Abstract
Maintaining a balanced bile acids (BAs) metabolism is essential for lipid and cholesterol metabolism, as well as fat intake and absorption. The development of obesity may be intricately linked to BAs and their conjugated compounds. Our study aims to assess how BAs influence the obesity indicators by Mendelian randomization (MR) analysis. Instrumental variables of 5 BAs were obtained from public genome-wide association study databases, and 8 genome-wide association studies related to obesity indicators were used as outcomes. Causal inference analysis utilized inverse-variance weighted (IVW), weighted median, and MR-Egger methods. Sensitivity analysis involved MR-PRESSO and leave-one-out techniques to detect pleiotropy and outliers. Horizontal pleiotropy and heterogeneity were assessed using the MR-Egger intercept and Cochran Q statistic, respectively. The IVW analysis revealed an odds ratio of 0.94 (95% confidence interval: 0.88, 1.00; P = .05) for the association between glycolithocholate (GLCA) and obesity, indicating a marginal negative causal association. Consistent direction of the estimates obtained from the weighted median and MR-Egger methods was observed in the analysis of the association between GLCA and obesity. Furthermore, the IVW analysis demonstrated a suggestive association between GLCA and trunk fat percentage, with a beta value of −0.014 (95% confidence interval: −0.027, −0.0004; P = .04). Our findings suggest a potential negative causal relationship between GLCA and both obesity and trunk fat percentage, although no association survived corrections for multiple comparisons. These results indicate a trend towards a possible association between BAs and obesity, emphasizing the need for future studies.
Keywords: bile acids, causal association, Mendelian randomization, obesity, obesity indicators
1. Introduction
Obesity is defined as the abnormal accumulation of fat, leading to a disruption in energy metabolism.[1,2] Over 2 billion individuals globally, constituting 30% of the world’s population, are afflicted by overweight or obesity, based on statistical data.[3] This condition significantly contributes to a range of cardiovascular and metabolic ailments,[4,5] such as diabetes, which is associated with various complications.[6–11] Therefore, early detection and diagnosis of obesity are paramount. While body mass index (BMI) serves as a widely utilized tool for obesity assessment in clinical practice,[3,12–18] it fails to consider factors such as body fat distribution and muscle mass, which may contribute to the obesity paradox[19–26] (in patients with preexisting cardiovascular disease, individuals who are overweight or obese exhibit a more favorable prognosis compared to those who are non-overweight/nonobese).[4,27] To address this limitation, it is essential to incorporate other obesity-related biomarkers for a comprehensive evaluation. The biological indicators considered in our study encompass body fat percentage, BMI, hip circumference, trunk fat mass, trunk fat percentage, waist circumference (WC), and whole-body fat mass.
Furthermore, previous studies have found that bile acids (BAs) may influence obesity by altering fatty acid metabolism.[28] BAs, derived from cholesterol biosynthesis, are synthesized in the adult liver at a daily conversion rate of around 500 milligrams.[29] The main function of bile salts is to emulsify fats. Primary BAs, including cholate (CA) and chenodeoxycholate, are synthesized by the liver and stored as bile salts in the gallbladder, where they function during digestion.[30] The intestinal microbiota has the capacity to metabolize primary BAs into secondary BAs with increased hydrophobicity, such as deoxycholate (DCA) and lithocholate (LCA), which possess enhanced lipolytic properties and facilitate the digestion and absorption of fats.[31] Glycine or taurine conjugation precedes the departure of most BAs from hepatocytes, giving rise to conjugated BAs like glycochenodeoxycholate (GCDCA), glycolithocholate (GLCA), taurochenodeoxycholate (TCDCA), and others.[32]
While epidemiological evidence has indicated a potential association of BAs and their conjugates with obesity, BMI, and WC, the findings remain inconclusive. To overcome this uncertainty, we conducted a Mendelian randomization (MR) analysis, employing genetic variations as instrumental variables (IVs). This method effectively addresses confounding factors and reverse causality biases, allowing us to derive more robust and compelling causal conclusions.[33,34] The clinical significance of this research lies in its guidance for tackling obesity and metabolic diseases, as well as its contribution to understanding the underlying mechanisms of these disorders.
2. Methods
2.1. Study design
We employed MR to examine the correlation between genetically predicted bile acids and indicators of obesity. Single nucleotide polymorphisms (SNPs) are frequently employed as IVs in MR analysis, a methodology utilized to ascertain causal associations between traits and diseases. Before conducting MR analysis, it is crucial to verify that the chosen SNP meets 3 assumptions: (1) The selected SNP must demonstrate a robust association with the exposure variables (bile acids). (2) The chosen SNP should influence the outcome measures (indicators of obesity) solely via the exposure factors (bile acids). (3) No confounding exists regarding the impact of the chosen SNP on the outcome measures (indicators of obesity).
