Abstract
CD8+ T cells are critical for the clearance of acute polyomavirus infection and the prevention of polyomavirus-induced tumors, but the antigen-presenting cell(s) involved in generating polyomavirus-specific CD8+ T cells have not been defined. We investigated whether dendritic cells and macrophages are permissive for polyomavirus infection and examined their potential for inducing antiviral CD8+ T cells. Although dendritic cells and macrophages both supported productive polyomavirus infection, dendritic cells were markedly more efficient at presenting the immunodominant viral epitope to CD8+ T cells. Additionally, infected dendritic cells, but not infected macrophages, primed anti-polyomavirus CD8+ T cells in vivo. Treatment with Flt3 ligand, a hematopoietic growth factor that dramatically expands the number of dendritic cells, markedly enhanced the magnitude of virus-specific CD8+ T-cell responses during acute infection and the pool of memory anti-polyomavirus CD8+ T cells. These findings suggest that virus-infected dendritic cells induce polyomavirus-specific CD8+ T cells in vivo and raise the potential for their use as cellular adjuvants to promote CD8+ T cell surveillance against polyomavirus-induced tumors.
CD8+ T cells are important components of host immunity against viral infections (15) and malignancies (21). CD8+ T lymphocytes recognize peptides derived from endogenously synthesized proteins complexed with major histocompatibility complex (MHC) class I molecules on the surface of infected or neoplastic cells (44). While recognition of class I MHC-peptide complexes triggers target cell lysis by CD8+ cytotoxic T lymphocytes (CTL), priming of naive CD8+ T cells requires two distinct signals (30). The first signal originates from ligation of the T-cell-receptor (TCR) complex with the cognate class I MHC-peptide complex on the antigen-presenting cell (APC), and the second signal is provided either by soluble factors, such as interleukin-2 (IL-2), or ligation of cell surface molecules, such as B7 on the APC with CD28 on the T cell, that provide essential costimulatory signals to the T cell (24, 30).
A number of studies have investigated the ability of different APC types to stimulate antigen-specific CD8+ T-cell responses (18, 19, 64). Dendritic cells (DC), macrophages (Mφ), and B cells are designated “professional” APC (18) by virtue of their capacity to provide both the specific MHC-peptide complexes and the nonspecific costimulatory signals to generate primary T-cell responses (25, 62). Of these three cell types, DC are particularly suited to prime antiviral CTL. DC capture and process antigen in early peripheral sites of virus infection, such as skin and epithelial mucosa, then traffic to T-cell-rich areas of secondary lymphoid organs (3). Several studies also suggest that Mφ are as efficient as DC in inducing primary CD8+ T-cell responses (19, 59), while others suggest that DC and Mφ may interact to generate CTL-mediated immune responses (20, 46).
DC are a rare population of bone marrow-derived cells found throughout nonlymphoid tissue and T-cell-dependent areas of lymphoid tissue (3). Research on the role DC play in the generation and regulation of immune responses has been hampered by the rarity of cells that meet the morphological and immunophenotypic characteristics for classification as DC; for example, DC constitute fewer than 1% of mononuclear spleen cells in the mouse (32). Conventional use of granulocyte-Mφ colony-stimulating factor (GM-CSF) alone or in combination with other growth factors, such as IL-4 and tumor necrosis factor alpha, to expand DC progenitors in vitro (11, 52) still provide low cell yields even after extensive culture. A novel approach to generate large numbers of functional DC in vivo emerged with the recent discovery of Flt3 ligand (FL), a cytokine that induces the proliferation and differentiation of hematopoietic stem cells (38). FL administration in vivo causes a dramatic increase in the number of DC in a variety of lymphoid and nonlymphoid tissues. These DC are as efficient as DC isolated from spleens of untreated mice at generating antigen-specific T-cell responses in vitro and in vivo (41, 56). Recent evidence suggests that FL treatment induces vigorous antitumor immune responses that protect against tumor challenges and mediate the regression of established tumors (8, 39, 45). The impact of FL treatment on virus-specific T-cell responses, however, has not been investigated.
Polyomavirus is a natural murine papovavirus that causes a broad array of tumors when injected into immunocompromised adult mice or neonatal mice of particular inbred strains (13). Several lines of evidence suggest a role for virus-specific CTL in the prevention of polyomavirus-induced tumor development. CD8+ T-cell depletion of virus-immune mice has been shown to block rejection of a polyomavirus tumor challenge (31), and immunization with a synthetic MHC class I-binding peptide corresponding to a polyomavirus protein sequence protected mice from a challenge with a polyomavirus DNA-transfected lymphoma (5). β2-microglobulin knockout mice are highly susceptible to polyomavirus-induced tumors (16). Finally, mice susceptible to polyomavirus-induced tumors are selectively deficient in polyomavirus-specific CD8+ T cells (35, 37).
We recently identified the immunodominant epitope for polyomavirus-specific CTL in H-2k mice as the Dk-restricted peptide derived from amino acids 389 to 397 of the viral oncoprotein, middle T (MT) (37). Using tetrameric complexes of Dk molecules containing the MT389-397 peptide, we found that approximately 20% of splenic CD8+ T cells are specific for the immunodominant CTL epitope during acute infection and that high levels of MT389-397-specific CD8+ T cells are maintained in memory (36). This dramatic expansion of polyomavirus-specific CD8+ T cells during polyomavirus infection suggests highly efficient presentation of virus-derived class I MHC-restricted T-cell epitopes by professional APC. Because DC are the most potent stimulators of primary T-cell responses (3), we hypothesized that virus-infected DC play a major role in the generation of antipolyomavirus CTL responses. The tropism of polyomavirus for cells of epithelial and mesenchymal origin is well established (13), but little is known of the virus' ability to infect cells of hematopoietic origin. B lymphocytes do not support polyomavirus replication (10), and some evidence suggests that Mφ are permissive for polyomavirus infection (13, 47). In this report, we evaluated the permissivity of Mφ and DC for polyomavirus infection and examined the ability of each of these APC to generate virus-specific CD8+ T-cell responses. In addition, we examined the effect of FL treatment on the generation of polyomavirus-specific CTL during acute and persistent virus infection.
MATERIALS AND METHODS
Mice and cell lines.
C3H/HeNCr mice (6 to 10 weeks old) were purchased from the Frederick Cancer Research and Development Center of the National Cancer Institute (Frederick, Md.). AG104A cells (61) were maintained in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS; HyClone, Inc., Logan, Utah). BALB/3T3 clone A31 cells were obtained from the American Type Culture Collection (ATCC, Manassas, Va.) and maintained in DMEM containing 5% bovine calf serum (Summit Biotechnology, Ft. Collins, Colo.). H8-1.18 hybridoma cells were maintained in DMEM containing 10% FBS, 4 mM l-glutamine, 50 μM 2-mercaptoethanol (2-ME), 1 mM nonessential amino acids, and 1 mM sodium pyruvate. CTLL cells were maintained in RPMI 1640 containing 10% FBS, 4 mM l-glutamine, 1 mM sodium pyruvate, 50 μM 2-ME, and 80 U of recombinant human IL-2 per ml.
Virus and virus inoculation.
Polyomavirus strain A2 was molecularly cloned, and virus stocks were prepared on baby ICR mouse kidney cells as previously described (37). Virus stocks heat inactivated by incubation at 70°C for 45 min were negative for infectious virus by plaque assay, and no T proteins were detected by immunoblotting of NP-40-solubilized AG104A cells pulsed with heat-inactivated virus (data not shown). Mice were inoculated subcutaneously (s.c.) in each hind footpad with 106 PFU of virus.
