Abstract
Background:
Time-restricted eating (TRE), without calorie counting, has become a popular weight loss strategy, yet long-term randomized trials evaluating its efficacy are limited.
Objective:
To determine whether TRE is more effective for weight control and cardiometabolic risk reduction compared with calorie restriction (CR) or control.
Design:
12-month randomized controlled trial. (ClinicalTrials.gov: NCT04692532)
Setting:
University of Illinois Chicago from January 2021 to September 2022.
Participants:
90 adults with obesity.
Intervention:
8-hour TRE (eating between noon and 8:00 p.m. only, without calorie counting), CR (25% energy restriction daily), or control (eating over a period of 10 or more hours per day). Participants were not blinded.
Measurements:
Change in body weight, metabolic markers, and energy intake by month 12.
Results:
Seventy-seven persons completed the study. Mean age was 40 years (SD, 11), 33% were Black, and 46% were Hispanic. Mean reduction in energy intake was −425 kcal/d (SD, 531) for TRE and −405 kcal/d (SD, 712) for CR. Compared with the control group, weight loss by month 12 was −4.61 kg (95% CI, −7.37 to −1.85 kg; P ≤ 0.01) (−4.87% [CI, −7.61% to −2.13%]) for the TRE group and −5.42 kg (CI, −9.13 to −1.71 kg; P ≤ 0.01) (−5.30% [CI, −9.06% to −1.54%]) for the CR group, with no statistically significant difference between TRE and CR (0.81 kg [CI, −3.07 to 4.69 kg; P = 0.68]) (0.43% [CI, −3.48% to 4.34%]).
Limitation:
Not blinded, not powered to detect relatively large differences in weight loss, and lack of adjustment for multiple comparisons.
Conclusion:
Time-restricted eating is more effective in producing weight loss when compared with control but not more effective than CR in a racially diverse population.
Time-restricted eating (TRE) has become a popular weight loss regimen (1–3). The sudden increase in popularity of TRE is mostly likely due to its sheer simplicity and the fact that it does not require persons to count calories to lose weight. Participants are simply asked to consume all food within a specified time frame and fast with energy-free beverages for the remaining hours of the day. Evidence shows that when persons with obesity limit their eating window to 6 to 8 hours per day, they naturally reduce energy intake by 350 to 500 calories (4, 5). From a clinical standpoint, these findings are paramount. One of the main reasons for participant attrition with traditional dieting–that is, daily calorie restriction (CR)–is frustration with having to count calories every day (6, 7). Time-restricted eating regimens can sidestep this requirement by allowing participants to simply “watch the clock” instead of monitoring calories, while still producing weight loss and cardiometabolic health improvements (8–10). This feature of TRE has the potential to improve long-term adherence to this eating plan, and in turn, produce lasting weight control in adults with obesity.
However, few long-term trials have evaluated the efficacy of TRE for weight loss. In the study by Liu and colleagues (11), adults with obesity were randomly assigned to either early TRE (consuming all food between 8:00 a.m. and 4:00 p.m.) combined with intentional CR or CR alone. After 12 months, both groups lost a similar amount of weight (7% to 9% from baseline) (11). Although these findings are valuable to the field, the study is limited in that it did not examine how TRE alone–that is, without calorie counting–affects body weight. In addition, the real-life applicability of the study is questionable given that an early eating window was used. Evidence from a recent large-scale observational study of nearly 800 000 adults shows that Americans who engage in TRE place their eating window in the afternoon or evening so that they can continue to eat dinner with family and friends (12). Moreover, the generalizability of the findings is uncertain given that the trial was done solely in Chinese adults. Thus, it is unclear if these findings would be reproducible in a diverse American population. Indeed, conventional nutrition strategies are often difficult to execute in historically marginalized communities because they require a high level of numeracy, literacy, cost, and time to change the food composition in the home. All these factors limit the ability of traditional CR protocols to support weight reduction in these high-risk cohorts.
Accordingly, we conducted a 1-year randomized controlled trial to compare the effects of TRE (eating all food between noon and 8:00 p.m., without calorie counting) versus CR (25% energy restriction daily) and a control group eating over a period of 10 or more hours per day on body weight and cardiometabolic risk factors in a racially and ethnically diverse group of American adults with obesity. We hypothesized that the TRE group would achieve greater weight loss and have more pronounced improvements in insulin sensitivity over 12 months than the CR and control groups.
Methods
Design Overview
This is a 12-month prospective, parallel-group, randomized controlled trial done at the University of Illinois Chicago. Participants were randomly assigned in a 1:1:1 ratio to a TRE, CR, or control group. Participants were not blinded. Assessments were done at baseline, month 6, and month 12 (end of intervention). Trial recruitment occurred from 1 January to 30 September 2021, with the last follow-up on 30 September 2022.
The protocol was approved by the Office for the Protection of Research Subjects at the University of Illinois Chicago (Protocol #2020–1512). All participants provided signed informed consent. The trial is registered at ClinicalTrials.gov (NCT04692532). The full study protocol (available at Annals.org) and the CONSORT check-list (Supplement, available at Annals.org) are available. All authors had access to the study data and have reviewed and approved the final manuscript.
Setting and Participants
Participants were recruited by means of flyers placed around the University of Illinois Chicago campus and surrounding area. Recruitment was not targeted toward certain racial or ethnic groups. Participants were screened via a questionnaire, body mass index (BMI) assessment, pregnancy test, and a 7-day food record. No adjustments were made in the BMI eligibility criteria for Asian participants. Inclusion criteria were female, male, age between 18 and 65 years, and BMI between 30 and 50 kg/m2. Exclusion criteria were history of diabetes mellitus, use of weight loss medications, weight unstable for 3 months before the beginning of the study (>4 kg weight loss or gain), eating within less than a 10-hour window, perimenopausal or otherwise irregular menstrual cycle, nightshift workers, pregnant or trying to become pregnant, and current smokers.
Randomization and Interventions
Participants were randomly assigned in a 1:1:1 ratio to a TRE, CR, or control group. Randomization was done by a stratified random sampling procedure by sex, age (18 to 42 years and 43 to 65 years), and BMI (30 to 40 kg/m2 and 40.1 to 50 kg/m2). The trial duration was 1 year, and participants were provided compensation for their time and transportation costs.
Participants in all 3 groups were instructed not to change their physical activity habits throughout the trial to avoid potential confounding. The TRE and CR interventions consisted of a weight loss phase (6 months) and a weight maintenance phase (6 months) (Supplement Figure 1, available at Annals.org). Trained registered dietitians delivered dietary counseling to TRE and CR intervention participants (by telephone or Zoom [Zoom Video Communications]) every week during the first 3 months of the study, then biweekly from months 4 to 6. During these sessions, participants were taught how to make general healthy food choices to conform with American Diabetes Association nutrition guidelines (13). During the weight maintenance phase between months 6 and 12, participants in the TRE and CR (but not control) groups met individually with the dietitian every month to learn cognitive behavioral strategies to prevent weight regain (14).
TRE Dietary Strategy
During the 6-month weight loss phase, participants in the TRE group were instructed to eat ad libitum from noon to 8:00 p.m. daily and fast from 8:00 p.m. to noon. During the 8-hour eating window, participants were not required to monitor caloric intake, and there were no restrictions on types of or quantities of food consumed. During the 16-hour fasting window, participants were encouraged to drink plenty of water and were permitted to consume energy-free drinks, such as black tea, coffee, and diet sodas (limit 2 diet sodas per day).
