Keywords: action potential, delayed rectifier, Kv2.1, reflexes, synaptic transmission
Abstract
Diversity in the functional expression of ion channels contributes to the unique patterns of activity generated in visceral sensory A-type myelinated neurons versus C-type unmyelinated neurons in response to their natural stimuli. In the present study, Kv2 channels were identified as underlying a previously uncharacterized delayed rectifying potassium current expressed in both A- and C-type nodose ganglion neurons. Kv2.1 and 2.2 appear confined to the soma and initial segment of these sensory neurons; however, neither was identified in their central presynaptic terminals projecting onto relay neurons in the nucleus of the solitary tract (nTS). Kv2.1 and Kv2.2 were also not detected in the peripheral axons and sensory terminals in the aortic arch. Functionally, in nodose neuron somas, Kv2 currents exhibited frequency-dependent current inactivation and contributed to action potential repolarization in C-type neurons but not A-type neurons. Within the nTS, the block of Kv2 currents does not influence afferent presynaptic calcium influx or glutamate release in response to afferent activation, supporting our immunohistochemical observations. On the other hand, Kv2 channels contribute to membrane hyperpolarization and limit action potential discharge rate in second-order neurons. Together, these data demonstrate that Kv2 channels influence neuronal discharge within the vagal afferent-nTS circuit and indicate they may play a significant role in viscerosensory reflex function.
NEW & NOTEWORTHY We demonstrate the expression and function of the voltage-gated delayed rectifier potassium channel Kv2 in vagal nodose neurons. Within sensory neurons, Kv2 channels limit the width of the broader C-type but not narrow A-type action potential. Within the nucleus of the solitary tract (nTS), the location of the vagal terminal field, Kv2 does not influence glutamate release. However, Kv2 limits the action potential discharge of nTS relay neurons. These data suggest a critical role for Kv2 in the vagal-nTS reflex arc.
INTRODUCTION
The sensory arm of the vagus nerve innervates a variety of distal peripheral targets. Activation of vagal sensory neurons produces an increase in discharge in myelinated (A-type) and unmyelinated (C-type) sensory processes that project centrally through the nodose ganglia. A-type nodose visceral sensory neurons exhibit a reproducible discharge in response to peripheral stimuli signaling normal physiological functions such as lung inflation and arterial pressure (1–4). C-type neurons on the other hand have receptors that respond with a more irregular firing pattern and discharge at lower frequencies (1, 4–8). They have low or no baseline activity but discharge to signal specific information that falls outside the normal physiological range such as elevated arterial pressure and airway irritants.
Differences among expression of several ion channels in A-type versus C-type visceral sensory neurons have been previously identified by several groups and have been shown to contribute to shaping their unique patterns of natural activity (for review, see Ref. 9). Of those channels, potassium channels are critical to setting membrane potential and controlling excitability. Both A- and C-type nodose neurons express several non- or slowly-inactivating voltage-dependent potassium channels that regulate excitability and action potential duration. Specifically, α-dendrotoxin-sensitive channels (α-DTx), Kv1.1, Kv1.2, Kv1.6, and a margatoxin-sensitive channel (MgTx), Kv1.3, are important in regulating the discharge of visceral sensory neurons in response to depolarization (10, 11). Present in these neurons is an additional delayed rectifying current that is sensitive to tetraethylammonium but insensitive to α-DTx, MgTx, or low concentrations of 4-aminopyridine (4-AP; 2.5–5.0 mM). Candidates for the current include the Kv2 channels, which, in neurons, have been implicated in diverse functions including the regulation of excitability (12, 13) and vesicular release of transmitter (14). A- and C-vagal afferents terminate in the brainstem nucleus tractus solitarii (nTS), where Kv channels contribute to synaptic integration and neuronal activity (11, 15). The present study identified the presence of Kv2 channels in the viscerosensory-nTS circuit and demonstrates that they contribute to the activity profile in the soma of sensory C-type neurons as well as nTS relay neurons.
MATERIALS AND METHODS
Ethical Considerations
All experiments were performed on tissue collected from neonatal [postnatal day (P)0–P5] of either sex and male juvenile (P28–P42) Sprague-Dawley rats in accordance with the Case Western Reserve University Animal Research Committee and University of Missouri Animal Care and Use Committee guidelines.
Identification of Kv2.1 and 2.2 via Western Blots and Immunohistochemistry
The following antibodies were used for Western blot and/or immunohistochemistry, as detailed in results. To identify Kv2.1, the following intracellular-targeted antibodies were used: rabbit polyclonal against amino acids 837–853 (Upstate Biotechnology, lot 16092, 24074, and 29511; or Alomone Labs, lot AN-02). To examine Kv2.2, we used a rabbit polyclonal against amino acids 859–873 (Alomone Labs, lot AN-01). Western blots used a goat anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for loading controls. In nTS or aortic arch immunohistochemistry, respectively, a mouse monoclonal anti-synaptophysin (clone SVP-38, Sigma, cat. no. S5768) or goat polyclonal anti-peripherin (C-19, Santa Cruz Biotechnology, cat. no. SC-760) was also used to confirm location.
Western Blots
Frozen nodose ganglia from CO2-anesthetized neonatal (N = 32, tissue was pooled to have a repeat of 2 for P0 and P5) and halothane-anesthetized juvenile rats (N = 6) were homogenized in RIPA buffer containing freshly added protease inhibitor. The homogenate was incubated on ice for 1 h and then centrifuged at 14,000 g for 10 min, and the insoluble debris was discarded. The protein concentration of the lysate was measured by the BCA method (Pierce Chemical Co.). Equal amounts of protein (50 µg/sample) were separated on 7.5% polyacrylamide SDS gels and transferred to PVDF membranes. The PVDF membranes were blocked with 5% nonfat dry milk in PBS-0.1% Tween-20 (PBS-T; Fisher Scientific) overnight at 4°C and then incubated with primary antibodies against Kv2.1 (rabbit polyclonal, Upstate, 1:500) or Kv2.2 (rabbit polyclonal, Alomone, 1:2,000). Loading controls utilized a goat anti-β-actin antibody (Santa Cruz Biotechnology, 1:4,000). After being washed, the blots were incubated for 1 h at room temperature with anti-rabbit or anti-goat horseradish peroxidase-linked secondary antibodies in a blocking buffer. Following the wash, the blots were developed using the ECL-PLUS kit (Amersham), and the images were captured on Hyperfilm-ECL.