2.2. Data sources
In order to comply with the fundamental principles of a two-sample MR design, exposure and outcome data were sourced from separate European populations. The genome-wide association study (GWAS) datasets for 5 exposures, namely CA, DCA, GCDCA, GLCA, and TCDCA were extracted from a previous study conducted by Chen et al.[35] Additionally, the summary statistics of 8 outcomes related to obesity and its indicators including body fat percentage, BMI, hip circumference, trunk fat mass, trunk fat percentage, WC, and whole-body fat mass were obtained from the UK Biobank, Genetic Investigation of ANthropometric Traits, and FinnGen. Detailed information regarding the utilized GWAS datasets is provided in Table S1, Supplemental Digital Content, http://links.lww.com/MD/M910.
2.3. Selection of IVs
IVs were chosen for the MR analysis based on rigorous selection criteria. The inclusion criteria involved establishing a strong genetic association between the IVs and the exposure of interest, as determined by a P-value < 1 × 10-5. We employed clumping method within a genomic window of 10 megabases to identify independent IVs that exhibited low levels of linkage disequilibrium, denoted by an R2 value below 0.001, as reported previously.[36–38] Consistent with prior research findings, we restricted our analysis to IVs possessing minor allele frequencies exceeding 0.01. We calculated F-statistics as indicators of IV strength; values above or equal to ten signified minimal susceptibility to weak instrument bias.[39]
2.4. Statistical method
The primary method used for the MR analysis was the inverse-variance weighted (IVW) method. Additionally, we employed both the weighted median and MR-Egger methods as alternative approaches. We conducted an MR-Egger intercept test to assess potential horizontal pleiotropy. We incorporated outlier-corrected data from MR-PRESSO to account for potential outliers. We assessed heterogeneity by calculating the Cochrane Q value. A leave-one-out sensitivity analysis was performed to examine individual IV’s influence on causal relationships and validate result reliability. Causal effects in the MR analyses were evaluated using regression coefficients (Beta), while odds ratios along with their corresponding 95% confidence intervals (CIs) were employed for assessing dichotomous variable as outcome. We performed multiple comparisons with a false discovery rate threshold set at 5%. The TwoSampleMR package in R was utilized for all MR analyses.
3. Results
3.1. Assessment of the IVs
This study employed MR analysis to investigate the associations between 5 BAs and 8 indicators of obesity and its related factors. The F-statistics for the IVs of 5 BAs ranged from 19.55 to 38.06, showing good instrument strength (Table S2, Supplemental Digital Content, http://links.lww.com/MD/M911).
