Abstract
Objectives
Muscle function and size decline with age, but long-term effects of resistance training in older adults are largely unknown. Here, we explored the long-lasting (3 years) effects of 1 year of supervised resistance training with heavy loads.
Methods
The LIve active Successful Ageing (LISA) study was a parallel group randomised controlled trial at a university hospital in Denmark. Older adults (n=451) at retirement age were randomised to 1 year of heavy resistance training (HRT), moderate-intensity training (MIT) or a non-exercising control group (CON). Primary outcome measure was leg extensor power. Secondary outcomes included maximal isometric quadriceps torque (isometric leg strength) and body composition (dual-energy X-ray absorptiometry (DXA)). Participants completed test procedures at baseline, following the 1-year intervention, and 2 and 4 years post study start.
Results
At the 4-year assessment, 369 participants attended (mean age=71 years, 61% women). The main finding was that across all four time points, there was a significant group×time interaction in isometric leg strength (F6,1049=8.607, p<0.001, =0.05). Individuals in HRT maintained baseline performance in isometric leg strength (Baseline: 149.7±51.5 Nm, 4 years: 151.5±51.1 Nm, t(1050)=1.005, p=1.00) while participants in CON and MIT decreased.
Conclusion
In well-functioning older adults at retirement age, 1 year of HRT may induce long-lasting beneficial effects by preserving muscle function.
Trial registration number
Keywords: Training, Aging, Body composition, Exercise, Skeletal muscle
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Worldwide, the ageing population is growing. Unfortunately, skeletal muscle function and autonomy decrease with increased age. Thus, a challenge for society is to promote a healthy lifespan without age-related diseases and loss of autonomy.
WHAT THIS STUDY ADDS
Despite relatively healthy and well-functioning participants, 1 year of heavy resistance training at retirement age resulted in maintained strength 4 years after the study started. We propose that higher load resistance training may play an important role to induce long-lasting adaptations.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides evidence that resistance training with heavy loads at retirement age can have long-term effects over several years. The results, therefore, provide means for practitioners and policy-makers to encourage older individuals to engage in heavy resistance training.
Introduction
Skeletal muscle function declines with advancing age.1–3 Although resistance training may partly counteract loss of muscle mass and function, shorter training studies (6–9 months duration) only show somewhat preserved muscle mass and function at 6–12 months follow-up.4 5 Unfortunately, long-term follow-ups are sparse.6 In one study, strength gains following high-intensity resistance training, and not low-intensity training, were preserved after 48 weeks of detraining.7 The LIve active Successful Ageing (LISA) study, a large-scale randomised controlled trial (n=451), showed that strength can be maintained over 12 months following 1 year of heavy resistance training (HRT), but not after moderate training.8 Thus, to gain long-lasting effects of resistance training in ageing one could speculate that high intensity or heavy loads are required. Here, we investigated whether there would be long-lasting effects of a 1-year supervised resistance training regimen with heavy loads, 3 years following the training in older individuals at retirement age.
Methods
Intervention
The current manuscript is an interim analysis of the LISA study, and additional follow-ups are planned (7-year and 10-year follow-ups). For details of intervention, recruitment and power calculations, see previous publications.9 10 Briefly, 451 older adults were stratified according to sex, body mass index (BMI) and chair-rise test performance and randomised to 1 year of training with either heavy loads (HRT, n=149), moderate-intensity training (MIT, n=154) or a control condition (CON, n=148). At a commercial gym, HRT performed a supervised full body programme three times per week, with 6–8 weeks of initial habituation. The periodisation programme was machine based and each exercise included 3 sets of 6–12 repetitions at ~70%–85% of 1 RM, which was estimated using the prediction equation according to methods by Brzycki.11 12 The moderate training in MIT was performed as circuit training with body weight and resistance bands once per week at the hospital and two times per week at home. Exercises in MIT progressed with the load of resistance bands (TheraBand, Akron, Ohio, USA) and mimicked the exercises in HRT but were performed with 3 sets of 10–18 repetitions at ~50%–60% of 1 RM. Both training programmes were created to comply with recommended guidelines13 and included nine exercises—see published study protocol for full details.10 Individuals in CON were encouraged to maintain their habitual physical activity level and were invited to regular cultural and social activities. In general, participants did not receive advice on healthy behaviour but were aware of the study timeline and planned follow-ups.
