Abstract
Study design
Cross-sectional retrospective observational study.
Objective
To evaluate the reliability and clinical utility of the Modic changes (MC) grading score.
Method
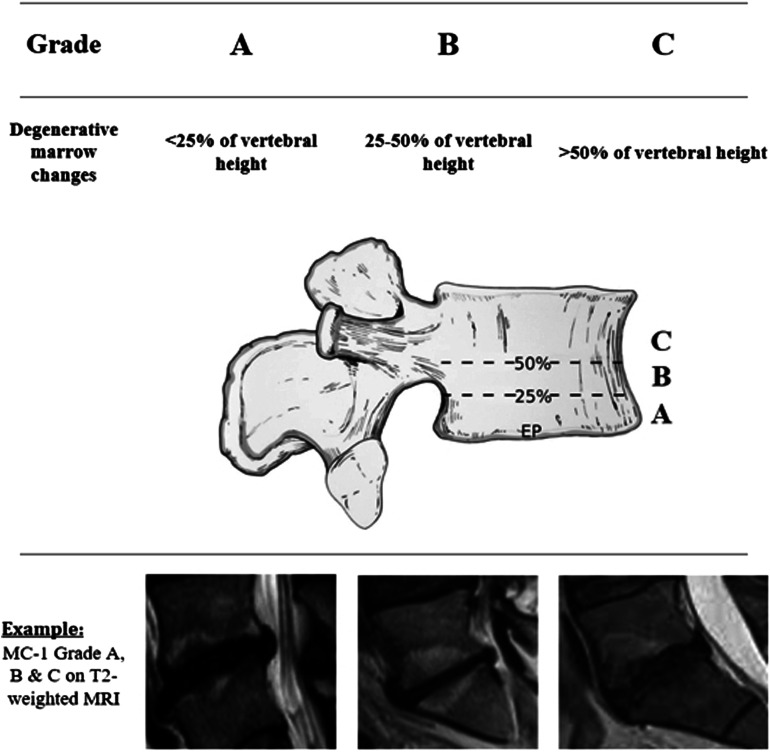
Patients from the Danish national spine registry, DaneSpine, scheduled for lumbar discectomy were identified. MRI of patients with MC were graded based on vertical height involvement: Grade A (<25%), Grade B (25%-50%), and Grade C (>50%). All MRIs were reviewed by 2 physicians to evaluate the reliability of the MC grade.
Results
Of 213 patients included, 142 patients had MC, 71 with MC-1 and 71 with MC-2; 34% were Grade A, 45% were Grade B, and 21% were Grade C. MC grade demonstrated substantial intra-rater (κ = .68) and inter-rater (κ = .61) reliability. A significantly higher proportion (n = 40, 57%) of patients with MC-1 had a severe MC grade compared to patients with MC-2 (n = 30, 43%, P < .001). Severe MC grade was associated with the presence of severe lumbar disc degeneration (DD) (Pfirrmann grade = V, P = .024), worse preoperative ODI (52.49 vs 44.17, P = .021) and EQ-5D scores (.26 vs .46, P = .053). MC alone including type was not associated with a significant difference in patient-reported outcomes (P > .05).
Conclusion
The MC grade score was demonstrated to have substantial intra- and inter-observer reliability. Severe MC grade was associated with both severe DD and MC type, being more prevalent in patients with MC-1. The MC grade was also significantly associated with worse disability and reduced health-related quality of life. Results from the study suggest that MC grade is more clinically important than MC type.
