Abstract
Immunoglobulin A (IgA) is the primary immune response induced in the intestine by rotavirus infection, but vaccination with virus-like particles induces predominantly IgG, not IgA. To definitively assess the role of IgA in protection from rotavirus infection, IgA knockout mice, which are devoid of serum and secretory IgA, were infected and then rechallenged with murine rotavirus at either 6 weeks or 10 months. Following primary rotavirus infection, IgA knockout mice cleared virus as effectively as IgA normal control mice. Rotavirus-infected IgA knockout mice produced no serum or fecal IgA but did have high levels of antirotavirus serum IgG and IgM and fecal IgG, whereas IgA normal control mice made both serum IgA and IgG and fecal IgA. Both IgA normal and IgA knockout mice were totally protected from rotavirus challenge at 42 days. Ten months following a primary infection, both IgA normal and knockout mice still had high levels of serum and fecal antirotavirus antibody and were totally protected from rotavirus challenge. To determine if compensatory mechanisms other than IgG were responsible for protection from rotavirus infection in IgA knockout mice, mice were depleted of CD4+ T cells or CD8+ T cells. No changes in the level of protection were seen in depleted mice. These data show that fecal or systemic IgA is not essential for protection from rotavirus infection and suggest that in the absence of IgA, IgG may play a significant role in protection from mucosal pathogens.
Rotaviruses are the leading cause of severe gastroenteritis in young children worldwide, making rotavirus a good model for understanding mucosal immunity for enteric viral infections. Although much effort has focused on determining the immune correlate(s) of protection from rotavirus infection, it remains to be determined whether (i) immunoglobulin A (IgA) is absolutely necessary for protection, (ii) in the absence of IgA, other immunologic mediators can provide protection, (iii) a specific IgG subclass can mediate protection in the absence of IgA, and (iv) protection is mediated through a polarized TH1 or TH2 immune response.
Immune knockout mice have been used to dissect correlates of protective immune responses to rotavirus. When β2-microglobulin knockout mice, which are deficient in major histocompatibility complex class I expression and therefore lack CD8+ T cells, were infected with rotavirus, they shed virus slightly longer than normal mice but were immune to reinfection, suggesting that CD8+ T cells play a role in clearance of primary rotavirus infection but are not necessary to achieve protection from a second rotavirus infection (9, 10). When JHD or μMT antibody knockout mice were infected with rotavirus, they cleared a primary infection but were susceptible to reinfection, indicating that antibody is not needed for clearance but is critical in long-term protection (9, 16, 17). Further analyses showed that JHD mice are almost completely protected from rotavirus challenge up to 3 weeks following primary infection and are partially protected 6 weeks following primary infection (10). Following primary infection with rotavirus, mice and rabbits show long-term antibody production and are completely protected from rotavirus challenge for at least 2 years following primary infection. Taken together, these results for small-animal models suggest that antibody is dispensable in virus clearance but is of primary importance in long-term protection from secondary rotavirus infection.
Because rotavirus infections generally remain localized to the villus epithelial cells of the small intestine, it was assumed that IgA is the most important immunoglobulin isotype in protection from challenge. Supporting this idea, titers of serum and intestinal IgA have been shown to correlate with protection from rotavirus challenge after oral infection with virus in children (6, 13, 22) and in mice (8). However, in most of these studies, IgA was the only fecal immunoglobulin tested. In piglets, oral infection with a human rotavirus was shown to induce both IgA and IgG antibody-secreting cells (ASC) in the intestine, but protection from challenge correlated only with the number of intestinal IgA ASC as detected by enzyme-linked immunospot assay (27). However, protection does not always correlate with IgA. Parenteral immunization with nonreplicating inactivated rotavirus and virus-like particles (VLPs) induces high levels of fecal IgG in rabbits but no detectable fecal IgA, and the rabbits are protected from challenge (3, 5). Following intranasal administration of VLPs to mice, protection correlates with serum antibody (P < 0.001) and fecal IgG (P < 0.001) but does not correlate with fecal IgA (P = 0.575) (21). Therefore, whether IgA is necessary for protection from rotavirus infection is uncertain.
IgA knockout mice which have a deletion of the entire IgA switch region, as well as the 5′ half of the constant region, can serve as a system to test whether IgA is required for protection from rotavirus infection. These mice have no detectable IgA in their serum or in any secretions, nor do they have detectable IgA ASC cells (11). Higher levels of IgG and IgM are seen in serum and gastrointestinal secretions of IgA knockout mice compared to IgA normal mice, but levels of CD4+ and CD8+ T cells are the same.
IgA knockout mice show normal clearance of a primary infection with influenza virus as well as equivalent levels of protection to secondary infection with influenza virus compared to IgA normal mice (15). Similar results have also been seen with vaginal herpes simplex virus type 2 (HSV-2) and gastric Helicobacter pylori infections (1, 23). In the present study, IgA knockout mice were used to determine if IgA is absolutely necessary for protection from localized rotavirus infection of the gastrointestinal mucosa.
MATERIALS AND METHODS
Virus.
Wild-type murine rotavirus ECwt (P[16], G3) was obtained from Harry Greenberg (Stanford University Medical School, Palo Alto, Calif.). A stock of ECwt was prepared in suckling mice, and the titer of ECwt in adult mice was determined to be 4 × 107 50% shedding doses (SD50)/ml (21). Plaque-purified SA11 clone 3 (P[2], G3) rotavirus was cultivated in fetal rhesus monkey kidney (MA104) cells in the presence of trypsin as previously described (3, 5) and used for enzyme-linked immunosorbent assay (ELISA).
