Abstract
Cancer immunotherapy has flourished over the last 10–15 years, transforming the practice of oncology and providing long-term clinical benefit to some patients. During this time, three distinct classes of immune checkpoint inhibitors, chimeric antigen receptor-T cell therapies specific for two targets, and two distinct classes of bispecific T cell engagers, a vaccine, and an oncolytic virus have joined cytokines as a standard of cancer care. At the same time, scientific progress has delivered vast amounts of new knowledge. For example, advances in technologies such as single-cell sequencing and spatial transcriptomics have provided deep insights into the immunobiology of the tumor microenvironment. With this rapid clinical and scientific progress, the field of cancer immunotherapy is currently at a critical inflection point, with potential for exponential growth over the next decade. Recognizing this, the Society for Immunotherapy of Cancer convened a diverse group of experts in cancer immunotherapy representing academia, the pharmaceutical and biotechnology industries, patient advocacy, and the regulatory community to identify current opportunities and challenges with the goal of prioritizing areas with the highest potential for clinical impact. The consensus group identified seven high-priority areas of current opportunity for the field: mechanisms of antitumor activity and toxicity; mechanisms of drug resistance; biomarkers and biospecimens; unique aspects of novel therapeutics; host and environmental interactions; premalignant immunity, immune interception, and immunoprevention; and clinical trial design, endpoints, and conduct. Additionally, potential roadblocks to progress were discussed, and several topics were identified as cross-cutting tools for optimization, each with potential to impact multiple scientific priority areas. These cross-cutting tools include preclinical models, data curation and sharing, biopsies and biospecimens, diversification of funding sources, definitions and standards, and patient engagement. Finally, three key guiding principles were identified that will both optimize and maximize progress in the field. These include engaging the patient community; cultivating diversity, equity, inclusion, and accessibility; and leveraging the power of artificial intelligence to accelerate progress. Here, we present the outcomes of these discussions as a strategic vision to galvanize the field for the next decade of exponential progress in cancer immunotherapy.
Keywords: Education, Immune modulatory, Immune related adverse event - irAE, Solid tumor, Hematologic Malignancies
Introduction
The idea that an interplay existed between the immune system and cancer was initially posed well over 100 years ago, receiving mixed interest and support. Subsequently, in the late 19th and early 20th centuries, incremental findings began to support this concept.1 Dr. William Coley and colleagues reported that bacterial infections may contribute to cancer regression,2 and the cancer immunosurveillance hypothesis was introduced and refined during the mid-1900s to early 2000s.3 4 While the proposal to therapeutically harness the immune system to effectively fight or even cure cancer initially seemed like a far-off reality, tantalizing reports began to suggest substantial therapeutic promise. High-dose interleukin-2 in patients with advanced melanoma and renal cell carcinoma caused dramatic, complete tumor regressions in approximately 7% of patients.5 The first proof of concept trials testing expanded, adoptively transferred autologous tumor-infiltrating lymphocytes (TILs) demonstrated response rates of 50%–70% in patients with treatment-refractory metastatic melanoma.6 Sipuleucel-T immunotherapy was approved by the US Food and Drug Administration (FDA) in 2010 for patients with castration-resistant metastatic prostate cancer, providing the first evidence that therapeutic cancer vaccines can confer an overall survival benefit for patients.7 Ultimately, after decades of work to understand mechanisms of immunosurveillance and T cell activation and control through T cell receptors (TCRs)8 and immune checkpoint pathways,9 10 cancer immunotherapy reached an exciting inflection point. The first immune checkpoint inhibitor (ICI), the anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody ipilimumab, was approved by the FDA in 2011 for patients with advanced melanoma based on improved overall survival.11 Concurrently, based on promising phase II data for anti-programmed cell death protein 1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) ICIs, the phase III studies that led to the first anti-PD-(L)1 ICI approvals began to enroll patients. During this same period, early clinical trials using engineered chimeric antigen receptor (CAR)-T cells revealed sustained remissions in patients with refractory B cell malignancies.12 13
At this critical juncture in the field, the Society for the Immunotherapy of Cancer (SITC) leadership recognized the importance of developing a roadmap to ensure continued progress in immunotherapy research. SITC convened a summit of international experts representing diverse organizations in cancer immunotherapy in 2009 and 2010. This group collaboratively defined nine major hurdles to progress in the landmark white paper, “Defining the critical hurdles in cancer immunotherapy”14: (1) limitations of current animal models to predict efficacy of cancer immunotherapy strategies in humans; (2) prolonged time to obtain approval to initiate clinical trials; (3) complexity of cancer, tumor heterogeneity, and immune escape; (4) limited availability of reagents for combination immunotherapy studies; (5) limited funds available to translate science into patients; (6) lack of definitive biomarker(s) for the assessment of clinical efficacy of cancer immunotherapies; (7) conventional clinical response criteria that do not take into consideration differences between response patterns to cytotoxic agents and immunotherapies; (8) paucity of teams of scientists and clinicians dedicated to translational research in cancer immunotherapy; and (9) insufficient exchange of information critical to advancing the field.
Galvanized by a shared roadmap, SITC supported the cancer immunotherapy community as the field continued to expand and flourish. A plethora of diverse immunotherapies—including three distinct ICIs, multiple ICI combinations, CAR-T cell therapies, two distinct classes of bispecific T cell engagers, cytokines, a vaccine, and an oncolytic virus—are now FDA-approved, transforming cancer treatment for both solid and hematologic malignancies. Immunotherapy is now a standard of care for many cancers, offering long-term clinical benefits to patients, including those with cancers previously associated with dismal prognoses. Many therapeutic successes over the last decade were achieved by overcoming the hurdles described in the 2011 manuscript. For example, one hurdle described was the lack of radiographic response criteria that captured the unique response patterns seen with immunotherapy, such as delayed responses, pseudoprogression, and/or hyperprogression.15 Several radiographic response criteria have since been developed that incorporate these response patterns, allowing for more accurate assessments of the clinical response to cancer immunotherapy.15 In addition, researchers have made rapid progress in understanding tumor heterogeneity and immune escape through new technologies such as single-cell sequencing16 17 and spatial transcriptomics,18–20 among others. These studies provided insights leading to a more comprehensive understanding of the diverse spatial composition of the tumor microenvironment (TME) and to more refined genotypic and phenotypic characterization of distinct classes of immune cells and their activated and/or suppressed states. As a result, targeting immune cells beyond T cells, such as NK cells21 and macrophages,22 23 by engineering the cells or by administering agents that target their unique regulatory pathways, are now being tested in early-phase clinical trials.
Progress in cancer immunotherapy over the last 10 years has been awe-inspiring, and the collaborative field-wide refocusing in 2011 undoubtedly contributed to that rapid progress. 13 years later, cancer immunotherapy has reached another critical inflection point, creating a mandate to again convene leaders in the field to both revisit the original challenges and identify new challenges and opportunities that have emerged with the astounding advances of the last decade. For example, the number of agents that show efficacy in preclinical testing yet fail in early-phase human studies remains unacceptably high, in large part due to animal models that inadequately recapitulate human tumors, the human immune system, and the interplay between the two. The development of mouse models with humanized immune systems24 25 represents some progress in this area, but interspecies variability remains a major challenge. Unfortunately, the lack of preclinical models that accurately reflect the tumor immunobiology of patients with cancer remains a significant limiting factor in investigating the mechanisms of antitumor activity, toxicity, and therapeutic resistance associated with immunotherapy. Other remaining challenges that continue to slow progress are challenges related to data sharing, and the continuing need for more effective predictive biomarkers of response, and new predictive biomarkers of toxicity and therapeutic resistance. Limited funding continues to be a major obstacle to progress, particularly considering the expense of developing and using cutting-edge technologies like single-cell sequencing and spatial transcriptomics to advance the field. In addition to the expense of generating the highly complex datasets associated with these technologies, expertise in the use and analysis of complex datasets is essential and in short supply, further compounding the cost burdens. Additionally, the plethora of new immunotherapy agents and combinations for testing in clinical trials has strained the clinical research ecosystem. Although innovative clinical trial designs—including adaptive trials, basket trials, and multiarm umbrella trials—have powered the clinical development of new immunotherapy agents and combinations, they have also introduced new challenges for clinical researchers, clinical research sites, regulators, and funding bodies.26 27
Today, the cancer immunotherapy field is poised for another phase of exponential growth. Innovative, next-generation agents with great potential to expand the number of patients with cancer who benefit from immunotherapy are in early clinical trials. A wealth of translational and clinical data is being collected by researchers across the globe. Integration of this creative and productive community will no doubt accelerate progress. It is also imperative to include patients and their advocates as key stakeholders in determining the path forward. Given this evolving landscape of old and new challenges along with the potential for exponential progress over the next decade, SITC leadership recognized the need for a contemporary roadmap for the field to refocus and galvanize the community around the most pressing current opportunities for advancing lifesaving cancer immunotherapies. Accordingly, SITC organized a multistakeholder consensus meeting with experts in cancer immunotherapy, including representation from academic, pharmaceutical, biotechnology, patient advocacy, and regulatory institutions, with the goal of defining and prioritizing present-day opportunities and challenges.