3.2. Results of the MR analysis
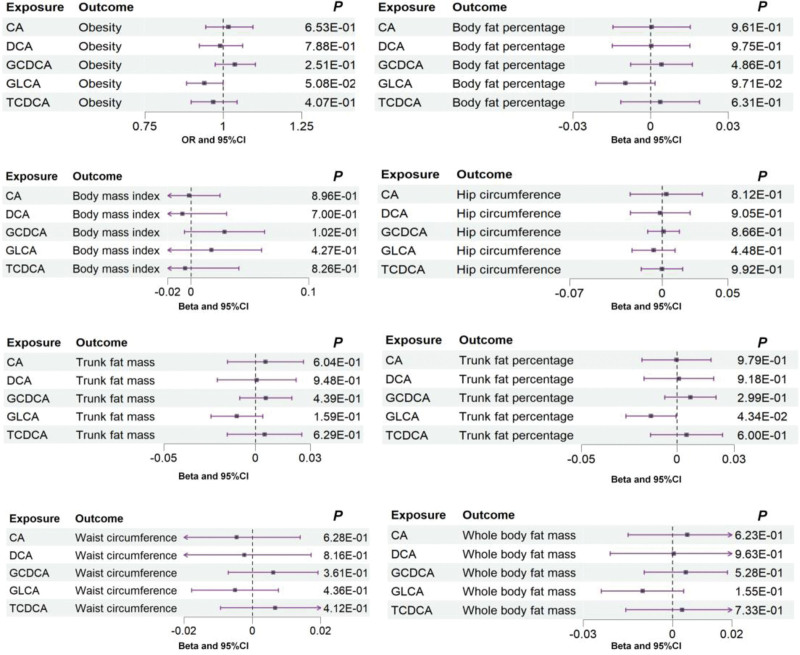
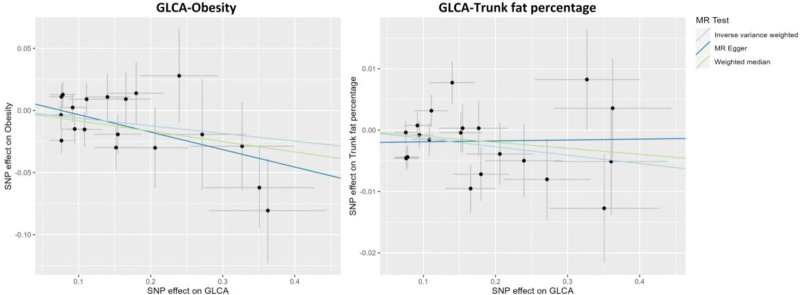
The IVW method in MR analysis demonstrated a suggestive negative causal association between GLCA and trunk fat percentage (Beta = ‐0.014; 95% CI: −0.027, −0.0004; P = .04) (Fig. 1; Table S3, Supplemental Digital Content, http://links.lww.com/MD/M912 and Table S4, Supplemental Digital Content, http://links.lww.com/MD/M913). However, we found that GLCA showed an association with trunk fat percentage with a different direction from IVW analysis when analyzed using MR-Egger techniques (Table S3, Supplemental Digital Content, http://links.lww.com/MD/M912 and Table S4, Supplemental Digital Content, http://links.lww.com/MD/M913). Furthermore, the IVW method suggested a potential negative association of marginal significance between GLCA and obesity (odds ratio = 0.94; 95% CI: 0.88, 1.00; P = .05) (Fig. 1; Table S3, Supplemental Digital Content, http://links.lww.com/MD/M912), and same association direction was observed using the MR-Egger and weighted median methods (Table S3, Supplemental Digital Content, http://links.lww.com/MD/M912 and Table S4, Supplemental Digital Content, http://links.lww.com/MD/M913). However, the above 2 associations lost statistical significance after adjusting multiple comparisons. Figure 2 displays the scatter plot showing the causal relationships between GLCA and obesity, as well as trunk fat percentage.
Figure 1.
Associations between genetically predicted 5 bile acids and obesity and its related indicators examined by IVW method. CA = cholate; CI = confidence interval; DCA = deoxycholate; GCDCA = glycochenodeoxycholate; GLCA = glycolithocholate; IVW = inverse-variance weighted; OR = odds ratio; P = P-value; TCDCA = taurochenodeoxycholate.
Figure 2.
Scatter plots showing the causal effects of GLCA on obesity and trunk fat percentage. GLCA = glycolithocholate; MR = Mendelian randomization; SNP = single nucleotide polymorphism.
3.3. Results of the sensitivity analysis
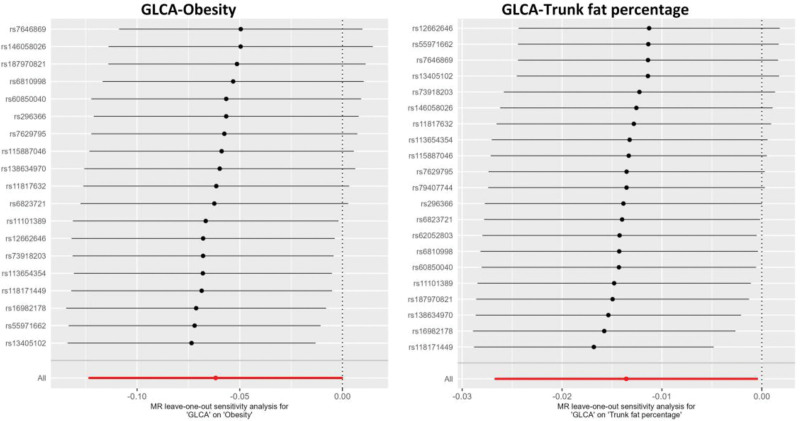
Funnel plots of the MR analyses and heterogeneity tests were also performed (Fig. S1, Supplemental Digital Content, http://links.lww.com/MD/M909; Table S5, Supplemental Digital Content, http://links.lww.com/MD/M914). The analysis did not indicate significant evidence of horizontal pleiotropy according to the testing of the MR-Egger intercept term (Table S6, Supplemental Digital Content, http://links.lww.com/MD/M915). This finding is consistent with results obtained from MR-PRESSO, where no outlier IV was identified. Figure 3 illustrates the results of a leave-one-out analysis, indicating that the results were generally the same when removing IVs one-by-one. The sensitivity analysis method described above provides evidence of the reliability of the MR results.
Figure 3.