Animals.
All experiments were performed in genetically matched IgA normal and IgA knockout mice (C57BL/6 × 129 background). Mice were 1 month old at the time of primary infection. IgA knockout mice were constructed and characterized to lack IgA (11). Briefly, targeted deletions of the IgA switch and constant regions were made in mouse strain 129 embryonic stem cells using homologous recombination. The embryonic stem cells were introduced into C57BL/6 blastocysts and inserted into foster mothers. Heterozygous offspring were bred to obtain homozygous mice lacking the IgH IgA locus.
Mice were confirmed to be rotavirus antibody free and, in the case of IgA knockout mice, IgA free by ELISA prior to infection. All mice were housed in microisolator cages throughout the experiments.
Virulent murine rotavirus challenge.
Ten IgA knockout mice and ten IgA normal mice were intragastrically inoculated with 105 SD50 of ECwt murine virus on 0 day postinoculation (dpi) and with the same dose of virus at either 42 dpi (five mice per group) or 10 months postinfection (five mice per group). Following both primary infection and challenge, fecal samples from individual mice were collected daily for 11 days, starting on the day of inoculation. Fecal samples were processed, stored, and tested for the presence of rotaviral antigen in fecal samples by ELISA as previously described (21).
Sample collection and processing.
Serum and fecal antibody samples were collected from each mouse 0, 42, and 56 dpi. Mice that were not challenged until 10 months following the primary infection also had serum and fecal samples collected 10 months following the primary infection. Serum was collected by tail bleed and stored at −20°C until use. Fecal samples were processed and stored as previously described (21).
ELISAs. (i) To measure rotavirus excretion.
The level of viral antigen in fecal samples was measured by ELISA as previously described (21). Fecal viral shedding was expressed as the net optical density (OD) value (OD from the fecal sample minus the background OD from wells containing fecal samples collected on the day of challenge). To compare the magnitude and duration of virus shedding between groups, viral shedding curves of each animal were plotted, and the mean areas under the curves for each group were calculated and compared statistically. Percent reduction in shedding was calculated for each animal by comparing the area under the curve for each individual animal to the mean of the areas under the curves of the control group. Percent reduction in shedding was then calculated for each depletion group by determining the mean percent reduction of each test group compared to the control group (1 − mean area of test group/mean area of control group).
(ii) To measure total IgA, IgG, or IgM in serum and fecal samples.
All antibodies were tested for specificity prior to use and found to react with only the specified isotype at the dilutions used. Ninety-six-well polyvinyl chloride microtiter plates (Dynatech, McLean, Va.) were coated by diluting the appropriate antibody in carbonate-bicarbonate buffer (pH 9.6) and incubating the plates overnight at room temperature. Coating antibodies used were goat anti-mouse IgA (Sigma, St. Louis, Mo.), goat anti-mouse IgG (Sigma), or goat anti-mouse IgM (Sigma). Plates were blocked with 200 μl of 5% BLOTTO (5% [wt/vol] Carnation powdered milk in phosphate-buffered saline [PBS]) for 2 h at 37°C. Following each step after the block, plates were washed three times with 0.05% Tween 20 in PBS, using an Ultrawasher Plus plate washer (Dynatech). Serum or fecal samples were added to the plates in duplicate and diluted twofold. Purified mouse IgA (Sigma), mouse IgG (Southern Biotechnology Associates, Birmingham, Ala.), and mouse IgM (Sigma) were also added in duplicate and serially diluted twofold on each plate. One of the following conjugates was then added to the plates: goat anti-mouse IgA-horseradish peroxidase (HRP) (Sigma), goat anti-mouse IgG-HRP (Southern Biotechnology), or goat anti-mouse IgM-HRP (Sigma). The substrate used in all assays was TMB Microwell ELISA substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). The substrate was allowed to react for 10 min at room temperature, and the reaction was stopped by addition of an equivalent volume of 1 M H3PO4. ODs were determined at 450 nm with a Flow Titertech Multiscan Plus plate reader (Flow Laboratories, Inc., McLean, Va.).
Standard curves were plotted using the results from the purified immunoglobulins, and the concentration of immunoglobulin isotypes in a given sample was then calculated using the standard curve. For every assay, the standard curve had an r value greater than or equal to 0.95, and the OD of the antibody dilution of the sample used to determine the antibody concentration was in the linear range of the standard curve. Concentrations of each immunoglobulin isotype were determined by comparison of samples from IgA-normal and IgA-deficient mice to standard curves of purified mouse IgM (Sigma), IgG (Southern Biotechnology), or IgA (Sigma).
ELISAs to measure IgA, IgG, and IgM serum and fecal antirotavirus antibody.
IgA, IgG, and IgM antirotavirus antibodies in serum and fecal samples were measured as previously described (21), with the modification that three different conjugates (goat-anti mouse IgA-HRP [Sigma], goat anti-mouse IgG-HRP [Southern Biotechnology], and goat anti-mouse IgM-HRP [Sigma]) were used depending on the isotype of antibody being measured. Antibody titers were defined as the reciprocal of the highest dilution giving a net OD value (OD with SA11 minus OD with 0.5% BLOTTO) greater than or equal to 0.1.
In vivo depletion of CD4+ T cells or CD8+ T cells.