Keywords: modic changes, endplate, disc, degeneration, magnetic resonance imaging, low back pain
Introduction
Low back pain (LBP) is one of the most common causes of reduced health-related quality of life and disability.1,2 Most episodes of LBP are self-limiting with a short duration, and are classified as non-specific.3,4 Unfortunately, for many patients, LBP is a recurrent or chronic condition associated with both increased opioid use, physical disability, depression and increased mortality.1,3,5,6 A great deal of effort including government funded initiatives have focused on reducing the global burden of the back pain pandemic.3,7-11 This effort has also included extensive research in risk factors for developing LBP such as genetic disposition, disc degeneration (DD) and Modic changes (MC).12-15
Degenerative changes in the spine are unavoidable with advanced age and include changes in the sagittal/frontal plane like HIZ-lesions and disc protrusion/herniation; and in the transverse plane such as endplate changes (EC) including Schmorl’s nodes (SN) and MC.16-23 In a clinical setting it is of importance to differentiate between and understand the significance and association of these potential causes of LBP.24,25
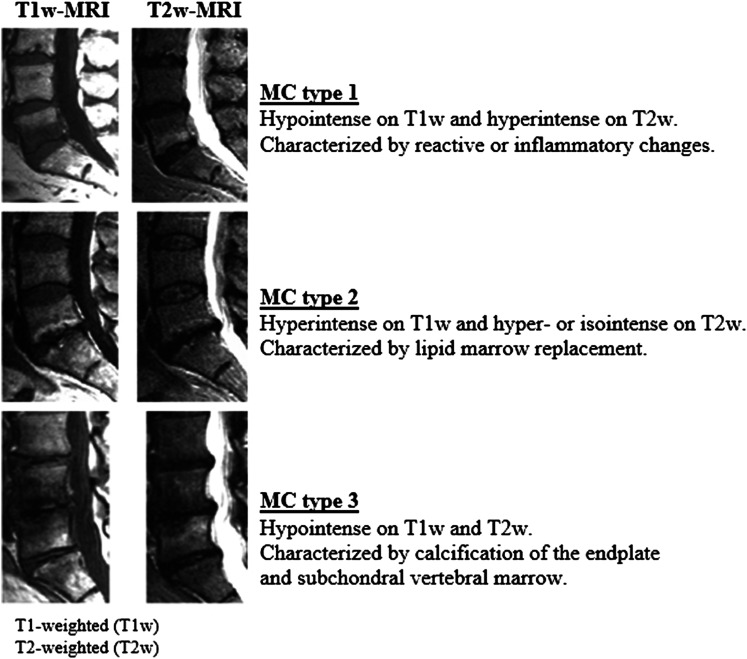
MC are non-neoplastic subchondral bone marrow changes within the vertebral body and endplates visible on magnetic resonance imaging (MRI).26-29 First described by Assheuer, 26 de Roos, 27 and Modic et al. 28 MC are now classified into 3 types- Modic changes type 1-3 (MC-1/MC-2/MC-3 – see Figure 1). 29 MC in the low back, are found in 5%-22% the background population.15,30 There is an increased presence of MC in patients with LBP and lumbar disc herniation (LDH).15,31,32 LDH can cause endplate-junction failure which can possibly explain the increased prevalence of MC and the increased risk of LBP in sciatica patients with MC.33–36
Figure 1.
MRI of Modic changes.
MC-1 is characterized by a reactive change in response to biomechanical stressors, reparative change, and/or inflammation; MC-2 is characterized by fatty infiltration, and MC-3 is associated with bony sclerosis of trabeculae and crowding out of marrow signal.28,29
In systematic reviews, the prevalence of MC in LBP patients and its association with patient-reported outcomes (PROs) demonstrated large between-study variations,37,38 specifically in the interpretation of the actual term “MC”. 37 This variation and the heterogeneity of MC nomenclature has been proposed as a possible cause of phenotype variation across studies.36,38,39 In addition, the extent of MC involvement has not generally been taken into account.40-42 The clinical relevance of the size of the MC have been investigated by Määttä43,44 and Weishaupt, 42 who found that increased vertical extent of MC (proximal or distal) was associated with the type of MC (MC-1 > MC-2). Severe degenerative changes at the functional spinal unit (FSU) were also associated with an increased risk of a positive discography and worse PROs.42,44 Based on these results, previous studies have suggested that MC grade, based on the vertical extent of MC in the vertebral body, should be included in the description and analysis of MC.36,37,40,43,45 The purpose of this study was to evaluate the reliability and clinical utility of the MC grading score in a clinically relevant population.
Methods
Ethical Considerations, Approvals, and Registration
Approval for data collection was obtained from the Danish National Data Protection Agency. The study was approved by The Regional Committees on Health Research Ethics (S-20192000-112). All patients gave informed consent for the use of their data including questionnaires for research purposes before registration in the registry.
This is a registry-based comparative cohort study on patients who underwent primary elective discectomy for LDH at L4-L5 or L5-S1 at a single institution between January 2014 and July 2017. 46 Patients were consecutively included based on the preoperative MRI findings. As patients with isolated MC-3 were few, these cases were excluded.
