Abstract
Background:
The identification of oncogenic mutations in diffuse large B-cell lymphoma (DLBCL) has led to the development of drugs that target essential survival pathways. Based on drug synergy studies, we hypothesized that targeting multiple survival pathways may be curative in DLBCL.
Methods:
We performed a single-center phase Ib/II trial of venetoclax, ibrutinib, prednisone, obinutuzumab, and lenalidomide (ViPOR) in relapsed or refractory (R/R) DLBCL. In phase Ib, which included DLBCL and indolent lymphomas, 4-dose levels of venetoclax were evaluated to identify the recommended phase II dose (RP2D) with fixed doses of other study agents. A phase II expansion in germinal center B-cell (GCB) and non-GCB DLBCL was performed. ViPOR was administered every 21 days for 6 cycles.
Results:
Phase Ib in 20 patients (10 DLBCL) showed a single dose-limiting toxicity of grade 3 intracranial hemorrhage, establishing venetoclax 800 mg as the RP2D. Phase II included 40 patients with DLBCL. Toxicity in all patients included grade 3-4 neutropenia, thrombocytopenia, anemia, and febrile neutropenia in 24%, 23%, 7%, and 1% of cycles, respectively. Objective responses occurred in 54% of 48 evaluable DLBCL patients, with complete responses (38%) occurring exclusively in non-GCB DLBCL and HGBCL-DH-BCL2. Circulating-tumor DNA was undetectable in 33% of patients at the end-of-therapy. With a median follow-up of 40 months, 2-year progression-free survival and overall survival were 34% (95% CI: 21-47%) and 36% (95% CI: 23-49%), respectively.
Conclusions:
ViPOR achieved durable remissions in specific molecular DLBCL subtypes and was associated with mainly reversible adverse events.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is a molecularly heterogenous disease for which chemoimmunotherapy can be curative.1-7 Patients with early relapse or refractory (R/R) disease, however, have a poor outcome with conventional treatments.8,9 While anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy has improved outcomes, only approximately 30-40% of R/R patients are cured with such therapy.10-13
Genetic and functional genomic studies have revealed oncogenic driver pathways in DLBCL, leading to the development of drugs against actionable targets. Although many of these agents are active as monotherapy,14-17 they rarely induce deep responses or cure, although certain molecular subtypes may occasionally and exceptionally respond to these agents.18-20 Based on our identification of synergy of targeted drugs in DLBCL models,21,22 we hypothesized that inhibiting multiple survival pathways simultaneously could be curative in certain DLBCL molecular subtypes.
Survival pathways in the activated B-cell (ABC) subtype of DLBCL include chronic active B-cell receptor (BCR) signaling to NF-κB and phosphoinositide 3-kinase (PI3K), prevention of apoptosis by BCL2, and expression of the lineage-restricted transcription factors IRF4, Ikaros (IKZF1) and Aiolos (IKZF3).23,24 In the germinal center B-cell (GCB) subtype of DLBCL, cell viability is maintained by constitutive BCR-dependent PI3K signaling, BCL2, Ikaros, and Aiolos.25,26 These survival pathways can be blocked by targeted drugs, including the BTK inhibitor ibrutinib, which targets BCR-dependent NF-κB activation, glucocorticoids, which target proximal BCR signaling,27 the BCL2 inhibitor venetoclax, and lenalidomide, which targets Ikaros, Aiolos and, indirectly, IRF4. Preclinical studies have demonstrated synergistic killing of DLBCL cell lines by combinations of these drugs.21,22
We developed a 5-drug regimen (ViPOR) that targets these pathways and includes obinutuzumab to trigger innate immune responses to malignant B-cells. (Fig. 1A). To maximize potential synergy while minimizing cumulative drug toxicities, we administered all agents in non-continuous cycles for a fixed duration.21,22 Herein, we present the results of a single-center phase Ib study of ViPOR in R/R B-cell lymphomas and efficacy analysis for the phase II expansion in R/R DLBCL.
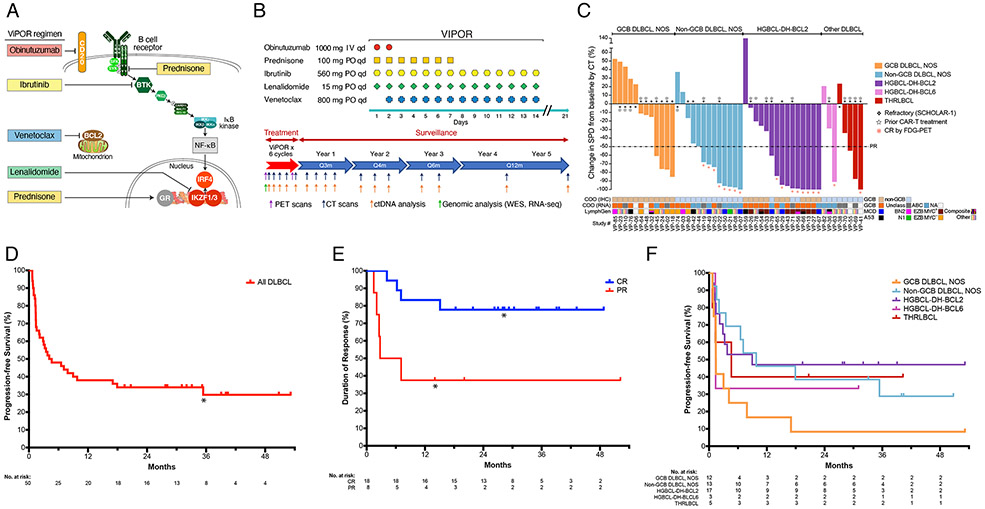
Figure 1: ViPOR Treatment Regimen and Clinical Activity.