Tissue Immunocytochemistry
Anti-Kv2.1 and anti-Kv2.2 antibodies were used to identify immunoreactivity in cultured nodose neurons and tissue sections of nodose ganglia, in aortic arch baroreceptor terminals, and in brainstem containing the nTS. Antibody-treated tissues were imaged with the Leica SP2 spectral confocal microscope.
Nodose ganglia and isolated neurons.
Ganglia were harvested from halothane anesthetized rats (N = 5) and processed for sections in one of two ways. Fresh frozen nodose ganglia were cryosectioned (6–16 μm), collected on slides, and postfixed with 2% paraformaldehyde-lysine-periodate (PLP) containing 0.1% Triton X-100 for 5–10 min or 4% paraformaldehyde. Alternatively, nodose ganglia were immersion fixed in 4% paraformaldehyde overnight at 4°C. They were cryoprotected with 5% sucrose in PBS for several hours, followed by 30% sucrose in PBS. The tissue was frozen in OCT immersed in isopentane chilled on dry ice and then cryosectioned. Individual neurons from ganglia harvested from halothane-anesthetized rats (N = 15) were isolated and cultured as previously described (10). Cultured cells were placed on glass coverslips that were rinsed in Dulbecco’s PBS two times and then fixed with the 2% PLP with 0.1% Triton X-100 or 4% paraformaldehyde for 10 min.
Ganglion sections on slides and isolated nodose neurons on glass coverslips were blocked with PBS containing 5–10% normal donkey serum, 1% bovine serum albumin, and 0.03% TritonX-100 for at least 30 min. If Triton X-100 was not used in the fixative, 0.3% was added to the blocking solution. Primary Kv2.1 and Kv2.2 antibodies were diluted 1:100. As a negative control, a blocking solution followed by the appropriate secondary antibody was used. The immunizing peptides for Alomone anti-Kv2.1 and Alomone anti-Kv 2.2 were used to preabsorb each respective antibody at a 1:1-μg concentration as a second negative control. After overnight incubation at 4°C, the antibodies were visualized with anti-rabbit IgG tagged with fluorescein isothiocyanate or Rhodamine Red-X (Jackson ImmunoResearch) diluted 1:200 and incubated for 1.5 h at room temperature. The tissue was rinsed and coverslipped with Vectashield antifade solution (Vector laboratories). Cell diameter was measured using FIJI ImageJ software.
Aortic arch.
Aortic arches from halothane-anesthetized juvenile Sprague-Dawley rats (N = 8) were isolated and fixed in 4% paraformaldehyde. The adventitia containing the baroreceptors was separated from the arterial wall (16) and placed in paraformaldehyde overnight. The tissue was blocked for nonspecific staining in PBS containing 10% normal donkey serum, 0.3% Triton X-100 (Pierce, Rockford, IL), and 1% BSA (Jackson ImmunoResearch, West Grove, PA) overnight followed by a 3-day incubation in a primary antibody against either Kv2.1 or Kv2.2 channel (1:100) or peripherin (1:300) to aid in the localization of the nerve terminals. The secondary antibodies were tagged with FITC, rhodamine Red-X, or Cy5. The adventitia was mounted with Vectashield or 50% glycerol in PBS with 2% N-propyl gallate on glass slides and coverslipped for viewing.
nTS.
Juvenile rats (N = 6) were deeply anesthetized via intraperitoneal injection of a cocktail of ketamine (25%), xylazine (25%), and acepromazine (50%) at a dosage of 1.2 mL/kg followed by perfusion with paraformaldehyde fixative. The medulla was isolated, frozen, and cryosectioned. Serial horizontal nTS sections (8 μm) containing the solitary tract and the nTS were collected on glass slides and fixed with cold 4% paraformaldehyde for 30 min. Sections were blocked with PBS containing 1% BSA, 10% normal donkey serum, and 0.3% Triton X-100 for at least 30 min followed by an incubation in the presence of primary antibodies Kv2.1 or Kv2.2 (1:100) and synaptophysin (1:500) overnight at 8°C. Slides were rinsed in PBS, and secondary antibodies were added for 90 min. Sections were rinsed and mounted in Vectashield (Vector laboratories) for viewing.
Nodose Electrophysiology
Neonatal rats (P0–P2; N = 14) were anesthetized with CO2, or juvenile rats (N = 15) were anesthetized with halothane or isoflurane and decapitated. Nodose ganglia were extracted, and the neurons dissociated and cultured as previously described (10, 17, 18). Electrophysiological experiments were performed on isolated neurons 4–24 h after plating onto glass coverslips (19). A gravity-fed perfusion system was used to exchange extracellular solutions at a rate of ∼0.5 mL/min. Recordings were done at room temperature using a whole cell patch configuration under voltage- or current-clamp conditions. Data were obtained with an Axopatch-1C or Axopatch 200B patch-clamp amplifier at a low-pass filter frequency of 5,000 Hz (voltage clamp) or 500 Hz (current clamp) and then digitized at a sampling rate of 10–20 kHz and analyzed using pClamp software programs. For current-clamp studies, the following solutions were used (in mM): bath, 137 NaCl, 5.4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH 7.4; pipette (in mM), 145 K-aspartate, 5 NaCl, 1.95 CaCl2, 2.2 EGTA, 2 MgCl2, 10 glucose, and 5 HEPES, pH 7.3. For voltage-clamp studies, K+ currents were isolated by replacing Na+ in the bath solution with N-methyl-d-glucamine (NMDG) and reducing Ca2+ to 0.02 mM. In voltage-clamp experiments, the series resistance (4–8 MΩ) was ∼70% compensated. The voltage error was approximately ±3 mV. Stromatoxin (ScTx; Alomone Labs, cat. no. STS-350) and intracellular introduction of an anti-Kv2.1 antibody (Alomone Labs, cat. no. APC-012) via the patch pipette were used to characterize the K+ currents (20). ScTx inhibits Kv2.1, Kv2.2, as well as the transient Kv4.2 channel (21, 22). It is unknown whether a subgroup of nodose neurons expresses the transient A-type Kv4.2 channel. If so, it would presumably interfere with the calculation of the activation tau of Kv2.1. We did not encounter this problem in the cells we studied.