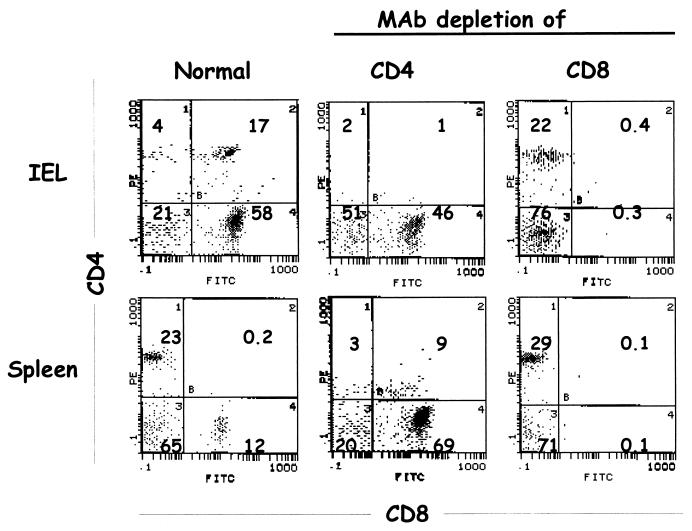
Mice were depleted of CD8+ or CD4+ T cells by administration of ascites fluid containing either rat anti-mouse CD8 monoclonal antibody (MAb) 2.43 or rat anti-mouse CD4 MAb GK 1.5, obtained from the American Type Culture Collection, as previously described (9). Each mouse received 0.5 ml of ascites fluid intraperitoneally 5, 4, and 3 days prior to rotavirus infection, on the day of rotavirus infection, and on days 3, 6, and 9 after infection. On the day of rotavirus infection, two depleted and nondepleted IgA normal and knockout control mice were killed to verify depletion in spleen and among intraepithelial lymphocytes (IEL) by flow cytometry (9, 10). Depletion of CD8+ T cells was verified using fluorescein isothiocyanate (FITC)-conjugated anti-CD8+ MAb clone 53-6.7 (Pharmingen, San Diego, Calif.); depletion of CD4+ T cells was verified using R-phycoerythrin (PE)-conjugated anti-CD4+ MAb clone RM4-4 (Pharmingen).
Statistical analysis.
Statistical analyses were performed using SPSS version 7.0 for Windows (SPSS, Inc., Chicago, Ill.). Antibody titers between groups were compared by the Kruskal-Wallis test followed by the Mann-Whitney U test.
RESULTS
IgA knockout mice have no IgA.
To verify the IgA-negative status of the IgA knockout mice in our experiments, total non-antigen-specific IgA, IgG, and IgM levels were determined in serum and fecal samples from IgA normal and knockout mice that were not included in the rotavirus infection experiments (data not shown). While IgA was readily detected in the serum from IgA normal mice, no IgA was detected in the serum of IgA knockout mice. Serum and fecal samples from IgA knockout mice had elevated concentrations of IgG and IgM compared to IgA normal mice. Fecal IgG antibody was elevated in IgA knockout mice. IgA was readily detected in fecal samples from IgA normal mice but not in the feces of IgA knockout mice. These results confirm that although IgA knockout mice have no IgA in the serum or intestine, they do have compensatory changes in other antibody isotypes as reported previously (11).
IgA knockout mice clear primary rotavirus infection.
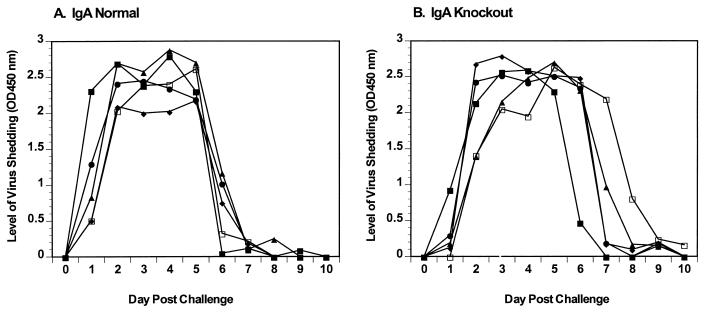
Analysis of virus clearance following primary rotavirus infection was performed in two separate experiments (five mice per group); results of a representative experiment are shown (Fig. 1). IgA normal and knockout mice were inoculated with 105 SD50 of ECwt, and clearance of virus antigen monitored in fecal samples (Fig. 1). IgA normal and knockout mice shed rotavirus for 5 to 7 days (means = 6.2 and 6.3 days, respectively), and all cleared the infection by 10 dpi (Fig. 1). Although clearance of rotavirus infection in IgA-deficient mice appears to be slightly delayed, there was no significant difference in the mean duration of virus shedding calculated by comparing the number of days of virus shedding in individual animals. Because no significant difference was seen in the mean duration of virus shedding, onset of virus shedding, or the timing of clearance between IgA normal and knockout mice (P > 0.05), IgA is not required for viral clearance.
FIG. 1.
Rotavirus shedding curves following primary infection with ECwt. IgA normal (A) and knockout (B) mice were orally infected with 105 SD50 of ECwt. Fecal samples were collected for 11 days, and levels of virus antigen shedding were determined by ELISA. The y axis represents the level of virus shed as determined by ELISA.
IgA knockout mice develop serum and fecal antibody.