Leave-one-out sensitivity analyses using the IVW method to investigate the causal estimates of GLCA on obesity and trunk fat percentage after excluding a particular SNP from the analysis. The IVW estimate of all SNPs on each outcome was shown by the red line. IVW = inverse-variance weighted; GLCA = glycolithocholate; MR = Mendelian randomization; SNP = single nucleotide polymorphism.
In sum, our findings suggest a potential negative causal relationship between GLCA and obesity, as well as truncal fat percentage, although no association survived corrections for multiple comparisons. These results indicate a trend towards a possible association between bile acids and obesity.
4. Discussion
We utilized a two-sample MR analysis to examine the causal relationship between BAs and obesity and its related indicators. The analysis integrated summary statistics from GWAS available in public databases. The findings revealed a suggestive causal association between GLCA and trunk fat percentage (P < .05) reported by IVW analysis, as well as marginally significant causative connections between GLCA and obesity (P = .05). None of the other exposures revealed any significant associations with obesity or its related metrics.
4.1. Potential mechanisms underlying the impact of GLCA on obesity
In an animal study, cultivating live P. distasonis (LPD) in high-fat mice, resulting in higher levels of LCA and succinic acid in the intestines and improving obesity and obesity-related functional impairments.[40] LCA is one of the most toxic BAs, most of which exists in the form of GLCA.[41,42] GLCA is the product of glycine coupling to LCA, in which the carboxylate group of LCA combines with glycine to form bile salts,[41,43,44] which are allowed to dissolve lipids in the small intestine, increasing the lipid surface area to be more easily absorbed, and thus lipid reduction affects obesity.[43–48] Following oral administration of GLCA to rats, a swift reduction in phospholipid and cholesterol secretion was observed, reaching 25% and 50% of their initial levels, respectively, as reported by Kuipers et al.[49]
The transportation of bile phospholipids to the canalicular membrane occurs via a calcium-dependent microtubule-mediated vesicle pathway.[50,51] A review of the literature shows that cells are able to maintain low concentrations of intracellular free calcium due to the efficient operation of calcium pumps located in the plasma membrane and mitochondria.[52] There is speculation that GLCA disrupts the intracellular calcium balance, thereby impeding lipid transport.[53] GLCA has cytotoxicity and can cause apoptosis.[54] LCA carboxylate group in GLCA forms bile salts with glycine, which cause toxic damage to mitochondria, leading to disruption of the intracellular calcium balance.[44,45,54–56]
Furthermore, most lipids are hydrolyzed in the small intestine and then proceed to the large intestine after gastric preprocessing, where they are influenced by the gut microbiota for lipid utilization.[32,57,58] The Bacteroidetes and Firmicutes phyla, which dominate the human gut, play a pivotal role in regulating inflammation, obesity, and insulin sensitivity.[10,59] Additionally, the abundance of Firmicutes and Bacteroidetes exhibits a positive correlation with plasma GLCA levels.[60] LCA binds to glycine in the liver to produce GLCA and is secreted into the small intestine, where they play an important role in the digestion process by acting as detergents.[43,61] The main role of bile salt hydrolases in bacteria is to detoxify the bound bile acids, thus promoting the colonization of bacteria in the harsh intestinal environment.[62,63] Bacteroides is one of the major members of the animal microbiota, particularly within the digestive system.[64–66] It can be inferred from a review of the literature that GLCA is a binding bile acid that can be detoxified by bile salt hydrolases to promote intestinal bacteroides colonization.[41,43,62,64,67]
Turicibacteraceae, Turicibacterales, and Turicibacter have been established in previous literature to be positively correlated with GLCA.[68] Nonetheless, individuals at a higher risk of obesity exhibit lower levels of Turicibacterales and Turicibacteraceae compared to their healthier counterparts.[69] Simultaneously, lower peripheral GLCA and TLCA levels in periparturient cows undergoing excessive lipolysis result in diminished expression of G protein-coupled bile acid receptor 1, a crucial mediator in the neural mechanisms that counteract diet-induced obesity.[70,71] These findings align with the potential negative association observed in this study, albeit with marginal significance, between GLCA and obesity. The effects of BAs and their conjugate forms on lipid metabolism are complex, as changes in the chemical forms of different BAs may affect their physiological properties. Additional extensive investigations are required to shed light on this intricate phenomenon.
4.2. The function of CA, DCA, GCDCA, TCDCA
Researchers report that conjugates of BAs potentially impact the development of obesity. For example, bile salt—CA or DCA—microparticles, show enhanced efficacy in breaking down adipocytes both in vitro and in vivo settings.[72] Furthermore, stronger associations were found between conjugated primary or secondary BAs (excluding GLCA) and higher BMI, larger WC, as well as elevated energy expenditure, comparing with their nonconjugated counterparts.[73] Notably, these outcomes contradict the results obtained from our investigation. This contradiction may imply that observational studies might face limitations regarding their capacity to control for confounding variables effectively while also considering reverse causality as a plausible explanation for this disparity.
