Abstract
Anemia is common in patients with rheumatoid arthritis (RA), and it is unknown whether the dietary inflammatory index (DII) is linked to anemia. This study aimed to clarify the prevalence of anemia in RA patients and its association with the DII. The data utilized in this study were collected from the National Health and Nutrition Examination Survey database from 1999 to 2018. The prevalence of anemia in RA patients was estimated by ethnicity, sex, and age. Weighted multivariate logistic regression was utilized to explore the correlation between anemia risk and DII. The most crucial dietary factors related to the risk of anemia in RA patients were screened by stepwise regression. A nomogram model was established according to key dietary factors. A total of 10.25% (confidence interval, 8.58–11.92%) of RA patients will develop anemia, with the lowest prevalence around the age of 60. In addition, higher DII levels were discovered in anemic patients than in nonanemic patients. In multivariate regression models, an important positive association was revealed between anemia and growing quartiles of DII (Q4 vs Q1: odds ratio = 1.98; confidence interval, 1.25–3.15). In the subgroup analysis, the adjusted relation of DII with anemia in females, Mexicans, smokers, nondrinkers, and age groups ≥ 60 years was statistically significant. The same association was observed in the sensitivity analysis. A nomogram model based on stepwise regression screening of key dietary factors showed good discriminatory power to identify anemic risk in RA patients (area under the curve: 0.707). In patients with RA, high DII levels were associated with the risk of anemia. More attention should be given to controlling dietary inflammation to better prevent and treat anemia.
Keywords: anemia, dietary inflammatory index, inflammation, NHANES, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by persistent synovitis and systemic chronic inflammation. Although it mainly targets synovial tissues of joints, it is a systemic disease that can influence multiple systems.[1] Anemia is the most common hematological complication in RA patients,[2] and it has been reported that the probability of anemia in RA patients within 1 year after diagnosis is 5%.[3] Moreover, it has a close correlation with an incremental risk of physical disability and early death.[4] Anemia not only aggravates joint destruction and systemic symptoms in patients with RA but is also an independent reference factor in determining the prognosis of the disease.[5]
Diet is a controllable factor in the environment that significantly influences inflammation and immune function.[6–8] Research has shown that dietary intake, particularly anti-inflammatory diets, is crucial in managing chronic diseases, such as RA,[9,10] anemia,[11] and hypertension.[12] Recent studies have reported that many anti-inflammatory food components, including n-3 fatty acids,[13] probiotics,[14] and anti-inflammatory dietary patterns, including the Mediterranean diet[14] and anti-inflammatory portfolio diet,[15] can diminish systemic inflammation levels and relieve disease activity. Research indicated that higher consumption of propolis can decrease reactive oxygen species and interleukin-17 production, leading to decreased inflammation in patients with RA.[10] A review has shown that high consumption of fish or supplements containing n-3 polyunsaturated fatty acids can achieve excellent anti-inflammatory effects and should be standard of care along with medication in the treatment of RA patients.[13]
It is believed that the consumption of anti-inflammatory foods has effects in regulating systemic inflammation, which can affect the risk of anemia in RA patients. This is because many studies have found that the development of anemia in patients with RA is largely influenced by inflammatory cytokines. These cytokines influence iron metabolism and inhibit bone marrow erythropoiesis, which together result in anemia in RA patients.[16–19] Interleukin-6 (IL-6) is a broad class of inflammatory cytokines that can mediate chronic inflammation-induced anemia.[20] It decreases total iron-binding capacity and hemoglobin (Hb), whereas it elevates iron-regulatory hormone, leading to anemia.[21]
The dietary inflammation index (DII)[22] is a literature-derived method to assess the potential inflammatory levels of our daily diet. DII has been shown in nutritional epidemiological research to correlate with levels of inflammatory and anti-inflammatory markers like IL-1β, IL-4, IL-6, IL-10, CRP, and TNF-α, predicting the connection between overall diet and these markers.[23] As recently documented in several studies,[11,24] the DII is strongly connected with RA and anemia. However, to our knowledge, no study has specifically investigated the correlation between DII and anemia in RA patients. Although earlier research has highlighted the significance of inflammatory cytokines in the pathogenesis of anemia among RA patients, the relationship between DII and anemia in this specific population remains unexplored.
Therefore, our study aims to bridge this knowledge gap by examining the association between DII and anemia in RA patients. By utilizing data from the National Health and Nutrition Examination Survey (NHANES) spanning the years 1999 to 2018, we conducted a comprehensive population-based analysis to investigate this association.
2. Materials and methods
2.1. Study population
NHANES is administered by the National Center for Health Statistics, which uses a sophisticated, multistage probability sampling method to collect nationally representative health and nutrition-related data in the American population. The public can obtain information on the design and data of the survey at https://www.cdc.gov/nchs/nhanes/ (accessed on July 1st, 2023). Every 2 years, the continuous data of the NHANES will be made available for public use. The Research Ethics Review Board of National Center for Health Statistics authorized the study protocols for the NHANES, and participants provided signed informed permission.
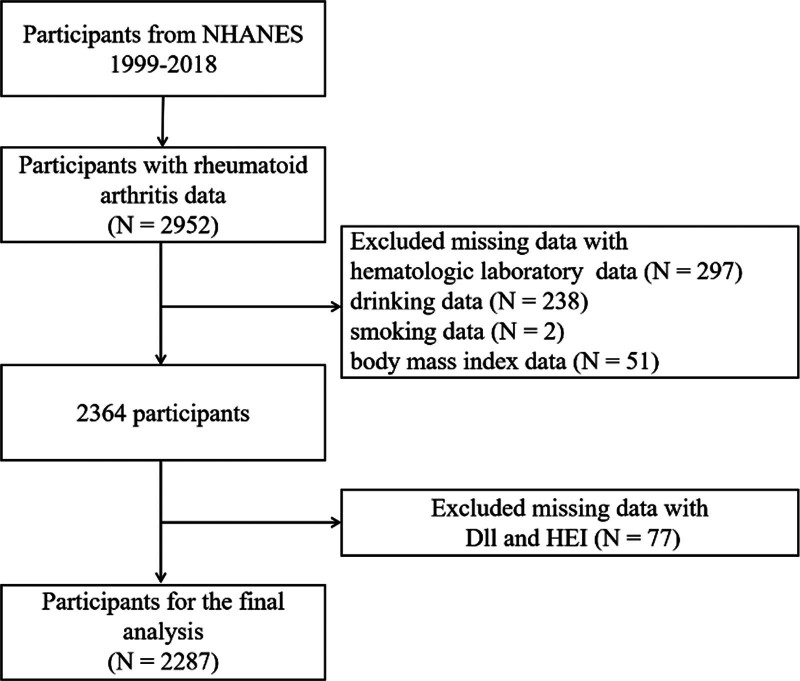
Questionnaires about the medical conditions provided data from a personal and self-reported interview that can be utilized to acquire the condition of RA. Participants who responded with self-reported “RA” were involved in our research. Participants who chose “yes” as the answer to the question “Has a doctor ever told you that you have arthritis?” were first selected. By selecting those who then chose “Rheumatoid arthritis” in response to the question “What kind of arthritis was it?,” we were able to identify the RA patients. Initially, 2952 RA patients were identified. After removing those who had missing related data, 2287 participants were eventually included in our study (Fig. 1).