FL treatment.
Mice were injected intraperitoneally (i.p.) for 10 consecutive days with 200 μl of Hanks balanced salt solution (HBSS) containing 20 μg of Chinese hamster ovary (CHO) cell-derived human FL. Mock controls received 200 μl of HBSS using the same injection schedule. Where indicated, mice were infected with polyomavirus on day 8 or 9 of FL administration.
Synthetic peptides.
MT389-397 (RRLGRTLLL) and gag88-96 (RRKGKYTGL) peptides were synthesized by 9-fluorenylmethoxy carbonyl (F moc) chemistry as described previously (36).
APC.
Mφ were harvested by peritoneal lavage 3 days after i.p. injection of 2.5 ml of thioglycolate medium (Sigma, St. Louis, Mo.). Unless otherwise indicated, Mφ were collected as adherent cells after 24 h of incubation at 37°C. Mφ were cultured in DMEM containing 10% FBS.
Splenic DC were prepared as follows. Adherent cells were isolated from red blood cell (RBC)-depleted spleen cells after incubation in tissue culture-treated dishes (Nunc, Naperville, Ill.) at 37°C for 2 h. After 18 to 24 h of culture in DC media (Iscove's modified Eagle medium [IMDM] containing 10% FBS, 4 mM l-glutamine, 50 μM 2-ME, and 10 ng of GM-CSF [Intergen, Purchase, N.Y.] per ml), nonadherent cells were purified on Percoll step gradients as described elsewhere (51) and maintained in DC media.
Generation of polyomavirus-specific class I MHC-restricted hybridoma H8-1.18.
The MT389-397 peptide-specific CTL clone 8-1 (37) was fused to a CD8α gene transfected αβ TCR− BW5147 fusion partner (22) using polyethylene glycol (50%, Mr = 1,500), and hybridomas were selected in hypoxanthine-aminopterin-thymidine (HAT) medium as described elsewhere (27). The CD8+Vβ6+ H8-1.18 hybridoma secretes IL-2 when cocultured with syngeneic polyomavirus-infected or MT389-397 peptide-pulsed cells but not when cocultured with uninfected cells or cells pulsed with the Dk-binding Gag88-96 peptide (14) (data not shown).
To assay for presentation of the MT389-397 CD8+ T cell epitope by infected DC and Mφ, 5 × 104 H8-1.18 cells/well in flat-bottom 96-well microtiter plates (Costar, Cambridge, Mass.) were cocultured with the indicated number of APC for 18 to 24 h at 37°C. Freshly explanted, thioglycolate-elicited peritoneal Mφ or CD11+ DC sorted by fluorescence-activated cell sorting (FACS) were infected with polyomavirus at a multiplicity of infection (MOI) of 3 for 24 h before the addition of H8.1-18 cells. IL-2 release by H8.1-18 cells was assayed by [3H]thymidine uptake by the IL-2-dependent CTLL cell line. Values represent the means of three or four replicate wells; unless indicated, the standard errors of the mean (SEMs) were <5% of mean values and were omitted.
Flow cytometry.
Cells were stained with the following fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or allophycocyanin-conjugated monoclonal antibodies (MAbs). Anti-CD80 (1G10), anti-CD86 (GL1), and anti-I-Ek (14-4-4S) MAbs were obtained from the ATCC. Anti-CD3 (145-2C11), anti-Vβ6 (RR4-7), anti-CD16/32 (2.4G2), anti-CD11b (M1/70), and biotin-conjugated CD11c (HL3) MAbs were obtained from PharMingen (San Diego, Calif.). Anti-CD8α (CT-CD8a), anti-Dk (CTDk), and anti-Kk (CTKk) MAbs were obtained from Caltag Laboratories (South San Francisco, Calif.). Anti-CD11a and anti-CD44 MAbs were obtained from Beckman Coulter, Inc. (Fullerton, Calif.). PE-conjugated streptavidin (Molecular Probes, Eugene, Oreg.) was used to detect binding of biotinylated anti-CD11c.
Dk tetramers containing the MT389-397 peptide were prepared and intracellular gamma interferon (IFN-γ) staining performed as previously described (36). Samples were acquired on a FACScan (Becton Dickinson, San Jose, Calif.) flow cytometer using CELLQuest software (Becton Dickinson) and analyzed using FlowJo software (Tree Star, Inc., San Carlos, Calif.). Where indicated, DC isolated from the spleens of FL-treated mice as described above were surface stained for CD11c and sorted on a FACSVantage (Becton Dickinson) flow cytometer using CELLQuest software.
Titers of splenic polyomavirus.
Spleens were mechanically homogenized, and titers were determined by plaque assay using BALB/3T3 clone A31 cells as described elsewhere (16).
Western immunoblotting.
Uninfected or polyomavirus-infected (MOI = 3) Mφ and FACS-sorted DC were NP-40 solubilized as described earlier (34). Then, 50 μg of protein was resolved on a 12% reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to nitrocellulose membranes, and immunoblotted with MAb F4 against polyomavirus T proteins (43) or rabbit anti-VP1 antisera (63). Membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) or HRP-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch, West Grove, Pa.), followed by treatment with Renaissance chemiluminescence reagent (DuPont NEN, Boston, Mass.). Gels were visualized by autoradiography.
51Cr release assay.
51Cr-labeled polyomavirus-infected and peptide-pulsed AG104A target cells were prepared as described elsewhere (37) and aliquoted at 5 × 103 cells/well into 96-well U-bottom microtiter plates (Costar). RBC-lysed nonadherent spleen cells were plated at the indicated effector/target ratios. After 4 to 4.5 h at 37°C, samples were counted in a 1470 Wallac Wizard gamma counter (Turku, Finland). The percent specific lysis in each well was calculated as described previously (37). Values represent the means of three or four replicate wells, with the SEM values (all <5%) omitted.
Electron microscopy.
CD11c+ cells were FACS sorted from the spleens of FL-treated mice, infected by polyomavirus in vitro for 36 h (MOI = 3), fixed in 4% buffered glutaraldehyde, and then postfixed in 1% osmium tetroxide. Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined using a Philips EM201 transmission electron microscope.
APC priming of antipolyomavirus CTL.
For in vitro primary generation of polyomavirus-specific CTL, uninfected, virus-infected (MOI = 3), or MT389-397 peptide-pulsed (10 μM) APC at the indicated cell numbers were cultured with naive T cells isolated from spleens using T-cell columns (R&D Systems). Six days later, T cells were restimulated with syngeneic, polyomavirus-infected, irradiated splenocytes in the presence of T-cell medium (IMDM supplemented with 10% FBS, 8% conditioned medium of concanavalin A-pulsed rat splenocytes, 4 mM l-glutamine, 5 mM α-methylmannoside, and 50 μM 2-ME), prepared as previously described (37). After 7 days, T cells were restimulated with infected stimulator cells prepared as described above and then assayed for cytolytic activity against 51Cr-labeled AG104A targets 5 days after the last stimulation. Where indicated, CD40 cross-linking was performed as described earlier (50) using anti-CD40 MAb (3/23; PharMingen) and goat anti-rat IgG (Jackson Immunoresearch).