nTS Slice Electrophysiology
Brainstem slices containing the nTS were prepared from 5-wk-old isoflurane-anesthetized rats (N = 5) as previously reported (23, 24). Following decapitation, the brainstem was removed and placed in ice-cold artificial cerebrospinal fluid (aCSF) containing the following (in mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 10 MgSO4, 30 NaHCO3, 20 HEPES, 25 d‐glucose, 5 l‐ascorbic acid, 2 thiourea, 3 sodium pyruvate, and 0.5 CaCl2. The pH was adjusted to 7.4 with ∼93 mM HCl, osmolarity of 295–310 mosmol/L, and aerated with 95% O2 + 5% CO2. Horizontal nTS slices (∼280 μm) were generated using a vibratome (VT 1200, Leica, Wetzlar, Germany). Sequentially, the slices were incubated at 35°C for 12 min in NMDG‐cutting solution and then transferred and maintained for 30 min at room temperature (∼22°C) in recording aCSF containing the following (in mM): 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d‐glucose, 0.4 l‐ascorbic acid, and 2 CaCl2, aerated with 95% O2 + 5% CO2, pH 7.4, osmolarity of 295–310 mosmol/L. Tissue sections were placed in a superfusion chamber mounted on a fixed stage Olympus BX-51WI microscope, secured with a nylon harp, and superfused at a flow rate of ∼3 mL/min with aCSF at ∼32°C. All recordings were made from cells in the medial and commissural subnuclei of the nTS that receive a high density of cardiorespiratory afferent fiber termination from the nodose (25, 26).
Data were recorded using a MultiClamp 700B amplifier, filtered at 2 kHz, and sampled at 10 kHz using pClamp programs (Molecular Devices). Afferent [solitary tract (TS)]-evoked excitatory postsynaptic currents (EPSCs) were generated by placing a concentric bipolar stimulating electrode (F. Haer, Bowdoinham, ME) on the TS and stimulating with a negative current pulse (0.01–0.3 mA and 0.1-ms duration) with an isolated programmable stimulator (AMPI, Jerusalem, Israel). Recordings were made in voltage-clamp mode while the TS was stimulated at 0.5, 1, 5, and 10 Hz. Resting membrane potential (RMP) and current-evoked action potential (AP) discharge were recorded in current-clamp mode with no holding current (I = 0). AP discharge was also evoked by depolarizing current steps (0 to 120 pA, 100 ms). Cells were rejected if the resting potential was more positive than −45 mV under the current-clamp mode upon initial membrane rupture. No leak subtractions, liquid junction potential corrections, or series resistance compensations were performed. Experiments were discarded if series resistance was not stable. ScTx was used to characterize the influence of Kv2 potassium currents on EPSC and membrane potential properties. Only one cell was examined with ScTx per slice.
nTS Slice Calcium Imaging
Imaging was performed as previously described (23, 24). Briefly, 4-wk-old rats (N = 3) were anesthetized with isoflurane, and using aseptic techniques, the nodose was exposed through a midline incision in the neck. A micropipette containing Cal520-dextran (AATBioquest, 15% with 1% Triton-X100 in distilled water) was connected to a Picospritzer (model II, General Valve Corp., Fairfield, NJ). Pressure pulses of 2–10 PSI were used to expel Cal520 from the pipette and fill the ganglion. The total injected volume was ∼100 nL. The incision was closed with a 4-0 suture. Upon recovery from the anesthesia, animals were returned to their home cage for 5 days to allow anterograde transport of the dye.
Coronal brain slices (∼250 µm) containing the nTS were generated as in the electrophysiological protocols above and stored at room temperature until needed. The use of coronal sections from several animals allowed for the study of the role that Kv2 plays in TS-induced changes in calcium fluorescence across several slices from the same animal. A bipolar electrode was placed on the visible TS and stimulated with an isolated stimulator (−0.2 mA, 0.1-ms duration, and 20 stimuli at 10 Hz). Changes in Cal520 fluorescence were analyzed from the medial and commissural nTS subnuclei. Time-lapse confocal calcium imaging was acquired via a Yokagawa CSU-W1 confocal system (3i, Denver, CO) using ×40 water-immersion objective, Prime 95B sCMOS camera (Photometrics), and a 488-nm excitation laser. ScTx was used to characterize the influence of Kv2 potassium currents on intraterminal Ca2+. ScTx was only applied once per slice.
Data Analysis
Data were analyzed by Clampfit 10 (Molecular Devices), OriginPro (OriginLab, Northampton, MA), and Microsoft Excel. In nodose neurons, we examined the current in both neonatal and juvenile isolated neurons. Stromatoxin (ScTx) was used to isolate Kv2.1/Kv2.2 current by subtracting current in the presence of ScTx from that before the addition of ScTx. The resulting current, the ScTx-sensitive current, was examined for the following characteristics for comparison to the exogenous expression of Kv2.1. The activation tau was obtained from a single exponential fit (Clampfit) to the current response to a 400-ms step to +20 mV from −80 mV after ignoring the initial delay as has been done for other delayed rectifiers (27, 28). The activation curve was obtained from a Boltzmann fit (Origin) to the peak of the inward tail currents elicited immediately after a return to the holding potential of −80 mV from voltages of −70 to +20 mV in 10-mV increments (2-s intersweep interval). The V1/2, the midpoint on the resulting current/voltage curve, and “k,” the slope factor of the curve, were calculated. The deactivation tau was a single exponential fit to the decay of the tail current at −80 mV following the 400-ms step to +20 mV. These electrophysiological data (activation tau, deactivation tau, and the V1/2 and k slope of activation curve) of ScTx-sensitive current in neonate neurons (n = 4) compared with juvenile neurons (n = 4) did not statistically differ (Student’s t test, P > 0.05) and thus were grouped together for analysis. Nodose sensory neurons are typed on the basis of conduction velocity (fastest to slowest) of their axon into A-type, Ah-type with myelinated axons, and C-type neurons with unmyelinated axons. The electrophysiological characteristics of these three groups have been applied to the identification of isolated neurons. In general, A-type neurons have larger diameters with higher membrane capacitance, and narrower action potentials than C-type neurons, most of which display a hump on the repolarization phase of the action potential (29). The smaller A-type neuron (Ah) has intermediate characteristics (29, 30). In the following electrophysiological studies, we used action potential duration at 0 mV (a voltage in which the Kv2 channel is open and functional) or cell capacitance to separate A- versus C-type cells realizing that Ah cells might fall into either category.