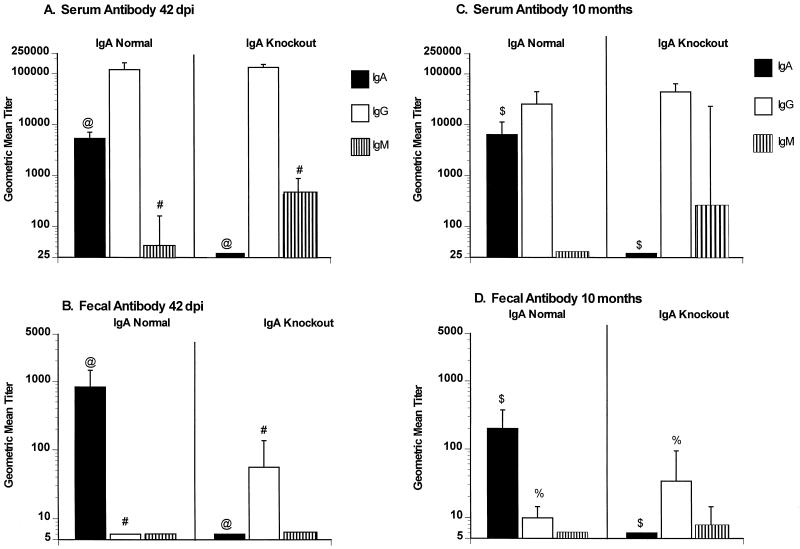
Antirotavirus IgA, IgG, and IgM were determined in serum and fecal samples 42 dpi (Fig. 2). IgA normal mice had high levels of antirotavirus serum IgA and IgG but almost no IgM following primary infection with rotavirus (Fig. 2A). IgA knockout mice had no detectable antirotavirus serum IgA but had high levels of serum IgG and IgM (Fig. 2A). As is typically observed following rotavirus infection, IgA normal mice had high levels of antirotavirus fecal IgA but no detectable fecal IgG or IgM (Fig. 2B). IgA knockout mice had high levels of antirotavirus fecal IgG but no detectable fecal IgA or IgM (Fig. 2B). Serum and fecal neutralizing antibody responses were also determined 42 dpi (data not shown). The geometric mean titers (GMT) of serum neutralizing antibody (all isotypes) in IgA normal and knockout mice were identical (1,600). IgA normal mice had slightly higher fecal neutralizing antibody (GMT = 16) than IgA knockout mice (GMT = 4), but the difference was not significant (P > 0.05).
FIG. 2.
Serum and fecal rotavirus-specific antibody responses in IgA normal and knockout mice at 42 dpi and 10 months following primary infection with 105 SD50 of ECwt. Serum (A and C) and fecal (B and D) antirotavirus IgA, IgG, and IgM antibodies (as indicated) were measured 42 days (A and B) or 10 months (C and D) following infection with ECwt. Antibody titers were measured for individual mice, and results are plotted as GMTs of the groups (n = 10 per group). Error bars represent 1 standard error of the mean. Significant differences in GMT are indicated (@, #, $, and %, P = 0.001); Mann-Whitney U test).
IgA knockout mice maintain their antibody response for at least 10 months.
Serum and fecal antirotavirus antibody responses were also determined 10 months following the primary infection (Fig. 2C and D). IgA normal mice maintained stable serum IgA and IgG responses, while IgA knockout mice maintained stable serum IgG responses for at least 10 months, compared to titers at 42 dpi. Fecal antibody responses also were maintained out to 10 months after primary infection, although IgA antibody titers dropped approximately two- to threefold in IgA normal mice. At 10 months after primary infection, IgA knockout mice had significantly higher levels of fecal IgG than IgA normal mice (P = 0.001).
IgA knockout mice are protected from rotavirus challenge at 42 dpi.
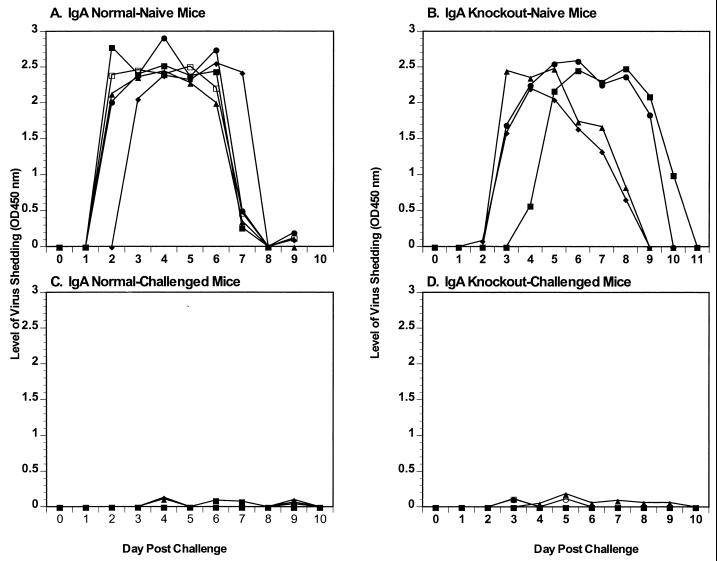
IgA normal and knockout mice received a primary infection or were challenged with 105 SD50 of ECwt at 42 dpi, and virus antigen shedding in the feces was monitored (Fig. 3). The naive control mice (Fig. 3A and B) shed normal levels of virus antigen (means = 6.5 and 6.3 days, respectively) compared to infection of younger mice (Fig. 1A and B). As expected, IgA normal mice were totally protected from rotavirus challenge at 42 dpi (Fig. 3C). IgA knockout mice were also totally protected from rotavirus challenge at 42 dpi (Fig. 3D). Although the OD of all fecal samples fell below the negative cutoff in the antigen ELISA, we confirmed that the low OD readings seen in the fecal samples from the IgA knockout mice did not represent low levels of infectious virus shedding. Two processed fecal samples (100 μl), from two mice, which had low OD readings were orally inoculated into two rotavirus naive mice, and virus shedding was monitored for 10 dpi (data not shown). No virus shedding was seen in either of the mice inoculated with processed fecal samples, confirming that even in the absence of fecal IgA, the IgA knockout mice were totally protected from rotavirus challenge.
