Abstract
Introduction
Gaps in antimicrobial resistance (AMR) surveillance and control, including implementation of national action plans (NAPs), are evident internationally. Countries’ capacity to translate political commitment into action is crucial to cope with AMR at the human–animal–environment interface.
Methods
We employed a two-stage process to understand opportunities and challenges related to AMR surveillance and control at the human–animal interface in Argentina. First, we compiled the central AMR policies locally and mapped vital stakeholders around the NAP and the national commission against bacterial resistance. Second, we conducted qualitative interviews using a semistructured questionnaire covering stakeholders’ understanding and progress towards AMR and NAP. We employed a mixed deductive–inductive approach and used the constant comparative analysis method. We created categories and themes to cluster subthemes and determined crucial relationships among thematic groups.
Results
Crucial AMR policy developments have been made since 1969, including gradually banning colistin in food-producing animals. In 2023, a new government decree prioritised AMR following the 2015 NAP launch. Our qualitative analyses identified seven major themes for tackling AMR: (I) Cultural factors and sociopolitical country context hampering AMR progress, (II) Fragmented governance, (III) Antibiotic access and use, (IV) AMR knowledge and awareness throughout stakeholders, (V) AMR surveillance, (VI) NAP efforts and (VII) External drivers. We identified a fragmented structure of the food production chain, poor cross-coordination between stakeholders, limited surveillance and regulation among food-producing animals and geographical disparities over access, diagnosis and treatment. The country is moving to integrate animal and food production into its surveillance system, with most hospitals experienced in monitoring AMR through antimicrobial stewardship programmes.
Conclusion
AMR accountability should involve underpinning collaboration at different NAP implementation levels and providing adequate resources to safeguard long-term sustainability. Incorporating a multisectoral context-specific approach relying on different One Health domains is crucial to strengthening local AMR surveillance.
Keywords: Public health, Health policy, QUALITATIVE RESEARCH
STRENGTHS AND LIMITATIONS OF THIS STUDY.
We used a hybrid approach consisting of a historical synthesis of regulations regarding antimicrobial resistance (AMR) and antibiotic usage across animals and humans and qualitative analyses of the potential challenges and facilitators towards the national action plan to reduce AMR across key stakeholders.
We used a mixed deductive–inductive approach alongside the constant comparative analysis method for qualitative analysis, enabling a deep integration and comprehensive understanding of complex datasets through the emergence of new themes and patterns.
The primary limitation of our study includes the varying levels of interviewees’ involvement in AMR policies and the under-representation of certain sectors, notably the private industry.
Introduction
Antimicrobial resistance (AMR) represents a global public health threat driven by inter-related human, animal and environmental factors and requires multidisciplinary and cross-government action.1–3 National and international endeavours have collectively helped reduce AMR over recent years.4 The WHO launched the Global Action Plan on AMR in 2015, soliciting countries to elaborate a national action plan (NAP) to confront AMR.5 Similar initiatives have come through the United Nations (UN)6 and the European Commission7 to develop multisectoral strategies involving human and animal health to fortify innovation stages and shape the global health agenda towards AMR NAPs. Despite 152 countries having published NAPs,8 challenges exist in implementing NAPs locally, limiting the progress towards addressing AMR.9 Contrasting cultures, policies, incentives and behaviours of relevant sectors and stakeholders have made the implementation of NAPs an arduous process.4 For instance, the lack of surveillance and epidemiological data, the variety of methods used to collect data and the limited understanding of the clinical and social burden of AMR pose challenges for the consummation of NAPs internationally.10 Locally, policy design, including governance and stakeholder involvement and cross-sectoral coordination, is critical to fulfilling the NAP’s objectives while adapting the alternating demands of each local subgroup.4 11
Recent literature suggests that low- and middle-income countries (LMICs) are likely to face the most significant challenges in NAP implementation.4 12–20 Among LMICs in Asia, a review found that accountability—a sense of ownership of organisations or people requiring responsibility to other stakeholders—has been omitted in most NAPs.4 Indications of unmet goals and lack of clarity in the stakeholders’ role remain significant obstacles to AMR prevention and control.14 15 18 In the Americas, 29 countries (89%) have reported developing NAPs to combat AMR since the beginning of 2020.21 However, most countries including Argentina have not focused sufficiently yet on One Health components; active surveillance of human health is not integrated with surveillance in animals or the environment.4 18 Using a One Health approach is critical for effective NAP implementation because it optimises the health of different sectors, including natural environments which play a crucial role in AMR evolution and transmission, while preventing zoonotic diseases and improving food safety and security. Argentina implemented a multisectoral NAP strategy in 2015,22 and progress has been made, including the prohibition of colistin usage in 2019 and the banning of antibiotics as growth promoters among livestock.23 24 A recent study measuring global response to AMR by employing a governance framework on NAP contents highlighted that Argentina can make improvements in standards to control AMR.18 The study stated Argentina’s moderate efforts towards monitoring and evaluating AMR in humans and animals and modest AMR policy design (ie, lack of accountability). However, the study used the Tripartite Antimicrobial Resistance Country Self-Assessment survey, which could be influenced by the exclusion of publicly and privately accessible documents relevant for AMR monitoring locally, and it lacked data sources that could invite heterogeneity (eg, interviews with multiple experts/stakeholders), all of which are considerably important in LMICs. A more direct way to explore Argentina’s response to AMR policy is to obtain current data from the stakeholders involved.
This study aims to better understand the stakeholder and regulatory landscape and the challenges and opportunities Argentina faces in implementing its NAP. We examined Argentina’s case because it is one of the first countries to have a NAP and has vast experience in AMR surveillance in the human sphere, which might indicate a good model for the Latin American region. We use the One Health25 approach to assess policy priorities in action plans, and the governance framework26 to evaluate inter-related dynamics between the One Health actors to improve critical areas: policy design, implementation tools and monitoring and evaluation. The need to provide technical and financial support for implementing One Health integrated NAPs has been recognised globally through the AMR Multi-Partner Trust Fund. Still, the donor base for this remains a few countries.27 28 Global action may be based on shared goals, but there is a further need to understand the needs and priorities enabling or hindering those actions at national levels. We explore Argentina as a case study to understand the complex landscape.
Methods
Study aims and setting
This article draws on literature and qualitative interview data from a study conducted in Argentina between September 2022 and February 2023. Argentina is an upper-middle income country that is endowed with highly fertile soils and great potential for renewable energy (hydroelectric, wind and solar energy).29 It is a major food-producing country with an extensive agriculture and livestock industry; however, the country faces a high fiscal deficit with poverty and inflation rates of about 39% and 94.8% in 2022, respectively. The project’s scope was to comprehend the context of AMR policy locally and among relevant stakeholders towards AMR control and surveillance, focusing on food-producing animals. Relevant stakeholders included government (those with political/administrative duties and those having scientific-technical functions), academic, international, non-governmental organisations (NGOs) and private (chamber of producers, commercial laboratories and producers) institutions.
Study design
We divided our study into two stages. First, we explored the legal framework within the AMR NAP scope and mapped vital organisations, particularly related to food-producing animals (ie, cattle, chicken and pigs). We searched the literature, including government documents, academic articles, stakeholder’s websites and grey literature, to capture the most relevant AMR policies and critical actors and their role over time. Most sources were identified from the website of the National Commission for AMR control (CoNaCRA, ‘Comisión Nacional de Control de la Resistencia Antimicrobiana’),30 the National Institute of Infectious Diseases (INEI)31 and the government website for national laws (http://www.infoleg.gob.ar/). A recent systematic literature review was also used to support evidence synthesis and mapping.32 Moreover, expert knowledge was consulted for main organisations related to the AMR NAP and food-producing animals, and their interactions with stakeholders. Second, after identifying and mapping relevant stakeholders, we conducted qualitative semistructured interviews between September 2022 and January 2023 to gather information on stakeholder’s views and experiences of NAP implementation, the role of different organisations and the nature of coordination and decision making between organisations. Interviews were held in person or online lasting 45–90 min and were conducted by a bilingual (English–Spanish) social scientist. The stakeholder mapping developed in stage 1 was used to inform recruitment. A range of participants were sought from government, private and academic organisations. Potential participants were sent a formal request letter via email inviting them to take part in the interview study. Semistructured interviews were performed using an interview topic guide designed to explore five main items, with questions and objectives detailed below (box 1). Stakeholders were encouraged to discuss their own views openly. After obtaining informed consent, interviews were audiorecorded in Spanish and transcribed and translated to produce English and Spanish transcripts. Transcripts were checked for accuracy by the interviewer and anonymised. Translation bias was minimised by analysing both original and translated versions by Spanish native speakers (ie, KA and a qualified third-party translator from Argentina). Back-translation was performed with a reduced sample (20%) of interviews to validate the accuracy of the initial translation (no discrepancies were found). Special attention was given to cultural references, idiomatic expressions and regional dialects to ensure that translations were contextually appropriate and culturally sensitive.33 The consent form, participant information sheet and participants’ interview topic guide are included in online supplemental material.