A). Schematic of the survival pathways targeted by the ViPOR regimen; B). Schematic of ViPOR agent dosing and post-treatment surveillance monitoring; C). Waterfall plot of DLBCL patients showing the percent change in the sum of the product of the diameters (SPD) following ViPOR therapy as compared to baseline as assessed by computed tomography (CT); D). Kaplan-Meier analysis of progression-free survival starting at the on-study date for all DLBCL patients. *One non-GCB DLBCL, NOS patient died in remission at 31 months from COVID-19 infection; E). Kaplan-Meier analysis of duration of response starting at the response date and separated by complete or partial response to ViPOR. *Two patients (1 in complete response and 1 in partial response) died without disease relapse or progression and were censored at the time of death; F). Kaplan-Meier analysis of progression-free survival starting at the on-study date and separated by histologic DLBCL subtype.
METHODS
Patients
R/R B-cell lymphoma patients were eligible for the phase Ib and R/R DLBCL (including high-grade B-cell lymphoma “double-hit” (HGBCL-DH) and T-cell/histocyte-rich large B-cell lymphoma (THRLBCL)) for the phase II expansion (Supplementary Methods). Inclusion criteria included age ≥18 years, ECOG performance status ≤2, and adequate organ function unless secondary to lymphoma. Prior anthracycline was required for DLBCL patients, and all patients required prior anti-CD20 antibody. Exclusions included active CNS disease, pregnant or breastfeeding women, use of strong CYP3A4 inducers/inhibitors, and HIV infection. Prior treatment with one study agent (i.e., venetoclax, ibrutinib, or lenalidomide) was allowed. The study was approved by the National Cancer Institute Institutional Review Board, and all patients provided written informed consent in accordance with the Declaration of Helsinki (protocol available at nejm.org). ClinicalTrials.gov identifier: NCT03223610.
Treatment
In phase Ib, a “3+3” design was used to determine the recommended phase 2 dose (RP2D) of venetoclax at 4 dose-levels (DLs) (200-800 mg) days 2-14 (starting cycle 2 for DL1) (Table S1) in combination with fixed-dose ibrutinib 560 mg days 1-14, prednisone 100 mg days 1-7, obinutuzumab 1000 mg days 1-2, and lenalidomide 15 mg days 1-14 (Fig. 1B). A 12-day initial ramp-up of venetoclax was used in all patients (Table S2) with serial tumor lysis syndrome laboratory monitoring and prophylaxis based on pre-treatment tumor lysis syndrome risk (Supplementary Methods). Dose-limiting toxicity (DLT) criteria are provided in the Supplementary Appendix.
Phase II expansion cohorts, 20 patients each with GCB and non-GCB DLBCL, were assessed at the RP2D. Six cycles of ViPOR every 21-days were administered unless progression or unacceptable toxicity. All patients received growth factor support and pneumocystis jirovecii prophylaxis, with venous thromboembolism prophylaxis given if indicated (Supplementary Methods). Pre-treatment biopsies were analyzed for whole-exome and transcriptome sequencing (Supplementary Methods). Baseline computed tomography (CT), fluorodeoxyglucose positron-emission tomography (FDG-PET), and bone marrow biopsy/aspirate were performed with restaging CT and PET scans during treatment and in follow-up (Fig. 1B and Supplementary Methods). Plasma samples were collected for circulating-tumor DNA (ctDNA) analysis using clonoSEQ (Fig. 1B),28 as well as for pharmacokinetic (PK) analyses (Supplementary Methods). Response to therapy was per Lugano classification criteria.29
Statistical Analysis
In phase Ib, a maximum of 30 patients could be treated to assess all 4 DLs of venetoclax and allow for a potential DL(−1). In phase II, an additional 40 R/R DLBCL patients were treated at the RP2D. With 40 R/R DLBCL patients, an exact binomial test with a 0.10 one-sided significance level has 87% power to rule out 40% and target a 60% overall response rate. Response duration, progression-free survival, and overall survival were determined using the Kaplan-Meier method, with the median and a 95% confidence interval (95% CI) reported for each analysis, and a data cutoff date of July 11, 2023. Response duration was calculated in all responding patients (N=26) from the date of first response to date of relapse/progression or last follow-up. Progression-free and overall survival were calculated in all DLBCL patients (N=50) from the on-study date to date of relapse/progression, death, or last follow-up, as appropriate. PET and minimal residual disease (MRD) analyses based on data obtained after cycles 1 or 2, or at the end-of-therapy began at that respective timepoint. Additional statistical methods are described in the Supplementary Appendix.