During the 6-month weight maintenance phase, participants were instructed to maintain their body weight and to widen their eating window to 10:00 a.m. to 8:00 p.m. and fast from 8:00 p.m. to 10:00 a.m. This maintenance eating window was chosen because our previous TRE trials (4, 5) showed that eating within a 10-hour window resulted in no change in body weight in this population group. Thus, we hypothesized that this would be an ideal eating window for sustained weight loss. As in the weight loss phase, participants were not required to monitor caloric intake and could eat food as desired. During the 14-hour fasting window, participants were encouraged to drink plenty of water and were permitted to consume energy-free drinks.
Calorie Counting Dietary Strategy
During the 6-month weight loss phase, participants in the CR group were instructed to reduce their energy intake by 25% every day. Total energy expenditure was calculated by the Mifflin–St. Jeor equation (15) and multiplied by the appropriate activity factor for each participant. Participants in the CR group met with the study dietitian at the beginning of the trial to develop individualized weight loss meal plans. The plans included menus, portion sizes, and food lists that were consistent with the participant’s food preferences and prescribed calorie levels for weight loss. The food lists contained examples of healthy foods that should be purchased to make the meals–for example, lean proteins (chicken, turkey, fish, and tofu), fruits, vegetables, nuts, and low-fat dairy products. Participants were asked to fill half their plates with fruits or vegetables at every meal and to consume roughly 50% of energy as carbohydrates, 30% of energy as fat, and 20% of energy as protein.
During the weight maintenance phase, CR participants were instructed to consume 100% of their energy needs every day. Total daily energy expenditure was recalculated at the beginning of this period for all participants. The net caloric reduction from baseline was approximately 15%.
Control
Control participants were instructed to maintain their weight, physical activity habits, and baseline eating window of 10 or more hours per day throughout the trial. This eating window was chosen because our previous TRE studies (4, 5) indicated that persons in the Chicago area typically eat within 10 or more hours each day. Control participants received no food or dietary counseling but visited the research center at the same frequency as the intervention participants to provide outcome measurements. Control participants who completed the 12-month trial received free weight loss counseling at the end of the study.
Outcomes and Follow-up
The primary outcome was absolute change in body weight between the TRE, CR, and control groups by month 12. Prespecified secondary outcome measures included relative change in body weight; change in fat mass, lean mass, visceral fat mass, bone density, blood pressure, heart rate, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, fasting glucose, fasting insulin, insulin resistance, insulin sensitivity, hemoglobin A1c, energy intake, and steps per day; and dietary adherence between the TRE, CR, and control groups by month 12. Occurrence of general adverse events were assessed by a questionnaire at 3-month intervals. Adverse events were not graded. Analytical methods are detailed in the full protocol.
Statistical Analysis
For the sample size calculation, we estimated that the TRE and CR groups would reduce body weight by 8 kg (extrapolated from our pilot data) and 5 kg (16), respectively, by month 12 versus the control group (no change in body weight). We calculated that 26 participants per group would provide 90% power to detect a statistically significant difference in body weight between the TRE, CR, and control groups by month 12 using an overall F-test from a one-way analysis of variance with α = 0.05, effect size of 0.4125, and a common SD of 8 kg. We anticipated a dropout rate of 12%. Thus, we aimed to recruit 90 participants (30 per group), assuming that 78 participants (26 per group) would complete the trial.
Data are shown as mean (95% CI) unless otherwise noted. A Bonferroni-adjusted 2-tailed P value of less than 0.017 was considered statistically significant for pairwise group comparisons of body weight. P values generated from analyses of secondary outcomes were not adjusted for multiplicity and are considered descriptive. We conducted an intention-to-treat analysis, which included data from all 90 participants who were randomly assigned. Results are reported by intention-to-treat analysis unless indicated otherwise.
A linear mixed model was used to assess time, group, and time-by-group effects for each outcome. Linear mixed models for longitudinal data analysis account for missing outcome data using maximum likelihood principles. Thus, these models provide unbiased estimates of time and treatment effects under a missing at random assumption. The inclusion of time in the model allows for changes in the outcome over time that are unrelated to the intervention. In each model, time and group effects (and their interaction) were estimated without imposing a linear time trend. In models for body weight, which was measured at 13 time points (baseline plus 12 months of follow-up), time was modeled with cubic splines. In models of all other outcome variables, which were measured at 3 time points (baseline plus month 6 plus month 12), time was modeled as a categorical variable.
For each outcome variable, linear modeling assumptions were assessed with residual diagnostics. To account for the potential for nonuniform variances (heteroskedasticity) between treatment groups due to random chance, all CIs and P values from linear mixed models were calculated using robust variance estimators (sandwich estimators) (17–19). Intraclass correlation coefficients from each linear mixed effect were also calculated. To assess the effect of loss to follow-up on study findings, we conducted a sensitivity analysis using multiple imputation. All analyses were done using R, version 4.3.1 (R Foundation).
Role of the Funding Source
The funders had no influence on the study design, data collection, statistical analysis, preparation of the manuscript, or on the decision to publish.
Results
Trial Participants
Of the 126 participants who were screened, 90 (71%) were randomly assigned to the intervention or control groups, and 77 (86% of those assigned) completed the study (Figure 1). All dropouts occurred during the first 6 months of the study: 13% in the TRE group, 17% in the CR group, and 13% in the control group. Reasons for participant attrition were scheduling conflicts, personal reasons, and inability to contact. No participants reported dropping out due to dislike of the TRE or CR interventions. All baseline characteristics had similar distributions between the TRE, CR, and control groups (Table 1). The participants were primarily non-Hispanic Black and Hispanic women with insulin resistance. The participants who dropped out of the study were generally younger, heavier, and more insulin resistant when compared with those who completed the study (Supplement Table 1, available at Annals.org).
Figure 1. Participant flow diagram.