To evaluate the capacity of DC to elicit polyomavirus-specific CD8+ T cells in vivo, FACS-sorted CD11c+ DC either were left untreated or were pulsed with MT389-397 peptide (10 μM) for 2 h at 37°C, washed thoroughly, resuspended in HBSS, and then injected s.c. at a dose of 5 × 105 cells into each rear flank. Alternatively, freshly explanted, thioglycolate-elicited peritoneal Mφ or FACS-sorted CD11c+ DC were left uninfected or were infected (MOI = 3) for 2 h, washed extensively, and then injected s.c. at a dose of 2.5 × 105 cells into each hind footpad. For both protocols, single spleen cell suspensions were prepared 6 days later, and CD8+ T cells were analyzed by flow cytometry for MT389-397 peptide-stimulated intracellular IFN-γ production.
RESULTS
DC and Mφ are permissive for polyomavirus infection.
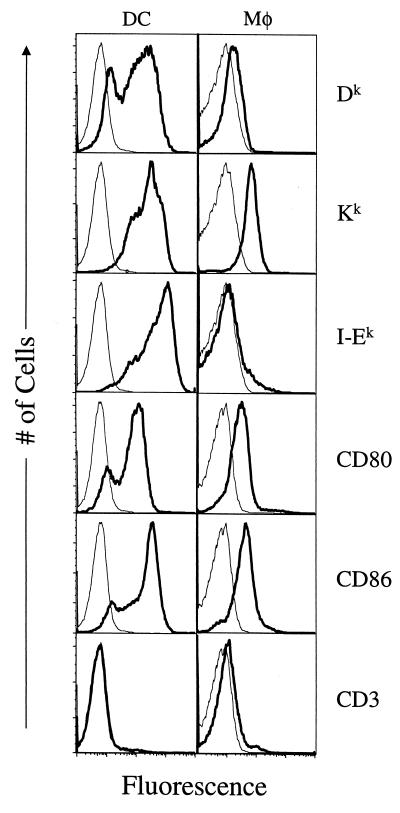
FL treatment of C3H/HeN mice reproducibly increased the percentage of spleen cells expressing the DC marker CD11c from approximately 1% up to 16 to 20% (data not shown), as reported for other inbred mouse strains (41, 48). FL-expanded DC and thioglycolate-elicited Mφ (positive for the monocyte marker CD11b) were analyzed by flow cytometry for surface expression of MHC and costimulatory molecules. As shown in Fig. 1 and consistent with reports by others (41), FL-expanded CD11c+ DC expressed high levels of CD80, CD86, Dk, Kk, and I-Ek. Expression of MHC and costimulatory molecules by the FL-expanded DC was 10- to 50-fold higher than those of Mφ (Fig. 1).
FIG. 1.
Cell surface phenotypic analysis of DC and Mφ. Percoll-purified FL-expanded DC were costained with biotin-conjugated anti-CD11c MAb and PE-conjugated streptavidin and the indicated FITC-conjugated MAbs. Freshly explanted thioglycolate-elicited peritoneal Mφ were costained with PE-conjugated anti-CD11b and the indicated FITC-conjugated MAbs. Plots represent the cell number versus the log fluorescence of CD11c-gated DC and CD11b-gated Mφ. Thin lines represent the staining by FITC-conjugated isotype control MAbs.
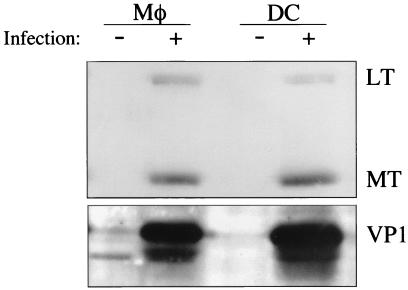
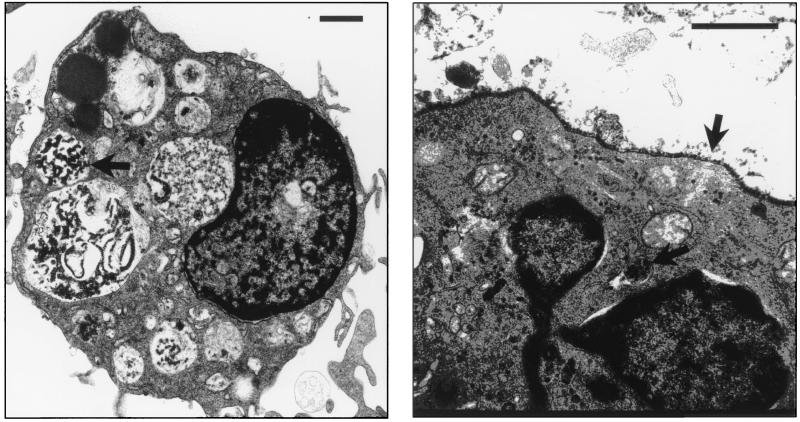
To examine whether Mφ and DC are permissive for polyomavirus infection, Mφ and FACS-sorted CD11c+ DC were infected in vitro and analyzed for expression of polyomavirus proteins by Western immunoblotting. By 24 h postinfection, both Mφ and DC expressed the early region nonstructural middle T (MT) and large T (LT) proteins and the major viral capsid protein, VP1 (Fig. 2), indicating productive virus infection. Virus infection of Mφ and DC did not alter levels of cell surface expression of MHC and costimulatory molecules (data not shown). Ultrastructural analysis of DC at 36 h postinfection revealed intact virions throughout the cytoplasm, as well as lining the cell surface (Fig. 3). This distribution of polyomavirus virions within smooth cytoplasmic vesicles and large cytoplasmic vacuoles, within cytoplasmic lamellar structures, together with separation of nuclear membranes and densely stained aggregates in enlarged nuclei, are features characteristic of productive infection by polyomavirus and simian virus 40 (6, 12, 42). In addition, by 48 h after infection, nearly all DC had undergone cytopathic effect, while the uninfected DC remained entirely viable (data not shown).
FIG. 2.
DC and Mφ are permissive for polyomavirus infection. Whole-cell protein lysates from uninfected and virus-infected (MOI = 3) FACS-sorted CD11c+ DC or thioglycolate-elicited, adherent macrophages were electrophoresed on a 12% reducing SDS-PAGE gel and immunoblotted by using the anti-T protein MAb F4 (top panel) or rabbit anti-VP1 antisera (bottom panel). U, uninfected; I, infected.
FIG. 3.
Ultrastructural analysis of polyomavirus-infected DC. FACS-sorted CD11c+ DC were infected with polyomavirus (MOI = 3) and prepared for electron microscopy at 36 h postinfection. The length of each inserted line represents 1 μm. Arrows point toward polyomavirus virions within cytoplasmic vacuoles (left panel) and packed along the cellular surface and within a cytoplasmic lamellar structure (right panel). Left panel magnification, ×6,500; right panel magnification, ×43,850.
Virus-infected DC and Mφ present the immunodominant CD8+ T-cell epitope.
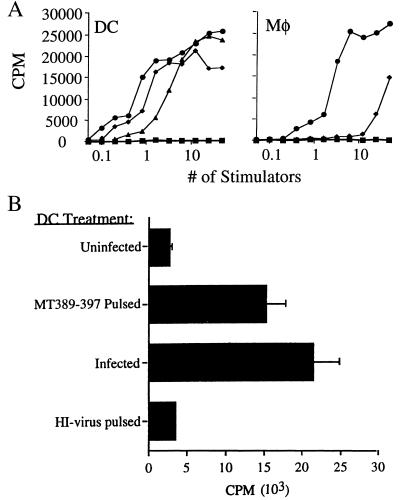
We next investigated the capacity of virus-infected DC and Mφ to present the immunodominant Dk-restricted epitope, MT389-397, to polyomavirus-specific T cells. Purified Mφ and FACS-sorted DC were infected in vitro with polyomavirus and assayed for their ability to stimulate IL-2 production by the MT389-397-specific T-cell hybridoma H8-1.18. Infected Mφ were found to be considerably less potent than MT389-397 peptide-pulsed Mφ on a cell-to-cell basis in their capacity to stimulate the H8.1-18 hybridoma; in contrast, infected and peptide-pulsed DC stimulated H8.1-18 to comparable levels (Fig. 4A). That nearly 10-fold higher numbers of peptide-pulsed Mφ than DC were needed to trigger half-maximal stimulation of H8-1.18 (Fig. 4A) correlates with the ∼1-log-lower Dk cell surface expression by Mφ (Fig. 1). These findings suggest that infected DC are more efficient than infected Mφ at presenting the MT389-397 epitope.