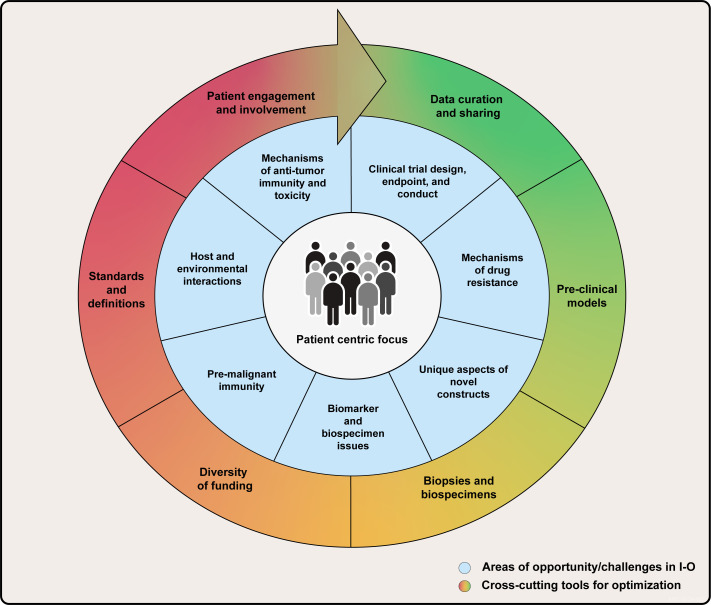
This manuscript is the first in a special review series in the Journal for ImmunoTherapy of Cancer (JITC) that will describe the current state of the field, address pressing challenges, and identify the greatest opportunities for high clinical impact in the scientific, clinical, and regulatory arenas of cancer immunotherapy (figure 1). While this overarching manuscript will introduce these broad areas of opportunity, identify high-impact cross-cutting tools relevant to several of them, and define the priorities with the highest potential for significant clinical impact, the upcoming topic-focused manuscripts will take a deeper dive into the current state of the field by area of opportunity and report on the expert-identified challenges and opportunities within each area. The special series will provide insights on the roadmap to success from some of the most experienced leaders in cancer immunotherapy, serving as a resource for the scientific community working to overcome the challenges facing the field and effectively capitalize on current and future opportunities for rapid progress.
Figure 1.
Areas of opportunity in cancer immunotherapy.
An update to the challenges and opportunities in cancer immunotherapy
The updated areas of opportunity identified by the consensus panel encompass challenges across the scientific, regulatory, and clinical arenas and include the following: (1) mechanisms of antitumor activity and toxicity; (2) mechanisms of drug resistance; (3) biomarkers and biospecimens; (4) unique aspects of novel therapeutics (new); (5) host and environmental interactions (expanded); (6) premalignant immunity, immune interception, and immunoprevention (new); and (7) clinical trial design, endpoints, and conduct. Notably, four of these challenges were identified in the 2011 roadmap. Although impressive progress in cancer immunotherapy was achieved by addressing elements of these four challenges, the overall challenge remains a hurdle that continues to merit focused attention. The three new or expanded areas of opportunity are captured here by figures that summarize their key elements. During the exploration of the areas of opportunity, certain superimposed themes emerged that affected multiple opportunities. The consensus panel characterized these emergent themes as cross-cutting tools for optimization that, if addressed, would be of highest impact on the field.
For example, improved preclinical models came up consistently as a major unmet need required to accelerate understanding of important scientific areas, such as the TME and its immune contexture, and mechanisms of immunotherapy activity, toxicity, and resistance. Improved and innovative preclinical in vivo and in vitro models were also deemed essential for accelerating drug development, particularly to facilitate generating relevant preclinical data for novel agents that can be effectively translated into early-phase human studies. Additionally, the need for developing and improving the uptake of definitions and standards for the consistent identification, collection, analysis, and reporting of data related to adverse events and therapeutic resistance was cited as a challenge contributing to our current lack of understanding of mechanisms of immune-related (ir)-toxicity and resistance to therapy. Box 1 lists these examples and other cross-cutting tools for optimization and deployment across the prioritized areas of opportunity.
Box 1. Cross-cutting tools for optimization in cancer immunotherapy.
Preclinical models
Data curation and sharing
Biopsies and biospecimens
Diversification of funding sources
Definitions and standards
Patient engagement and involvement
The following sections explore each area of opportunity, providing an overview of the state of the field, current-day challenges from the researcher, clinician, regulator, and patient perspectives, and potential strategies to overcome the key challenges within each area. Additionally, future papers in this JITC special series will dive even deeper into each prioritized area of opportunity with actionable steps for diverse stakeholders within the scientific community, all with the intention to catalyze novel ideas and foster cross-disciplinary collaboration to ensure rapid clinical progress.
Areas of opportunity in cancer immunotherapy
Mechanisms of antitumor activity, toxicity, and therapeutic resistance
State of the field and needs
As mentioned above, immunotherapy has transformed cancer treatment, with unprecedented durable clinical benefit in some patients. However, response rates are highly variable depending on the unique features of the patient and their cancer, averaging 20%–30% in the metastatic setting.28–30 As most tumors do not respond, bringing the durable benefit of immunotherapy to more patients is a high priority for the field. A better understanding of the regulatory mechanisms underlying immunotherapeutic response and resistance is key to achieving this goal. Moreover, ir-toxicities (immune-related adverse events (irAEs)) are unique and mostly related to on-target/off-tumor immune activation that causes damage to normal host tissues.31 These events are generally manageable if detected and treated early. However, ir-toxicities can be severe, life-changing, or even life-threatening, particularly with pure immunotherapy combinations or immunotherapy combined with other cancer therapies. Thus, it is critical for the field to define the immune and non-immune variables that determine effective antitumor immunity, undesirable ir-toxicity, and therapeutic resistance, map areas of overlap and divergence, and develop strategies for maximizing antitumor activity, minimizing ir-toxicity, and circumventing resistance to therapy. Identifying predictive biomarkers for immunotherapy response, resistance, and risk for ir-toxicity (and which irAEs) is a high priority for the field.32–34 More detailed knowledge will inform treatment choice, guide response assessment, and inform the monitoring and management of ir-toxicity, thus maximizing clinical benefit and minimizing harm. Actively engaging patients to contribute their insights about their cancer immunotherapy experience is essential to improve both clinical outcomes and patient satisfaction.
Current challenges for antitumor activity, toxicity, and therapeutic resistance
Three major challenges for dissecting mechanisms of antitumor response, ir-toxicity, and therapeutic resistance are as follows: (1) access to clinically relevant tumor models for forward and reverse translation; (2) availability of matched, longitudinal (baseline/on-treatment/post-treatment) biospecimens acquired from patients being treated with immunotherapy; and (3) adoption of standard definitions and frameworks for study.30 Challenges in these three areas are described below.
Clinically relevant tumor models
Preclinical in vivo models are expensive and time-consuming to use. In addition, their clinical relevance is limited by interspecies variability between the models and patients with cancer, with clear interspecies differences in genetic diversity, pathways of immunoregulation, cell-surface immune targets, and the microbiome (among other factors). Moreover, the pace of tumor development and the evolution of antitumor immune responses are very different, being fast in models and slow in humans; significant ir-toxicity in preclinical models is quite rare. Given the diversity of irAEs observed in patients, it remains unclear whether immunotherapy-induced autoimmunity can be effectively modeled in animals, particularly when it involves multiple organ systems.31 Tumor models on autoimmunity-prone backgrounds may address some of these challenges, though these are not readily available and require development.
Longitudinal biospecimens
Biospecimen-based models, such as organoids or ex vivo human tumor slice cultures, may alleviate the expense of in vivo models and accelerate the identification of relevant human therapeutic targets for clinical translation.30 However, they are limited by their ex vivo nature, and more studies are needed to understand their potential value. Also, human biospecimens may be difficult to collect due to provider and patient reluctance, the costs of obtaining them, and the expense and infrastructure required for maintaining a biospecimen repository. Biospecimen protocols for collection (methods and timing), processing, and annotation of clinical data are not standardized across institutions; for irAEs, biospecimens are not often collected. Other challenges related to establishing irAE databases are that the diagnosis is mostly clinical and subjective, consensus definitions have not been adopted, and standard data elements have not been defined.