Figure 1.
Flow diagram of the screening and enrollment of study participants.
2.2. Measurement of the Hb count and definition of anemia
Blood samples were collected after at least 8 hours of an overnight fast to evaluate the levels of Hb. Hb is measured in g/dL. Procedures for collecting blood biochemical measurements were described in detail on the NHANES website.[25] The diagnosis of anemia was carried out according to the latest diagnostic criteria specified by the WHO https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1.
2.3. Dietary information
The collection of dietary information was conducted by the Nutrition Methodology Working Group of the NHANES by holding 24-hour dietary recall interviews for participants. Similar to previously conducted research, the DII is a scoring algorithm that is used to evaluate dietary inflammation levels dependent on 28 dietary food components.[26,27] Importantly, it is believed that DII scores are still considered reliable even when fewer than 30 nutrients are included in the calculation.[28,29] This index is a literature-derived tool developed by analyzing the influence of nutrients on inflammatory biomarkers.[30] According to their impact on 6 well-known inflammatory biomarkers (IL-4, IL-6, IL-10, IL-1β, TNF-α, and CRP), the inflammatory potential of nutrients is evaluated by scoring between ‐1 (anti-inflammatory) and +1 (pro-inflammatory).[31] Additionally, FFQ-derived dietary data were specifically described by Shivappa et al, and they were used to determine DII scores.[32] First, DII was evaluated as a continuous variable. Next, participants were separated evenly into 4 groups based on the DII score: low DII (Q1), medium-low DII (Q2), medium-high DII (Q3), and high DII (Q4).
The healthy eating index (HEI) was also evaluated in our study. It reveals the overall dietary quality by assessing the alignment of the diet with the Dietary Guidelines for Americans. The HEI ranges from 0 to 100, and the higher the score is, the better the quality of the diet.[33] Similarly, the HEI is also first examined as a continuous variable and then grouped into 4 quartiles.
2.4. Covariates
Social demography, health status, and lifestyle factors were gathered via questionnaires and interviews. In our analysis, several variables from the following major aspects were identified as potential confounding factors: (1) baseline information: age, sex, and ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, others); (2) obesity status: divided by body mass index (BMI), thin (BMI < 18.50 kg/m2), normal weight (18.5 < BMI < 24.9 kg/m2), overweight (25.0 < BMI < 29.9 kg/m2), and obesity (≥30 kg/m2); and (3) lifestyle factors: smoking status (no and yes) and alcohol status (no and yes), which were obtained from questionnaires. The public can acquire information in detail at http://www.cdc.gov/nchs/nhanes/.
2.5. Statistical analysis
To derive estimates that applied to the population of the U.S., all analyses used weighted samples and took the stratification and clustering of the design into account. A 20-year, weight variable sample was established by taking two-tenths of the 4-year weight for each individual sampled from 1999 to 2002 and one-tenth for the 2-year weight for each person sampled from 2003 to 2018 to obtain estimates for the entire 20 years. The mean ± standard deviation (normal distribution) can be used to present continuous variables. Categorical variables are shown as the number of participants (weighted percentages). We compared the prevalence of anemia across age ranges and various races for both sexes. Furthermore, by using independent t-tests, chi-square tests, and Mann–Whitney U tests, we compared baseline traits among people with and without anemia. The Spearman method was used to study the correlation between the DII and HEI. The correlation between anemic risk and DII was examined with weighted multivariate logistic regression after excluding confounding factors (age, sex, race, smoking status, and drinking status). Subgroup analysis, stratified by the above confounding factors, aimed to assess the heterogeneity among various populations. In the sensitivity analysis, we adjusted for antirheumatic medication, family poverty income ratio (PIR), and C-reactive protein (CRP) as covariates in weighted multivariate logistic regressions to minimize their potential impact on the association between the DII and anemia in RA patients.[34–36] We carried out stepwise regression and the nomogram model to further identify the association between anemia and DII. The receiver operating characteristic curve was performed to determine the discriminatory ability of the nomogram model in identifying anemic risk.
We calculated 95% confidence intervals (CIs) for all effect estimates. R version 4.2.2 software (CRAN team, Vienna, Australia) was implemented to carry out statistical analyses. Two-sided P < .05 was regarded as statistically significant.
3. Results
3.1. Prevalence of anemia in RA patients among US adults
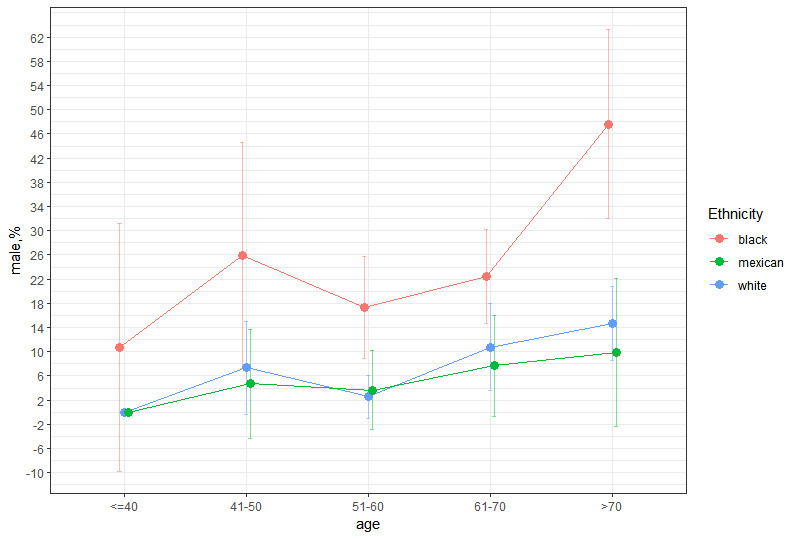
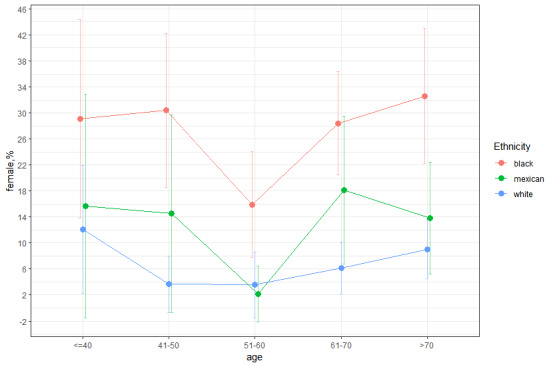
We estimated the prevalence of anemia in RA patients to be approximately 10.25% (95% CI, 8.58–11.92%) by weighting the data. Approximately 77,000 individuals in the US suffered from both RA and anemia from 1999 to 2018, and females were slightly more prevalent than males (11.09%, 95% CI 8.92–13.27% vs 9.07%, 95% CI 6.87–11.27%). We also found that blacks had significantly higher rates of anemia than whites and Mexicans across all 5 age groups in the graph, regardless of whether they were males or females (Appendix Fig. 1, Supplemental Digital Content, http://links.lww.com/MD/M863 and Appendix Fig. 2, Supplemental Digital Content, http://links.lww.com/MD/M864). The results among males, blacks, and whites showed similar trends in prevalence with age. The results among females, blacks, and Mexicans showed similar trends in prevalence up to age 70. Regardless of sex, the prevalence of anemia was the lowest around the age of 60, and the anemia rate rose again after the age of 60. The distribution of anemia prevalence was roughly U-shaped and almost V-shaped among females.