FIG. 4.
Virus-infected DC and Mφ process and present the immunodominant antipolyomavirus CTL epitope. (A) Uninfected, MT389-397 peptide-pulsed or virus-infected thioglycolate-elicited Mφ or FACS-sorted CD11c+ DC were cocultured with the MT389-397-specific T-cell hybridoma H8-1.18 for 24 h, and IL-2 levels measured by determining [3H]thymidine incorporation by CTLL cells. Splenic CD11c+ DC were also FACS sorted at day 2 postinfection and assayed for their capacity to stimulate H8-1.18. Stimulators: ■, uninfected; ⧫, in vitro infected; ●, MT389-397 pulsed; ▴, in vivo infected. (B) DC (5 × 104/well) pulsed with heat-inactivated (HI) virus, pulsed with MT389-397 peptide, infected by polyomavirus, or left untreated were cultured for 24 h with H8-1.18 cells, and IL-2 production was measured by CTLL bioassay.
We then examined whether splenic DC freshly isolated from acutely infected mice presented the MT389-397 epitope. DC were FACS sorted from the spleens of FL-treated C3H/HeN mice 2 days after polyomavirus infection and then tested for their capacity to stimulate H8-1.18. As shown in Fig. 4A (left panel), these ex vivo DC readily stimulated H8-1.18 and did so in a cell-dose-dependent profile only twofold lower than in vitro-infected DC. In addition, peritoneal Mφ harvested 2 days after i.p. polyomavirus inoculation stimulated H8-1.18 at similar cell doses as in vitro-infected Mφ (data not shown). Thus, splenic DC efficiently process and present the immunodominant antipolyomavirus CTL epitope in virus-infected H-2k mice.
A prominent feature of DC is their capacity to uptake and process extracellular proteins for loading onto newly synthesized class I MHC molecules (3). Although MT is a nonstructural protein, the viral preparations used here are crude lysates of polyomavirus-infected primary murine kidney epithelial cells; thus, the lysates potentially contain MT protein or peptide fragments that could be presented by DC through this alternative class I MHC processing pathway. Despite our inability to detect full-length or carboxy-truncated T proteins by Western immunoblotting using an MAb recognizing an epitope common to all three T proteins (43) (data not shown), we asked whether viral lysates rendered noninfectious by heat inactivation could sensitize DC for recognition by H8-1.18. As shown in Fig. 4B, DC exposed to heat-inactivated viral lysate under conditions identical to those of the infectious lysate failed to stimulate H8-1.18.
DC induce antigen-specific CTL in vivo.
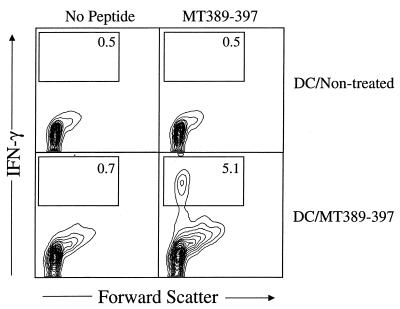
FL-expanded DC have been reported to be as efficient as DC from nontreated mice for eliciting primary antigen-specific CD4+ T-cell responses in vivo (40, 41). To determine whether DC isolated from FL-treated mice also induce functional CD8+ T cells in vivo, nontreated or MT389-397 peptide-pulsed DC were injected s.c., and 6 days later ex vivo splenic CD8+ T cells were assayed for antigen-specific activation. Figure 5 shows that >5% of the CD8+ T cells in the spleens of recipients of MT389-397 peptide-pulsed DC stain for intracellular IFN-γ after a 6-h in vitro stimulation with MT389-397 peptide; no IFN-γ production was detected in the absence of in vitro peptide stimulation. No MT389-397 stimulation of intracellular IFN-γ was detected by splenic CD8+ T cells from mice injected with untreated DC (Fig. 5). The increase in forward scatter by splenic CD8+ T cells in recipients of MT389-397 peptide-pulsed DC, but not those of untreated DC, may indicate that in vivo generation of mature, functional MT389-397-specific CD8+ T cells by peptide-pulsed DC nonspecifically triggers CD8+ T-cell blastogenesis (Fig. 5).
FIG. 5.
MT389-397 peptide-pulsed DC prime an antigen-specific CD8+ T-cell response in vivo. Untreated or MT389-397 peptide-pulsed DC were injected s.c. into naive mice. Six days later, spleen cells were stimulated with MT389-397 for 6 h and then stained for surface CD8 and intracellular IFN-γ. The plots are gated on CD8+ cells, and the values indicate the percentage of cells in the indicated regions. Both axes are log scale.
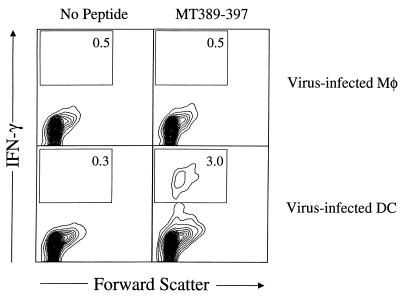
To determine whether virus-infected DC generate polyomavirus-specific CD8+ T cells in vivo, DC infected in vitro with polyomavirus were injected s.c. into naive syngeneic mice, and splenic CD8+ T cells were assayed 6 days later for MT389-397-specific activation. Figure 6 shows that 3% of splenic CD8+ T cells in recipients of virus-infected DC stained for intracellular IFN-γ after 6-h stimulation with MT389-397; no IFN-γ was produced in the absence of peptide or in the presence of control gag88-96 peptide (data not shown). Notably, MT389-397-specific CD8+ T cells were not detected in the spleens of recipients of polyomavirus-infected Mφ (Fig. 6).
FIG. 6.
Virus-infected DC, but not infected Mφ, prime antipolyomavirus CD8+ T cells in vivo. Uninfected or virus-infected DC and Mφ were injected s.c. in the hind footpads. Six days later, spleen cells were stimulated with MT389-397 for 6 h and then stained for surface CD8 and intracellular IFN-γ. The plots are gated on CD8+ cells, and the values indicate the percentage of cells in the indicated regions. Both axes are log scale.
FL treatment enhances the polyomavirus-specific CD8+ T-cell response to virus infection.
