carbon nanomaterials, followed by works in which impurities are explicitly considered in the experimental design and, finally, reports that highlight the role of impurities on the applicability of the nanomaterial.
Metal catalysts used in synthesizing carbon nanomaterials retain impurities that enhance or inhibit electrocatalytic processes.2 For example, Mazánek and co-workers reported that the electrocatalytic activity at elevated temperature was almost negligible in ultrapure graphene prepared by the chemical reduction of graphene oxide. At the same time, the rate of heterogeneous electron transfer was slightly slowed down.19 It is crucial to enhance the mobility of graphene for electronic and sensor applications, but Coulomb impurities84 present in graphene affect the intrinsic electronic properties. The long-range scattering by charged impurities and short-range scattering by atomic impurities degrade the mobility and transport properties. Martin and co-workers used a Monte Carlo simulation to show that the effects of the impurities also depend on the substrate. Pure graphene on hexagonal boron nitride (h-BN) exhibits the best performance. In contrast, in the presence of Coulomb impurities, graphene on SiC shows the best results in diffusion coefficient, drift velocity, and low field mobility due to better screening of such impurities.85
Impurities can directly affect surface defects, such as wrinkles, cracks, and microstructural defects (MSD), thus playing an essential role in determining the crystal quality and electronic properties of graphene. Zhang and co-workers described how the growth mechanism of an MSD depends on the impurities present on the Cu substrate surface, such as Cu particles and silicon dioxide particles introduced during the chemical vapor deposition (CVD) growth of graphene.86 Nucleation initiates from the surface impurities on the substrate, and finally, MSDs form around those impurities. Carbon impurities on the substrate surface can damage the graphene, decrease stability, and direct nucleation, depending on the etching environment. In a weak etching growth environment, the carbon impurities act as the primary nucleus for graphene growth, while in a robust etching atmosphere, they inhibit nucleation.16 Zang and Li reported that isotope impurity in single-walled carbon nanotubes could reduce conductance by 60% and change temperature-dependent behaviors.87
Impurities often lead to misinterpretation and confusion in the practical implementation of carbon materials, as specific impurities and concentrations vary from sample to sample. One example of this principle is in CNT sensors; because a significant deviation has been observed in electrochemical responses in similar sensors, Jones and co-workers used CNTs from Phoenix Nano-Systems, synthesized with metal-free catalysts,88 to avoid impurity-related misinterpretation. Further misinterpretation can be caused by secondary impurities, which originate from the synthesis reaction or reaction media, if these impurities affect the properties of the nanomaterial. For example, FCDs have gained attention due to their unique tunable fluorescence and high photostability. Still, recent reports are showing that the origin of fluorescence may be due to the organic byproduct, imidazo[1,2-a] pyridine-7-carboxylic acid, 1,2,3,5-tetrahydro-5-oxo- (IPCA) formed alongside FCD (Figure 7).89 It is clear that IPCA is the true source of Et-CDs fluorescence, synthesized using N-ethylmethane-1,2-diamine (Et-EDA) and citric acid. Essner and co-workers described a purification procedure of unfractionated samples by membrane dialysis90 to revisit the misconception about FCD photophysical properties and fluorescent applications (bioimaging, sensing, and heavy metal detection).
Figure 7.
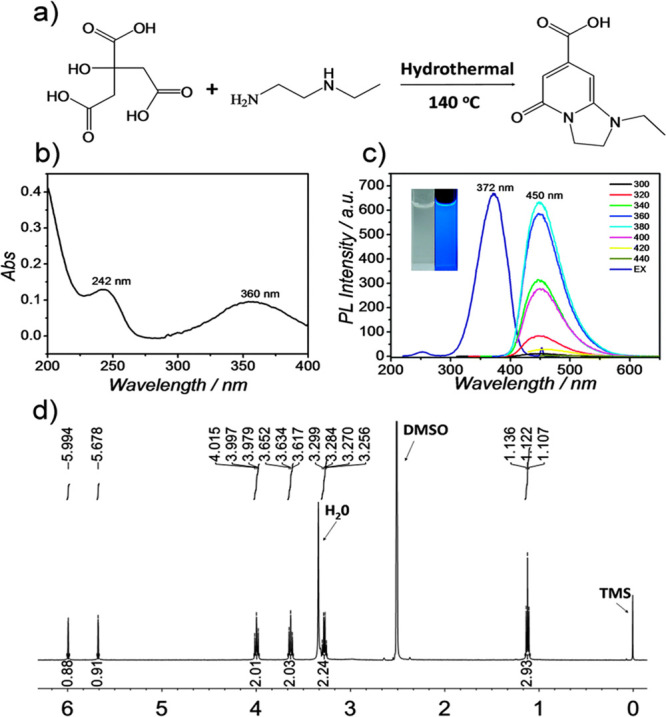
(a) Schematic synthesis of PL Et-CDs using citric acid and N-ethylmethane-1,2-diamine (Et-EDA). Panels (b)–(d) represent characterization of these CDs: (b) UV–vis absorption spectrum, (c) PL spectra (with inset photographs of Et-CDs solution under ambient and UV illumination), and (d) 1H NMR spectrum of 1-ethyl-5-oxo-1,2,3,5-tetrahydroimidazo[1,2-a]pyridine-7-carboxylic acid (Et-IPCA), a product of the hydrothermal reaction of CA and Et-EDA separated from Et-CDs). Adapted with permission from ref (89). Copyright 2015 Royal Society of Chemistry.
As purification may not always be practical, researchers must explore other creative ways to deal with impurities. Graphene conductivity depends on charged impurities.91 To reduce the effect of the charged impurities, Chen, Xia, and Tao introduced the ionic screening method using NaF as ion sources, as both the Na+ and F– ions are chemically inert toward adsorption onto the graphene surface and neutralize charged impurities.92
Remnant impurities following synthesis can enhance or prevent their target application. Recently, the enhanced electrochemical properties of graphene/polylactic acid (PLA) 3D-printing filament have been related to the presence of Fe, Ti, and Al impurities.14 While metal impurities have shown both positive and negative effects on the electrocatalytic performance of carbon nanomaterials, their toxicity makes them detrimental to biomedical applications.93 These metal impurities severely affect cell viability due to the excessive production of reactive oxygen species (ROS) in the A549 cell line.11
5. Semiconductor Nanomaterials
The modern study of nanoscience, nanotechnology, and nanobiotechnology would not be possible without the enormous contribution of semiconductor QDs and their access to the quantum confinement effect,94,95 as recognized by a recent Nobel prize.96 Since their inception, significant advances have been achieved in synthetic procedures for QDs to control their optical, magnetic, and electronic properties.97 This understanding has strengthened the backbone of the nanoworld, bringing chemistry, physics, biology, and technology together and allowing for the harmonization of QDs with inorganic, organic, and biomolecules. In addition, the unique properties of QDs have extended their applications in optoelectronics, photovoltaics, telecommunication, spintronics, quantum computing, biosensing, and bioimaging.98−101 Many of these advances derive from the intentional incorporation of impurities in the crystal structure (doping) to tune the photophysical properties of QDs.102 This section presents studies that encountered or sought evidence of impurities affecting the morphology of semiconductor nanomaterials and their photophysical properties.
Since the first report of CdSe light emitting diode (LED) nanocrystals by Alivisatos and co-workers,103 much work has been dedicated to their improvement using impurities.104 The growth, morphology, and optical properties of QDs can be readily influenced by added impurities from impure reagents or doping.105 The growth kinetics and morphology of CdSe QDs depend on the supplier of tri-n octylphosphine oxide (TOPO), commonly used as a ligand in nanocrystal synthesis.1,106−108 Technical grade TOPO contains alkylphosphonic and phosphinic acid of various chain lengths, and is reported to include at least ten phosphorus-containing impurities.109,110 These impurities bind strongly to cadmium ions and lead to rod formation instead of a CdSe spheres.111,112 Wang, Tang, and Buhro probed how common impurities (e.g., dinoctylphosphine oxide, DOPO; di-n-octylphosphinic acid, DOPA; mono-n octylphosphinic acid, MOPA; and n-octylphosphinic acid, OPA) present in commercially available TOPO assist in nearly isotropic QD formation.1 DOPA, MOPA, and OPA regulate the generation of quantum rods (QRs), with DOPA promoting quantum wire (QWs) growth. The phosphonic acids play an even more prominent role depending on their chain length; short-chain phosphonic acids preferentially bind to the CdSe or CdTe facets with densest electron deficient surface site packing (i.e., {111}ZB facets of zinc-blend (ZB) lattice structure and {001}W facets of wurtzite (W) lattice structure). As {111}ZB facets outnumber {001}W, the facet-to-surface energy ratio of the ZB nanocrystal is higher than that of the wurtzite nanocrystal. As a result, short-chain alkylphosphonic acid favors ZB phase of CdSe and CdTe nanocrystals, whereas long-chain phosphonic acids favor the wurtzite phase.111,113 Even high-purity commercially available TOPO can be decomposed into DOPA, MOPA, and OPA at elevated temperatures in the presence of residual oxygen in the reaction mixture, leading to the formation of QRs.107
Another popular solvent for colloidal CdSe nanocrystal synthesis is octadecene (ODE), though it forms poly(ODE) impurities at temperatures above 120 °C which inhibit charge transfer in resulting nanocrystal films.114 Alternative solvents, like n-hexadecane and n-octadecane, can be used to avoid these problems, though some researchers opt to instead separate the nanocrystal from poly(ODE) by exchanging it with a polar capping agent [such as (6-[2-[2-(2-methoxy-ethoxy)-ethoxy]-ethoxy] hexyl) phosphonic acid]. In these cases, the now polar nanocrystals can be separated from poly(ODE) by precipitation with the addition of hexane. Similarly, the growth kinetics of QDs can be affected by intentionally introducing impurities in the crystal, altering the particle growth and dissolution rate. Tuinenga and co-workers reported that In3+ impurities promote larger CdSe NP growth (Figure 8) by increasing the conversion of magic-size nanoclusters (MSNCs)115 to monomers.116 Also, the surface energy changes as +2 charged Cd is replaced by +3 charged In. This enhances the attraction of monomers to the particle surface above the rate of monomer desorption.
Figure 8.
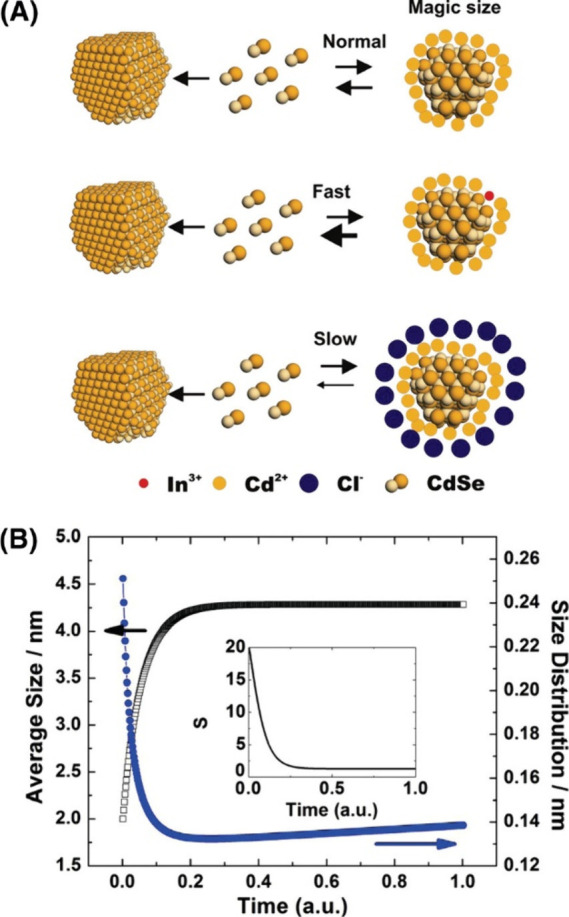
Proposed kinetic model of heterogeneous growth without adding any impurities establishes an equilibrium between the dissolution of the magic-size cluster and the free monomers in the solution. Adding In to the particle surface increases the dissolution rate of magic-size clusters to provide monomers for growth, while chloride ions stabilize the magic-size cluster against dissolution. Reproduced from ref (116). Copyright 2008 American Chemical Society.
