Abstract
Background
Asthma is a heterogeneous disease with a prevalence and severity that differs between male and female patients.
Question
What are differences between male and female patients with asthma with regard to asthma control, lung function, inflammation and exacerbations?
Methods
We performed a post hoc analysis in the ATLANTIS (Assessment of Small Airways Involvement in Asthma) study, an observational cohort study including patients with asthma from nine countries with a follow-up of 1 year during which patients were characterised with measures of large and small airway function, questionnaires, inflammation and imaging. We compared differences in baseline characteristics and longitudinal outcomes between male and female patients with asthma.
Results
773 patients were enrolled; 450 (58%) of these were female. At baseline, female patients with asthma were in higher Global Initiative for Asthma (GINA) steps (p=0.042), had higher Asthma Control Questionnaire 6 (F: 0.83; M: 0.66, p<0.001) and higher airway resistance as reflected by uncorrected impulse oscillometry outcomes (ie, R5-R20: F: 0.06; M: 0.04 kPa/L/s, p=0.002). Male patients with asthma had more severe airway obstruction (forced expiratory volume in 1 s/forced vital capacity % predicted: F: 91.95; M: 88.33%, p<0.01) and more frequently had persistent airflow limitation (F: 27%; M: 39%, p<0.001). Blood neutrophils were significantly higher in female patients (p=0.014). With Cox regression analysis, female sex was an independent predictor for exacerbations.
Interpretation
We demonstrate that female patients are in higher GINA steps, exhibit worse disease control, experience more exacerbations and demonstrate higher airway resistance compared with male patients. The higher exacerbation risk was independent of GINA step and blood eosinophil level. Male patients, in turn, have a higher prevalence of persistent airflow limitation and more severe airflow obstruction. These findings show sex can affect clinical phenotyping and outcomes in asthma.
Trial registration number
Keywords: asthma, asthma epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
It is well recognised that the clinical presentation of asthma may differ across the sexes; however, how exactly sex affects severity of symptoms, large and small function, inflammation and exacerbation has not been systematically investigated in large clinical studies.
WHAT THIS STUDY ADDS
We use available data of the large and well-characterised ATLANTIS (Assessment of Small Airways Involvement in Asthma) study.
We show sex to have a significant impact on the clinical expression of asthma.
Female sex is associated with more severe symptoms, large and small airway function and bronchial hyperresponsiveness.
In addition, it is a risk factor for exacerbations independent of blood eosinophil levels.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Sex influences the clinical expression of asthma; therefore, it may be important for physicians and researchers to take into account this factor in daily clinical practice and in future studies, including those focusing on pharmacological treatment of asthma.
Introduction
In 2019, it was estimated that 262 million people worldwide were affected by asthma.1 Asthma is a heterogeneous disease with distinctive phenotypes and endotypes.2 Childhood-onset asthma is often atopic, whereas adult-onset asthma is frequently non-atopic and more severe.3 The prevalence and severity of asthma also differ between male and female patients, and this ratio changes during the lifetime. Asthma is more common and severe in boys during childhood, but this changes around puberty after which asthma becomes more prevalent and severe in female patients.4 5 Consequently, female patients with asthma have an increased risk of exacerbations and asthma-related mortality in adulthood compared with male patients.4 6
It is already known that the sex disparity in asthma is multifactorial. Endogenous sex hormones are one of the widely studied factors; their fluctuations throughout life, such as in puberty, the menstrual cycle and menopause, play an important role in the increased prevalence and severity of asthma in female adults.6–9 Additionally, male and female patients with asthma may also experience and report symptoms differently and throughout life may be exposed to different social and environmental factors.6–9 Thus, sex disparity in asthma is highly complex and may have an impact on asthma severity as well as control and management. Therefore, it is important to gain more insight in the clinical differences between male and female patients with asthma, as this might ultimately lead to optimisation of precision asthma treatment.
Previous studies on sex differences in asthma lack extensive clinical characterisation or a broad spectrum of asthma severities and often did not take the presence and extent of small airways dysfunction (SAD) into account. The aim of this post hoc study was to investigate sex differences related to asthma control, lung function and exacerbations in extensively clinically characterised patients, including parameters of both large and small airway function.10
Methods
ATLANTIS study design
The ATLANTIS study is an observational cohort including patients with asthma across all severities. Recruitment started 30 June 2014 and lasted until 3 March 2017 and took place in 9 countries across 29 centres.10 A complete overview of the study design, including a list of all inclusion and exclusion criteria, can be found in online supplemental files 1 and 2. In short, the inclusion criteria were an age between 18 and 65 years and a confirmed asthma diagnosis according to Global Initiative for Asthma (GINA) 2012 guidelines,11 without any recent (8 weeks) changes to their maintenance asthma medication. Participants were either non-smokers, current smokers or past smokers who had quit at least 12 months before inclusion. The main exclusion criteria were a smoking history of >10 packyears, an asthma exacerbation <8 weeks before inclusion, pregnancy or a confirmed diagnosis of chronic obstructive pulmonary disease (COPD). The study is registered on clinicaltrials.gov (NCT02123667). The names of the review boards are included in the online supplemental file.
bmjresp-2024-002316supp001.pdf (248.3KB, pdf)
Participants were characterised at the baseline visit including multiple questionnaires, such as the Asthma Control Questionnaire 6 (ACQ-6),12 Mini Asthma Quality of Life Questionnaire (Mini AQLQ)13 and asthma control test (ACT),14 and lung function tests, such as fractional exhaled nitric oxide (FeNO), body plethysmography, impulse oscillometry (IOS), multiple breath nitrogen washout (MBNW) and prebronchodilator and postbronchodilator spirometry according to American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines.15 Airway hyperresponsiveness (AHR) was tested using a methacholine challenge test in a subset of patients. Blood sample collection was done at baseline and during follow-up. Thoracic CT scans and sputum inductions were performed at baseline at selected sites. After the baseline visit, patients had follow-up phone calls at 3 and 9 months and physical visits at 6 and 12 months. Exacerbations were recorded throughout the study and defined as a deterioration of asthma requiring a systemic course of corticosteroids (≥3 days) and/or hospitalisation and/or emergency room attendance. During inclusion, participants received routine medical care provided by their own healthcare provider. Changes in medication were recorded.