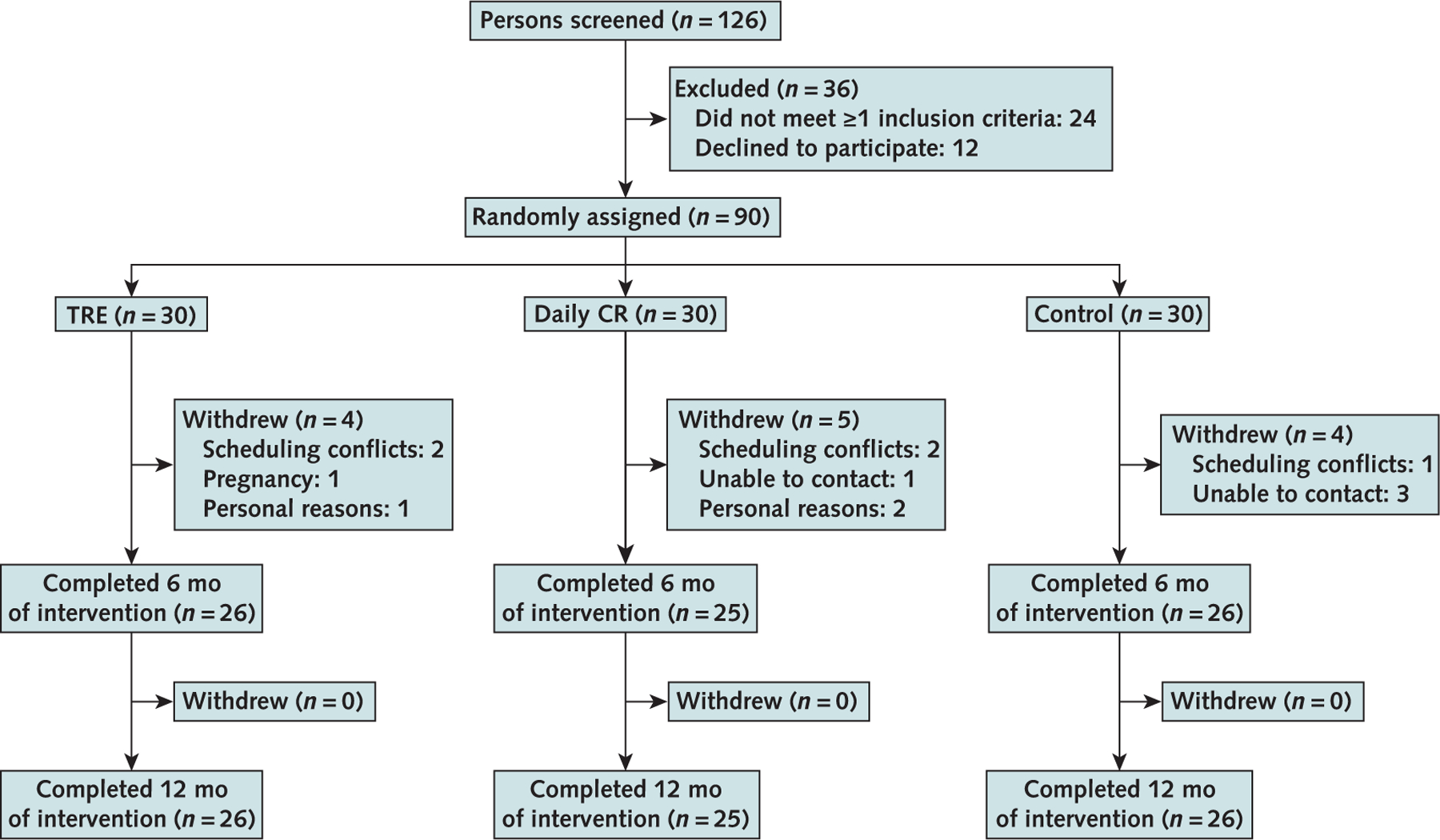
CR = calorie restriction; TRE = time-restricted eating.
Table 1.
Baseline Characteristics of the Participants
| Characteristic | TRE | Daily CR | Control |
|---|---|---|---|
| Participants, n | 30 | 30 | 30 |
| Mean age (SD), y | 44 (12) | 44 (9) | 44 (13) |
| Sex, n (%) | |||
| Female | 25 (83) | 24 (80) | 25 (83) |
| Male | 5 (17) | 6 (20) | 5 (17) |
| Race or ethnic group, n (%) | |||
| Black | 11 (37) | 9 (30) | 10 (33) |
| Asian | 3 (10) | 3 (10) | 0 (0) |
| Hispanic | 13 (43) | 11 (37) | 17 (57) |
| White | 3 (10) | 7 (23) | 3 (10) |
| Body composition | |||
| Mean body weight (SD), kg | 100 (17) | 102 (18) | 102 (17) |
| Mean fat mass (SD), kg | 46 (11) | 47 (11) | 47 (10) |
| Mean lean mass (SD), kg | 50 (10) | 50 (9) | 51 (8) |
| Mean visceral fat mass (SD), kg | 1.6 (0.6) | 1.6 (0.8) | 1.7 (0.8) |
| Mean waist circumference (SD), cm | 109 (13) | 110 (14) | 110 (13) |
| Mean height (SD), cm | 164 (9) | 166 (9) | 165 (7) |
| Mean BMI (SD), kg/m2 | 37 (6) | 37 (5) | 38 (5) |
| Bone parameters | |||
| Mean bone mineral density (SD), g/cm2 | 1.28 (0.14) | 1.29 (0.10) | 1.29 (0.16) |
| Mean bone mineral content (SD), g | 2610 (429) | 2680 (390) | 2700 (449) |
| Blood pressure and heart rate | |||
| Mean systolic blood pressure (SD), mm Hg | 124 (16) | 125 (14) | 126 (14) |
| Mean diastolic blood pressure (SD), mm Hg | 84 (10) | 83 (9) | 85 (10) |
| Mean heart rate (SD), beats/min | 75 (12) | 75 (13) | 74 (13) |
| Plasma lipids | |||
| Mean total cholesterol (SD) | |||
| mmol/L | 4.79 (0.80) | 4.71 (0.96) | 4.61 (0.83) |
| mg/dL | 185 (31) | 182 (37) | 178 (32) |
| Mean low-density lipoprotein cholesterol (SD) | |||
| mmol/L | 2.77 (0.70) | 2.85 (0.85) | 2.64 (0.73) |
| mg/dL | 107 (27) | 110 (33) | 102 (28) |
| Mean high-density lipoprotein cholesterol (SD) | |||
| mmol/L | 1.42 (0.36) | 1.42 (0.28) | 1.27 (0.34) |
| mg/dL | 55 (14) | 55 (11) | 49 (13) |
| Mean triglycerides (SD) | |||
| mmol/L | 1.30 (0.53) | 0.99 (0.36) | 1.59 (0.85) |
| mg/dL | 115 (47) | 88 (32) | 141 (75) |
| Glucoregulatory factors | |||
| Mean fasting glucose (SD) | |||
| mmol/L | 4.94 (0.67) | 4.88 (0.72) | 4.83 (0.67) |
| mg/dL | 89 (12) | 88 (13) | 87 (12) |
| Mean fasting insulin (SD), pmol/L | 118.06 (76.40) | 76.40 (41.67) | 118.06 (69.45) |
| Mean insulin resistance (SD) (HOMA-IR) | 3.6 (2.8) | 2.6 (1.4) | 3.6 (2.6) |
| Mean insulin sensitivity (SD) (QUICKI) | 0.33 (0.03) | 0.34 (0.03) | 0.33 (0.03) |
| Mean hemoglobin A1c, % | 5.5 (0.5) | 5.4 (0.5) | 5.5 (0.4) |
BMI = body mass index; CR = calorie restriction; HOMA-IR = homeostasis model assessment of insulin resistance; QUICKI = quantitative insulin sensitivity check index; TRE = time-restricted eating.
Weight Loss and Body Composition
Compared with the control group, absolute weight loss (primary outcome) was −4.61 kg (95% CI, −7.37 to −1.85 kg; P ≤ 0.01) for the TRE group and −5.42 kg (CI, −9.13 to −1.71 kg; P ≤ 0.01) for the CR group, with no statistically significant difference between the TRE and CR groups (0.81 kg [CI, −3.07 to 4.69]; P = 0.68]) by month 12 (Figure 2, top and middle, and Table 2). Relative to the control group, weight loss (secondary outcome) as a percentage of baseline body weight was −4.87% (CI, −7.61% to −2.13%) for the TRE group and −5.30% (CI, −9.06% to −1.54%) for the CR group, with no notable difference between the TRE and CR groups (0.43% [CI, −3.48% to 4.34%]) by month 12.
Figure 2. Change in body weight and energy intake between groups over 12 months.