As previously mentioned, commercially accessible OAm is found to have notable impurities, including the trans isomer, shorter and unsaturated amines, and various luminescent components (e.g., complexes of nitroalkanes and aminoalkanes).117 Indeed, these impurities alter the solvent properties of the OAm and provide a barrier for luminescence-sensitive characterization techniques like Raman spectroscopy. Purification techniques must be included before or after synthesis to remove luminescent impurities that can obscure the optical properties of the nanomaterial. Baranov and co-workers found that purified OAm allows selective precipitation of PbS quantum dots before remnant lead chloride starting materials during nanocrystal washing.118 OAm impurities can also complicate thermal treatments. For example, Sperry and Luscombe identified a pyrolyzed graphene oxide fine-grain layer (FGL) underneath a layer of synthesized Cu2ZnSnS4 (CZTS) nanocrystals following air-free synthesis and annealing (225 °C) in OAm medium.119
Anionic impurities from the precursor metal salts can alter the properties of metal chalcogenide NPs. Metal acetylacetonate, halide, acetate, oxide, nitrate, hydrate, sulfates, and mixed salts are the most commonly used salts for the synthesis of Cu(In,Ga)S2 (CIGS), Cu2ZnSnS4 (CZTS), Cu(In,Ga)(S,Se)2 (CIGSSe), and Cu2ZnSn(S,Se)4, (CZTSSe) NPs. Anionic impurities, such as chlorides, can behave as n-type impurities in the device.120 While this problem can be avoided for CIGSSe using acetylacetonate salts, indium acetylacetonate and acetate form indium oxide due to heating in the absence of a sulfur source.121 Formation of such oxides can drastically obstruct the performance of photovoltaic devices. This can be particularly problematic for highly stable and high bandgap oxides like gallium oxide.122 This oxide can behave as an insulating layer, significantly hindering the solar cell performance. Depositing CIGS on aluminum-doped zinc oxide (AZO) by the low-temperature pulsed electron deposition technique (LTPED)123 can prevent the formation of Ga2O3. The LTPED approach allows for high-quality CIGS layer formation on various surfaces and substrates. To avoid the problems arising from the anionic contaminant, Deshmukh and co-workers introduced a new pathway for metal chalcogenide NPs by combining an amine-thiol-based precursor approach and colloidal NP synthesis.124 They dissolved the pure metals in 1,2-ethanedithiol in the desired amount to make binary, ternary, and quaternary metal thiolate precursors free from the previously mentioned anionic precursors.
Transition metal doping in QDs imparts new properties, such as thermal and environmental stability and Stokes shifts, which help to avoid self-quenching and extend excited-state lifetimes. Indeed, Mn2+ and Cu2+ are among the most common transition metal impurities imposed in QDs. For Mn2+-doped QDs, the altered optical properties depend on the relative position of the Mn2+ energy levels; in CdS, ZnS, and ZnSe hosts, the Mn2+ ligand field excited state lies within the bandgap of the semiconductor nanocrystal. Eilers and co-workers reported that increasing Mn2+ concentration in a ZnTe host decreases the excitonic luminescence intensity (∼430 nm) and increases the Mn2+6A1 ← 4T1 ligand field transition intensity (∼560 nm).125 This observation was attributed to semiconductor-to-Mn energy transfer. Also, the lifetime of the doped semiconductor was enhanced up to 200 μs because of the spin-forbidden Mn2+6A1 ← 4T1 transition (Figure 9). Beaulac and co-workers observed that both the bandgap and the relative position of the Mn2+ energy levels in CdSe changed with nanocrystal size.126 For smaller QDs with higher bandgaps, the Mn energy levels lie within the bandgap and exhibit two luminescence peaks; one comes from CdSe exciton emission and another due to the 6A1 ← 4T1 transition (Figure 9). Only one photoluminescence (PL) peak is observed in larger nanocrystals, as Mn energy levels lie above the host bandgap. In the case of Mn2+ doped ZnO QDs, the PL intensity decreases with increasing dopant concentration. Additionally, no additional peak arises due to the d-d transition in the Mn center. The presence of a sub-bandgap photoionization state leads to nonradiative relaxation back to the ground state (Figure 9). Sivasankar and co-workers reported that the optical bandgap of CdSe nanocrystals increases with the concentration of dopant Cu2+ ion.127 They proposed incorporating copper ions and creating defect levels in the bandgap to increase the carrier concentration. Cu-doped CdSe exhibits room-temperature ferromagnetism, as the localized magnetic ions in semiconductors give rise to an exchange interaction that increases the bandgap between the s-p electron of the host and the Cu d-electron. The extent of the magnetic moment increases with the Cu concentration and reaches the optimum value for 8% Cu-doped CdSe. Jabeen and co-workers reported that noble metal-doped ZnS QDs show redshift in their absorption spectra.128 Au-doped ZnS exhibits green light emission along with blue light, but the crystallinity of the doped ZnS decreased compared to the pure ZnS. Rekha and co-workers129 reported reduced photocatalytic activity when ZnO is doped with Mn.
Figure 9.
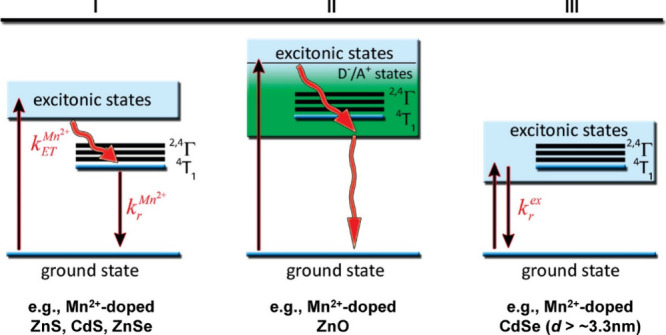
Schematic representation of different electronic structures related to photoluminescence is observed in colloidal Mn2+-doped semiconductor nanocrystals. In the first scenario (I), efficient energy transfer (kETMn2+) quenches excitonic emission and sensitizes Mn2+6A1 ← 4T1 luminescence. In scenario (II), excitons are quenched by Mn2+ photoionization states (dopant-bound excitons) that relax nonradiatively to the ground state. Finally, in scenario (III), all Mn2+ excited states are located outside the bandgap, and the nanocrystals show excitonic emission. Examples of Mn2+-doped II–VI diluted magnetic semiconductor (DMS) nanocrystals of each type are provided. Reproduced from ref (126). Copyright 2008 American Chemical Society.
6. Perovskite
In spite of their short history,130,131 perovskites nanomaterials are currently an exceedingly popular research area, with their tunable bandgap,132 large absorption cross sections,133 negligible electron–phonon coupling,134 narrow emission line width, and simple and cost-effective solution synthesis garnering widespread attention. However, perovskite nanomaterials are unstable and intolerant of harsh conditions, limiting their commercial use.135 Similar to semiconductor QDs, doping has become quite common to tune the properties of perovskites, though recent works have shown that some impurities can accelerate their degradation.136,137 This section presents studies that encountered or sought evidence of impurities affecting, positively or negatively, the synthesis of perovskite nanomaterials, followed by works in which impurities are explicitly considered in the experimental design.
Zhao and co-workers reported that doping alkali metals, such as K+ or Na+, can improve grain size, increase luminescence intensity, increase the photoluminescence quantum yield (PLQY), and decrease the carrier lifetime trap state.138 Because fewer trap states are equivalent to less nonradiative recombination, doping the alkaline earth metal Sr2+ in an organic–inorganic hybrid perovskite, methylammonium lead iodide (MAPbI3), can decrease the bandgap and increase thermal stability.139 A similar effect can be observed by replacing MA+ with Cs+ in MAPbBr3 nanocrystals due to lattice shrinkage and reduced defect density.140 Li and co-workers dug deep into this to understand the reason behind such improved chemical, thermal, and optoelectronic properties. X-ray photoelectron spectroscopy (XPS) measurements have shown that the binding energies of Br, Pb, and N (from the methylammonium cation) atoms increase monotonically with increasing Cs+ dopant concentration. This phenomenon indicates that Cs+, an isoelectric impurity, impacts atomic interaction as Cs+ is more electronegative than MA+. Thus, Cs+ impurities present in the CsxMA(1-x)PbBr3 lattice enhance stability, lead to charge polarization, and can increase the photocurrent in CsxMA(1-x)PbBr3 photodetectors.141
Transition metal doping is being extensively investigated to disseminate new optical, magnetic, and electronic properties on perovskite nanocrystals. For example, Liu and co-workers first reported Mn2+-doped CsPbCl3 with tunable bandgap and color.142 Ni2+ is another emerging dopant, though unlike Mn2+, it does not exhibit dual emission but decreases the perovskite crystal size and distribution with increasing Ni2+ concentration.143 Bi3+ doped lead halide perovskites (LHP) exhibit a broad (850- 1600 nm) NIR photoluminescence emission due to the dopant,144 but the host’s photoluminescence intensity decreases both with increasing Bi3+ concentration and over time. Also, the Bi3+ doped MAPbI3 solar cell performance dropped significantly in the presence of trace amounts of Bi3+. Figure 10 shows that with increasing Bi3+ amount the short circuit current density (Jsc) and the fill factor (FF) decrease. This is because Bi3+ generates deep and shallow electronic states near the bandgap and causes a reduction in charge carriers’ mobility and defective recombination.20 Elemental lead is a byproduct from the decomposition of PbI2 that accelerated the degradation of perovskite.136 Cr2+ has a high diffusion barrier but a low Cri (interstitial site) formation energy. Hence, the incorporation of Cr2+ in the MAPbI3 layer is kinetically hindered. Mo and W are the most compatible metals since they have higher Moi and Wi formation energies and diffusion barrier. Even though Cu and Ag can diffuse quickly into the MAPbI3 layer, they do not introduce any deep levels that cause nonradiative recombination. Meanwhile, Au trapped in the Pb vacancy causes significant nonradiative recombination. In contrast, Au, Ag, and Cu can easily diffuse in CsSnBr3, but, therein, Au does not cause nonradiative recombination.24 Lineva and co-workers showed that a small amount of LiBO3, LiPO4, and Bi2O3 doped in Li0.33La0.57TiO3 (LLTO) perovskite can downturn the sintering temperature.17 LiBO3 and LiPO4 present in LLTO raised the total conductivity by 1 order of magnitude due to the reduction of the Schottky barrier height.145 However, impurities (e.g., oxygen vacancy Sb, La, and K) in BaSnO3 perovskite decreased the thermal conductivity via different phonon scattering pathways, such as acoustic and low-frequency optical phonon scattering.146
Figure 10.
JV hysteresis loops starting from 1.2 V were measured at various voltage sweep rates measured at solar cells with different Bi concentrations. The reduced JSC and FF upon adding Bi are due to a strong dependence on charge extraction on the electric field. Adapted with permission from ref (20). Copyright 2019 Royal Society of Chemistry.
In the case of MAPbI3 solar cells, noble and transition metal electrodes can introduce unintentional impurities and cause device degradation. Irrespective of the presence of a hole transport layer (HTL), it has been seen that some metal ions can diffuse into the absorption layer. Du and co-workers investigated the energy and kinetics of the electrode materials based on DFT calculations and found that the diffusion barrier is positively correlated with oxidation charge, a guideline for selecting a metal as an electrode in a solar cell.13 While point and intrinsic defects were being blamed for the inferior performance of MAPbI3-based solar cells, Liang and co-workers147 showed using DFT studies that atomic hydrogen interstitial, Hi+, serves as an electrically active negative-U defect.148 But molecular hydrogen plays a chemically inert role. The Hi+ defects can be easily incorporated from the reaction media, deprotonation of organic HTL in the solar cell, and the organic cations (e.g., MA+) in organic–inorganic hybrid perovskite. High-density Hi+ in MAPbI3 can lead to poor solar cell performance due to polarization, hysteresis, and a photogenerated field-screening effect.
