Abstract
Study Design
Systematic review and meta-analysis.
Objective
The aim of this study was to determine the prevalence of asymptomatic cervical spinal cord compression (CSCC) in individuals with lumbar spinal stenosis (LSS).
Methods
A systematic electronic search was conducted in Medline, EMBASE, Scopus, and Web of Science without language restriction, with no starting date limit to June 8, 2023, to define the prevalence of asymptomatic CSCC in symptomatic LSS patients. Asymptomatic CSCC was defined based on radiographic studies. All types of studies were included in the review. Meta-analysis was performed on the reported prevalence of asymptomatic CSCC in LSS.
Results
The database search yielded 10,272 articles. After a full-text review, five studies were included in the final review, comprising a total of 1043 cases. Two studies had a low risk for bias, two moderate, and one estimated to be high risk. The range of prevalence of asymptomatic CSCC in LSS in the five included studies was between 24% and 61%. Meta-analysis on the reported prevalence of asymptomatic CSCC patients with symptomatic LSS demonstrated that the random pooled prevalence was 35% (95% CI: 23 to 48).
Conclusions
Asymptomatic CSCC appears to occur in a high number of patients, with this study noting its presence in one-third of patients with LSS. Based on these findings, we strongly recommend that spine surgeons exercise particular caution during the positioning of patients who are undergoing surgery for lumbar stenosis. Furthermore, it is imperative to monitor individuals with symptomatic LSS closely for any potential signs of emerging myelopathy.
Keywords: spinal cord compression, asymptomatic, systematic review, magnetic resonance imaging, lumbar canal stenosis, tandem spinal stenosis
Introduction
Degenerative Cervical Myelopathy (DCM) represents a progressive affliction of the spinal cord that may arise from various sources, including mechanical stress or degenerative alterations. 1 The degenerative processes can include osteophytes, disc herniation, enlargement, or ossification of the ligamentum flavum, and translation or instability. 1 A multifactorial interplay of mechanical forces, such as shear, tension, and compression, contribute to a comprehensive understanding of DCM. 2 Individual predispositions, such as age, genetic factors, cardiovascular, gastrointestinal, and neural health, and exposure duration, also have a significant bearing on the susceptibility to spinal cord injury. 2 Symptoms of DCM manifest as pain, numbness in limbs, coordination impairment, balance issues, and bladder complications. 3
A key challenge within the DCM poulation is the accurate diagnosis and prediction of disease progression. 4 The global AO Spine RECODE-DCM initiative, collaborating with multiple stakeholders, aims to create a research toolkit that fosters rapid knowledge acquisition and augments DCM outcomes. 5 These endeavors emphasize the crucial role of early surgical intervention, currently the only disease-modifying treatment available, which can arrest further spinal cord injury and deliver significant benefits, though full recovery may remain elusive.6-9 Optimal surgical outcomes and the prevention of irreversible damage hinge on the early identification of patients exhibiting symptomatic myelopathy. Nevertheless, the inherent surgical risks warrant that in patients with Cervical Spinal Cord Compression (CSCC) lacking symptomatic myelopathy, surgical intervention is discouraged. 8
The occurrence of asymptomatic CSCC varies between 8% and 57%.10-12 Those with CSCC stand at risk for DCM development. In a 4-year study involving 66 asymptomatic CSCC patients, Bednarik et al. discovered that 19.7% manifested clinical DCM symptoms. 13 Spinal stenosis, a condition more prevalent in individuals with smaller spinal canals, arises from degenerative changes, and this degenerative sequence, coupled with resultant canal stenosis, can provoke neuronal compression in both cervical and lumbar regions, a condition known as Tandem Spinal Stenosis (TSS). 14
Tandem spinal stenosis prevalence is reported to range from 7.6% to 60%, with asymptomatic cases, where the patient exhibits no discernible symptoms, found in 23.7%-61.4% of instances.15-18 Recognizing asymptomatic CSCC, which can be present in patients with symptomatic Lumbar Spinal Stenosis (LSS), is important since the condition can evolve into myelopathy.13,19-22 Early diagnosis and thorough follow-ups can benefit CSCC patients. Moreover, during surgical operations, asymptomatic CSCC patients should be carefully positioned and further monitored for any signs of myelopathy.17,23
Appreciating the prevalence of asymptomatic CSCC in symptomatic LSS patients can inform more effective clinical decision-making. In this research, our objective is to define the prevalence of asymptomatic CSCC in patients diagnosed with symptomatic LSS.
Methods
The protocol for this study was registered with PROSPERO (CRD42023430201). 24 The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocol for systematic reviews and meta-analysis. 25 The Ethics Committee of Tehran University of Medical Sciences, approved the study, and the reference number is IR.TUMS.SINAHOSPITAL.REC.1402.003.