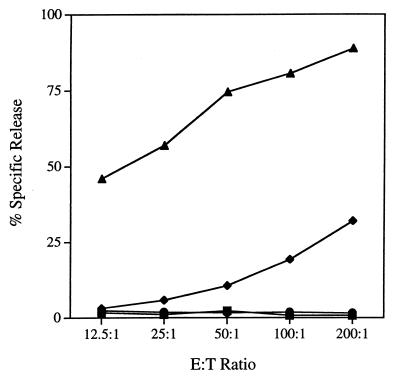
Having demonstrated that DC isolated from virus-infected, FL-treated mice present the immunodominant anti-polyomavirus CTL epitope (Fig. 4A, left panel), we sought to determine whether FL treatment altered the magnitude of the MT389-397-specific CD8+ T-cell response to polyomavirus infection. C3H/HeN mice received a 10-day course of i.p.-administered FL and were inoculated s.c. with polyomavirus on the eighth day of FL treatment. Our previous work established that MT389-397-specific cytotoxic activity was readily detected in freshly explanted spleens from acutely infected adult C3H/HeN mice and that peak antigen-specific cytotoxicity was achieved by days 7 to 9 postinfection (36). In contrast to MT389-397 peptide-coated syngeneic targets, we have consistently observed only low-level lysis of polyomavirus-infected syngeneic targets by MT389-397-specific CTL clones and lines and by spleens of C3H/HeN mice at days 7 to 8 postinfection (reference 37 and data not shown). This difference in target cell sensitivity to virus-specific CTL lysis, possibly due to inefficient processing and/or presentation of class I MHC epitopes by infected cells, has been described in other viral systems (54, 60). Therefore, to optimize sensitivity for detecting antigen-specific CTL responses, we compared ex vivo MT389-397-specific cytotoxic activity in the spleens of mock- and FL-treated C3H/HeN mice at day 8 after polyomavirus infection. As shown in Fig. 7, spleen cells from infected FL-treated mice exhibited a striking enhancement of cytotoxicity against syngeneic target cells pulsed with the MT389-397 peptide. No lysis of unpulsed targets or of targets coated with the gag88-96 peptide (data not shown) was exhibited by spleen cells from untreated and FL-treated infected mice. Although of considerably lower magnitude than for peptide-pulsed targets, an increase in ex vivo specific cytotoxicity against infected targets was also seen in FL-treated mice, and this increase was generally proportional to the FL-induced increase against MT389-397 peptide-pulsed targets (data not shown).
FIG. 7.
FL treatment enhances ex vivo MT389-397 epitope-specific cytolytic activity during acute polyomavirus infection. Nonadherent spleen cells from virus-infected, nontreated or FL-treated mice were assayed at day 8 postinfection for lysis of 51Cr-labeled virus-infected or MT389-397 peptide (10 μM)-pulsed AG104A target cells. Spleen cells from uninfected nontreated and FL-treated mice did not lyse MT389-397 peptide-pulsed targets (data not shown). Results with effectors from HBSS-injected mice with (as target) no peptide (■) or MT389-397 (⧫) and with effectors from FL-treated mice with (as target) no peptide (●) or MT389-397 (▴) are as indicated.
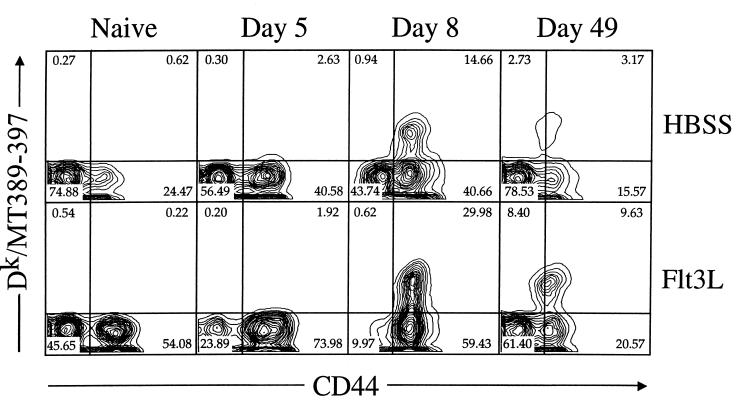
This marked increase in antigen-specific cytotoxicity suggested that FL treatment boosted the size of the MT389-397-specific CD8+ T-cell population in the spleens of acutely infected mice. To directly quantify polyomavirus-specific CD8+ T cells in vivo during infection, we constructed Dk tetramers containing the MT389-397 peptide (36). Figure 8 illustrates the rapid kinetics and large-scale expansion of Dk/MT389 tetramer+ CD8+ T cells in acutely infected C3H/HeN mice. Consistent with our previous results (36), by day 8 postinfection, when antigen-specific cytotoxicity is maximal, 15 to 20% of splenic CD8+ T cells in non-FL-treated mice stain with the Dk/MT389 tetramer (Fig. 8 and Table 1). All of the Dk/MT389 tetramer+ CD8+ T cells at day 8 postinfection exhibit upregulated expression of CD44, a finding indicative of prior TCR engagement of cognate MHC-peptide ligand. Polyomavirus infection of FL-treated mice elicited a major increase in the frequency of antigen-specific CD8+ T cells by day 8 postinfection, at which point roughly 30% of the splenic CD8+ T cells bound the Dk/MT389 tetramer, and all of these expressed the CD44high phenotype. Interestingly, by day 8 of infection nearly all splenic CD8+ T cells in FL-treated mice, regardless of their specificity, are CD44high and CD11ahigh (Fig. 8 and data not shown), while in non-FL-treated mice approximately 40% remain CD44low.
FIG. 8.
Visualization of polyomavirus-specific CD8+ T cells during virus infection in FL-treated mice. Spleen cells from naive or infected FL-treated or HBSS control mice at the indicated day postinfection were stained with PE-conjugated anti-CD8α, allophycocyanin-conjugated Dk/MT389 tetramers, and FITC-conjugated anti-CD44 and then analyzed by flow cytometry. Both axes are log scale. The plots shown are representative of three individual naive and polyomavirus-inoculated mice at each of the indicated postinfection time points.
TABLE 1.
Quantitation of MT389-397 epitope-specific CD8+ T cells during polyomavirus infection of nontreated and FL-treated micea
| Time postinfection | Dk/MT389 tetramer+ cells (mean ± SEM)
|
|
|---|---|---|
| Total (10−3) | % CD8+ T cells | |
| Nontreated | ||
| Naive | 25.1 | 0.89 |
| Day 5 | 433 ± 107 | 5.0 ± 0.4 |
| Day 8 | 1,672 ± 196 | 17.0 ± 1.6 |
| Day 49 | 423 ± 42 | 11.8 ± 0.3 |
| FL treated | ||
| Naive | 15.2 | 0.2 |
| Day 5 | 189 ± 36 | 1.8 ± 0.4 |
| Day 8 | 12,184 ± 733 | 28.9 ± 1.2 |
| Day 49 | 1,587 ± 287 | 14.8 ± 2.1 |
Spleen cells of naive or polyomavirus-inoculated, nontreated or FL-treated mice at the indicated days postinfection were stained with PE-conjugated anti-CD8α, FITC-conjugated anti-CD44, and allophycocyanin-conjugated Dk/MT389 tetramers and then analyzed by flow cytometry. Values for virus-infected mice at each time point postinfection represent the mean ± the SEM of the gated CD8+ population of three mice. Values from naive mice are from one uninfected nontreated or FL-treated mouse.
The impact of FL treatment on the extent of the antiviral CD8+ T-cell expansion is dramatized by the increase in the total number of MT389-397-specific CD8+ T cells in the spleens of infected mice. At day 8 postinfection, FL-treated mice possess nearly 10-fold more CD8+ T cells directed to the immunodominant epitope than nontreated mice (Table 1). Interestingly, at day 5 postinfection, nontreated and FL-treated mice possess low numbers of MT389-397-specific splenic CD8+ T cells. Although the numbers of antigen-specific CD8+ T cells at day 5 postinfection in FL-treated mice were approximately half that of the nontreated mice, this difference was not statistically significant (P < 0.05 by two-tailed Student t test). These findings suggest that FL treatment augments the magnitude but does not accelerate the onset of expansion of antipolyomavirus CD8+ T cells.