RESULTS
Patients
Sixty patients with R/R B-cell lymphoma enrolled between February 2018 and June 2021, including 20 patients (10 DLBCL) in phase Ib and 40 patients (all DLBCL) in phase II (Table 1). In 50 DLBCL patients, the median (range) age was 61 (29-77) years, with stage III/IV disease in 92%, elevated LDH in 86%, ≥2 extranodal sites in 56%, and international prognostic index (IPI) ≥3 in 68% of patients. Seventeen (34%) DLBCL patients had transformed lymphoma. Median (range) prior systemic therapies was 3 (1-9), including 20 patients (40%) with prior CAR-T and 29 (58%) with refractory disease per SCHOLAR-1 criteria.9 Twenty-five patients had DLBCL, NOS, including 12 GCB and 13 non-GCB subtypes by immunohistochemistry.30,31 Twenty patients had HGBCL-DH, including 17 with MYC/BCL2 translocations (HGBCL-DH-BCL2) and 3 with MYC/BCL6 translocations (HGBCL-DH-BCL6), and five patients had THRLBCL (Tables 1 and S3).31
Table 1:
Baseline Patient Characteristics
| Characteristic | Phase Ib | Phase II | All DLBCL (N=50) |
All Patients (N=60) |
|---|---|---|---|---|
| DL1-DL4 (N=20) |
DLBCL Cohort (N=40) |
|||
| Median (range) age – yr. | 58 (34-76) | 59 (29-77) | 61 (29-77) | 58 (29-77) |
| Male sex – no. (%) | 13 (65%) | 26 (65%) | 33 (66%) | 39 (65%) |
| Race and ethnicity – no. (%) | ||||
| Caucasian | 15 (75%) | 25 (63%) | 33 (66%) | 40 (67%) |
| African American | 3 (15%) | 10 (25%) | 11 (22%) | 13 (22%) |
| Asian | 2 (10%) | 1 (3%) | 2 (4%) | 3 (5%) |
| Hispanic | 0 (0%) | 4 (10%) | 4 (8%) | 4 (7%) |
| Disease histology – no. (%) | ||||
| Non-GCB DLBCL, NOS | 4 (20%) | 9 (23%) | 13 (26%) | 13 (22%) |
| HGBCL-DH-BCL6 | 0 (0%) | 3 (8%) | 3 (6%) | 3 (5%) |
| THRLBCL | 0 (0%) | 5 (13%) | 5 (10%) | 5 (8%) |
| GCB DLBCL, NOS | 4 (20%) | 8 (20%) | 12 (24%) | 12 (20%) |
| HGBCL-DH-BCL2 | 2 (10%) | 15 (38%) | 17 (34%) | 17 (28%) |
| FL | 8 (40%) | 0 (0%) | 0 (0%) | 8 (13%) |
| MZL | 2 (10%) | 0 (0%) | 0 (0%) | 2 (3%) |
| Transformed lymphoma – no. (%) a, b | 4 (40%) | 13 (33%) | 17 (34%) | -- |
| ECOG ≥2 – no. (%) | 3 (15%) | 4 (10%) | 7 (14%) | 7 (12%) |
| Stage III/IV – no. (%) | 18 (90%) | 37 (93%) | 46 (92%) | 55 (92%) |
| Elevated LDH – no. (%) | 13 (65%) | 35 (88%) | 43 (86%) | 48 (80%) |
| Extranodal sites ≥2 – no. (%) | 12 (60%) | 22 (55%) | 28 (56%) | 34 (57%) |
| IPI ≥3 – no. (%) a | 7 (70%) | 27 (68%) | 34 (68%) | -- |
| Median (range) prior therapies | 3 (1-7) | 3 (1-9) | 3 (1-9) | 3 (1-9) |
| Prior therapies- no. (%) | ||||
| One prior study agentc | 6 (30%) | 14 (35%) | 18 (36%) | 20 (33%) |
| ASCT | 4 (20%) | 3 (8%) | 5 (10%) | 7 (12%) |
| Allo-HSCT | 1 (5%) | 0 (0%) | 1 (2%) | 1 (2%) |
| CAR-T | 3 (15%) | 17 (43%) | 20 (40%) | 20 (33%) |
| Refractory status - no. (%) | ||||
| Primary refractory | 9 (45%) | 27 (68%) | 30 (60%) | 36 (60%) |
| Refractory per SCHOLAR-1a | 6 (60%) | 23 (58%) | 29 (58%) | -- |
Aggressive B-cell lymphoma patients only (N=50);
Seventeen patients had transformed DLBCL from an underlying indolent lymphoma, 15 from follicular lymphoma and 2 from marginal zone lymphoma, including 9 who transformed to HGBCL-DH-BCL2, 5 to GCB DLBCL, NOS, and 3 to non-GCB DLBCL, NOS;
Includes 12 patients who received prior lenalidomide, 7 patients who received prior ibrutinib or acalabrutinib, and 1 patient who received prior venetoclax;
Abbreviations: Non-GCB DLBCL, NOS, non-germinal center B-cell diffuse large B-cell lymphoma, not otherwise specified; HGBCL-DH-BCL6, high-grade B-cell lymphoma, double-hit, with MYC and BCL6 rearrangements; THRLBCL, T-cell/histiocyte-rich large B-cell lymphoma; GCB DLBCL, NOS, germinal center B-cell diffuse large B-cell lymphoma, not otherwise specified; HGBCL-DH-BCL2, high-grade B-cell lymphoma, double-hit, with MYC and BCL2 rearrangements; FL, follicular lymphoma; MZL, marginal zone lymphoma; ECOG, Eastern Cooperative Oncology Group performance score; LDH, lactate dehydrogenase; IPI, international prognostic index; ASCT, autologous stem cell transplantation; Allo-HSCT, allogeneic hematopoietic stem cell transplantation; CAR-T, chimeric antigen receptor T-cell therapy; DL, dose level.