Literature Search
An electronic search was conducted to identify research that determined the prevalence of patients with asymptomatic CSCC in patients with symptomatic LSS using Medline, EMBASE, Scopus, and Web of Science with no language restriction, with no starting date limit to June 8, 2023. The search strategy for this study is included in Appendix 1.
Selection Criteria
Studies had to report the prevalence of asymptomatic CSCC in known cases of lumbar stenosis. Asymptomatic was defined as those patients without neck pain, signs or symptoms of myelopathy, and cervical radiculopathy. Radiculopathy was considered arm pain or paresthesia in the dermatomal distribution or muscle weakness relating to the affected nerve. Myelopathic symptoms were defined as neck or upper limb pain, weakness, sensory loss, loss of dexterity, paresthesia, imbalance, falls, and autonomic dysfunction. Studies that met any of the following criteria were excluded: (1) Studies that reported the prevalence of symptomatic CSCC in patients with LSS. (2) Studies that reported asymptomatic cervical and thoracic cord compression in patients with LSS without mentioning separate prevalence for cervical and thoracic cord compression cases. (3) Reviews, case reports, and case series with less than ten patients. (4) Spinal compressions due to non-degenerative, oncologic, or traumatic causes.
Selection Process
Search results were imported to EndNote (X9, Thomason Reuters), duplicates were removed, and two reviewers independently screened titles and abstracts. Any discrepancies between the reviewers were resolved in a session with the senior author. After obtaining the full text of included articles, the full texts were checked against inclusion and exclusion criteria for including studies by two reviewers. Any disagreements were resolved by the senior author.
Data Extraction
A data collection spreadsheet was designed and the following items were extracted from the included studies: authors’ names, publication date, study date, country, study design, sample size, and characteristics, percentage of patients with LSS, percentage of patients with asymptomatic CSCC, BMI, level of lesions, primary lesion type, cervical lesion location, and tandem percentage. Data were extracted and rechecked by two investigators.
Quality Assessment
The ROBINS-I tool was used for risk of bias assessment, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions. 26 This was also done by two independent authors and conflicts were resolved by the senior author. The class of evidence was based on the Journal of Bone and Joint Surgery for studies investigating treatment results for assessing methodological quality. 27
Outcomes
The variable of interest was the prevalence of imaging findings of CSCC in patients with symptomatic LSS that did not have any signs or symptoms of myelopathy or cervical radiculopathy.
Statistical Analysis