Patient and public involvement
Initiation of the original ATLANTIS study was driven by unmet needs identified by patients. However, for these post hoc analyses, patients were not involved in the study design, recruitment or conduct of the study. Participants were not informed of the results of the post hoc analyses.
Definitions
Persistent airflow limitation (PAL) was defined as the postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) below the lower limit of normal. Early onset asthma was defined as an age of onset <18 years of age. IOS was used to measure resistance and reactance in the airways. It involves three separate measurements of breathing at tidal volume through a mouthpiece with an integrated speaker. The equipment measures the returning sound waves and calculates the resistance and reactance at different anatomical locations in the lung: R5 reflects the total airway resistance, R20 reflects the large airway resistance and R5-20 is thereby the calculated small airway resistance. At the time of writing, no reference equations endorsed by the ERS or ATS were available. We have applied the reference equations proposed by Oostveen et al.16
Statistical analysis
Statistical analyses were done using R (V.4.1.1)17 and RStudio (V.1.3.959).18 Baseline characteristics were stratified by sex using the R package TableOne (V.0.13.2).19 Normality of distribution of data was assessed using histograms and QQ plots. Differences in baseline clinical characteristics, lung function or inflammatory parameters between sexes were tested using a Mann-Whitney U test, t-test or χ2 test as appropriate.
Analysis and visualisation of time to first exacerbation were done using survival (V.3.3-1),20 survminer (V.0.4.9)21 and ggplot2 (V.3.3.6).22 Subjects were censored after their first exacerbation or after their last visit. Cox proportional hazard analysis was performed in a model with age, sex, GINA step 4–5 (yes/no), blood eosinophils and FEV1 % predicted. First, baseline characteristics, blood cell count and pulmonary function variables with p value <0.05 in a univariate Cox regression analysis with exacerbations were selected. Thereafter, we decided on the final Cox model with backward selection, taking collinearity into account. Furthermore, a Poisson regression model was used to analyse the effect of the interaction term between sex and GINA step for the exacerbation rate during follow-up.
Lastly, the sex differences we found made us question whether male and female patients received similar medical treatment at the same asthma severity in terms of lung function, symptoms, exacerbations and AHR. Therefore, we performed a logistic regression for medication (ie, inhaled corticosteroid (ICS), long-acting beta-2 agonist, etc) prescriptions as a dependent variable. The independent variables were sex, different measures for severity of disease and an interaction term of this measure of severity and sex. We chose more subjective (ie, ACQ-6 score and number of exacerbations prior to and during inclusion) and objective (ie, FEV1 % predicted and AHR) parameters of asthma severity.
Results
Patients
In total, 773 patients were included at baseline, of which 450 were female and 323 were male. Baseline characteristics are presented in table 1. Male patients were younger at diagnosis and more likely to have early onset asthma (age of onset <18 years), with a prevalence of 44% in male patients and 35% in female patients (p=0.017). Male patients had a significantly higher number of packyears than female patients (M: 6 vs F: 3 packyears; p<0.001). While mean body mass index (BMI) was not significantly different between male and female patients, female patients were more often in either the normal or obese BMI category and male patients were more often in the overweight category.
Table 1.
Baseline characteristics
| Female patients with asthma | Male patients with asthma | P value | |
| N | 450 | 323 | |
| Age, years (mean (SD)) | 44.86 (13.02) | 43.62 (12.94) | 0.192 |
| Age at diagnosis, years (median (IQR)) | 26.04 (10.29, 41.97) | 22.00 (7.12, 39.55) | 0.028 |
| Age at diagnosis <18 years, n (%) | 158 (35.3) | 142 (44.1) | 0.017 |
| Duration of disease, years (median (IQR)) | 15.66 (4.99, 29.15) | 18.56 (6.54, 29.39) | 0.079 |
| BMI, kg/m2 (mean (SD)) | 27.25 (6.46) | 27.20 (4.97) | 0.902 |
| BMI groups, n (%) | <0.001 | ||
| ≤18 kg/m2 | 8 (1.8) | 0 (0.0) | |
| >18, ≤25 kg/m2 | 197 (43.8) | 110 (34.1) | |
| >25, ≤30 kg/m2 | 117 (26.0) | 151 (46.7) | |
| >30, ≤40 kg/m2 | 107 (23.8) | 52 (16.1) | |
| >40 kg/m2 | 21 (4.7) | 10 (3.1) | |
| Smoking status, n (%) | 0.184 | ||
| Current smoker | 15 (3.3) | 12 (3.7) | |
| Past smoker | 81 (18.0) | 75 (23.2) | |
| Never smoker | 354 (78.7) | 236 (73.1) | |
| Ever smoker, n (%) | 0.085 | ||
| Current or past smoker | 96 (21.3) | 87 (26.9) | |
| Never smoker | 354 (78.7) | 236 (73.1) | |
| Packyears past and current smokers (median (IQR)) | 3.00 (1.15, 5.12) | 6.00 (3.50, 8.85) | <0.001 |
| Positive IgE screening for inhaled allergens, n (%) | 265 (78.6) | 189 (83.3) | 0.211 |
| ACQ-6 score (median (IQR)) | 0.83 (0.33, 1.67) | 0.66 (0.16, 1.20) | <0.001 |
| Mini AQLQ score (median (IQR)) | 5.40 (4.47, 6.20) | 5.93 (5.07, 6.50) | <0.001 |
| ACT score (median (IQR)) | 20.00 (17.00, 23.00) | 22.00 (20.00, 24.00) | <0.001 |
| MMAS score (median (IQR)) | 6.00 (4.50, 7.00) | 5.75 (4.00, 7.00) | 0.044 |
| GINA treatment step, n (%) | 0.042 | ||
| 1 | 72 (16.0) | 63 (19.5) | |
| 2 | 41 (9.1) | 44 (13.6) | |
| 3 | 117 (26.0) | 90 (27.9) | |
| 4 | 188 (41.8) | 112 (34.7) | |
| 5 | 32 (7.1) | 14 (4.3) | |
| Current use of | |||
| ICS, n (%) | 368 (81.8) | 262 (81.1) | 0.888 |
| LABA, n (%) | 311 (69.1) | 212 (65.6) | 0.347 |
| LAMA, n (%) | 20 (4.4) | 9 (2.8) | 0.315 |
| Montelukast, n (%) | 97 (21.6) | 47 (14.6) | 0.018 |
| Biologic, n (%) | 20 (4.4) | 12 (3.7) | 0.75 |
| Systemic corticosteriods, n (%) | 16 (3.6) | 6 (1.9) | 0.238 |
| Daily ICS dose in subjects on ICS (beclometasone equivalent), µg (mean (SD)) | 831.67 (547.31) | 842.60 (705.17) | 0.832 |
| Daily ICS dose including patients not on (daily) ICS (median (IQR)) | 500.00 (362.50, 1000.00) | 500.00 (200.00, 1000.00) | 0.187 |
Univariable analyses of baseline characteristics of subjects with asthma in the ATLANTIS study, stratified by sex.