Box 1. Interview’s main sections including objectives, items and questions.
-
Participant’s current role. These questions seek to understand what experiences our participants have and how they might be relevant to implementing the NAP to combat AMR.
What your job/role is, and what tasks do you and your organisation mainly perform?
What are your (or department/organisation) interests and responsibilities concerning AMR?
-
Understanding AMR among stakeholders. This group of questions aim to understand employees’ and organisations’ views on AMR; its main drivers, change overtime and priority areas within organisations to help tackle AMR.
How do you feel (or what are your personal and department’s concerns) about antimicrobial resistance in humans? Does that differ from antimicrobial resistance in animals or any other source, including the environment (how)?
Do you think the view about antimicrobial resistance has changed over the years? How?
What are the priority areas within your organisation to increase AMR awareness and comply with the NAP? Do you feel your organisation helps to contribute to any of the areas detailed in the national plan (how, which)?
What are the cornerstones for increasing AMR awareness while complying with the NAP within your organisation?
Which cornerstones do you feel are most relevant within your department (organisation)? Why?
-
Information channels and flow within stakeholders/departments. These questions attempt to improve organisations decision making towards better AMR surveillance by identifying how the information is channelled within and between organisations.
How do you feel about AMR-related information and communication flow within your organisation and among all stakeholders?
What do you think about communicational interactions, networking and educational or getting-to-know instances between your organisation and other stakeholders and within departments of your organisation? Would you believe (and how) that the information pathways vary between specific organisation’s fields/disciplines, public/private institutions or certain other groups?
Could you identify which organisms (organisations), and how, are involved in your organisation’s decision making and strategy towards improving AMR surveillance and control?
-
Challenges in the implementation of the national action plan. These questions attempt to answer what factors or challenges might be key to increasing AMR awareness and improving AMR surveillance in food-producing animals and agriculture.
Do you feel there is any challenge that your organisation faces in complying with the AMR NAP and helping contribute to better animal AMR surveillance? (Political priorities, monetary and non-monetary resources, communication, etc) What are the most important and what can be done to overcome these challenges if your organisation could prioritise resources to contribute more to AMR surveillance in animals?
Who else do you think has a critical role in helping with AMR surveillance in animals from the pool of stakeholders?
-
Future and other considerations. These questions aim to understand future steps to be taken within the industry and different key members to tackle AMR.
How organisations might be helped to enhance cross-sectoral communication and teamwork? How can we ensure organisations make progress and collaborate to meet NAP criteria?
Which organisations, at the national and international levels, do you think are most important to talk to and direct efforts to address AMR knowledge gaps? Does the list differ from that necessary to improve the NAP?
Is there anything else important that you might want to share with us or that we are missing in the current interview?
AMR, antimicrobial resistance; NAP, national action plan.
bmjopen-2023-082156supp002.pdf (563.6KB, pdf)
Data analyses
First, we systematically organised Argentina’s main AMR regulations using a timeline frame and drew a map to delineate the main stakeholders directly or indirectly supporting the CoNaCRA directed by the coordination on the appropriate use of antimicrobials of the ministry of health, as the commission articulating the efforts for the implementation of the NAP. Second, we employed a systematic qualitative thematic analysis of the interviews using a mixed deductive–inductive approach34; deductive because it was guided by interview questions from a general topic to a more specific, but inductive as we draw data-driven conclusions derived from bottom-up reasoning. We followed the constant comparative method35 to favour participants’ comparability. Information and open data were classified into themes and subgroups using a coding scheme. Two investigators independently recorded the interviews (KA, EP) using Dedoose software (V.8.0.35, SocioCultural Research Consultants, Los Angeles, California, USA). Researchers frequently met to reconcile differences in code application and distinguish new themes emerging from the data analyses. After consolidating new themes, all interviews were recoded using an updated scheme (see online supplemental material). Subsequently, we identified interconnections between theme data to ascertain larger categories into which themes were clustered. For quotations, we reported descriptive characteristics, such as organisation type (academia, government, private, production system) and sex at birth, and assigned a random number to each interviewee. Quotes are reported in the text and Tables as ‘Q’ followed by ordered numbers. We reported descriptive statistics to facilitate the reader’s understandability from whom quotations were drawn and favour studies’ transparency while maintaining the anonymity of participants. We used the consolidated Criteria for Reporting Qualitative Research checklist to guide reporting of findings.36
Patient and public involvement
This study was focused on the views and experiences of professionals. Patient and public involvement was therefore not included but we ensured engagement with the community of interest. Public health experts from Argentina and the UK contributed feedback on our study design and research questions, ensuring relevance and applicability across diverse settings without direct involvement in the core research process.
Results
First stage: review of policies and mapping key stakeholders
The timeline containing established laws and regulations related to AMR is found in online supplemental material section I. Briefly, antimicrobial regulation started in the early 1960s with the first law of medications and enforced prescriptions for antibiotic acquisition. In 2007–2009, new decrees were introduced instituting required prescription for antibiotic sale and compliance for dispensation of medicines, including the registration book of veterinary medicines usage in food-producing animals (2009–2011, decree number 26514). In 2013, Argentina’s National Food Safety and Quality Service (SENASA) created the traceability system for veterinary medicines, which links sellers and purchasers where entities take full responsibility for antibiotic possession. Consecutively, Argentina’s National Commission for the Control of AMR (CoNaCRA) was established and led the launch of the NAP in 2015 (see online supplemental material section II for a summary). This was concurrent with the abolition of antibiotic usage as an animal growth promoter (2015). The national law towards gradual prohibition of colistin usage in any form/ingredient took place in 2019. Recently, there was a new law encompassing a One Health AMR agenda in the future (decree number 27680) aiming to foster and promote AMR control, prevention, research, regulation and awareness.24
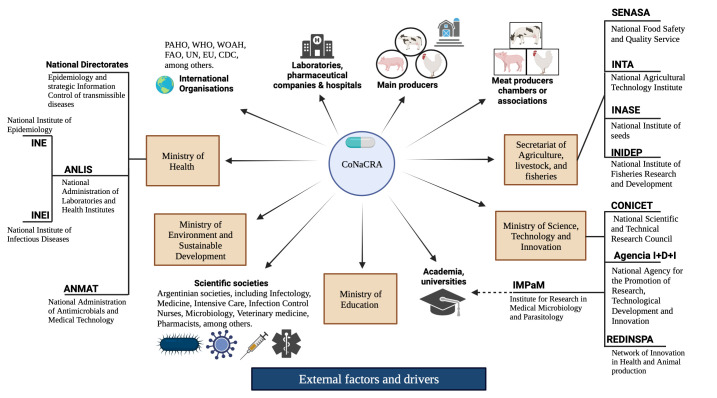
Finally, figure 1 shows the mapping of key AMR surveillance and control stakeholders. We organised it starting from the CoNaCRA as the commission responsible for the implementation of the NAP and those relevant organisations surrounding them (main governmental divisions in brown boxes).
Figure 1.
Stakeholders within the human and food-producing animal interfaces for the implementation of the NAP for the control of AMR in Argentina. ANLIS, Argentina’s National Administration of Laboratories and Institutes of Health; ANMAT, Argentina’s National Administration of Drugs, Food and Medical Devices; CoNaCRA, National Commission for the Control of Antimicrobial Resistance; CONICET, Argentina’s National Scientific and Technical Research Council; EU, European Union; FAO, Food and Agriculture Organization; INE, National Institute of Epidemiology; INEI, National Institute of Infectious Diseases; INTA, National Agricultural Technology Institute; PAHO, Pan American Health Organization; SENASA, National Food Safety and Quality Service; UN, United Nations; WOAH, World Organization of Animal Health. All information on the government’s ministries and structures is available online (https://www.argentina.gob.ar/organismos).