In brain slice studies, Clampfit templates were used to detect EPSCs, with spontaneous EPSC threshold set at two times the root‐mean‐square noise level (23, 24). Short-term depression of afferent-evoked TS-EPSC amplitude was analyzed from 5 replicates of 10 consecutive stimuli at 1, 5, and 10 Hz. Asynchronous EPSCs (aEPSCs) were designated as those events occurring after the TS stimulation, relative to the baseline spontaneous EPSCs occurring before the train. Peak aEPSCs were classified as those occurring 1 s after the train. Average aEPSC were designated as those events occurring 4 s after the train (31). RMP was recorded during 1 min with no holding current (I = 0). The number of APs generated with step depolarization was manually identified. In Ca2+ imaging studies, FIJI ImageJ and Microsoft Excel were used to analyze the fluorescence. The peak of calcium fluorescence of Cal520 signals in response to afferent TS activation was subtracted from its initial prestimulus baseline (ΔF) and quantified as a change from baseline (ΔF/F).
Statistical analysis was performed with GraphPad Prism 9 or 10 (GraphPad Software, La Jolla, CA) or OriginPro software (Origin Labs, Northampton, MA). Outliers were identified by the Rout test and normality was evaluated using the Shapiro-Wilk test. Effects of a ScTx were tested by Student’s t test, or one/two-way ANOVA where appropriate and noted in the text. Holm-Sidak multiple comparison post hoc test identified individual differences. All data are presented as means ± SD Results were considered significantly different at P < 0.05. “N” denotes the number of rats whereas “n” marks the number of cells examined in a given protocol.
RESULTS
Detection of Kv2.1 and Kv2.2 Channels in Nodose Ganglia
Protein from nodose ganglia was examined by Western blot for the presence of Kv2.1 and Kv2.2 (Fig. 1). In membrane lysate from neonatal and juvenile nodose ganglia (Fig. 1A), the Kv2.1 antiserum revealed a prominent band ∼105 kDa. Weaker bands were present just below 105 kDa. The predicted molecular mass of Kv2.1 is 95 kDa, and the increase of the molecular mass to 105 kDa and above has been attributed to the phosphorylation of the subunit (32). In immunohistochemical experiments, Kv2.1 (N = 3) in nodose ganglion sections was strongly localized to intracellular regions and the initial segment (Fig. 1B) with weaker staining at the membrane. Kv2.1 was widely distributed across nodose neurons (Fig. 1C). In acutely isolated neurons, similar Kv2.1 staining occurred (Fig 1Biii). The Kv2.2 band in nodose (Fig. 1D) was located near 120 kDa as previously described (33). The anti-Kv2.2 antibody was more diffusively distributed in the soma (Fig. 1, E and F) and was also located in the initial segment. Thus Kv2.1 and Kv2.2 are localized in vagal sensory neurons.
Figure 1.
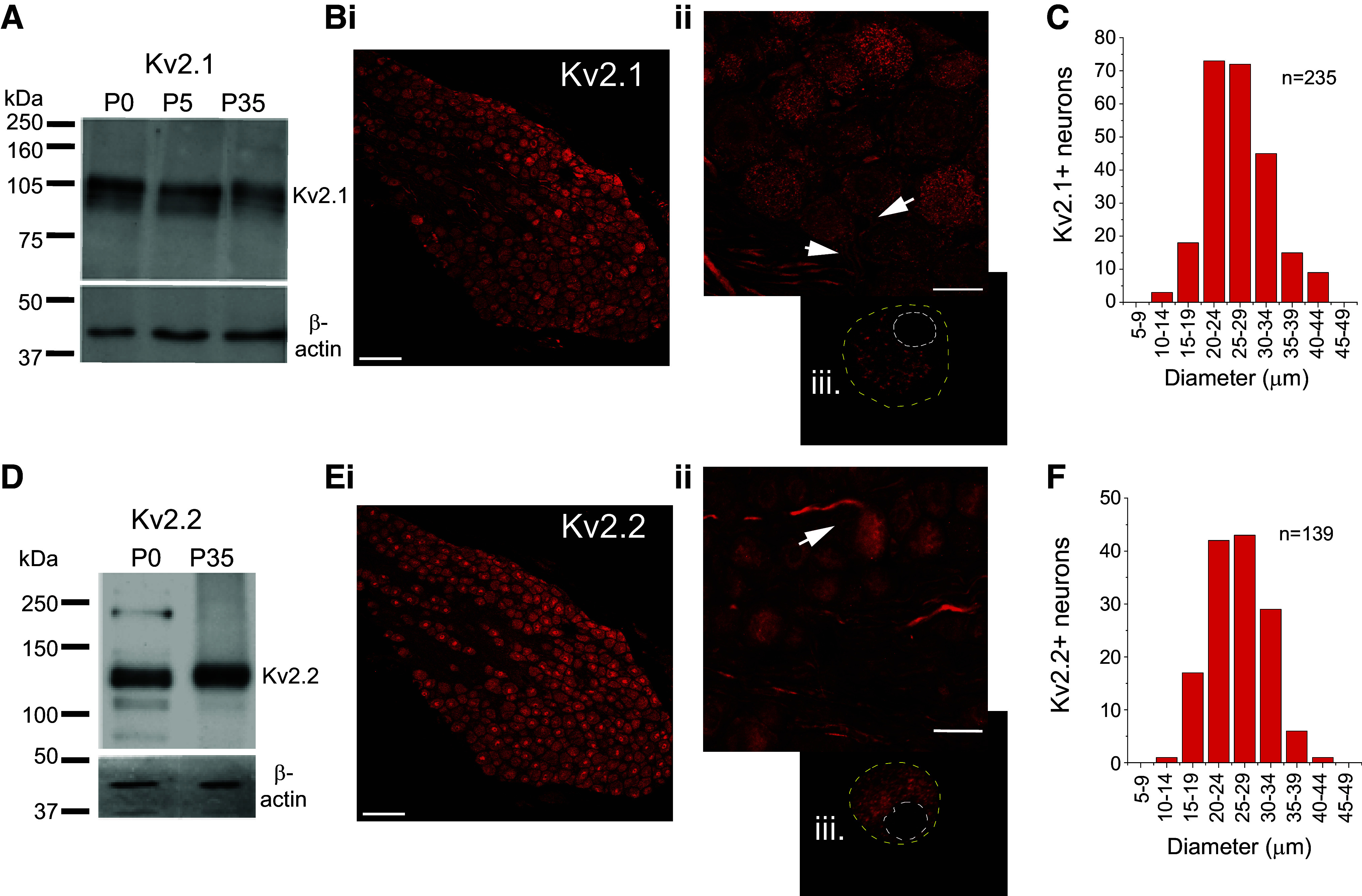