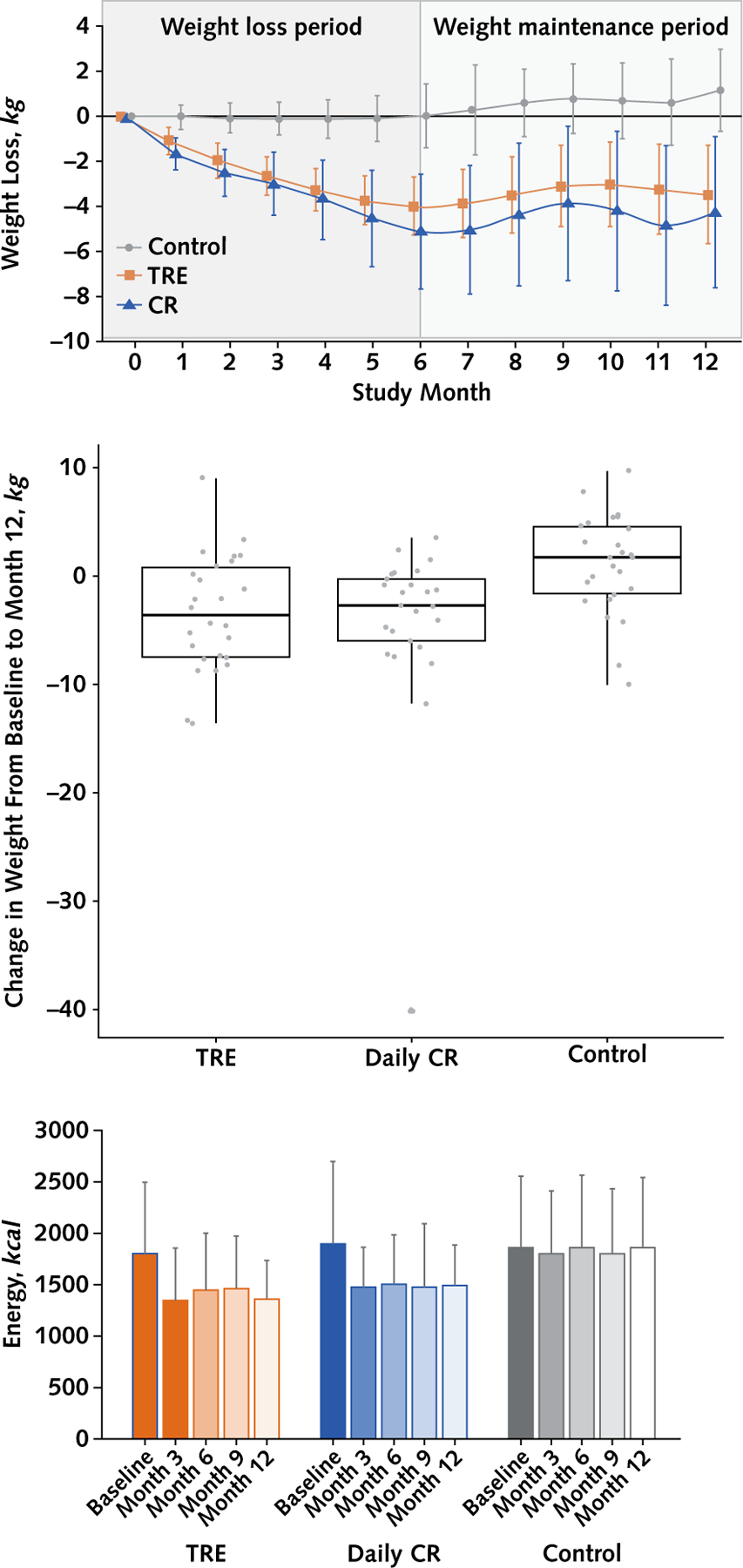
CR = calorie restriction; TRE = time-restricted eating. Top. Body weight: Data were included for 90 participants; means were estimated using an intention-to-treat analysis using a linear mixed model. Error bars indicate 95% CIs for each parameter from baseline by diet group. Middle. Box plot of change in absolute weight from baseline to month 12 by treatment group. Participants with missing values for weight at month 12 are excluded from the figure. Bottom. Energy intake: Data are expressed as mean (SD); only observed values included. A total of 19 of 30 TRE participants returned all food records; 17 of 30 CR participants returned all food records. The widths of the CIs have not been adjusted for multiplicity and should therefore not be used to reject or not reject treatment effects.
Table 2.
Change in Body Weight and Body Composition From Baseline and Between Intervention Groups*
| Variables | Participants, n | Change From Baseline (95% CI) |
Differeernce Between Groups (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| TRE | Daily CR | Control | TRE vs. CR | TRE vs. Control | CR vs. Control | ||
| Primary outcome | |||||||
| Body weight, kg | |||||||
| 12 mo | 77 | −3.49 (−5.65 to −1.32) | −4.30 (−7.63 to −0.96) | 1.12 (−0.69 to 2.94) | 0.81 (−3.07 to 4.69) P = 0.68 |
−4.61 (−7.37 to −1.85) P ≤ 0.01 |
−5.42 (−9.13 to −1.71) P ≤ 0.01 |
| Secondary outcomes | |||||||
| Body weight, kg | |||||||
| 6 mo | 66 | −4.00 (−5.31 to −2.70) | −5.14 (−7.66 to −2.62) | 0.00 (−1.40 to 1.40) | 1.14 (−1.63 to 3.91) | −4.00 (−5.87 to −2.13) | −5.14 (−7.95 to −2.33) |
| Body weight, % | |||||||
| 6 mo | 66 | −4.27 (−5.69 to −2.84) | −5.06 (−7.56 to −2.57) | −0.03 (−1.47 to 1.40) | 0.80 (−2.01 to 3.61) | −4.23 (−6.21 to −2.26) | −5.03 (−7.84 to −2.22) |
| 12 mo | 77 | −3.76 (−5.89 to −1.64) | −4.20 (−7.59 to −0.80) | 1.11 (−0.72 to 2.94) | 0.43 (−3.48 to 4.34) | −4.87 (−7.61 to −2.13) | −5.30 (−9.06 to −1.54) |
| Fat mass, kg | |||||||
| 6 mo | 68 | −2.68 (−3.75 to −1.61) | −2.25 (−4.62 to 0.13) | −0.13 (−1.33 to 1.08) | −0.43 (−2.96 to 2.10) | −2.55 (−4.12 to −0.98) | −2.12 (−4.71 to 0.46) |
| 12 mo | 76 | −2.20 (−3.88 to −0.52) | −2.61 (−5.97 to 0.74) | 0.57 (−1.14 to 2.27) | 0.42 (−3.24 to 4.07) | −2.77 (−5.10 to −0.43) | −3.18 (−6.85 to 0.49) |
| Lean mass, kg | |||||||
| 6 mo | 68 | −0.12 (−0.97 to 0.72) | −0.23 (−1.07 to 0.61) | 0.23 (−0.26 to 0.72) | 0.11 (−1.05 to 1.27) | −0.36 (−1.31 to 0.60) | −0.46 (−1.41 to 0.48) |
| 12 mo | 76 | −0.41 (−0.91 to 0.08) | −0.74 (−1.44 to −0.03) | 0.39 (−0.51 to 1.29) | 0.32 (−0.52 to 1.16) | −0.81 (−1.81 to 0.20) | −1.13 (−2.24 to −0.01) |
| Visceral fat mass, kg | |||||||
| 6 mo | 68 | −0.22 (−0.30 to −0.14) | −0.19 (−0.34 to −0.03) | −0.05 (−0.15 to 0.05) | −0.03 (−0.20 to 0.14) | −0.17 (−0.29 to −0.05) | −0.14 (−0.32 to 0.04) |
| 12 mo | 76 | −0.14 (−0.23 to −0.04) | −0.12 (−0.29 to 0.06) | −0.03 (−0.16 to 0.10) | −0.02 (−0.22 to 0.17) | −0.11 (−0.27 to 0.06) | −0.08 (−0.30 to 0.13) |
| Waist circumference, cm | |||||||
| 6 mo | 76 | −5.54 (−7.51 to −3.57) | −4.69 (−7.75 to −1.63) | −0.70 (−2.29 to 0.88) | −0.85 (−4.40 to 2.70) | −4.83 (−7.30 to −2.37) | −3.98 (−7.35 to −0.62) |
| 12 mo | 76 | −6.44 (−8.65 to −4.24) | −3.77 (−7.46 to −0.08) | −1.46 (−3.77 to 0.84) | −2.67 (−6.86 to 1.52) | −4.98 (−8.09 to −1.87) | −2.30 (−6.55 to 1.94) |
| BMI, kg/m2 | |||||||
| 6 mo | 65 | −1.48 (−2.00 to −0.96) | −1.95 (−2.94 to −0.97) | 0.02 (−0.53 to 0.57) | 0.47 (−0.61 to 1.55) | −1.50 (−2.24 to −0.76) | −1.97 (−3.07 to −0.88) |
| 12 mo | 76 | −1.29 (−2.09 to −0.50) | −1.62 (−2.98 to −0.26) | 0.40 (−0.29 to 1.08) | 0.33 (−1.21 to 1.87) | −1.69 (−2.71 to −0.67) | −2.02 (−3.50 to −0.53) |
| Bone mineral density, g/cm2 | |||||||
| 6 mo | 68 | 0.00 (−0.01 to 0.01) | 0.00 (−0.01 to 0.01) | 0.01 (0.00 to 0.02) | 0.00 (−0.02 to 0.01) | −0.01 (−0.02 to 0.01) | −0.01 (−0.02 to 0.01) |
| 12 mo | 76 | 0.01 (0.00 to 0.02) | −0.01 (−0.02 to 0.01) | 0.00 (−0.01 to 0.02) | 0.01 (0.00 to 0.03) | 0.00 (−0.01 to 0.02) | −0.01 (−0.03 to 0.01) |
| Bone mineral content, g | |||||||