4.3. The function and potential mechanism of BAs for obesity
BAs can exert an influence on obesity by regulating fatty acid metabolism.[28] These effects primarily occur through the modulation of multiple signaling pathways, which contribute to the maintenance of homeostasis in vivo by controlling triglyceride balance, cholesterol levels, glucose regulation, and energy expenditure.[74] As a potential underlying signaling pathway, BAs activate the farnesoid X receptor in the liver, leading to the induction of short heterodimer chaperone expression.[75] Consequently, this inhibits liver receptor homologue-1 and liver X receptor-α activity, further suppressing transcriptional activation of cholesterol 7-hydroxylase (CYP7A1) gene encoding a rate-limiting enzyme essential for bile acid-mediated cholesterol synthesis.[75–77] Moreover, Short heterodimer partner disrupts sterol regulatory element-binding protein 1c synthesis—a transcription factor crucial for controlling genes associated with fatty acid synthesis such as acetyl-CoA carboxylase, fatty acid synthase, and acetyl-CoA synthetase.[78] Additionally, BAs enhance lipoprotein lipase activity thereby promoting plasma triglyceride clearance.[79] Furthermore, BAs downregulate hepatic phosphoenolpyruvate carboxykinase and glucose-6-phosphatase expression, resulting in reduced hepatic gluconeogenesis and inhibition of triglyceride synthesis.[80] Our investigation has not established a definitive causal relationship between obesity and BAs, excluding GLCA. Further research is warranted to explore the reasons and underlying mechanisms.
4.4. Strengths and limitations
As an advantage of our research, we employ the MR methods to verify causal relationships. Compared to traditional observational studies, this approach reduces biases related to potential confounding factors and reverse causality interference. As a limitation of our study, the restriction of our sample to the European population distinctly hampers the applicability of our research findings to other races. Moreover, the relatively small sample size of the exposure used in our study may contribute to the lack of significant causal relationships.
5. Conclusion
Our findings indicate a potential negative causal relationship between GLCA and both obesity and trunk fat percentage. However, this relationship lost significance after correction for multiple comparisons. Nonetheless, the results still suggest a trend of association between bile acid and obesity, highlighting the need for future studies with expanded sample sizes.
Author contributions
Conceptualization: Sen Li, Meihua Bao.
Writing – original draft: Chunxia Huang, Shuling Xu, Rumeng Chen, Yining Ding, Qingming Fu, Binsheng He, Ting Jiang, Bin Zeng, Meihua Bao.
Writing – review & editing: Sen Li.
Supplementary Material
Abbreviation:
- BAs
- bile acids
- BMI
- body mass index
- CA
- cholate
- CI
- confidence interval
- DCA
- deoxycholate
- GCDCA
- glycochenodeoxycholate
- GLCA
- glycolithocholate
- GWAS
- genome-wide association study
- IVW
- inverse-variance weighted
- LCA
- lithocholate
- IVs
- instrumental variables
- MR
- Mendelian randomization
- SNP
- single nucleotide polymorphism
- TCDCA
- taurochenodeoxycholate
- WC
- waist circumference
This study was supported by the BUCM Precision Cultivation Program (Grant No. JZPY-202205) and the BUCM Research Development Fund (Grant No. 2021-ZXFZJJ-052).
The GWASs included in this work were approved by their relevant review board, and informed consent were given by all participants.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Huang C, Xu S, Chen R, Ding Y, Fu Q, He B, Jiang T, Zeng B, Bao M, Li S. Assessing causal associations of bile acids with obesity indicators: A Mendelian randomization study. Medicine 2024;103:25(e38610).
CH, SX, and RC contributed equally to this work.
Contributor Information
Chunxia Huang, Email: huangchunxia@csmu.edu.cn.
Shuling Xu, Email: xushuling_ahmu@163.com.
Rumeng Chen, Email: Chenrm2020@163.com.
Yining Ding, Email: 20192110675@stu.gzucm.edu.cn.
Qingming Fu, Email: 178380220@qq.com.
Binsheng He, Email: hnaios@163.com.
Ting Jiang, Email: 1332818360@qq.com.
Bin Zeng, Email: 271808980@qq.com.
Meihua Bao, Email: mhbao78@163.com.