Inclusion criteria were patients who had surgery for LDH due to bilateral or unilateral radiculopathy with MRI findings concordant with the level and side of the radiculopathy. All patients had an MRI of the lumbar spine within 6 months before their discectomy. All patients underwent lumbar discectomy for persistent radiculopathy (at least 6 weeks) and after failure of nonsurgical treatment. Exclusion criteria were incomplete preoperative data and preoperative lumbar MRIs unavailable for review. Patients with motor deficits (motor grade <3/5), cauda equina, suspicion of malignancy, fractures, disc rupture due to trauma, and a history of previous spine surgery were also excluded.
Based on previous studies,38,47,48 it was estimated that approximately 45% of included patients would have MC on the preoperative MRI. Three predefined groups of similar size were selected for inclusion: MC-1, MC-2, and patients without MC (no-MC). The total number of patients within each cohort would depend on the least common MC finding in regards to these 3 predefined groups
Study participants completed questionnaires within 2 weeks before planned surgery including the Oswestry Disability Index (ODI), 49 European Quality of Life – 5 Dimensions (EQ-5D), Visual Analogue Scale (VAS) 0 (no pain)-100 (maximal pain) for back pain (VAS-BP), and leg pain (VAS-LP). 50
MRI Technique
The MRI system used was a Philips 1.5 Tesla (Philips Healthcare, The Netherlands). The standard imaging protocol consisted of a sagittal T1-weighted turbo spin-echo (TSE), and axial T2-weighted TSE of the lumbar segmental levels including S1. Sagittal short-tau inversion recovery (STIR) imaging was not included in the analysis. Imaging parameters for the sagittal images used in the study were: T1: repetition time 728 milliseconds (ms), echo time 10 ms, matrix 212 × 413, echo train length 6; T2: repetition time 3000 ms, echo time 90 ms, matrix 244 × 216, echo train length 24. Field of view was 196 mm × 196 mm and slice thickness/spacing was 4.0 mm/.4 mm for T1 and 3.0 mm/.3 mm for T2.
MRI Assessment
Modic Change
Modic changes were classified in accordance with the original studies by de Roos 27 and Modic et al.28,29 and the Modic grading score by Udby et al. 36 MC types were defined by T1/T2 weighted characteristics alone; type 1 as clearly hypo-intense on T1 and hyper-intense on T2, type 2 as hyperintense on T1, and iso- or hyperintense on T2 (Figure 1). If mixed MC or several types were present, the MC at the index level with the lowest classification, MC-1 > MC-2 > MC-3, was described. 36 If not present at the index level, MC was described as above, since adjacent segment MC could be associated with clinical symptoms. This was done in order to avoid a type-2 error, failing to find an existing association, as previous studies have shown a consistent association between MC-1 and LBP.37,38
MRI Grade
Modic change grade was defined as Grade A - MC <25% vertebral vertical height involvement, Grade B - MC with 25%-50% vertebral vertical height involvement, and Grade C - MC >50% vertebral vertical height involvement (Figure 2). 36
Figure 2.
The grading score.
All MRIs were evaluated twice by 2 physicians, and thrice by a third physician if inconsistencies existed after the second evaluation. The evaluating physicians were blinded as to the clinical status and discectomy level. Lumbar MRI evaluation included: Presence, type (MC-1/2/3), and level of MC, MC grade, DD (Pfirrmann classification, Grade I (no disc degeneration) - V (most severe degree of disc degeneration)), 51 and level of disc herniation (L1-S1).
Statistical Analyses
All statistical analysis was performed using IBM SPSS v27.0 (Armonk, New York). Continuous data from the groups were compared using one-way ANOVA and the unpaired student t-tests. Categorical data were analyzed using Fisher’s exact test. Reliability tests were evaluated by weighted Cohen’s kappa (values ≤0 as indicating no agreement and .01-.20 as none to slight, .21-.40 as fair, .41- .60 as moderate, .61-.80 as substantial, and .81-1.00 as almost perfect agreement). 52 The threshold for statistical significance was established at P ≤ .05.
Results
A total of 620 patients met the criteria for inclusion in the study. 46 Of these, 290 (47%) had MC and 330 (53%) did not have MC on the preoperative MRI scan. According to the study protocol, MC-1 (being the least common finding in regards to the 3 predefined groups) would be the limiting factor for the number of patients included in each cohort for this study. The final cohort included 71 pts with MC-1, 71 pts with MC-2, and 71 pts without MC, a total of 213 patients.