Because the size of the memory virus-specific CD8+ T cell pool is directly related to the clonal burst size of antiviral CD8+ T cells to acute infection (23, 29), the higher-magnitude polyomavirus-specific CD8+ T-cell response at day 8 postinfection in FL-treated mice (Fig. 8) would be predicted to generate a larger pool of antipolyomavirus memory CD8+ T cells than in non-FL-treated, infected mice. Comparison of MT389-397-specific memory CD8+ T-cell numbers in untreated and FL-treated mice confirms this expectation. At day 49 postinfection, when infectious virus is below detectable limits (reference 36 and Fig. 9), approximately fivefold-higher numbers of Dk/MT389 tetramer+ CD8+ T cells reside in the spleens of FL-treated mice than in nontreated mice, constituting roughly 15% of all and 30% of activated splenic CD8+ T cells (Fig. 8 and Table 1).
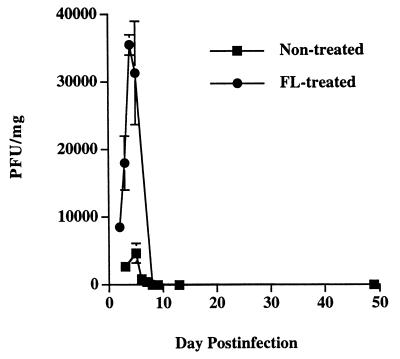
FIG. 9.
Comparison of splenic virus titers in FL-treated and untreated mice. Spleens from virus-infected mice at the indicated day postinfection were homogenized, and the titers of the infectious virus were determined by plaque assay. Each value represents the mean PFU/milligram of spleen ± the SEM of three mice.
We previously showed that emergence of polyoma-specific CD8+ T cells during acute infection correlates with elimination of infectious virus (36). To determine whether FL treatment alters the kinetics of virus clearance, we measured infectious virus in the spleens of C3H/HeN mice during primary polyomavirus infection. As shown in Fig. 9, splenic virus titers peaked at days 4 to 5 postinfection in both FL-treated and untreated mice but reached sixfold-higher levels in the FL-treated mice. Despite this increased viral load, mice of both groups cleared infectious virus at nearly equivalent rates, a phenomenon that may reflect the larger polyomavirus-specific CD8+ T-cell response in FL-treated mice.
DISCUSSION
In this report, we provide evidence that DC are susceptible to polyomavirus infection and that infected DC prime polyomavirus-specific CD8+ T cells in vivo. Because peptides bound to class I MHC molecules are generally derived from de novo-synthesized proteins (44), infection provides an efficient route for loading virus-derived peptides onto class I MHC molecules of DC, the APC pivotal for priming antiviral CD8+ T cells (33). DC may also utilize an alternative class I MHC processing pathway to process and present exogenous viral proteins to antiviral CTL (57). Extracellular viral proteins may be derived from infected cells undergoing virus-induced cytolysis or destroyed by NK cells or CTL (2, 28). The relative contribution of this cross-presentation to the classical endogenous pathway for providing class I MHC-restricted epitopes from viral proteins for display by DC in vivo remains to be defined. Although polyomavirus is known to infect a variety of epithelial and mesenchymal cells in vivo (13), this report provides the first evidence that DC are also susceptible to polyomavirus infection. These findings support the concept that processing of newly synthesized viral proteins by DC provides epitopes for generating polyomavirus-specific CD8+ T-cell responses and provides a plausible explanation for the dramatic expansion of polyomavirus-specific CD8+ T cells during acute infection (36).
Recent studies demonstrate that DC isolated from FL-treated mice share the same phenotypic and functional characteristics as DC from untreated mice. In parallel experiments using DC purified from the spleens of mice that received or did not receive FL in vivo, Maldonado-Lopez et al. (40) showed that both DC isolates expressed equivalent surface levels of class II MHC and costimulatory molecules and, when pulsed in vitro with keyhole limpet hemocyanin (KLH) and injected s.c., induced comparable KLH-specific T helper cell responses. In this and other studies (41, 48, 49), the ability of DC from FL-treated mice to efficiently take up soluble proteins for processing and presentation to class II MHC-restricted T cells and to possess phagocytotic activity indicates that these DC are not fully mature (3). This conclusion is further supported by evidence that DC purified from FL-treated mice home to draining lymph nodes (D. R. Drake, unpublished observations). In addition, FL-derived DC do not spontaneously secrete IL-12 but do so upon activation by Staphylococcus aureus Cowan + IFN-γ + GM-CSF (48) or by cross-linking surface CD40 molecules (D. R. Drake, unpublished observations). Thus, these studies justify the use of FL to increase the availability of this rare APC population and facilitate characterization of its in vivo function.
Although Mφ were also found to be permissive for polyomavirus infection, unlike infected DC, they were unable to generate primary antiviral CD8+ T-cell responses. Infected DC also displayed considerably higher efficiency than Mφ in presenting the immunodominant MT389-397 epitope to CD8+ T cells. Detection of MT389-397 epitope-bearing DC in the spleen within 2 days after s.c. virus inoculation further suggests that infected DC may migrate from the periphery to this secondary lymphoid organ. This possibility is supported by the presence of MT389-397-specific CD8+ T cells in the spleens of mice s.c. injected with polyomavirus-infected or MT389-397 peptide-pulsed DC. Taken together, these results indicate that infected DC rather than infected macrophages prime polyomavirus-specific CD8+ T-cell responses.
Recent evidence suggests that signaling through its surface CD40 receptor by interaction with CD40L on antigen-specific CD4+ T cells “licenses” DC cells to prime antigen-specific CD8+ T cells (4, 50, 53). DC infected by influenza virus have been shown to induce antigen-specific CD8+ T cells independent of CD40 cross-linking (50). Direct activation of DC by infection likely applies to other viruses known to infect DC, such as lymphocytic choriomeningitis virus (9), where induction of antiviral CD8+ CTL during acute infection is unimpaired in CD4+ T-cell-deficient mice (1). Polyomavirus infection of DC may similarly license these APC to prime polyomavirus-specific CD8+ T cells. Preliminary evidence suggests that CD4+ T-cell depletion does not affect the induction of functional antiviral CD8+ T cells during acute polyomavirus infection (J. Moser, unpublished observations). In addition, infected DC mediate the induction of polyomavirus-specific CTL from naive precursors in vitro, but MT389-397 peptide-pulsed DC do so only after CD40 cross-linking (data not shown).