3.2. Baseline characteristics
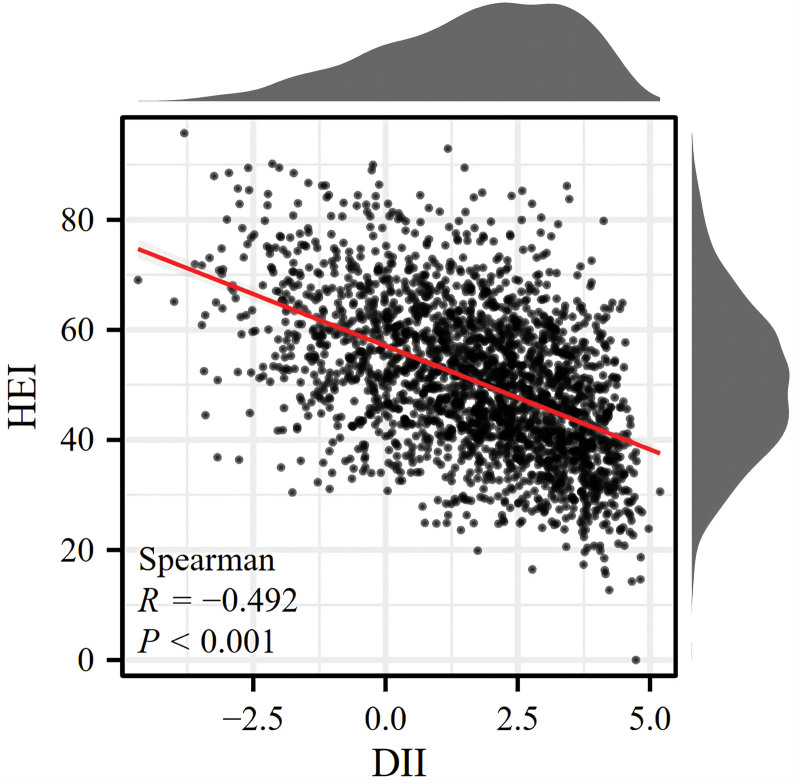
Table 1 represents the weighted and comprehensive baseline data of participants grouped by anemia status, and notable differences in baseline characteristics between anemic and nonanemic patients were observed. The average age of anemic patients was 60.73 years old (standard deviation = 1.09). Anemia was more common among male patients. (63.19% vs 36.81%). Moreover, it was highest among whites (46.58%), followed by blacks (39.33%), and lowest among Mexicans (5.90%). People over the age of 60 were more likely to suffer from anemia (56.72%). The occurrence of anemia was significantly related to drinking (P = .006) and smoking, but the significance was not large (P = .042). Notably, the subjects with anemia had a substantially higher DII (2.00 (0.13) vs 1.55 (0.07), P = .004) than participants without anemia. Anemic patients were more likely to have high DII or higher middle DII. Figure 2 illustrates the distribution of HEI and DII among all RA patients and the association between HEI and DII. The inverse correlation between HEI and DII was demonstrated (R = −0.492; P < .001). However, the differences in HEI scores between the anemia group and the nonanemia group (P = .214) were not significant. Therefore, we speculated that DII may be a better indicator of anemia in RA patients because of its potential correlation with systemic inflammation.
Table 1.
Weighted distributions of characteristics of participants.†
| Characteristic | Total (N = 2287) |
No anemia (N = 1965) |
Anemia (N = 322) |
P value |
|---|---|---|---|---|
| Age | 57.42 (0.38) | 57.05 (0.41) | 60.73 (1.09) | .002 ** |
| Sex | .181 | |||
| Male | 957 (41.61) | 826 (42.15) | 131 (36.81) | |
| Female | 1330 (58.39) | 1139 (57.85) | 191 (63.19) | |
| Ethnicity | <.001 *** | |||
| Non-Hispanic black | 651 (15.70) | 478 (13.00) | 173 (39.33) | |
| Non-Hispanic white | 980 (67.90) | 894 (70.34) | 86 (46.58) | |
| Mexican American | 370 (6.30) | 336 (6.34) | 34 (5.90) | |
| Other races | 286 (10.10) | 257 (10.32) | 29 (8.19) | |
| Age group | .004 ** | |||
| <60 | 928 (54.40) | 834 (55.67) | 94 (43.28) | |
| ≥60 | 1359 (45.60) | 1131 (44.33) | 228 (56.72) | |
| Drinking status | <.001 *** | |||
| No | 1004 (38.50) | 836 (37.06) | 168 (51.11) | |
| Yes | 1283 (61.50) | 1129 (62.94) | 154 (48.89) | |
| Smoking status | .043 * | |||
| No | 1738 (73.30) | 1475 (72.51) | 263 (80.16) | |
| Yes | 549 (26.70) | 490 (27.49) | 59 (19.84) | |
| Body Mass Index | .355 | |||
| Normal weight | 483 (23.18) | 404 (22.80) | 79 (26.52) | |
| Overweight | 1071 (45.51) | 915 (45.46) | 156 (45.94) | |
| Obesity | 707 (30.09) | 623 (30.43) | 84 (27.07) | |
| Thin | 26 (1.22) | 23 (1.31) | 3 (0.47) | |
| DII | 1.60 (0.06) | 1.55 (0.07) | 2.00 (0.13) | .004 ** |
| HEI | 50.08 (0.40) | 50.23 (0.43) | 48.82 (1.04) | .214 |
Bold values represent significant differences with P < 0.05.
DII = dietary inflammatory index, HEI = healthy eating index.
Categorical variables are shown as the number of participants (weighted percentages), and continuous variables are shown as weighted means (SDs).
P < .05.
P < .01.
P < .001.
Figure 2.
Association between the DII and HEI. DII = dietary inflammatory index, HEI = healthy eating index.
To investigate the effects of dietary factors on influencing DII between anemia and nonanemia patients, scores of each dietary component are shown in Table 2. Higher inflammatory scores in dietary fibers, vitamin B1, vitamin B2, vitamin B6, vitamin D, niacin, magnesium, zinc, selenium, and alcohol and lower inflammatory scores in energy, protein, carbohydrate, and vitamin B12 were found in anemia patients. Iron plays an important role in most anemic patients; however, it did not have a significant effect on DII scores in RA patients suffering from anemia.
Table 2.