As removal of impurities may not always be practical or desirable, researchers have been considering the effect of impurities as part of experimental design and exploring creative ways to deal with them. Liang and co-workers147 used DFT calculations to predict the outcomes of atomic and molecular hydrogen in MAPbI3 and MASnI3 perovskites. They concluded that a slight increase in the amount of iodine or tin in the synthesis suppresses damage from atomic hydrogen. Similarly, Wang and co-workers137 studied the preparation of HTL using nickel oxide in a perovskite solar cell; they demonstrated that the efficiency and stability of the solar cell could be improved by preventing the absorption of nitrate ion, which is simply done by adding [BMIM]BF4 IL, since the resulting [BMIM]+ can preferentially absorb on the surface of Ni(OH)2.
7. Oxide Nanomaterials
Metal oxides have unique physical, chemical, electronic, and magnetic structures and properties compared with other nanomaterials. Their distinctive features have made them popular in diverse fields like sensors, magnetic imaging, photocatalysis, solar cell design, batteries, magnetic storage media, energy conversion, optoelectronics, and electronics.149−154 However, like other nanomaterials, impurities significantly impact the synthesis of metal oxide NPs. This section presents studies that encountered or sought evidence of impurities affecting the structure of metal oxide nanomaterials and their photocatalytic properties.
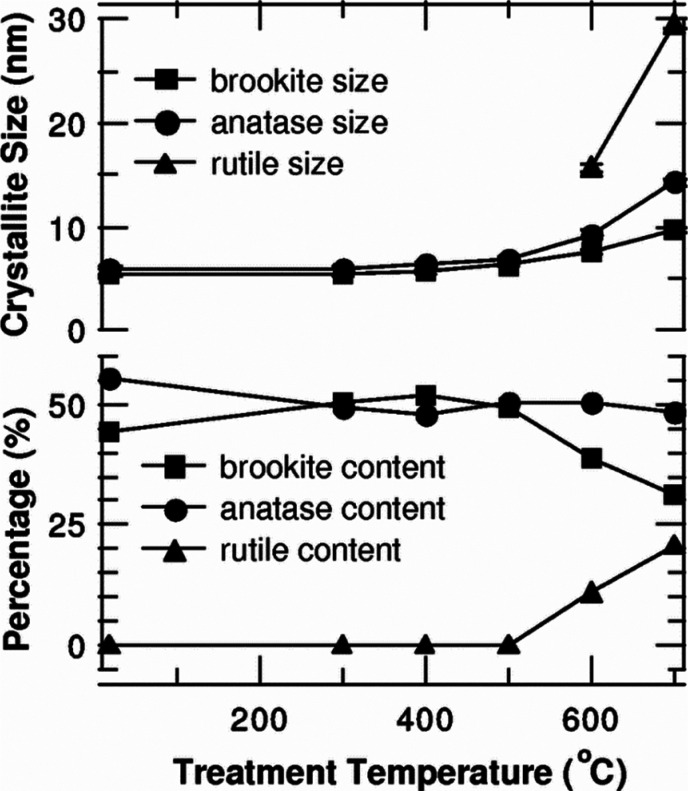
Surface impurities affect the crystal growth and phase transformation of the NPs. Grena and co-workers used Car–Parrinello dynamics as a relaxation tool, combined with a standard DFT-based structural optimization method, to show the stabilizing effect of surface impurities on ZrO2 nanoclusters.23 When chemisorbed water was introduced as an impurity, it was observed that the clusters with greater impurity concentrations expressed more ordered and crystalline structures than those when impurities were absent. This ordering was proportional to the amount of water impurity. Thus, they proposed that the nanoclusters have an affinity to bind with impurities because the formation of the Zr–O bond is energetically favorable. These computational data are supported by the experimental data obtained from the X-ray total scattering pair distribution function (PDF). Another example is the well-reported phase transformation in TiO2 NPs that structurally incorporate impurities. Dopants with > +4 charge favor the anatase to rutile phase change, while dopants having < +4 charge slow the process.155 However, Y3+ is an exception that inhibits rutile transformation (Figure 11). Y3+ surface dopants increase the activation energy of anatase to rutile transformation by lowering the anatase surface energy of TiO2 NPs.15
Figure 11.
Crystallite size in TiO2 NPs as a function of the treatment temperature (top). Percentage of constituent structures as a function of treatment temperature (bottom). Adapted with permission from ref (15). Copyright 2007 American Physical Society.
Xiong and co-workers provided a fine correlation between particle size and impurities to the photoluminescence of ZnO NPs.156 ZnO NPs synthesized via a plasma synthesis technique have shown smaller particles to possess higher carboxylate (COO−) and hydroxyl (−OH) group impurities and vice versa. These impurities originated from the carbon-containing plasma species, and hydroxyl groups are present due to the hygroscopic nature of ZnO. These near-surface impurities serve as the nonradiative recombination centers or trapping centers, which leads to a decrease in the radiative recombination intensity for smaller-size particles.157 Annealing the samples in a vacuum can reduce the carboxylate and hydroxyl impurities, but that can increase the oxygen-deficient defects. Ga2O3 nanostructures synthesized without annealing tend to exhibit elevated interstitial impurities derived from oxygen atoms.158 Depending on the impurity concentration, the intensity of green emission can either decline or surge. As interstitial oxygen defects accumulate, there is an augmented electron population at the impurity level stemming from the transfer of electrons from the Ga2O3 conduction band. In another study, Calcabrini and co-workers showed fatty acid-stabilized oxide nanocrystal formation from metal nitrate precursor without adding any fatty acid to the reaction mixture.159 The authors discovered that metal nitrate precursors (e.g., Ce(NO3)3·6H2O) form complexes (e.g., [Ce-(RNH2)n(NO3)3]) upon reacting with OAm, the intended NP ligand. The nitrates then oxidize OAm to create fatty acid impurities which then become the primary capping ligands. The same phenomenon was observed during the preparation of the NiO, ZnO, and CeO2-x NPs.
The doping of TiO2 has also been used to enhance its photocatalytic efficiency.160,161 For example, Al and Cu impurities decreased the bandgap value of TiO2 and increased its photocatalytic activity by trapping electrons and prolonging the recombination time of photogenerated electrons and holes.162 Xing and co-workers showed that Ti3+ self-doped TiO2 exhibits enhanced visible light absorption and photocatalytic efficiency without weakening UV-light photocatalytic activity.163 Furthermore, adsorbed chloride ions onto the TiO2 NP surface can also influence densification, phase transformation, and grain growth during the sintering of NPs.7 Impurities do not only affect the physical properties of the host nanocrystal; considering the kind of chemical reaction intended, the catalyst can be altered by selecting a particular doping material. For example, doping In2O3 nanocrystals with Bi atoms creates Lewis acidic/basic Bi3+–O2– pairs which enhance the adsorption and activation of CO2 during photocatalytic hydrogenation.164 An important n-type semiconductor bismuth tungstate (Bi2WO6) has shown an increased nonlinear absorption upon La doping due to the charge separation efficiency of photogenerated charge carriers.165 Doping La causes contraction in the Bi2O3 lattice, and the defect sites present in the crystalline structure act as centers to reduce photogenerated electron–hole pair recombination. An enhanced nonlinear absorption has also been seen in nanostructures of Cr-doped NiO (Ni0.98Cr0.02O).165 The excellent nonlinear optical properties of the doped materials make them superior candidates for fabricating various optical devices. Impurities can also affect the localized surface plasmon resonance (LSPR) of semiconductor nanocrystals, as revealed by a systematic study using Sn, Ce, Ti, or Zr as a dopant in In2O3 nanocrystals. Tandon and co-workers reported that properties inherent to the dopant, like electropositivity, atomic radius, and stability of aliovalent oxidation state affect carrier concentration and damping but does not affect surface depletion, which seems to depend only on the characteristic of the In2O3 nanocrystal.166
8. Thermoelectric Materials
Thermoelectric materials can uniquely convert temperature differences into electrical energy or vice versa, making them a promising technology for a wide range of applications, from power generation to cooling and heating devices.167−169 However, the performance of thermoelectric materials is strongly influenced by the presence of impurities, which affect the electrical conductivity, thermal conductivity, and carrier concentration. Therefore, understanding the effect of impurities on thermoelectric materials is critical for optimizing their performance and developing more efficient and reliable thermoelectric devices. In this context, researchers are exploring various strategies, such as doping and nanostructuring, to mitigate the negative impact of impurities on thermoelectric materials, to improve their thermoelectric performance, and to widen their potential applications. This section presents an analysis of the theoretical effect of impurities on the properties of thermoelectric materials, followed by examples of the effects of oxygen or magnetic impurities and a summary of works seeking to improve thermoelectric materials by exploiting the role of impurities.
The figure of merit,
defines the efficiency of a thermoelectric material, where S is the Seebeck coefficient, σ is the electrical conductivity, κ is the thermal conductivity, and T is the absolute temperature.169,170 The higher the ZT value, the better the energy conversion efficiency. The impurities can change the density of states (DOS) around the materials’ Fermi level, which can alter the ZT value. Ionized impurities are charged atoms or molecules that have lost or gained one or more electrons, creating a charged defect in the material’s crystal lattice. When a carrier (either an electron or a hole) moves through the lattice, it can interact with the charged impurities and scatter off. This scattering process can affect the carrier mobility and, therefore, the material’s electrical conductivity. In the case of thermoelectric materials, ionized impurity scattering can also affect the Seebeck coefficient, which is a measure of the material’s ability to generate a voltage in response to a temperature difference. The Seebeck coefficient is related to the difference in energy between the Fermi level and the energy level of the carrier, and ionized impurities can shift the Fermi level, affecting the Seebeck coefficient.
Atmospheric oxygen can oxidize transition-metal-based thermoelectric materials to produce oxide impurities. Oxygen incorporation can degrade the crystalline quality of ScN film and increase the bandgaps from 2.2 to 3.1 eV. Higher oxygen contamination can also result in non-Arrhenius behavior of ScN film resistivity vs temperature, ultimately leading to a degenerate n-type conductivity.171 Thus, ScN fabrication requires a low oxygen environment or ultrahigh vacuum conditions. A reduction in the oxygen impurity under 3% in the reaction environment can decrease electrical resistivity 4-fold without affecting the Seebeck coefficient value of ScN film. This resulted in an estimated power factor of 3.2 Å ∼ 10–3 W m–1 K–2 at room temperature.172 The ZT of a material can be enhanced either by lowering the lattice thermal conductivity or by increasing the power factor S2·σ. Introducing impurities or dopants can change the DOS, the most traditional method to influence thermoelectric properties. Bi2Te3 demonstrates superior thermoelectric performance within the temperature range of 25–100 °C. Manzano and co-workers investigated how commercially available Te powder, and the impurities present can influence the material’s thermoelectric properties.173 Bi2Te3 film made with Te powder had alkali metal impurities (i.e., Li, K, and Na). These alkali metal ions acted as p-type dopants. On the other hand, Te powder with selenium impurity improved the Seebeck coefficient and the electrical properties of the film, behaving as an n-type dopant.
Magnetic
elements, as dopants, can influence the figure of merit
of Bi2Te3, Sb2Te3, and
Bi2Se3 single crystals by changing the concentration
of electrons and holes.174 Introducing
magnetic impurities is a natural and effective method for exposing
the topological properties of Dirac points such as linear dispersion,175 chirality,176 Berry
phase,177 topological protection,178 and Dirac cones.179 Magnetic impurity doping of Bi2Te3, Sb2Te3, and Bi2Se3 causes an
increase in the effective scattering parameter r, indicating a shift
in the primary electron (or hole) scattering mechanism from acoustic
phonon scattering to impurity scattering in Fe- and Cr-doped samples.