Second stage: qualitative analyses
Descriptive characteristics of study participants
We approached 27 individuals initially (non-response levels=33%), but our final sample consisted of 18 participants (6 women, 33%) mostly based in Buenos Aires city (88%) and from diverse institutions including government (n=9), academia (n=3), private (n=2), NGOs3 and international organisations (n=1).
Thematic categories and codes
Barriers, opportunities and state-of-art information contributing to human and food-producing animal AMR were clustered into seven thematic categories organised by specificity level (from less and more specific themes towards AMR policy). Themes were established based on a number of mentions and repetitiveness. Two themes emerged from Argentina’s embedded values and political context impacting AMR indirectly: (I) Cultural factors and country context and (II) Governance. Four themes were directly associated with AMR: (III) Antibiotic access and use, (IV) AMR knowledge and awareness, (V) AMR surveillance and (VI) NAP efforts. The remaining theme was linked to external factors indirectly affecting all chain’s decision-making: (VII) External drivers. The full definition of themes—ordered from less to more specific except for external drivers, which affect them all—with their respective subthemes, are shown in table 1.
Table 1.
Main themes, definitions and subthemes identified from the interview analysis (N=18 people)
| Themes* | Definitions | Subthemes | Times mentioned (n)† |
| I. Cultural factors and sociopolitical country context | Understanding the importance of cultural, country context, including personal relationships | (1) Cultural features | (1) 21 |
| (2) National context and sociodemographic characteristics | (2) 18 | ||
| II. Governance | Government attributes related to political priorities, federalisation, nature of institutions/groups, accountability, human and economic/budgetary resource available, data systems and capacity, importance of changing political will, regulations and communication between stakeholders. | (1) System governance | (1) 23 |
| (2) Resources | (2) 22 | ||
| (3) Stakeholder communication | (3) 39 | ||
| (4) Information and data flow | (4) 39 | ||
| (5) Regulation and compliance | (5) 38 | ||
| (6) Political context and agenda | (6) 11 | ||
| III. Antibiotic access and use | Referring to antibiotic consumption and access, prescription control and alternatives being developed among animals and humans. | (1) Antibiotic access | (1) 15 |
| (2) Antibiotic consumption | (2) 11 | ||
| (3) Antibiotic regulations including prescriptions. | (3) 14 | ||
| (4) Alternatives to antibiotics | (4) 9 | ||
| IV. AMR knowledge and awareness | Related to public and professional awareness—the challenge of awareness and steps taken to address it including seminars, conferences, courses, stewardship programmes, advertising campaigns using mass media, etc. | (1) Challenges related to public and professional awareness. | (1) 41 |
| (2) Existing training and learning opportunities | (2) 15 | ||
| V. AMR surveillance | Perceived progress and challenges, inclusive of AMR surveillance. Reference to food production markets including differences by animal species and the role of the veterinary sector on AMR surveillance. | (1) AMR surveillance | (1) 34 |
| (2) Food production systems and specific surveillance | (2) 35 | ||
| (3) Veterinary sector, agent of change | (3) 14 | ||
| VI. National action plan (NAP) efforts | Implementation of NAP and professional roles, including views on progress over time | (1) NAP progress including challenges, barriers and opportunities | (1) 22 |
| VII. External drivers | Referring to external factors contributing to the acceleration of AMR, potential opportunities and good practice, including international actors and role in/influence on national AMR | (1) Context of COVID-19 | (1) 16 |
| (2) International actors and policies | (2) 12 |
*Themes are ordered from less to more specific levels (except for external drivers which affect them all), see figure SM2, online supplemental material, for visual hierarchy.
†Codes can be mentioned more than once per interview.
AMR, antimicrobial resistance.
Theme I. Cultural factors and sociopolitical country context
This category involved country characteristics that determine the response to AMR. Interviewees described that country’s economic and political shifts, including goods shortages and high inflation rates, had jeopardised the health system, bringing instability over time to AMR control. Most political decisions in a resource-constrained country were said to be difficult to manage but high reliance on people and relationships was essential. For instance, a participant described frustration and uncertainty but ability to cope with challenges despite the economic circumstances.
Argentina has 30 years of experience on AMR surveillance, we live economic shortages and political shifts fiercely in Argentina; a state of crisis, and we are somewhat used to this dynamic trying to cope with it as best we can. We have developed good coordinating links between teams centralised on good communication skills, but we cannot guarantee sustainability. It will depend on future leader’s coordination since monetary resources are limited, a lot relies upon the projects or people’s willingness to contribute but we believe it will perpetuate.—Participant from a public institution (ID=9), female.
Most participants agreed that changing people’s attitudes and behaviour, especially among food-producing animal producers, is challenging due to embedded values (Q1, table 2). For instance, a participant described it as follows:
Table 2.
Quote examples by themes and subthemes and identification of challenges and opportunities
| Themes* | Subtheme | Representative quote examples | Challenge/ opportunity |
| I. Cultural factors and sociopolitical country context | (1) | Q1: ‘AMR is a cross-cutting issue, but with older-generation low-educated producers living mostly in remote areas (a large quantity), it has been difficult to make them switch their mindset and understand that antibiotics are not necessary for food production, if no bacterial disease is present, and that antibiotic misuse and AMR affect humans and animal well-being.’—Veterinary, public institution (ID=3), male. | Challenge |
| (2) | Q2: ‘Today, in Argentina, we have 52% poverty and talking about AMR is challenging, considering that 95% of the population is deprived. Telling people to buy cage- and antibiotic-free eggs for a double price seem problematic, considering that overcrowding, hunger, and lack of sewers are constant daily challenges.’—Participant from an NGO (ID=13), male. | Challenge | |
| II. Governance | (1) | Q3: ‘SENASA is very dynamic but there is lack of accountability from each participating institution when combatting AMR. It is worrisome that SENASA’s and other public organisation’s labours are highly centralised in the capital, because there is a high heterogeneity in access to treatment, diagnosis, quality standards for animal products, and antibiotic prescribing throughout the country. We know SENASA, for instance, is impeccable but our surveillance system is not punitive, whereas it mostly teaches producers as we do not know where those AMR pathogens came from (reservoirs).’—Participant from an NGO (ID=12), male. | Challenge and opportunity |
| (2) | Q4: ‘I think that the most important difficulty is economic and human resources, because especially in public institutions, it is challenging for people to have exclusive and fully compensated dedication.’—Participant from a public institution (ID=11), female. | Challenge | |
| (3) | Q5: ‘We have solid communication channels between governmental institutions (SENASA, INTA, MALBRAN, CoNaCRA) and somewhat with international and academic institutions, but we should employ a strategy of involvement between technical scientific groups, hospital and veterinary leaders, the private industry, including pharmaceutical, and meat producers and related organisations. We need to tell them what our problem is, for them to tell us theirs, and finally come up with integrative solutions that are beneficial for all parties.’—Participant, from a public institution (ID=6), male. | Challenge and opportunity | |
| (4) | Q6: ‘For some diagnostics we report directly to the health informatics system, but we lack perhaps of articulating all the information flows more horizontally facilitating access above all the political decision-makers. Additionally, most systems are not integrated.’—Participant from a public institution (ID=11), female. | Challenge and opportunity | |
| (5) | Q7: ‘Our legislation should regulate and control sales and consumption of antibiotics, including misuse (prohibition, for example, colistin in veterinary in 2017). Now, it is approved the Antimicrobial Use Law, which legislates on the request of archived prescriptions, and it is stricter than the former law (only monitored required prescriptions and it had poor compliance). We believe antibiotic consumption might decrease. However, regulations are not yet standardised across country regions organically and the informal market is ample.’—Participant from a public institution (ID=6), male. | Challenge and opportunity | |
| (6) | Q8: ‘At the ministerial level, sometimes AMR does not receive the importance and continuity it requires. Today we have a Minister of Health to whom AMR was a priority on their political agenda and most policy attempts entails, and do not omit, reducing AMR and antibiotic misuse. It is helpful to work in favour of that, but it is not frequently the case.’—Participant from an international institution (ID=17), female. | Challenge and opportunity | |
