Abstract
Background
Dystonia is a genetic or non-genetic movement disorder with typical patterned and twisting movements due to abnormal muscle contractions that may be associated with tremor. Genetic and phenotypic heterogeneity leads to variable clinical presentation.
Methodology
Next-generation sequencing technologies are being currently used in the workup of patients with inherited dystonia to determine the specific cause in the individuals with autosomal dominant, recessive, X-linked or mitochondrial inheritance patterns. Calcium voltage-gated channel subunit alpha1 A (CACNA1A) gene variants are rare in dystonias.
Results
We here present a 20-year-old man with a history of delayed milestones, flexor posturing, dysarthria, dysphagia and a negative family history from consanguineous parents. Neurological examination revealed right lateral scoliosis of the neck and generalised dystonic posturing affecting both upper and lower limbs. MRI of the brain was unremarkable. Molecular genetic results revealed a heterozygous variant in the CACNA1A gene (CHR19: NM_023035.2, c. 1602G>A; p. Met534Ile). Segregation analyses in both the parents revealed wild-type CACNA1A gene suggesting de novo nature of the variant with a likely pathogenic classification.
Conclusion
Dystonia is one of the clinical phenotypes that can be associated with CACNA1A gene mutations and we recommend that this gene either be included in the dystonia panel offered or tested when the initial primary genetic result is negative.
Keywords: DYSTONIA, GENETICS
Introduction
Calcium voltage-gated channel subunit alpha1 A (CACNA1A, OMIM#601011) gene encodes the transmembrane pore-forming subunit of the P/Q-type ‘high-voltage activated’ calcium channel, which not only mediates the entry of Ca(2+) ions into excitable cells but is also involved in a variety of processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death.1 The CACNA1A C-terminal polypeptide (alpha-1ACT) generated using an internal ribosomal entry site in the CACNA1A gene transcript functions as a transcription factor mediating cerebellar development.2 Alpha-1A subunit is predominantly expressed in cerebellum, cerebral cortex, thalamus and hypothalamus.3
At the molecular level, CACNA1A gene shows polymorphic (CAG)n-repeat variation occurring in both the 3' UTR and coding region with multiple transcripts encoding different isoforms.4 CACNA1A (CAG)n-repeat expansion (21–33 vs 4–18 as normal) in the coding region is known to be associated with spinocerebellar ataxia 6.4 Studies in human subjects have revealed large-scale deletions, single-nucleotide deletions leading to a frameshift, exonic deletions, splice site variants and missense variants in CACNA1A gene for familial hemiplegic migraine, Naito-Oyanagi disease, autosomal dominant cerebellar ataxia, migraine with aura, idiopathic generalised epilepsy and episodic ataxia.5–7 DREAM repression, dynorphin expression and TCR signalling are among CACNA1A-related pathways with monoatomic ion channel activity and voltage-gated calcium channel activity among its gene ontology annotations. CACNA1A gene mutation in dystonia is rare and very few cases of dystonia, including focal and generalised forms, have been reported thus far.8–11 We present here a case of generalised dystonia carrying a de novo heterozygous CACNA1A variant (NM_023035.2, c. 1602G>A; p. Met534Ile), thereby providing a valuable addition to the CACNA1A-related dystonia. We recommend CACNA1A gene to be part of molecular workup of patients with dystonia.