Weighted comparison of DII scores of each component between anemia patients and nonanemia patients.†
| Variables | Non-Anemia (n = 1965) | Anemia (n = 322) | P value |
|---|---|---|---|
| Energy | ‐0.03 (0.00) | ‐0.06 (0.01) | .005 ** |
| Protein | 0.00 (0.00) | ‐0.01 (0.00) | <.001 *** |
| Carbohydrate | ‐0.03 (0.00) | ‐0.04 (0.01) | .287 |
| Dietary fiber | 0.26 (0.01) | 0.33 (0.03) | .038 * |
| Total fatty acid | 0.00 (0.01) | ‐0.03 (0.02) | .153 |
| Total saturated fatty acid | ‐0.09 (0.01) | ‐0.12 (0.02) | .098 |
| MUFA | 0.00 (0.00) | 0.00 (0.00) | .063 |
| PUFA | ‐0.03 (0.01) | 0.00 (0.02) | .186 |
| Cholesterol | ‐0.03 (0.00) | ‐0.03 (0.01) | .367 |
| Vitamin A | 0.19 (0.01) | 0.21 (0.01) | .236 |
| b-Carotene | 0.35 (0.01) | 0.37 (0.03) | .366 |
| Vitamin B1 | 0.02 (0.00) | 0.03 (0.00) | .022 * |
| Vitamin B2 | ‐0.01 (0.00) | 0.00 (0.00) | <.001 *** |
| Niacin | 0.05 (0.00) | 0.08 (0.01) | .004 ** |
| Vitamin B6 | ‐0.05 (0.01) | ‐0.01 (0.02) | .019 * |
| Folate | 0.12 (0.00) | 0.12 (0.01) | .556 |
| Vitamin B12 | ‐0.02 (0.00) | ‐0.03 (0.00) | .011 * |
| Vitamin C | 0.20 (0.01) | 0.20 (0.02) | .976 |
| Vitamin D | 0.20 (0.01) | 0.26 (0.02) | .031 * |
| Vitamin E | 0.08 (0.01) | 0.12 (0.03) | .231 |
| Magnesium | 0.09 (0.01) | 0.16 (0.02) | <.001 *** |
| Iron | 0.00 (0.00) | 0.00 (0.00) | .186 |
| Zinc | 0.01 (0.01) | 0.08 (0.02) | .001 ** |
| Selenium | ‐0.07 (0.00) | ‐0.05 (0.01) | .015 * |
| Caffeine | 0.08 (0.00) | 0.08 (0.00) | .052 |
| Alcohol | 0.18 (0.01) | 0.22 (0.01) | .003 ** |
| n3 polyunsaturated fatty acid | 0.27 (0.00) | 0.27 (0.00) | .709 |
| n6 polyunsaturated fatty acid | ‐0.05 (0.00) | ‐0.04 (0.01) | .182 |
Bold values represent significant differences with P < 0.05.
MUFAs = monounsaturated fatty acids; PUFAs = polyunsaturated fatty acids.
Data are presented as the weighted means (SDs).
P < .05.
P < .01.
P < .001.
3.3. Association between DII and anemia
3.3.1. Multiple regression model
We established 3 weighted univariate and multivariate regression models: model 1, unadjusted; model 2, adjusted for sex, ethnicity, smoking status, and drinking status; and model 3, adjusted for sex, ethnicity, smoking status, drinking status, age, and BMI (Table 3). With increasing DII quartiles (Q4 vs Q1: odds ratio [OR] = 2.16; 95% CI, 1.36–3.42; P = .001), a significantly close relationship was revealed between DII scores and anemia in the unadjusted model. Additionally, this correlation persisted in model 2 (Q4 vs Q1: OR = 1.87; 95% CI, 1.16–3.00; P = .010) and model 3 (Q4 vs Q1: OR = 1.98; 95% CI, 1.25–3.15; P = .004) after confounding factors were adjusted. We found that the higher quartile of DII (compared to Q1) increased the risk of anemia (P for trend < .05).
Table 3.
Association of DII level with anemia.†
| DII | Model 1 | P value | Model 2 | P value | Model 3 | P value |
|---|---|---|---|---|---|---|
| Q1 (≤0.49) | Reference | Reference | Reference | |||
| Q2 (0.49–1.93) | 1.41 (0.86,2.31) | .172 | 1.26 (0.76,2.10) | 0.360 | 1.29 (0.78,2.15) | .323 |
| Q3 (1.93–3.13) | 1.55 (0.89,2.69) | .121 | 1.45 (0.82,2.56) | 0.204 | 1.47 (0.82,2.63) | .199 |
| Q4 (>3.13) | 2.16 (1.36,3.42) | .001 ** | 1.87 (1.16,3.00) | 0.010 * | 1.98 (1.25,3.15) | .004 ** |
| P for trend | .002 | .009 | .004 |
Bold values represent significant differences with P < 0.05.
Model 1: crude model. Model 2: adjusted for sex, race, smoking status, and drinking status. Model 3: as model 2 and additionally adjusted for age and BMI.
DII = dietary inflammatory index; Q1 = low DII; Q2 = medium-low DII; Q3 = medium-high DII; Q4 = high DII.
Data are presented as odds ratios (ORs) [95% confidence intervals (CIs)].
P < .05.
P < .01.
P < .001.
3.3.2. Subgroup analysis and sensitivity analysis
Participants were stratified by sex, race, smoking status (yes or no), drinking status (yes or no), and age (<60 years, ≥60 years) to perform subgroup analyses for investigating the correlation between DII and anemia risk in various populations. In the sex-stratified subgroup analyses, we discovered a substantial correlation between DII levels and the risk of anemia in females (Q4 vs Q1: OR = 2.15; 95% CI, 1.14–4.08) but not in males. In addition, the correlation between DII and anemia risk was not detected in participants who did not smoke or drink alcohol. In contrast, this association was only present in the smoking and nondrinking populations. Therefore, this linear relationship is only evident among females, smoking, and nondrinking populations (P for trend = .01, .003, .001, respectively). The interaction of stratified analysis according to sex, smoking, and drinking status was not significant, but the P-value of the interaction of stratified analysis according to a cutoff value of 60 years was significant, indicating inconsistent results between the different age groups (Table 4).
Table 4.
Subgroup analyses for the association between DII and anemia risk. Sex, ethnicity, smoking, drinking, age, and BMI were adjusted to carry out weighted multivariable logistic analyses among various populations.†
| DII | Q1 | Q2 | Q3 | Q4 | P for trend | P for interaction |
|---|---|---|---|---|---|---|
| Female | Reference | 1.31 (0.65,2.60) | 1.51 (0.71,3.22) | 2.15 (1.14,4.08) | .011 | .905 |
| Male | Reference | 1.17 (0.56,2.43) | 1.36 (0.54,3.40) | 1.59 (0.77,3.26) | .223 | |
| White | Reference | 0.91 (0.40,2.09) | 1.33 (0.51,3.50) | 2.14 (1.00,4.06) | .045 | 0.331 |
| Black | Reference | 1.53 (0.82,2.84) | 1.38 (0.64,2.95) | 1.52 (0.85,2.74) | .282 | |
| Mexican | Reference | 1.38 (0.33,5.83) | 4.74 (1.17,19.10) | 5.07 (1.46,17.61) | .003 | |
| Other races | Reference | 3.25 (0.76,13.89) | 1.03 (0.26, 4.04) | 2.22 (0.57, 8.68) | .615 | |
| Smokers | Reference | 0.46 (0.13,1.63) | 1.54 (0.46,6.04) | 0.77 (0.27,2.22) | .854 | 0.125 |
| Nonsmokers | Reference | 1.58 (0.89,2.79) | 1.42 (0.76,2.66) | 2.74 (1.43,4.26) | .003 | |
| Drinkers | Reference | 1.18 (0.60,2.32) | 2.23 (1.08,4.59) | 2.61 (1.34,5.08) | .001 | .076 |
| Nondrinkers | Reference | 1.32 (0.62,2.81) | 0.89 (0.37,2.17) | 1.46 (0.80,2.68) | .425 | |
| Age < 60 | Reference | 1.33 (0.55,3.21) | 2.96 (1.10,7.98) | 1.83 (0.76,4.43) | .077 | .028 |
| Age ≥ 60 | Reference | 1.25 (0.70,2.26) | 0.86 (0.48,1.56) | 2.19 (1.17,4.11) | .050 |
Bold values represent significant differences with P < 0.05.