| III. Antibiotics access and use | (1) | Q9: ‘Laboratories distribute veterinary products. Laboratories have the approval of SENASA to produce and commercialise the products, as well as the distributors. The commercialisation path is laboratory>distributor> production, through veterinarians, but sometimes is the owner himself selling and applying them due to lack of regulation on access, even over the counter. Among cattle, there is a large part of the production that is still extensive and pharmaceutical companies are often far away from feedlot farms. Hence, they keep first-aid kits, including antibiotics, which are applied to wounds or with no diagnostic or under no veterinary supervision. The law obliges establishments to have a treatment book where animal treatments and antibiotics used are recorded, but in most remote areas is not reliably fulfilled and control is limited.’—Participant from an NGO linked to animals (ID=4), male. | Challenge |
| (2) | Q10: ‘I work in swine production; our main concern relies upon prophylactic consumption of the antibiotic. Indiscriminate antibiotic consumption is present in two ways within swine production. First, meta-phylactic, when there is a percentage of animals that are indeed sick within the flock, but since they are living with them, it is very likely that others are incubating the disease, hence they are treated. The second is the prophylactic, where there is no sick animal, but there are factors of potential stressors that could make those animals sick. This is our biggest concern, because antibiotic is then incorporated into food, whose biological matrix restricts bioavailability of the active ingredient. Additionally, when antibiotics are applied, withdrawal times are not monitored, which promotes the development of AMR mechanisms further.’—Veterinarian, academia (ID=14), male. | Challenge | |
| (3) | Q11: ‘Laboratories producing medicines for veterinary use must comply with good manufacturing practice ‘GMP’ standards. They register the product, present all the documentation on waste, among other features. We have traceability in some products, such as ketamine, each bottle is identified and we follow-up until the final user. Laboratory production of medicines is well monitored, but there are limited regulations over usage registration, because products are not often administered by health professionals. For instance, final users do not necessarily respect the restriction period or animal treatment is incomplete, for which regulation is scarce.’—Participant from an NGO (ID=4), male. | Challenge | |
| (4) | Q12: ‘We employ tannins, organic acids, probiotics, and prebiotics instead of antibiotics. We proved their efficacy for Escherichia coli and Salmonella using a mixture of probiotics and prebiotics in sentinel farms, which were boosted animal growth. We have produced alternative to antibiotics for a long-time including animal vaccines against pneumonia, and it was company’s initiative.’—Participant from a private company (ID=5), male. | Opportunity | |
| IV. AMR knowledge and awareness | (1) | Q13: ‘One of the main challenges is the access to information/ communication, the awareness of responsible antibiotic use, and lack of commitment from the private sector. Technical and general education on antibiotics and AMR should be promoted to help set a change of consciousness in the consumer, prescriber, and sellers by letting them know the actual effects/impacts of AMR on population health and animal businesses (among producers). The profitability of the sector is compromised if producers are not willing to pay a differential for meat production that could produce higher costs in the future if not committed.’—Engineer from an NGO (ID=7), male. | Challenge |
| (2) | Q14: ‘The Argentine population is much more aware of what human health is and what antibiotics are designed for. For instance, we had the World Week of Awareness in November, and we held the AMR awareness race alongside the Ministry of Health and Sports, which exhibits interrelationships between different groups.’—Participant from an international organisation (ID=17), female | Challenge and opportunity | |
| V. AMR surveillance | (1) | Q15: ‘We created the infection surveillance program in 2004 for human hospitals, which is an essential part of national surveillance reporting the annual prevalence/incidence of most critical pathogens. A ministerial resolution from 2018 recommends establishing hospital prevention and control programs, but adherence is optional. We have more than 200 added institutions reporting the appropriate use of antimicrobials yearly, including whether it was empirical, directed, surgical prophylaxis, community-acquired infection, etc. In-hospital software, part of the National Antimicrobial Resistance Surveillance Network ‘WHONET’, clinicians load the data, computes the analyses (automatically) and feeds the information to the epidemiology department. However, since 2021 (ministerial resolution), prevention and control programs must be certified depending on international guidelines. We use a federal criterion to enrol national institutions and federal hospitals, but private organisations are poorly represented. Also, some institutions did not have stewardship programs actively functioning. We updated the last referendum to include antimicrobial stewardship as a section for infection control (2021), assigning importance to protected areas for infectious disease specialist and pharmacists within hospitals.’—Participant from a public institution (ID=11), female | Challenge |
| (2) | Q16: ‘We have two main problems. First, we detect and observe a lot of animal cases experiencing neonatal diarrhoea produced by multi-resistant E. coli, even encountering septicaemia, which presents a serious health problem. Secondly, we see multi-resistant bacterial strains more frequently associated with bovine’s respiratory complex. We identify bronchopneumonia or pneumonia as the two main infectious syndromes. We receive multiple samples from animal lungs, mainly originated in fattening animals in the pen or outbreaks of pneumonia. Quite predominantly, we observe the presence of bacterial resistance to a large majority of the antibiotics routinely used for treating respiratory conditions. That shows some inefficiency from fragmented production systems lacking vertical structure, reduced biosecurity, and poor vaccination rates, especially among cattle.’—Veterinarian, public organisation (ID=8), male. | Challenge | |
| (3) | Q17: ‘I do believe that veterinarians have a very important multisectoral role to play there as training and awareness agents to contain AMR burden.’— Participant from an NGO (ID=4), male. | Opportunity | |
| VI. National action plan (NAP) efforts | (1) | Q18: ‘Progress has been made if we compare it with five years ago. Regulations on the use of antibiotics to prevent their misuse, such as growth promoters, and vast existing (AMR control in animals) and new initiatives to start controlling AMR reservoirs in soil and water demonstrates we are working towards a better integrated system by taking into consideration the One Health approach.’—Veterinarian, public organisation (ID=8), male. | Opportunity |
| VII. External drivers | (1)† | Q19: ‘Something the pandemic left us was the effectiveness of virtual meetings, for example, plan out objectives and do training with the veterinarians and farm owners.’—Participant from an NGO (ID=4), male. | Opportunity |
| (2) | Q20: ‘We performed projects alongside the European Union, WHO and Centre for Disease Control, which supported Argentina through international funding to perform AMR surveillance and control, even in food products and wastewater.’—Participant from a public institution (ID=1), female. | Opportunity |
*Subthemes descriptions are found in table 1.
†There are challenges associated as well, but we included an opportunity.
AMR, antimicrobial resistance; CoNaCRA, National Commission for the Control of Antimicrobial Resistance; INE, National Institute of Epidemiology; INEI, National Institute of Infectious Diseases; INTA, National Agricultural Technology Institute; NGO, non-governmental organisation; SENASA, National Food Safety and Quality Service.
Inappropriate antibiotic use, driven by cultural norms like self-medication and seeking quick remedies is often influenced by limited healthcare access. This extends into food production, where profit motives can override caution. A shift in cultural perspective is crucial, educating on responsible use and the benefits of animal welfare and sustainable practices across the sectors.—Participant, from a public institution (ID=6), male.
Theme II. Governance
Participants reported constraints in the administration system that limited or enhanced their ability to perform improvements towards tackling AMR. Most participants recognised that SENASA conducts extensive and well-articulated labour; however, they emphasised the lack of auditing and accountability in decentralised administrations (regions) in Argentina, which hampers AMR control due to the inherent variability in the quality of care and health access (Q3, table 2). Efforts to homogenise quality of care and access to antibiotics in humans and animals are ongoing, but monetary and non-monetary resources are bounded (Q4, table 2). Although resources were finite, stakeholders’ communication was often seen as a local strength and the cornerstone of policy making. Good interpersonal relationships within the public, academic, NGOs and international organisations were reported (Q5, table 2). However, a demand for more integrated services and decision-making was stated (Q5, table 2), translating into the need to foster horizontal information flows with mutually integrated systems and organisations throughout the country (Q6, table 2).
Regarding the regulation subtheme, there are positive views towards the new law on antimicrobial use, despite the former law introducing some restrictions but lacking control on usage, prescribing and storage (most frequent among animals) (Q7, table 2). On top of all previously discussed factors, the political context and agenda were considered critical for AMR control but conflicting depending on the country’s obstacles and people in charge (Q8, table 2).