Doping with magnetic impurities causes a significant increase in the
Seebeck coefficient for p-Bi2-xFexTe3 and Sb2-xCrxTe3 (and
a decrease for Bi2-xFexSe3), primarily due to changes in carrier
concentration and Fermi energy level, and the heat conductivity decreases
upon doping. This resulted in an enhancement in ZT value in n-Bi2-xFexSe3 (T < 100 K) and Sb2-xCrxTe3 (T > 150 K). Other n-type Mg3Sb2-based
materials have recently been reported to exhibit promising thermoelectric
properties at a low- to midtemperature range; they are the competitive
alternatives to conventional and environmentally harmful thermoelectric
materials like Bi2Te3 or PbTe.180−183 These materials are regarded as p-type compounds due to the Mg-vacancies
as calculated by Tamaki and co-workers.183 Electrical conductivity of Te-doped Mg3Sb2 exhibits an anomalous temperature dependency because of the same
point defects arising from the Mg-vacancies.184 Therefore, it is crucial to control these point defects to enhance
the thermoelectric properties, and that can be achieved by tuning
the synthetic conditions like hot-pressing temperature and holding
time. As described by Ren’s group,182 a sample of Mg3.2Sb1.5Bi0.49Te0.01 hot pressed at 1073 K (∼128 cm2 V–1 s–2) showed ∼228% increase
in Hall mobility at room temperature than the sample prepared at 923
K (∼39 cm2 V–1 s–2). This leads to a dramatic enhancement in the power factor from
∼6 to ∼20 μW cm–1 K–2. There was an almost 4-fold increase in the room temperature electrical
conductivity as well. Boosting carrier mobility (μ = 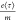 , where e is the electron
charge, m is the effective mass, and ⟨τ⟩
is the average relaxation time) can provide another route to improve
thermoelectric properties, in addition to the conventional method
of enhancing the Seebeck coefficient.181 The same group achieved an improved room temperature Hall mobility
of ∼81 cm2 V–1 s–1 from ∼16 cm2 V–1 s–1 and a power factor of ∼13 μW cm–1 K–2 from ∼5 μW cm–1 K–2 by doping transition metal elements (i.e.,
Fe, Co, Hf, and Ta). This also decreased the thermal conductivity,
and a ZT value of ∼1.7 was obtained in Mg3.1Co0.1Sb1.5Bi0.49Te0.01. In a
different example, Ga doping in PbTe creates two types of impurity
states, a shallow-level Ga3+ state, and a deep-level Ga+ state.185 This weakens the electron–phonon
scattering, resulting in higher carrier mobility, and decreases lattice
thermal conductivity.
, where e is the electron
charge, m is the effective mass, and ⟨τ⟩
is the average relaxation time) can provide another route to improve
thermoelectric properties, in addition to the conventional method
of enhancing the Seebeck coefficient.181 The same group achieved an improved room temperature Hall mobility
of ∼81 cm2 V–1 s–1 from ∼16 cm2 V–1 s–1 and a power factor of ∼13 μW cm–1 K–2 from ∼5 μW cm–1 K–2 by doping transition metal elements (i.e.,
Fe, Co, Hf, and Ta). This also decreased the thermal conductivity,
and a ZT value of ∼1.7 was obtained in Mg3.1Co0.1Sb1.5Bi0.49Te0.01. In a
different example, Ga doping in PbTe creates two types of impurity
states, a shallow-level Ga3+ state, and a deep-level Ga+ state.185 This weakens the electron–phonon
scattering, resulting in higher carrier mobility, and decreases lattice
thermal conductivity.
Various strategies have been applied for ZT value improvement, defect engineering,186−188 band-engineering,189,190 nanostructuring,191,192 modulation doping,193,194 including resonant level,195 and ionized impurity scattering.196 All of these methods mentioned here can regulate the point defect in the lattice. However, dealing with line, planar, and bulk defects presents greater difficulties. These issues can be mitigated by utilizing a more precise and regulated synthetic approach at the NP level. Fiedler and co-workers suggested a technique for converting precisely defined powder into a compact inorganic thermoelectric material using solution processing to achieve specific features.197 Colloidal synthesis of thermoelectric materials in the presence of organic ligands will result in more controlled and organized microstructure formation.
9. Perspective and Conclusion
The presence of impurities is considered a problem in synthesis; even if adverse effects are not directly observed, the uncertain nature of impurities can lead to misinterpretation of the results. For this reason, efforts have been made to reduce or remove impurities. However, as doping has shown, impurities are not always avoidable or undesirable: they must simply be known and controlled for a synthesis to be considered successful and understandable.
As discussed herein, impurities influence the course of nanomaterial research worldwide. Attempts to work around impurities have bolstered our capability to decipher their role and invent creative ways to correct for their negative effects. Bridges between theoretical work and experimental design are working to account for the role of impurities and combat misinterpretation. Indeed, a major milestone toward alleviating this misinterpretation is the understanding that vendors prepare reagents in varying ways and with a diverse precursor lineup; this knowledge has tempered the expectation that nanoscience will be reproducible vendor-to-vendor and even lot-to-lot. Perhaps this discovery is disheartening to some, but the variability it imposes also increases the likelihood of new discoveries: how could we come to know that irradiation of Ag ions in the presence of acetate would yield nanoparticles without significant acetate impurities in PVAc?
The presence of impurities can even be exploited as a pseudointernal standard for the quantification of nanomaterials during synthesis and use.198 On the other hand, we know that mass purification of reagents to ultrapure levels can be impractical for scalability and sustainability. For example, the great demand for pure carbon in synthesizing carbon nanomaterials has forced an inquiry into more abundant carbon sources (though even the highest quality coal has up to 8% impurities).199 Instead, impurities are an omnipresent variable that researchers will need to work with, particularly as we move toward a more green and sustainable future.
Author Contributions
CRediT: Angira Roy writing-original draft, writing-review & editing; Ciaran P Healey writing-review & editing; Nathaniel E. Larm writing-review & editing; Piyuni Ishtaweera writing-original draft; Maryuri Roca conceptualization, writing-original draft, writing-review & editing; Gary A. Baker conceptualization, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
References
- Wang F.; Tang R.; Buhro W. E. The trouble with TOPO; identification of adventitious impurities beneficial to the growth of cadmium selenide quantum dots, rods, and wires. Nano Letters. 2008, 8 (10), 3521–3524. 10.1021/nl801692g. [DOI] [PubMed] [Google Scholar]
- Pumera M. Materials electrochemists’ never-ending quest for efficient electrocatalysts: The devil is in the impurities. ACS Catalysis. 2020, 10 (13), 7087–7092. 10.1021/acscatal.0c02020. [DOI] [Google Scholar]
- Abutbul R. E.; Golan Y. ‘Beneficial impurities’ in colloidal synthesis of surfactant coated inorganic nanoparticles. Nanotechnology. 2021, 32 (10), 102001. 10.1088/1361-6528/abc0c7. [DOI] [PubMed] [Google Scholar]
- Shin J.; Kim Y.; Lee K.; Lim Y. M.; Nho Y. C. Significant effects of sodium acetate, an impurity present in poly(vinyl alcohol) solution on the radiolytic formation of silver nanoparticle. Radiat. Phys. Chem. 2008, 77 (7), 871–876. 10.1016/j.radphyschem.2007.12.006. [DOI] [Google Scholar]
- Rayavarapu R. G.; Ungureanu C.; Krystek P.; van Leeuwen T. G.; Manohar S. Iodide impurities in hexadecyltrimethylammonium bromide (CTAB) products: Lot–Lot variations and influence on gold nanorod synthesis. Langmuir. 2010, 26 (7), 5050–5055. 10.1021/la100166f. [DOI] [PubMed] [Google Scholar]
- Lazarus L. L.; Riche C. T.; Malmstadt N.; Brutchey R. L. Effect of ionic liquid impurities on the synthesis of silver nanoparticles. Langmuir. 2012, 28 (45), 15987–15993. 10.1021/la303617f. [DOI] [PubMed] [Google Scholar]
- Dittmann R.; Wintermantel E.; Graule T. Sintering of nano-sized titania particles and the effect of chlorine impurities. J. Eur. Ceram Soc. 2013, 33 (15), 3257–3264. 10.1016/j.jeurceramsoc.2013.05.028. [DOI] [Google Scholar]
- Amri N. E.; Roger K. Polyvinylpyrrolidone (PVP) impurities drastically impact the outcome of nanoparticle syntheses. J. Colloid Interface Sci. 2020, 576, 435–443. 10.1016/j.jcis.2020.04.113. [DOI] [PubMed] [Google Scholar]
- Lang E. N.; Pintro C. J.; Claridge S. A. Trans and saturated alkyl impurities in technical-grade oleylamine: Limited miscibility and impacts on nanocrystal growth. Chem. Mater. 2022, 34 (11), 5273–5282. 10.1021/acs.chemmater.2c00945. [DOI] [Google Scholar]
- Liz-Marzán L. M.; Kagan C. R.; Millstone J. E. Reproducibility in nanocrystal synthesis? watch out for impurities!. ACS Nano 2020, 14 (6), 6359–6361. 10.1021/acsnano.0c04709. [DOI] [PubMed] [Google Scholar]
- Ge C.; Li Y.; Yin J.; et al. The contributions of metal impurities and tube structure to the toxicity of carbon nanotube materials. NPG Asia Mater. 2012, 4 (12), e32 10.1038/am.2012.60. [DOI] [Google Scholar]
- El-Nagar G.; El-Deab M.; Mohammad A. M.; El-Anadouli B. Promoting effect of hydrocarbon impurities on the electro-oxidation of formic acid at Pt nanoparticles modified GC electrodes. Electrochim. Acta 2015, 180, 268–279. 10.1016/j.electacta.2015.08.119. [DOI] [Google Scholar]
- Ming W.; Yang D.; Li T.; Zhang L.; Du M. Formation and diffusion of metal impurities in perovskite solar cell material CH3NH3PbI3: Implications on solar cell degradation and choice of electrode. Adv. Sci. 2018, 5 (2), 1700662. 10.1002/advs.201700662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne M. P.; Pumera M. Impurities in graphene/PLA 3D-printing filaments dramatically influence the electrochemical properties of the devices. Chem. Commun. 2019, 55 (58), 8374–8377. 10.1039/C9CC03774H. [DOI] [PubMed] [Google Scholar]
- Chen B.; Zhang H.; Gilbert B.; Banfield J. F. Mechanism of inhibition of nanoparticle growth and phase transformation by surface impurities. Phys. Rev. Lett. 2007, 98 (10), 106103. 10.1103/PhysRevLett.98.106103. [DOI] [PubMed] [Google Scholar]