References
- [1].Wang T, Gao Q, Yao Y, et al. Causal relationship between obesity and iron deficiency anemia: a two-sample Mendelian randomization study. Front Public Health. 2023;11:1188246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang K, Ma J, Li Y, et al. Effects of essential oil extracted from Artemisia argyi leaf on lipid metabolism and gut microbiota in high-fat diet-fed mice. Front Nutr. 2022;9:1024722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Caballero B. Humans against obesity: who will win? Adv Nutr. 2019;10(suppl_1):S4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neeland IJ, Poirier P, Després J-P. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137:1391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Piché M-E, Tchernof A, Després J-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126:1477–500. [DOI] [PubMed] [Google Scholar]
- [6].Yang YY, Shi L-X, Li J-H, Yao L-Y, Xiang D-X. Piperazine ferulate ameliorates the development of diabetic nephropathy by regulating endothelial nitric oxide synthase. Mol Med Rep. 2019;19:2245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xu Z, Zhang P, Chen Y, Jiang J, Zhou Z, Zhu H. Comparing SARC-CalF with SARC-F for screening sarcopenia in adults with type 2 diabetes mellitus. Front Nutr. 2022;9:803924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Luo M, Cao Q, Wang D, et al. The impact of diabetes on postoperative outcomes following spine surgery: a meta-analysis of 40 cohort studies with 2.9 million participants. Int J Surg. 2022;104:106789. [DOI] [PubMed] [Google Scholar]
- [9].Yu T, Xu B, Bao M, et al. Identification of potential biomarkers and pathways associated with carotid atherosclerotic plaques in type 2 diabetes mellitus: a transcriptomics study. Front Endocrinol (Lausanne). 2022;13:981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Su M, Hu R, Tang T, Tang W, Huang C. Review of the correlation between Chinese medicine and intestinal microbiota on the efficacy of diabetes mellitus. Front Endocrinol (Lausanne). 2022;13:1085092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen J, Li X, Liu H, et al. Bone marrow stromal cell-derived exosomal circular RNA improves diabetic foot ulcer wound healing by activating the nuclear factor erythroid 2-related factor 2 pathway and inhibiting ferroptosis. Diabet Med. 2023;40:e15031. [DOI] [PubMed] [Google Scholar]
- [12].Bray GA. Beyond BMI. Nutrients. 2023;15:2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gandham A, Zengin A, Bonham MP, et al. Incidence and predictors of fractures in older adults with and without obesity defined by body mass index versus body fat percentage. Bone. 2020;140:115546. [DOI] [PubMed] [Google Scholar]
- [14].Schumann R, Eipe N. Body mass index, obesity, and ambulatory surgery-thoughts, words, and actions? Anesth Analg. 2022;134:e34–5. [DOI] [PubMed] [Google Scholar]
- [15].Shaw KA, Zello GA, Crizzle AM. Body mass index and waist circumference correlates with lifestyle and health in long-haul truck drivers. J Occup Environ Med. 2023;65:1051–7. [DOI] [PubMed] [Google Scholar]
- [16].Zhang Y, Abdin E, Sambasivam R, et al. Changes in body mass index and its association with socio-demographic characteristics between 2010 and 2016 in Singapore. Front Public Health. 2024;12:1374806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiang X, Yan M. Comparing the impact on the prognosis of acute myocardial infarction critical patients of using midazolam, propofol, and dexmedetomidine for sedation. BMC Cardiovasc Disord. 2021;21:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huo R, Liu Y, Xu H, et al. Associations between carotid atherosclerotic plaque characteristics determined by magnetic resonance imaging and improvement of cognition in patients undergoing carotid endarterectomy. Quant Imaging Med Surg. 2022;12:2891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bosello O, Vanzo A. Obesity paradox and aging. Eat Weight Disord. 2021;26:27–35. [DOI] [PubMed] [Google Scholar]
- [20].Donini LM, Pinto A, Giusti AM, Lenzi A, Poggiogalle E. Obesity or BMI paradox? Beneath the tip of the iceberg. Front Nutr. 2020;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dramé M, Godaert L. The obesity paradox and mortality in older adults: a systematic review. Nutrients. 2023;15:1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–50. [DOI] [PubMed] [Google Scholar]
- [23].Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61:151–6. [DOI] [PubMed] [Google Scholar]
- [24].Simati S, Kokkinos A, Dalamaga M, Argyrakopoulou G. Obesity paradox: fact or fiction? Curr Obes Rep. 2023;12:75–85. [DOI] [PubMed] [Google Scholar]
- [25].Tutor AW, Lavie CJ, Kachur S, Milani RV, Ventura HO. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2023;78:2–10. [DOI] [PubMed] [Google Scholar]