We assessed the heterogeneity of the studies with the Chi2 test and the I2 statistics. The “Metaprop” command in Stata was applied for meta-analyses of effect sizes. Random effects model was used to combine the effect sizes. Also, the effect sizes with 95% confidence intervals were plotted. Stata version 14.0 (Stata Corp, College Station, TX) was used for analysis.
Results
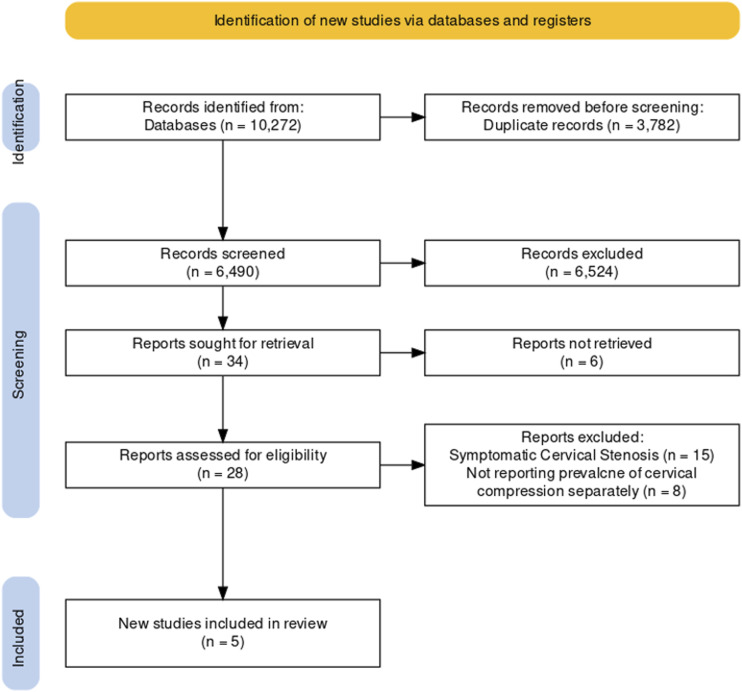
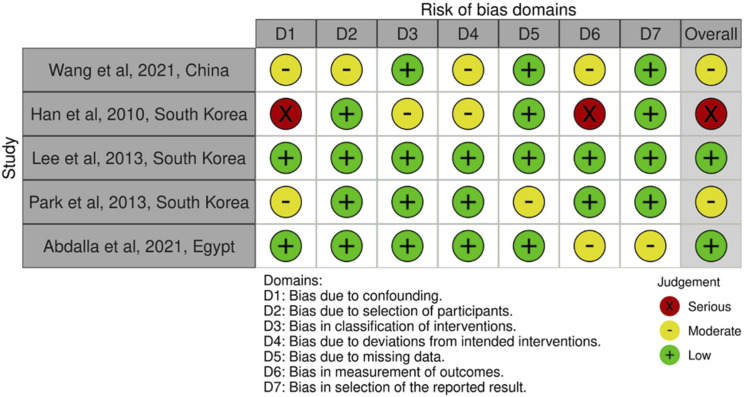
The PRISMA chart of this study is presented in Figure 1. The database search yielded 10,272 clinical cases. After screening relevant titles and abstracts, 28 manuscripts were included for further assessment. After a full-text review of these 28 records, five studies were included in the final analysis. Of these five studies, four were retrospective and one was prospective. In total, there were 1043 cases included. The included study characteristics are presented in Table 1. The ROBIN-I tool was used to assess the risk of bias in the included studies, and the results are presented in Figure 2. Two studies assessed low risk, two moderate risks, and one serious risk for bias.
Figure 1.
Flowchart of studies based on the PRISMA statement for the prevalence of asymptomatic cervical spinal cord compression in individuals presenting with symptomatic lumbar spinal stenosis.
Table 1.
Characteristics of Included Studies.
| First Author, Year | Country | Study Type | N (Female%) | Age (SD) | Pathology | Outcome Measurement | Level of Evidence |
|---|---|---|---|---|---|---|---|
| Wang, 2021 18 | China | Retrospective | 114 (49%) | 61.37 (9.1) | Lumbar spinal stenosis | Radiological assessment included evaluation of redundant nerve roots (RNRs), the dural sac cross-sectional area (DCSA), the facet joint angle (FJA), the lumbar lordosis angle (LLA), the Pelvic incidence (PI), the Torg-Pavlov ratio (TPR) of the cervical spine and lumbosacral transitional vertebrae (LSTV), cervical stenosis index, lumbar stenosis index | Level III |
| Han, 2010 28 | South Korea | Retrospective | 306 (52%) | 56.3 (NA) | Degenerative spinal diseases | Whole spine sagittal T2-weighted images | Level III |
| Lee, 2010 17 | South Korea | Retrospective | 93 (59%) | 69.9 (NA) | Lumbar spinal stenosis | Percentage of central canal compression, lumbar stenosis index, cervical cord compression index | Level III |
| Park, 29 2013 | South Korea | Retrospective | 460 (NA) | N/A | Asymptomatic spondylotic cervical and thoracic stenosis, lumbar spinal stenosis | Anterior epidural stenosis, posterior epidural stenosis | Level III |
| Abdalla, 30 2021 | Egypt | Cross-sectional | 70 (34.29%) | 55.7 (9.8) | Surgical lumbar canal stenosis | Cervical spine MRI | Level III |
Figure 2.
Risk of bias assessment of included studies for the prevalence of asymptomatic cervical spinal cord compression in individuals presenting with symptomatic lumbar spinal stenosis.