DII = dietary inflammatory index; Q1 = low DII; Q2 = medium-low DII; Q3 = medium-high DII; Q4 = high DII.
Data are presented as odds ratios (ORs) [95% confidence intervals (CIs)].
To evaluate the robustness of the risk of DII and anemia in RA patients, we also included antirheumatic medication, PIR, and CRP as covariates. After adjusting for antirheumatic drugs in weighted multivariate logistic regression, the relationship between the DII and anemia risk in RA patients remained significant (Q4 vs Q1, P value = .004) (Appendix Table 1, Supplemental Digital Content, http://links.lww.com/MD/M865). After making further adjustments to the PIR and CRP, we still found the same association (Appendix Table 2, Supplemental Digital Content, http://links.lww.com/MD/M866).
3.3.3. Stepwise regression and the nomogram model
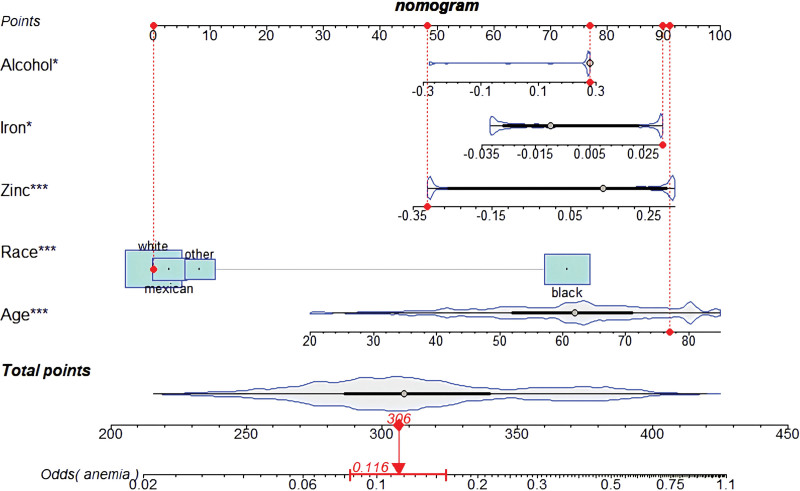
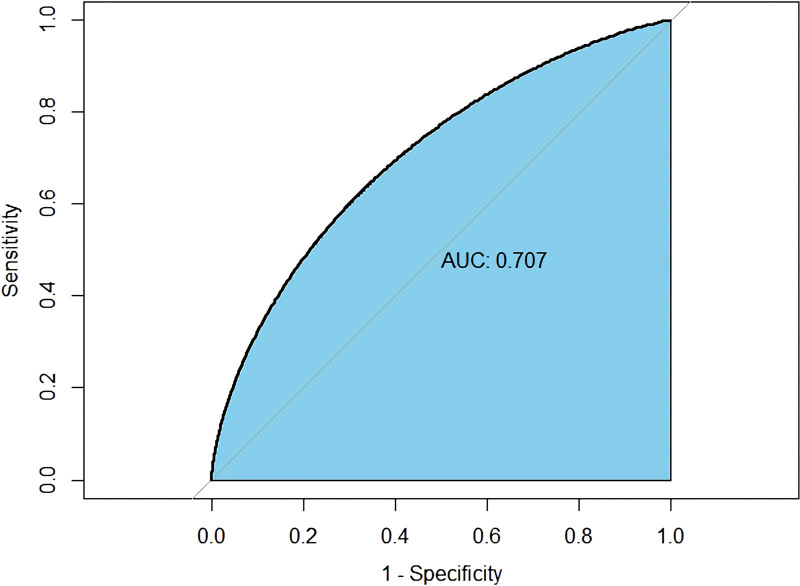
To address issues of collinearity, we performed stepwise multivariate logistic regression analysis to screen for the key dietary factors mostly related to anemia in RA patients. In the original stepwise regression model, 28 dietary factors used to establish the DII were included. Moreover, baseline characteristics, including age, sex, BMI, and race, were also included. We constructed the crude nomogram model by using 10 variables (protein, total fatty acid, β-carotene, vitamin E, BMI, alcohol, iron, zinc, age, and race). Furthermore, among those variables, 6 (BMI, alcohol, iron, zinc, age, race) were eventually included because their contribution to the model was significant (Fig. 3). A receiver operating characteristic curve with an area under the curve of 70.07% indicated that this nomogram model has great discriminatory power (Fig. 4).
Figure 3.
The nomogram model was established depending on the key dietary components screened by stepwise multivariate logistic regression analysis (the red points display an example).
Figure 4.
The ROC curve was designed to evaluate the diagnostic power of the nomogram model. ROC = receiver operating characteristic.
4. Discussion
A growing body of evidence has determined the association between dietary inflammation levels and RA; nonetheless, the relationship between DII and anemia in RA patients remains intriguing. In the present cross-sectional study, we tried to fill this knowledge gap. A total of 2287 participants from NHANES were included, and the main discoveries are as follows: (1) the prevalence of anemia in patients with RA in the US during 1999 to 2018 was 10.25% (95% CI, 8.58–11.92%). The prevalence showed a U-shaped distribution across age groups, with the lowest prevalence at approximately 60 years of age. In addition, this U-shaped distribution did not vary by race or sex. (2) A higher mean DII was found in anemia patients than in nonanemia patients. (3) The DII was inversely related to the HEI; however, the HEI showed no significant difference between anemia and nonanemia patients. (4) The DII was positively associated with anemia risk in RA patients after covariates were adjusted. The subgroup analysis and sensitivity analysis further confirmed the validity of the results. (5) Alcohol, iron, and zinc were the most determinant dietary factors that correlated with anemia. (6) According to those determinant dietary factors, we created a nomogram model that demonstrated strong diagnostic power in evaluating anemia risk in RA patients.
Few studies have systematically assessed the incidence of RA in the US population. This study represents the largest examination of the prevalence of anemia in RA patients in the US over the past ten years. Approximately 10.25% of RA patients in this analysis were diagnosed with anemia according to the latest WHO criteria, which is in line with the discoveries of research in 2009.[37] Anemia in RA patients showed a U-shaped distribution, with the lowest prevalence around the age of 60, and the prevalence was slightly lower in men than in women, which is in accordance with the results of Frederick et al.[38] Furthermore, our study examined the incidence of anemia in RA patients by ethnicity. We found that the U-shaped distribution did not vary with ethnicity and sex, although ethnicity and sex are major contributors to variation in Hb counts (Appendix Fig. 1, Supplemental Digital Content, http://links.lww.com/MD/M863 and Appendix Fig. 2, Supplemental Digital Content, http://links.lww.com/MD/M864).