- [26].Wang S, Ren J. Obesity paradox in aging: from prevalence to pathophysiology. Prog Cardiovasc Dis. 2018;61:182–9. [DOI] [PubMed] [Google Scholar]
- [27].Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–32. [DOI] [PubMed] [Google Scholar]
- [28].Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–94.e3. [DOI] [PubMed] [Google Scholar]
- [29].Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. [DOI] [PubMed] [Google Scholar]
- [30].Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–93. [DOI] [PubMed] [Google Scholar]
- [31].Li R, Andreu-Sánchez S, Kuipers F, Fu J. Gut microbiome and bile acids in obesity-related diseases. Best Pract Res Clin Endocrinol Metab. 2021;35:101493. [DOI] [PubMed] [Google Scholar]
- [32].Macierzanka A, Torcello-Gómez A, Jungnickel C, Maldonado-Valderrama J. Bile salts in digestion and transport of lipids. Adv Colloid Interface Sci. 2019;274:102045. [DOI] [PubMed] [Google Scholar]
- [33].Fu Q, Chen R, Ding Y, et al. Sodium intake and the risk of various types of cardiovascular diseases: a Mendelian randomization study. Front Nutr. 2023;10:1250509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jiang Y, Chen R, Xu S, et al. Assessing causal associations of hyperparathyroidism with blood counts and biochemical indicators: a Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1295040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen Y, Lu T, Pettersson-Kymmer U, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet. 2023;55:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fu Q, Chen R, Xu S, et al. Assessment of potential risk factors associated with gestational diabetes mellitus: evidence from a Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1276836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jiang Y, Chen R, Xu S, et al. Endocrine and metabolic factors and the risk of idiopathic pulmonary fibrosis: a Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1321576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen R, Xu S, Ding Y, et al. Dissecting causal associations of type 2 diabetes with 111 types of ocular conditions: a Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1307468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Burgess S, Thompson SG; CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64. [DOI] [PubMed] [Google Scholar]
- [40].Wang K, Liao M, Zhou N, et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019;26:222–35.e5. [DOI] [PubMed] [Google Scholar]
- [41].Bansal S, Lau AJ. Inhibition of human sulfotransferase 2A1-catalyzed sulfonation of lithocholic acid, glycolithocholic acid, and taurolithocholic acid by selective estrogen receptor modulators and various analogs and metabolites. J Pharmacol Exp Ther. 2019;369:389–405. [DOI] [PubMed] [Google Scholar]
- [42].Kirkpatrick RB, Belsaas RA. Formation and secretion of glycolithocholate-3-sulfate in primary hepatocyte cultures. J Lipid Res. 1985;26:1431–7. [PubMed] [Google Scholar]
- [43].Zhang F, Duan Y, Wei Y, et al. The inhibition of hepatic Pxr-Oatp2 pathway mediating decreased hepatic uptake of rosuvastatin in rats with high-fat diet-induced obesity. Life Sci. 2020;257:118079. [DOI] [PubMed] [Google Scholar]
- [44].Durník R, Šindlerová L, Babica P, Jurček O. Bile acids transporters of enterohepatic circulation for targeted drug delivery. Molecules. 2022;27:2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Peng J, Fan M, Huang KX, et al. Design, synthesis, computational and biological evaluation of novel structure fragments based on lithocholic acid (LCA). Molecules. 2023;28:5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kelly KR, Navaneethan SD, Solomon TPJ, et al. Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Med Sci Sports Exerc. 2014;46:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gonzalez FJ. Nuclear receptor control of enterohepatic circulation. Compr Physiol. 2012;2:2811–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sievänen E. Exploitation of bile acid transport systems in prodrug design. Molecules. 2007;12:1859–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kuipers F, Derksen JP, Gerding A, Scherphof GL, Vonk RJ. Biliary lipid secretion in the rat. The uncoupling of biliary cholesterol and phospholipid secretion from bile acid secretion by sulfated glycolithocholic acid. Biochim Biophys Acta. 1987;922:136–44. [DOI] [PubMed] [Google Scholar]
- [50].Barnwell SG, Lowe PJ, Coleman R. The effects of colchicine on secretion into bile of bile salts, phospholipids, cholesterol and plasma membrane enzymes: bile salts are secreted unaccompanied by phospholipids and cholesterol. Biochem J. 1984;220:723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kockx M, Guo DL, Huby T, et al. Secretion of apolipoprotein E from macrophages occurs via a protein kinase A and calcium-dependent pathway along the microtubule network. Circ Res. 2007;101:607–16. [DOI] [PubMed] [Google Scholar]