FIG. 3.
Rotavirus antigen shedding curves following challenge with ECwt. IgA normal and knockout mice were orally infected with 105 SD50 of ECwt. Rotavirus antigen shedding curves for IgA normal (A) and knockout (B) control mice receiving a primary infection on 42 dpi and IgA normal (C) and knockout (D) mice challenged on 42 dpi are shown. Fecal samples were collected for 11 days, and levels of virus antigen shedding were determined by ELISA. The y axis represents the level of virus shed as determined by ELISA.
IgA knockout mice are protected from challenge at 10 months.
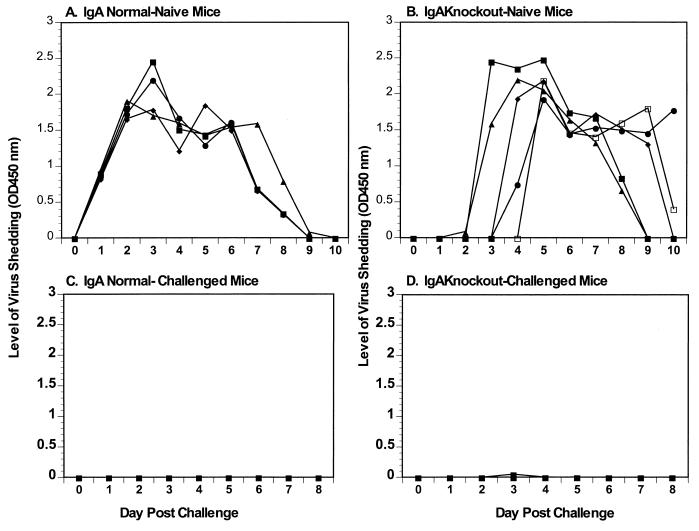
To determine if the protection observed in the IgA knockout mice was durable, five IgA normal and five IgA knockout control mice that underwent a primary infection at 42 dpi were maintained for 10 months and then challenged with 105 SD50 of ECwt (Fig. 4C and D). Age-matched controls were not available, but five 3-month-old IgA normal and knockout mice received a primary infection on this day (Fig. 4A and B) and shed normal levels of virus (5 to 7 days). All IgA normal and knockout mice were totally protected from rotavirus challenge 10 months following a primary infection (Fig. 4C and D). Therefore, rotavirus infection of IgA knockout mice results in induction of a long-lived immunologic mediator, resulting in a protective memory immune response.
FIG. 4.
Rotavirus antigen shedding curves following challenge infection with ECwt. (A and B) Rotavirus antigen shedding curves for primary infection of 3-month-old IgA normal (A) and knockout (B) control mice. IgA normal (C) and IgA knockout (D) mice were orally challenged with 105 SD50 of ECwt 10 months following the primary infection. Fecal samples were collected for 11 days, and levels of virus antigen shedding were determined by ELISA. The y axis represents the level of virus antigen shed as determined by ELISA.
CD4+ and CD8+ T cells are not responsible for the protection seen in IgA knockout mice.
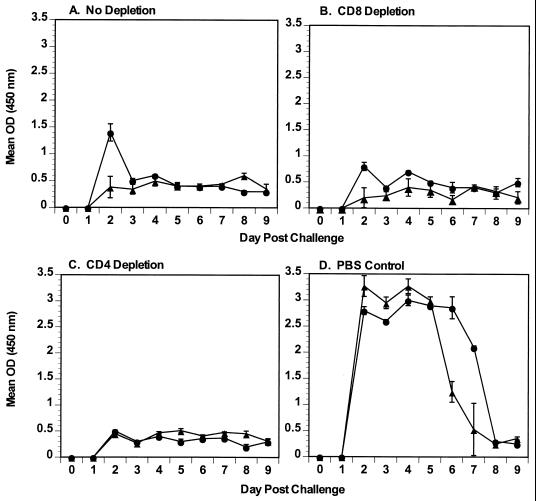
Although compensatory increases in overall numbers of CD4+ and CD8+ T cells are not seen in IgA knockout mice (11), these cells might have been responsible for the protection seen in the absence of IgA. To evaluate the role of T cells in protection in IgA normal and knockout mice, mice underwent normal primary infection with rotavirus and were depleted of either CD4+ or CD8+ T cells before and after challenge with rotavirus. Depletion of CD4+ or CD8+ T cells was confirmed in spleen and IELs on the day of rotavirus challenge (Fig. 5). In spleens and IELs, CD4+ and CD8+ T cells were depleted at least 92 and 99%, respectively. Once depletion of CD4+ and CD8+ T cells was confirmed, the remaining mice were challenged with 105 SD50 of ECwt and virus shedding was monitored for 9 days. Mean virus shedding was similar between IgA knockout and normal mice treated identically (Fig. 6). Virus shedding in T-cell-nondepleted and -depleted challenged mice (Fig. 6A to C) was compared to virus shedding in concurrently infected, previously rotavirus naive, control mice (Fig. 6D). Mean protection from rotavirus reinfection of all groups ranged from 74 to 84%, while protection in individual mice ranged from 65 to 91%. There was no statistical difference in mean protection (P > 0.05) between IgA normal and knockout mice treated identically. Therefore, neither CD4+ nor CD8+ T cells mediated the protection from rotavirus challenge seen in IgA knockout mice.
FIG. 5.