Test procedures
Day 1 included a health screening. On day 2, participants were dual-energy X-ray absorptiometry (DXA) scanned. Visceral fat mass was estimated by scanner software (Lunar iDXA, GE HealthCare—enCORE software V.16). Isometric leg strength (quadriceps) was assessed in a Good Strength chair (Bluetooth V.3.14, Metitur) and maximal isometric quadriceps torque (Newton metres) was measured during a minimum of 3 attempts per leg.14 15 Day 3 included MRI of the brain and thigh (two-dimensional T1-weighted, 3.0 Tesla Phillips Achieva). Blinded assessors determined CSA of m. vastus lateralis using JIM software (Xinapse systems).
Daily physical activity was assessed as daily step count between days 2 and 3, by an accelerometer (activPAL micro, PAL Technologies) worn by the participants for five consecutive days. The test procedures were performed at baseline, postintervention (year 1) and at 2-year and 4-year follow-ups.
Patient and public involvement
Participants were informed of study progress through newsletters, and received overviews of personal results after tests at each time point. Additionally, participants were invited to an information evening, where the general study results at the time were presented.
Statistical analysis
Statistical analyses were performed in R V.4.1.1 and Rstudio 2021.09.0 using ‘psych’,16 ‘emmeans’17 and ‘sjstats’18 packages. Figures were created in GraphPad Prism V.10.0.3.
Descriptive data are presented as mean±SD. Student’s paired t-tests tested changes from baseline to year 4 in sample characteristics. Two-way mixed-model analyses of variance (ANOVAs), adjusted for age and sex, were used to test group×time interaction effects on strength (power, isometric leg strength and handgrip), and on body composition (lean body mass, lean leg mass, CSA of m. vastus lateralis, fat percentage and visceral fat content) including all four available time points. For Δchanges from baseline to year 4, one-way mixed-model ANOVAs were used to test group differences. The ANOVAs were controlled for sex and age. Significant interactions were further examined with post hoc tests. Effect sizes are reported in the form of eta squared ( ). The significance level was p<0.006 after Bonferroni correction for multiple comparisons (eight tests).
Results
At year 4, 369 participants attended follow-up assessments (HRT, n=128; MIT, n=126; CON, n=115). 82 older adults dropped out primarily due to lack of motivation or severe illness. These individuals had higher body weight, BMI and waist circumference at baseline compared with participants who were still part of the study at year 4. However, there was no difference in the response to the intervention in all outcomes at year 1 assessments between participants and individuals subsequently lost to follow-up. On average, participants were 71 years old (range: 64–75 years), 61% women and still active based on the daily physical activity (table 1). There was no difference in sample characteristics between groups at baseline or at follow-up.
Table 1.
Sample characteristics (mean±SD), n=369 unless otherwise specified
| Baseline | 4 years | T-test | |
| Sex (men/women, %) | 39/61 | 39/61 | – |
| Age (years) | 66.4±2.5 | 70.5±2.5 | – |
| Body weight (kg) | 75.7±13.6 | 75.3±14.0 | P=0.09 |
| BMI (kg/m2) | 25.8±4.0 | 25.9±4.3 | P=0.10 |
| Waist circumference (cm) (n=367) | 92.7±11.5 | 92.5±12.2 | P=0.66 |
| Daily physical activity (steps/day) (n=349) | 9548±3446 | 9590±3387 | P=0.79 |
BMI, body mass index.