Theme III. Antibiotics
Thematic III encompasses efforts to improve antibiotic access, consumption and regulation while accounting for potential alternatives. Access to antibiotics was indicated as better regulated on the human side, where hospitals and labs work collaboratively; however, a few participants expressed concerns about the applicability of mandatory prescriptions for sales and a mismatch between prescribed treatments and antibiotic package dosing:
Antibiotics are still sold without a prescription either for human or animal use (more frequent among animals), even if prescriptions are mandatory by law, including keeping track of their usage by health professionals (electronic sales). Another issue is the dosage; antibiotics are usually sold in dosages greater than needed, which incentivises inappropriate utilisation maximising commercial interests.—Participant, academia (ID=14), female.
In addition, one participant expressed worrying views towards antibiotic access and prophylactic use in animals and its relationship with AMR evolution, including reduced capacity for monitoring usage and withdrawal (Q10, table 2). On the animal side, the route for antibiotic acquisition could have been more cohesive; vets do not necessarily monitor antibiotic purchase, application and storage, and limited local regulations are in place (Q9, table 2). Such practices can be reflected early in the regulation system and antibiotic dispensing from the first stage of the purchasing chain. Most medicines for veterinary use follow international manufacturing standards, but traceability of compounds and usage is restricted once purchase is made (Q11, table 2). Finally, some participants working on antibiotic replacement produce alternatives for antibiotic use in food-producing animals. They were optimistic over vaccine production offering an efficacious alternative, tackling respiratory diseases in animals and using additives and plant-based biomolecules to improve food conversion efficiency and eradicate antibiotic use as growth promoters in animal farms (Q12, table 2).
Theme IV. AMR knowledge and awareness
Theme IV incorporated progress made towards AMR knowledge and awareness targeting different settings and communities. Participants were inclined to say that awareness has increased, and impacts on human health are acknowledged (Q13, table 2), primarily among leading institutions including government, but awareness campaigns only have limited reach to the wider population, including meat producers, as they tend to be specific and attend professional needs:
There is a problem with vets’ knowledge of antibiotics, specifically in terms of pharmacokinetic and pharmacodynamic aspects, the correct calculation of the dose administration. It is the duration of treatment in the form of clinical criteria and corresponding to specific physiological situations. All these aspects are related to rational antibiotic usage. If you forget these aspects, you can reach a therapeutic failure despite having chosen the correct antibiotic based on what the laboratory said; and this is largely prevalent in the country.— Veterinarian, academia (ID=14), male.
Notwithstanding, participants reported initiatives to raise the human population’s awareness generally, including social events and educational seminars, having positive perceived conceptions from participants but their effectiveness remains unclear (Q14, table 2).
Theme V. AMR surveillance
Theme V comprised all barriers or opportunities that made the implementation of surveillance and AMR control over time arduous or fruitful. Participants expressed that surveillance of AMR has exhibited a lot of progress over time, especially among humans, and most recently in animals. Antibiotic stewardship and infection surveillance programmes in humans, including the regular monitoring of a consolidated network of +200 hospitals, primarily public, are some of the positive views perceived by stakeholders towards implementation (Q15, table 2). Antibiotic consumption control is a central priority of the government now and a countrywide programme is being developed to strengthen AMR surveillance:
We have implemented a national surveillance system for the national consumption of antibiotics in humans, which we did not have until a short time ago. Now, it is time to access the information related to disaggregated statistics on antibiotic sales through collaboration with Pharmacology in human and animal side.—Pharmacist, public organisation (ID=6), female
However, policies around AMR surveillance and control are moving forward among livestock production systems but at slower rates. One participant indicated that testing for critical animal pathogens is routine and usually comes from public organisations. Still, animal diagnostics are difficult to access sometimes, and surveillance does not yet clearly account for different locations, species and seasonal components:
INTA monitors some animal production chains, but surveillance is the primary task of SENASA, for example, in dairy, we evaluate animals experiencing a mastitis disease and track AMR and potential environmental reservoirs with technology developed locally. Another example, we detect Salmonella in animals and utilise microbiological analyses, including phenotyping and genotyping to analyse AMR and evolution, as part of surveillance routines hand by hand with SENASA. However, most surveillance comes from the governmental side, sampling seasonality is not often captured due to limited resources, the quantity of livestock farms is massively distributed throughout the country, and local producer’s veterinary diagnostics are often sent to private labs where traceability is missing.—Veterinarian, public organisation (ID=15), male.
Challenges identified by participants on AMR surveillance among animals are mirrored mainly in the animal industry, which constitutes many producers and actors through the production chain, making it complex to supervise (figure 2 shows a brief description and example of cattle production based on interview content and additional sources for broader context). As reported by one participant, industry’s main challenges rely on controlling early stages within the production chain, including improved hygiene and sanitation and vaccination strategies before animals are stressed while moving from breeding to fattening stages (Q16, table 2).
Figure 2.
Brief description of some features within the cattle industry in Argentina. (A) Feed supplied in the trough for cattle, livestock farm, Buenos Aires, Argentina. (B) Cattle pens, livestock farm, Buenos Aires, Argentina. (C) Characteristics of beef production and relationship with AMR control. AMR, antimicrobial resistance; NAP, national action plan. Most information was derived from our interviews; exact quotes are shown on request.
Theme VI. NAP efforts
All participants expressed a positive attitude towards progress made on the NAP even if it is slow. Participants agreed on improvements, such as the institutionalisation of CoNaCRA, recently launched law on established AMR network and surveillance (which does not depend on political will), a new research centre (IMPaM) for environmental surveillance of AMR reservoirs including water, more control over companion and food-producing animals since 2015 from National Agricultural Technology Institute and SENASA, and that prohibition of some antibiotics has been crucial (Q18, table 2). Another participant highlighted its value as a premier platform for interdisciplinary engagement:
The NAP has significantly advanced and enhanced interdisciplinary and interdepartmental cooperation between animal and human health sectors, primarily driven by the CoNaCRA, which has facilitated knowledge sharing. However, the challenge of synchronising NAP initiatives across 24 distinct provinces in a federal system underscores the imperative for more effective inter- and intra-level cooperation.—Participant from a public institution (ID=9), female.
Theme VII. External drivers
Theme VII comprises external factors, identified by participants, that have had a direct or indirect role affecting AMR control and surveillance. Most participants (60%) recognised that COVID-19 limited the progress of AMR control and policy due to reallocation of human, economic and other resources towards the pandemic response. For instance, one participant described it as follows:
Teams were absolutely overwhelmed during COVID-19, all the artillery was dedicated to diagnosis and containment of COVID. We observed an overuse of antimicrobials during these two years, which have accelerated the appearance of new AMR mechanisms and their transmissibility. At the microbiological level, pandemic lineages have appeared, which changes local epidemiology.—Infectious disease doctor, public organisation (ID=1), female.
However, positive lessons were drawn from the pandemic, including the effectiveness of virtual meetings as an advantage of multisectoral collaboration (Q19, table 2). Additionally, prioritisation of personal hygiene and care and hand washing was understood to be improved due to increasing awareness of communicable diseases and human health among citizens, as one participant reflected:
The pandemic has taught us, it’s to prioritize our personal hygiene. Hand washing and personal care help us not get sick from diseases, and if we don’t get sick, we do not require antibiotics.—Veterinary, public organisation (ID=3), male.
Finally, the second external factor relies on international collaborations. Participants described solid relationships with international actors within the Americas and abroad, which has helped fund local projects for improving AMR control and surveillance (Q20, table 2).