Toxicity and Pharmacokinetics
Eighteen of 20 patients in phase Ib were evaluable for DLTs (2 patients did not complete the DLT window due to progression). One of 18 evaluable patients experienced a DLT of grade 3 intracranial hemorrhage at DL1 in the setting of concomitant enoxaparin and aspirin. No other DLTs occurred, and venetoclax 800 mg was identified as the RP2D.
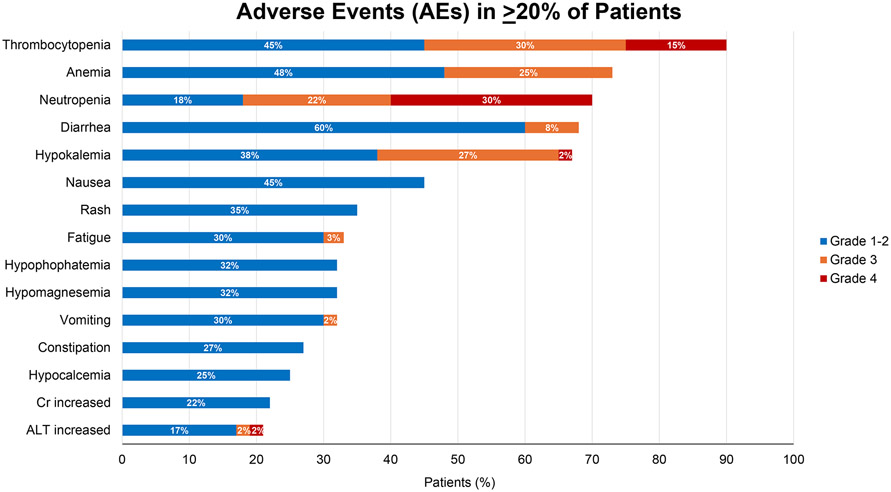
Hematologic adverse events of any grade occurred in over two-thirds of patients (Fig. 2), with grade 3-4 neutropenia, thrombocytopenia, and anemia observed in 24%, 23%, and 7% of cycles, respectively (Tables S4-S5). Furthermore, only 3 episodes of grade 3 febrile neutropenia occurred, and T-cell lymphocyte subsets were generally unaffected following therapy (Fig. S1, Table S6, and Supplementary Results). Although hypokalemia was the only non-hematologic grade 3-4 adverse event observed in ≥10% of patients (28%), 67% of patients experienced hypokalemia of any grade which required electrolyte replacement. Other non-hematologic adverse events included diarrhea (68%), nausea (45%), rash (35%), and fatigue (33%) (Fig. 2). Grade 3 atrial fibrillation occurred in 3 patients, and 1 major hemorrhage event occurred. One grade 4 tumor lysis syndrome event occurred, resolved, and did not recur despite continued treatment. Serious adverse events occurred in 42% of patients (Tables S7-S8), with non-neutropenic fever most common.
Figure 2: Adverse Events.
Depicted are all adverse events with an attribution of at least possibly related to ViPOR therapy and occurring in ≥20% of patients. Adverse events are depicted in descending order of frequency and broken down by grade of toxicity encountered.
Dose reductions and delays for toxicity occurred in 17% and 25% of patients, respectively, and 5 patients prematurely discontinued study therapy due to an unacceptable adverse event or intercurrent illness (Supplementary Results). PK analysis of venetoclax, ibrutinib, and lenalidomide was comparable to published single-agent data, indicating no significant drug interactions (Fig. S2, Table S9, and Supplementary Results).32-34
Clinical Outcomes
Forty-eight DLBCL patients were evaluable for response (2 HGBCL-DH-BCL2 patients discontinued therapy for toxicity prior to restaging). Response rate was 54% (26/48) with 38% (18/48) complete response (Fig. 1C and Table 2). Median (range) time to response was 0.66 (0.59-4.34) months, with 72% of complete responses ongoing without consolidation (Supplementary Results). Complete responses were obtained in 62% (8/13) of non-GCB DLBCL, NOS, 53% (8/15) of HGBCL-DH-BCL2, 33% (1/3) of HGBCL-DH-BCL6, and 20% (1/5) of THRLBCL, while no complete responses (0/12) were observed in GCB DLBCL, NOS (33% partial response, 4/12) (Table 2).
Table 2:
Response and Survival
| Overall Response Rate (ORR) |
Complete Response Rate (CR) |
Progression-free Survival (PFS) [2-year (95% CI)] |
Overall Survival (OS) [2-year (95% CI)] |
|
|---|---|---|---|---|
| All DLBCL | 54% (26/48) | 38% (18/48) | 34% (21-47%) | 36% (23-49%) |
| Disease histology | ||||
| Non-GCB DLBCL, NOS | 62% (8/13) | 62% (8/13) | 39% (14-63%) | 39% (14-63%) |
| HGBCL-DH-BCL6 | 33% (1/3) | 33% (1/3) | 33% (1-77%) | 33% (1-77%) |
| THRLBCL | 60% (3/5) | 20% (1/5) | 40% (5-75%) | 40% (5-75%) |
| GCB DLBCL, NOS | 33% (4/12) | 0% (0/12) | 8% (1-31%) | 17% (3-41%) |
| HGBCL-DH-BCL2 | 67% (10/15) | 53% (8/15) | 47% (23-68%) | 47% (23-68%) |
| Line of therapy | ||||
| Second-line therapy | 80% (12/15) | 73% (11/15) | 60% (32-80%) | 60% (32-80%) |
| Third or later-line therapy | 42% (14/33) | 21% (7/33) | 23% (11-38%) | 25% (13-41%) |
| Transformed lymphoma | 47% (7/15) | 33% (5/15) | 29% (11-51%) | 28% (10-51%) |
| Prior CAR-T | 45% (9/20) | 20% (4/20) | 30% (12-50%) | 30% (12-50%) |
| Refractory per SCHOLAR-1 | 44% (12/27) | 19% (5/27) | 21% (8-37%) | 24% (11-41%) |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; Non-GCB DLBCL, NOS, non-germinal center B-cell diffuse large B-cell lymphoma, not otherwise specified; HGBCL-DH-BCL6, high-grade B-cell lymphoma, double-hit, with MYC and BCL6 rearrangements; THRLBCL, T-cell/histiocyte-rich large B-cell lymphoma; GCB DLBCL, NOS, germinal center B-cell diffuse large B-cell lymphoma, not otherwise specified; HGBCL-DH-BCL2, high-grade B-cell lymphoma, double-hit, with MYC and BCL2 rearrangements; CAR-T, chimeric antigen receptor T-cell therapy.