Comparison of the Three Groups
The demographics were similar for all 3 groups (No-MC, MC-1, MC-2). No significant differences were found in regards to sex, age, body mass index, smoking status, or baseline PROs (ODI, EQ-5D, and pain scores), P > .05 (Table 1).
Table 1.
Preoperative Characteristics for All Groups.
| No-MC (n = 71) | MC-1 (n = 71) | MC-2 (n = 71) | P-value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 51.72 (11.56) | 50.95 (10.88) | 50.23 (14.61) | .521 |
| Females, N (%) | 35 (49%) | 36 (51%) | 35 (49%) | .833 |
| Body mass index (kg/m2) | 26.89 (4.54) | 27.43 (5.73) | 26.77 (8.31) | .357 |
| Smoker, N (%) | 18 (25%) | 20 (28%) | 18 (24%) | .426 |
| ODI score a , mean (SD) | 47.48 (18.91) | 49.12 (18.72) | 46.76 (17.63) | .534 |
| EQ5D UK score b , mean (SD) | .32 (.33) | .36 (.28) | .38 (.34) | .211 |
| VAS score for back pain c , mean (SD) | 49.31 (29.50) | 49.40 (22.63) | 46.39 (27.42) | .352 |
| VAS score for leg pain, mean (SD) | 68.82 (23.49) | 69.01 (26.75) | 70.09 (22.33) | .698 |
Plus-minus values are means±standard deviation.
Years: yr; Number: no; Kilo: kg; meter: m.
aScores on the Oswestry Disability Index (ODI) range from 0 to 100, with higher scores indicating more severe disability.
bScores on the European Quality of Life–5 Dimensions (EQ-5D) range from 0 to 1, with higher scores indicating better quality of life.
cScores on the visual-analog scales (VASs) for back pain and leg pain range from 0 to 100, with higher scores indicating more severe pain.
Impact of the MC Grade
A scatter-plot showed substantial overlap in PRO-scores between patients with MC Grade B and Grade C. Thus, patients with MC were stratified into 2 groups – Grade A patients (non-severe MC grade) vs Grade B-C patients (severe MC grade). Patients with a severe MC grade had worse ODI scores, 52.49 vs 44.17 (P = .021), and worse EQ-5D scores of .26 vs .46 (P = .053). A similar but non-significant finding was observed for VAS-BP, 55.91 vs 44.85 (P = .102). A more severe MC grade was seen in patients with MC-1 (40, 57%) compared to MC-2 (30, 43%, P > .001) (Table 2).
Table 2.
Preoperative Characteristics for MC Patients ± Severe MC Grade.
| Grade A (n = 72) | Grade B + C (n = 70) | P-value | |
|---|---|---|---|
| Age, years, mean (SD) | 49.08 (10.21) | 50.32 (12.55) | .423 |
| Females, N (%) | 35 (48) | 36 (52) | .196 |
| Body mass index (kg/m2) | 25.72 (5.43) | 28.27 (6.82) | .175 |
| Smoker, N (%) | 18 (25) | 20 (28) | .078 |
| ODI score a , mean (SD) | 44.17 (18.31) | 52.49 (17.72) | .021 |
| EQ5D UK score b , mean (SD) | .46 (.31) | .26 (.22) | .053 |
| VAS score for back pain c , mean (SD) | 44.85 (29.30) | 55.91 (27.02) | .102 |
| VAS score for leg pain, mean (SD) | 69.30 (24.61) | 70.12 (28.43) | .738 |
Plus-minus values are means ± standard deviation.
Years : yr; Number: no; Kilo: kg; meter: m.
aScores on the Oswestry Disability Index (ODI) range from 0 to 100, with higher scores indicating more severe disability.
bScores on the European Quality of Life–5 Dimensions (EQ-5D) range from 0 to 1, with higher scores indicating better quality of life.
cScores on the visual analog scales (VASs) for back pain and leg pain range from 0 to 100, with higher scores indicating more severe pain.
Reliability of the Modic Change Grading Score
The results showed substantial reliability in the radiological MRI evaluation of the MC grade with an intra-observer Kappa of .68 (95%CI: .52-.76) and inter-observer Kappa of .61 (95%CI: .47-.69).