ACQ-6, Asthma Control Questionnaire 6; ACT, asthma control test; AQLQ, Asthma Quality of Life Questionnaire; BMI, body mass index; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; LABA, long-acting beta-2 agonist; LAMA, long-acting muscarinic antagonists; MMAS, Morisky Medication Adherence Scale.
At baseline, female patients reported significantly worse asthma control as reflected by higher ACQ scores than their male counterparts (F: 0.83 vs M: 0.66 points; p<0.001) (table 1). This was also the case for the ACT score, which was lower in female patients (p<0.001). Quality of life, as measured by the Mini AQLQ, was significantly lower in female patients with asthma (p<0.001).
Lung function
Postbronchodilator FEV1 % predicted and reversibility in FEV1 were not significantly different between sexes (table 2). Male patients had a lower prebronchodilator and postbronchodilator FEV1/FVC ratio (postbronchodilator: F: 0.76 (91.95 % predicted) vs M: 0.71 (88.33 % predicted); p<0.001) and more frequently had PAL. The results for Z-scores and % predicted for spirometry yielded similar outcomes, with Z-scores included in the online supplemental table S1. The total lung capacity (TLC), residual volume (RV) and RV/TLC ratio % predicted were not significantly different between male and female patients. This was also the case for the forced expiratory flow at 50% and 25%–75% of FVC % predicted. Female patients more often had moderate and severe rather than mild AHR (p=0.017). FeNO levels were higher in male patients, trending towards significance (F: 23 vs M: 26 parts per billion; p=0.069). Male and female patients with asthma were similar in their MBNW outcomes; both Scond and Sacin did not differ. In contrast, all unadjusted IOS parameters were significantly different between male and female patients with asthma at baseline. Resistance at 5 Hz (R5), 20 Hz (R20) and between 5 and 20 Hz (R5-R20), as well as the area under the curve of reactance between 5 Hz and resonant frequency (AX) were significantly higher in female patients, indicating higher resistance in both the small and large airways. The reactance at 5 Hz (X5) was significantly more negative in female patients (p<0.001). We included reference equations by Oostveen et al,16 which found similar R5, AX, X5 % predicted but a higher % predicted of R20 in male patients with asthma.
Table 2.
Lung function
| Female patients with asthma | Male patients with asthma | P value | |
| N | 450 | 323 | |
| Spirometry | |||
| Prebronchodilator FEV1 % predicted (mean (SD)) | 82.09 (17.63) | 79.90 (17.67) | 0.092 |
| FEV1 reversibility, % change from baseline (median (IQR)) | 8.47 (4.58, 16.24) | 9.84 (4.73, 17.31) | 0.178 |
| Postbronchodilator FEV1 % predicted (mean (SD)) | 89.88 (15.76) | 88.64 (16.24) | 0.295 |
| Postbronchodilator FVC % predicted (mean (SD)) | 98.01 (15.00) | 100.90 (14.37) | 0.008 |
| Prebronchodilator FEV1/FVC (mean (SD)) | 0.71 (0.11) | 0.67 (0.11) | <0.001 |
| Prebronchodilator FEV1/FVC % predicted (mean (SD)) | 86.31 (12.10) | 82.71 (12.57) | <0.001 |
| Postbronchodilator FEV1/FVC (mean (SD)) | 0.76 (0.10) | 0.71 (0.11) | <0.001 |
| Postbronchodilator FEV1/FVC % predicted (mean (SD)) | 91.95 (11.38) | 88.33 (12.42) | <0.001 |
| Postbronchodilator FEF25-75 % predicted (mean (SD)) | 67.21 (32.17) | 64.97 (29.06) | 0.338 |
| Postbronchodilator FEF50 % predicted (mean (SD)) | 82.54 (32.62) | 79.81 (30.88) | 0.268 |
| PAL (%) | 122 (27.11) | 126 (39.00) | <0.001 |
| Body plethysmography | |||
| TLC, % of predicted (mean (SD)) | 105.41 (15.47) | 103.40 (11.90) | 0.065 |
| RV, % predicted (mean (SD)) | 110.96 (28.78) | 110.78 (27.77) | 0.938 |
| RV/TLC, (mean (SD)) | 0.35 (0.09) | 0.31 (0.08) | <0.001 |
| RV/TLC, % predicted (mean (SD)) | 101.43 (23.69) | 98.79 (24.23) | 0.153 |
| Airway hyperresponsiveness | |||
| Airway hyperresponsiveness category (%) | 0.017 | ||
| Very mild (PC20 ≥4 and <16 mg/mL, PD20 ≥0.5 and <2 mg) | 70 (21.3) | 76 (33.2) | |
| Mild (PC20 ≥1 and <4 mg/mL, PD20 ≥0.13 and <0.5 mg) | 105 (31.9) | 62 (27.1) | |
| Moderate (PC20 ≥0.25 and <1 mg/mL, PD20 ≥0.03 and <0.13 mg) | 83 (25.2) | 52 (22.7) | |
| Severe (PC20 <0.25 mg/mL, PD20 <0.03 mg) | 71 (21.6) | 39 (17.0) | |
| Impulse oscillometry | |||
| X5, kPa/L/s (median (IQR)) | −0.12 (−0.17,–0.09) | −0.08 (−0.12,–0.06) | <0.001 |
| X5 % predicted* (mean (SD)) | 103.55 (42.16) | 103.62 (52.10) | 0.984 |
| AX, Hz*kPa/L/s (median (IQR)) | 0.41 (0.22, 0.86) | 0.23 (0.12, 0.54) | <0.001 |
| AX % predicted* (mean (SD)) | 178.24 (173.01) | 214.27 (291.26) | 0.077 |
| R5, kPa/L/s (mean (SD)) | 0.42 (0.14) | 0.35 (0.14) | <0.001 |
| R5 % predicted* (mean (SD)) | 125.16 (38.24) | 131.51 (48.08) | 0.065 |
| R20, kPa/L/s (mean (SD)) | 0.35 (0.09) | 0.30 (0.08) | <0.001 |
| R20 % predicted* (mean (SD)) | 103.02 (24.98) | 231.60 (65.75) | <0.001 |
| R5-20 kPa/L/s (median (IQR)) | 0.06 (0.02, 0.11) | 0.04 (0.02, 0.08) | 0.002 |
| Multiple breath nitrogen washout and FeNO | |||
| Scond, 1/L (median (IQR)) | 0.03 (0.02, 0.05) | 0.03 (0.02, 0.04) | 0.138 |
| Scond, % predicted (median (IQR)) | 86.73 (44.93, 136.10) | 75.64 (45.42, 114.21) | 0.183 |
| Sacin, 1/L (median (IQR)) | 0.10 (0.06, 0.15) | 0.10 (0.06, 0.15) | 0.33 |
| Sacin, % predicted (median (IQR)) | 121.44 (73.11, 172.51) | 106.63 (66.84, 143.50) | 0.16 |
| FeNO, ppb (median (IQR)) | 23.00 (15.00, 36.50) | 26.00 (16.75, 40.00) | 0.069 |
Univariable analyses of lung function results at baseline of subjects with asthma in the ATLANTIS study, stratified by sex.