Standard definitions and frameworks
The unique patterns of response to immunotherapy—initial progression followed by response, pseudoprogression, and stable disease followed by delayed responses—provide one high-level framework for investigating mechanisms of antitumor immunity.35 In addition, early reports describe hyperprogression (rapid response on treatment) in 10%–30% of patients36; this phenomenon needs to be validated in randomized trials that include patient experience research. There has been less attention to patterns of resistance. To address this gap, SITC convened an Immunotherapy Resistance Task Force to develop consensus definitions of immunotherapy resistance based on patterns of clinical response: (1) primary resistance (never responders to immunotherapy), (2) secondary resistance (initial response followed by treatment resistance), and (3) progression after treatment discontinuation.37 38 These consensus definitions still require both validation and plain language patient materials that effectively solicit patient input.
Opportunities for the field: mechanisms of antitumor immunity and toxicity
Effectively addressing these challenges has the potential to rapidly accelerate progress toward safer, more effective cancer immunotherapies. Leveraging existing databases, such as genome-wide association studies (GWAS) that demonstrate potential links between genomic variants and autoimmune disorders, may identify patients at higher risk for ir-toxicity with immunotherapy.31 Sophisticated technologies will continue to provide breakthroughs in our understanding of cancer immunobiology through big data initiatives that mine very large, complex datasets. Machine learning and artificial intelligence will efficiently enable the community to extract insights from translational and clinical data that otherwise might be missed. New data and technologies may lead to innovative clinical trial designs. Bringing together rheumatologists and other medical subspecialists with experts in immuno-oncology (I-O) and regulators will pool diverse talents to address the complex challenge of unraveling antitumor immunity and treatment-related, tissue-specific autoimmunity, growing an increasingly sophisticated immunotherapy workforce with a broad array of talents. As with other tools, care must be taken to verify data accuracy, and data and biospecimens must reflect the diversity of patients with cancer.
Opportunities for the field: mechanisms of drug resistance with immunotherapy
A major challenge to understanding immunotherapy resistance is the inconsistent use of standard definitions. It is imperative to educate the field on the importance of using consistent language to make progress.38 In clinical trials, it is essential to stratify patients for primary and secondary resistance to immunotherapy to facilitate an understanding of clinical activity in these distinct groups for researchers, providers, and patients alike. In addition, the collection of matched, longitudinal (baseline/on-treatment) biospecimens from patients being treated with immunotherapy should be undertaken whenever possible. In immunotherapy combination studies, it may be difficult both to tease out single-agent activity and to address dose and sequencing considerations. The development of clinical endpoints that account for diverse interlesional responses is also needed. Biologically, immunotherapy resistance may be due to tumor cell-intrinsic factors and/or extrinsic factors related to the TME.30 Tumor cell-intrinsic factors include defects in antigen presentation machinery, genetic alterations that converge on the interferon-γ signaling pathway, oncogenic signaling, and epigenetic reprogramming. Tumor cell-extrinsic factors may be related to intratumoral immune cells, fibroblasts, vessels, the microbiome, or hormonal and neuronal signals. A major challenge for dissecting mechanisms of immunotherapy resistance is access to clinically relevant preclinical tumor models for forward and reverse translation that reflect these variables. These advances will likely identify novel therapeutic targets that can overcome resistance to immunotherapy and that may have greater activity than the current standard frontline therapy. Addressing the challenge of therapeutic resistance has the potential to rapidly accelerate progress in I-O, leading to safer and more effective treatments for patients who need them.
Biomarkers and biospecimens
State of the field and needs
Predictive biomarkers of response, resistance, and toxicity are urgently needed to select patients with the highest likelihood of clinical benefit from immunotherapy and to identify those at highest risk of experiencing an irAE due to treatment with immunotherapy.32–34 In addition, pharmacodynamic (PD) biomarkers that provide very early evidence of on-treatment response or resistance are needed to optimize therapeutic decision-making. It is currently standard clinical practice to use biomarkers for selecting patients to receive immunotherapy in some clinical scenarios.39 These include assessment of PD-L1 expression by immunohistochemistry (IHC), detection of microsatellite instability by IHC or next-generation sequencing (NGS), and measurement of tumor mutational burden (TMB) by NGS for patients treated with ICI. In addition, B cell aplasia is a biomarker of CAR-T function/persistence in children with B cell leukemia. However, the use of immunotherapy is not biomarker-driven for most patients, potentially exposing them to drugs with limited to no benefit along with a risk of substantial drug-related toxicity. Furthermore, there are currently no established predictive biomarkers of toxicity or early (on-treatment) PD biomarkers of response or resistance (such as non-invasive molecular imaging).
Patients are key stakeholders in efforts to develop biomarkers. Teaching patients about the role of biomarkers in selecting an immunotherapy treatment for their unique clinical situation and for integrating biospecimens in clinical trials is key to accelerating progress in immunotherapy biomarker development. In addition, developing minimally invasive biomarker strategies (eg, liquid biopsy40) and non-invasive imaging technologies (immuno-positron emission tomography and others41) will make the routine use of biomarkers and biomarker research more acceptable to both patients and providers.
Current challenges
Advances in biomarker technologies both create the opportunity for more precise, in-depth understanding of tumor immunobiology and create greater complexity for the real-world application of biomarker testing. Rapidly growing areas of biomarker research include multiplex IHC, NGS-based testing to identify both gene mutations and gene expression signatures, epigenetic mapping to characterize higher-order gene structures, and metabolic profiling to characterize the energy state of the tumor. Integrating these approaches can provide a multidimensional portrait of the tumor. For example, combining NGS with multiplex IHC and imaging can reveal the spatial distribution of gene expression in the context of cell-cell interactions within the tumor. This explosion of innovative technologies creates multiple urgent questions and challenges.
First, for clinical use, what level of biomarker complexity (number and pattern of target molecules) is required to balance assay feasibility and cost with the value of the information gained?
Second, what is the best approach to assay development, validation, standardization, and deployment to the real world?
Third, what is the best way to obtain correlative data of response—invasive tumor biopsies, minimally invasive blood collection (liquid biopsy), non-invasive “biopsy” (molecular imaging)—in a way that captures tumor heterogeneity across diverse disease sites within a given patient?
Fourth, what is the best way to assemble, curate, and interrogate the large datasets that will be generated with sophisticated biomarker technologies?
Fifth, what is the best way to manage the cost of developing novel biomarker assays, test them in appropriately sized clinical trials, navigate the regulatory process, and deploy them into the clinical setting?
Opportunities for the field
Sophisticated technologies will continue to evolve and provide breakthroughs in our understanding of cancer immunobiology through big data initiatives that mine very large, complex datasets.42 Machine learning and artificial intelligence may efficiently extract insights from translational and clinical data that otherwise might be missed.43 New data and technologies may lead to innovative clinical trial designs, accelerating the pace of developing safe and effective new cancer immunotherapies. Investigators in these diverse areas will continue to come together with regulators, pooling their talents to address the complex challenge of developing predictive biomarkers of response, toxicity, and resistance, creating a diverse cancer immunotherapy workforce with a broad array of talents. Engaging patients and patient advocates in biomarker development will help prioritize areas of focus, ensure incorporation of critical biomarker research in clinical trials, and facilitate deployment of validated biomarkers into the standard of care.
Unique aspects of novel therapeutics
State of the field and needs
Immunotherapy with antibodies specifically blocking CTLA-4 or PD-(L)1 provided proof of principle for modern human cancer immunotherapy,44 and adoptive cellular therapy with CAR-T cells transformed the management of some hematologic malignancies.29 The race is on to improve the efficacy of these therapies and extend the benefit of immunotherapy to patients with cancers that are currently unresponsive. These successes highlight the need for novel engineered therapeutics that promote tumor immunity in unique ways to both expand the applicability of immunotherapy to more patients and further improve clinical outcomes.45
Current challenges
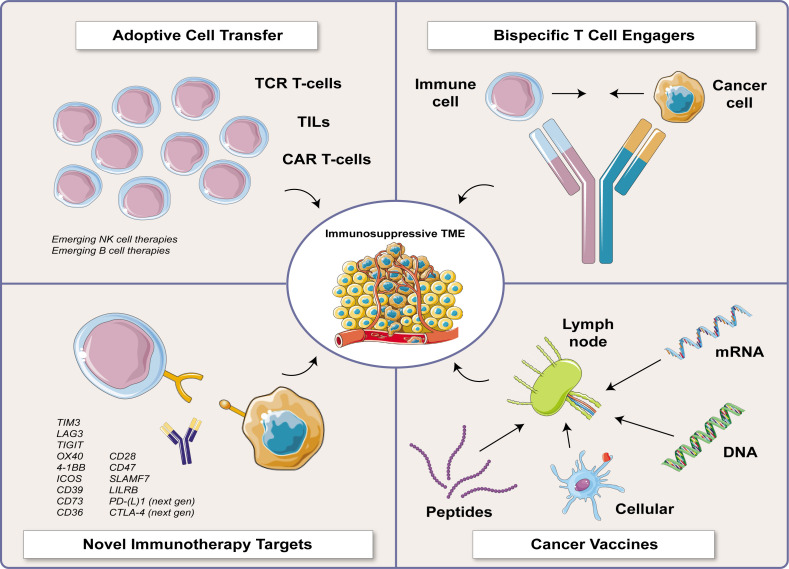
The most promising innovative immunotherapies target novel immune checkpoints44; activate or induce cancer immunity through vaccination intended to treat, intercept, or prevent cancer (cancer vaccines)46; passively provide antitumor immune cells through adoptive transfer (adoptive cellular therapy with TILs, CAR-T cells, or TCR-T cells)29; or anchor T cells to tumor cells for immune attack (bispecific T cell engagers and other synthetic molecules) (figure 2).47 Other novel immunotherapy agents include oncolytic viruses, engineered cytokines, and some antibody-drug conjugates. These agents have many common challenges.