With a median follow-up of 40 months (interquartile range: 32-51), 2-year progression-free survival was 34% (95% CI: 21-47%) in all DLBCL patients (Fig. 1D). In responding patients, 2-year response duration was 65% overall, including 78% of patients with complete responses and 38% of patients with partial responses to ViPOR (Fig. S3 and 1E). By subtype, 2-year progression-free survival was 39% in non-GCB DLBCL, NOS, 47% in HGBCL-DH-BCL2, 33% in HGBCL-DH-BCL6, 40% in THRLBCL, and 8% in GCB DLBCL, NOS (Fig. 1F). Overall survival largely reflected progression-free survival both overall and within DLBCL subtypes (Fig. S4). DLBCL patients who received ViPOR as second-line therapy had improved progression-free survival compared to those who received it as third or later-line therapy [log-rank hazard ratio (HR) 0.33 (95% CI: 0.17-0.66)] (Table 2 and Fig. S6). Four patients experienced systemic disease relapse after achieving complete response, all within 18 months of treatment, and one patient died in remission 31 months after treatment from COVID-19 infection (Fig. S7). Response and survival data for additional DLBCL subsets, including transformed, post-CAR-T, refractory, and molecular DLBCL subtypes are shown in Table 2 and in the Supplementary Appendix (Fig. S5 and Supplementary Results).
PET Imaging and Minimal Residual Disease
Baseline PET imaging was available in all 50 DLBCL patients with 41 having a paired end-of-therapy scan (Table S10 and Supplementary Results). Patients with a Deauville score of 5 at end-of-therapy had inferior progression-free survival [log-rank HR 0.22 (95% CI: 0.10-0.48)] and overall survival [log-rank HR 0.19 (95% CI: 0.08-0.43)] compared to those with a Deauville score of 1-4 (Supplementary Results). Additionally, patients with elevated total metabolic tumor volume (TMTV) or total lesion glycolysis (TLG) above the median at baseline were less likely to achieve complete response and had inferior progression-free and overall survival compared to those below the baseline median (Fig. S8-S9).
Of 50 DLBCL patients, 90% (45/50) had detectable ctDNA at baseline for MRD analysis (Table S11 and Supplementary Results). Following ViPOR, ctDNA concentration decreased rapidly with 33% of all patients and 93% of patients in complete response by PET having undetectable ctDNA at the end-of-therapy (Fig. S10). Elevated baseline ctDNA concentration ≥2.5 log10 hGE/mL was associated with inferior progression-free and overall survival to ViPOR, whereas undetectable ctDNA after cycle 1, cycle 2, or at end-of-therapy was associated with improved progression-free and overall survival compared to those with detectable ctDNA (Fig. S11-S12). Of 15 patients in complete response with serial ctDNA samples, 11 remained progression-free with undetectable ctDNA, whereas among 4 patients who relapsed, 3 developed molecular relapse before clinical relapse appeared by imaging (Fig. S13).