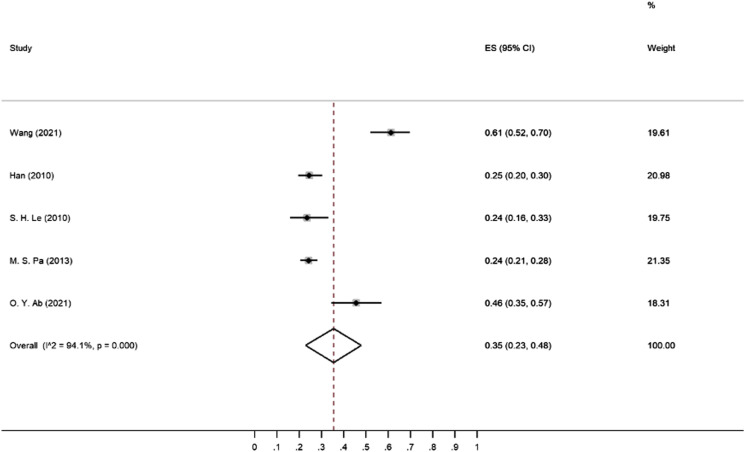
The range of prevalence of asymptomatic CSCC in patients with LSS in these five studies was between 24 to 61 percent. In the Wang et al study, 18 141 cases of LSS with a mean age of 61 ± 9.1 years were evaluated for CSCC, and the reported prevalence was 61 percent (95% CI: 52 to 70). This study found that LSS symptom duration, the presence of redundant nerve root, dural sac cross-sectional area, and pelvic incidence were significantly associated with the risk of CSCC. Additionally, a strong positive linear relationship was reported between the lumbar stenosis index (LSI) and the degree of CSCC. In the Han et al study, 28 the prevalence of CSCC in patients with LSS was reported as 25%. In contrast to the Wang et al study, there was a significant difference in the prevalence of CSCC in patients under and over 40 years old.18,28 In the Lee et al study, the prevalence of CSCC in patients with LSS was reported as 24%. 17 Similar to the Wang et al study, a positive linear relationship was found between LSI and CSCC.17,18 In the Park et al study, the prevalence of CSCC in TSS was also reported as 24%. 29 Abdalla et al study noted a 46% incidence. 30 In two of the five studies, the prevalence was reported as 24 percent and a third study reported the prevalence as 25%. The random pooled prevalence is 35% (95% CI: 23 to 48) as seen in Figure 3 and Table 2
Figure 3.
Meta-analysis of the prevalence of asymptomatic cervical spinal cord compression (CSCC) in individuals with lumber spinal stenosis (LSS).
Table 2.
Table With Pooled Values of 5 Included Studies.
| Study | Prevalence | 95% CI | % Weight |
|---|---|---|---|
| Wang (2021) | 0.61 | 0.52 to 0.70 | 19.61 |
| Han (2010) | 0.25 | 0.20 to 0.30 | 20.98 |
| Le (2010) | 0.24 | 0.16 to 0.33 | 19.75 |
| Park (2013) | 0.24 | 0.21 to 0.28 | 21.35 |
| Abdalla (2021) | 0.46 | 0.35 to 0.57 | 18.31 |
| Random pooled prevalence | 0.35 | 0.23 to 0.48 | 100.00 |
Discussion
In the current study, there was a pooled prevalence of asymptomatic CSCC in symptomatic LSS patients of 35% (range: 24-61). Asymptomatic CSCC is not uncommon and prevalence rates are known to increases with age, with a reported prevalence between 25%-60% in patients over 60 years old.12,31 However, in LSS patient, asymptomatic CSCC may be related to factors beyond the simple aging process. 17 There is little data on the natural history of asymptomatic cervical stenosis, but one study with a 4-year follow-up indicated that 19.7% of these patients became symptomatic. 13 Additionally, pain in the lower limb in LSS may be caused by CSCC as pressure on the cervical spinal cord can stimulate the nerve track connecting the spinothalamic tract of the spinal cord to the lower limb.17,21,31 In these patients, surgical treatment of LSS may not result in sufficient clinical outcomes if the source of lower limb pain is CSCC.14,32-34 Furthermore, prolonged surgical positioning in individuals with LSS, including the commonly employed prone or lateral positions during spine operations, can lead to worsening of cervical compression and possible neurologic injury.17,23 Hence, it is imperative to exercise increased caution when initially positioning LSS patients at the outset of surgery. Furthermore, regular positional reassessments during extended procedures are recommended. This approach will help prevent circumstances that could potentially result in heightened compression within the cervical spine.
This meta-analysis presented the specific prevalence rates of asymptomatic CSCC among different demographic groups. According to one analysis, the prevalence of CSCC without signs and symptoms of myelopathy in a healthy population was 24.2%, and it increases to 35.3% in individuals over 60 years of age. 35 Interestingly the pooled prevalence of asymptomatic CSCC in patients with symptomatic LSS is 35%, which is close to their findings for patients over 60 years old. While we were not able to perform subgroup analysis based on age, these findings might suggest that although healthy patients under 60 years old have asymptomatic cervical cord compression in about 24%, However, the prevalence could be higher in patients with LSS in the same age group, making them at higher risk for developing DCM as they have long years to live.
Thus the progression rate of cervical myelopathy in patients with CSCC is between 1%-5% per year, and this risk is cumulative over time, putting younger patients at a higher risk.13,36,37 Thus, a patient with CSCC has a 50% chance of progression of myelopathy over 20 years. 38 Additionally, certain patient factors, such as younger age and a lack of access to healthcare, may further increase this risk. It is important to take these factors into account when deciding on treatment and follow-up options.