Flow cytometric analyses of IgA knockout mice depleted of CD4+ or CD8+ T cells. In IgA knockout mice depleted of CD4+ or CD8+ T cells, spleen and IELs were isolated, and flow cytometry was performed to confirm depletion. PE-stained CD4+ T cells are shown on the vertical axis; FITC-stained CD8+ T cells are shown on the horizontal axis. Numbers in quadrants indicate percentages of stained cells in the compartments.
FIG. 6.
Protection from rotavirus challenge in IgA normal and knockout mice depleted of CD4+ or CD8+ T cells. Mice were infected with 105 SD50 of ECwt on day 0 and then challenged at 42 dpi with the same dose of ECwt rotavirus. Individual stool samples were collected daily and quantitated for levels of rotavirus antigen by ELISA. Results are plotted as the mean OD reading for each group of mice (four or five mice per group). The y axis represents the mean level of virus antigen shed by IgA normal (●) and knockout (▴) mice as determined by ELISA. (A) Mice infected with rotavirus, not depleted of any cell type, and then challenged with rotavirus; (B) mice infected with rotavirus, depleted of CD8 cells, and then challenged with rotavirus; (C) mice infected with rotavirus, depleted of CD4 cells, and then challenged with rotavirus; (D) nondepleted mice that received their primary infection on the same day that the other groups were challenged with rotavirus. Error bars represent the standard error of the mean.
It is not clear why protection in both IgA knockout and normal mice in this experiment was not 100%, as was seen in previous experiments (Fig. 3 and 4). Additional experiments performed with IgA normal and knockout mice (data not shown) result in high levels of protection from infection (70 to 100%), but 100% protection from reinfection was not always obtained. However, since the differences in mean protection between IgA normal and knockout mice that were treated identically (rotavirus reinfection without depletion or rotavirus reinfection following depletion of CD4+ or CD8+ T cells) did not differ significantly, our conclusion that neither CD4 or CD8 T cells mediate protection in IgA knockout mice is valid.
DISCUSSION
IgA deficiency is the most common immune deficiency in humans, affecting up to 1 in 500 individuals in Western populations (19). These individuals do not have increased incidences of gastrointestinal infections, possibly due to compensatory increases of IgM or IgG at mucosal surfaces. These observations from IgA-deficient humans are consistent with the results we report here for IgA knockout mice.
IgA knockout mice were developed previously to assess the role of IgA in immunity (11). Compared to IgA normal mice, IgA knockout mice have compensatory or altered expressions of immunoglobulin isotypes (no IgA, increased levels of IgG and IgM, and decreased levels of IgE in serum and secretions) and IgG subclasses (increased IgG2b and decreased IgG3 in serum and secretions, and variable changes in IgG1 and IgG2a between serum and secretions) (11). We used IgA knockout mice to assess the requirement for intestinal IgA in clearance of primary and protection from secondary rotavirus infection. Both IgA normal and IgA knockout mice cleared primary rotavirus infection in the same time period. The normal clearance of a primary infection with murine rotavirus seen in the IgA knockout mice is not surprising, because these mice have equivalent levels of CD8+ and CD4+ T cells compared to IgA normal mice (11). Therefore, even in the absence of IgA, CD8+ and CD4+ T cells are likely to be fully functional to clear primary rotavirus infection (9, 10, 16, 17).
Previous studies with rotavirus infections in knockout mice indicate that antibody is necessary for complete long-term protection (9, 10, 16), but direct studies have not been performed to determine which antibody isotypes are able to mediate protection from rotavirus challenge. In natural and experimental rotavirus infections, IgA antibody in the intestine is likely the important protective effector molecule. Following natural or experimental rotavirus infection in children (6, 13, 22), mice (8), and piglets (27), serum and intestinal IgA or IgA ASC have been shown to correlate with protection from rotavirus challenge. However, in the absence of IgA due to immune deficiency (19) or when limited levels of IgA are induced by immunization such as with VLPs or inactivated virus (3, 5, 21; K. K. Lee, M. Ciarlet, and M. E. Connor, unpublished data) other effector antibody molecules present in the intestine may provide protection. Our results showing complete protection from rotavirus challenge in IgA knockout mice indicate that IgA is not absolutely necessary for protection from rotavirus reinfection and that other immunologic mediators can provide protection in the absence of IgA.
We propose that in situations when an immunogen fails to induce an IgA response but does induce an IgG response, the IgG response can protect the intestinal or other mucosal surfaces from subsequent challenge. IgA knockout mice have significantly enhanced levels of intestinal rotavirus-specific IgG following primary infection compared to IgA normal mice. A role for IgG in protection is further supported by other studies in rabbits and mice. (i) Passive oral administration of IgG to mice results in protection from diarrhea (14, 20, 25). (ii) Protection in rabbits following parenteral immunization with live or inactivated rotavirus or VLPs induces high levels of fecal IgG but no detectable fecal IgA, nor is IgA detected in intestinal fragment cultures (3, 5; Lee et al., unpublished data). (iii) In mice, intranasal or parenteral immunization with inactivated rotavirus or VLPs induces high level of rotavirus-specific IgG ASC in the lamina propria or fecal IgG, but no or only low levels of IgA ASC in lamina propria or fecal IgA (4, 21). (iv) Following intranasal administration of VLPs, protection correlated with serum antibody and fecal IgG but did not correlate with fecal IgA (21). (v) Preliminary results in IgA knockout mice immunized intranasally with VLPs suggest that protection is not dependent on the presence of intestinal IgA (K. L. Warfield, C. M. O'Neal, and M. E. Conner, unpublished data). Taken together, these results strongly suggest that IgG is able to mediate protection from rotavirus challenge.