The DII is a tool that was developed from the literature for assessing the inflammatory level of diet. Recent research has shown a clear positive correlation between DII and the risk of anemia. Reducing the intake of pro-inflammatory foods may be one of the effective measures to prevent the development of anemia.[11] Xiang et al discovered a positive correlation between DII and RA in Americans. They concluded that to prevent RA, simply eating anti-inflammatory foods is not enough. Consuming pro-inflammatory foods, such as protein, energy, and total saturated acids, may yield better results.[24] Consuming moderate dietary fiber may provide therapeutic benefits for inflammation and research has indicated a strong link between high intake of cereal fiber and the low prevalence of RA. The DII plays a key mediating role in this relationship, underscoring the significance of dietary strategies in preventing and treating RA.[39] Previous studies have mainly focused on the relationship between DII and RA; nevertheless, the association between DII and the development of anemia in RA patients has not been reported. Based on 2275 adults from NHANES, our results showed that DII levels were significantly increased in anemic subjects. The risk of anemia in RA patients in the highest DII quartile was approximately one time higher than that in RA patients in the lowest quartile (Table 2). Furthermore, this relationship remained stable in fully adjusted models with additional adjustments for a great number of covariates (1.98, CI = 1.25–3.15). Notably, the modest decrease in the OR value after adjusting for sex, race, smoking status, and drinking status suggested that some of these factors might be protective (1.87 vs 2.16). The minor variations in the odds ratio between model 2 and the fully adjusted model might mean that some risk factors for anemia (such as BMI and age) might not be very important in this situation (1.87 vs 1.98). However, because of the possibility of residual confounding, it should be interpreted with caution. Several studies have found inverse associations of serum Hb levels with antirheumatic medication, PIR, and CRP.[34–36] To eliminate the possibility of antirheumatic medication, PIR, and CRP affecting our results, we adjusted for them in the sensitivity analysis. We found that there was still an association between the DII and the risk of anemia.
After analyzing the participants’ inflammatory diets, we discovered that anemic patients have higher inflammatory scores in niacin, dietary fiber, vitamin D, vitamin B1, vitamin B2, vitamin B6, selenium, magnesium, and zinc, most of which are well-known anti-inflammatory substances. Moreover, we found that they had lower inflammatory scores for energy, protein, and carbohydrates. The higher inflammatory scores for these anti-inflammatory dietary factors indicate a lower than global average intake of these anti-inflammatory nutrients in anemic patients. The high DII diet of anemic participants was characterized by higher pro-inflammatory scores, particularly with respect to various vitamins. Epidemiological and clinical evidence has suggested that vitamin D deficiency is quite common in RA patients.[40] In addition, the concentration of vitamin D is negatively associated with disease development and severity,[41] potentially because vitamin D can downregulate proinflammatory mediators in monocyte-derived macrophages.[42] Notably, a deficiency of 25-hydroxyvitamin D has been linked to inflammatory anemia and vitamin D supplementation may help to some extent.[43] The potential reason underlying this condition is that vitamin D inhibits hepcidin synthesis, thereby increasing the availability of iron for erythropoiesis. Given that the majority of instances of anemia in RA patients are marked by inflammation-related anemia[44] and our results revealed that anemic patients have higher inflammatory scores in vitamin D, we speculated that vitamin D levels may have a relationship with anemia in RA patients. However, further laboratory investigation and clinical trials are necessary to confirm this hypothesis. Additionally, other food components with higher inflammatory scores in our anemic patients, including dietary fiber, niacin, vitamin B1, vitamin B2, vitamin B6, magnesium, selenium, and zinc, were reported to have anti-inflammatory abilities, but their effects on anemia in RA patients remain unclear. Biological agents are the main method of treating anemia in RA patients and have been studied more in recent years.[45,46] However, these treatment methods are expensive and inconvenient and even have side effects. Therefore, our findings may provide new insights into anemia treatment in RA patients, namely, by reducing dietary inflammation levels.
It was interesting to note that an adjusted association of DII levels with anemia was statistically significant in females but not males in our subgroup analyses, which may be related to hormones and gender-related factors[47] (Table 4). Moreover, Mexicans with high DII levels had a substantially increased risk of anemia, but there was no such association among other races. This may be attributed to the different genetic backgrounds among various races. Therefore, Mexican patients with RA should be more careful with their dietary inflammation level to lower their risk of anemia. We also found that the association between DII and anemia risk was significant only in the smoker group but not in the nonsmoker group, which suggested that smoking may strengthen the positive correlation between DII and anemia. That is, people taking high-DII food could reduce their risk of recurrent anemia by quitting smoking. In addition, we discovered that the association between DII and anemia risk was not significant, which may be due to the anti-inflammatory effects of moderate alcohol consumption.[48] This also explains why participants with a drinking history were excluded, but participants with a smoking history were included in a cross-sectional study that investigated the correlation between DII and RA.[49] In addition, they considered that drinking alcohol might alter the nutritional status of patients. Notably, we found a significant interaction between different age groups and DII on the development of anemia in subjects. In patients over 60 years of age, the highest DII score increased the risk of developing anemia by 1.19-fold. Conversely, anti-inflammatory diets may attenuate the adverse effects of advanced age on anemia. However, given that this study was a cross-sectional study, we can only draw correlational conclusions. The cause-effect relationship between DII and anemia must be further researched through more prospective studies.
Although the DII has been shown to reflect dietary inflammatory potential and systemic inflammation levels, it is not utilized to predict anemia risk in RA patients. We aimed to screen for the dietary factors most associated with anemia risk. Therefore, using stepwise regression analysis, we identified protein, total fatty acid, β-carotene, vitamin E, alcohol, iron, and zinc as key dietary components that are strongly associated with anemia. According to the significant contribution to the prediction model, alcohol, iron, and zinc were eventually included in the stepwise logistic regression model (Fig. 3). The effects of zinc on the pathogenesis of RA have been well elucidated within the last 2 years. Although there is no evidence at the genetic level to support a causal relationship between zinc intake and the development of rheumatoid arthritis,[50] zinc supplementation has been shown to potentially modestly improve some of the clinical outcomes in RA patients.[51] It has been shown that zinc finger protein A20 can be protective against RA by inhibiting NLRP3 inflammasome-mediated pyroptosis.[52] Although the direct relationship between zinc and anemia in RA remains intriguing, given the critical role of zinc in inflammation in RA patients and the close relationship between inflammation and anemia, we suspect that zinc may be a determinant factor in the pathogenesis of anemia in RA patients. Further basic experimental studies are warranted to analyze the important effects of these dietary components in the pathogenesis of anemia in RA patients.
Our study has some advantages and limitations. First, to our knowledge, this is the first study to analyze the prevalence of anemia in RA patients in the past 20 years from 1999 to 2018. Furthermore, this is the first food-based DII score to link diet-related inflammation and anemia risk in RA patients. We used a large, well-defined cohort and appropriately weighted survey participants, allowing the findings to be broadly applicable to the U.S. population. In addition, we compared the correlation between anemia risk and DII among various populations and analyzed the interaction of relevant confounders with DII as a way to determine the robustness of our results and to help us identify special populations. Finally, we performed stepwise regression analysis to identify the most critical dietary components correlated with anemia and developed a nomogram model with good discriminatory power.