Several screening tools for cervical myelopathy (DCM) have been proposed,39–41 with one method suggested by Nouri et al. 41 targeting patients with lumbar radiculopathy or LSS. This method assigns points based on the presence of signs or symptoms of DCM, as well as comorbidities that predispose or are frequently associated with cervical myelopathy. Patients with a score of ≥3 points are recommended to undergo a cervical MRI examination. Preliminary results of this screening method showed that out of 97 patients screened, 26 screened positive (≥3 points) and 18 had a subsequent cervical MRI. Of the 18 patients, 7 (38.9%) were diagnosed with cervical myelopathy. 41 This suggests that if this screening method were used in a population of 100 patients with lumbar symptoms, only about 7% would be diagnosed with DCM based on their cervical MRIs. However, our findings indicate that if all 100 patients with lumbar symptoms underwent cervical MRI, even without any DCM symptoms, about 35% of them should have CSCC in their imaging. This suggests that while the screening method proposed by Nouri et al. 41 may be cost-effective, one can also consider screening patients with symptomatic LSS.
In comparison to another meta-analysis by Smith et al., 35 which found a 24.2% prevalence of asymptomatic CSCC in the general population, our focused review revealed a higher prevalence (35%) in patients with symptomatic LSS. This difference could be attributed to the presence of degenerative spinal processes in LSS patients or congenital stenosis, potentially making them more prone to develop similar conditions in other parts of the spine. While Smith et al.’s study provides a valuable epidemiological insight into the prevalence of spinal cord compression; it does not specifically address the value of cervical MRI screening in patients already undergoing MRI for LSS. Our data suggests the potential benefit of such an approach in detecting co-existing pathologies early, possibly improving patient outcomes, but more studies are required to substantiate these preliminary findings.
Prior research indicates that patients with imaging signs of spinal cord compression but no evident myelopathy symptoms, can still show electrophysiological markers of cervical radiculopathy or central conduction deficit.42,43 These markers may herald the onset of myelopathy. Furthermore, the AOSpine Clinical Practice Guideline for DCM suggests that such patients be informed about the potential risk. 8 It even proposes considering either surgical treatments or non-operative approaches such as routine monitoring or structured rehabilitation as options. 8 However, a considerable knowledge gap exists in our understanding regarding the risk of developing myelopathy in asymptomatic CSCC patients who also exhibit symptomatic LSS, compared to CSCC patients without LSS. This study underscores the necessity for further research in this area. It remains to be seen whether these patients carry similar or even higher progression risks compared to patients with electrophysiological abnormalities, particularly since an additional symptomatic degenerative pathology may be present in another part of the spine. This could potentially alter the risk prediction and treatment strategies in this subset of patients.
Quantitative MRI methodologies such as Diffusion Tensor Imaging (DTI) may serve as a tool for categorizing patients according to risk levels. A multitude of research efforts have unanimously indicated a correlation between fractional anisotropy and mean diffusivity with disease severity, along with clinical parameters in DCM. 44 Furthermore, fractional anisotropy has been observed to be diminished at the compression site in asymptomatic patients. 45 Additionally, it has been proposed that the duration of symptoms warrants deeper exploration concerning its impact on DTI parameters in upcoming DCM research. 46 This insight could be integrated into clinical procedures, implying that DTI parameters or alternative quantitative imaging techniques might aid in better understanding the progression risk in asymptomatic CSCC amongst patients with symptomatic LSS.
While this study has presented insightful findings, certain limitations should be factored into the interpretation of its results. To begin with, the analysis incorporated a rather limited set of studies, totaling five, which may not offer a comprehensive view of the subject matter. However, the 95% CI of the pooled prevalence for asymptomatic CSCC in symptomatic LSS patients lies between 23% and 48%. This indicates that future investigations might yield results within this interval. Therefore, even at the lower limit, nearly one-quarter of symptomatic LSS patients might present with asymptomatic CSCC. This represents a sizable patient group worthy of consideration.
An additional limitation of this study is that it exclusively comprises retrospective and cross-sectional studies, rated as level III evidence. This classification extends to the pooled analysis, making it level III evidence as well. Although more robust evidence levels would have been preferable, our primary objective was to resolve a clinical question: how many patients undergoing medical examination and imaging for LSS concurrently present with asymptomatic CSCC? Until more rigorous studies emerge, cross-sectional or retrospective studies provide an appropriate framework to answer this query. Hence, we maintain that even with the level III evidence, this pooled analysis carries significant clinical relevance and is sufficiently supported by this evidence tier.