- [52].Kodavanti PR. Measurement of calcium buffering by intracellular organelles in brain. Methods Mol Med. 1999;22:171–6. [DOI] [PubMed] [Google Scholar]
- [53].Oelberg DG, Lester R. Cellular mechanisms of cholestasis. Annu Rev Med. 1986;37:297–317. [DOI] [PubMed] [Google Scholar]
- [54].Pike CM, Tam J, Melnyk RA, Theriot CM. Tauroursodeoxycholic acid inhibits Clostridioides difficile toxin-induced apoptosis. Infect Immun. 2022;90:e0015322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010;42:409–18. [DOI] [PubMed] [Google Scholar]
- [56].Barrasa JI, Olmo N, Lizarbe MA, Turnay J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol In Vitro. 2013;27:964–77. [DOI] [PubMed] [Google Scholar]
- [57].Jiao N, Baker SS, Nugent CA, et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol Genomics. 2018;50:244–54. [DOI] [PubMed] [Google Scholar]
- [58].Wang K, Zhou M, Gong X, et al. Starch-protein interaction effects on lipid metabolism and gut microbes in host. Front Nutr. 2022;9:1018026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda). 2016;31:283–93. [DOI] [PubMed] [Google Scholar]
- [60].Osuna-Prieto FJ, Xu H, Ortiz-Alvarez L, et al. The relative abundance of fecal bacterial species belonging to the Firmicutes and Bacteroidetes phyla is related to plasma levels of bile acids in young adults. Metabolomics. 2023;19:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sun Z, Wang Y, Su X, Yang X, Luo Q. Proteomic characterization of human gut habitual bacteroides intestinalis against common intestinal bile acid stress. Adv Gut Microbiome Res. 2023;2023:8395946. [Google Scholar]
- [62].McMillan AS, Foley MH, Perkins CE, Theriot CM. Loss of Bacteroides thetaiotaomicron bile acid-altering enzymes impacts bacterial fitness and the global metabolic transcriptome. Microbiol Spectr. 2024;12:e0357623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Thomas F, Hehemann J-H, Rebuffet E, Czjzek M, Michel G. Environmental and gut bacteroidetes: the food connection. Front Microbiol. 2011;2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bornet E, Westermann AJ. The ambivalent role of Bacteroides in enteric infections. Trends Microbiol. 2022;30:104–8. [DOI] [PubMed] [Google Scholar]
- [66].Béchon N, Ghigo JM. Gut biofilms: bacteroides as model symbionts to study biofilm formation by intestinal anaerobes. FEMS Microbiol Rev. 2022;46:fuab054. [DOI] [PubMed] [Google Scholar]
- [67].Foley MH, O'Flaherty S, Barrangou R, Theriot CM. Bile salt hydrolases: gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog. 2019;15:e1007581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sun N, Zhang J, Wang J, et al. Abnormal gut microbiota and bile acids in patients with first-episode major depressive disorder and correlation analysis. Psychiatry Clin Neurosci. 2022;76:321–8. [DOI] [PubMed] [Google Scholar]
- [69].Yuan X, Chen R, Zhang Y, Lin X, Yang X, McCormick KL. Gut microbiota of Chinese obese children and adolescents with and without insulin resistance. Front Endocrinol (Lausanne). 2021;12:636272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gu F, Zhu S, Tang Y, et al. Gut microbiome is linked to functions of peripheral immune cells in transition cows during excessive lipolysis. Microbiome. 2023;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Castellanos-Jankiewicz A, Guzmán-Quevedo O, Fénelon VS, et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021;33:1483–92.e10. [DOI] [PubMed] [Google Scholar]
- [72].Safari H, Kaczorowski N, Felder ML, et al. Biodegradable, bile salt microparticles for localized fat dissolution. Sci Adv. 2020;6:eabd8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Heianza Y, Zhou T, He H, et al. Changes in bile acid subtypes and long-term successful weight-loss in response to weight-loss diets: the POUNDS lost trial. Liver Int. 2022;42:363–73. [DOI] [PubMed] [Google Scholar]
- [74].Xu Y. Recent progress on bile acid receptor modulators for treatment of metabolic diseases. J Med Chem. 2016;59:6553–79. [DOI] [PubMed] [Google Scholar]
- [75].Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–15. [DOI] [PubMed] [Google Scholar]
- [76].Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. [DOI] [PubMed] [Google Scholar]
- [77].Brendel C, Schoonjans K, Botrugno OA, Treuter E, Auwerx J. The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol Endocrinol. 2002;16:2065–76. [DOI] [PubMed] [Google Scholar]
- [78].Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sagar NM, McFarlane M, Nwokolo C, Bardhan KD, Arasaradnam RP. Mechanisms of triglyceride metabolism in patients with bile acid diarrhea. World J Gastroenterol. 2016;22:6757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.