PAL was defined as postbronchodilator FEV1/FVC lower than the lower limit of normal.
*Calculated using the IOS reference values proposed by Oostveen et al. 16
AX, area of reactance; FEF50, forced expiratory flow at 50% of FVC; FEF25-75, forced expiratory flow at 25%–75% of FVC; FeNO, ractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IOS, impulse oscillometry; PAL, persistent airflow limitation; R5, resistance at 5 Hz; R20, resistance at 20 Hz; R5-20, resistance at 5 Hz–resistance at 20 Hz; RV, residual volume; Sacin, ventilation homogeneity of the acinar zone of the lungs corrected for tidal volume; Scond, ventilation heterogeneity in the conductive zone of the lungs corrected for tidal volume; TLC, total lung capacity; X5, reactance at Hz.
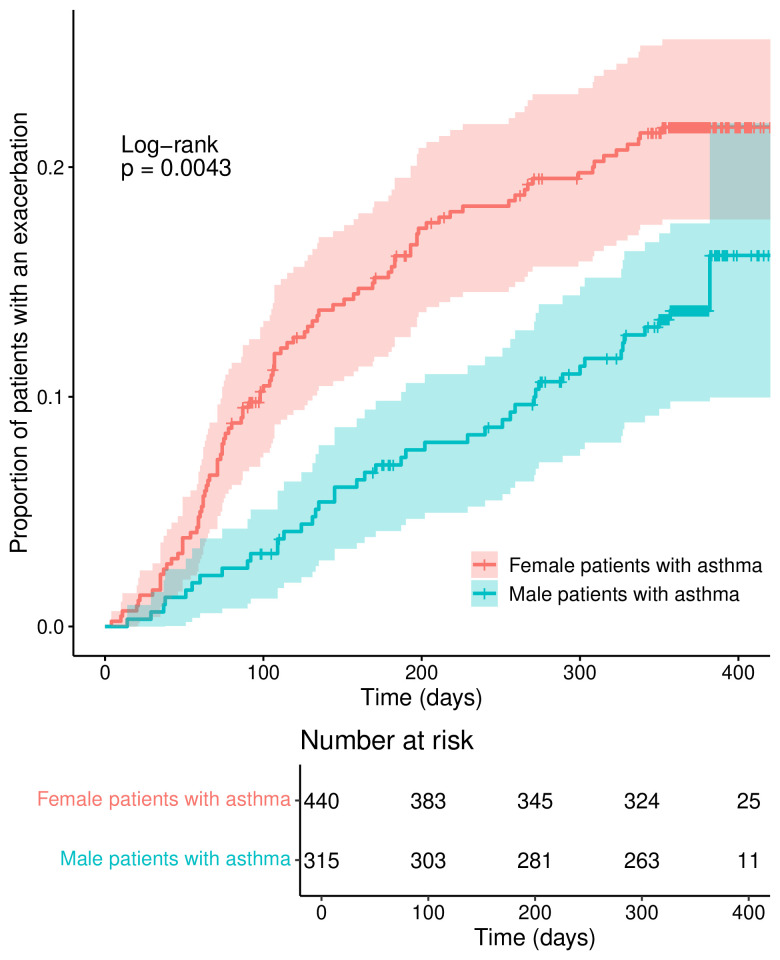
Exacerbations
755 patients were included in the analysis of the time to first exacerbation. A total of 136 first exacerbations were included in the analysis, with a median follow-up duration (to end of follow-up or censoring) of 365 days (range 0–564). Proportionally, fewer male patients experienced exacerbations during the 1-year follow-up period, as illustrated in figure 1. In a multivariable Cox regression model for exacerbations, as depicted in table 3, male sex was still associated with a lower exacerbation risk (HR 0.61 (95% CI 0.42 to 0.88, p=0.008)) adjusted for age, blood eosinophils at baseline, GINA steps 4 and 5 and FEV1 % predicted. For reference, we have added the exacerbation rate (0, 1, 2+ per year during follow-up) per GINA step by sex to the supplement (online supplemental table S2).
Figure 1.
Exacerbations during follow-up, stratified by sex.
Table 3.
Cox regression analysis for exacerbations
| HR (95% CI) | P value | |
| Male sex | 0.61 (0.42 to 0.88) | 0.008 |
| Age, years | 1.01 (1.00 to 1.03) | 0.044 |
| GINA steps 4–5 | 2.26 (1.52 to 3.37) | <0.001 |
| Blood eosinophil counts | 3.29 (1.95 to 5.54) | <0.001 |
| FEV1 % predicted (post-BD) | 0.98 (0.97 to 0.99) | 0.001 |
BD, bronchodilator; FEV1, forced expiratory volume in 1 s; GINA, Global Initiative for Asthma.