However, it is also important to point out several limitations of this study. First, we had to remove some patients due to the absence of relevant covariates, which may have had some impact on the estimated incidence of anemia. Second, even after accounting for possible confounding factors, there is a possibility of remaining confounding factors that could influence the development of anemia in patients with RA. For example, the presence of anemia of chronic disease (ACD) in RA patients is a crucial confounding factor that needs consideration.[53–55] ACD, often linked to RA disease activity and inflammation, can suppress red blood cell production. Disease activity and medication history play a pivotal role in the context of anemia and could greatly affect our study’s outcomes. The severity of RA and the specific medications patients are taking can influence Hb levels, and potential drug interactions may further complicate the analysis. Without access to data on disease activity, blood markers such as ESR and CRP, and detailed medication records, it may be difficult to accurately isolate the impact of DII on anemia risk. However, due to the lack of data on ACD, disease activity, and inflammatory markers such as ESR in NHANES itself, this inevitably has some impact on our results. We recognize the importance of addressing these limitations and are exploring various avenues to obtain such data in future studies. Third, many of the variables in this study were obtained based on questionnaires and are susceptible to recall bias. Since the diagnoses are primarily based on self-report questionnaire data, there is an inherent risk of inaccuracies due to patients’ limited medical knowledge or possible misunderstanding of their conditions. In addition, patients may not be able to accurately recall or report their dietary habits and health history, which may affect the validity of the findings. Fourth, given the type of study that was a cross-sectional study, a causal association could not be determined. To explore the exact correlation between DII and anemia, more prospective studies are needed. Last but not least, there are significant racial differences in diet, genetic variation, and susceptibility to anemia. Therefore, further research must be done in future work to determine whether conclusions based on the US population can be applied to other populations.
5. Conclusion
In conclusion, we retrospectively analyzed 2287 adults from the NHANES in the US, and we estimated the prevalence of anemia in RA patients to be 10.25% (95% CI, 8.58–11.92%). Additionally, we also discovered that a rising DII was strongly related to an increased risk of anemia. The nomogram model based on stepwise regression screening of key dietary factors in this study showed good diagnostic power in predicting the risk of anemia. As proinflammatory diets might be modifiable risk factors for anemia in RA patients, we expect additional research in this area.
Acknowledgments
The authors thank the participants of the NHANES databases.
Author contributions
Conceptualization: Jingjing Song, Yujun Zhang, Jie Peng, Chulin Zhou, Yang Wu, Wentao Zhao.
Data curation: Jie Peng, Wentao Zhao.
Funding acquisition: Rui Wu, Hui Li.
Supervision: Hui Li.
Visualization: Chulin Zhou, Xifu Cheng.
Writing – original draft: Jingjing Song, Yujun Zhang.
Writing – review & editing: Ao Li, Zhen Zong.
Supplementary Material
Abbreviations:
- ACD
- anemia of chronic disease
- BMI
- body mass index
- CRP
- C-reactive protein
- DII
- dietary inflammatory index
- Hb
- hemoglobin
- HEI
- healthy eating index
- IL-6
- interleukin-6
- NHANES
- National Health and Nutrition Examination Survey
- OR
- odds ratio
- PIR
- poverty income ratio
- RA
- rheumatoid arthritis
This research was financially supported by “TCM scientific research project of Jiangxi Provincial Health and Family Planning Commission” (Grant Number: 2018B038) and “Research subject of educational reform of Nanchang University” (NCUJGLX-2022-160-96).
The protocols of NHANES were approved by the institutional review board of the National Center for Health Statistics, CDC (https://www.cdc.gov/nchs/nhanes/irba98.htm). NHANES obtained written informed consent from all participants.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
How to cite this article: Song J, Zhang Y, Li A, Peng J, Zhou C, Cheng X, Wu Y, Zhao W, Zong Z, Wu R, Li H. Prevalence of anemia in patients with rheumatoid arthritis and its association with dietary inflammatory index: A population-based study from NHANES 1999 to 2018. Medicine 2024;103:25(e38471).
JS and YZ contributed equally to this work.
Contributor Information
Jingjing Song, Email: 4206120060@email.ncu.edu.cn.
Yujun Zhang, Email: 4206121036@email.ncu.edu.cn.
Ao Li, Email: ndyfy04408@ncu.edu.cn.
Jie Peng, Email: 4203119216@email.ncu.edu.cn.
Chulin Zhou, Email: 19916095307@163.com.
Xifu Cheng, Email: xifu_cheng@email.ncu.edu.cn.
Yang Wu, Email: tcmclinic@163.com.
Wentao Zhao, Email: zhaowt2001@163.com.
Zhen Zong, Email: ndefy16133@ncu.edu.cn.
Rui Wu, Email: tcmclinic@163.com.
References
- [1].Mitrović J, Hrkač S, Tečer J, et al. Pathogenesis of extraarticular manifestations in rheumatoid arthritis—a comprehensive review. Biomedicines. 2023;11:1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jahid M, Khan KU, Rehan Ul H, Ahmed RS. Overview of rheumatoid arthritis and scientific understanding of the disease. Mediterr J Rheumatol. 2023;34:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bowman SJ. Hematological manifestations of rheumatoid arthritis. Scand J Rheumatol. 2002;31:251–9. [DOI] [PubMed] [Google Scholar]
- [4].Chen YF, Xu SQ, Xu YC, et al. Inflammatory anemia may be an indicator for predicting disease activity and structural damage in Chinese patients with rheumatoid arthritis. Clin Rheumatol. 2020;39:1737–45. [DOI] [PubMed] [Google Scholar]
- [5].Islam MR, Islam MS, Sultana MM. Anemia of chronic disease in rheumatoid arthritis and its relationship with disease activities. TAJ J Teach Assoc. 2020;33:85–93. [Google Scholar]
- [6].Pahlavani N, Malekahmadi M, Sedaghat A, et al. Effects of melatonin and propolis supplementation on inflammation, oxidative stress, and clinical outcomes in patients with primary pneumosepsis: a randomized controlled clinical trial. Complement Med Res. 2022;29:275–85. [DOI] [PubMed] [Google Scholar]
- [7].Putera HD, Doewes RI, Shalaby MN, et al. The effect of conjugated linoleic acids on inflammation, oxidative stress, body composition and physical performance: a comprehensive review of putative molecular mechanisms. Nutr Metab (Lond). 2023;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Barghchi H, Dehnavi Z, Nattagh-Eshtivani E, et al. The effects of Chlorella vulgaris on cardiovascular risk factors: a comprehensive review on putative molecular mechanisms. Biomed Pharmacother. 2023;162:114624. [DOI] [PubMed] [Google Scholar]
- [9].Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nattagh-Eshtivani E, Pahlavani N, Ranjbar G, et al. Does propolis have any effect on rheumatoid arthritis? A review study. Food Sci Nutr. 2022;10:1003–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ma H, Deng W, Chen H, Ding X. Association between dietary inflammatory index and anemia in US adults. Front Nutr. 2023;10:1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pant A, Chew DP, Mamas MA, Zaman S. Cardiovascular disease and the mediterranean diet: insights into sex-specific responses. Nutrients. 2024;16:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Parolini C. The role of marine n-3 polyunsaturated fatty acids in inflammatory-based disease: the case of rheumatoid arthritis. Mar Drugs. 2023;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shekhar KV, Pathak MM, Pisulkar G. Diet and lifestyle impact on rheumatoid arthritis: a comprehensive review. Cureus. 2023;15:e48625. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [15].Vadell AKE, Bärebring L, Hulander E, Gjertsson I, Lindqvist HM, Winkvist A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)—a randomized, controlled crossover trial indicating effects on disease activity. Am J Clin Nutr. 2020;111:1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Calisto Pérez C, León R, León F, Ng SL. Rheumatoid arthritis and anemia: the impact of different anti-inflammatory therapies on hemoglobin levels. An observational study. Bol Asoc Med P R. 2012;104:34–41. [PubMed] [Google Scholar]