Discussion
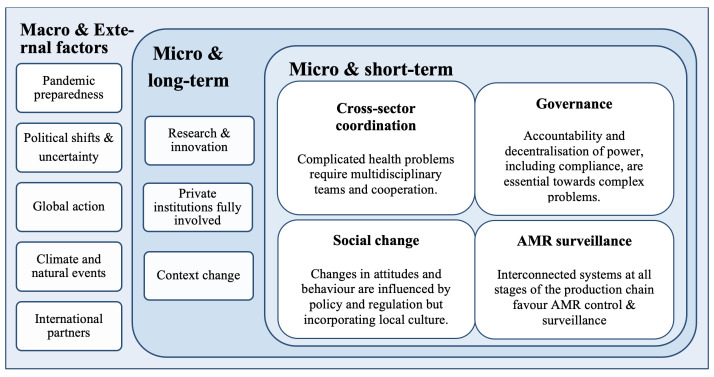
We employed a context and qualitative analysis to understand the main gaps related to the progress of AMR control in implementing AMR policy and regulation in Argentina. Our findings revealed that overall stakeholders’ perceptions towards AMR policy are positive, including the development of personal relationships enabling progress, and that the NAP along with current emerging legislation is essential in formalising the first steps to a multisectoral and better integrated AMR surveillance system. Interviewees stated that main challenges on the way forward are related, but not limited to, commitment and accountability, monetary and non-monetary resources, cultural factors implying behaviour change, fragmented food-production systems and global governance. We have summarised these below in figure 3 in relation to whether at macrolevel or microlevel and short-term or long-term.
Figure 3.
Major challenges and opportunities related to AMR policy in Argentina within a One Health scope in the short-term and long-term. AMR, antimicrobial resistance.
One of the most immediate short-term challenges identified by stakeholders was governance, which implied needing more accountability and resources through different AMR actors. Clearer administration systems for successful improvement and conveying AMR policies are crucial to moving forward in the AMR agenda.26 37 Argentina’s administration system is divided into provinces. Still, political decisions are highly centralised in the capital city, which hampers regulation of antibiotic access, AMR testing and delivery of consistent access to healthcare and hospital infrastructure in humans and animals. Although the CoNaCRA has provincial representatives, the AMR agenda had limited alignment with subnational and local governments for NAP implementation posing significant challenges in rural and remote areas.38 Likewise in Brazil,20 these areas are often highly exposed to AMR risks due to health deprivation. Readministering monetary and non-monetary resources to meet local needs and capacities and empowering provincial sentinel organisations are crucial to making AMR policies accountable.
Cross-sector coordination including animal, environmental and human sectors was highlighted as crucial by interviewees and constituted an essential element of short-term action towards tackling AMR and improving policy design. Although communication was perceived as positive due to interpersonal relations between colleagues, adequate governance must be established, including mechanisms to link organisations across sectors through formal channels to foster continuity. Effectively tackling these challenges is essential for the One Health approach, particularly given the COVID-19 pandemic’s revelation of significant complexities and gaps in intersectoral collaboration, underscoring the need for integrated, human-uncentered, policies.39 Literature has focused on countries’ need for better engagement and advocacy from various stakeholders.9 14 40 Governments, policymakers and NGOs are essential to AMR control, supported by budgetary commitment and political authority to meet objectives.19 Current interventions in Argentina remain sector-specific, which could be attributed to differences in priorities for AMR or insufficiently well-defined roles in the NAP. Developing a monitoring or evaluation system for all implementation plans is recommended to determine policy effectiveness. Broad cross-field participation is also crucial if no public budget is allocated to address AMR nationally. Insufficient funding provided by the annual national budgets negatively impacts NAP implementation, generating more constraints on AMR action. The COVID-19 pandemic garnered global attention disrupting national resources and health services that were indirectly assigned to AMR control, and reallocating those funds has posed challenges to AMR reduction measures. One Health although theoretically a useful framework, was not considered in NAP implementation—it could have accounted for the cobenefits of addressing both risks concurrently. That is why the United Nations Environment Programme established the Multi-Partner Trust Fund to help LMICs improve delivery of multisectoral NAPs.28
AMR awareness has increased over time in Argentina. Recent efforts have included various seminars and activities, including a long-distance foot race to spread the word on AMR.41 However, AMR comprises stakeholders with diverse comprehension of the AMR phenomenon, and we evidenced a mismatch between scientific and non-scientific domains, including general public. Social change promoting human health via shifts in society’s behaviour should be prioritised to ensure the sustainability of human development and their environments.42 For instance, public engagement is overlooked in Argentina’s NAP. Strategies to evaluate attitudes, behaviours, necessities and practices of socioeconomically and culturally diverging communities, drawing particular attention to those most vulnerable to AMR infections, are crucial to design public health interventions to combat AMR.4
Strengthening AMR surveillance and control is vital, with different challenges depending on the species spectrum, as highlighted previously in LMICs.4 19 20 43–45 In humans, laboratory surveillance has been based for decades on the connectivity of a well-established hospital network (eg, WHONET-Argentina46), which has helped monitor AMR locally with institutionalised infection prevention and control (IPC) policies, led by the Antimicrobial Agents Division of INEI-ANLIS (Argentina’s National Administration of Laboratories and Institutes of Health), the National and Regional Reference Laboratory for AMR and the National Hospital Infection Surveillance Program, respectively. However, there are still gaps regarding the effectiveness of preserving antibiotics through stewardship programmes, although consumption levels and inappropriate usage rates decreased before COVID-19.47 Yet, quality control among stewardship programmes, antimicrobial sales with necessary prescriptions and targeted local efforts in differing regions are still challenging throughout the country.48 In animals, the main surveillance challenges that were reported were concentrated around the unification of production systems (dispersed via multiple chains and actors potentially favouring the dissemination of AMR), whose current fragmented status hampers regulation, with differing control levels depending on animal species. Systems’ capacity to ensure prescription and consumption data is compromised and policies should coordinate and harmonise AMR surveillance while regulating the usage of antimicrobials in animal production at all production stages.32 Globally, antimicrobial stewardship by farm owners and health professionals (eg, veterinarians) is relatively weak within agricultural systems; developing efforts towards stewardship programmes in veterinary services, bolstering the veterinary role as a critical change agent, and companion animal practice remains crucial.49–51 Argentina’s chambers of producers play an active role in agglomerating food producers and understanding their needs (eg, Camara Argentina de Feedlots); their job should be directed towards better integration and prioritisation of educational services and improved production standards.
A recent WHO report on integrated surveillance of AMR in foodborne diseases indicated that ineffective public health AMR surveillance systems often lack broader regulation and laboratory infrastructure, limited biosecurity and inadequate data management capacity at government levels.52 We observed reduced capacity for data monitoring and sharing among animal stakeholders, whereby surveillance of antibiotic sales/usage and AMR rates by animal species could be better reported. Despite limited public control due to fragmentation of the production chain, food producers are perceived to prioritise profitability and local needs, regardless of the effects of AMR on population health. Moreover, creating an integrated One Health approach combining animal and human systems, including environmental sources, might help reduce the AMR burden and prevent animal infections in farming communities,25 53 ensuring sustainability over time and lowering the risks associated with political shifts and global uncertainties. The role of the private sector, not only restricted to food producers, in supporting AMR surveillance should be encouraged to provide a holistic whole-system integration, including a whole food-chain approach.54 55 This should involve data access and optimising contemporary treatments and diagnostics through more research and technology to elucidate the transmission pathways of the most critical microorganisms for animal and human health.
We identified potential opportunities that could help contribute to progressing action to reduce AMR locally. Most stakeholders favoured agricultural non-antimicrobial drug products as an antibiotic replacement for animal growth promoters. Using tannins and natural plant-based medicines could supersede antibiotics, reducing selective pressure and AMR burden.56 57 Nevertheless, most of these products are difficult to access locally with limited legislation and high reliance on a few international companies. The provision of replacement routes from the government for antibiotic-free additives usage in animals, including appropriate stakeholder education and countrywide support through public pharmacies, is something the authorities should leverage. Furthermore, the new law establishing the AMR agenda as a constituted programme, regardless of political change, presents substantial progress towards national recognition of the AMR problem.24 The initiative brings a long-term perspective to AMR policy, which could be used for the creation of an AMR policy database containing information on NAP implementation accountability and cross-species and environment AMR surveillance for policy advisors.
Our study has some shortcomings. First, we were not able to speak to stakeholders from all areas of the stakeholder mapping and cannot generalise the views of participants to others but have confidence in the transferability of findings and common themes that arose among the diverse stakeholders who participated. Nevertheless, common themes arose from speaking to a range of stakeholders and our sample reached saturation with a narrow range of interviews, considered an appropriate sample size for qualitative research.58 Second, we could not represent private hospitals for human AMR, and differences between production chains, including a broader scope of animal species, dairy products and final animal product providers, which remains a future study. Third, the extent of interviewees’ actual involvement in AMR policies differed; however, we ensured respondents best authority through collaborative local work and expert knowledge. Fourth, the authorship group includes people involved in AMR policy in Argentina which could either favour (facilitate information flow) or bias (sampling, selection and confounding) our study results.