Alternatively, protection from rotavirus may be independent of any antibody response. When B-cell-deficient μMT mice were administered double-layered rotavirus particles or VP6 expressed in Escherichia coli fused to maltose binding protein, protection from rotavirus challenge was observed in the absence of an antibody response even when mice were depleted of CD8+ T cells (2, 18). These results suggest that protection against rotavirus infection induced by a subunit vaccine may occur through antibody-independent mechanisms. However, it is not yet clear how these results can be reconciled with previous results suggesting the necessity of antibody in long-term protection from rotavirus infection (9, 10, 16). Further studies with VLPs are needed to determine directly whether intranasally administered VLPs induce protection by antibody-dependent or -independent mechanisms.
A role for IgM in protection from rotavirus challenge cannot be ruled out but is unlikely. Antirotavirus IgM was detected in the serum of IgA knockout mice, but rotavirus-specific IgM was detected in fecal samples from only 1 of 10 mice at the time of challenge, either 42 dpi or 10 months. IgA knockout mice have a compensatory approximately 25-fold increase in total IgM in intestinal secretions (11), but a compensatory increase in rotavirus-specific IgM was not induced. Antirotavirus IgM antibodies or ASC have been detected in mice 10 to 60 dpi (12, 24), but longer-term persistence of rotavirus-specific serum IgM in mice had not previously been evaluated. Our findings of serum IgM in IgA knockout mice 10 months following primary infection with rotavirus indicates the existence of a memory IgM response in IgA knockout mice. Dogma has been that affinity maturation of IgM antibody or long-lived IgM memory response does not occur. However, this idea has been challenged by recent observation of persistence of serum IgM for 2 years in mice orally immunized with dinitrophenyl-keyhole limpet hemocyanin with cholera toxin (26), as well as results reported here.
CD8+ T cells can mediate total short-term (<3 weeks) and partial long-term (≤3 months) protection from rotavirus challenge, but CD8+ T cells alone provide little to no protection at 8 months after primary infection (9, 10). Because CD8+ T cells might have partially mediated the observed protection at 42 dpi, IgA normal and IgA knockout mice were challenged 10 months following primary rotavirus infection. Both IgA normal and IgA knockout mice were totally protected from challenge. Therefore, it was unlikely that the protection seen in the IgA knockout mice was due to CD8+ T cells. It was also unlikely that the protection seen in the IgA knockout mice was due to CD4+ T cells because CD4+ T-cell-deficient mice are protected from rotavirus challenge and produce low levels of rotavirus-specific IgA and IgG (10). Our results strongly suggested that complete protection from rotavirus challenge seen in the IgA knockout mice at 10 months was due to antibody, most likely IgG.
To directly determine the role of CD4+ or CD8+ T cells in protection of IgA knockout mice, previously infected IgA knockout mice were depleted of either CD4+ or CD8+ T cells before and after challenge with rotavirus. Mice depleted of either CD4+ or CD8+ T cells, as well as nondepleted mice, showed equivalent levels of protection from rotavirus challenge. These results indicate that in the absence of IgA neither CD4+ nor CD8+ T cells provide a compensatory mechanism of protection. Therefore, IgG is the most likely immunologic mediator providing the long-term protection seen in the absence of IgA. Undoubtedly in normal children and animals infected with rotavirus, IgA is the mediator of protection. However, when low levels of IgA are induced such as following immunization with nonreplicating inactivated virus (5) or VLPs (3, 21; Lee et al., unpublished data) or in the absence of IgA due to IgA deficiency (19), other antibody isotypes, in particular IgG, are able to mediate protection from rotavirus challenge.
Are our results with rotavirus an exception to the idea that IgA is necessary to protect mucosal surfaces? No, clearance of or protection from influenza virus, HSV-2, or H. pylori infections is also observed in the absence of IgA; no differences were observed between IgA normal and knockout mice infected or challenged with these agents (1, 15, 23). Protection in all three systems was attributed to IgG alone or in combination with IgM (influenza virus) or CD4+ T cells (H. pylori). The isotype of the immunogen-specific immunoglobulin response in IgA knockout mice appears to be influenced by the immunogen. In contrast to IgA knockout mice immunized with killed whole influenza virus which have elevated influenza virus-specific IgM, but not IgG, in secretions (10), rotavirus-infected IgA knockout mice had elevated rotavirus-specific IgG, but not IgM, in the intestine. The results in IgA knockout mice with influenza virus and HSV-2 are not so unexpected because IgG is known to comprise a major portion of the immunoglobulin response in the lungs and vaginal tract. However in the intestine, IgA is the predominant immunoglobulin. Our work provides the first direct evidence that IgA is not absolutely required to protect the intestine from a localized enteric virus infection and supports a role for immunogen-specific IgG in protection of this mucosal surface.
Most current rotavirus vaccine studies use serum and intestinal IgA as markers for protection; our results suggest that IgA is not the sole mediator of protection and should not be the sole marker assayed in vaccine trials. Current live attenuated or future subunit vaccines which have limited to no ability to replicate will likely induce decreased IgA and increased IgG rotavirus-specific intestinal antibody titers compared to infection with replicating virus. These vaccines may still provide protection due to the rotavirus-specific IgG or to both IgA and IgG responses in the intestine. Results from our study and others (1, 15, 23) suggest that at mucosal surfaces (small intestinal, gastric, respiratory, and vaginal), protective mechanisms other than IgA may be important and should be pursued.
ACKNOWLEDGMENTS
We thank Juan Alvarado and Jessica Esparza for assistance in processing and assaying of fecal samples. We also thank Mary Estes and Dorothy Lewis for critical evaluation of the manuscript.