Mechanistic Foundation of ViPOR Efficacy
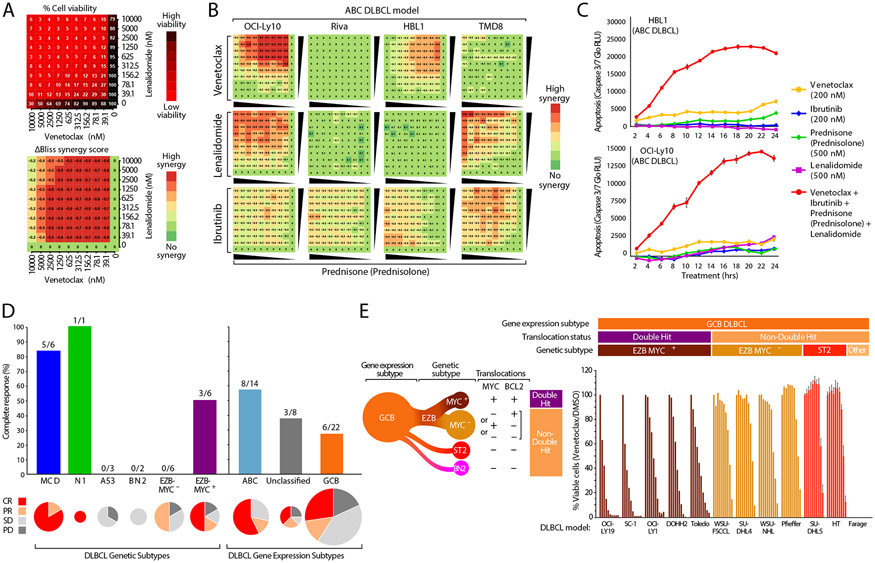
To explore the basis for ViPOR sensitivity, we employed high-throughput drug screens to measure the cytotoxicity of the 4 small molecule ViPOR drugs in pair-wise dose-response titrations in ABC cell line models (N=4), and used the ΔBliss metric to quantify drug synergy (Fig. 3A).21 Interestingly, while synergistic cytotoxicity was observed with all drug doublets, the pattern of synergy varied across ABC models. For example, the combination of prednisolone, the active metabolite of prednisone, with venetoclax was synergistically toxic in two of the four ABC models, while prednisolone plus lenalidomide was synergistic in a different pair of models, and prednisolone plus ibrutinib was synergistic in all four models (Fig. 3B). Furthermore, this was in contrast to the lack of synergistic cytotoxicity observed in most GCB cell line models (Fig. S14). Using a quantitative measure of apoptosis, we tested each ViPOR drug individually and as a 4-drug combination over a 2-to-24-hour time course. In two ABC models, the 4-drug combination produced more rapid and profound apoptosis than any individual ViPOR agent (Fig. 3C). Thus, while individual DLBCL models respond differentially to ViPOR drug doublets, the combination of all four ViPOR drugs overcame this functional heterogeneity.
Figure 3: Molecular Correlates of ViPOR Response.
A). The response to combinations of venetoclax and lenalidomide in a 10 × 10 matrix for the OCI-Ly10 ABC DLBCL cell line using normalized cell viability (top) or the calculated ΔBliss synergy score (bottom); B). Heatmaps displaying the calculated ΔBliss synergy score for each combination of indicated ViPOR drug doublets in four ABC DLBCL cell lines in a 10 × 10 matrix; C). Relative luminescence units (RLU) are displayed for cleaved caspase 3/7 measured in the indicated ABC DLBCL cell lines in culture with ViPOR agents singly or in combination for 2 to 24 hours; D). The complete response rate of ViPOR-treated patients is displayed for tumor biopsy grouped by LymphGen genetic subtype (left), or by cell-of-origin gene expression-assigned subtype (right). Pie charts are scaled to the total number of biopsies assigned to each subtype and display the percent of patients with complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD); E). Normalized cell viability of indicated GCB DLBCL cell lines treated with dilutions of the BCL2 inhibitor venetoclax (0.01-10.0uM). Assignment of GCB cell lines to LymphGen genetic subtypes is indicated. The EZB subtype can by further subdivided into EZB-MYC+ with both MYC and BCL2 translocations and EZB-MYC− that has only one or neither of these translocations.
We analyzed whole-exome and RNA-sequencing from ViPOR tumor samples to assign tumors to DLBCL genetic subtypes using the LymphGen algorithm3 and to cell-of-origin gene expression subtypes (Fig. S15-S16). Patients with the MCD subtype (N=6) had a considerably higher complete response rate [5/6, 83% (95% CI: 53-100%)] compared to patients with all other genetically classified DLBCL subtypes [4/18, 22% (95% CI: 3-41%)] and in all non-MCD patients [12/38; 32% (95% CI: 22-42%)] (Fig. 3D). Additionally, the 1 patient with N1 DLBCL also achieved a complete response. Among the gene-expression subtypes, complete response rate was highest in ABC (8/14, 57%) and unclassified DLBCL (3/8, 38%), and lowest in GCB DLBCL (6/22, 27%). Together, these data support the hypothesis that the MCD and possibly N1 genetic subtypes of ABC DLBCL likely benefitted from ViPOR due to their exceptional reliance on BCR-dependent NF-κB activation.18,19
Patients with HGBCL-DH-BCL2 had a high rate of durable complete response following ViPOR. Such tumors are classified as EZB-MYC+ in the DLBCL genetic taxonomy, whereas other GCB DLBCLs are classified into the EZB-MYC−, ST2, and BN2 subtypes (Fig. 3E).3 The complete response rate was higher in EZB-MYC+ (3/6, 50%), than in EZB-MYC− (0/6, 0%) with ViPOR, suggesting the dual translocations of MYC and BCL2 might confer sensitivity to ViPOR. To test this hypothesis, we measured the cytotoxicity of the 4 VIPOR agents in 12 GCB DLBCL cell line models, including EZB-MYC+(N=5), EZB-MYC− (N=4), ST2 (N=2), and genetically unclassified GCB (N=1) models. EZB-MYC+ lines were strikingly more sensitive to venetoclax than EZB-MYC− or other GCB models, whereas these lines did not differ consistently to the other ViPOR agents (Fig. 3E). This finding suggests that BCL2 inhibition with venetoclax likely contributes prominently to the observed durable complete response rate to ViPOR in patients with EZB-MYC+DLBCL.