Finally, participants’ beliefs (interviews held between December 2022 and January 2023) might be subject to change in the forthcoming years due to the implementation of the newly introduced national law on AMR prevention and control (August 2022).24 Tighter measures regarding antibiotic usage and sales (only underfilled prescriptions) and promoting the One Health approach via implementing cross-sector policies while accounting for organisation-specific responsibilities for their listed tasks are examples of the expected outcomes the law might enforce.
Conclusion
Our study results draw attention to the main strengths, opportunities and challenges in the process towards improved AMR awareness, control and surveillance across the human–animal frontier in Argentina. The country has been one of the leaders in the region with an established AMR surveillance network for human health in the latest 40 years. However, AMR governance requires a multidisciplinary focus to help stakeholders at all levels deal with knowledge uncertainties and resulting differences in framing the AMR problem. We found critical areas that should be strengthened, including accountability, sustainable engagement, integrity and equity, sociobehavioural change, international cooperation and consolidation of environmental and animal departments. Cross-cutting interventions incorporating these areas through different One Health domains should be accounted for if progressing towards AMR is noted. The recent law on AMR prevention and control serves as a good example, which identifies potential pathways to overcome challenges with direct implications for LMICs in the Latin American region.
bmjopen-2023-082156supp001.pdf (94.2KB, pdf)
Supplementary Material
Acknowledgments
All authors attest they meet the ICMJE criteria for authorship and have reviewed and approved the final article. The authors are grateful to all participants for volunteering time as part of interviews and workshops.
Footnotes
@kasim_allel, @Lisa_A_Boden, @EPitchforth
Collaborators: AMR Policy Research Group Argentina: Rodolfo Luzbel de la Sota (Facultad de Ciencias Veterinarias, Universidad Nacional de la Plata, Argentina); Sonia Gómez (Servicio Antimicrobianos, Instituto Nacional de Enfermedades Infecciosas, ANLIS “Dr Carlos G. Malbrán”, Buenos Aires, Argentina); Sergio Sánchez Bruni (Facultad de Ciencias Veterinarias, Universidad Nacional del Centro de la Provincia de Buenos Aires, Argentina); Kristen Reyher (Bristol Veterinary School, University of Bristol, UK); Helen West (University of Nottingham, UK); Peers Davies (Institute of Infection, Veterinary and Ecological Sciences, Liverpool University, UK); Dominic Moran (The Royal (Dick) School of Veterinary Studies, University of Edinburgh, UK).
Contributors: Conceptualisation (KA, EP, WG, MF-M and AP); methodology (KA, EP and LBo); formal analysis (KA); writing—original draft preparation (KA); writing—review, editing and commenting (KA, EP, WG, MF-M, AP, LBo, LBa, AC and FL); supervision (EP, MF-M and AP); securing funding for the project (RL, SG, SSB, AP, MF-M, KR, HW, PD, DM and WG). All authors have read and approved the final version of the manuscript. EP acts as guarantor for the conduct of the study and content.
Funding: This research is cofunded by the UK Department of Health and Social Care as part of the Global AMR Innovation Fund (GAMRIF)(BB/T004452/1) and includes funding under the GAMRIF UK: Argentina AMR Pan-Programme Integration Project (PPIP). This is a UK aid programme that supports early-stage innovative research in underfunded areas of antimicrobial resistance (AMR) research and development for the benefit of those in LMICs, who bear the greatest burden of AMR. The views expressed in this publication are those of the author(s) and not necessarily those of the UK Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: AMR Policy Research Group Argentina, Rodolfo Luzbel de la Sota, Sonia Gómez, Sergio Sánchez Bruni, Kristen Reyher, Helen West, Peers Davies, and Dominic Moran
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
Ethical approval was obtained from the Human Ethical Review Committee at the University of Edinburgh (Ref: HERC_696_21). Participants gave informed consent to participate in the study before taking part.
References
- 1. Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016;387:176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 2. Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399:629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allel K, Day L, Hamilton A, et al. Global antimicrobial-resistance drivers: an ecological country-level study at the human–animal interface. Lancet Planet Health 2023;7:e291–303. 10.1016/S2542-5196(23)00026-8 [DOI] [PubMed] [Google Scholar]
- 4. Charani E, Mendelson M, Pallett SJC, et al. An analysis of existing national action plans for antimicrobial resistance—gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Glob Health 2023;11:e466–74. 10.1016/S2214-109X(23)00019-0 [DOI] [PubMed] [Google Scholar]
- 5. Mendelson M, Matsoso MP. The world health organization global action plan for antimicrobial resistance. S Afr Med J 2015;105:325. 10.7196/SAMJ.9644 [DOI] [PubMed] [Google Scholar]
- 6. Humphreys G, Fleck F. United Nations meeting on antimicrobial resistance. Bull World Health Organ 2016;94:638–9. 10.2471/BLT.16.020916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Commission E . A European one health action plan against antimicrobial resistance (AMR). European Commission; 2017. [Google Scholar]
- 8. World Health Organization . Library of AMR national action plans. 2023. Available: https://www.who.int/teams/surveillance-prevention-control-AMR/national-action-plan-monitoring-evaluation/library-of-national-action-plans
- 9. Frumence G, Mboera LEG, Sindato C, et al. The governance and implementation of the national action plan on antimicrobial resistance in Tanzania: a qualitative study. Antibiotics (Basel) 2021;10:273. 10.3390/antibiotics10030273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wernli D, Jørgensen PS, Harbarth S, et al. Antimicrobial resistance: the complex challenge of measurement to inform policy and the public. PLoS Med 2017;14:e1002378. 10.1371/journal.pmed.1002378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Organization WH . Monitoring and evaluation of the global action plan on antimicrobial resistance: framework and recommended indicators. 2019.