CT scan parameters
304 patients had a CT scan at baseline (table 4). The wall area divided by the total area (WA%) was not significantly different between sexes (F: 63.2% vs M: 62.7%; p=0.226). The voxel index at −950 Hounsfield units (VI 950) was significantly higher in male patients (F: 2.73% vs M: 5.38%; p<0.001).
Table 4.
CT scan parameters
| Female patients with asthma | Male patients with asthma | P value | |
| N | 184 | 120 | |
| CT scan-derived parameters | |||
| Median lumen area, mm2 (mean (SD)) | 18.15 (4.27) | 22.11 (5.53) | <0.001 |
| Median wall area, mm2 (mean (SD)) | 31.11 (5.08) | 36.91 (6.46) | <0.001 |
| Median total area, mm2 (mean (SD)) | 49.59 (8.61) | 59.37 (11.14) | <0.001 |
| WA% (wall area/total area), % (mean (SD)) | 63.22 (3.36) | 62.73 (3.63) | 0.226 |
| Pi10, mm (mean (SD)) | 7.14 (0.90) | 7.29 (1.06) | 0.195 |
| VI 856, % (median (IQR)) | 7.83 (2.49, 18.18) | 8.40 (2.58, 20.64) | 0.897 |
| VI 950, % (median (IQR)) | 2.73 (1.11, 6.06) | 5.38 (2.27, 10.19) | <0.001 |
CT scan parameters at baseline of subjects with asthma in the ATLANTIS study, stratified by sex.
Pi10, 10 mm internal luminal perimeter; VI 856, voxel index at −856 Hounsfield units; VI 950, voxel index at −950 Hounsfield units.
Inflammatory cells in blood and sputum
Blood eosinophil counts did not differ between male and female patients (F: 0.22×109/L vs M: 0.24×109/L; p=0.088) (table 5). Sputum eosinophil percentages were similar in male and female patients (F: 0.5% vs M: 0.4%; p=0.433). Blood neutrophils were significantly higher in female patients (p=0.014), but this was not the case for sputum neutrophils. Lastly, blood monocytes were significantly higher in male patients (p<0.001).
Table 5.
Inflammatory cell counts in blood and proportions in sputum at baseline of subjects with asthma in the ATLANTIS study, stratified by sex
| Female patients with asthma | Male patients with asthma | P value | |
| N | 450 | 323 | |
| Blood cell counts | |||
| Blood cell counts available, n | 450 (100%) | 317 (98.1%) | |
| Eosinophils 109/L (median (IQR)) | 0.22 (0.12, 0.37) | 0.24 (0.16, 0.38) | 0.088 |
| Neutrophils 109/L (median (IQR)) | 3.80 (3.04, 4.89) | 3.56 (2.86, 4.41) | 0.014 |
| Monocytes 109/L (median (IQR)) | 0.44 (0.36, 0.54) | 0.50 (0.40, 0.60) | <0.001 |
| Sputum cell proportions | |||
| Sputum cell proportions available, n (%) | 116 (25.8%) | 112 (34.7%) | |
| Bronchial cells % (median (IQR)) | 1.50 (0.68, 4.03) | 1.85 (1.00, 3.95) | 0.165 |
| Lymphocytes, % (median (IQR)) | 0.70 (0.30, 1.52) | 0.50 (0.30, 1.22) | 0.230 |
| Eosinophils, % (median (IQR)) | 0.50 (0.00, 1.93) | 0.40 (0.10, 4.00) | 0.433 |
| Macrophages, % (median (IQR)) | 38.30 (19.02, 60.80) | 33.85 (18.00, 57.12) | 0.669 |
| Neutrophils, % (median (IQR)) | 50.50 (26.17, 71.50) | 52.40 (31.18, 70.43) | 0.968 |
Prescribed medication
When stratifying for sex, we found the distribution of GINA steps to be skewed towards higher GINA steps in female patients (p=0.042). The prescription and dose of daily ICS were similar between male and female patients. Female patients were more likely to have been prescribed montelukast (F: 21.6% vs M: 14.6%; p=0.018). Female patients scored higher on Morisky Medication Adherence Scale score, reflecting a higher medication adherence.
Furthermore, we assessed whether differences in asthma characteristics between male and female patients, as detailed above, were attributable to variations in their treatment regimens. We also investigated whether male and female patients were treated similarly despite having similar disease severity. This was done by assessing the prescribed medication by sex, an indicator of asthma severity and the interaction between sex and the indicator of asthma severity (online supplemental tables S3-S7). FEV1 % predicted, ACQ6 score, AHR and exacerbations were used as indicators for severity of disease. The only significant finding was that female patients were more likely to use montelukast than male patients when they had milder disease with regard to FEV1 % predicted. In general, male and female patients were treated similarly at baseline when adjusting for severity of disease during inclusion, and differences in asthma characteristics between the sexes could not be explained by difference in prescribed medication.
Discussion
We show that female patients with asthma exhibit poorer disease control, a higher risk of exacerbations and greater airway resistance, as evidenced by worse impulse oscillometry results. In contrast, male patients with asthma suffer from more severe airflow obstruction and more frequently experience PAL. The latter finding was previously described in a separate publication.23
IOS has previously been suggested to be more suitable for detecting SAD than spirometry.10 24 We found that female patients had worse results for all uncorrected IOS parameters, while FEV1 % predicted was similar. These findings of a higher resistance in the central and peripheral airways may reflect more large airways dysfunction and SAD and IOS might be more sensitive than FEV1. However, it should be noted that proper reference values are currently lacking.25 Alternatively, it could be speculated that our results are merely based on an anatomical difference, that is, dysanapsis. Dysanapsis is a mismatch between the size of the airway lumen in relation to the lung parenchyma. This could explain the differences between male and female patients with asthma rather than a clinically significantly higher level of SAD. This would be in accordance with literature on dysanapsis, which suggests that adult female patients have higher small airways resistance than male patients.26 We have applied reference equations by Oostveen et al,16 which, in contrast to unadjusted IOS values, suggest a higher peripheral airway resistance in male patients (R20) while other IOS parameters did not differ (R5, AX and X5). These results need to be interpreted with caution as these reference equations have not been widely accepted. In 2020, the ERS task force on technical recommendations for oscillometry acknowledged that large studies are necessary to determine normal values.25 Clearly, sex is one of the factors that should be taken into account.