Figure 2.
Promising and novel immunotherapeutic strategies. CAR, chimeric antigen receptor; CTLA-4, cytotoxic T lymphocyte antigen-4; ICOS, inducible costimulator; LAG3, lymphocyte-activation gene 3; LILRB, leukocyte immunoglobulin-like receptor family; mRNA, messenger RNA; NK cell, natural killer cell; OX40, tumor necrosis factor receptor superfamily member 4; PD-(L)1, PD-1/PD-L1 axis; SLAMF7, SLAM family member 7; TCR, T cell receptor; TIGIT, T cell immunoreceptor with Ig and ITIM domains; TIM3, T cell immunoglobulin and mucin domain-containing protein 3; TME, tumor microenvironment; 4-1BB, tumor necrosis factor receptor superfamily member 9.
First, despite the impressive clinical activity of ICIs specific for CTLA-4, PD-(L)1, and lymphocyte-activation gene 3 (LAG-3) in combination with PD-1, only a minority of eligible tumors respond, and some tumor histologies do not respond at all. Lack of response to ICIs is more common in immune-excluded and cold tumors, but inflamed tumors may also be resistant. There are intense efforts to identify novel ICIs that can be targeted therapeutically. ICIs may promote ir-toxicities that can be chronic or even acutely life-threatening. Again, high-performance predictive biomarkers of response, resistance, and toxicity are urgently needed for optimal patient selection for ICI therapy.
Second, cancer vaccines induce and/or expand antigen-specific T cells. The success of vaccination for infectious disease (eg, recent success of mRNA vaccines for COVID-19) illustrates the profound impact that cancer vaccines could have on global public health. Challenges include defining the most specific and potent tumor-associated or disease-associated antigens to target,48 developing the most active vaccine platforms and adjuvants, and identifying the most appropriate patient populations for vaccination. The manufacturing of personalized and/or cell-based vaccines poses additional challenges, including manufacturing process, time, and cost, and optimal testing strategies for confirming potency and release testing. These challenges in the manufacturing process pose new dilemmas for patients to navigate, as patients may have to contend with long wait times to access the novel agents and face the possibility of progression of their disease while they are waiting.
Third, CAR-T cells are engineered, major histocompatibility complex (MHC)-independent antigen-specific T cells.29 As antigen-dependent therapeutics, CAR-T cells share some of the same antigen-related challenges as cancer vaccines and bispecific T cell engagers. CAR-T cells specific for CD19 or B cell maturation antigen (BCMA) have significant clinical activity in B cell leukemia/lymphoma or multiple myeloma, respectively, with serious side effects such as cytokine release syndrome (CRS), immune cell-associated neurotoxicity syndrome, and chronic hypogammaglobulinemia. Efforts to expand the use of CAR-T to solid tumors have been less successful than with hematologic malignancies, likely due to tumor heterogeneity, poor T cell trafficking and persistence, and the suppressive TME of solid tumors. Practical challenges include patient access49 and manufacturing time, cost, and release criteria. Other types of cellular therapies include TILs and TCR-T cells and emerging work with engineered NK cells, macrophages, or B cells that express receptors such as TCRs, NK cell receptors, or CARs.
Fourth, bispecific T cell engagers are linked recombinant proteins with two distinct antigen-binding domains that target a tumor antigen and a T cell-activating molecule (such as CD3 or TCR). They activate T cells through a tight immune synapse that efficiently induces tumor lysis.47 Multiple bispecific T cell engagers are currently FDA-approved, and at the time of manuscript preparation targets include CD19 (B cell leukemia), BCMA or G protein-coupled receptor, class C, group 5, member D (GPRC5D, multiple myeloma),50 CD20 (B cell lymphoma),51–53 the tumor antigen gp-100 complexed to MHC Class 1 (uveal melanoma)54, and Delta-like ligand 3 (DLL3, small cell lung cancer)55 . Challenges include drug delivery issues related to the short half-life of these agents, on-target/off-tumor toxicity, CRS, and neurotoxicity.
In addition to the challenges mentioned above, drug dosing and delivery, immunogenicity, trial designs, and regulatory issues related to manufacturing science and release testing may be unique to these agents.
Opportunities for the field
Overlapping challenges related to the development of novel constructs present an opportunity to leverage progress in one drug class for the benefit of another. New agents and knowledge may allow novel combinations of agents designed to initiate or expand an immune response and allow effector cells to remain functional in the immunosuppressive TME.
Host and environmental interactions with tumor immunotherapy
State of the field and needs
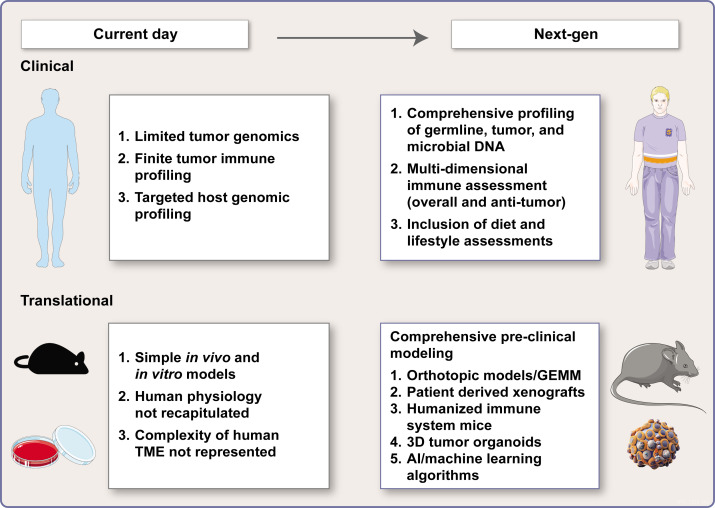
Though the majority of cancer immunotherapy research to date has focused on the TME, there is a growing appreciation that factors outside of the TME—both within and outside of the host—contribute to clinical response, therapeutic resistance, and ir-toxicity (figure 3).32 This includes intrinsic host factors, such as systemic immune fitness, comorbid diseases, host genomics (host germline gene mutations and polymorphisms), endogenous hormones, and the host metabolic state (obesity and anorexia). It also includes factors extrinsic to the host, such as diet and lifestyle factors, medications, environmental exposures (such as tobacco), sleep quality, and other psychosocial factors.56 External factors also include the commensal microbes that coexist in various niches within the host, particularly the gastrointestinal tract and the TME itself.57
Figure 3.
Host and environmental interactions that influence tumor immunotherapy. AI, artificial intelligence; GEMM, genetically engineered mouse model; TME, tumor microenvironment.
Genomic testing to identify germline mutations can both assess cancer risk58 and guide personalized cancer treatment. The information gained from the use of standard gene panel testing and/or NGS to assess both germline and tumor somatic mutations can be leveraged to guide therapy, as in the case of BRCA1/2 mutations and PARP inhibitors.59 This information can also be used to inform strategies designed to prevent cancer, such as prophylactic total gastrectomy in individuals with germline mutations of CDH1 60 and risk-reducing mastectomy and/or oophorectomy in individuals with germline mutations of BRCA1/2.61 There is increasing interest in defining germline modifiers of the immune TME that drive cancer risk and modulate response to immunotherapy in patients with cancer.62
Environmental exposures may also influence the efficacy of cancer immunotherapy. For example, there is a definitive risk of multiple cancers associated with exposure to cigarette smoke. Consistent with this, there is a clear association between the presence of a genomic smoking signature, specific genetic mutations related to smoking detected by NGS and reflected by TMB, and clinical response to ICIs in non-small cell lung cancer.63 Much remains to be learned about how environmental exposures influence tumor immunity and response to immunotherapy.