Additionally, the lack of age-specific data on the prevalence of CSCC in the included studies makes it difficult to draw conclusions about specific age groups. Furthermore, the inconsistent definition of asymptomatic patients across studies may have resulted in underreporting or over reporting of the condition. Another important limitation to be aware of is that some of the included studies included patients who had undergone surgery for LSS, which may mean that these patients had a more severe degenerative disease and it is not possible to determine how the results vary based on disease severity.
Conclusions
Approximately a third of patients with symptoms of LSS may also have concurrent radiographic cervical stenosis. A careful physical evaluation should be performed on these LSS patients to confirm no myelopathy symptoms due to the high rate of cervical stenosis in this population. These patients may develop new neurological symptoms in the form of DCM in the future. Based on these findings, we also strongly recommend that spine surgeons exercise particular caution during the positioning of patients who are undergoing surgery for lumbar stenosis.
Supplemental Material
Supplemental Material for The Prevalence of Asymptomatic Cervical Spinal Cord Compression in Individuals Presenting With Symptomatic Lumbar Spinal Stenosis: A Meta-Analysis by Farzin Farahbakhsh, Sepehr Khosravi, Vali Baigi, Masoud Pourghahramani Koltapeh, Amirmahdi Khayyamfar, Zahra Eskandari, Zahra Ghodsi, James Harrop, and Vafa Rahimi-Movaghar in Global Spine Journal
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Tehran University of Medical Sciences, [grant number is 1401-4-101-63630].
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Farzin Farahbakhsh https://orcid.org/0000-0003-4435-9034
Sepehr Khosravi https://orcid.org/0000-0002-5372-0787
Vali Baigi https://orcid.org/0000-0003-1882-7340
Masoud Pourghahramani Koltapeh https://orcid.org/0000-0001-8066-5739
Amirmahdi Khayyamfar https://orcid.org/0000-0001-9117-8207
Zahra Eskandari https://orcid.org/0000-0002-9278-4193
Zahra Ghodsi https://orcid.org/0000-0001-8662-2342
Vafa Rahimi-Movaghar https://orcid.org/0000-0001-7347-8767
References
- 1.Banerjee A, Mowforth OD, Nouri A, et al. The prevalence of degenerative cervical myelopathy-related pathologies on magnetic resonance imaging in healthy/asymptomatic individuals: A meta-analysis of published studies and comparison to a symptomatic cohort. J Clin Neurosci. 2022;99:53-61. doi: 10.1016/j.jocn.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 2.Davies BM, Mowforth O, Gharooni A-A, et al. A new framework for investigating the biological basis of degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 5]: Mechanical stress, vulnerability and time. Global Spine J. 2022;12(1_suppl):78S-96S. doi: 10.1177/21925682211057546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milligan J, Ryan K, Fehlings M, Bauman C. Degenerative cervical myelopathy: Diagnosis and management in primary care. Can Fam Physician. 2019;65(9):619-624. [PMC free article] [PubMed] [Google Scholar]
- 4.Nouri A, Cheng JS, Davies B, Kotter M, Schaller K, Tessitore E. Degenerative cervical myelopathy: A brief review of past perspectives, present developments, and future directions. J Clin Med. 2020;9(2):535. doi: 10.3390/jcm9020535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies BM, Khan DZ, Mowforth OD, et al. RE-CODE DCM (REsearch objectives and common data elements for degenerative cervical myelopathy): A consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Global Spine J. 2019;9(1 Suppl):65s-76s. doi: 10.1177/2192568219832855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grodzinski B, Stubbs DJ, Davies BM. Most degenerative cervical myelopathy remains undiagnosed, particularly amongst the elderly: Modelling the prevalence of degenerative cervical myelopathy in the United Kingdom. J Neurol. 2023;270(1):311-319. doi: 10.1007/s00415-022-11349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope DH, Mowforth OD, Davies BM, Kotter MRN. Diagnostic delays lead to greater disability in degenerative cervical myelopathy and represent a health inequality. Spine. 2020;45(6):368-377. doi: 10.1097/brs.0000000000003305 [DOI] [PubMed] [Google Scholar]
- 8.Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: Recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(3 Suppl):70s-83s. doi: 10.1177/2192568217701914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grodzinski B, Durham R, Mowforth O, Stubbs D, Kotter MRN, Davies BM. The effect of ageing on presentation, management and outcomes in degenerative cervical myelopathy: A systematic review. Age Ageing. 2021;50(3):705-715. doi: 10.1093/ageing/afaa236 [DOI] [PubMed] [Google Scholar]