- Ge X.; Zhang Y.; Chen Z.; et al. Effects of carbon-based impurities on graphene growth. Phy Chem. Chem. Phys. 2018, 20 (22), 15419–15423. 10.1039/C8CP00944A. [DOI] [PubMed] [Google Scholar]
- Lineva B. A.; Kobylyanskaya S. D.; Kovalenko L. L.; V’yunov O. I.; Belous A. G. Effect of impurities on the electrical properties of the defect perovskite Li0.33La0.57TiO3. Inorgac Mater. 2017, 53 (3), 326–332. 10.1134/S0020168517030074. [DOI] [Google Scholar]
- Jessl S.; Tebbe M.; Guerrini L.; Fery A.; Alvarez-Puebla R.; Pazos-Perez N. Silver-assisted synthesis of gold nanorods: The relation between silver additive and iodide impurities. Small. 2018, 14 (20), 1703879. 10.1002/smll.201703879. [DOI] [PubMed] [Google Scholar]
- Mazánek V.; Luxa J.; Matějková S.; et al. Ultrapure graphene is a poor electrocatalyst: Definitive proof of the key role of metallic impurities in graphene-based electrocatalysis. ACS Nano 2019, 13 (2), 1574–1582. 10.1021/acsnano.8b07534. [DOI] [PubMed] [Google Scholar]
- Yavari M.; Ebadi F.; Meloni S.; et al. How far does the defect tolerance of lead-halide perovskites range? the example of bi impurities introducing efficient recombination centers. J. Mater. Chem. A 2019, 7 (41), 23838–23853. 10.1039/C9TA01744E. [DOI] [Google Scholar]
- Zhang Y.; Sui Y.; Chen Z.; et al. Role of hydrogen and oxygen in the study of substrate surface impurities and defects in the chemical vapor deposition of graphene. Carbon. 2021, 185, 82–95. 10.1016/j.carbon.2021.09.016. [DOI] [Google Scholar]
- Ying S.; Guan Z.; Ofoegbu P. C.; et al. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. 10.1016/j.eti.2022.102336. [DOI] [Google Scholar]
- Grena R.; Masson O.; Portal L.; et al. Stabilization effect of surface impurities on the structure of ultrasmall ZrO2 nanoparticles: An ab-initio study. J. Phys. Chem. C 2015, 119 (27), 15618–15626. 10.1021/acs.jpcc.5b00684. [DOI] [Google Scholar]
- Liang Y.; Cui X.; Li F.; Stampfl C.; Ringer S. P.; Zheng R. Electrode-induced impurities in tin halide perovskite solar cell material CsSnBr3 from first principles. npj Comput. Mater. 2021, 7 (1), 63. 10.1038/s41524-021-00533-5. [DOI] [Google Scholar]
- Faraday M. S. The Bakerian lecture—Experimental relations of gold (and other metals) to light. Philos. Trans R Soc. Lond A 1857, 147, 145–181. 10.1098/rstl.1857.0011. [DOI] [Google Scholar]
- Sankaran K. J.; Kunuku S.; Sundaravel B.; et al. Gold nanoparticle-ultrananocrystalline diamond hybrid structured materials for high-performance optoelectronic device applications. Nanoscale. 2015, 7 (10), 4377–4385. 10.1039/C4NR07030E. [DOI] [PubMed] [Google Scholar]
- Yildirim M. A.; Kasif T. Self-powered silicon carbide ultraviolet photodetector via gold nanoparticle plasmons for sustainable optoelectronic applications. Phys. Scr. 2022, 97 (11), 115804. 10.1088/1402-4896/ac9562. [DOI] [Google Scholar]
- Pradhan N.; Pal A.; Pal T. Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf. Physicochem Eng. Aspects. 2002, 196 (2), 247–257. 10.1016/S0927-7757(01)01040-8. [DOI] [Google Scholar]
- Thompson D. T. Using gold nanoparticles for catalysis. Nano Today. 2007, 2 (4), 40–43. 10.1016/S1748-0132(07)70116-0. [DOI] [Google Scholar]
- Bindhu M. R.; Umadevi M. Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim Acta A 2014, 128, 37–45. 10.1016/j.saa.2014.02.119. [DOI] [PubMed] [Google Scholar]
- Lee K.; El-Sayed M. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 2006, 110 (39), 19220–19225. 10.1021/jp062536y. [DOI] [PubMed] [Google Scholar]
- Sahu S.; Sharma S.; Kurrey R.; Ghosh K. K. Recent advances on gold and silver nanoparticle-based colorimetric strategies for the detection of different substances and SARS-CoV-2: A comprehensive review. Environ. Sci-Nano. 2022, 9 (10), 3684–3710. 10.1039/D2EN00503D. [DOI] [Google Scholar]
- Zhou Y.; Kong Y.; Kundu S.; Cirillo J. D.; Liang H. Antibacterial activities of gold and silver nanoparticles against escherichia coli and bacillus calmette-guérin. J. Nanobiotechnol. 2012, 10 (1), 19. 10.1186/1477-3155-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh S.; Grijalva M.; Debut A.; de la Torre B. G.; Albericio F.; Cumbal L. H. Peptides conjugated to silver nanoparticles in biomedicine - a “value-added” phenomenon. Biomater Sci. 2016, 4 (12), 1713–1725. 10.1039/C6BM00688D. [DOI] [PubMed] [Google Scholar]
- Smith D. K.; Korgel B. A. The importance of the CTAB surfactant on the colloidal seed-mediated synthesis of gold nanorods. Langmuir. 2008, 24 (3), 644–649. 10.1021/la703625a. [DOI] [PubMed] [Google Scholar]
- Ye X.; Gao Y.; Chen J.; Reifsnyder D. C.; Zheng C.; Murray C. B. Seeded growth of monodisperse gold nanorods using bromide-free surfactant mixtures. Nano Lett. 2013, 13 (5), 2163–2171. 10.1021/nl400653s. [DOI] [PubMed] [Google Scholar]
- Millstone J. E.; Wei W.; Jones M. R.; Yoo H.; Mirkin C. A. Iodide ions control seed-mediated growth of anisotropic gold nanoparticles. Nano Lett. 2008, 8 (8), 2526–2529. 10.1021/nl8016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T. H.; Koo H.; Chung B. H. Shape-controlled syntheses of gold nanoprisms and nanorods influenced by specific adsorption of halide ions. J. Phys. Chem. C 2007, 111 (3), 1123–1130. 10.1021/jp066454l. [DOI] [Google Scholar]
- Liu M.; Guyot-Sionnest P. Mechanism of silver(I)-assisted growth of gold nanorods and bipyramids. J. Phys. Chem. C 2005, 109 (47), 22192–22200. 10.1021/jp054808n. [DOI] [PubMed] [Google Scholar]
- Taladriz-Blanco P.; Spuch-Calvar M.; Prado Ad; et al. Impurities in polyvinylpyrrolidone: The key factor in the synthesis of gold nanostars. Nanoscale Adv. 2022, 4 (2), 387–392. 10.1039/D1NA00711D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan N.; Reifsnyder D.; Xie R.; Aldana J.; Peng X. Surface ligand dynamics in growth of nanocrystals. J. Am. Chem. Soc. 2007, 129 (30), 9500–9509. 10.1021/ja0725089. [DOI] [PubMed] [Google Scholar]
- Boles M. A.; Ling D.; Hyeon T.; Talapin D. V. The surface science of nanocrystals. Nat. Mater. 2016, 15 (2), 141–153. 10.1038/nmat4526. [DOI] [PubMed] [Google Scholar]
- Lang E. N.; Claridge S. A. Cow-to-cow variation in nanocrystal synthesis: Learning from technical-grade oleylamine. Nanotechnology. 2022, 33 (8), 082501. 10.1088/1361-6528/ac39cb. [DOI] [PubMed] [Google Scholar]
- Antonietti M.; Kuang D.; Smarsly B.; Zhou Y. Ionic liquids for the convenient synthesis of functional nanoparticles and other inorganic nanostructures. Angew. Chem. Int. Ed. 2004, 43 (38), 4988–4992. 10.1002/anie.200460091. [DOI] [PubMed] [Google Scholar]
- Hajalilou A.; Ferreira L. P.; Melo Jorge M. E.; Reis C. P.; Cruz M. M. Superparamagnetic Ag-Fe3O4 composites nanoparticles for magnetic fluid hyperthermia. J. Magn Magn Mater. 2021, 537, 168242. 10.1016/j.jmmm.2021.168242. [DOI] [Google Scholar]
- El-Bassuony A.; Gamal W. M.; Abdelsalam H. K. Fascinating study of adding nanocomposite cobalt nano ferrite to silver nanoparticles accompanied magnetite impurity. J. Mater. Sci: Mater. Electron. 2022, 33 (20), 16219–16235. 10.1007/s10854-022-08516-y. [DOI] [Google Scholar]
- Panizon E.; Bochicchio D.; Rossi G.; Ferrando R. Tuning the structure of nanoparticles by small concentrations of impurities. Chem. Mater. 2014, 26 (11), 3354–3356. 10.1021/cm501001f. [DOI] [Google Scholar]
- Karadaghi L. R.; Malmstadt N.; Van Allsburg K. M.; Brutchey R. L. Techno-economic analysis of recycled ionic liquid solvent used in a model colloidal platinum nanoparticle synthesis. ACS Sustain Chem. Eng. 2021, 9 (1), 246–253. 10.1021/acssuschemeng.0c06993. [DOI] [Google Scholar]
- Wagle D. V.; Rondinone A. J.; Woodward J. D.; Baker G. A. Polyol synthesis of magnetite nanocrystals in a thermostable ionic liquid. Cryst. Growth Des. 2017, 17 (4), 1558–1567. 10.1021/acs.cgd.6b01511. [DOI] [Google Scholar]
- Adhikari L.; Larm N. E.; Bhawawet N.; Baker G. A. Rapid microwave-assisted synthesis of silver nanoparticles in a halide-free deep eutectic solvent. ACS Sustain Chem. Eng. 2018, 6 (5), 5725–5731. 10.1021/acssuschemeng.8b00050. [DOI] [Google Scholar]
- Balasubramanian S. K.; Yang L.; Yung L. L.; Ong C.; Ong W.; Yu L. E. Characterization, purification, and stability of gold nanoparticles. Biomaterials. 2010, 31 (34), 9023–9030. 10.1016/j.biomaterials.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Hammond G. B.; Xu B. Cationic gold catalyst poisoning and reactivation. Org. Lett. 2014, 16 (13), 3452–3455. 10.1021/ol501663f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarabelli L.; Sánchez-Iglesias A.; Pérez-Juste J.; Liz-Marzán L. M. A “Tips and tricks” practical guide to the synthesis of gold nanorods. J. Phys. Chem. Lett. 2015, 6 (21), 4270–4279. 10.1021/acs.jpclett.5b02123. [DOI] [PubMed] [Google Scholar]
- Uboldi C.; Bonacchi D.; Lorenzi G.; et al. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part Fibre Toxicol. 2009, 6 (1), 18. 10.1186/1743-8977-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilany A. M.; Nagaria P. K.; Hexel C. R.; Shaw T. J.; Murphy C. J.; Wyatt M. D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small. 2009, 5 (6), 701–708. 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J.; Paulus U. A.; Gasteiger H. A.; Behm R. J. The oxygen reduction reaction on a pt/carbon fuel cell catalyst in the presence of chloride anions. J. Electroanal. Chem. 2001, 508 (1), 41–47. 10.1016/S0022-0728(01)00499-5. [DOI] [Google Scholar]
- Osawa M.; Komatsu K.; Samjeské G.; et al. The role of bridge-bonded adsorbed formate in the electrocatalytic oxidation of formic acid on platinum. Angew. Chem. Int. Ed. 2011, 50 (5), 1159–1163. 10.1002/anie.201004782. [DOI] [PubMed] [Google Scholar]
- Chen Y. X.; Heinen M.; Jusys Z.; Behm R. J. Bridge-bonded formate: Active intermediate or spectator species in formic acid oxidation on a pt film electrode?. Langmuir. 2006, 22 (25), 10399–10408. 10.1021/la060928q. [DOI] [PubMed] [Google Scholar]
- Ashraf M.; Ahmad M. S.; Inomata Y.; Ullah N.; Tahir M. N.; Kida T. Transition metal nanoparticles as nanocatalysts for suzuki, heck and sonogashira cross-coupling reactions. Coord. Chem. Rev. 2023, 476, 214928. 10.1016/j.ccr.2022.214928. [DOI] [Google Scholar]