The microbiome, particularly the gut microbiome, has emerged as both a significant determinant of response to immunotherapy and a novel therapeutic target.57 Distinct signatures derived from gut microbes distinguish patients with cancer from healthy individuals and immunotherapy responders from non-responders. Accordingly, there is intense interest in developing microbiome-based treatment strategies to modulate gut microbes both to enhance the response to immunotherapy and to mitigate its associated ir-toxicity.
Tools for measuring and modulating host and environmental factors are emerging, but the optimal means of identifying, measuring, and manipulating these factors intentionally to optimize the clinical activity of immunotherapy and overall clinical care remain incompletely understood. Nonetheless, it seems clear that modulating critical host and environmental factors that impact tumor immunity offers the opportunity to maximize the efficacy of immunotherapy for patients across the continuum of cancer risk, initiation, and progression.
Current day challenges
The assessment of host and environmental factors that impact cancer risk, response to immunotherapy, and ir-toxicity is complex due to the diverse intrinsic and extrinsic variables involved and the various methods of assessment utilized. Major challenges are summarized below.
First, although genomic testing can lend important clinical insights, it may impose financial and potential psychological burdens on the individual and family, incur social stigma, and impact insurability. Testing may present challenges related to the speed of testing and the efficiency of data analysis. The need to revisit the risk to an individual and family as data accumulate defining new germline pathogenic variants and their relative risk of disease also creates challenges related to clinical follow-up.
Second, current preclinical models fail to recapitulate the impact of host and environmental interactions on cancer initiation, development, and response to immunotherapy. This may, in part, explain their shortcomings in assessing mechanisms of response and resistance to immunotherapy and ir-related toxicity. The community needs innovative models that better capture the complex interactions between the host and environment as they shape the interplay between the tumor and immune system.
Third, innovative tools to measure and integrate intrinsic and extrinsic host factors that impact tumor immunity and response to immunotherapy should be more broadly utilized. Wearable devices can measure physiologic variables, such as heart rate and its variability, respiratory rate and oxygenation, sleep and exercise quantity and quality, and stress.64 Tools that measure immune fitness and systemic inflammation are currently available or under development.65 Tools also exist to characterize metabolic factors, capturing dietary intake and host metabolomics.66 Technology to characterize the taxonomic features and functional activities of microbes localized to the gut or other physiologic niches is also available.57 However, well-established standards for measurements, quality control, and integrated use of such data do not exist.
Fourth, patient engagement at every stage of translational and clinical research focused on host and environmental variables and response to immunotherapy is crucial as we move forward as a field.
Opportunities for the field
Multiple untapped opportunities to optimize immunotherapy, transform cancer care, and promote overall health by effectively modulating host and environmental interactions can lead to synergistic gains in global public health. Developing standards for capturing data related to host and environmental factors, ensuring its quality, and integrating it with clinical data are a priority to advance research in this area. Engaging patients in this research for their critical perspective will be essential for progress in understanding the role of these factors in cancer care and in effectively deploying therapeutic and preventative lifestyle modifications in the clinic.
Premalignant immunity
State of the field and needs
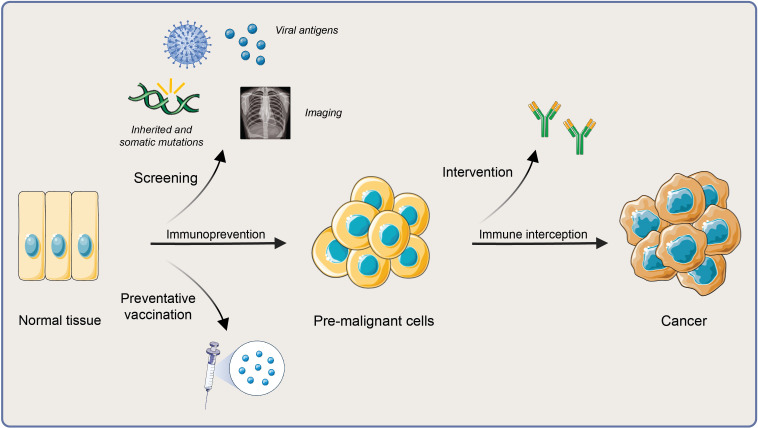
Studies of host-tumor interactions have historically focused on primary invasive cancer and established metastasis, paving the way for successful immunotherapy in both early-stage and late-stage invasive disease. With the success of immunotherapy in frank malignancy, there is growing interest in elucidating the immunobiology of preinvasive lesions during their evolution to invasive cancer to facilitate immune-based interventions that intercept cancer development or even prevent cancer (figure 4).67 Characterizing both clonal tumor evolution (using whole exome sequencing and NGS) and its immune contexture (using CyTOF, flow cytometry, and functional immune assays) should both identify antigens of premalignancy to target and capture the relative balance of antitumor immunity and protumorigenic inflammation across the process of tumorigenesis.68–70 Advances in discovery research, bioengineering, technology/big data, and translational/clinical science have created tangible potential for improving global public health by preventing or intercepting cancer development using novel immune-based interventions, including vaccines.
Figure 4.
Premalignant immunity, immunoprevention, and immune-interception.
Current day challenges
Despite the clear opportunity for meaningful progress in cancer immune interception and prevention, significant hurdles remain.
First, identifying patients without cancer who may be candidates for an immune intervention is difficult and requires validated screening procedures accepted by patients and providers alike. Cancer risk factors may include strong family history, exposure to an infectious agent or carcinogen, or the presence of germline mutations. Screening procedures may include a medical history, genetic testing, radiologic procedures (mammography for breast cancer or low-dose CT for lung cancer), and other assays or procedures (fecal occult blood testing and/or colonoscopy for colon cancer).
Second, identifying the optimal antigens to target remains a major challenge. Tumors driven by viral infection or inherited and/or acquired gene mutations may have (neo)antigens readily recognized by the immune system. Shared tumor antigens may offer a more generalizable solution71 in some cases. It is imperative to interrogate premalignant lesions for novel, potent antigens to target. However, lesions present at the earliest stages of tumorigenesis (hyperplasia, atypical hyperplasia, and carcinoma in situ) are often very small and provide limited tissue for analysis. Moreover, they are often difficult (or maybe impossible) to access, requiring interventional procedures (such as colonoscopy for colon lesions or bronchoscopy for pulmonary lesions) conducted by trained specialists to collect tissue.
Third, tools for studying premalignant immunobiology are lacking. Genetically engineered animal models that spontaneously develop tumors and have an intact immune system may be useful, but even these models fail to accurately reproduce the coevolution of cancer and the associated immune response found in human patients. Innovative models that more faithfully reproduce the complexity of premalignant biology in humans are clearly needed.72
Fourth, advances in regulatory science that include novel, efficient clinical trial designs for immunoprevention, new surrogate trial endpoints (such as blood-based biomarkers), and novel tools for assessing the impact of immune interventions on the quality of life of healthy individuals and communities at risk for cancer will be essential for success. Employing cost-effective and scalable technology will ensure that immune interventions are widely deployed for maximal impact.
Finally, as patients and their families will directly benefit most from effective strategies for immune interception and prevention, patient and community engagement are critical to success. Importantly, this requires expanding the educational tools, activities, and communication channels adapted to the public and also learning from patients about their challenges and preferences with these approaches.
Opportunities for the field
Effectively tackling these challenges presents tremendous opportunities to transform patient care and improve public health around the globe by harnessing the immune system to effectively eradicate cancer through immunoprevention and immune interception strategies. Identifying the best antigens to target for immune interception and prevention, developing effective screening strategies, and employing cost-effective technology that can be delivered to underserved communities are high-priority areas for development.
Clinical trial design, endpoints, and conduct
State of the field and needs
Clinical trials are complex and increasingly costly research endeavors implemented in distinct phases of planning, activation, and execution. Compounding their inherent complexity, each trial phase is composed of multiple steps. Successful clinical trial completion requires the collaboration of multiple stakeholders, including clinical investigators, trial staff, administrators, institutional review boards, pharmaceutical sponsors, patient advocates, contract research organizations, the National Cancer Institute (NCI), the FDA, and other major oncology professional organizations. This diverse group ultimately serves patients and their caregivers, engaging them in the process of creating effective new cancer therapies for future patients. Accordingly, the system should rightfully be centered around patients, their advocates, and their communities.73 Having lost sight of this patient-centric and community-centric view, the current clinical trial ecosystem is in a state of crisis, with staffing shortages, administrative burdens, process inefficiencies, and outdated clinical trial business models substantially slowing progress in drug development. This situation poses a significant threat to clinical trial access and the ultimate development of novel, effective cancer therapeutics, with potential to affect the clinical landscape well into the future.