In patients with MC, severe disc degeneration in the lumbar spine (defined as Pfirrmann grade 5 at any lumbar level) was predominantly found in those with a severe MC Grade, 15 patients (83%) with a MC Grade ≥B vs 3 patients (17%) with a MC Grade A (P = .024).
Discussion
Low back pain is the most common cause of disability worldwide. 1 Millions of spine MRIs are performed annually in order to evaluate patients with back pain. 53 Typically, pathological findings, including those found on MRI, are described and graded according to structure, size, and extent, of involvement. 54 For example, DD grade on MRI is typically graded using the Pfirrmann classification, with grade V indicating maximum amount of DD, and is associated with the severity of DD.51,55
In a systematic review, Herlin et al. 37 found inconsistent results on MC and the association with LBP and activity limitation. Overall, the studies included in the systematic review were inadequate in regards to the definition and grading of MC. This made it impossible to analyse the impact of different phenotypes of MC.
The extent/volumne of MC, have been associated with increased biomechanical strain in the area of involvement, degenerative sagittal/coronal plane changes (facet joint degeneration, disc protrusion, high-intensity zones, disc degeneration), and PROs.18,42,43,46 Weishaupt et al. 42 found that in normal discs (no/minimal DD) a provocative discography was in general not painful. However, in patients with MC (MC-1 or MC-2) and a vertical extent of >25% a positive provocative discography was present in all patients. Määttä et al.43,44,56 found that a vertical extent of the MC >25% was associated with MC-1, overall DD, a worse total disc displacement score, and an increased number of SN in the lumbar spine overall, and worse disability scores. Recently, a subgroup analysis of patients with MC and LBP treated with antibiotics, demonstrated that a larger intervertebral MC size on MRI STIR is associated with decreased disability after treatment. 45 These findings have established the foundation and need for implementing a useable MC grading score that can be applied when reviewing MRIs.
The current study found that it is possible to grade MC according to the vertical extent (Figure 2). The grading score is reproducible and reliable in a clinically relevant population. A severe MC grade was associated with worse disability scores, reduced health-related quality of life, and the presence of severe lumbar disc degeneration. Overall, the results from this study are consistent and in agreement with the results from previous studies.42,44,57
These findings may indicate a paradigm shift in terms of understanding spine phenotypes. Previous studies have primarily viewed MC type as the significant spine phenotype. Results from this study suggest that the MC grade is a better clinical predictor of disability and spine degeneration than the MC type.
As with any clinical study, limitations and strengths existed with our study. Limitations of this study include the lack of a follow-up MRI. Also, as the patient cohort consisted of patients scheduled for discectomy due to LDH, some patients might have very little or no back pain. A selection bias is possible since only patients with complete datasets available in DaneSpine were included in the study. The evaluation was performed by experienced clinical physicians with a research background, no musculoskeletal radiologists participated in the MRI evaluation.
The strengths of this study include a sizeable relevant study population of patients scheduled for discectomy due to LDH at the same institution. Relevant confounders such as smoking and BMI were comparable between the 2 groups. Only patients with L4-L5 or L5-S1 discectomy and MC at these levels were included, in order to avoid an interpatient comparison bias. All MRIs were reviewed twice by 2 experienced physicians adhering to a verified classification system and a predefined protocol on MC definition and description.
The results from this study indicate that the adherence to a strict definition and grading of MC on MRI can improve future studies making these less heterogeneous and the results comparable in systematic reviews. It is essential that we proceed with a valid, reproducible, and systematic classification and grading of MC. Overall, future studies on the etiology, natural course, and clinical association of MC in patients with LBP are needed.