A number of factors may be responsible for the massive expansion of MT389-397-specific CD8+ CTL in FL-treated mice. A straightforward explanation is that FL-induced expansion of DC (41, 56) enlarges the pool of professional APC available to prime antiviral CD8+ T-cell precursors for differentiation into CTL effectors. Although infected DC may directly present class I MHC-restricted polyomavirus epitopes, uninfected DC which have taken up exogenous viral proteins for class I MHC presentation may also contribute to the induction of polyomavirus-specific CD8+ T cells. Since polyomavirus is a cytopathic virus, such cross-presentation conceivably may represent a mechanism used by DC to prolong the availability of class I MHC-restricted epitopes for priming polyomavirus-specific CD8+ T-cell responses. Moreover, because polyomavirus infects a variety of cells of epithelial and mesenchymal lineages (13), these infected cells may further drive expansion of polyomavirus-specific CD8+ CTL. The increased viral load in the spleens of FL-treated mice may originate from the expansion of splenic DC that become targets for polyomavirus infection but, because FL also expands splenic myeloid cell numbers (38), it is possible that infected macrophages may contribute to the high viral burden as well. Additional evidence that FL-driven expansion of DC is responsible for the elevated splenic virus levels is that polyomavirus titers were approximately 10-fold higher in the spleens and livers of FL-treated mice, a magnitude increase that closely correlates with the FL expansion of DC in these organs (41); a minimal difference was seen between FL-treated and untreated mice in the virus titers in the kidney (data not shown), an organ with few bone-marrow-derived cells. FL administration in vivo and adoptive transfer of FL-expanded DC have also been shown to augment the number and activity of NK cells (45, 55), an effect that may be attributable to direct contact between NK cells and DC (17). In addition to their role in providing early host resistance against some viral infections (58), NK cells may facilitate expansion of virus-specific CD8+ T cells (7, 26). The increased viral load in the spleens of FL-treated mice at days 3 to 4 postinfection, a time preceding emergence of polyomavirus-specific CD8+ T cells, may indicate little direct involvement by NK cells in eliminating infected cells. In preliminary studies, we find that NK cytotoxic activity is induced early during acute polyomavirus infection; work in progress is directed toward determining whether and, if so, by what mechanism(s) NK cells contribute to polyomavirus clearance.
Several lines of evidence also suggest that FL bolsters bystander CD8+ T-cell activation. More than half of the splenic CD8+ T cells in naive FL recipients upregulated expression of the activation markers CD44 and CD11a, a phenotype expressed by only a quarter of naive nontreated mice. At the peak expansion of MT389-397-specific CD8+ T cells during acute polyomavirus infection, just over half of the CD8+ T cells in nontreated mice expressed the activated phenotype, but nearly 90% of those in the FL-treated mice were CD44high. Despite this marked shift toward CD44high CD8+ T cells in FL-treated mice by day 8 postinfection, the proportion of CD44high CD8+ T cells that bind to those that do not bind the Dk/MT389 tetramer is roughly the same as for untreated mice (Fig. 8). These findings suggest that the inflammatory response to polyomavirus infection markedly accentuates the nonspecific CD8+ T-cell activation induced by FL administration. The increased size of splenic CD8+ T cells in recipients of MT389-397 peptide-pulsed, but not unpulsed DC, also raises the possibility that MT389-397-specific CD8+ T cells may themselves contribute to bystander CD8+ T-cell activation.
In view of the comparable rates of viral clearance in FL-treated and untreated mice, the larger antipolyomavirus CD8+ T-cell response in mice given FL appears to reflect the host response to control the higher infection level. The increased viral load in FL-treated mice may also raise questions about the potential therapeutic use of this cytokine for augmenting antigen-specific T-cell responses during an active virus infection, particularly by viruses that are capable of productively infecting DC. The enhanced MT389-397-specific CD8+ T-cell burst size in FL-treated mice during acute infection is associated with an enlarged pool of memory antipolyomavirus CD8+ T cells. Since polyomavirus DNA and transcripts persist in tumor-resistant mice (16), continuous monitoring for and elimination of transformed cells by antipolyomavirus CTL is likely essential to prevent tumors. The numerical increase in memory polyomavirus-specific CD8+ T cells in FL-treated mice should provide improved surveillance against polyomavirus-induced neoplasia.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants CA71971 (to A.E.L.) and AI42373 (to J.D.A.).
We thank Robert Karaffa for his expertise in flow cytometry and Robert Santoianni for electron microscopy.
REFERENCES
- 1.Ahmed R, Butler L D, Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988;62:2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Bennett S R M, Carbone F R, Karamalis F, Flavell R A, Miller J F A P, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 5.Berke Z, Palmer S, Bergman T, Wester D, Svedmyr J, Linder S, Jornvall H, Dalianis T. A short peptide eluted from the H-2Kb molecule of a polyomavirus-positive tumor corresponds to polyomavirus large T antigen peptide at amino acids 578 to 585 and induces polyomavirus-specific immunity. J Virol. 1996;70:3093–3097. doi: 10.1128/jvi.70.5.3093-3097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biczysko W, Solter D, Pienkowski M, Koprowski H. Interaction of early mouse embryos with oncogenic viruses-simian virus 40 and polyoma. Ultrastructural studies. J Natl Cancer Inst. 1973;51:1945–1954. doi: 10.1093/jnci/51.6.1945. [DOI] [PubMed] [Google Scholar]
- 7.Biron C A. Role of early cytokines, including alpha and beta interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 8.Borges L, Miller R E, Jones J, Ariail K, Whitmore J, Fanslow W, Lynch D H. Synergistic action of fms-like tyrosine kinase 3 ligand and CD40 ligand in the induction of dendritic cells and generation of antitumor immunity in vivo. J Immunol. 1999;163:1289–1297. [PubMed] [Google Scholar]
- 9.Borrow P, Evans C F, Oldstone M B A. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected cells results in generalized immunosuppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell B A, Villarreal L P. Lymphoid and other tissue-specific phenotypes of polyomavirus enhancer recombinants: positive and negative combinational effects on enhancer specificity and activity. Mol Cell Biol. 1986;6:2068–2079. doi: 10.1128/mcb.6.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 12.Clayson E T, Jones Brando L V, Compans R W. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J Virol. 1989;63:2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawe C J, Freund R, Mandel G, Ballmer-Hofer K, Talmage D A, Benjamin T L. Variations in polyoma virus genotype in relation to tumor induction in mice: characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987;127:243–261. [PMC free article] [PubMed] [Google Scholar]
- 14.de Bergeyck V, De Plaen E, Chomez P, Boon T, Van Pel A. An intracisternal A-particle sequence codes for an antigen recognized by syngeneic cytolytic T lymphocytes on a mouse spontaneous leukemia. Eur J Immunol. 1994;24:2203–2212. doi: 10.1002/eji.1830240941. [DOI] [PubMed] [Google Scholar]
- 15.Doherty P C, Allan W, Eichelberger M, Carding S R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 16.Drake D R, III, Lukacher A E. β2-microglobulin knockout mice are highly susceptible to polyoma virus tumorigenesis. Virology. 1998;252:275–284. doi: 10.1006/viro.1998.9455. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez N C, Lozier A, Flament C, Ricciardi-Castognoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cell directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs E J, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 19.Gagliardi M C, De Petrillo G, Salemi S, Boffa L, Longobardi M G, Dellabona P, Casorati G, Tanigaki N, Harris R, Lanzavecchia A. Presentation of peptides by cultured monocytes or activated T cells allows specific priming of human cytotoxic T lymphocytes in vitro. Int Immunol. 1995;7:1741–1752. doi: 10.1093/intimm/7.11.1741. [DOI] [PubMed] [Google Scholar]