REFERENCES
- 1.Blanchard T G, Czinn S J, Redline R W, Sigmund N, Harriman G R, Nedrud J G. Antibody-independent protective mucosal immunity to gastric helicobacter infection in mice. Cell Immunol. 1999;191:74–80. doi: 10.1006/cimm.1998.1421. [DOI] [PubMed] [Google Scholar]
- 2.Choi A H-C, Basu M, McNeal M M, Clements J D, Ward R L. Antibody-independent protection against rotavirus infection of mice stimulated by intranasal immunization with chimeric VP4 or VP6 protein. J Virol. 1999;73:7574–7581. doi: 10.1128/jvi.73.9.7574-7581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciarlet M, Crawford S E, Barone C, Bertolotti-Ciarlet A, Ramig R F, Estes M K, Conner M E. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J Virol. 1998;72:9233–9246. doi: 10.1128/jvi.72.11.9233-9246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffin S E, Klinek M, Offit P A. Induction of virus-specific antibody production by lamina propria lymphocytes following intramuscular inoculation with rotavirus. J Infect Dis. 1995;172:874–878. doi: 10.1093/infdis/172.3.874. [DOI] [PubMed] [Google Scholar]
- 5.Conner M E, Crawford S E, Barone C, Estes M K. Rotavirus vaccine administered parenterally induces protective immunity. J Virol. 1993;67:6633–6641. doi: 10.1128/jvi.67.11.6633-6641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulson B S, Grimwood K, Hudson I L, Barnes G L, Bishop R F. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol. 1992;30:1678–1684. doi: 10.1128/jcm.30.7.1678-1684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ericson B L, Graham D Y, Mason B B, Estes M K. Identification, synthesis, and modifications of simian rotavirus SA11 polypeptides in infected cells. J Virol. 1982;42:825–839. doi: 10.1128/jvi.42.3.825-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng N, Burns J W, Bracy L, Greenberg H B. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol. 1994;68:7766–7773. doi: 10.1128/jvi.68.12.7766-7773.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco M A, Greenberg H B. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–7806. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco M A, Tin C, Greenberg H B. CD8+ T cells can mediate almost complete short-term and partial long-term immunity to rotavirus in mice. J Virol. 1997;71:4165–4170. doi: 10.1128/jvi.71.5.4165-4170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harriman G R, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike I N. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol. 1999;162:2521–2529. [PubMed] [Google Scholar]
- 12.Khoury C A, Moser C A, Speaker T J, Offit P A. Oral inoculation of mice with low doses of microencapsulated, noninfectious rotavirus induces virus-specific antibodies in gut-associated lymphoid tissue. J Infect Dis. 1995;172:870–874. doi: 10.1093/infdis/172.3.870. [DOI] [PubMed] [Google Scholar]
- 13.Matson D O, O'Ryan M L, Herrera I, Pickering L K, Estes M K. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis. 1993;167:577–583. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- 14.Matsui S M, Offit P A, Vo P T, Mackow E R, Benfield D A, Shaw R D, Padilla-Noriega L, Greenberg H B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J Clin Microbiol. 1989;27:780–782. doi: 10.1128/jcm.27.4.780-782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mbawuike I N, Pacheco S, Acuna C L, Switzer K C, Zhang Y, Harriman G R. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J Immunol. 1999;162:2530–2537. [PubMed] [Google Scholar]
- 16.McNeal M M, Barone K S, Rae M N, Ward R L. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology. 1995;214:387–397. doi: 10.1006/viro.1995.0048. [DOI] [PubMed] [Google Scholar]
- 17.McNeal M M, Rae M N, Ward R L. Evidence that resolution of rotavirus infection in mice is due to both CD4 and CD8 cell-dependent activities. J Virol. 1997;71:8735–8742. doi: 10.1128/jvi.71.11.8735-8742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeal M M, Rae M N, Bean J A, Ward R L. Antibody-dependent and -independent protection following intranasal immunization of mice with rotavirus particles. J Virol. 1999;73:7565–7573. doi: 10.1128/jvi.73.9.7565-7573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogens K, Russell M W. Function of mucosal immunoglobulins. In: Ogra P L, Lamm M E, McGee J R, Mestecky J, Strober W, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. p. 134. [Google Scholar]
- 20.Offit P A, Clark H F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985;54:58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus VLPs administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Ryan M L, Matson D O, Estes M K, Pickering L K. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J Infect Dis. 1994;169:504–511. doi: 10.1093/infdis/169.3.504. [DOI] [PubMed] [Google Scholar]
- 23.Parr M B, Harriman G R, Parr E L. Immunity to vaginal HSV-w infection in immunoglobulin A knockout mice. Immunology. 1998;95:208–213. doi: 10.1046/j.1365-2567.1998.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw R D, Merchant A A, Groene W S, Cheng E H. Persistence of intestinal antibody response to heterologous rotavirus infection in a murine model beyond 1 year. J Clin Microbiol. 1993;31:188–191. doi: 10.1128/jcm.31.2.188-191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheridan J F, Smith C C, Manak M M, Aurelian L. Prevention of rotavirus-induced diarrhea in neonatal mice born to dams immunized with empty capsids of simian rotavirus SA-11. J Infect Dis. 1984;149:434–438. doi: 10.1093/infdis/149.3.434. [DOI] [PubMed] [Google Scholar]
- 26.Vajdy M, Lycke N. Mucosal memory B cells retain the ability to produce IgM antibodies 2 years after oral immunization. Immunology. 1995;86:336–342. [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan L, Ward L A, Rosen B I, To T L, Saif L J. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]