- Bhoyate S. D.; Kim J.; de Souza F. M.; et al. Science and engineering for non-noble-metal-based electrocatalysts to boost their ORR performance: A critical review. Coord. Chem. Rev. 2023, 474, 214854. 10.1016/j.ccr.2022.214854. [DOI] [Google Scholar]
- Bell T. E.; Torrente-Murciano L. H2 production via ammonia decomposition using non-noble metal catalysts: A review. Top Catal. 2016, 59 (15), 1438–1457. 10.1007/s11244-016-0653-4. [DOI] [Google Scholar]
- Dutta S.; Yu I. K. M.; Tsang D. C. W.; et al. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. 10.1016/j.cej.2019.04.199. [DOI] [Google Scholar]
- Zhang T.; Doert T.; Schwedtmann K.; Weigand J. J.; Ruck M. Facile synthesis of tellurium nano- and microstructures by trace HCl in ionic liquids. Dalton T. 2020, 49 (6), 1891–1896. 10.1039/C9DT04604F. [DOI] [PubMed] [Google Scholar]
- LêAnh M.; Wolff A.; Kaiser M.; et al. Mechanistic exploration of the copper(I) phosphide synthesis in phosphonium-based and phosphorus-free ionic liquids. Dalton T. 2017, 46 (43), 15004–15011. 10.1039/C7DT03083E. [DOI] [PubMed] [Google Scholar]
- Kido O.; Kamitsuji K.; Kurumada M.; et al. Morphological alteration upon phase transition and effects of oxygen impurities of chromium nanoparticles. J. Cryst. Growth. 2005, 275 (1), e1745–e1750. 10.1016/j.jcrysgro.2004.11.161. [DOI] [Google Scholar]
- Kimoto K.; Nishida I. A. An electron diffraction study on the crystal structure of a new modification of chromium. J. Phys. Soc. Jpn. 1967, 22, 744–756. 10.1143/JPSJ.22.744. [DOI] [Google Scholar]
- Peter T.; Polonskyi O.; Gojdka B.; et al. Influence of reactive gas admixture on transition metal cluster nucleation in a gas aggregation cluster source. J. Appl. Phys. 2012, 112 (11), 114321. 10.1063/1.4768528. [DOI] [Google Scholar]
- Acsente T.; Negrea R. F.; Nistor L. C.; et al. Synthesis of flower-like tungsten nanoparticles by magnetron sputtering combined with gas aggregation. Eur. Phys. J. D 2015, 69 (6), 161. 10.1140/epjd/e2015-60097-4. [DOI] [Google Scholar]
- Kooi B. J.; Palasantzas G.; De Hosson J. T. M. Gas-phase synthesis of magnesium nanoparticles: A high-resolution transmission electron microscopy study. Appl. Phys. Lett. 2006, 89 (16), 161914. 10.1063/1.2358860. [DOI] [Google Scholar]
- Krishnan G.; de Graaf S.; ten Brink G. H.; Persson P. O. Å.; Kooi B. J.; Palasantzas G. Strategies to initiate and control the nucleation behavior of bimetallic nanoparticles. Nanoscale. 2017, 9 (24), 8149–8156. 10.1039/C7NR00916J. [DOI] [PubMed] [Google Scholar]
- Krishnan G.; de Graaf S.; ten Brink G. H.; Verheijen M. A.; Kooi B. J.; Palasantzas G. Shape and structural motifs control of MgTi bimetallic nanoparticles using hydrogen and methane as trace impurities. Nanoscale. 2018, 10 (3), 1297–1307. 10.1039/C7NR06771B. [DOI] [PubMed] [Google Scholar]
- Kroto H. W.; Heath J. R.; O’Brien S. C.; Curl R. F.; Smalley R. E. C60: Buckminsterfullerene. Nature. 1985, 318 (6042), 162–163. 10.1038/318162a0. [DOI] [Google Scholar]
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991, 354 (6348), 56–58. 10.1038/354056a0. [DOI] [Google Scholar]
- Sun Y.; Zhou B.; Lin Y.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128 (24), 7756–7757. 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
- Xu X.; Ray R.; Gu Y.; et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126 (40), 12736–12737. 10.1021/ja040082h. [DOI] [PubMed] [Google Scholar]
- Hayashi T.; Kim Y. A.; Natsuki T.; Endo M. Mechanical properties of carbon nanomaterials. ChemPhysChem. 2007, 8 (7), 999–1004. 10.1002/cphc.200700077. [DOI] [PubMed] [Google Scholar]
- Jariwala D.; Sangwan V. K.; Lauhon L. J.; Marks T. J.; Hersam M. C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42 (7), 2824–2860. 10.1039/C2CS35335K. [DOI] [PubMed] [Google Scholar]
- Peng Z.; Liu X.; Zhang W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. 10.1016/j.envint.2019.105298. [DOI] [PubMed] [Google Scholar]
- Debnath S. K.; Srivastava R. Drug delivery with carbon-based nanomaterials as versatile nanocarriers: Progress and prospects. Front Nanotechnology. 2021, 3, 3. 10.3389/fnano.2021.644564. [DOI] [Google Scholar]
- Jana P.; Dev A. Carbon quantum dots: A promising nanocarrier for bioimaging and drug delivery in cancer. Mater. Today Commun. 2022, 32, 104068. 10.1016/j.mtcomm.2022.104068. [DOI] [Google Scholar]
- Sheoran K.; Thakur V. K.; Siwal S. S. Synthesis and overview of carbon-based materials for high performance energy storage application: A review. Mater. Today Proc. 2022, 56, 9–17. 10.1016/j.matpr.2021.11.369. [DOI] [Google Scholar]
- Maduraiveeran G.; Jin W. Carbon nanomaterials: Synthesis, properties and applications in electrochemical sensors and energy conversion systems. Mater. Sci. Eng. B 2021, 272, 115341. 10.1016/j.mseb.2021.115341. [DOI] [Google Scholar]
- Shahzad N.; Lutfullah; Perveen T.; et al. Counter electrode materials based on carbon nanotubes for dye-sensitized solar cells. Renew Sust Energ Rev. 2022, 159, 112196. 10.1016/j.rser.2022.112196. [DOI] [Google Scholar]
- Pereira V. M.; Nilsson J.; Castro Neto A. H. Coulomb impurity problem in graphene. Phys. Rev. Lett. 2007, 99 (16), 166802. 10.1103/PhysRevLett.99.166802. [DOI] [PubMed] [Google Scholar]
- Rengel R.; Pascual E.; Martín M. J. Influence of the substrate on the diffusion coefficient and the momentum relaxation in graphene: The role of surface polar phonons. Appl. Phys. Lett. 2014, 104 (23), 233107. 10.1063/1.4882238. [DOI] [Google Scholar]
- Zhang Y.; Zhang H.; Li F.; et al. Invisible growth of microstructural defects in graphene chemical vapor deposition on copper foil. Carbon. 2016, 96, 237–242. 10.1016/j.carbon.2015.09.041. [DOI] [Google Scholar]
- Zhang G.; Li B. Thermal conductivity of nanotubes revisited: Effects of chirality, isotope impurity, tube length, and temperature. J. Chem. Phys. 2005, 123 (11), 114714. 10.1063/1.2036967. [DOI] [PubMed] [Google Scholar]
- Jones C. P.; Jurkschat K.; Crossley A.; Compton R. G.; Riehl B. L.; Banks C. E. Use of high-purity metal-catalyst-free multiwalled carbon nanotubes to avoid potential experimental misinterpretations. Langmuir. 2007, 23 (18), 9501–9504. 10.1021/la701522p. [DOI] [PubMed] [Google Scholar]
- Song Y.; Zhu S.; Zhang S.; et al. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3 (23), 5976–5984. 10.1039/C5TC00813A. [DOI] [Google Scholar]
- Essner J. B.; Kist J. A.; Polo-Parada L.; Baker G. A. Artifacts and errors associated with the ubiquitous presence of fluorescent impurities in carbon nanodots. Chem. Mater. 2018, 30 (6), 1878–1887. 10.1021/acs.chemmater.7b04446. [DOI] [Google Scholar]
- Chen J. H.; Jang C.; Adam S.; Fuhrer M. S.; Williams E. D.; Ishigami M. Charged-impurity scattering in graphene. Nat. Phys. 2008, 4 (5), 377–381. 10.1038/nphys935. [DOI] [Google Scholar]
- Chen F.; Xia J.; Tao N. Ionic screening of charged-impurity scattering in graphene. Nano Lett. 2009, 9 (4), 1621–1625. 10.1021/nl803922m. [DOI] [PubMed] [Google Scholar]
- Saleemi M. A.; Hosseini Fouladi M.; Yong P. V. C.; Chinna K.; Palanisamy N. K.; Wong E. H. Toxicity of carbon nanotubes: Molecular mechanisms, signaling cascades, and remedies in biomedical applications. Chem. Res. Toxicol. 2021, 34 (1), 24–46. 10.1021/acs.chemrestox.0c00172. [DOI] [PubMed] [Google Scholar]
- Ekimov A. I.; Efros A. L.; Onushchenko A. A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56 (11), 921–924. 10.1016/S0038-1098(85)80025-9. [DOI] [Google Scholar]
- Brus L. E. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80 (9), 4403–4409. 10.1063/1.447218. [DOI] [Google Scholar]
- Manna L. The bright and enlightening science of quantum dots. Nano Lett. 2023, 23 (21), 9673–9676. 10.1021/acs.nanolett.3c03904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García de Arquer F. P.; Talapin D. V.; Klimov V. I.; Arakawa Y.; Bayer M.; Sargent E. H. Semiconductor quantum dots: Technological progress and future challenges. Science. 2021, 373 (6555), eaaz8541 10.1126/science.aaz8541. [DOI] [PubMed] [Google Scholar]
- Nozik A. J.; Beard M. C.; Luther J. M.; Law M.; Ellingson R. J.; Johnson J. C. Semiconductor quantum dots and quantum dot arrays and applications of multiple exciton generation to third-generation photovoltaic solar cells. Chem. Rev. 2010, 110 (11), 6873–6890. 10.1021/cr900289f. [DOI] [PubMed] [Google Scholar]
- Biju V.; Itoh T.; Anas A.; Sujith A.; Ishikawa M. Semiconductor quantum dots and metal nanoparticles: Syntheses, optical properties, and biological applications. Anal Bioanal Chem. 2008, 391 (7), 2469–2495. 10.1007/s00216-008-2185-7. [DOI] [PubMed] [Google Scholar]
- Vajner D. A.; Rickert L.; Gao T.; Kaymazlar K.; Heindel T. Quantum communication using semiconductor quantum dots. Adv. Quantum Technol. 2022, 5 (7), 2100116. 10.1002/qute.202100116. [DOI] [Google Scholar]
- Kamimura M.Semiconductor quantum dots for NIR bioimaging. In Transparency in biology: Making the invisible visible; Soga K, Umezawa M, Okubo K, Eds.; Springer: Singapore, 2021; pp 73–84. 10.1007/978-981-15-9627-8_4 [DOI] [Google Scholar]
- Xu L.; Yuan S.; Zeng H.; Song J. A comprehensive review of doping in perovskite nanocrystals/quantum dots: Evolution of structure, electronics, optics, and light-emitting diodes. Mater. Today Nano. 2019, 6, 100036. 10.1016/j.mtnano.2019.100036. [DOI] [Google Scholar]
- Colvin V. L.; Schlamp M. C.; Alivisatos A. P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature. 1994, 370 (6488), 354–357. 10.1038/370354a0. [DOI] [Google Scholar]
- Luo D.; Wang L.; Qiu Y.; Huang R.; Liu B. Emergence of impurity-doped nanocrystal light-emitting diodes. Nanomaterials. 2020, 10 (6), 1226. 10.3390/nano10061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu Y.; Ishizumi A. Luminescence properties of impurity-doped semiconductor nanoparticles. J. Lumin. 2006, 119–120, 161–166. 10.1016/j.jlumin.2005.12.026. [DOI] [Google Scholar]
- Wang F.; Buhro W. E. Morphology control of cadmium selenide nanocrystals: Insights into the roles of di-n-octylphosphine oxide (DOPO) and di-n-octylphosphinic acid (DOPA). J. Am. Chem. Soc. 2012, 134 (11), 5369–5380. 10.1021/ja300135c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott A.; Fitzmorris R. C.; Muzaffery O.; Zhang J. Z. CdSe quantum rod formation aided by in situ TOPO oxidation. Chem. Mater. 2010, 22 (9), 2814–2821. 10.1021/cm903740e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Cohen A.; Donakowski M. D.; Knowles K. E.; Weiss E. A. The effect of a common purification procedure on the chemical composition of the surfaces of CdSe quantum dots synthesized with trioctylphosphine oxide. J. Phys. Chem. C 2010, 114 (2), 897–906. 10.1021/jp909492w. [DOI] [Google Scholar]