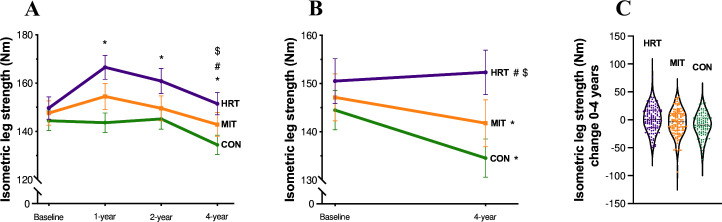
For isometric leg strength, there was a significant group×time interaction across the four time points (F6,1049 = 8.607, p<0.001, = 0.05; figure 1A). In HRT, the strength was unaltered after 4 years (Baseline: 149.7±51.5 Nm, 4 years: 151.5±51.1 Nm, t(1050)=1.005, p=1.00), unlike the CON group in which strength decreased (Baseline: 144.4±43.3 Nm, 4 years: 134.5±42.2 Nm, t(1050)=−5.261, p<0.001). The decrease in MIT was not significant (Baseline: 147.6±54.9 Nm, 4 years: 142.9±54.6 Nm, t(1050)=−2.594, p=0.28). For the Δchanges over the 4 years, HRT significantly differed from MIT and CON (HRT>MIT, t(350) = 3.273, p=0.003; HRT>CON, t(350) = 3.655, p<0.001).
Figure 1.
(A–C) Isometric strength (mean±SEM) across 4 years for the different groups (heavy resistance training, HRT, moderate-intensity training, MIT and control group, CON). (A) (n=353) Isometric leg strength (Nm) trajectories for all time points separated by group. (B) Baseline and 4-year follow-up data (n=362), each group shown separately. (C) Individual data points showing the distribution of change from baseline to year four separated by group. *Significantly different from baseline (A): HRT 1 year, p<0.001; MIT 1 year, p=0.01; HRT 2 years, p<0.001; CON 4 years, p<0.001) (B): MIT 4 years, p=0.01; CON 4 years, p<0.001). #Change from baseline significantly different from change in MIT (A): HRT 4 years, p=0.003) (B): HRT 4 years, p=0.03). $Change from baseline significantly different from change in CON (A): HRT 4 years, p<0.001) (B): HRT 4 years, p<0.001).
In the change from baseline to year 4 (figure 1B), muscle strength was decreased in MIT (t(122)=1.98, p=0.01) and in CON (t(113)=1.98, p<0.001), whereas it was maintained in HRT (t(124)=1.98, p=0.37).
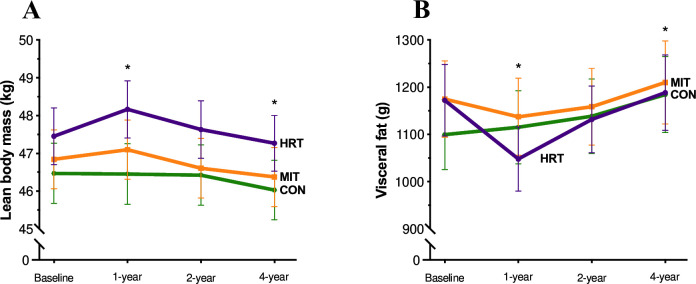
There was a significant group×time interaction for lean body mass (F6,1085 = 5.353, p<0.001, = 0.03), see figure 2A, again in favour of HRT (Baseline: 47.5±8.5 kg, 4 year: 47.3±8.3 kg, t(1086)=−1.813, p=0.81), compared with MIT (Baseline: 46.8±8.7 kg, 4 years: 46.4±8.6 kg, t(1086)=−4.506, p<0.001) and CON (Baseline: 46.5±8.5 kg, 4 years: 46.0±8.5 kg, t(1086)=−4.075, p=0.003).
Figure 2.
(A–B) Lean body mass and visceral fat (mean±SEM) across 4 years for the different groups (heavy resistance training, HRT, moderate-intensity training, MIT and control group, CON). (A) (n=365) Lean body mass (kg) trajectories for all time points separated by group. (B) Visceral fat (g) trajectories (n=365), for all time points separated by group. *Significantly different from baseline (A): HRT 1 year, p<0.001; MIT 4 years, p<0.001; CON 4 years, p=0.003) (B): HRT 1 year, p=0.01; CON 4 years, p=0.04).