Conclusion
This is the first study to investigate the clinical relevance and reliability of the MC grading score. The intra- and interobserver reliability for the MC grading score is substantial in a clinically relevant population. A severe MC grade is associated with both severe DD and MC-1. The MC grade is also statistically significantly associated with increased disability and reduced health-related quality of life. Results from this study suggest that MC grade is more clinically important than MC type. We advocate for future studies on MC to implement the MC grading score in order to advance the current knowledge in this field.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Peter M. Udby https://orcid.org/0000-0001-9675-9123
Signe Elmose https://orcid.org/0000-0003-1522-072X
Leah Y. Carreon https://orcid.org/0000-0002-7685-9036
Mikkel Ø. Andersen https://orcid.org/0000-0001-8478-8218
Dino Samartzis https://orcid.org/0000-0002-7473-1311
References
- 1.Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545-1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoy D, March L, Brooks P, et al. The global burden of low back pain: Estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(6):968-974. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder R, van Tulder M, Öberg B, et al. Low back pain: A call for action. Lancet. 2018;391(10137):2384-2388. doi: 10.1016/S0140-6736(18)30488-4. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Huffman LH, American Pain Society. American College of Physicians . Clinical guidelines annals of internal medicine nonpharmacologic therapies for acute and chronic low back pain: A review of the evidence for an American pain society/American college of physicians clinical practice guideline. Ann Intern Med. 2007;147(7):492-504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Roseen EJ, Rajendran I, Stein P, et al. Association of back pain with mortality: A systematic review and meta-analysis of cohort studies. J Gen Intern Med. 2021;36(10):3148-3158. doi: 10.1007/s11606-021-06732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiltenwolf M, Akbar M, Hug A, et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician. 2014;17(1):9-19. doi: 10.1016/j.spinee.2011.08.290. [DOI] [PubMed] [Google Scholar]
- 7.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990GÇô2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163-2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356-2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 9.Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet. 2018;391(10137):2368-2383. doi: 10.1016/S0140-6736(18)30489-6. [DOI] [PubMed] [Google Scholar]
- 10.Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84(1):95-103. doi: 10.1016/S0304-3959(99)00187-6. [DOI] [PubMed] [Google Scholar]
- 11.Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35-E70. [PubMed] [Google Scholar]
- 12.Karppinen J, Daavittila I, Solovieva S, et al. Genetic factors are associated with Modic changes in endplates of lumbar vertebral bodies. Spine (Phila Pa 1976). 2008;33(11):1236-1241. doi: 10.1097/BRS.0b013e318170fd0e. [DOI] [PubMed] [Google Scholar]
- 13.Livshits G, Popham M, Malkin I, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK twin spine study. Ann Rheum Dis. 2011;70(10):1740-1745. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freidin M, Kraatari M, Skarp S, et al. Genome-wide meta-analysis identifies genetic locus on chromosome 9 associated with Modic changes. J Med Genet. 2019;56(7):420-426. doi: 10.1136/jmedgenet-2018-105726. [DOI] [PubMed] [Google Scholar]
- 15.Kjaer P, Korsholm L, Bendix T, Sorensen JS, Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15:1312-1319. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong AY, Karppinen J, Samartzis D. Low back pain in older adults: risk factors, management options and future directions. Scoliosis Spinal Disord. 2017;12(1):1-23. doi: 10.1186/s13013-017-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan D, Song Y, Sham P, Cheung KMC. Genetics of disc degeneration. Eur Spine J. 2006;15(Suppl_3):S317-S325. doi: 10.1007/s00586-006-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udby PM, Ohrt-Nissen S, Bendix T, Brorson S, Carreon LY, Andersen MØ. The association of MRI findings and long-term disability in patients with chronic low back pain. Glob Spine J. 2021;11(5):633-639. doi: 10.1177/2192568220921391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mok FPS, Samartzis D, Karppinen J, Luk KDK, Fong DYT, Cheung KMC. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: A population-based study of 2449 individuals. Spine (Phila Pa 1976). 2010;35(21):1944-1952. doi: 10.1097/BRS.0b013e3181d534f3. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Videman T, Battié MC. Lumbar vertebral endplate lesions: Prevalence, classification, and association with age. Spine (Phila Pa 1976). 2012;37(17):1432-1439. doi: 10.1097/BRS.0b013e31824dd20a. [DOI] [PubMed] [Google Scholar]