Current challenges
SITC adopted the crisis in clinical trials as a major strategic priority, issuing an urgent call to action by convening a Virtual Summit on the Crisis in Clinical Research on August 17, 2022, to engage key stakeholders in open conversation about the situation.74 In addition to operational inefficiencies, challenges that span clinical science, funding, and patient engagement were also identified:
First, the current clinical trial ecosystem is dated and inefficient. Centralizing resources, standardizing and simplifying data collection, streamlining clinical trial operations by incorporating automation and artificial intelligence,75–77 implementing modern business models, diversifying and decentralizing clinical research sites to optimally serve patients and communities,73 78 and supporting the activities and professional development of the clinical trial workforce were identified as high priorities for change. Efforts to develop in-person and decentralized procedures would increase trial access to more patients, especially patients of low socioeconomic status.
Second, the design of clinical trials themselves requires modernization.79–81 Novel study designs, surrogate clinical endpoints that correlate with survival benefit, better predictive biomarkers associated with response or clinical benefit, and determining the optimal treatment duration are pressing clinical needs. Approaches that include adaptive designs and master protocols are to be encouraged.
Third, new funding models for clinical trials are needed, particularly for multisite, multicohort trials not funded by pharma. Continuation of federal support of the cooperative groups is necessary to fund research into key clinical questions that impact patients’ quality of life and may generate cost-savings, such as treatment de-escalation, toxicity management, and biomarker-driven trials that may limit the use of therapy to a biomarker-selected population.
Fourth, more effectively engaging patient advocates in trial design and effectively communicating with patients who are trial candidates about the drug under study, the trial design, biospecimen collection for biomarker development, and the potential benefit relative to the burden of trial participation is essential.82 Optimizing patient education and engagement to enhance accrual rates, combined with efforts toward decentralization, have great potential to improve the efficiency of the clinical trial ecosystem and bring new advances to patients faster.
Opportunities for the field
We have made enormous progress in elucidating the mechanisms of cancer pathogenesis and the immune response to malignancy and in developing powerful new technologies to characterize cancer and antitumor immunity. Accordingly, we are now currently poised to make transformational progress in drug development by delivering an expanded portfolio of innovative cancer immunotherapies with the potential to prolong life. The inefficiencies in the clinical research ecosystem pose a significant risk to this progress. SITC has engaged a community of diverse stakeholders to address the crisis in clinical research, with the goal of defining a path forward that restores the patient and community to the center of the clinical trial ecosystem. Although the nature and interrelationships of the hurdles embedded in the clinical research ecosystem are daunting, they also present tangible opportunities to transform the system and accelerate the development of cancer immunotherapies that result in better outcomes for patients.
Summary and conclusions
The SITC-sponsored strategic meeting on cancer immunotherapy was convened to take stock of our transformative progress in cancer immunotherapy. Over the last 10–15 years, our efforts achieved unprecedented durable clinical benefit in some patients with advanced disease. Adjuvant immunotherapy is now a standard of care for melanoma, non-small cell lung cancer, bladder cancer, and renal cell carcinoma. More recently, the incorporation of immunotherapy into neoadjuvant cancer treatment suggests the potential for even better clinical outcomes, and the neoadjuvant setting is a powerful platform for translational science as well.83 This clinical progress suggests that deploying immune-based interventions earlier in cancer development is likely most effective, setting the stage for the application of immune-based interventions to intercept and even prevent cancer.84 The consensus meeting included a diverse group of leaders from all sectors of the cancer immunotherapy community who collaboratively developed a next-generation strategy for the field to catalyze and accelerate the next decade of progress. This group identified seven areas of opportunity for future progress, including four core areas that have proven fruitful and remain high priority, and three new or expanded areas not highlighted in the previous roadmap that are at the leading edge of progress (figure 1). The group’s deliberations around the critical scientific areas of priority in cancer immunotherapy also identified multiple shared roadblocks to progress as cross-cutting tools for optimization with potential to impact every scientific priority area of opportunity. These are listed in Box 1 and are captured in greater detail in figure 5.
Figure 5.
Cross-cutting tools for optimizing impact in cancer immunotherapy.
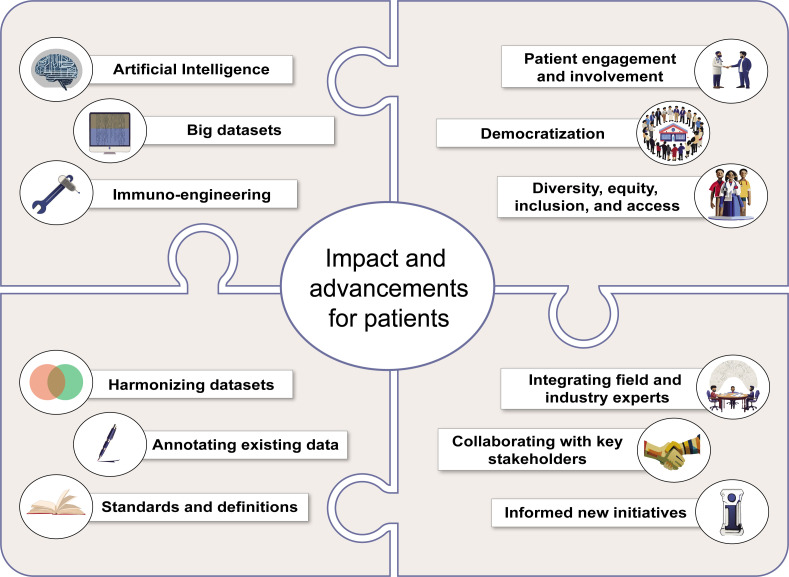
During the deliberations of the leadership group, three foundational concepts emerged as key guiding principles for the next decade of progress. First and foremost, the patient is central to our efforts. Engaging patients in clinical development and implementation is essential to ensure we optimally realize the potential of cancer immunotherapy. Second, prioritizing diversity across our stakeholder community and integrating it into our research endeavors are critical. This will ensure that our work is democratized to have the broadest possible impact on all patients. Third, optimally leveraging the rapidly growing area of artificial intelligence will accelerate our efforts. Informing our way forward with the patient voice; a mindset of diversity, equity, inclusion, and accessibility; and the power of artificial intelligence will ensure maximal progress toward realizing our vision of a cancer-free world through cancer immunotherapy.
The SITC leadership group looks forward to the next decade with great anticipation of exciting new developments. We expect to broaden the science of cancer immunology and immunotherapy and markedly expand the proportion of patients with cancer that enjoy clinical benefit and good quality of life with potent and safe immunotherapies. This consensus paper provides a roadmap to the future, which will be further detailed in an upcoming series of manuscripts that will provide a deeper dive into the seven identified areas of opportunity. Moving forward, SITC leadership will continue to gauge progress, identify emerging opportunities and roadblocks, and engage diverse stakeholders across the drug development enterprise. The future of cancer immunotherapy is very bright, and we have a lot of collective work to do.
jitc-2024-009063supp001.pdf (69.2KB, pdf)
Acknowledgments
The authors wish to acknowledge the SITC 2023 Strategic Retreat attendees (online supplemental materials) for their participation in identifying and articulating the challenges and opportunities discussed in this manuscript series. The authors also wish to thank SITC staff including Emily Gronseth, PhD, for the medical writing support, as well as all SITC staff who provided support to the SITC 2023 Strategic Retreat and/or manuscript development (online supplemental materials). The authors would also like to thank Manoj Chelvanambi, PhD (MD Anderson Cancer Center), for the assistance on figure development. Lastly, the authors thank SITC for supporting the manuscript development.