| 6 mo | 68 | 5.86 (−17.30 to 29.01) | 22.84 (−0.75 to 46.44) | −10.14 (−21.30 to 1.02) | −16.99 (−49.14 to 15.16) | 15.99 (−9.02 to 41.01) | 32.98 (7.62 to 58.35) |
| 12 mo | 76 | 10.18 (−9.36 to 29.71) | 13.32 (−17.73 to 44.37) | 1.86 (−18.75 to 22.46) | −3.14 (−38.88 to 32.59) | 8.32 (−19.36 to 35.99) | 11.46 (−24.84 to 47.76) |
BMI = body mass index; CR = calorie restriction; TRE = time-restricted eating.
Data were included for 90 participants; means were estimated using an intention-to-treat analysis using a linear mixed model. Error bars indicate 95% CIs for each parameter from baseline by diet group. The widths of the CIs have not been adjusted for multiplicity and should therefore not be used to reject or not reject treatment effects.
At month 12, both TRE and CR led to reductions in fat mass, waist circumference, and BMI but not lean mass, visceral fat mass, bone mineral density, and bone mineral content compared with control (Table 2).
Glucoregulatory Factors, Blood Pressure, and Plasma Lipids
Fasting plasma glucose, fasting insulin, insulin resistance, and hemoglobin A1c were not associated with treatment group in any pairwise comparisons at month 12 (Table 3; Supplement Table 2, available at Annals.org). Time-restricted eating was associated with increases in insulin sensitivity compared with control but not compared with CR. In addition, we found a negative correlation between body weight and insulin sensitivity (Supplement Figure 2, available at Annals.org). Blood pressure, heart rate, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride concentrations were not associated with treatment group in any pairwise comparisons at month 12.
Table 3.
Change in Metabolic Disease Risk Variables From Baseline and Between Intervention Groups*
| Secondary Outcomes | Participants, n | Change From Baseline (95% CI) |
Difference Between Groups (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| TRE | Daily CR | Control | TRE vs. CR | TRE vs. Control | CR vs. Control | ||
| Fasting glucose | |||||||
| 6 mo | 74 | ||||||
| mmol/L | −0.09 (−0.36 to 0.18) | −0.04 (−0.31 to 0.22) | 0.16 (−0.15 to 0.47) | −0.05 (−0.42 to 0.32) | −0.25 (−0.65 to 0.15) | −0.20 (−0.60 to 0.20) | |
| mg/dL | −1.65 (−6.54 to 3.25) | −0.81 (−5.62 to 4.00) | 2.88 (−2.73 to 8.48) | −0.84 (−7.53 to 5.85) | −4.52 (−11.78 to 2.73) | −3.69 (−10.89 to 3.52) | |
| 12 mo | 74 | ||||||
| mmol/L | 0.16 (−0.06 to 0.38) | 0.32 (0.07 to 0.58) | 0.35 (0.09 to 0.60) | −0.17 (−0.49 to 0.16) | −0.19 (−0.52 to 0.14) | −0.02 (−0.38 to 0.33) | |
| mg/dL | 2.82 (−1.15 to 6.79) | 5.83 (1.25 to 10.40) | 6.26 (1.63 to 10.90) | −3.01 (−8.91 to 2.90) | −3.44 (−9.39 to 2.51) | −0.43 (−6.78 to 5.91) | |
| Fasting insulin, pmol/L | |||||||
| 6 mo | 74 | −20.07 (−42.43 to 2.36) | −6.32 (−21.11 to 8.40) | 4.51 (−16.32 to 25.28) | −13.68 (−39.86 to 12.43) | −24.52 (−54.31 to 5.21) | −10.83 (−35.70 to 14.03) |
| 12 mo | 74 | −19.65 (−37.09 to −2.29) | −0.83 (−15.42 to 13.68) | 10.00 (−16.11 to 36.18) | −18.82 (−40.91 to 3.33) | −29.66 (−60.28 to 0.90) | −10.90 (−40.07 to 18.27) |
| Insulin resistance (HOMA-IR) | |||||||
| 6 mo | 74 | −0.66 (−1.61 to 0.29) | −0.28 (−0.83 to 0.27) | 0.33 (−0.50 to 1.15) | −0.38 (−1.45 to 0.69) | −0.99 (−2.21 to 0.24) | −0.61 (−1.58 to 0.36) |
| 12 mo | 74 | −0.49 (−1.23 to 0.24) | 0.07 (−0.47 to 0.61) | 0.54 (−0.33 to 1.41) | −0.56 (−1.45 to 0.32) | −1.03 (−2.14 to 0.07) | −0.47 (−1.46 to 0.53) |
| Insulin sensitivity (QUICKI) | |||||||
| 6 mo | 74 | 0.02 (0.00 to 0.03) | 0.00 (−0.01 to 0.01) | 0.00 (−0.01 to 0.01) | 0.02 (0.00 to 0.03) | 0.02 (0.00 to 0.04) | 0.01 (−0.01 to 0.02) |
| 12 mo | 74 | 0.01 (0.00 to 0.02) | 0.00 (−0.02 to 0.01) | −0.01 (−0.02 to 0.00) | 0.01 (0.00 to 0.03) | 0.02 (0.01 to 0.04) | 0.01 (−0.01 to 0.02) |
| Hemoglobin A 1c , % | |||||||
| 6 mo | 74 | 0.00 (−0.14 to 0.14) | −0.05 (−0.17 to 0.07) | 0.05 (−0.04 to 0.13) | 0.05 (−0.13 to 0.23) | −0.05 (−0.21 to 0.11) | −0.10 (−0.24 to 0.05) |
| 12 mo | 74 | 0.00 (−0.15 to 0.14) | 0.05 (−0.07 to 0.18) | 0.07 (−0.03 to 0.18) | −0.06 (−0.25 to 0.13) | −0.08 (−0.25 to 0.10) | −0.02 (−0.18 to 0.14) |