- Wang F.; Tang R.; Kao J. L. F.; Dingman S. D.; Buhro W. E. Spectroscopic identification of tri-n-octylphosphine oxide (TOPO) impurities and elucidation of their roles in cadmium selenide quantum-wire growth. J. Am. Chem. Soc. 2009, 131 (13), 4983–4994. 10.1021/ja900191n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosky M.; Vialle J.; Cotel T. Determination of trioctylphosphine oxide and its impurities by reversed-phase high-performance liquid chromatography. J. Chromatogr A 1984, 299, 436–444. 10.1016/S0021-9673(01)97861-6. [DOI] [Google Scholar]
- Shi A.; Claridge S. A. Lipids: An atomic toolkit for the endless frontier. ACS Nano 2021, 15 (10), 15429–15445. 10.1021/acsnano.1c07428. [DOI] [PubMed] [Google Scholar]
- Peng X.; Manna L.; Yang W.; et al. Shape control of CdSe nanocrystals. Nature. 2000, 404 (6773), 59–61. 10.1038/35003535. [DOI] [PubMed] [Google Scholar]
- Huang J.; Kovalenko M. V.; Talapin D. V. Alkyl chains of surface ligands affect polytypism of CdSe nanocrystals and play an important role in the synthesis of anisotropic nanoheterostructures. J. Am. Chem. Soc. 2010, 132 (45), 15866–15868. 10.1021/ja105132u. [DOI] [PubMed] [Google Scholar]
- Dhaene E.; Billet J.; Bennett E.; Van Driessche I.; De Roo J. The trouble with ODE: Polymerization during nanocrystal synthesis. Nano Lett. 2019, 19 (10), 7411–7417. 10.1021/acs.nanolett.9b03088. [DOI] [PubMed] [Google Scholar]
- Singh V.; Priyanka; More P. V.; Hemmer E.; Mishra Y. K.; Khanna P. K. Magic-sized CdSe nanoclusters: A review on synthesis, properties and white light potential. Mater. Adv. 2021, 2 (4), 1204–1228. 10.1039/D0MA00921K. [DOI] [Google Scholar]
- Tuinenga C.; Jasinski J.; Iwamoto T.; Chikan V. In situ observation of heterogeneous growth of CdSe quantum dots: Effect of indium doping on the growth kinetics. ACS Nano 2008, 2 (7), 1411–1421. 10.1021/nn700377q. [DOI] [PubMed] [Google Scholar]
- Constantinou C. P.; Mukundan T.; Chaudhri M. M.; Cooper J.; Field J. E.; Gray P. Sensitization of nitrocompounds by amines. Philos. Trans R Soc. Lond A 1992, 339 (1654), 403–417. 10.1098/rsta.1992.0046. [DOI] [Google Scholar]
- Baranov D.; Lynch M. J.; Curtis A. C.; et al. Purification of oleylamine for materials synthesis and spectroscopic diagnostics for trans isomers. Chem. Mater. 2019, 31 (4), 1223–1230. 10.1021/acs.chemmater.8b04198. [DOI] [Google Scholar]
- Sperry B. M.; Luscombe C. K. Ligand pyrolysis during air-free inorganic nanocrystal synthesis. Chem. Mater. 2021, 33 (1), 136–145. 10.1021/acs.chemmater.0c03088. [DOI] [Google Scholar]
- Lany S.; Zhao Y. J.; Persson C.; Zunger A. N-type doping principles for doping CuInSe2 and CuGaSe2 with Cl, Br, I, Mg, Zn, and Cd. IEEE Xplore 2005, 343–346. 10.1109/PVSC.2005.1488139. [DOI] [Google Scholar]
- Selishcheva E.; Parisi J.; Kolny-Olesiak J. Copper-assisted shape control in colloidal synthesis of indium oxide nanoparticles. J. Nanopart. Res. 2012, 14 (2), 711. 10.1007/s11051-011-0711-6. [DOI] [Google Scholar]
- Cavallari N.; Pattini F.; Rampino S.; et al. Low temperature deposition of bifacial CIGS solar cells on al-doped zinc oxide back contacts. Appl. Surf. Sci. 2017, 412, 52–57. 10.1016/j.apsusc.2017.03.242. [DOI] [Google Scholar]
- Rampino S.; Armani N.; Bissoli F.; et al. 15% efficient Cu(In,Ga)Se2 solar cells obtained by low-temperature pulsed electron deposition. Appl. Phys. Lett. 2012, 101 (13), 132107. 10.1063/1.4755772. [DOI] [Google Scholar]
- Deshmukh S. D.; Ellis R. G.; Sutandar D. S.; Rokke D. J.; Agrawal R. Versatile colloidal syntheses of metal chalcogenide nanoparticles from elemental precursors using amine-thiol chemistry. Chem. Mater. 2019, 31 (21), 9087–9097. 10.1021/acs.chemmater.9b03401. [DOI] [Google Scholar]
- Eilers J.; Groeneveld E.; de Mello Donegá C.; Meijerink A. Optical properties of Mn-doped ZnTe magic size nanocrystals. J. Phys. Chem. Lett. 2012, 3 (12), 1663–1667. 10.1021/jz300300g. [DOI] [PubMed] [Google Scholar]
- Beaulac R.; Archer P. I.; Liu X.; et al. Spin-polarizable excitonic luminescence in colloidal Mn2+-doped CdSe quantum dots. Nano Lett. 2008, 8 (4), 1197–1201. 10.1021/nl080195p. [DOI] [PubMed] [Google Scholar]
- Sivasankar J.; Mallikarjana P.; Rigana Begam M.; Madhusudhana Rao N.; Kaleemulla S.; Subrahmanyam J. Structural, optical and magnetic properties of Cu doped CdSe powders prepared by solid state reaction method. J. Mater. Sci: Mater. Electron. 2016, 27 (3), 2300–2304. 10.1007/s10854-015-4025-9. [DOI] [Google Scholar]
- Jabeen U.; Adhikari T.; Shah S. M.; et al. Synthesis, characterization and photovoltaic applications of noble metal—doped ZnS quantum dots. Chinese J. Phys. 2019, 58, 348–362. 10.1016/j.cjph.2019.01.012. [DOI] [Google Scholar]
- Rekha K.; Nirmala M.; Nair M. G.; Anukaliani A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Physica B: Condens Matter. 2010, 405 (15), 3180–3185. 10.1016/j.physb.2010.04.042. [DOI] [Google Scholar]
- Ananthakumar S.; Moorthy Babu S. Progress on synthesis and applications of hybrid perovskite semiconductor nanomaterials—A review. Synth. Met. 2018, 246, 64–95. 10.1016/j.synthmet.2018.10.003. [DOI] [Google Scholar]
- Weidman M. C.; Goodman A. J.; Tisdale W. A. Colloidal halide perovskite nanoplatelets: An exciting new class of semiconductor nanomaterials. Chem. Mater. 2017, 29 (12), 5019–5030. 10.1021/acs.chemmater.7b01384. [DOI] [Google Scholar]
- Song Z.; Chen C.; Li C.; Awni R. A.; Zhao D.; Yan Y. Wide-bandgap, low-bandgap, and tandem perovskite solar cells. Semicond. Sci. Technol. 2019, 34 (9), 093001. 10.1088/1361-6641/ab27f7. [DOI] [Google Scholar]
- Puthenpurayil J.; Cheng O. H.; Qiao T.; Rossi D.; Son D. H. On the determination of absorption cross section of colloidal lead halide perovskite quantum dots. J. Chem. Phys. 2019, 151 (15), 154706. 10.1063/1.5126039. [DOI] [PubMed] [Google Scholar]
- Wright A. D.; Verdi C.; Milot R. L.; et al. Electron-phonon coupling in hybrid lead halide perovskites. Nat. Commun. 2016, 7 (1), 11755. 10.1038/ncomms11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou S.; Xuan T.; Wang J. (INVITED) stability: A desiderated problem for the lead halide perovskites. Opt Mater. X. 2019, 1, 100023. 10.1016/j.omx.2019.100023. [DOI] [Google Scholar]
- Liang J.; Hu X.; Wang C.; et al. Origins and influences of metallic lead in perovskite solar cells. Joule. 2022, 6 (4), 816–833. 10.1016/j.joule.2022.03.005. [DOI] [Google Scholar]
- Wang S.; Li Y.; Yang J.; et al. Critical role of removing impurities in nickel oxide on high-efficiency and long-term stability of inverted perovskite solar cells. Angew. Chem. Int. Ed. 2022, 61 (18), e202116534 10.1002/anie.202116534. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Yao Z.; Yu F.; Yang D.; Liu S. Alkali metal doping for improved CH3NH3PbI3 perovskite solar cells. Adv. Sci. 2018, 5 (2), 1700131. 10.1002/advs.201700131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas J.; Sánchez-Coronilla A.; Gallardo J. J.; et al. New insights into organic-inorganic hybrid perovskite CH3NH3PbI3 nanoparticles. an experimental and theoretical study of doping in Pb2+ sites with Sn2+, Sr2+, Cd2+ and Ca2+. Nanoscale. 2015, 7 (14), 6216–6229. 10.1039/C5NR00041F. [DOI] [PubMed] [Google Scholar]
- Ding J.; Du S.; Zhou T.; et al. Cesium decreases defect density and enhances optoelectronic properties of mixed MA1-xCsxPbBr3 single crystal. J. Phys. Chem. C 2019, 123 (24), 14969–14975. 10.1021/acs.jpcc.9b03987. [DOI] [Google Scholar]
- Li L.; Su M.; Qiu X.; et al. Lattice relaxation effect in CsxMA(1-x)PbBr3 single crystal to enhance optoelectronic performance of perovskite photodetectors. Ceram. Int. 2022, 48 (1), 436–445. 10.1016/j.ceramint.2021.09.119. [DOI] [Google Scholar]
- Liu W.; Lin Q.; Li H.; et al. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content. J. Am. Chem. Soc. 2016, 138 (45), 14954–14961. 10.1021/jacs.6b08085. [DOI] [PubMed] [Google Scholar]
- Yong Z.; Guo S.; Ma J.; et al. Doping-enhanced short-range order of perovskite nanocrystals for near-unity violet luminescence quantum yield. J. Am. Chem. Soc. 2018, 140 (31), 9942–9951. 10.1021/jacs.8b04763. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Yong Z.; Zhang W.; et al. Ultra-broadband optical amplification at telecommunication wavelengths achieved by bismuth-activated lead iodide perovskites. J. Mater. Chem. C 2017, 5 (10), 2591–2596. 10.1039/C6TC05539G. [DOI] [Google Scholar]
- Tung R. T. The physics and chemistry of the schottky barrier height. Appl. Phys. Rev. 2014, 1 (1), 011304. 10.1063/1.4858400. [DOI] [Google Scholar]
- Chen L.; Zhang Y.; Wang X.; Jalan B.; Chen S.; Hou Y. Roles of point defects in thermal transport in perovskite barium stannate. J. Phys. Chem. C 2018, 122 (21), 11482–11490. 10.1021/acs.jpcc.8b00653. [DOI] [Google Scholar]
- Liang Y.; Cui X.; Li F.; Stampfl C.; Ringer S. P.; Zheng R. Atomic and molecular hydrogen impurities in hybrid perovskite solar cells. J. Phys. Chem. C 2022, 126 (4), 1721–1728. 10.1021/acs.jpcc.1c10339. [DOI] [Google Scholar]
- Watkins G. D. Negative-U properties for point defects in silicon. Mater. Res. Soc. Symp. Proc. 1980, 2 (1), 21. 10.1557/PROC-2-21. [DOI] [Google Scholar]
- Dey A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. 10.1016/j.mseb.2017.12.036. [DOI] [Google Scholar]
- Liu B.; Liu J. Sensors and biosensors based on metal oxide nanomaterials. TrAC Trend Anal Chem. 2019, 121, 115690. 10.1016/j.trac.2019.115690. [DOI] [Google Scholar]
- Kumar A.; Gupta G.; Bapna K.; Shivagan D. D. Semiconductor-metal-oxide-based nano-composites for humidity sensing applications. Mater. Res. Bull. 2023, 158, 112053. 10.1016/j.materresbull.2022.112053. [DOI] [Google Scholar]
- Gupta J.; Hassan P. A.; Barick K. C. Core-shell Fe3O4@ZnO nanoparticles for magnetic hyperthermia and bio-imaging applications. AIP Adv. 2021, 11 (2), 025207. 10.1063/9.0000135. [DOI] [Google Scholar]