Footnotes
@EmensLeisha, @AnaAndersonlab, @BcellBruno, @CapitiniMD, @deborahcollyar, @gulleyj1, @PatrickHwuMD, @IAmDrDex, @annwsilkmd, @JenWargoMD
Contributors: LAE contributed to the drafting, conception, design, and development of the manuscript and as such is listed as the first author. JAW served as chair of SITC’s Strategic Retreat Committee and provided guidance for the conception, design, and development of the manuscript and as such is listed as the last author. PJR provided additional guidance on the design and overall development of the work and therefore is listed as the second author. All other authors participated equally in the manuscript development by providing conceptual feedback and critical review of intellectual content in the manuscript. Thus, they are listed alphabetically. All authors provided final approval for the publication of this manuscript. Figures were drafted and/or developed by JAW, Manoj Chelvanambi, PhD (MD Anderson Cancer Center), and Emily Gronseth, PhD (SITC). Parts of the figures were created using adapted images from Servier Medical Art and Biorender.com. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: LAE, employee, Ankyra Therapeutics; researcher, AbbVie, AstraZeneca, Bolt Therapeutics, Bristol Meyers Squibb, Compugen, Corvus, CytomX, EMD Serono, Genentech, F Hoffman La Roche, Immune Onc, Merck, Next Cure, Silverback, Takeda, and Tempest; consultant/advisor/speaker, AstraZeneca, BioLineRx, DNAMx, Genentech, F Hoffman La Roche, GPCR, Gilead, Immune Onc, Immunitas, Immutep, Lilly, Macrogenics, Mersana, and Shionogi; royalty and patent beneficiary, potential for royalties in the future from Molecuvax; publicly traded stocks, potential for stock options in the future from Ankyra Therapeutics; Other, NSABP Foundation, Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, NCI, Department of Defense, Johns Hopkins University, University of California San Francisco, Cornell University, Dana-Farber Cancer Institute, and Stand Up to Cancer. These are grants from non-industry entities. TCB, advisory board, Kalivir, Tabby; consultant, Galvanize, Attivare, Mestag, and Tallac. CMC, consultant/advisor/speaker, Bayer, Elephas, Novartis, Nektar Therapeutics, and WiCell Research Institute. ACA, member of the SAB for Tizona Therapeutics, Trishula Therapeutics, Compass Therapeutics, Zumutor Biologics, Excepgen, and ImmuneOncia, which have interests in cancer immunotherapy. ACA is also a paid consultant for iTeos Therapeutics and Larkspur Biosciences. ACA is an inventor on patents related to the checkpoint receptor Tim-3. DC, executive role, ORIEN Patient Advisory Council. JLG, royalty and patent beneficiary, Bethesda Handbook of Clinical Oncology (royalty) and UpToDate (royalty); JITC interim editor-in-chief. PH, consultant/advisor/speaker, Dragonfly SAB and Immatics SAB. ADP, researcher, Astellas; consultant/advisor/speaker, ImmunoACT, Stromatis Pharma, GO Therapeutics, Astellas, and MaxCyte. PJR, employee, Novigenix and SA; researcher, Roche, pRED, Schilieren, and CH; consultant/advisor/speaker, Enterome, Transgene, and Maxivax. AWS, researcher, Biohaven Pharmaceuticals, Replimune, Morphogenesis, Shattuck Laboratories, Regeneron, and Merck; consultant/advisor/speaker, InStil Bio, Signatera, Merck, and Regeneron; royalty and patent beneficiary, UpToDate; and publicly traded stocks, Illumina. JAW reports compensation for speaker’s bureau and honoraria from PeerView and serves as a consultant and/or advisory board member for Gustave Roussy Cancer Center, EverImmune, OSE Immunotherapeutics, Bayer Therapeutics, James Cancer Center OSU, Daiichi Sanyko. SITC staff: EG, nothing to disclose. JW, nothing to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Decker WK, da Silva RF, Sanabria MH, et al. Cancer immunotherapy: historical perspective of a clinical revolution and emerging preclinical animal models. Front Immunol 2017;8:829. 10.3389/fimmu.2017.00829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. with a report of ten original cases. 1893. Clin Orthop Relat Res 1991;3–11. [PubMed] [Google Scholar]
- 3. Burnet M. Cancer: a biological approach. III. viruses associated with neoplastic conditions. Br Med J 1957;1:841–7. 10.1136/bmj.1.5023.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329–60. 10.1146/annurev.immunol.22.012703.104803 [DOI] [PubMed] [Google Scholar]
- 5. Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994;271:907–13. [PubMed] [Google Scholar]
- 6. Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008;26:5233–9. 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 8. Allison JP, McIntyre BW, Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol 1982;129:2293–300. [PubMed] [Google Scholar]
- 9. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–6. 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- 10. Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature 1987;328:267–70. 10.1038/328267a0 [DOI] [PubMed] [Google Scholar]
- 11. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–18. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–33. 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fox BA, Schendel DJ, Butterfield LH, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med 2011;9:214. 10.1186/1479-5876-9-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berz AM, Dromain C, Vietti-Violi N, et al. Tumor response assessment on imaging following immunotherapy. Front Oncol 2022;12:982983. 10.3389/fonc.2022.982983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caligola S, De Sanctis F, Canè S, et al. Breaking the immune complexity of the tumor microenvironment using single-cell technologies. Front Genet 2022;13:867880. 10.3389/fgene.2022.867880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bai Z, Lundh S, Kim D, et al. Single-cell multiomics dissection of basal and antigen-specific activation States of CD19-targeted CAR T cells. J Immunother Cancer 2021;9:e002328. 10.1136/jitc-2020-002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu B, Sajid M, Lv R, et al. A review of spatial profiling technologies for characterizing the tumor microenvironment in immuno-oncology. Front Immunol 2022;13:996721. 10.3389/fimmu.2022.996721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larroquette M, Guegan J-P, Besse B, et al. Spatial transcriptomics of macrophage infiltration in non-small cell lung cancer reveals determinants of sensitivity and resistance to anti-PD1/PD-L1 antibodies. J Immunother Cancer 2022;10:e003890. 10.1136/jitc-2021-003890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh LA, Quail DF. Decoding the tumor microenvironment with spatial technologies. Nat Immunol 2023;24:1982–93. 10.1038/s41590-023-01678-9 [DOI] [PubMed] [Google Scholar]
- 21. Chu J, Gao F, Yan M, et al. Natural killer cells: a promising immunotherapy for cancer. J Transl Med 2022;20:240. 10.1186/s12967-022-03437-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther 2021;6:127. 10.1038/s41392-021-00506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Truxova I, Cibula D, Spisek R, et al. Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma. J Immunother Cancer 2023;11:e005968. 10.1136/jitc-2022-005968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allen TM, Brehm MA, Bridges S, et al. Humanized immune system mouse models: progress, challenges and opportunities. Nat Immunol 2019;20:770–4. 10.1038/s41590-019-0416-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chuprin J, Buettner H, Seedhom MO, et al. Humanized mouse models for immuno-oncology research. Nat Rev Clin Oncol 2023;20:192–206. 10.1038/s41571-022-00721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michaeli DT, Michaeli T, Albers S, et al. Clinical benefit, development, innovativeness, trials, epidemiology, and price for cancer drugs and indications with multiple special FDA designations. SSRN Journal 2023. 10.2139/ssrn.4539269 [DOI] [PubMed] [Google Scholar]
- 27. Le Tourneau C, André F, Helland Å, et al. Modified study designs to expand treatment options in personalised oncology: a multistakeholder view. Eur J Cancer 2023;194:113278. 10.1016/j.ejca.2023.113278 [DOI] [PubMed] [Google Scholar]
- 28. Finck A, Gill SI, June CH. Cancer immunotherapy comes of age and looks for maturity. Nat Commun 2020;11:3325. 10.1038/s41467-020-17140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finck AV, Blanchard T, Roselle CP, et al. Engineered cellular immunotherapies in cancer and beyond. Nat Med 2022;28:678–89. 10.1038/s41591-022-01765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharma P, Goswami S, Raychaudhuri D, et al. Immune checkpoint therapy-current perspectives and future directions. Cell 2023;186:1652–69. 10.1016/j.cell.2023.03.006 [DOI] [PubMed] [Google Scholar]
- 31. Mangani D, Yang D, Anderson AC. Learning from the nexus of autoimmunity and cancer. Immunity 2023;56:256–71. 10.1016/j.immuni.2023.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morad G, Helmink BA, Sharma P, et al. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2022;185. 10.1016/j.cell.2022.01.008 [DOI] [PubMed] [Google Scholar]
- 33. Goodman RS, Jung S, Balko JM, et al. Biomarkers of immune checkpoint inhibitor response and toxicity: challenges and opportunities. Immunol Rev 2023;318:157–66. 10.1111/imr.13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakamura Y. Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Front Med (Lausanne) 2019;6:119. 10.3389/fmed.2019.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borcoman E, Nandikolla A, Long G, et al. Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educ Book 2018;38:169–78. 10.1200/EDBK_200643 [DOI] [PubMed] [Google Scholar]