Apart from a significantly higher use of leukotriene modifiers in female patients, we found no differences in asthma medication prescription between male and female patients. Nevertheless, female patients reported more symptoms and were classified in higher GINA treatment steps and male patients more frequently had PAL and had worse airflow obstruction (FEV1/FVC). Therefore, we questioned whether male and female patients in the same class of asthma severity received similar medical treatment or whether either one of the sexes was undertreated. To determine whether this was the case, we performed a subgroup analysis to assess whether male and female patients with asthma classified by quartiles of disease severity (ie, FEV1, AHR) and subjective outcomes (ie, ACQ-6, annual exacerbation rate prior to inclusion/during follow-up) received similar medical treatment. Overall, these analyses did not show major differences in pattern of medication prescriptions by asthma severity between male and female patients with asthma.
We found significantly higher blood neutrophil counts in female patients with asthma. This was an expected result as neutrophilic inflammation is predominantly present in patients with late-onset and severe asthma, who are more frequently female.27 Possibly, the higher blood neutrophil counts are explained by the higher prevalence of obesity in female patients. Previous studies show that blood neutrophilia is associated with increased exacerbation risk and lower quality of life.28 In addition, a significantly higher number of female patients in our study were obese, which is also known to be associated with neutrophilic infiltration in the airways.29
Male patients with asthma tended to have higher blood eosinophil counts and FeNO levels, both indicators of type 2 inflammation which is often predominant in patients with early onset asthma.7 30 Blood eosinophil counts were also higher in men in the general population, so this finding may not be specific for asthma.31 We also found higher blood monocyte counts in male patients. The role of blood monocytes in asthma is less well elucidated. Although absolute monocyte counts are infrequently elevated in asthma, it has now been described that there are three subsets of blood monocytes (ie, classical C14++CD16−, non-classical CD14+CD16+, intermediate CD14++CD16+) of which higher proportions of intermediate monocytes have been found in severe patients with asthma.32–34 In the current study, we did not perform detailed flow cytometry to identify monocyte subsets and therefore we cannot make a definite conclusion about the monocyte subsets in our study. Interestingly, all aforementioned differences in blood leucocyte counts could not be replicated in sputum.
Analysis of CT scans showed that male and female patients with asthma have similar thickness of the airway walls as reflected by WA% and Pi10 (ie, the average wall thickness for a hypothetical airway of 10 mm lumen perimeter). Both the airway lumens as well as the airway walls, as reflected by median wall and lumen area, were significantly smaller in female patients with asthma, but this is logically explained by the fact that women overall have smaller lungs and therefore smaller airways. Quantitative CT scanning has made a lot of progress in the past decades, but bronchial wall parameters might need reference values, that also take sex into account, something that has recently been explored in a systematic review and meta-analysis by Dudurych et al.35 Lastly, VI 950 was significantly higher in male patients with asthma, indicating more emphysema-like lung on CT. This may be a physiological phenomenon, as the percentage of emphysema-like lung was relatively low and Hoffman et al previously showed that respiratory-healthy male never-smokers have a higher percentage of emphysema-like lung than female never-smokers.36 However, male current-smokers and past-smokers in ATLANTIS had a significantly higher number of packyears, which was also associated with a higher VI 950. Thus, some degree of overlap with COPD in these subjects cannot be excluded even though all subjects had a confirmed diagnosis if asthma and a smoking history of >10 packyears was an exclusion criterium in the ATLANTIS study.
Our findings of a significantly higher risk of exacerbations and more severe AHR in female patients with asthma are in accordance with literature.6 37 38 Sex hormones might play a role in both the increased exacerbation risk as well as the more severe AHR in female patients.7 It has, for example, been shown that AHR is increased during the luteal phase of the menstrual cycle and that the risk of exacerbations increases during pregnancy.39 40 In contrast, androgens, such as testosterone, may actually reduce asthma incidence and symptoms.7 However, a lot is still unknown about the exact mechanisms by which sex hormones influence asthma pathogenesis and more research is needed in this area. Lastly, contrary to previous findings, current smoking was not a significant predictor of exacerbations in our study, likely due to the small number of current smokers in this cohort.41
We found multiple differences between female and male patients in key features of asthma, some of which can be integrated. The clearest interplay between our findings are mechanical in nature; a smaller airway lumen size in female patients can increase both airway resistance as well as induce more severe AHR. Furthermore, there is a known interplay between sex hormones, differences in body composition, airway inflammation and exacerbations. The exact mechanisms of the latter interplay are beyond the scope of this study. Lastly and most hypothetically, an interplay can be observed between the development of asthma earlier in life in male patients, a more dominant type 2 inflammation, lower therapy adherence and impaired lung function with ageing with a higher prevalence of PAL.
A strength of this study is the fact that it covers a large cohort of patients with asthma, from a varied range of countries, with extensive clinical characterisation including small and large airway function and across all asthma severities. A limitation of our study was the fact that this cohort was not specifically designed to unravel sex differences in asthma and therefore the post hoc analyses performed were exploratory.
Interpretation
We find that in asthma, female patients experience worse disease control, have a higher risk of exacerbations and potentially have more small airways and less large airways dysfunction compared with male patients with asthma, who have more severe airflow obstruction and a higher prevalence of PAL. These findings are significant because they highlight the potential importance of precision treatment of patients with asthma, possibly taking sex into account. We hope these results increase awareness among clinicians.
Footnotes
TMK and SM contributed equally.
Presented at: Oral presentation: ‘Sex differences in asthma control, lung function and exacerbations: the ATLANTIS study’ at ATS 2023, session titled ‘All that wheezes: translational studies in asthma’ held on 22 May 2023.
Contributors: SM, TMK, MvdB, MCN and HAMK did the data curation, formal analysis and writing of the original draft. SM, TMK and MvdB had access to and verified the data, were responsible for the decision to submit. MK, SS, LMF, KFR, AP, CB, DS, TvdM and MvdB were involved in study design and data collection of ATLANTIS. All authors had access to the raw data, MvdB is the guarantor. Contributed to interpretation of results and reviewing and editing of this manuscript.
Funding: The ATLANTIS study was funded by Chiesi Farmaceutici. The funder of the study contributed to the data collection, but had no role in data analysis, data interpretation or writing of the report. The submitted work was co-financed by the Dutch Ministry of Economic Affairs and Climate Policy by means of the public-private partnership programme and University of Groningen/University Medical Centre Groningen.