Case presentation
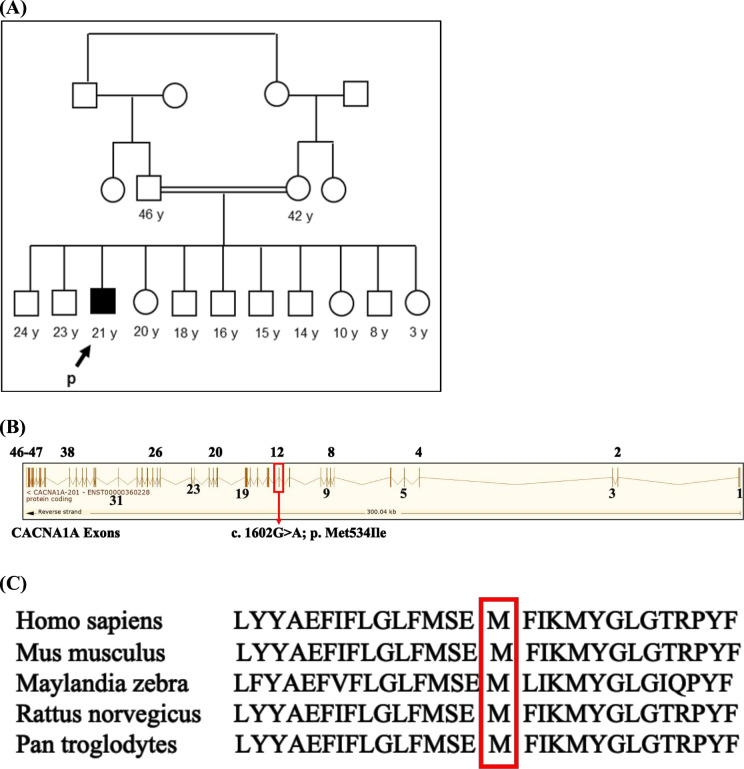
A 20-year-old man presented to the movement disorder clinic for evaluation of dystonia. He is the product of an uncomplicated spontaneous vaginal delivery; however, he had delayed attainment of motor milestones, in which did not start to walk till age of 21 months and started to crawl at age of 14 months. However, his cognitive function including speech is like his sibling and he completed school. At around age 8, he started to have flexor posturing of the left wrist, which progressed to the right side. Later, he also experienced posturing of the neck. In addition, he had dysarthria and dysphagia. His medical history was notable for diabetes mellitus. His family history was notable for consanguinity in his parents; however, all other male and female siblings were apparently healthy (figure 1A).
Figure 1.
Family pedigree, exon map of CACNA1A gene showing the variant in exon 12 and conservation of mutated nucleotide across species. (A) Extended pedigree showing consanguineous marriage with the arrows pointing to the affected case (solid box) including other healthy siblings (open box). (B) Detailed exon map of the CACNA1A gene (reverse strand) showing all the 47 exons (numbered 1–23) including the variant c.1602G>A; p.M534I in exon12. (C) Part of the CACNA1A amino acid sequence obtained from NCBI showing the conservation of the mutated residue (M534) across species. CACNA1A, calcium voltage-gated channel subunit alpha1 A.
On neurological examination, he had a normal performance in Mini-Mental State Examination (29±1), dysarthria was exhibited during conversation, saccadic smooth pursuits were noted during extraocular muscle examination. The patient had right latero-collis of the neck and dystonic posturing affecting both upper and lower limbs. He also had dysmetria noticed on finger-to-nose and heel-to-shin testing. Gait examinations revealed difficulty with tandem gait.
Investigations
Renal function, liver function and enzymes, creatine kinase, thyroid function, and MRI of the brain were unremarkable. In addition to that, genetic testing using next-generation sequencing (NGS) and copy number variant analysis revealed a heterozygous missense variant involving CACNA1A gene (c. 1602G>A; p.Met534Ile: NM_023035.2). Segregation analysis for the targeted variant in the parents of the case revealed wild-type CACNA1A gene suggesting de novo nature of the variant. The American College of Medical Genetics and Genomics (ACMG) classification criteria12 with cosegregation data indicate CACNA1A gene variant to be likely pathogenic and the cause of this patient’s clinical condition.
Treatment, outcome and follow-up
The patient is receiving regular follow-up in the movement disorder clinic, with symptomatic management of his condition including carbidopa-levodopa, clonazepam, trihexyphenidyl, tetrabenazine, baclofen and botox injections. The patient’s condition is still worsening, affecting his everyday life activity and resulting in recurrent falls, with no improvement from physical and medical therapy.
Discussion
We report a case of a patient who presented with childhood dystonia and was found to have a de novo heterozygous variant in the CACNA1A gene through the use of NGS, which is currently the first-line molecular genetic testing used in the workup of patients with inherited dystonia.