Kv2.1 and Kv2.2 detection in nodose ganglia. A: Western blot analysis of the Kv2.1 protein from nodose ganglia at postnatal day (P)0 (N = 2), P5–7 (N = 2), and P35 (N = 4) blotted with an antibody against Kv2.1 (Upstate Biotechnology, rabbit polyclonal). The antibody recognizes a principal band of 105-kDa molecular mass and a lighter lower band of about 100 kDa, presumably representing different phosphorylation states of the protein. β-Actin was used as a loading control. B, i and ii, confocal projection of an optical slice of Kv 2.1 immunostaining (Upstate, rabbit polyclonal; N = 3) in a juvenile nodose section. Note Kv2.1 in the initial axonal segments (arrow). Biii: dissociated nodose neuron demonstrating Kv2.1 labeling (Alomone Labs, rabbit polyclonal, N = 5) clustered around the nucleus. C: Kv2.1 somal size distribution. D: Western blot analysis of the Kv2.2 protein from neonate and juvenile nodose ganglia. Kv2.2 protein was detected in P0 (N = 2) and P35 (N = 2) nodose ganglia. Antibody (Alomone Labs, rabbit polyclonal) recognized a principal band near the 120 kDa of molecular mass that correlated with the molecular mass reported for the protein. E: anti-Kv 2.2 immunostaining (Alomone Labs, rabbit polyclonal, N = 3) in juvenile nodose section is diffuse throughout the neuron (i) and at initial segment (arrow, ii). Eiii: dissociated nodose neuron (N = 5). F: Kv2.2 somal size distribution. In Bi and Ei, immunostaining was performed for Kv2.1 and Kv2.2 (dual labeling), and each is pseudocolored red. Scale for B and E: i = 100 µm; ii and iii = 25 µm.
Kv2 in the nTS and Aortic Arch
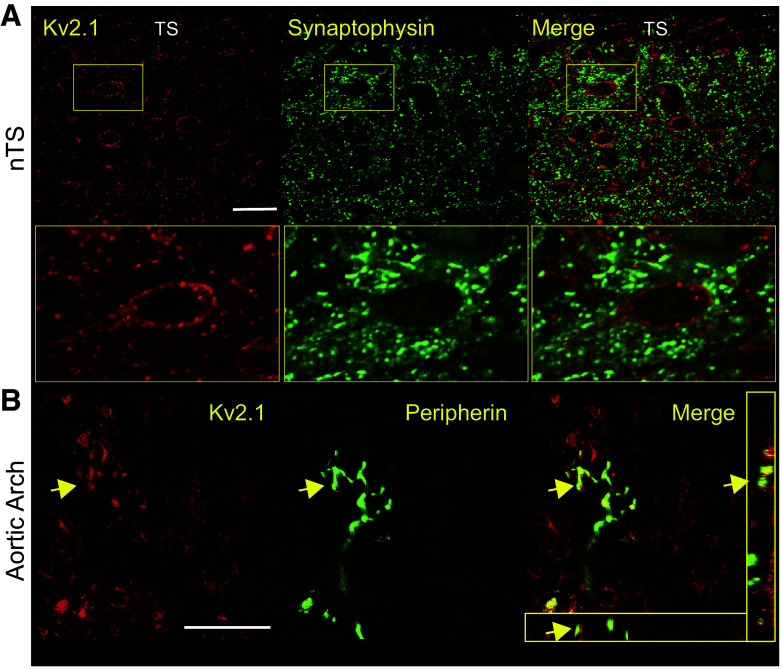
We further examined the expression of nodose Kv2 in their peripheral and central processes across several animals (N = 5 for nTS; N = 8 for aortic arch). Neither Kv2.1 (Fig. 2A) nor Kv2.2 (not shown) was present in the synaptophysin-identified presynaptic terminals located in the nucleus of the solitary tract (nTS). By contrast, postsynaptic relay nTS neurons expressed Kv2.1 in clusters (Fig. 2A) as previously described in other central neurons (34). We also examined the arterial baroreceptors of the aortic arch. Anti-Kv2.1 (Fig. 2B) and Kv2.2 (not shown) were not detected in the peripheral sensory terminals. Altogether, the distribution of Kv2.1 and Kv2.2 protein appeared confined to the nodose and nTS soma and nodose initial segment.
Figure 2.
Kv2.1 is localized to nucleus of the solitary tract (nTS) somas. A: confocal mass projection of Kv2.1 immunoreactivity (Upstate, rabbit polyclonal) with labeling in nTS neurons. Box illustrates a staining pattern of the Kv2.1 in the neurons of clusters in the membrane. Overlay of Kv2.1 image with synaptophysin-identified synaptic terminals to demonstrate they are closely aligned but not overlapping. N = 4 repeats; scale = 30 µm. B: stacked series of confocal images from a whole mount of adventitia of the aortic arch showing a group of arterial baroreceptor fibers identified with anti-peripherin staining. Anti-Kv2.1 (Alomone Labs, rabbit polyclonal) does not label the fine processes of aortic baroreceptors but is present in surrounding satellite cells, including Schwann cells (82, 83). Examples of Kv2.1 staining Schwann cells that overlay on the nerve fibers are indicated by the arrows. Inset: orthogonal view illustrating close association but not overlap between peripherin and Kv2.1. N = 3 repeats; scale = 30 µm.