- 12. Jimah T, Ogunseitan O. National action plan on antimicrobial resistance: stakeholder analysis of implementation in Ghana. J Glob Health Rep 2020;4:e2020067. 10.29392/001c.13695 [DOI] [Google Scholar]
- 13. Khan MS, Durrance-Bagale A, Mateus A, et al. What are the barriers to implementing national antimicrobial resistance action plans? A novel mixed-methods policy analysis in Pakistan. Health Policy Plan 2020;35:973–82. 10.1093/heapol/czaa065 [DOI] [PubMed] [Google Scholar]
- 14. Chua AQ, Verma M, Hsu LY, et al. An analysis of national action plans on antimicrobial resistance in Southeast Asia using a governance framework approach. Lancet Reg Health West Pac 2021;7:100084. 10.1016/j.lanwpc.2020.100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harant A. Assessing transparency and accountability of national action plans on antimicrobial resistance in 15 African countries. Antimicrob Resist Infect Control 2022;11:1–15. 10.1186/s13756-021-01040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nair M, Zeegers MP, Varghese GM, et al. India’s national action plan on antimicrobial resistance: a critical perspective. J Glob Antimicrob Resist 2021;27:236–8. 10.1016/j.jgar.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 17. Amin ET, Omeichu AA, Shu DM, et al. Control of antimicrobial resistance in Cameroon: feasibility of implementing the national action plan. Tropical Med Int Health 2021;26:1231–9. 10.1111/tmi.13649 [DOI] [PubMed] [Google Scholar]
- 18. Patel J, Harant A, Fernandes G, et al. Measuring the global response to antimicrobial resistance, 2020–21: a systematic governance analysis of 114 countries. Lancet Infect Dis 2023;23:706–18. 10.1016/S1473-3099(22)00796-4 [DOI] [PubMed] [Google Scholar]
- 19. Shabangu K, Essack SY, Duma SE. Barriers to implementing national action plans on antimicrobial resistance using a one health approach: policy-makers. J Glob Antimicrob Resist 2023;33:130–6. 10.1016/j.jgar.2023.02.007 [DOI] [PubMed] [Google Scholar]
- 20. Corrêa JS, Zago LF, Da Silva-Brandão RR, et al. The governance of antimicrobial resistance in Brazil: challenges for developing and implementing a one health agenda. Global Public Health 2023;18. 10.1080/17441692.2023.2190381 [DOI] [PubMed] [Google Scholar]
- 21. da Silva JB, Espinal M, Ramón-Pardo P. Antimicrobial resistance: time for action. Rev Panam Salud Publica 2020;44:e131. 10.26633/RPSP.2020.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ministry of Health . National strategy for the control of antimicrobial resistance. 2015. Available: https://www.boletinoficial.gob.ar/pdf/linkQR/YzB6b2o4Qy9ZRDFycmZ0RFhoUThyQT09
- 23. República Argentina - Poder Ejecutivo Nacional . Resolución 22/19. Productos Veterinarios: prohibición de elaboración, distribución, importación, USO Y Tenencia. 2019. Available: http://www.senasa.gob.ar/sites/default/files/ARBOL_SENASA/INFORMACION/NORMATIVA/RESOL_Y_ANEXOS/2019/r_senasa_22-2019.pdf
- 24. Nacion ANCPdl . Boletin Oficial de la REPUBLICA de Argentina. Ley de Prevención Y control de la Resistencia a Los Antimicrobianos, Ley 27680. 2022. Available: www.boletinoficial.gob.ar/detalleAviso/primera/270118/20220824?busqueda=1
- 25. Ogyu A, Chan O, Littmann J, et al. National action to combat AMR: a one-health approach to assess policy priorities in action plans. BMJ Glob Health 2020;5:e002427. 10.1136/bmjgh-2020-002427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson M, Schulze K, Cassini A, et al. A governance framework for development and assessment of national action plans on antimicrobial resistance. Lancet Infect Dis 2019;19:e371–84. 10.1016/S1473-3099(19)30415-3 [DOI] [PubMed] [Google Scholar]
- 27. Pitchforth E, Smith E, Taylor J, et al. Global action on antimicrobial resistance: lessons from the history of climate change and tobacco control policy. BMJ Glob Health 2022;7:e009283. 10.1136/bmjgh-2022-009283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. United Nations . Antimicrobial resistance multi-partner trust fund countering antimicrobial resistance with a ‘one health’ approach. 2022. Available: https://mptf.undp.org/fund/amr00
- 29. World Bank . World Bank data. 2023. Available: https://data.worldbank.org/country/argentina
- 30. Ministry of Health . Comisión nacional de control de la resistencia antimicrobiana (Conacra). 2024. Available: https://www.argentina.gob.ar/salud/epidemiologiaysituacion/conacra
- 31. Instituto nacional de enfermedades infecciosas (INEI) – ANLIS “DR. CARLOS G. MALBRAN. Servicio Antimicrobianos. 2024. Available: http://antimicrobianos.com.ar/servicio-antimicrobianos
- 32. Prack McCormick B, Quiroga MP, Álvarez VE, et al. Antimicrobial resistance dissemination associated with intensive animal production practices in Argentina: a systematic review and meta-analysis. Revista Argentina de Microbiología 2023;55:25–42. 10.1016/j.ram.2022.07.001 [DOI] [PubMed] [Google Scholar]
- 33. Temple B, Young A. Qualitative research and translation dilemmas. Qual Res 2004;4:161–78. 10.1177/1468794104044430 [DOI] [Google Scholar]
- 34. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 35. Boeije H. A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant 2002;36:391–409. 10.1023/A:1020909529486 [DOI] [Google Scholar]
- 36. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 37. Rochford C, Sridhar D, Woods N, et al. Global governance of antimicrobial resistance. Lancet 2018;391:1976–8. 10.1016/S0140-6736(18)31117-6 [DOI] [PubMed] [Google Scholar]
- 38. Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob Health 2019;4:e002104. 10.1136/bmjgh-2019-002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen J, He J, Bergquist R. Challenges and response to pandemics as seen in a one health perspective. Science in One Health 2022;1:100010. 10.1016/j.soh.2023.100010 [DOI] [Google Scholar]
- 40. Castro-Sánchez E, Iwami M, Ahmad R, et al. Articulating citizen participation in national anti-microbial resistance plans: a comparison of European countries. Eur J Public Health 2018;28:928–34. 10.1093/eurpub/cky128 [DOI] [PubMed] [Google Scholar]
- 41. With a massive race, PAHO Argentina spreads the word to prevent antimicrobial resistance [press release]. 2022.
- 42. Hernando-Amado S, Coque TM, Baquero F, et al. Antibiotic resistance: moving from individual health norms to social norms in one health and global health. Front Microbiol 2020;11:1914. 10.3389/fmicb.2020.01914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lota MMM, Chua AQ, Azupardo K, et al. A qualitative study on the design and implementation of the national action plan on antimicrobial resistance in the Philippines. Antibiotics (Basel) 2022;11:820. 10.3390/antibiotics11060820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sirijatuphat R, Chayangsu S, Srisompong J, et al. Feasibility, challenges, and benefits of global antimicrobial resistance surveillance system implementation: results from a multicenter quasi-experimental study. Antibiotics (Basel) 2022;11:348. 10.3390/antibiotics11030348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sumpradit N, Wongkongkathep S, Poonpolsup S, et al. New chapter in tackling antimicrobial resistance in Thailand. BMJ 2017;358:j3415. 10.1136/bmj.j2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rossi MA, Tokumoto M, Couto E, et al. Survey of the levels of antimicrobial resistance in Argentina: WHONET program-1991 to 1994. Int J Antimicrob Agents 1995;6:103–10. 10.1016/0924-8579(95)00028-8 [DOI] [PubMed] [Google Scholar]
- 47. Dapás JI, Quirós RE. Antimicrobial stewardship in low-and middle-income countries. Curr Treat Options Infect Dis 2018;10:17–27. 10.1007/s40506-018-0141-4 [DOI] [Google Scholar]
- 48. Boni S, Marin GH, Campaña L, et al. Disparities in antimicrobial consumption and resistance within a country: the case of beta-lactams in Argentina. Rev Panam Salud Publica 2021;45:e76. 10.26633/RPSP.2021.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brodbelt D. Breaking new ground in antimicrobial stewardship in companion animal veterinary practice. Nat Commun 2021;12:2445. 10.1038/s41467-021-22542-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nind LS. International trends Impacting antimicrobial stewardship in animal health. Aust Vet J 2019;97:362–4. 10.1111/avj.12839 [DOI] [PubMed] [Google Scholar]
- 51. Singleton DA, Rayner A, Brant B, et al. A randomised controlled trial to reduce highest priority critically important antimicrobial prescription in companion animals. Nat Commun 2021;12:1593. 10.1038/s41467-021-21864-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. World Health Organization . Integrated surveillance of antimicrobial resistance in foodborne bacteria: application of a one health approach: guidance from the WHO advisory group on integrated surveillance of antimicrobial resistance (AGISAR). 2017.
- 53. Pinto Jimenez CE, Keestra SM, Tandon P, et al. One health WASH: an AMR-smart integrative approach to preventing and controlling infection in farming communities. BMJ Glob Health 2023;8:e011263. 10.1136/bmjgh-2022-011263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mendelson M. BSAC vanguard series: inequality and antibiotic resistance. J Antimicrob Chemother 2022;77:277–8. 10.1093/jac/dkab426 [DOI] [PubMed] [Google Scholar]
- 55. Blake LJ, Häsler B, Bennani H, et al. The UK antimicrobial resistance strategy 2013–18: a qualitative study of international and domestic policy and action related to livestock and the food chain. Front Sustain Food Syst 2022;6:102. 10.3389/fsufs.2022.819158 [DOI] [Google Scholar]
- 56. Huang Q, Liu X, Zhao G, et al. Potential and challenges of Tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr 2018;4:137–50. 10.1016/j.aninu.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Farha AK, Yang Q-Q, Kim G, et al. Tannins as an alternative to antibiotics. Food Biosci 2020;38:100751. 10.1016/j.fbio.2020.100751 [DOI] [Google Scholar]
- 58. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med 2022;292:114523. 10.1016/j.socscimed.2021.114523 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-082156supp002.pdf (563.6KB, pdf)
bmjopen-2023-082156supp001.pdf (94.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.