Additionally, there was a significant group×time interaction for visceral fat (F6,1085 = 3.120, p=0.005, = 0.02), see figure 2B. HRT (Baseline: 1172.4±854.6 g, 4 years: 1188.5±898.3 g, t(1086)=0.676, p=1.00) and MIT (Baseline: 1175.0±897.4 g, 4 years: 1210.1±972.9 g, t(1086)=1.450, p=0.95) did not change over the 4 years, while in CON, there was an increase in visceral fat content (Baseline: 1099.6±794.7 g, 4 years: 1184.4±862.5 g, t(1086)=3.387, p=0.04).
Significant group×time interactions for CSA of m. vastus lateralis and the percentage of total body fat (table 2) were driven by 1-year and 2-year changes, which have been reported previously.8 9
Table 2.
Outcome variables (mean±SD) at baseline and at 4 years separated by group
| HRT | MIT | CON | F (group×time) |
F (time) |
||||
| Baseline | 4 years | Baseline | 4 years | Baseline | 4 years | |||
| Power (W) |
195.3±65.1 | 185.3±60.2 | 193.2±64.0 | 179.4±64.1 | 187.7±61.8 | 171.2±57.2 | F6,1067=1.054 p=0.39, =0.006 |
F3,1067=43.651 p<0.001, =0.11 |
| Handgrip (kg) |
35.6±10.5 | 34.3±9.9 | 34.3±10.4 | 32.7±10.2 | 34.9±10.2 | 32.8±9.9 | F6,1064=0.554 p=0.77, =0.003 |
F3,1064=27.789 p<0.001, =0.07 |
| LLM (kg) |
17.0±3.3 | 16.3±3.1 | 16.7±3.5 | 15.9±3.3 | 16.4±3.4 | 15.7±3.2 | F6,1085=1.841 p=0.09, =0.01 |
F3,1085=249.742 p<0.001, =0.41 |
| CSA (mm2) |
1403.7±339.7 | 1306.2±328.4 | 1371.4±358.5 | 1284.3±352.1 | 1359.5±330.7 | 1243.1±293.8 | F6,908=3.654 p=0.001, =0.02 |
F3,908=36.068 p<0.001, =0.11 |
| Body fat (%) |
34.0±7.9 | 33.8±8.2 | 33.5±7.6 | 33.7±6.4 | 32.7±8.3 | 33.1±8.5 | F6,1085=3.813 p<0.001, =0.02 |
F3,1085=16.964 p<0.001, =0.04 |
Leg extensor power (power), handgrip strength (handgrip), lean leg mass (LLM), CSA of m. vastus lateralis (CSA) and total body fat (body fat). F-statistics for group×time interaction and effect of time.
CON, control group; HRT, heavy resistance training; MIT, moderate intensity training.
For leg extensor power, handgrip strength and lean leg mass, there was a main effect of time, with decreases over the 4 years across all groups, but no interaction effects or significant group differences for the Δchange over 4 years (table 2).
Discussion
Resistance training with heavy loads induced long-lasting beneficial effects on muscle strength in a sample of older adults. We observed a difference between groups in leg strength, whereas handgrip strength, a measure of overall muscle strength,19 was not influenced by any of the training regimes. Notably, benefits in leg strength were present despite lowered leg lean mass. Neural adaptations influence the response to resistance training.20 21 The present results suggest that these adaptations might play a role even as lean leg mass and thigh CSA decrease. This is in line with a recent report showing that prolonged training across the lifespan is associated with permanently elevated acetylcholine receptors and improved neuromuscular function.22 Resistance training may, therefore, be beneficial for function beyond the influence of muscle size itself.