| Systolic blood pressure, mm Hg | |||||||
| 6 mo | 77 | −0.59 (−5.01 to 3.84) | −5.54 (−10.15 to −0.92) | 0.06 (−4.18 to 4.30) | 4.95 (−1.29 to 11.19) | −0.65 (−6.63 to 5.34) | −5.60 (−11.71 to 0.52) |
| 12 mo | 77 | −1.78 (−6.80 to 3.24) | −4.62 (−8.92 to −0.31) | 0.06 (−4.52 to 4.64) | 2.84 (−3.62 to 9.29) | −1.84 (−8.47 to 4.79) | −4.68 (−10.81 to 1.46) |
| Diastolic blood pressure, mm Hg | |||||||
| 6 mo | 77 | −1.71 (−4.71 to 1.30) | −0.33 (−3.61 to 2.94) | 1.96 (−1.23 to 5.15) | −1.37 (−5.71 to 2.97) | −3.67 (−7.95 to 0.61) | −2.30 (−6.76 to 2.16) |
| 12 mo | 77 | −0.82 (−4.70 to 3.05) | 0.99 (−2.13 to 4.10) | 2.85 (0.10 to 5.59) | −1.81 (−6.66 to 3.04) | −3.67 (−8.30 to 0.97) | −1.86 (−5.91 to 2.18) |
| Heart rate, beats/min | |||||||
| 6 mo | 77 | −2.60 (−6.47 to 1.27) | 2.15 (−3.10 to 7.40) | 0.26 (−5.23 to 5.76) | −4.76 (−11.12 to 1.61) | −2.86 (−9.43 to 3.70) | 1.89 (−5.53 to 9.31) |
| 12 mo | 77 | −3.83 (−8.31 to 0.64) | 1.63 (−3.32 to 6.59) | −2.74 (−7.06 to 1.59) | −5.47 (−11.98 to 1.05) | −1.10 (−7.17 to 4.98) | 4.37 (−2.05 to 10.79) |
| Total cholesterol | |||||||
| 6 mo | 74 | ||||||
| mmol/L | −0.03 (−0.22 to 0.17) | −0.11 (−0.37 to 0.15) | 0.02 (−0.20 to 0.23) | 0.08 (−0.24 to 0.40) | −0.04 (−0.33 to 0.24) | −0.13 (−0.46 to 0.21) | |
| mg/dL | −1.09 (−8.56 to 6.39) | −4.20 (−14.37 to 5.96) | 0.64 (−7.70 to 8.98) | 3.11 (−9.17 to 15.40) | −1.73 (−12.63 to 9.18) | −4.84 (−17.64 to 7.96) | |
| 12 mo | 74 | ||||||
| mmol/L | −0.04 (−0.25 to 0.16) | −0.04 (−0.25 to 0.17) | −0.02 (−0.19 to 0.16) | −0.00 (−0.29 to 0.29) | −0.03 (−0.29 to 0.24) | −0.03 (−0.29 to 0.24) | |
| mg/dL | −1.69 (−9.73 to 6.36) | −1.64 (−9.80 to 6.52) | −0.67 (−7.37 to 6.03) | −0.05 (−11.21 to 11.11) | −1.02 (−11.22 to 9.18) | −0.97 (−11.25 to 9.31) | |
| Low-density lipoprotein cholesterol | |||||||
| 6 mo | 74 | ||||||
| mmol/L | 0.02 (−0.18 to 0.22) | −0.16 (−0.40 to 0.09) | 0.03 (−0.14 to 0.21) | 0.17 (−0.13 to 0.48) | −0.01 (−0.27 to 0.24) | −0.19 (−0.48 to 0.10) | |
| mg/dL | 0.70 (−6.93 to 8.33) | −6.00 (−15.37 to 3.36) | 1.21 (−5.53 to 7.96) | 6.71 (−5.06 to 18.47) | −0.51 (−10.43 to 9.41) | −7.22 (−18.46 to 4.02) | |
| 12 mo | 74 | ||||||
| mmol/L | −0.02 (−0.24 to 0.19) | −0.03 (−0.19 to 0.12) | 0.06 (−0.12 to 0.24) | 0.01 (−0.25 to 0.27) | −0.08 (−0.36 to 0.19) | −0.10 (−0.33 to 0.14) | |
| mg/dL | −0.86 (−9.14 to 7.42) | −1.33 (−7.47 to 4.81) | 2.37 (−4.51 to 9.24) | 0.47 (−9.57 to 10.52) | −3.22 (−13.71 to 7.26) | −3.70 (−12.67 to 5.28) | |
| High-density lipoprotein cholesterol | |||||||
| 6 mo | 74 | ||||||
| mmol/L | −0.05 (−0.13 to 0.04) | 0.03 (−0.08 to 0.13) | −0.02 (−0.07 to 0.03) | −0.07 (−0.21 to 0.06) | −0.03 (−0.12 to 0.07) | 0.05 (−0.07 to 0.16) | |
| mg/dL | −1.78 (−5.04 to 1.48) | 1.01 (−3.17 to 5.19) | −0.76 (−2.76 to 1.23) | −2.79 (−7.96 to 2.38) | −1.02 (−4.74 to 2.71) | 1.77 (−2.74 to 6.29) | |
| 12 mo | 74 | ||||||
| mmol/L | −0.04 (−0.12 to 0.04) | −0.00 (−0.12 to 0.11) | −0.07 (−0.14 to −0.01) | −0.04 (−0.17 to 0.10) | 0.03 (−0.06 to 0.13) | 0.07 (−0.06 to 0.20) | |
| mg/dL | −1.53 (−4.51 to 1.44) | −0.17 (−4.53 to 4.19) | −2.88 (−5.40 to −0.36) | −1.36 (−6.50 to 3.77) | 1.35 (−2.45 to 5.15) | 2.71 (−2.19 to 7.61) | |
| Triglycerides | |||||||
| 6 mo | 74 | ||||||
| mmol/L | 0.00 (−0.25 to 0.25) | 0.08 (−0.06 to 0.22) | −0.01 (−0.21 to 0.20) | −0.08 (−0.36 to 0.21) | 0.01 (−0.31 to 0.33) | 0.08 (−0.16 to 0.33) | |
| mg/dL | 0.15 (−22.23 to 22.52) | 6.84 (−5.42 to 19.10) | −0.66 (−18.80 to 17.48) | −6.69 (−31.55 to 18.16) | 0.81 (−27.26 to 28.87) | 7.50 (−13.83 to 28.84) | |
| 12 mo | 74 | ||||||
| mmol/L | 0.04 (−0.12 to 0.20) | −0.00 (−0.15 to 0.14) | −0.00 (−0.20 to 0.20) | 0.04 (−0.17 to 0.25) | 0.04 (−0.21 to 0.29) | 0.00 (−0.24 to 0.24) | |
| mg/dL | 3.60 (−10.22 to 17.42) | −0.02 (−12.85 to 12.81) | −0.08 (−17.95 to 17.78) | 3.62 (−14.76 to 21.99) | 3.68 (−18.33 to 25.69) | 0.07 (−21.36 to 21.49) | |
CR = calorie restriction; HOMA-IR = homeostasis model assessment of insulin resistance; QUICKI = quantitative insulin sensitivity check index; TRE = time-restricted eating.