- 10.Martin AR, Tadokoro N, Tetreault L, et al. Imaging evaluation of degenerative cervical myelopathy: Current state of the art and future directions. Neurosurg Clin N Am. 2018;29(1):33-45. doi: 10.1016/j.nec.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki A, Daubs MD, Hayashi T, et al. Patterns of cervical disc degeneration: Analysis of magnetic resonance imaging of over 1000 symptomatic subjects. Global Spine J. 2018;8(3):254-259. doi: 10.1177/2192568217719436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teresi LM, Lufkin RB, Reicher MA, et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology. 1987;164(1):83-88. doi: 10.1148/radiology.164.1.3588931 [DOI] [PubMed] [Google Scholar]
- 13.Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical cord compression. Spine. 2004;29(20):2260-2269. doi: 10.1097/01.brs.0000142434.02579.84 [DOI] [PubMed] [Google Scholar]
- 14.Dagi TF, Tarkington MA, Leech JJ. Tandem lumbar and cervical spinal stenosis. Natural history, prognostic indices, and results after surgical decompression. J Neurosurg. 1987;66(6):842-849. doi: 10.3171/jns.1987.66.6.0842 [DOI] [PubMed] [Google Scholar]
- 15.Iizuka H, Takahashi K, Tanaka S, Kawamura K, Okano Y, Oda H. Predictive factors of cervical spondylotic myelopathy in patients with lumbar spinal stenosis. Arch Orthop Trauma Surg. 2012;132(5):607-611. doi: 10.1007/s00402-012-1465-z [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Oya T, Abe Y, et al. Spinal stenosis due to ossified lumbar lesions. J Neurosurg Spine. 2005;3(4):262-270. doi: 10.3171/spi.2005.3.4.0262. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Kim KT, Suk KS, et al. Asymptomatic cervical cord compression in lumbar spinal stenosis patients: A whole spine magnetic resonance imaging study. Spine. 2010;35(23):2057-2063. doi: 10.1097/BRS.0b013e3181f4588a [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Rong Y, Tang P, et al. Prevalence and predictive factors of asymptomatic spondylotic cervical spinal stenosis in patients with symptomatic lumbar spinal stenosis. World Neurosurg. 2021;151:e1051-e1058. doi: 10.1016/j.wneu.2021.05.054 [DOI] [PubMed] [Google Scholar]
- 19.Kudo T, Sato Y, Kowatari K, Nitobe T, Hirota K. Postoperative transient tetraplegia in two patients caused by cervical spondylotic myelopathy. Anaesthesia. 2011;66(3):213-216. doi: 10.1111/j.1365-2044.2010.06562.x [DOI] [PubMed] [Google Scholar]
- 20.Ghobrial GM, Oppenlander ME, Maulucci CM, et al. Management of asymptomatic cervical spinal stenosis in the setting of symptomatic tandem lumbar stenosis: A review. Clin Neurol Neurosurg. 2014;124:114-118. doi: 10.1016/j.clineuro.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Langfitt TW, Elliott FA. Pain in the back and legs caused by cervical spinal cord compression. JAMA. 1967;200(5):382-385. [PubMed] [Google Scholar]
- 22.Young IA, Burns SP, Little JW. Sudden onset of cervical spondylotic myelopathy during sleep: A case report. Arch Phys Med Rehabil. 2002;83(3):427-429. doi: 10.1053/apmr.2002.29621 [DOI] [PubMed] [Google Scholar]
- 23.Kim BS, Kim J, Koh HS, Han SY, Lee DY, Kim KH. Asymptomatic cervical or thoracic lesions in elderly patients who have undergone decompressive lumbar surgery for stenosis. Asian Spine J. 2010;4(2):65-70. doi: 10.4184/asj.2010.4.2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosravi S, Farahbakhsh F, Ghodsi Z, Rahimi-Movaghar V. The prevalence of asymptomatic cervical cord compression in IndividualsPresented with symptomatic lumbar stenosis: A systematic review and meta-analysis. PROSPERO 2023 CRD42023430201. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023430201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85(1):1-3. [PubMed] [Google Scholar]
- 28.Han IH, Suh SH, Kuh SU, Chin DK, Kim KS. Types and prevalence of coexisting spine lesions on whole spine sagittal MR images in surgical degenerative spinal diseases. Yonsei Med J. 2010;51(3):414-420. doi: 10.3349/ymj.2010.51.3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park MS, Moon SH, Kim TH, et al. Asymptomatic stenosis in the cervical and thoracic spines of patients with symptomatic lumbar stenosis. Global Spine J. 2015;5(5):366-371. doi: 10.1055/s-0035-1549031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdalla OY, Al-Shami H, Maghraby HM, Enayet A. The value of cervical MRI in surgical lumbar canal stenosis patients. Egypt J Neurol Psychiatry Neurosurg. 2021;57(1):10. doi: 10.1186/s41983-020-00249-1 [DOI] [Google Scholar]