- 21.Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723-3734. doi: 10.1007/s00586-016-4459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69-73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 23.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(3):403-408. http://www.ncbi.nlm.nih.gov/pubmed/2312537. Accessed August 31, 2011. [PubMed] [Google Scholar]
- 24.Cavanaugh JM, Ozaktay AC, Yamashita T, Avramov A, Getchell T V, King AI. Mechanisms of low back pain: A neurophysiologic and neuroanatomic study. Clin Orthop Relat Res. 1997;335:166-180. http://www.ncbi.nlm.nih.gov/pubmed/9020216. Accessed January 5, 2012. [PubMed] [Google Scholar]
- 25.Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: A systematic review and meta-analysis. Am J Neuroradiol. 2015;36(12):2394-2399. doi: 10.3174/ajnr.A4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assheuer J, Lenz G, Lenz W, Gottschlich K, Schulitz K. Fett-/Wassertrennung im Kernspintomogramm. RöFo. 1987;147(07):58-63. doi: 10.1055/s-2008-1048590. [DOI] [PubMed] [Google Scholar]
- 27.De Roos A, Kressel H, Spritzer C, Dalinka M. MR imaging of marrow changes adjacent to end plates in degenerative lumbar disk disease. Am J Roentgenol. 1987;149(3):531-534. doi: 10.2214/ajr.149.3.531. [DOI] [PubMed] [Google Scholar]
- 28.Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiol. 2016;168(1):177-186. doi: 10.1148/radiology16813289089. [DOI] [PubMed] [Google Scholar]
- 29.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1):193-199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 30.Jensen TS, Bendix T, Sorensen JS, Manniche C, Korsholm L, Kjaer P. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet Disord. 2009;10:81. doi: 10.1186/1471-2474-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen TS, Kjaer P, Korsholm L, et al. Predictors of new vertebral endplate signal (Modic) changes in the general population. Eur Spine J. 2010;19:129-135. doi: 10.1007/s00586-009-1184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtori S, Yamashita M, Yamauchi K, et al. Low back pain after lumbar discectomy in patients showing endplate modic type 1 change. Spine (Phila Pa 1976). 2010;35(13):596-600. doi: 10.1097/BRS.0b013e3181cd2cb8. [DOI] [PubMed] [Google Scholar]
- 33.Albert HB, Manniche C. Modic changes following lumbar disc herniation. Eur Spine J. 2007;16(7):977-982. doi: 10.1007/s00586-007-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zehra U, Cheung JPY, Bow C, Lu W, Samartzis D. Multidimensional vertebral endplate defects are associated with disc degeneration, modic changes, facet joint abnormalities, and pain. J Orthop Res. 2019;37(5):1080-1089. doi: 10.1002/jor.24195. [DOI] [PubMed] [Google Scholar]
- 35.Rajasekaran S, Bajaj N, Tubaki V, Kanna RM, Shetty AP. ISSLS prize winner: The anatomy of failure in lumbar disc herniation: An in vivo, multimodal, prospective study of 181 subjects. Spine (Phila Pa 1976). 2013;38(17):1491-1500. doi: 10.1097/BRS.0b013e31829a6fa6. [DOI] [PubMed] [Google Scholar]
- 36.Udby PM, Samartzis D, Carreon LY, Andersen MØ, Karppinen J, Modic M. A definition and clinical grading of Modic changes. J Orthop Res. 2022;40(2):301-307. doi: 10.1002/jor.25240. [DOI] [PubMed] [Google Scholar]
- 37.Herlin C, Kjaer P, Espeland A, et al. Modic changes—Their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS One. 2018;13(8):1-27. doi: 10.1371/journal.pone.0200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen TS, Karppinen J, Sorensen JS, Niinimäki J, Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17(11):1407-1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viswanathan VK, Shetty AP, Rajasekaran S. Modic changes - An evidence-based, narrative review on its patho-physiology, clinical significance and role in chronic low back pain. J Clin Orthop Trauma. 2020;11:761-769. doi: 10.1016/j.jcot.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen TS, Sorensen JS, Kjaer P. Intra- and interobserver reproducibility of vertebral endplate signal (modic) changes in the lumbar spine: the Nordic Modic consensus group classification. Acta Radiol. 2007;48(7):748-754. doi: 10.1080/02841850701422112. [DOI] [PubMed] [Google Scholar]