Data were included for 90 participants; means were estimated using an intention-to-treat analysis using a linear mixed model. Error bars indicate 95% CIs for each parameter from baseline by diet group. The widths of the CIs have not been adjusted for multiplicity and should therefore not be used to reject or not reject treatment effects.
Sensitivity Analyses Using Multiple Imputation
Sensitivity analyses incorporating multiple imputation of missing data (Supplement Table 3, available at Annals.org) showed the robustness of our primary results.
Adherence, Energy Intake, and Physical Activity
Energy intake decreased in the TRE and CR groups, relative to baseline, at months 3, 6, 9, and 12 (Figure 2, bottom). Over the 12-month study, the mean caloric deficit was −425 kcal/d (SD, 531) in the TRE group and −405 kcal/d (SD, 712) in the CR group, with no difference between groups. Participants in the TRE group reported being adherent with their eating window on average 6.1 days per week (SD, 0.8) (87% of days) over the course of the 12-month study (Supplement Figure 3, available at Annals.org). As for CR, 61% of participants reported being adherent with their prescribed calorie goal during the 12-month trial. The mean (SD) daily eating window in the TRE group decreased from baseline to month 12 (Table 4). The mean (SD) eating window in the CR and control groups was greater than 10 hours at baseline and did not change during the trial. Dietary intake did not differ over time within group or between groups (Table 4). Physical activity, measured as steps per day, did not differ over time within group or between groups (Table 4).
Table 4.
Dietary Intake and Physical Activity Between Groups Over 12 Months*
| Variable | TRE |
Daily CR |
Control |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 | Month 12 | Baseline | Month 6 | Month 12 | Baseline | Month 6 | Month 12 | |
| Mean daily eating window (SD), h:min | 10:25 (2:08) | 7:35 (1:40) | 7:51 (1:25) | 10:26 (1:46) | 10:09 (2:05) | 10:30 (2:00) | 10:32 (1:24) | 10:23 (1:51) | 10:11 (1:57) |
| Dietary intake | |||||||||
| Mean protein (SD), % | 17 (4) | 20 (6) | 19 (4) | 18 (5) | 21 (6) | 19 (4) | 18 (6) | 18 (6) | 18 (8) |
| Mean carbohydrates (SD), % | 43 (13) | 38 (15) | 41 (12) | 46 (9) | 41 (9) | 44 (8) | 39 (9) | 41 (6) | 41 (12) |
| Mean total sugar (SD), % | 16 (8) | 12 (6) | 13 (7) | 14 (5) | 17 (7) | 18 (8) | 14 (7) | 15 (5) | 17 (7) |
| Mean fat (SD), % | 40 (8) | 42 (5) | 40 (6) | 36 (10) | 38 (8) | 37 (6) | 43 (9) | 41 (5) | 41 (5) |
| Mean saturated fat (SD), % | 11 (5) | 12 (5) | 11 (5) | 12 (5) | 13 (5) | 13 (6) | 13 (3) | 12 (2) | 13 (6) |
| Mean cholesterol (SD), mg | 325 (144) | 321 (222) | 319 (144) | 270 (121) | 224 (112) | 241 (125) | 343 (211) | 313 (150) | 320 (108) |
| Mean fiber (SD), g | 14 (7) | 13 (5) | 12 (4) | 14 (6) | 14 (6) | 14 (6) | 14 (6) | 16 (7) | 14 (6) |
| Mean sodium (SD), mg/d | 3203 (1037) | 2870 (1187) | 2618 (848) | 2782 (985) | 2457 (912) | 2477 (821) | 3156 (1182) | 3142 (947) | 3094 (957) |
| Mean caffeine (SD), mg/d | 113 (111) | 81 (86) | 88 (83) | 74 (77) | 52 (69) | 54 (73) | 78 (90) | 88 (78) | 117 (98) |
| Mean alcohol (SD), g/d | 5 (7) | 3 (5) | 4 (8) | 4 (6) | 5 (11) | 4 (9) | 2 (4) | 4 (10) | 4 (8) |
| Physical activity | |||||||||
| Mean steps per day (SD), n | 6150 (2497) | 5440 (2421) | 5813 (2418) | 7022 (3171) | 7247 (2835) | 7148 (2815) | 6892 (2619) | 6624 (2408) | 6507 (2818) |
CR = calorie restriction; TRE = time-restricted eating.
Only observed values included. A total of 19 of 30 TRE participants returned all food records, and 17 of 30 CR participants returned all food records.
Adverse Events
No serious adverse events or deaths were reported during the year-long trial and did not differ substantially across groups (Supplement Table 4, available at Annals.org).
Discussion
Our randomized controlled trial shows that both an 8-hour TRE strategy without calorie counting and a CR strategy with calorie counting produced greater weight loss than no-intervention control at 12 months. Differences in weight loss were not statistically significantly different between the TRE and CR groups. Participants of both active interventions showed moderately high adherence and decreased energy intake to a meaningful level. No serious adverse events were detected in any group.
Several clinical trials have compared the effect of TRE combined with intentional energy restriction with that of daily CR on body weight. Liu and colleagues (11) found that 12 months of 8-hour TRE with calorie counting produced similar reductions in body weight (9%) as daily CR (7%) in 139 adults with obesity. Peeke and colleagues (20) found similar reductions in body weight by 10-hour TRE plus energy restriction (8%) when compared with daily CR (7%) after 2 months of intervention. Likewise, Thomas and colleagues (21) found that 3 months of 10-hour TRE combined with calorie counting produced similar weight loss (6%) as daily CR (5%) in 85 men and women with obesity. Taken together, TRE combined with intentional energy restriction may produce similar reductions in body weight as daily CR over 2 to 12 months.
Our trial is novel in that it compared the effects of TRE without intentional energy restriction with that of daily CR. We show here that limiting the eating window to 8 hours per day (noon to 8:00 p.m.) without calorie counting reduced body weight by 4.6 kg by month 12 versus control. These body weight reductions were not statistically significantly different compared with CR (5.4 kg), although our study was only powered to detect relatively large weight loss differences. Only a few controlled trials have assessed the effect of TRE with ad libitum food intake on body weight. Chow and colleagues (22) found that 3 months of 8-hour TRE without calorie counting reduced body weight by 4%, versus control in 20 adults with obesity. Likewise, Gabel and colleagues (5) showed that 8-hour TRE produced 3% weight loss after 3 months versus control. Cienfuegos and colleagues (4) also noted 3% reductions in body weight with 6-hour TRE after 2 months, relative to control, among 58 men and women with obesity. In contrast to these findings, Lowe and colleagues (23) reported no change in body weight after 3 months of 8-hour TRE in 116 adults with obesity relative to control. As for daily CR, body weight generally decreases by 4% to 5% after 3 months of intervention in persons with obesity (24). As such, the weight loss efficacy of TRE without calorie monitoring may be similar to that of daily CR over short periods of time (24).
Adherence to the TRE eating plan was high, with participants adhering to the 8-hour eating window on average 6.1 out of 7 days per week (87%) over 12 months. This finding is consistent with several studies of TRE (5, 22, 23, 25) but not all (26). In comparison, CR participants displayed moderately high adherence, with 61% of participants adhering to their prescribed calorie goals over 1 year. The adherence data for TRE and CR are difficult to compare because different metrics were used to assess adherence. However, given that the average degree of energy reduction achieved with TRE (425 kcal/d) and CR (405 kcal/d) seemed similar, it is likely that overall adherence was similar.