Competing interests: SM reports a travel grant from GSK outside of the submitted work. MK reports grants paid to her institution by National Institutes of Health, American Lung Association, Synairgen, Janssen, AstraZeneca and Sanofi; personal consulting fees from AstraZeneca, Sanofi, Chiesi, GSK, Kinaset, Genentech; presentation fees from Chiesi; support for attending the European Respiratory Society annual conference; one issued and two filed patents by RaeSedo; participation in data safety and monitoring board at ALung and past membership of the National Heart, Lung and Blood Advisory Council; current membership of Association of Professor of Medicine; equity ownership in RaeSedo and is a section editor for UptoDate. SS reports consulting fees from CSL Behring, AstraZeneca, GSK, Areteia Therapeutics, Novartis; speaker fees for presenting ATLANTIS data by Chiesi; support from ERS for attending ERS science council meetings and membership of the ALTANTIS scientific steering group. LMF reports being a consultant for Chiesi; consulting fees by Chiesi, GSK, AstraZeneca, Novartis, Alfasigma and participation in a board with Novartis and Chiesi. KFR reports presenter fees by AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, Sanofi, Regeneron, GSK, Berlin Chemie, Roche Pharma; participation in data safety and monitoring boards for AstraZeneca, Boehringer Ingelheim, Sanofi and Regeneron; leadership of German Center for Lung Research (DZL), German Chest Society (DGP) and American Thoracic Society. AP reports grants or contracts paid to institution by Chiesi, AstraZeneca, GSK, Sanofi, Agenzia Italiana del farmaco (AIFA); consulting fees from Chiesi, AstraZeneca, GSK, Novartis, Sanofi, Avillion, Elpen Pharmaceuticals; speaker fees from Chiesi, AstraZeneca, GSK, Menarini, Novartis, Zambon, Mundipharma, Sanofi, Edmond Pharma, Iqvia, Avillion, Elpen Pharmaceuticals, membership of advisory board for Chiesi, AstraZeneca, GSK, MSD, Novartis, Sanofi, Iqvia, Avillion, Elpen Pharmaceuticals. CEB reports grants paid to institution by GSK, AstraZeneca, Sanofi, Regeneron, Roche, Genentech, Boehringer Ingelheim, Chiesi, Novartis, Mologic, Areteia and consulting fees paid to institution by GSK, AstraZeneca, Sanofi, Regeneron, Roche, Genentech, Boehringer Ingelheim, Chiesi, Novartis, Mologic, Areteia. DS reports consulting fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, EpiEndo, Genentech, GSK, Glenmark, Gossamer Bio, Kinaset Therapeutics, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Synairgen, Teva, Theravance Biopharma, Verona Pharma. TvdM reports presenter fees paid to institution by Chiesi and GSK. MCN reports unrestricted research grants paid to institution: European Union’s H2020 Research and Innovation Programme under grant agreement, the Ministry of Economic Affairs and Climate Policy (the Netherlands) through a PPP allowance from the Top Sector Life Sciences & Health, GSK, Stevenage (UK), Netherlands Lung Foundation, the Chan Zuckerberg Initiative, The Stichting Astmabestrijding; support of travel costs by the Belgian Respiratory Society and unpaid leadership of the Lung Bionetwork of the Human Cell Atlas consortium. HAMK reports that his institution has received fees per patient for recruitment in trials from GSK, Novartis and FLUIDDA, grants for investigator-initiated studies from GSK, Novartis and Boerhinger. Additionally, his institution has received consultancy fees from Novartis, AstraZeneca, Boehringer Ingelheim, Chiesi and GSK. MvdB reports grants paid to the University from GSK, Chiesi, Teva, AstraZeneca, Genentech, outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The following review boards or institutions approved the protocol: Comitato Etico dell’Azienda Ospedaliera V. Cervello di Palermo (Palermo 2), Comitato Etico della Provincia di Ferrara, Comitato Etico dell’Azienda Ospedaliero-Universitaria di Parma, Comitato Etico di Area Vasta Nord-Ovest per la sperimentazione clinica, Comitato Etico per la Sperimentazione Clinica delle Province di Verona e Rovigo, Comitato Etico dell’Azienda Ospedaliera dei Colli, Comitato Etico della Fondazione Salvatore Maugeri, Comitato Etico dell’A.O.U Ospedali Riuniti di Foggia, NHS Health Research Authority NRES Committee North West—Greater Manchester South (for all UK sites), UMCG Medical Ethical Review Committee, Forest Medical School, Jeroen Bosch Ziekenhuis, Martini Ziekenhuis, Comité Ético de Investigación Clínica, Ethikkommission bei der Ärztekammer Schleswig-Holstein, Ethik-Kommission der Medizinischen Hochschule Hannover, Ethik-Kommission bei der Sächsischen Landesärztekammer, Institutional review board for human investigation (Cleveland, Ohio), Western Institutional Review Board, Institutional Review Board for Baylor College of Medicine and Affiliated Hospitals, University of Arizona Institutional Review Board, Biomedical Research Ethics Board of the McGill University Health Center, Comitê de Ética em Pesquisa com Seres Humanos da Universidade Federal de Santa Catarina, Comissão de Ética para Análise de Projetos de Pesquisa—CAPPesq do HCFMUSP, Coordinator EC—Comitê de Ética em Pesquisa da Fundação ABC—FMABC, Medical Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. Participants gave informed consent to participate in the study before taking part.
References
- 1. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet 2018;391:783–800. 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- 3. de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev 2013;22:44–52. 10.1183/09059180.00007112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schatz M, Camargo CA. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol 2003;91:553–8. 10.1016/S1081-1206(10)61533-5 [DOI] [PubMed] [Google Scholar]
- 5. Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep 2015;15:28. 10.1007/s11882-015-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zein JG, Denson JL, Wechsler ME. Asthma over the adult life course: gender and hormonal influences. Clin Chest Med 2019;40:149–61. 10.1016/j.ccm.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 7. Chowdhury NU, Guntur VP, Newcomb DC, et al. Sex and gender in asthma. Eur Respir Rev 2021;30:210067. 10.1183/16000617.0067-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jenkins CR, Boulet L-P, Lavoie KL, et al. Personalized treatment of asthma: the importance of sex and gender differences. J Allergy Clin Immunol Pract 2022;10:963–71. 10.1016/j.jaip.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 9. Pignataro FS, Bonini M, Forgione A, et al. Asthma and gender: the female lung. Pharmacol Res 2017;119:384–90. 10.1016/j.phrs.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 10. Postma DS, Brightling C, Baldi S, et al. Exploring the relevance and extent of small Airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med 2019;7:402–16. 10.1016/S2213-2600(19)30049-9 [DOI] [PubMed] [Google Scholar]
- 11. Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention. 2012.