Despite no group effects in lean leg mass, HRT maintained total lean mass, yet differences were minor. Interestingly, leg muscle strength was maintained from baseline in HRT, indicating that among individuals who already seemed to have a high physical activity level but were previously resistance training naive, implementing resistance training with heavy loads for 1 year may at group-level induce long-term health effects. Considering that muscle strength has been shown to predict mortality in apparently healthy populations,23 these results may be of particular relevance. It is somewhat surprising that there was no muscular effect of the moderate training at year 4, as the intervention improved both lean mass and function in MIT, although to a lesser extent than HRT.
Interestingly, the amount of visceral fat was maintained from baseline to year 4 in both training groups, implying that some parameters may not be load-dependent or intensity-dependent in the long term. Recent research suggests that visceral fat is positively affected by resistance training.24 Like visceral fat, the decrease over time in leg extensor power (primary outcome measure) was in line with our previous studies.8 9
The present study benefited from its large sample size, long intervention and multiple follow-ups. Further, study attendance remained high (82% at year 4). Of note, with almost 10 000 daily steps, the study sample is likely to be healthier and more active than the average ageing population. Even so, ≈80% of the participants had at least one chronic medical disease.9 In age-matched older individuals living in residential care facilities, high-intensity functional training has proven effective in improving independence in activities of daily living. Although over 4 months, these results show further evidence of the effectiveness of high-intensity training in older adults.25
In conclusion, we showed that in a group of well-functioning older adults around retirement age, 1 year of HRT may induce long-lasting beneficial effects by preserving muscle function.
Acknowledgments
The authors thank Sussi Larsen, Kenneth Hudlebusch Mertz, Christian Skou Eriksen and Andreas Kraag Ziegler for helping with data collection.
Footnotes
Contributors: Conceptualisation: MK; Methodology: MK and C-JB; Validation: MB-I, ATG, KK, ND, MK and C-JB; Formal analysis: MB-I and ND; Investigation: MB-I, ATG and KK; Visualisation: MB-I; Supervision: MK and C-JB; Project administration: MB-I and ATG; Writing–original draft: MB-I; Writing–review and editing: MB-I, ATG, KK, ND, MK and C-JB. All authors approved the final manuscript to be published.
Funding: Lundbeck Foundation (R380-2021-1269) and supported by Nordea Foundation (Grant from Center for Healthy Aging, University of Copenhagen, Denmark).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Regional ethics committee: Capital Region, Copenhagen, Denmark, No. H-3-2014-017. Participants gave informed consent to participate in the study before taking part.
References
- 1. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an Undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J Am Med Dir Assoc 2011;12:249–56. 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suetta C, Haddock B, Alcazar J, et al. The Copenhagen Sarcopenia study: lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J Cachexia Sarcopenia Muscle 2019;10:1316–29. 10.1002/jcsm.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernández-Lezaun E, Schumann M, Mäkinen T, et al. Effects of resistance training frequency on cardiorespiratory fitness in older men and women during intervention and follow-up. Exp Gerontol 2017;95:44–53. 10.1016/j.exger.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 5. Snijders T, Leenders M, de Groot L, et al. Muscle mass and strength gains following 6 months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp Gerontol 2019;121:71–8. 10.1016/j.exger.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 6. Kennis E, Verschueren SM, Bogaerts A, et al. Long-term impact of strength training on muscle strength characteristics in older adults. Arch Phys Med Rehabil 2013;94:2054–60. 10.1016/j.apmr.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 7. Fatouros IG, Kambas A, Katrabasas I, et al. Strength training and Detraining effects on muscular strength, anaerobic power, and mobility of inactive older men are intensity dependent. Br J Sports Med 2005;39:776–80. 10.1136/bjsm.2005.019117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gylling AT, Bloch-Ibenfeldt M, Eriksen CS, et al. Maintenance of muscle strength following a one-year resistance training program in older adults. Exp Gerontol 2020;139:111049. 10.1016/j.exger.2020.111049 [DOI] [PubMed] [Google Scholar]