- 31.Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184. [PubMed] [Google Scholar]
- 32.Hitselberger WE, Witten RM. Abnormal myelograms in asymptomatic patients. J Neurosurg. 1968;28(3):204-206. doi: 10.3171/jns.1968.28.3.0204 [DOI] [PubMed] [Google Scholar]
- 33.LaBan MM, Green ML. Concurrent (tandem) cervical and lumbar spinal stenosis: A 10-yr review of 54 hospitalized patients. Am J Phys Med Rehabil. 2004;83(3):187-190. doi: 10.1097/01.phm.0000113405.48879.45 [DOI] [PubMed] [Google Scholar]
- 34.Vogt MT, Cawthon PM, Kang JD, Donaldson WF, Cauley JA, Nevitt MC. Prevalence of symptoms of cervical and lumbar stenosis among participants in the osteoporotic fractures in men study. Spine. 2006;31(13):1445-1451. doi: 10.1097/01.brs.0000219875.19688.a6 [DOI] [PubMed] [Google Scholar]
- 35.Smith SS, Stewart ME, Davies BM, Kotter MRN. The prevalence of asymptomatic and symptomatic spinal cord compression on magnetic resonance imaging: A systematic review and meta-analysis. Global Spine J. 2021;11(4):597-607. doi: 10.1177/2192568220934496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenehan B, Boran S, Street J, Higgins T, McCormack D, Poynton AR. Demographics of acute admissions to a national spinal injuries unit. Eur Spine J. 2009;18(7):938-942. doi: 10.1007/s00586-009-0923-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsunaga S, Sakou T, Taketomi E, Komiya S. Clinical course of patients with ossification of the posterior longitudinal ligament: A minimum 10-year cohort study. J Neurosurg. 2004;100(3 Suppl Spine):245-248. doi: 10.3171/spi.2004.100.3.0245. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh Taha AM, Shue J, Lebl D, Girardi F. Considerations for prophylactic surgery in asymptomatic severe cervical stenosis: Review article. Hss J. 2015;11(1):31-35. doi: 10.1007/s11420-014-9426-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkoh K, Ohiorhenuan IE, Lee L, et al. The down questionnaire: A novel screening tool for cervical spondylotic myelopathy. Global Spine J. 2019;9(6):607-612. doi: 10.1177/2192568218815863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi H, Kikuchi S-i, Otani K, Sekiguchi M, Sekiguchi Y, Konno S-i. Development of a self-administered questionnaire to screen patients for cervical myelopathy. BMC Muscoskel Disord. 2010;11(1):268. doi: 10.1186/1471-2474-11-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nouri A, Molliqaj G, Gondar R, et al. Can screening for degenerative cervical myelopathy (SCREEN-DCM) be effectively undertaken based on signs, symptoms and known risk factors? Rationale and research protocol for a prospective, multicentre, observational study. BMJ Open. 2022;12(7):e060689. doi: 10.1136/bmjopen-2021-060689 [DOI] [Google Scholar]
- 42.Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical myelopathy: an updated predictive model. Eur Spine J. 2008;17(3):421-431. doi: 10.1007/s00586-008-0585-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson JR, Barry S, Fischer DJ, et al. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patient with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine. 2013;38(22 Suppl 1):S37-54. doi: 10.1097/BRS.0b013e3182a7f2e7 [DOI] [PubMed] [Google Scholar]
- 44.Martin AR, Tetreault L, Nouri A, et al. Imaging and electrophysiology for degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 9]. Global Spine J. 2022;12(1_suppl):130s-146s. doi: 10.1177/21925682211057484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin AR, De Leener B, Cohen-Adad J, et al. A novel MRI biomarker of spinal cord white matter injury: T2*-weighted white matter to gray matter signal intensity ratio. AJNR Am J Neuroradiol. 2017;38(6):1266-1273. doi: 10.3174/ajnr.A5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farahbakhsh F. Letter to the editor on “Imaging and electrophysiology for degenerative cervical myelopathy [AO Spine RECODE-DCM Research Priority Number 9]” by Allan R. Martin et al. Global Spine J. 2023;5:21925682231188629. doi: 10.1177/21925682231188629 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The Prevalence of Asymptomatic Cervical Spinal Cord Compression in Individuals Presenting With Symptomatic Lumbar Spinal Stenosis: A Meta-Analysis by Farzin Farahbakhsh, Sepehr Khosravi, Vali Baigi, Masoud Pourghahramani Koltapeh, Amirmahdi Khayyamfar, Zahra Eskandari, Zahra Ghodsi, James Harrop, and Vafa Rahimi-Movaghar in Global Spine Journal