- 12. Juniper EF, O’Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902–7. 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- 13. Juniper EF, Guyatt GH, Ferrie PJ, et al. Measuring quality of life in asthma. Am Rev Respir Dis 1993;147:832–8. 10.1164/ajrccm/147.4.832 [DOI] [PubMed] [Google Scholar]
- 14. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59–65. 10.1016/j.jaci.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 15. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022;60:2101499. 10.1183/13993003.01499-2021 [DOI] [PubMed] [Google Scholar]
- 16. Oostveen E, Boda K, van der Grinten CPM, et al. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J 2013;42:1513–23. 10.1183/09031936.00126212 [DOI] [PubMed] [Google Scholar]
- 17. RCoreTeam . A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria, 2021. [Google Scholar]
- 18. RStudioTeam . RStudio: Integrated Development Environment for R. Boston, MA: RStudio, PBC, 2020. [Google Scholar]
- 19. Yoshida K, Bartel A. “Tableone: create 'table 1' to describe baseline characteristics with or without propensity score weights. R package version 0.13.0”. 2021.
- 20. Therneau T. A package for survival analysis in R. 2021.
- 21. Kassambra A, Kosinski M, Biecek P. “Survminer: drawing survival curves using 'Ggplot2'. R package version 0.4.9”. 2021.
- 22. Wickham H. Ggplot2: Elegant graphics for data analysis. Cham: Springer-Verlag New York, 2016. [Google Scholar]
- 23. Kole TM, Vanden Berghe E, Kraft M, et al. Predictors and associations of the persistent airflow limitation phenotype in asthma: a post-hoc analysis of the ATLANTIS study. Lancet Respir Med 2023;11:55–64. 10.1016/S2213-2600(22)00185-0 [DOI] [PubMed] [Google Scholar]
- 24. Cottini M, Licini A, Lombardi C, et al. Prevalence and features of IOS-defined small airway disease across asthma severities. Respir Med 2021;176:106243. 10.1016/j.rmed.2020.106243 [DOI] [PubMed] [Google Scholar]
- 25. King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J 2020;55:1900753. 10.1183/13993003.00753-2019 [DOI] [PubMed] [Google Scholar]
- 26. Dominelli PB, Molgat-Seon Y, Bingham D, et al. Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol (1985) 2015;119:1105–13. 10.1152/japplphysiol.00409.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015;16:45–56. 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 28. Nadif R, Siroux V, Boudier A, et al. Blood granulocyte patterns as predictors of asthma phenotypes in adults from the EGEA study. Eur Respir J 2016;48:1040–51. 10.1183/13993003.00336-2016 [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Wan R, Hu C. Leptin/obR signaling exacerbates obesity-related neutrophilic airway inflammation through inflammatory M1 Macrophages. Mol Med 2023;29:100. 10.1186/s10020-023-00702-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turrin M, Rizzo M, Bonato M, et al. Differences between early- and late-onset asthma: role of comorbidities in symptom control. J Allergy Clin Immunol Pract 2022;10:3196–203. 10.1016/j.jaip.2022.08.007 [DOI] [PubMed] [Google Scholar]
- 31. Hartl S, Breyer M-K, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J 2020;55:1901874. 10.1183/13993003.01874-2019 [DOI] [PubMed] [Google Scholar]
- 32. Niessen NM, Baines KJ, Simpson JL, et al. Neutrophilic asthma features increased airway classical monocytes. Clin Exp Allergy 2021;51:305–17. 10.1111/cea.13811 [DOI] [PubMed] [Google Scholar]
- 33. Shrestha Palikhe N, Nahirney D, Laratta C, et al. Increased protease-activated receptor-2 (PAR-2) expression on CD14++CD16+ peripheral blood monocytes of patients with severe asthma. PLoS One 2015;10:e0144500. 10.1371/journal.pone.0144500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palikhe NS, Gandhi VD, Wu Y, et al. Peripheral blood intermediate monocyte protease-activated receptor-2 expression increases during asthma exacerbations and after inhalation allergen challenge. Ann Allergy Asthma Immunol 2021;127:249–56. 10.1016/j.anai.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 35. Dudurych I, Muiser S, McVeigh N, et al. Bronchial wall parameters on CT in healthy never-smoking, smoking, COPD, and asthma populations: a systematic review and meta-analysis. Eur Radiol 2022;32:5308–18. 10.1007/s00330-022-08600-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoffman EA, Ahmed FS, Baumhauer H, et al. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc 2014;11:898–907. 10.1513/AnnalsATS.201310-364OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Foresi A, Chetta A, Pelucchi A, et al. Bronchial responsiveness to inhaled propranolol in asthmatic children and adults. Eur Respir J 1993;6:181–8. [PubMed] [Google Scholar]
- 38. Leynaert B, Bousquet J, Henry C, et al. Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am J Respir Crit Care Med 1997;156:1413–20. 10.1164/ajrccm.156.5.9701060 [DOI] [PubMed] [Google Scholar]
- 39. Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol 2003;112:283–8. 10.1067/mai.2003.1516 [DOI] [PubMed] [Google Scholar]
- 40. Robijn AL, Bokern MP, Jensen ME, et al. Risk factors for asthma exacerbations during pregnancy: a systematic review and meta-analysis. Eur Respir Rev 2022;31:220039. 10.1183/16000617.0039-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blakey JD, Price DB, Pizzichini E, et al. Identifying risk of future asthma attacks using UK medical record data: a respiratory effectiveness group initiative. J Allergy Clin Immunol Pract 2017;5:1015–24. 10.1016/j.jaip.2016.11.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2024-002316supp001.pdf (248.3KB, pdf)
Data Availability Statement
Data are available on reasonable request.