- 20.Gong J L, McCarthy K M, Rogers R A, Schneeberger E E. Interstitial lung macrophages interact with dendritic cells to present antigenic peptides derived from particulates. Immunology. 1994;81:343–351. [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg P D. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumors. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 22.Gu J J, Gottlieb P D. Inducible functions in hybrids of a Lyt-2+ BW5147 transfectant and the 2C CTL line. Immunogenetics. 1992;36:283–293. doi: 10.1007/BF00215656. [DOI] [PubMed] [Google Scholar]
- 23.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 24.June C H, Bluestone J A, Nadler L M, Thompson C B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 25.Knight S C, Stagg A J. Antigen-presenting cell types. Curr Opin Immunol. 1993;5:374–382. doi: 10.1016/0952-7915(93)90056-x. [DOI] [PubMed] [Google Scholar]
- 26.Kos F J, Engelman E G. Immune regulation: a critical link between NK cells and CTLs. Immunol Today. 1996;17:174–176. doi: 10.1016/0167-5699(96)80616-5. [DOI] [PubMed] [Google Scholar]
- 27.Kruisbeek A M. Production of mouse T cell hybridomas. In: Coligan J, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 3.14.1–3.14.11. [DOI] [PubMed] [Google Scholar]
- 28.Kurts C, Miller J F, Subramaniam R M, Carbone F R, Heath W R. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 30.Liu Y, Linsley P S. Costimulation of T-cell growth. Curr Opin Immunol. 1992;4:265–270. doi: 10.1016/0952-7915(92)90075-p. [DOI] [PubMed] [Google Scholar]
- 31.Ljunggren G, Ljunggren H-G, Dalianis T. T cell subsets involved in immunity against polyoma virus-induced tumors. Virology. 1994;198:714–716. doi: 10.1006/viro.1994.1084. [DOI] [PubMed] [Google Scholar]
- 32.Lu L, Hsieh M, Oriss T B, Morel P A, Starzl T E, Rao A S, Thomson A W. Generation of DC from mouse spleen cell cultures in response to GM-CSF: immunophenotypic and functional analyses. Immunology. 1995;84:127–134. [PMC free article] [PubMed] [Google Scholar]
- 33.Ludewig B, Oehen S, Barchiesi F, Schwendener R A, Hengartner H, Zinkernagel R M. Protective antiviral cytotoxic T cell memory is most efficiently maintained by restimulation via dendritic cells. J Immunol. 1999;163:1839–1844. [PubMed] [Google Scholar]
- 34.Lukacher A E, Freund R, Carroll J P, Bronson R T, Benjamin T L. PyvS: a dominantly acting gene in C3H/BiDa mice conferring susceptibility to tumor induction by polyoma virus. Virology. 1993;196:241–248. doi: 10.1006/viro.1993.1472. [DOI] [PubMed] [Google Scholar]
- 35.Lukacher A E, Ma Y, Carroll J P, Abromson-Leeman S R, Laning J C, Dorf M E, Benjamin T L. Susceptibility to tumors induced by polyoma virus is conferred by an endogenous mouse mammary tumor virus superantigen. J Exp Med. 1995;181:1683–1692. doi: 10.1084/jem.181.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukacher A E, Moser J M, Hadley A, Altman J D. Visualization of polyomavirus-specific CD8+ T cells in vivo during infection and tumor rejection. J Immunol. 1999;163:3369–3378. [PubMed] [Google Scholar]
- 37.Lukacher A E, Wilson C S. Resistance to polyoma virus-induced tumors correlates with CTL recognition of an immunodominant H-2Dk-restricted epitope in the middle T protein. J Immunol. 1998;160:1724–1734. [PubMed] [Google Scholar]
- 38.Lyman S D. Biology of Flt3 ligand and receptor. Int J Hematol. 1996;62:63–73. doi: 10.1016/0925-5710(95)00389-a. [DOI] [PubMed] [Google Scholar]
- 39.Lynch D H, Andreasen A, Maraskovsky E, Whitmore J, Miller R E, Schuh J C. Flt3-ligand induces tumor regression and antitumor immune responses in vivo. Nat Med. 1997;3:625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 40.Maldonado-Lopez R, De Smedt T, Pajak B, Heirman C, Thielemans K, Oberdan L, Urbain J, Maliszewski C R, Moser M. Role of CD8α+ and CD8α− dendritic cells in the induction of primary immune responses in vivo. J Leukoc Biol. 1999;66:242–246. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 41.Maraskovsky E, Brasel K, Teepe M, Roux E R, Lyman S D, Shortman K, McKenna H J. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattern C F T, Takemoto K K, Daniel W A. Replication of polyoma virus in mouse embryo cells: electron microscopic observations. Virology. 1966;30:242–256. doi: 10.1016/0042-6822(66)90099-7. [DOI] [PubMed] [Google Scholar]
- 43.Pallas D C, Schley C, Mahoney M, Harlow E, Schaffhausen B S, Roberts T M. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986;60:1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 45.Peron J-M, Esche C, Subbotin V M, Maliszewski C, Lotze M T, Shurin M R. Flt3-ligand administration inhibits liver metastases: role of NK cells. J Immunol. 1998;161:6164–6170. [PubMed] [Google Scholar]
- 46.Pfeifer J D, Wick M J, Roberts R L, Findley K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 47.Piatti P G, Gottlieb K A, Taylor J A, Villarreal L P. Approaches to study interactions between small DNA viruses and differentiated tissue. Methods. 1998;16:62–82. doi: 10.1006/meth.1998.0645. [DOI] [PubMed] [Google Scholar]
- 48.Pulendran B, Lingappa J, Kennedy M K, Smith J, Teepe M, Rudensky A, Maliszewski C R, Maraskovsky E. Developmental pathways of dendritic cells in vivo. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- 49.Pulendran B, Smith J L, Jenkins M, Schoenborn M, Maraskovsky E, Maliszewski C R. Prevention of peripheral tolerance by a dendritic cell growth factor: Flt3 ligand as an adjuvant. J Exp Med. 1998;188:2075–2082. doi: 10.1084/jem.188.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 51.Ridge J P, Fuchs E J, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 52.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoenberger S P, Toes R E M, van der Voort E I H, Offringa R, Melief C J M. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 54.Selin L K, Welsh R M. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J Immunol. 1997;158:5366–5373. [PubMed] [Google Scholar]
- 55.Shaw S G, Maung A A, Steptoe R J, Thomson A W, Vanjanovic N L. Expansion of functional NK cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J Immunol. 1998;161:2817–2824. [PubMed] [Google Scholar]
- 56.Shurin M R, Pandharipande P P, Zorina T D, Haluszezak C, Subbotin V, Hunter O, Brumfield A, Storkus W J, Maraskovsky E, Lotze M T. FLT3-ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174–184. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- 57.Sigal L J, Crotty S, Andino R, Rock K L. Cytotoxic T-cell immunity to virus-infected non-hematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:26–27. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 58.Tay C H, Szomolanyi-Tsuda E, Welsh R M. Control of infections by NK cells. Curr Top Microbiol Immunol. 1998;230:193–220. doi: 10.1007/978-3-642-46859-9_12. [DOI] [PubMed] [Google Scholar]
- 59.Toujas L, Delcros J G, Diez E, Gervois N, Semana G, Corradin G, Jotereau F. Human monocyte-derived macrophages and dendritic cells are comparably effective in vitro in presenting HLA class I-restricted exogenous peptides. Immunology. 1997;91:635–642. doi: 10.1046/j.1365-2567.1997.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Most R G, Sette A, Oseroff C, Alexander J, Marali-Krishna K, Lau L L, Southjwood S, Sidney J, Chesnut R W, Matloubian M, Ahmed R. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- 61.Ward P L, Koeppen H, Hurteau T, Schreiber H. Tumor antigens defined by cloned immunological probes are highly polymorphic and are not detected on autologous normal cells. J Exp Med. 1989;170:217–225. doi: 10.1084/jem.170.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver C T, Unanue E R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990;11:49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- 63.Yi X, Peterson J, Freund R. Transformation and tumorigenic properties of a mutant polyomavirus containing a middle T antigen defective in Shc binding. J Virol. 1997;71:6279–6286. doi: 10.1128/jvi.71.9.6279-6286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zarling A L, Johnson J G, Hoffman R W, Lee D R. Induction of primary human CD8+ T lymphocyte responses in vitro using dendritic cells. J Immunol. 1999;162:5197–5204. [PubMed] [Google Scholar]