- 9. Gylling AT, Eriksen CS, Garde E, et al. The influence of prolonged strength training upon muscle and fat in healthy and chronically diseased older adults. Exp Gerontol 2020;136:110939. 10.1016/j.exger.2020.110939 [DOI] [PubMed] [Google Scholar]
- 10. Eriksen CS, Garde E, Reislev NL, et al. Physical activity as intervention for age-related loss of muscle mass and function: protocol for a randomised controlled trial (the LISA study). BMJ Open 2016;6:e012951. 10.1136/bmjopen-2016-012951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brzycki M. Scholastic coach and athletic director. In: Strength training -- A practical approach to strength training. 65. 1995: 86. [Google Scholar]
- 12. Wood TM, Maddalozzo GF, Harter RA. Accuracy of Seven Equations for Predicting 1-RM Performance of Apparently Healthy, Sedentary Older Adults. Lombardi, 1993. [Google Scholar]
- 13. American College of Sports Medicine . Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009;41:687–708. 10.1249/MSS.0b013e3181915670 [DOI] [PubMed] [Google Scholar]
- 14. Bieler T, Magnusson SP, Kjaer M, et al. Intra-Rater Reliability and agreement of muscle strength, power and functional performance measures in patients with hip osteoarthritis. J Rehabil Med 2014;46:997–1005. 10.2340/16501977-1864 [DOI] [PubMed] [Google Scholar]
- 15. Tiainen K, Sipilä S, Alen M, et al. Heritability of maximal Isometric muscle strength in older female twins. J Appl Physiol (1985) 2004;96:173–80. 10.1152/japplphysiol.00200.2003 [DOI] [PubMed] [Google Scholar]
- 16. Revelle W. R package version 2.3.6. Psych: Procedures for Psychological, Psychometric, and Personality Research. Evanston, Illinois: Northwestern University, 2023. Available: https://CRAN.R-project.org/package=psych [Google Scholar]
- 17. Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. The American Statistician 1980;34:216–21. 10.1080/00031305.1980.10483031 [DOI] [Google Scholar]
- 18. Lüdecke D. [10.5281/zenodo.1284472]. sjstats: Statistical Functions for Regression Models (Version 0.18.2), 2022. Available: https://CRAN.R-project.org/package=sjstats
- 19. Rijk JM, Roos PR, Deckx L, et al. Prognostic value of Handgrip strength in people aged 60 years and older: A systematic review and meta-analysis. Geriatr Gerontol Int 2016;16:5–20. 10.1111/ggi.12508 [DOI] [PubMed] [Google Scholar]
- 20. Häkkinen K, Kallinen M, Izquierdo M, et al. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985) 1998;84:1341–9. 10.1152/jappl.1998.84.4.1341 [DOI] [PubMed] [Google Scholar]
- 21. Walker S. Evidence of resistance training-induced neural adaptation in older adults. Exp Gerontol 2021;151:111408. 10.1016/j.exger.2021.111408 [DOI] [PubMed] [Google Scholar]
- 22. Soendenbroe C, Dahl CL, Meulengracht C, et al. Preserved stem cell content and Innervation profile of elderly human Skeletal muscle with lifelong recreational exercise. J Physiol 2022;600:1969–89. 10.1113/JP282677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. García-Hermoso A, Cavero-Redondo I, Ramírez-Vélez R, et al. Muscular strength as a Predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch Phys Med Rehabil 2018;99:2100–13. 10.1016/j.apmr.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 24. Wewege MA, Desai I, Honey C, et al. The effect of resistance training in healthy adults on body fat percentage, fat mass and visceral fat: a systematic review and meta-analysis. Sports Med 2022;52:287–300. 10.1007/s40279-021-01562-2 [DOI] [PubMed] [Google Scholar]
- 25. Toots A, Littbrand H, Lindelöf N, et al. Effects of a high-intensity functional exercise program on dependence in activities of daily living and balance in older adults with dementia. J Am Geriatr Soc 2016;64:55–64. 10.1111/jgs.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]