Our patient presented with lateral scoliosis of the neck and generalised dystonic posturing affecting both upper and lower limbs in childhood indicating a complex and severe disease course. Owing to consanguinity in the case presented here inherited dystonia, which has a distinct phenotype was a differential diagnosis, although phenotypic overlap with other forms of dystonia makes the classification difficult. Clinically, the classification of dystonia is based on age of onset, site of onset, the presence or absence of other neurological abnormalities and the presence of non-neurological abnormalities.13 Genetically, dystonia may be inherited in an autosomal dominant, recessive or mitochondrial mode of inheritance.13
Recent decades have seen an enormous expansion in the field of genetic testing such as NGS with different modalities, which has led to the discovery of new genetic variants in a huge number of diseases including dystonias that were believed not to be associated with any clear cause.14 15 Using NGS, a pathogenic variant of CACNA1A has been found to be associated with severe intellectual disabilities, epileptic encephalopathy, episodic ataxia, dystonia and autism spectrum disorders.4–7 In EA2, multiple investigators have reported a single-nucleotide deletion leading to a frameshift, splice site mutations, different exonic deletions, large-scale deletions in six families and a pathogenic duplication in CACNA1A gene.5–8 In familial hemiplegic migraine, different missense mutations have been reported.5 In SCA, CAG repeat expansion in CACNA1A gene has been reported.4 In idiopathic generalised epilepsy, single-nucleotide polymorphisms in CACNA1A gene association were suggested,7 and in DEE42 different heterozygous mutations in the CACNA1A gene were reported.5–7 A few cases of dystonia, including focal and generalised forms, have been found to be associated with a CACNA1A gene mutation.8–11 Dystonia noted in CACNA1A-related disorders can be both episodic and chronic.8–11 Unlike our patient, in most of these case reports, ataxia was also present during the initial clinical encounter (online supplemental table 1). For example, a genetic test of a female patient with activity-induced dystonia, cervical dystonia and mild ataxia revealed a heterozygous genetic mutation involving CACNA1A.10 However, in another study, a repeat expansion involving a CACNA1A gene mutation was discovered in a patient who initially presented with a cramp in his right hand, which was induced by writing; a few years later, the patient started to have difficulty walking, with abnormal balance.9 Another study in 2017 also showed that focal dystonia could be an early presentation of patients with a CACNA1A mutation; the researchers reported the case of a father who had abnormal posturing during writing and who later developed progressive cerebellar ataxia.10 Of note, the presence of dystonia in humans is not supersizing, as was noted in a mouse model with homozygous mutations in the CACNA1A gene.11
bmjno-2024-000710supp001.pdf (106.7KB, pdf)
CACNA1A gene located on chromosome 19 has 47 exons with 8647 base pairs and 2506 amino acid residues (figure 1). The variant NM_023035.2, c. 1602G>A; p. Met534Ile identified in our patient is located in exon 12 in the genomic location between 13 312 781 and 13 312 669 (figure 1). The gene variant c. 1602G>A; p. Met534Ile is absent in our in-house database (2564 cases) and the amino acid residue Met is conserved across species (figure 1C) suggesting its deleterious nature and possible contribution to the phenotype in our patients. The ACMG classification criteria applied included extremely low frequency in gnomAD population and KFMC in-house databases (PM2), missense variant in a gene with a low rate of benign missense mutations and for which missense mutation is a common mechanism of a disease (PP2) and various computational prediction tools such as PolyPhen, Sift and MutationTaster unanimously support a deleterious effect on the gene (PP3). Additionally, in conjunction with segregation data, the variant c. 1602G>A; p. Met534Ile is classified as likely pathogenic. It is, thus, evident that CACNA1A gene variant has an important role in the pathogenesis of dystonia in the current patient.
In conclusion, dystonia is one of the clinical phenotypes that can be associated with CACNA1A mutations. Although further studies may provide additional insights, we think that this gene should be tested when the initial primary dystonia panel is negative.
Acknowledgments
We thank KFMC Research Centre, Faculty of Medicine for their support.