- [17].Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eastgate J, Wood N, Di Giovine F, Symons J, Grinlinton F, Duff G. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988;332:706–9. [DOI] [PubMed] [Google Scholar]
- [19].Bertero MT, Caligaris-Cappio F. Anemia of chronic disorders in systemic autoimmune diseases. Haematologica. 1997;82:375–81. [PubMed] [Google Scholar]
- [20].Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- [21].Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013;15:R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hébert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: the Dietary Inflammatory Index (DII)-lessons learned, improvements made, and future directions. Adv Nutr. 2019;10:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shivappa N, Hébert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr. 2015;113:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xiang S, Wang Y, Qian S, et al. The association between dietary inflammation index and the risk of rheumatoid arthritis in Americans. Clin Rheumatol. 2022;41:2647–58. [DOI] [PubMed] [Google Scholar]
- [25].Lelijveld N, Benedict RK, Wrottesley SV, et al. Towards standardised and valid anthropometric indicators of nutritional status in middle childhood and adolescence. Lancet Child Adolesc Health. 2022;6:738–46. [DOI] [PubMed] [Google Scholar]
- [26].Wu L, Shi Y, Kong C, Zhang J, Chen S. Dietary inflammatory index and its association with the prevalence of coronary heart disease among 45,306 US adults. Nutrients. 2022;14:4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou N, Xie ZP, Liu Q, et al. The dietary inflammatory index and its association with the prevalence of hypertension: a cross-sectional study. Front Immunol. 2022;13:1097228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li A, Chen Y, Schuller AA, van der Sluis LWM, Tjakkes GE. Dietary inflammatory potential is associated with poor periodontal health: a population-based study. J Clin Periodontol. 2021;48:907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mazidi M, Shivappa N, Wirth MD, et al. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis. 2018;276:23–7. [DOI] [PubMed] [Google Scholar]
- [30].Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shivappa N, Hebert JR, Marcos A, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. 2017;61. doi: 10.1002/mnfr.201600707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sun Y, Liu J, Xin L, et al. Factors influencing the Sharp score of 1057 patients with rheumatoid arthritis and anemia: a retrospective study. J Int Med Res. 2022;50:3000605221088560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Padula AS, Pappas DA, Fiore S, et al. The effect of targeted rheumatoid arthritis therapeutics on systemic inflammation and anemia: analysis of data from the CorEvitas RA registry. Arthritis Res Ther. 2022;24:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rai A, Chan MT, Nambiar S. Social and ecological disparities in anaemia among adolescent girls 15–19 years old in Nepal. Public Health Nutr. 2023;26:2973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Furst DE, Chang H, Greenberg JD, et al. Prevalence of low hemoglobin levels and associations with other disease parameters in rheumatoid arthritis patients: evidence from the CORRONA registry. Clin Exp Rheumatol. 2009;27:560–6. [PubMed] [Google Scholar]
- [38].Wolfe F, Michaud K. Anemia and renal function in patients with rheumatoid arthritis. J Rheumatol. 2006;33:1516–22. [PubMed] [Google Scholar]
- [39].Wan H, Zhang Y, Ning Z, Liu M, Yang S. Associations of cereal fiber intake with rheumatoid arthritis mediated by dietary inflammatory index: insights from NHANES 2011-2020. Sci Rep. 2024;14:2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bose D, Ravi R, Maurya M, Legha R, Konwar M. Vitamin D deficiency in rheumatoid arthritis patients of India—a single-arm meta-analysis. Afr Health Sci. 2023;23:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Athanassiou L, Kostoglou-Athanassiou I, Koutsilieris M, Shoenfeld Y. Vitamin D and autoimmune rheumatic diseases. Biomolecules. 2023;13:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Neve A, Corrado A, Cantatore FP. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med. 2014;14:275–83. [DOI] [PubMed] [Google Scholar]
- [43].De la Cruz-Góngora V, Salinas-Rodríguez A, Flores-Aldana M, Villalpando S. Etiology of anemia in older Mexican adults: the role of hepcidin, vitamin A and vitamin D. Nutrients. 2021;13:3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Khalaf W, Al-Rubaie HA, Shihab S. Studying anemia of chronic disease and iron deficiency in patients with rheumatoid arthritis by iron status and circulating hepcidin. Hematol Rep. 2019;11:7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu J, Idborg H, Korotkova M, et al. Urinary prostanoids are elevated by anti-TNF and anti-IL6 receptor disease-modifying antirheumatic drugs but are not predictive of response to treatment in early rheumatoid arthritis. Arthritis Res Ther. 2024;26:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shirasugi I, Onishi A, Nishimura K, et al. Association of large joint involvement at the start of biological disease-modifying antirheumatic drugs and Janus kinase inhibitors with disease activity and drug retention in patients with rheumatoid arthritis: the ANSWER cohort study. Int J Rheum Dis. 2024;27:e15097. [DOI] [PubMed] [Google Scholar]
- [47].Jiang LQ, Zhang RD, Musonye HA, et al. Hormonal and reproductive factors in relation to the risk of rheumatoid arthritis in women: a prospective cohort study with 223 526 participants. RMD Open. 2024;10:e003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maldonado M, Romero-Aibar J, Calvo J. The melatonin contained in beer can provide health benefits, due to its antioxidant, anti-inflammatory and immunomodulatory properties. J Sci Food Agric. 2023;103:3738–47. [DOI] [PubMed] [Google Scholar]
- [49].Jandari S, Mosalmanzadeh N, Shadmand Foumani Moghadam MR, et al. Dietary inflammatory index and healthy eating index-2015 are associated with rheumatoid arthritis. Public Health Nutr. 2021;24:6007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yang M, Su Y, Xu K, et al. Iron, copper, zinc and magnesium on rheumatoid arthritis: a two-sample Mendelian randomization study. Int J Environ Health Res. 2023;34:2776–89. [DOI] [PubMed] [Google Scholar]
- [51].Turk MA, Liu Y, Pope JE. Non-pharmacological interventions in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Autoimmun Rev. 2023;22:103323. [DOI] [PubMed] [Google Scholar]
- [52].Zhao Z, Ma X, Dong S, Yin H, Yang Y, Xiong G. Regulatory effect of zinc finger protein A20 on rheumatoid arthritis through NLRP3/Caspase-1 signaling axis mediating pyroptosis of HFLS- RA cells. Cell Mol Biol (Noisy-le-grand). 2023;69:179–84. [DOI] [PubMed] [Google Scholar]
- [53].Youssef SR, Hassan EH, Morad CS, Elazab Elged AA, El-Gamal RA. Erythroferrone expression in anemic rheumatoid arthritis patients: is it disordered iron trafficking or disease activity? J Inflamm Res. 2021;14:4445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Patel A, Bhatt V, Edara M. A study of haematological profile in newly diagnosed rheumatoid arthritis and its correlation with disease activity. J Assoc Physicians India. 2022;70:11–2. [PubMed] [Google Scholar]
- [55].Swaak A. Anemia of chronic disease in patients with rheumatoid arthritis: aspects of prevalence, outcome, diagnosis, and the effect of treatment on disease activity. J Rheumatol. 2006;33:1467–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.