DISCUSSION
We provide evidence that the simultaneous targeting of multiple survival pathways is potentially curative in specific molecular subtypes of DLBCL. To optimize multi-agent drug synergy, we designed a schedule reminiscent of combination chemotherapy, whereby all targeted agents are concurrently administered in a non-continuous fashion for a maximum of 6 cycles. This strategy avoids the cumulative toxicity associated with continuous and/or indefinite drug dosing, thereby allowing the safe administration of multiple targeted agents. Although hematologic toxicities were common and occurred in over two-thirds of patients, serious hematologic adverse events occurred in less than a quarter of cycles with febrile neutropenia observed in only 1% of cycles. Intermittent drug dosing also resulted in less non-hematologic toxicities, such as high-grade rash, compared to other continuous targeted-therapy regimens.35-37 Despite an initial concern for possible tumor lysis syndrome and hyperkalemia, diarrhea and hypokalemia were more commonly observed following ViPOR, with most patients requiring anti-diarrheal and electrolyte support in contrast to the 1 tumor lysis syndrome event observed. Furthermore, adverse events frequently resolved during the off-treatment week and most patients received all cycles on schedule and without dose reductions.
Due to the molecular heterogeneity and complex aberrant genetic landscape of DLBCL, we hypothesized that the inhibition of multiple driver pathways is necessary for the curative potential of targeted therapy. ViPOR synergistically targets key survival pathways in non-GCB DLBCL, including BCR-dependent NF-κB signaling using the combination of ibrutinib, lenalidomide, and prednisone, 21,22,23,27 as well as the anti-apoptotic protein BCL2 with venetoclax. In line with this hypothesis, ViPOR achieved a higher complete response rate (62%) and 2-year progression-free survival (39%) in non-GCB DLBCL than in GCB DLBCL, NOS. Notably, MCD DLBCL had a higher complete response rate (83%) than other genetic DLBCL subtypes, which is in line with previous laboratory and clinical evidence of exceptional dependence of MCD tumors on BCR signaling.15,19,20,24,38
Unexpectedly, ViPOR was highly active in GCB tumors with MYC and BCL2 translocations (HGBCL-DH-BCL2), with a higher 2-year progression-free survival (47%) than in GCB tumors lacking both translocations (8%). Mechanistically, MYC can sensitize cells to apoptosis, but BCL2 can block this toxicity.39 Venetoclax was more toxic for cell line models of HGBCL-DH-BCL2 than for other GCB DLBCL lines, leading us to speculate that the inclusion of venetoclax in ViPOR may expose HGBCL-DH-BCL2 tumors to the toxicity of MYC.
Among post-CAR-T patients, ViPOR achieved a 2-year progression-free survival of 30%, suggesting it may alter the poor prognosis of these patients.40 Additionally, ViPOR allowed successful bridging to radiotherapy, CAR-T, or allogeneic transplant in over one-third of patients who achieved partial response. With a median follow-up of 40 months, over one-third of all DLBCL patients were progression-free at 2-years, with 78% of complete responses durable without consolidation. Not unexpectedly, ViPOR yielded a higher complete response rate and progression-free survival when administered as second-line therapy, suggesting it should be considered early in R/R DLBCL.
PET and molecular biomarkers of disease were prognostic of outcome following ViPOR. Elevated baseline metabolic (i.e., TMTV and TLG) or molecular (i.e., ctDNA) disease burden was associated with inferior progression-free and overall survival. During therapy, ctDNA provided an early predictor of long-term outcome as did undetectable ctDNA at the end-of-therapy.
In conclusion, this study shows that multiple targeted agents can be administered concurrently in a safe and tolerable fashion across patients of all ages with R/R DLBCL. Our study also shows that simultaneous targeting of multiple survival pathways is potentially curative in specific molecular subtypes of R/R DLBCL. ViPOR was not curative in GCB DLBCL, NOS, highlighting the need to develop and test agents targeting GCB-specific pathways. Notably, durable complete responses were observed in patients who were heavily pretreated with chemotherapy or CAR-T therapy. As with any phase II study, one must recognize the limitations of sample size and patient selection. While the efficacy of ViPOR in specific molecular subtypes limits the subset of DLBCL patients with potentially curable disease, this very specificity provides some confidence in the generalizability of our results. A multicenter phase II study is under development to confirm the activity of ViPOR in patients with R/R non-GCB DLBCL and HGBCL-DH-BCL2.
Supplementary Material
Acknowledgments:
The authors would like to acknowledge Lindsay Barrow for her technical assistance in replicating the original data for the cell viability experiments.
Funding:
Research support was provided by the Intramural Research Program of the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health. Research drug support for venetoclax and obinutuzumab was provided by Genentech, Inc., and research drug support for lenalidomide was provided by Bristol-Myers Squibb/Celgene through clinical trial agreements with the National Cancer Institute. Commercial drug support for ibrutinib and prednisone was provided by the National Institutes of Health Clinical Center Pharmacy Department.
(Funded by the Intramural Research Program of the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health and others; ClinicalTrials.gov Identifier: NCT03223610.)
Footnotes
Publisher's Disclaimer: This is an Author Accepted Manuscript, which is the version after external peer review and before publication in the Journal. The publisher’s version of record, which includes all New England Journal of Medicine editing and enhancements, is available at https://www.nejm.org/doi/full/10.1056/NEJMoa2401532
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Presented in part at the 15th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 18-22, 2019, the 61st Annual Meeting of the American Society of Hematology, Orlando, Florida, December 7-10, 2019, the 62nd Annual Meeting of the American Society of Hematology, San Diego, California, December 5-8, 2020, and the 65th Annual Meeting of the American Society of Hematology, San Diego, California, December 9-12, 2023.