Footnotes
Contributors: MA: senior resident who wrote the first draft. AA: clinical consultant who was involved in editing and writing the clinical part of the manuscript. OAA: clinical consultant who managed the patient and edited the clinical part of the manuscript. SB is a genetic counsellor who saw the case, counselled the family and provided genetic figures. AAP-Z was involved in editing and writing of the molecular genetic part of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data underlying the results are available as part of the article and no additional source data are required.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and written informed consent was obtained from the patient to publish this report. In addition, the study was approved by KFMC IRB and IRB log number: 2+114. Participants gave informed consent to participate in the study before taking part.
References
- 1. Kordasiewicz HB, Thompson RM, Clark HB, et al. C-termini of P/Q-type Ca(2+) channel Alpha1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum Mol Genet 2006;15:1587–99. 10.1093/hmg/ddl080 [DOI] [PubMed] [Google Scholar]
- 2. Du X, Wei C, Hejazi Pastor DP, et al. Alpha-1ACT is essential for survival and early cerebellar programming in a critical neonatal window. Neuron 2019;102:770–85. 10.1016/j.neuron.2019.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Du X, Wang J, Zhu H, et al. Second cistron in CACNA1A gene encodes a transcription factor mediating cerebellar development and SCA6. Cell 2013;154:118–33. 10.1016/j.cell.2013.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsuyama Z, Kawakami H, Maruyama H, et al. Molecular features of the CAG repeats of spinocerebellar ataxia 6 (SCA6). Hum Mol Genet 1997;6:1283–7. 10.1093/hmg/6.8.1283 [DOI] [PubMed] [Google Scholar]
- 5. Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial Hemiplegic migraine and episodic ataxia Type-2 are caused by mutations in the Ca(2+) channel gene CACNL1A4. Cell 1996;87:543–52. 10.1016/s0092-8674(00)81373-2 [DOI] [PubMed] [Google Scholar]
- 6. Labrum RW, Rajakulendran S, Graves TD, et al. Large scale calcium channel gene rearrangements in episodic ataxia and hemiplegic migraine: implications for diagnostic testing. J Med Genet 2009;46:786–91. 10.1136/jmg.2009.067967 [DOI] [PubMed] [Google Scholar]
- 7. Chioza B, Wilkie H, Nashef L, et al. Association between the Alpha-1A calcium channel gene CACNA1A and idiopathic generalized epilepsy. Neurology 2001;56:1245–6. 10.1212/wnl.56.9.1245 [DOI] [PubMed] [Google Scholar]
- 8. Muzaimi MB, Wiles CM, Robertson NP, et al. Task specific focal dystonia: a presentation of spinocerebellar ataxia type 6. J Neurol Neurosurg Psychiatry 2003;74:1444–5. 10.1136/jnnp.74.10.1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stampfl B, Fee D. Novel Mutation in CACNA1A associated with activity-induced dystonia, cervical dystonia, and mild ataxia. Case Rep Neurol Med 2021;2021:7797770. 10.1155/2021/7797770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spacey SD, Materek LA, Szczygielski BI, et al. Two novel CACNA1A gene mutations associated with episodic ataxia type 2 And Interictal dystonia. Arch Neurol 2005;62:314–6. 10.1001/archneur.62.2.314 [DOI] [PubMed] [Google Scholar]
- 11. Fletcher CF, Tottene A, Lennon VA, et al. Dystonia and cerebellar atrophy in CACNA1A null mice lacking P/Q calcium channel activity. FASEB J 2001;15:1288–90. 10.1096/fj.00-0562fje [DOI] [PubMed] [Google Scholar]
- 12. Richards S, Aziz N, Bale S, et al. ACMG laboratory quality assurance committee. standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. di Biase L, Di Santo A, Caminiti ML, et al. Classification of dystonia. Life (Basel) 2022;12:206. 10.3390/life12020206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Indelicato E, Boesch S. From genotype to phenotype: expanding the clinical spectrum of CACNA1A variants in the era of next generation sequencing. Front Neurol 2021;12:639994. 10.3389/fneur.2021.639994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hommersom MP, van Prooije TH, Pennings M, et al. The complexities of CACNA1A in clinical neurogenetics. J Neurol 2022;269:3094–108. 10.1007/s00415-021-10897-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjno-2024-000710supp001.pdf (106.7KB, pdf)
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.