- 36. Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol 2019;30:385–96. 10.1093/annonc/mdz003 [DOI] [PubMed] [Google Scholar]
- 37. Kluger HM, Tawbi HA, Ascierto ML, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC immunotherapy resistance taskforce. J Immunother Cancer 2020;8:e000398. 10.1136/jitc-2019-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tawbi HA, Sullivan RJ, Feltquate D, et al. Society for immunotherapy of cancer (SITC) checkpoint inhibitor resistance definitions: efforts to Harmonize terminology and accelerate immuno-oncology drug development. J Immunother Cancer 2023;11:e007309. 10.1136/jitc-2023-007309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pilard C, Ancion M, Delvenne P, et al. Cancer immunotherapy: it’s time to better predict patients' response. Br J Cancer 2021;125:927–38. 10.1038/s41416-021-01413-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sivapalan L, Murray JC, Canzoniero JV, et al. Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. J Immunother Cancer 2023;11:e005924. 10.1136/jitc-2022-005924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shankar LK, Schöder H, Sharon E, et al. Harnessing imaging tools to guide immunotherapy trials: summary from the National Cancer Institute cancer imaging steering committee workshop. Lancet Oncol 2023;24:e133–43. 10.1016/S1470-2045(22)00742-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Addala V, Newell F, Pearson JV, et al. Computational immunogenomic approaches to predict response to cancer immunotherapies. Nat Rev Clin Oncol 2024;21:28–46. 10.1038/s41571-023-00830-6 [DOI] [PubMed] [Google Scholar]
- 43. Prelaj A, Miskovic V, Zanitti M, et al. Artificial intelligence for predictive biomarker discovery in immuno-oncology: a systematic review. Ann Oncol 2024;35:29–65. 10.1016/j.annonc.2023.10.125 [DOI] [PubMed] [Google Scholar]
- 44. Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the Ipilimumab approval Decennial. Nat Rev Drug Discov 2022;21:509–28. 10.1038/s41573-021-00345-8 [DOI] [PubMed] [Google Scholar]
- 45. Dagher OK, Schwab RD, Brookens SK, et al. Advances in cancer immunotherapies. Cell 2023;186:1814. 10.1016/j.cell.2023.02.039 [DOI] [PubMed] [Google Scholar]
- 46. Sellars MC, Wu CJ, Fritsch EF. Cancer vaccines: building a bridge over troubled waters. Cell 2022;185:2770–88. 10.1016/j.cell.2022.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goebeler ME, Bargou RC. T cell-engaging therapies - bites and beyond. Nat Rev Clin Oncol 2020;17:418–34. 10.1038/s41571-020-0347-5 [DOI] [PubMed] [Google Scholar]
- 48. Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res 2009;15:5323–37. 10.1158/1078-0432.CCR-09-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Derman BA, Parker WF. Fair allocation of scarce CAR T-cell therapies for relapsed/refractory multiple myeloma. JAMA 2023. 10.1001/jama.2023.11846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moreau P, Garfall AL, van de Donk NWCJ, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med 2022;387:495–505. 10.1056/NEJMoa2203478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matarasso S, Assouline S. Mosunetuzumab and the emerging role of T-cell-engaging therapy in follicular lymphoma. Future Oncol 2023;19:2083–101. 10.2217/fon-2023-0274 [DOI] [PubMed] [Google Scholar]
- 52. Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol 2023;41:2238–47. 10.1200/JCO.22.01725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dickinson MJ, Carlo-Stella C, Morschhauser F, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2022;387:2220–31. 10.1056/NEJMoa2206913 [DOI] [PubMed] [Google Scholar]
- 54. Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 2021;385:1196–206. 10.1056/NEJMoa2103485 [DOI] [PubMed] [Google Scholar]
- 55. Ahn M-J, Cho BC, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. New England Journal of Medicine 2023;389:2063–75. 10.1056/NEJMoa2307980 [DOI] [PubMed] [Google Scholar]
- 56. Deshpande RP, Sharma S, Watabe K. The confounders of cancer immunotherapy: roles of lifestyle, metabolic disorders and sociological factors. Cancers (Basel) 2020;12:2983. 10.3390/cancers12102983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park EM, Chelvanambi M, Bhutiani N, et al. Targeting the gut and tumor microbiota in cancer. Nat Med 2022;28:690–703. 10.1038/s41591-022-01779-2 [DOI] [PubMed] [Google Scholar]
- 58. Esplin ED, Nielsen SM, Bristow SL, et al. Universal Germline genetic testing for hereditary cancer syndromes in patients with solid tumor cancer. JCO Precis Oncol 2022;6:e2100516. 10.1200/PO.21.00516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mastrodomenico L, Piombino C, Riccò B, et al. Personalized systemic therapies in hereditary cancer syndromes. Genes (Basel) 2023;14:684. 10.3390/genes14030684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gamble LA, Heller T, Davis JL. Hereditary diffuse gastric cancer syndrome and the role of CDH1: a review. JAMA Surg 2021;156:387–92. 10.1001/jamasurg.2020.6155 [DOI] [PubMed] [Google Scholar]
- 61. Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer. JAMA 2019;322:652. 10.1001/jama.2019.10987 [DOI] [PubMed] [Google Scholar]
- 62. Pagadala M, Sears TJ, Wu VH, et al. Germline modifiers of the tumor immune microenvironment implicate drivers of cancer risk and immunotherapy response. Nat Commun 2023;14:2744. 10.1038/s41467-023-38271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fonseka LN, Woo BK. Consumer wearables and the integration of new objective measures in oncology: patient and provider perspectives. JMIR Mhealth Uhealth 2021;9:e28664. 10.2196/28664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Herati RS, Silva LV, Vella LA, et al. Vaccine-induced ICOS(+)CD8(+) circulating Tfh are sensitive Biosensors of age-related changes in inflammatory pathways. Cell Reports Medicine 2021;2:100262. 10.1016/j.xcrm.2021.100262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clish CB. Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harb Mol Case Stud 2015;1:a000588. 10.1101/mcs.a000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol 2018;18:183–94. 10.1038/nri.2017.140 [DOI] [PubMed] [Google Scholar]
- 68. Spira A, Yurgelun MB, Alexandrov L, et al. Precancer atlas to drive precision prevention trials. Cancer Res 2017;77:1510–41. 10.1158/0008-5472.CAN-16-2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Unver N, Mohindroo C. Targets and strategies for cancer immunoprevention. Methods Mol Biol 2022;2435:7–17. 10.1007/978-1-0716-2014-4_2 [DOI] [PubMed] [Google Scholar]
- 70. Mohindroo C, Unver N. Mechanisms of antitumor immunity and immunosurveillance. Methods Mol Biol 2022;2435:1–6. 10.1007/978-1-0716-2014-4_1 [DOI] [PubMed] [Google Scholar]
- 71. Finn OJ. Human tumor antigens yesterday, today, and tomorrow. Cancer Immunol Res 2017;5:347–54. 10.1158/2326-6066.CIR-17-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chung AS, Mettlen M, Ganguly D, et al. Immune checkpoint inhibition is safe and effective for liver cancer prevention in a mouse model of hepatocellular carcinoma. Cancer Prevention Research 2020;13:911–22. 10.1158/1940-6207.CAPR-20-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li BT, Daly B, Gospodarowicz M, et al. Reimagining patient-centric cancer clinical trials: a multi-stakeholder International coalition. Nat Med 2022;28:620–6. 10.1038/s41591-022-01775-6 [DOI] [PubMed] [Google Scholar]
- 74. SITC crisis in clinical research virtual summit. n.d. Available: https://www.sitcancer.org/advocacy/crisis-in-clinical-research#265
- 75. Topaloglu U, Palchuk MB. Using a Federated network of real-world data to optimize clinical trials operations. JCO Clin Cancer Inform 2018;2:1–10. 10.1200/CCI.17.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Richard E, Reddy B. Text classification for clinical trial operations: evaluation and comparison of natural language processing techniques. Ther Innov Regul Sci 2021;55:447–53. 10.1007/s43441-020-00236-x [DOI] [PubMed] [Google Scholar]
- 77. Ismail A, Al-Zoubi T, El Naqa I, et al. The role of artificial intelligence in hastening time to recruitment in clinical trials. BJR Open 2023;5:20220023. 10.1259/bjro.20220023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mahoney ME, Sridhar SS. Clinical trial reform in the post-COVID era. Ther Adv Med Oncol 2023;15:17588359231183676. 10.1177/17588359231183676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med 2017;377:62–70. 10.1056/NEJMra1510062 [DOI] [PubMed] [Google Scholar]
- 80. The Adaptive Platform Trials Coalition . Adaptive platform trials C. adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov 2019;18:797–807. 10.1038/s41573-019-0034-3 [DOI] [PubMed] [Google Scholar]
- 81. Subbiah V, Burris HA, Kurzrock R. Revolutionizing cancer drug development: harnessing the potential of basket trials. Cancer 2024;130:186–200. 10.1002/cncr.35085 [DOI] [PubMed] [Google Scholar]
- 82. Michaud S, Needham J, Sundquist S, et al. Patient and patient group engagement in cancer clinical trials: a stakeholder charter. Curr Oncol 2021;28:1447–58. 10.3390/curroncol28020137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Topalian SL, Forde PM, Emens LA, et al. Neoadjuvant immune checkpoint blockade: a window of opportunity to advance cancer immunotherapy. Cancer Cell 2023;41:1551–66. 10.1016/j.ccell.2023.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stanton SE, Castle PE, Finn OJ, et al. Advances and challenges in immunoprevention and immune interception. J Immunother Cancer 2024;12:e007815. 10.1136/jitc-2023-007815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2024-009063supp001.pdf (69.2KB, pdf)