Changes in blood pressure, plasma lipids, and insulin sensitivity did not differ between TRE and CR, although the study was underpowered to detect differences in these metabolic outcomes. However, we did observe an increase in insulin sensitivity by month 12 when TRE was compared with control. Nevertheless, this finding should be viewed solely as hypothesis generating given the lack of correction for multiple testing.
Non-Hispanic Black and Hispanic adults have the highest age-adjusted prevalence of obesity in the United States (27). Effective nonpharmacologic weight loss regimens are critically needed in these populations. Our findings show that TRE is an effective and feasible regimen for sustained weight loss over 1 year in a sample population that was highly diverse in terms of its racial and ethnic makeup. Time-restricted eating is undoubtedly an attractive approach to weight loss in that it does not require the purchase of expensive food products, allows persons to continue consuming familiar foods, and omits complicated calorie tracking. Given the paucity of literature on nutrition strategies in this cohort (28), this study of a racially and ethnically diverse population may help to fill in critical knowledge gaps and improve the health of underrepresented racial and ethnic groups.
Our study has some important limitations. First, the study was small and not blinded. It was not powered to detect smaller but potentially clinically important differences between TRE and CR. Second, results from the secondary analyses should be viewed as hypothesis generating, given the fact that the type I error was not corrected for with multiple testing. Third, energy expenditure was not quantified in this trial. Measuring energy expenditure using the doubly labeled water technique would have provided more accurate assessments of the overall energy restriction produced by the intervention groups in relation to weight loss (29). Finally, the generalizability of our findings to patients with diabetes or cardiovascular diseases is limited by the enrollment.
In conclusion, TRE is effective for weight loss when compared with controls eating over a period of 10 or more hours but not more effective than daily CR in a racially diverse population. Future studies are needed to confirm our findings.
Supplementary Material
Primary Funding Source:
National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Grant Support:
By the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK128180).
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M23-0052.
Contributor Information
Shuhao Lin, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Sofia Cienfuegos, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Mark Ezpeleta, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Kelsey Gabel, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Vasiliki Pavlou, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Andrea Mulas, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Kaitie Chakos, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Mara McStay, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Jackie Wu, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Lisa Tussing-Humphreys, Department of Kinesiology and Nutrition and University of Illinois Cancer Center, University of Illinois Chicago, Chicago, Illinois.
Shaina J. Alexandria, Department of Preventative Medicine (Biostatistics), Northwestern University, Chicago, Illinois.
Julienne Sanchez, College of Medicine (Endocrinology), University of Illinois Chicago, Chicago, Illinois.
Terry Unterman, College of Medicine (Endocrinology), University of Illinois Chicago, and Jesse Brown VA Medical Center, Chicago, Illinois.
Krista A. Varady, Department of Kinesiology and Nutrition, University of Illinois Chicago, Chicago, Illinois.
Data Sharing Statement:
The authors have indicated they will not be sharing data. The subjects did not provide consent to share deidentified data at the time they were consented to participate in the study. As such, our institution’s institutional review board will not permit us to share deidentified data for this study.
References
- 1.O’Connor A Fasting diets are gaining acceptance. The New York Times. 7 March 2016. Accessed at https://archive.nytimes.com/well.blogs.nytimes.com/2016/03/07/intermittent-fasting-diets-are-gaining-acceptance/ on 29 March 2023. [Google Scholar]
- 2.Fanti M, Mishra A, Longo VD, et al. Time-restricted eating, intermittent fasting, and fasting-mimicking diets in weight loss. Curr Obes Rep. 2021;10:70–80. doi: 10.1007/s13679-021-00424-2 [DOI] [PubMed] [Google Scholar]
- 3.Varady KA, Cienfuegos S, Ezpeleta M, et al. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. 2022;18:309–321. doi: 10.1038/s41574-022-00638-x [DOI] [PubMed] [Google Scholar]
- 4.Cienfuegos S, Gabel K, Kalam F, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32:366–378.e3. doi: 10.1016/j.cmet.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4:345–353. doi: 10.3233/NHA-170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorling JL, Das SK, Racette SB, et al. ; CALERIE Study Group. Changes in body weight, adherence, and appetite during 2 years of calorie restriction: the CALERIE 2 randomized clinical trial. Eur J Clin Nutr. 2020;74:1210–1220. doi: 10.1038/s41430-020-0593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102:183–197. doi: 10.1016/j.mcna.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaix A, Manoogian ENC, Melkani GC, et al. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291–315. doi: 10.1146/annurevnutr-082018-124320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent fasting: a heart healthy dietary pattern? Am J Med. 2020;133:901–907. doi: 10.1016/j.amjmed.2020.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon S, Kang J, Kim SH, et al. Beneficial effects of time-restricted eating on metabolic diseases: a systemic review and meta-analysis. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu D, Huang Y, Huang C, et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386:1495–1504. doi: 10.1056/NEJMoa2114833 [DOI] [PubMed] [Google Scholar]
- 12.Torres L, Lee JL, Park S, et al. Retention, fasting patterns, and weight loss with an intermittent fasting app: large-scale, 52-week observational study. JMIR Mhealth Uhealth. 2022;10:e35896. doi: 10.2196/35896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731–754. doi: 10.2337/dci19-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalle Grave R, Sartirana M, Calugi S. Personalized cognitive-behavioural therapy for obesity (CBT-OB): theory, strategies and procedures. Biopsychosoc Med. 2020;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mifflin MD, St Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241 [DOI] [PubMed] [Google Scholar]
- 16.Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930–938. doi: 10.1001/jamainternmed.2017.0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. In: Le Cam LM, Neyman J, eds. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Berkeley, California, 21 June to 18 July 1965 and 27 December 965 to 7 January 1966. University of California Press; 1967:221–233. [Google Scholar]
- 18.Mansournia MA, Nazemipour M, Naimi AI, et al. Reflection on modern methods: demystifying robust standard errors for epidemiologists. Int J Epidemiol. 2021;50:346–351. doi: 10.1093/ije/dyaa260 [DOI] [PubMed] [Google Scholar]
- 19.White H A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980; 48:817–838. [Google Scholar]
- 20.Peeke PM, Greenway FL, Billes SK, et al. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: results of a randomized, controlled, virtual clinical trial. Nutr Diabetes. 2021;11:6. doi: 10.1038/s41387-021-00149-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas EA, Zaman A, Sloggett KJ, et al. Early time-restricted eating compared with daily caloric restriction: a randomized trial in adults with obesity. Obesity (Silver Spring). 2022;30:1027–1038. doi: 10.1002/oby.23420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow LS, Manoogian ENC, Alvear A, et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring). 2020;28: 860–869. doi: 10.1002/oby.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe DA, Wu N, Rohdin-Bibby L, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180:1491–1499. doi: 10.1001/jamainternmed.2020.4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rynders CA, Thomas EA, Zaman A, et al. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anton SD, Lee SA, Donahoo WT, et al. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Xing C, Zhang J, et al. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. 2021;19:148. doi: 10.1186/s12967-021-02817-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Center for Disease Control and Prevention. Adult obesity facts. Accessed at https://www.cdc.gov/obesity/data/adult.html on 1 March 2023. [Google Scholar]
- 28.Turner BE, Steinberg JR, Weeks BT, et al. Race/ethnicity reporting and representation in US clinical trials: a cohort study. Lancet Reg Health Am. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Jonge L, DeLany JP, Nguyen T, et al. Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. Am J Clin Nutr. 2007;85:73–79. doi: 10.1093/ajcn/85.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors have indicated they will not be sharing data. The subjects did not provide consent to share deidentified data at the time they were consented to participate in the study. As such, our institution’s institutional review board will not permit us to share deidentified data for this study.