- 41.Fields AJ, Battié MC, Herzog RJ, et al. Measuring and reporting of vertebral endplate bone marrow lesions as seen on MRI (Modic changes): Recommendations from the ISSLS Degenerative spinal phenotypes group. Eur Spine J. 2019;28(10):2266-2274. doi: 10.1007/s00586-019-06119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weishaupt D, Zanetti M, Hodler J, et al. Painful lumbar disk derangement: Relevance of endplate abnormalities at MR imaging. Radiology. 2001;218(2):420-427. doi: 10.1148/radiology.218.2.r01fe15420. [DOI] [PubMed] [Google Scholar]
- 43.Määttä JH, Karppinen JI, Luk KDK, Cheung KMC, Samartzis D. Phenotype profiling of Modic changes of the lumbar spine and its association with other MRI phenotypes: A large-scale population-based study. Spine J. 2015;15(9):1933-1942. doi: 10.1016/j.spinee.2015.06.056. [DOI] [PubMed] [Google Scholar]
- 44.Määttä JH, Karppinen J, Paananen M, et al. Refined phenotyping of modic changes. Med (United States). 2016;95(22):1-10. doi: 10.1097/MD.0000000000003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kristoffersen PM, Bråten LCH, Vetti N, et al. Oedema on STIR modified the effect of amoxicillin as treatment for chronic low back pain with Modic changes—subgroup analysis of a randomized trial. Eur Radiol. 2020;31:4285-4297. doi: 10.1007/s00330-020-07542-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Udby PM, Ohrt-Nissen S, Bendix T, et al. Are Modic changes associated with health-related quality of life after discectomy: A study on 620 patients with two-year follow-up. Spine (Phila Pa 1976). 2020;45(21):1491-1497. doi: 10.1097/BRS.0000000000003618. [DOI] [PubMed] [Google Scholar]
- 47.Laustsen AF, Bech-Azeddine R. Do Modic changes have an impact on clinical outcome in lumbar spine surgery? A systematic literature review. Eur Spine J. 2016;25(11):3735-3745. doi: 10.1007/s00586-016-4609-y. [DOI] [PubMed] [Google Scholar]
- 48.Rahme R, Moussa R, Bou-Nassif R, et al. What happens to Modic changes following lumbar discectomy? Analysis of a cohort of 41 patients with a 3- to 5-year follow-up period. J Neurosurg Spine. 2010;13(5):562-567. doi: 10.3171/2010.5.SPINE09818. [DOI] [PubMed] [Google Scholar]
- 49.Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Danish version of the Oswestry disability index for patients with low back pain. Part 1: Cross-cultural adaptation, reliability and validity in two different populations. Eur Spine J. 2006;15:1705-1716. doi: 10.1007/s00586-006-0117-9. [DOI] [PubMed] [Google Scholar]
- 50.Simony A, Hansen KH, Ernst C, Andersen MO. [Implementation of the Danish national database Danespine for spinal surgery]. Ugeskr Laeger. 2014;176(2A):V01130019. [PubMed] [Google Scholar]
- 51.Pfirrmann CWA, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873-1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 52.McHugh ML. Interrater reliability: The kappa statistic. Biochem Medica. 2012;22(3):276-282. doi: 10.11613/bm.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steffens D, Hancock MJ, Maher CG, Williams C, Jensen TS, Latimer J. Does magnetic resonance imaging predict future low back pain? A systematic review. Eur J Pain (United Kingdom). 2014;18(6):755-765. doi: 10.1002/j.1532-2149.2013.00427.x. [DOI] [PubMed] [Google Scholar]
- 54.Fardon DF, Williams AL, Dohring EJ, Murtagh FR, Gabriel Rothman SL, Sze GK. Lumbar disc nomenclature: Version 2.0 recommendations of the combined task forces of the North American spine society, the American society of spine radiology and the American society of neuroradiology. Spine J. 2014;14(11):2525-2545. doi: 10.1016/j.spinee.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Middendorp M, Vogl TJ, Kollias K, Kafchitsas K, Khan MF, Maataoui A. Association between intervertebral disc degeneration and the Oswestry disability index. J Back Musculoskelet Rehabil. 2017;30(4):819-823. doi: 10.3233/BMR-150516. [DOI] [PubMed] [Google Scholar]
- 56.Saukkonen J, Määttä J, Oura P, et al. Association between Modic changes and low back pain in middle age: A Northern Finland birth cohort study. Spine (Phila Pa 1976). 2020;45(19):1360-1367. doi: 10.1097/BRS.0000000000003529. [DOI] [PubMed] [Google Scholar]
- 57.Sherwood D, Haring RS, Gill B, et al. The interrater reliability of the novel Udby classification of Modic changes: A first estimate. Interven Pain Med. 2022;1:100092. doi: 10.1016/j.inpm.2022.100092. [DOI] [Google Scholar]