Characterization of a Nodose Neuron Kv2 Current
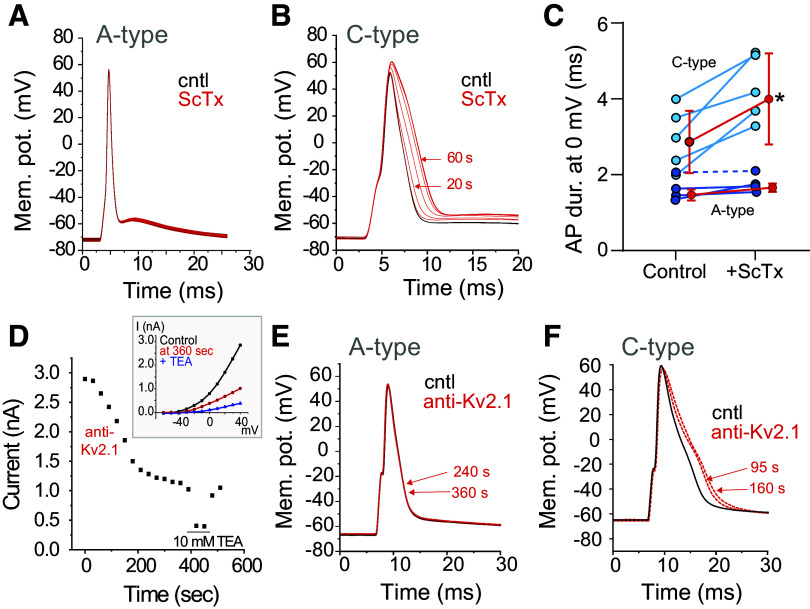
The functional expression of Kv2 channels and current in nodose neurons was determined in acutely dissociated neurons immersed in a potassium current isolating solution. We separated the potential Kv2 current from the total potassium current in voltage-clamp studies by subtracting the current following bath application of the selective Kv2.1/2.2 blocker, stromatoxin (ScTx; 100 nM), from the control current (Fig. 3A) to obtain the ScTx-sensitive current. Potassium current, measured at the end of a 400-ms pulse to +20 mV, was inhibited in response to application of ScTx with a time course illustrated in Fig. 3B. We examined the kinetics of the ScTx-sensitive current for comparison to those obtained in exogenous Kv2 expression systems and obtained parameters consistent with Kv2 current (activation tau, 17.7 ± 5.2 ms; deactivation tau, 16.0 ± 14.4 ms; V1/2, −16.9 ± 2.4 mV; k slope factor, 8.9 ± 6.7; N = 5; n = 8). Activation curves (Fig. 3C) were calculated from tail current measurements and illustrate threshold for current activation at −40 mV.
Figure 3.
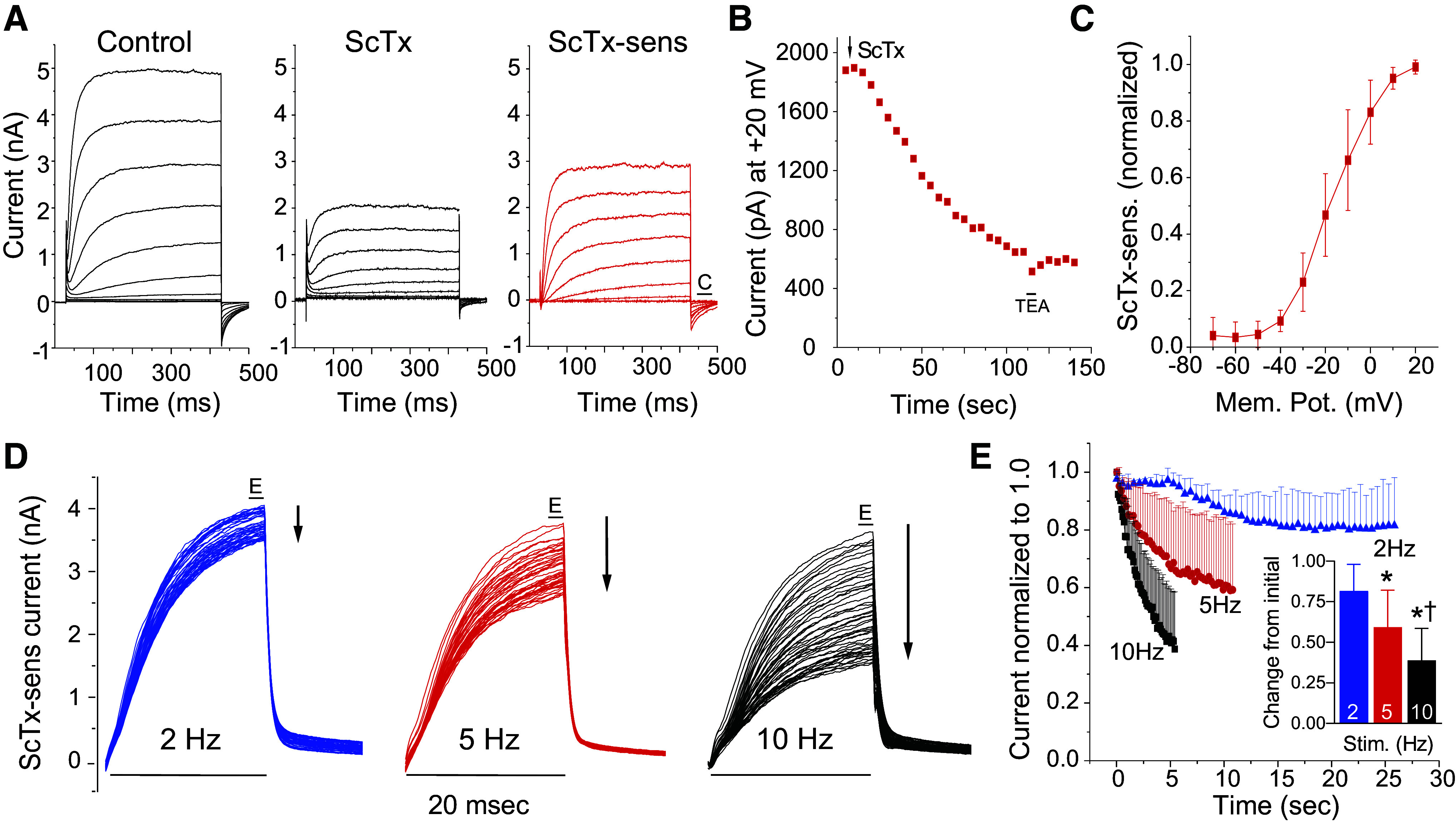
Functional expression and frequency dependence of Kv2 in nodose neurons. A: to isolate Kv2 currents a series of 400-ms depolarizing steps from −80 mV to +20 mV was followed by a return to −80 mV for 50 ms. The intertrace holding potential was −80 mV. Potassium current in the presence of 100 nM stromatoxin (ScTx) was subtracted from that in the absence of the toxin (control) to obtain the ScTx-sensitive current. “C” label in the ScTx-sensitive trace indicates location of tail currents for measurement of current-voltage relationship plotted in C. B: the time course of ScTx block, as measured at +20 mV at the end of the 400-ms step, from a single representative neuron (N = 1; n = 3). Tetraethylammonium (10 mM) is a potent and rapidly reversible blocker of Kv2.1 and was briefly added to confirm extensive block of the outward potassium current. C: activation curve (N = 4; n = 6) was obtained from tail current measurements at −80 mV following a series of 400-ms, 10-mV voltage steps from −70 mV to +20 mV. D: frequency-dependent inactivation of Kv2. ScTx-sensitive currents obtained following subtraction of the current in 100 nM ScTx from control current. The frequency-dependent decay in amplitude of Kv2 current was tested in 3 cells using a series of fifty 20-ms pulses delivered at frequencies of 2, 5, and 10 Hz from a holding potential of −60 mV to +40 mV with a return to −60 mV. “E” label denotes location of measurement of ScTx-sensitive. E: Kv2 current decreased with illustrated time course where the amplitude of the current at the end of each 20-ms depolarizing pulse was plotted against time for a series of frequencies from 2 to 10 Hz. Inset: change in current amplitude during the last events compared to the initial (N = 3; n = 3; *P < 0.05 vs. 2 Hz; †P < 0.05 vs. 5 Hz, one-way repeated measures ANOVA with Holm-Sidak).
Kv2 Current Shows Cumulative Frequency-Dependent Inactivation