- Yu X.; Marks T. J.; Facchetti A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15 (4), 383–396. 10.1038/nmat4599. [DOI] [PubMed] [Google Scholar]
- Liu X.; Iocozzia J.; Wang Y.; et al. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10 (2), 402–434. 10.1039/C6EE02265K. [DOI] [Google Scholar]
- Banfield J. F.; Bischoff B. L.; Anderson M. A. TiO2 accessory minerals: Coarsening, and transformation kinetics in pure and doped synthetic nanocrystalline materials. Chem. Geol. 1993, 110 (1), 211–231. 10.1016/0009-2541(93)90255-H. [DOI] [Google Scholar]
- Xiong G.; Pal U.; Serrano J. G.; Ucer K. B.; Williams R. T. Photoluminesence and FTIR study of ZnO nanoparticles: The impurity and defect perspective. Phys. Status Solidi C 2006, 3 (10), 3577–3581. 10.1002/pssc.200672164. [DOI] [Google Scholar]
- Abakumov V. N., Perel V. I., Yassievich I. N.. Nonradiative recombination in semiconductors, 1st ed.; Elsevier, 1991. [Google Scholar]
- Kumar A.; Singh L. R.; Bag A. Tailoring of structural and optical properties of electrosprayed β-Ga2O3 nanostructures via self-assembly. Opt Quant Electron. 2023, 55, 437. 10.1007/s11082-023-04707-x. [DOI] [Google Scholar]
- Calcabrini M.; Van den Eynden D.; Ribot S. S.; et al. Ligand conversion in nanocrystal synthesis: The oxidation of alkylamines to fatty acids by nitrate. JACS Au. 2021, 1 (11), 1898–1903. 10.1021/jacsau.1c00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa P. S.; Patil S. B.; Ganganagappa N.; Reddy K. R.; Raghu A. V.; Reddy C. V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy. 2020, 45 (13), 7764–7778. 10.1016/j.ijhydene.2019.07.241. [DOI] [Google Scholar]
- Prakash J.; Samriti; Kumar A.; et al. Novel rare earth metal-doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. 10.1016/j.mtsust.2021.100066. [DOI] [Google Scholar]
- Farzaneh A.; Javidani M.; Esrafili M. D.; Mermer O. Optical and photocatalytic characteristics of al and cu doped TiO2: Experimental assessments and DFT calculations. J. Phys. Chem. Solids. 2022, 161, 110404. 10.1016/j.jpcs.2021.110404. [DOI] [Google Scholar]
- Xing M.; Fang W.; Nasir M.; Ma Y.; Zhang J.; Anpo M. Self-doped Ti3+-enhanced TiO2 nanoparticles with a high-performance photocatalysis. J. Catal. 2013, 297, 236–243. 10.1016/j.jcat.2012.10.014. [DOI] [Google Scholar]
- Yan T.; Li N.; Wang L.; et al. Bismuth atom tailoring of indium oxide surface frustrated lewis pairs boosts heterogeneous CO2 photocatalytic hydrogenation. Nat. Commun. 2020, 11 (1), 6095. 10.1038/s41467-020-19997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon P. S.; Kunjumon J.; Bansal M.; et al. Role of surface defects in the third order nonlinear optical properties of pristine NiO and cr doped NiO nanostructures. Ceram. Int. 2023, 49 (4), 5815–5827. 10.1016/j.ceramint.2022.10.301. [DOI] [Google Scholar]
- Tandon B.; Gibbs S. L.; Zydlewski B. Z.; Milliron D. J. Quantitative analysis of plasmonic metal oxide nanocrystal ensembles reveals the influence of dopant selection on intrinsic optoelectronic properties. Chem. Mater. 2021, 33 (17), 6955–6964. 10.1021/acs.chemmater.1c01951. [DOI] [Google Scholar]
- Dresselhaus M.; Chen G.; Tang M.; et al. New directions for low-dimensional thermoelectric materials. Adv. Mater. 2007, 19 (8), 1043–1053. 10.1002/adma.200600527. [DOI] [Google Scholar]
- DiSalvo F. J. Thermoelectric cooling and power generation. Science. 1999, 285 (5428), 703–706. 10.1126/science.285.5428.703. [DOI] [PubMed] [Google Scholar]
- Snyder G. J.; Toberer E. S. Complex thermoelectric materials. Nat. Mater. 2008, 7 (2), 105–114. 10.1038/nmat2090. [DOI] [PubMed] [Google Scholar]
- Hsu K. F.; Loo S.; Guo F.; et al. Cubic AgPbmSbTe2+m: Bulk thermoelectric materials with high figure of merit. Science. 2004, 303 (5659), 818–821. 10.1126/science.1092963. [DOI] [PubMed] [Google Scholar]
- Moram M. A.; Barber Z. H.; Humphreys C. J. The effect of oxygen incorporation in sputtered scandium nitride films. Thin Solid Films. 2008, 516 (23), 8569–8572. 10.1016/j.tsf.2008.05.050. [DOI] [Google Scholar]
- le Febvrier A.; Tureson N.; Stilkerich N.; Greczynski G.; Eklund P. Effect of impurities on morphology, growth mode, and thermoelectric properties of (1 1 1) and (0 0 1) epitaxial-like ScN films. J. Phys. D 2019, 52, 035302. 10.1088/1361-6463/aaeb1b. [DOI] [Google Scholar]
- Manzano C. V.; Abad B.; Martín-González M. The effect of electrolyte impurities on the thermoelectric properties of electrodeposited Bi2Te3 films. J. Electrochem. Soc. 2018, 165 (14), D768. 10.1149/2.1131814jes. [DOI] [Google Scholar]
- Kulbachinskii V. A.; Kytin V. G.; Kudryashov A. A.; Tarasov P. M. Thermoelectric properties of Bi2Te3, Sb2Te3 and Bi2Se3 single crystals with magnetic impurities. J. Solid State Chem. 2012, 193, 47–52. 10.1016/j.jssc.2012.03.042. [DOI] [Google Scholar]
- Madsen P. A.; Murray R.; Sørensen O. R. A new form of the boussinesq equations with improved linear dispersion characteristics. Coast Eng. 1991, 15 (4), 371–388. 10.1016/0378-3839(91)90017-B. [DOI] [Google Scholar]
- Cayssol J. Introduction to dirac materials and topological insulators. Comptes Rendus Physique. 2013, 14 (9), 760–778. 10.1016/j.crhy.2013.09.012. [DOI] [Google Scholar]
- Zak J. Berry’s phase for energy bands in solids. Phys. Rev. Lett. 1989, 62 (23), 2747–2750. 10.1103/PhysRevLett.62.2747. [DOI] [PubMed] [Google Scholar]
- Blanco-Redondo A.; Bell B.; Oren D.; Eggleton B. J.; Segev M. Topological protection of biphoton states. Science. 2018, 362 (6414), 568–571. 10.1126/science.aau4296. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Liu C.; Xu C.; Qi X.; Zhang S. Magnetic impurities on the surface of a topological insulator. Phys. Rev. Lett. 2009, 102 (15), 156603. 10.1103/PhysRevLett.102.156603. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Song L.; Pedersen S. H.; Yin H.; Hung L. T.; Iversen B. B. Discovery of high-performance low-cost n-type Mg3Sb2-based thermoelectric materials with multi-valley conduction bands. Nat. Commun. 2017, 8 (1), 13901. 10.1038/ncomms13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.; Shuai J.; Song S.; et al. Manipulation of ionized impurity scattering for achieving high thermoelectric performance in n-type Mg3Sb2-based materials. Proc. Natl. Acad. Sci. 2017, 114 (40), 10548–10553. 10.1073/pnas.1711725114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.; Wu Y.; Song S.; et al. Defect engineering for realizing high thermoelectric performance in n-type Mg3Sb2-based materials. ACS Energy Lett. 2017, 2 (10), 2245–2250. 10.1021/acsenergylett.7b00742. [DOI] [Google Scholar]
- Tamaki H.; Sato H. K.; Kanno T. Isotropic conduction network and defect chemistry in Mg3+δSb2-based layered zintl compounds with high thermoelectric performance. Adv. Mater. 2016, 28 (46), 10182–10187. 10.1002/adma.201603955. [DOI] [PubMed] [Google Scholar]
- Mao J.; Wu Y.; Song S.; et al. Anomalous electrical conductivity of n-type te-doped Mg3.2Sb1.5Bi0.5. Mater. Today Phys. 2017, 3, 1–6. 10.1016/j.mtphys.2017.08.001. [DOI] [Google Scholar]
- Su X.; Hao S.; Bailey T. P.; et al. Weak electron phonon coupling and deep level impurity for high thermoelectric performance Pb1–xGaxTe. Adv. Energy Mater. 2018, 8 (21), 1800659. 10.1002/aenm.201800659. [DOI] [Google Scholar]
- Dismukes J. P.; Ekstrom L.; Steigmeier E. F.; Kudman I.; Beers D. S. Thermal and electrical properties of heavily doped Ge-Si alloys up to 1300 K. J. Appl. Phys. 1964, 35 (10), 2899–2907. 10.1063/1.1713126. [DOI] [Google Scholar]
- Kim S. I.; Lee K. H.; Mun H. A.; et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science. 2015, 348 (6230), 109–114. 10.1126/science.aaa4166. [DOI] [PubMed] [Google Scholar]
- Poudel B.; Hao Q.; Ma Y.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science. 2008, 320 (5876), 634–638. 10.1126/science.1156446. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Zhang X.; Wu H.; et al. Enhanced thermoelectric properties in the counter-doped SnTe system with strained endotaxial SrTe. J. Am. Chem. Soc. 2016, 138 (7), 2366–2373. 10.1021/jacs.5b13276. [DOI] [PubMed] [Google Scholar]
- Luo Z.; Cai S.; Hao S.; et al. Valence disproportionation of GeS in the PbS matrix forms Pb5Ge5S12 inclusions with conduction band alignment leading to high n-type thermoelectric performance. J. Am. Chem. Soc. 2022, 144 (16), 7402–7413. 10.1021/jacs.2c01706. [DOI] [PubMed] [Google Scholar]
- Pei Y.; Shi X.; LaLonde A.; Wang H.; Chen L.; Snyder G. J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature. 2011, 473 (7345), 66–69. 10.1038/nature09996. [DOI] [PubMed] [Google Scholar]
- Pei Y.; LaLonde A. D.; Wang H.; Snyder G. J. Low effective mass leading to high thermoelectric performance. Energy Environ. Sci. 2012, 5 (7), 7963–7969. 10.1039/c2ee21536e. [DOI] [Google Scholar]
- Zebarjadi M.; Joshi G.; Zhu G.; et al. Power factor enhancement by modulation doping in bulk nanocomposites. Nano Lett. 2011, 11 (6), 2225–2230. 10.1021/nl201206d. [DOI] [PubMed] [Google Scholar]
- Ibáñez M.; Luo Z.; Genç A.; et al. High-performance thermoelectric nanocomposites from nanocrystal building blocks. Nat. Commun. 2016, 7 (1), 10766. 10.1038/ncomms10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans J. P.; Jovovic V.; Toberer E. S.; et al. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science. 2008, 321 (5888), 554–557. 10.1126/science.1159725. [DOI] [PubMed] [Google Scholar]
- Wang S.; Yang J.; Wu L.; Wei P.; Zhang W.; Yang J. On intensifying carrier impurity scattering to enhance thermoelectric performance in Cr-doped CeyCo4Sb12. Adv. Funct Mater. 2015, 25 (42), 6660–6670. 10.1002/adfm.201502782. [DOI] [Google Scholar]
- Fiedler C.; Kleinhanns T.; Garcia M.; Lee S.; Calcabrini M.; Ibáñez M. Solution-processed inorganic thermoelectric materials: Opportunities and challenges. Chem. Mater. 2022, 34 (19), 8471–8489. 10.1021/acs.chemmater.2c01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiciński W.; Dyjak Sł. Transition metal impurities in carbon-based materials: Pitfalls, artifacts and deleterious effects. Carbon. 2020, 168, 748–845. 10.1016/j.carbon.2020.06.004. [DOI] [Google Scholar]
- Thomas R.; Manoj B. Electrochemical efficacies of coal derived nanocarbons. Int. J. Coal Sci. Technol. 2021, 8 (4), 459–472. 10.1007/s40789-020-00379-0. [DOI] [Google Scholar]