REFERENCES
- 1.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403(6769):503–11. DOI: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. The New England journal of medicine 2018;378(15):1396–1407. (In eng). DOI: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright GW, Huang DW, Phelan JD, et al. A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer cell 2020;37(4):551–568.e14. (In eng). DOI: 10.1016/j.ccell.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nature medicine 2018;24(5):679–690. (In eng). DOI: 10.1038/s41591-018-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacy SE, Barrans SL, Beer PA, et al. Targeted sequencing in DLBCL, molecular subtypes, and outcomes: a Haematological Malignancy Research Network report. Blood 2020;135(20):1759–1771. (In eng). DOI: 10.1182/blood.2019003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett NL, Wilson WH, Jung SH, et al. Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol 2019;37(21):1790–1799. (In eng). DOI: 10.1200/jco.18.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. The Lancet Haematology 2018;5(12):e609–e617. (In eng). DOI: 10.1016/S2352-3026(18)30177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28(27):4184–90. (In eng). DOI: 10.1200/jco.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130(16):1800–1808. (In eng). DOI: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. The Lancet Oncology 2019;20(1):31–42. (In eng). DOI: 10.1016/s1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. The Lancet Oncology 2021;22(10):1403–1415. (In eng). DOI: 10.1016/s1470-2045(21)00375-2. [DOI] [PubMed] [Google Scholar]
- 12.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. The New England journal of medicine 2022;386(7):640–654. (In eng). DOI: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 13.Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet (London, England) 2022;399(10343):2294–2308. (In eng). DOI: 10.1016/s0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 14.Davids MS, Roberts AW, Seymour JF, et al. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35(8):826–833. DOI: 10.1200/JCO.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nature medicine 2015;21(8):922–6. (In Eng). DOI: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2011;22(7):1622–7. (In eng). DOI: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 2011;117(22):5058–5066. DOI: 10.1002/cncr.26135. [DOI] [PubMed] [Google Scholar]
- 18.Younes A, Sehn LH, Johnson P, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37(15):1285–1295. (In eng). DOI: 10.1200/jco.18.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson WH, Wright GW, Huang DW, et al. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer cell 2021;39(12):1643–1653.e3. (In eng). DOI: 10.1016/j.ccell.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer cell 2017;31(6):833–843 e5. (In eng). DOI: 10.1016/j.ccell.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathews Griner LA, Guha R, Shinn P, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A 2014;111(6):2349–54. (Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural) (In eng). DOI: 10.1073/pnas.1311846111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Shaffer AL 3rd, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer cell 2012;21(6):723–37. (In Eng). DOI: 10.1016/j.ccr.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010;463(7277):88–92. (In eng). DOI: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelan JD, Young RM, Webster DE, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature 2018;560(7718):387–391. (In eng). DOI: 10.1038/s41586-018-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 2012;490(7418):116–20. (Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't) (In eng). DOI: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havranek O, Xu J, Köhrer S, et al. Tonic B-cell receptor signaling in diffuse large B-cell lymphoma. Blood 2017;130(8):995–1006. (In eng). DOI: 10.1182/blood-2016-10-747303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J, Ceribelli M, Phelan JD, et al. Molecular Targets of Glucocorticoids That Elucidate Their Therapeutic Efficacy in Aggressive Lymphomas. Cancer cell 2024; in press. DOI: 10.1016/j.ccell.2024.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roschewski M, Dunleavy K, Pittaluga S, et al. Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: a correlative biomarker study. Lancet Oncol 2015;16(5):541–9. DOI: 10.1016/S1470-2045(15)70106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(27):3059–68. (In Eng). DOI: 10.1200/jco.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103(1):275–82. [DOI] [PubMed] [Google Scholar]
- 31.Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood 2022;140(11):1229–1253. (In eng). DOI: 10.1182/blood.2022015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. The New England journal of medicine 2016;374(4):311–22. (In eng). DOI: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013;31(1):88–94. (Clinical Trial, Phase I Multicenter Study Research Support, Non-U.S. Gov't) (In eng). DOI: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blum W, Klisovic RB, Becker H, et al. Dose escalation of lenalidomide in relapsed or refractory acute leukemias. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28(33):4919–25. (In eng). DOI: 10.1200/jco.2010.30.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goy A, Ramchandren R, Ghosh N, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood 2019;134(13):1024–1036. (In eng). DOI: 10.1182/blood.2018891598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol 2018;5(3):e109–e116. DOI: 10.1016/S2352-3026(18)30018-8. [DOI] [PubMed] [Google Scholar]
- 37.Ujjani CS, Jung SH, Pitcher B, et al. Phase 1 trial of rituximab, lenalidomide, and ibrutinib in previously untreated follicular lymphoma: Alliance A051103. Blood 2016;128(21):2510–2516. (In eng). DOI: 10.1182/blood-2016-06-718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelan JD, Scheich S, Choi J, et al. Response to Bruton's tyrosine kinase inhibitors in aggressive lymphomas linked to chronic selective autophagy. Cancer cell 2024. (In eng). DOI: 10.1016/j.ccell.2023.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fanidi A, Harrington EA, Evan GI. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature 1992;359(6395):554–6. (In eng). DOI: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 40.Di Blasi R, Le Gouill S, Bachy E, et al. Outcomes of patients with aggressive B-cell lymphoma after failure of anti-CD19 CAR T-cell therapy: a DESCAR-T analysis. Blood 2022;140(24):2584–2593. (In eng). DOI: 10.1182/blood.2022016945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.