Kv2.1 has been shown in heterologous expression to undergo a frequency-dependent cumulative inactivation in which a series of high-frequency brief depolarizing pulses can produce more rapid inactivation than does a sustained depolarization (35–37). We examined the effect of fifty 20-ms depolarizing pulses delivered at 2, 5, and 10 Hz to +40 mV and returning to −60 mV between stimuli (Fig. 3D). The Kv2 component was obtained by subtracting the traces acquired in the presence of ScTx from control traces. At increasing frequencies above 2 Hz, the brief depolarization produced increasing inactivation of the Kv2 current (Fig. 3D). Further quantification of the Kv2 current at the end of the 50 pulses compared to the initial current revealed a frequency-dependent reduction (Fig. 3E).
Block of Kv2 Current Causes Action Potential Broadening in C- but Not A-Type Neurons
We examined the role of Kv2 current on repolarization of the action potential in current-clamp studies by two blocking methods, ScTx or inclusion of antibodies against Kv2.1 in the intracellular pipette solution (38). The normal stimulus to the soma of the sensory neurons arrives as an action potential (AP) from the periphery, and therefore, a brief depolarizing stimulus (400–1000 µs) delivered to the soma from the resting membrane potential eliciting a single AP is a practical test for the role of Kv2 channels in regulating the duration of the AP of A- and C-type neurons. C-type neurons were distinguished from A-type neurons based on the duration of the AP measured at 0 mV during a 1.0-Hz stimulation, a hump on the repolarization phase of the action potential, and, in two cells, on a test for the presence of TTX-resistant sodium current (9).
The influence of Kv2 on AP duration following pharmacological and antibody-mediated block is shown in Fig. 4. As shown in the representative tracing, block of Kv2 with ScTx (100 nM) increased the duration of the AP in C-type neurons during a 1-Hz stimulus (Fig. 4B). There was no change in the duration of the AP in A-type neurons with the addition of ScTx (Fig. 4A). The increase in AP duration of the neurons measured at 0 mV is shown in Fig. 4C and demonstrates those neurons with a wider AP duration, likely C-type neurons, increase their duration following block of Kv2. Utilizing an anti-Kv2.1 antibody within the patch pipette (100 nM; Ref. 20), we confirmed the presence of Kv2.1 channels in nodose neurons and their contribution to AP broadening. We first demonstrated that inclusion of the antibody reduced Kv2 currents (Fig. 4D, I/V plot in inset). When the APs of two A-type neurons were recorded, there was no change in the duration of the AP recorded over a 6-min time period with antibody in the pipette solution (example in Fig. 4E, AP duration; 2.2 ± 0.7 ms to 2.2 ± 0.7 ms; N = 2; n = 2). To the contrary, after gaining intracellular access with the antibody-containing pipette solution, the duration of the AP in C-type neurons increased and stabilized within 2–4 min (example in Fig. 4F, AP duration; 4.4 ± 1.3 to 5.3 ± 1.9 ms; 118 ± 19% of control; N = 3; n = 5). However, since it was not always possible to obtain an accurate initial duration as the AP duration already appeared to be increasing by the time the protocol was initiated (∼60 s after seal rupture), this increase did not reach statistical significance (P = 0.09, paired t test). There was no change in the duration of C-type APs during 6 min of recording in the absence of antibody (97 ± 4% of control; N = 4; n = 4).
Figure 4.
Block of Kv2.1 prolongs the action potential (AP) in C-type but not A-type neurons. A–C: effect of application of stromatoxin (ScTx) on AP duration. A: in current-clamp studies, a single action potential was elicited at 1 Hz in an A-type cell. Overlaying traces showed no change in duration over 60 s. B: example of the effects of ScTx on a C-type neuron elicited at 1 Hz illustrates an increase in the duration of the action potential. The increase in duration in this neuron stabilized at approximately 60 s after ScTx application. C: quantification of the significant increase in AP duration in C-type (light blue) but not A-type (dark blue) neurons. The dark blue neuron denoted by dashed line likely reflects an Ah-type neuron. *P < 0.05, paired t test for C-type only. Shown are individual neurons (blue) and mean ± SD for each group. N = 4; n = 9. D–F: intracellular application of anti-Kv2.1. D: anti-Kv2.1 in the pipette reduces Kv2 current. Brief tetraethylammonium (TEA) application illustrated substantial block of Kv2. Inset: current-voltage (I/V) curve during control (immediate break-in), ScTx, and TEA (N = 1; n = 3). E: a single action potential was elicited in an A-type cell 2 min after patch rupture. Three overlaying traces collected within 2 min of breaking into the cell and at 4 and 6 min (red) show no change in the action potential duration. F: an example of the effects of including anti-Kv2.1 antibody in the pipette while recording membrane potential of a C-type neuron illustrates an increase in the duration of the action potential; 80-pA step delivered at 3-s intervals recorded immediately after rupture and at 95 and 160 s later.
Kv2 is Not the Primary Channel Responsible for Frequency-Dependent Increase in Duration
A previous study illustrated the duration of the action potential of C-type nodose neurons (with unmyelinated axons) increased as stimulus frequency increased while that of A-type neurons did not (39). In view of the frequency dependence of inactivation of Kv2.1 (Fig. 3), we asked whether Kv2.1 also contributed to the prolongation of the action potential of C-type neurons versus A-type neurons that occurs with increased frequency (Fig. 5). The relationship between the increase in stimulation frequency and the increase in duration of the AP in A- and C-type cells is illustrated in Fig. 5, A and B. AP duration for C-type neurons across frequencies is quantified in Fig. 5C and illustrates cells with broader action potentials increase their duration when the frequency is increased to 5 and 10 Hz yet was unaltered at 1 and 2 Hz.
Figure 5.
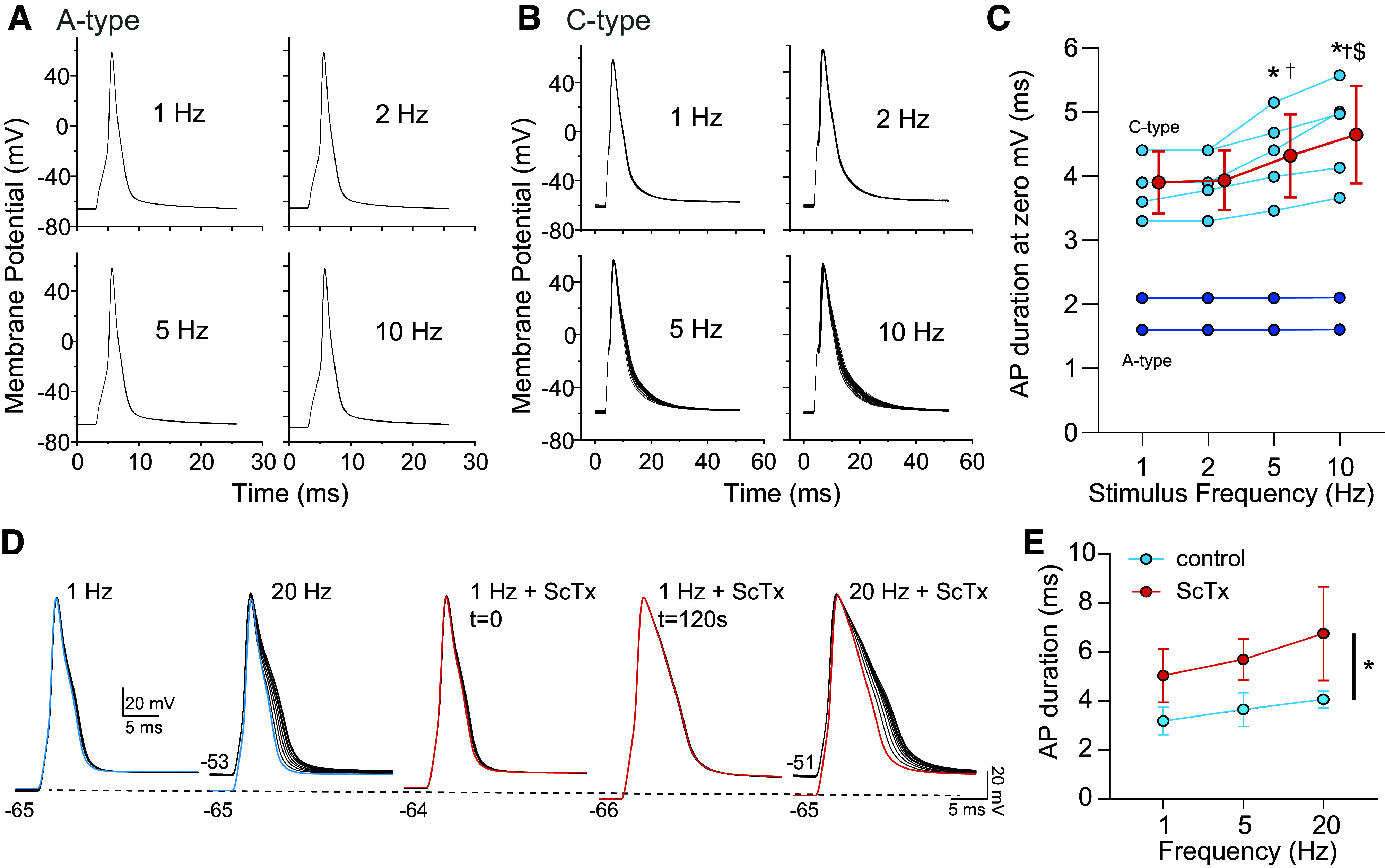
Frequency dependence of action potential (AP) duration in nodose neurons. A and B: increasing the frequency of AP generation leads to the broadening of C-type (B) but not A-type (A) neurons. APs induced by brief current injection. C: APs with the widest duration, indicative of C-type neurons (light blue), show increased AP duration upon elevated stimulation frequency. Conversely, narrow APs (dark blue) do not broaden upon augmented frequency. Shown are individual neurons (blue) and mean ± SD of the broader C-type neurons. N = 2; n = 7. *P < 0.05 vs. 1 Hz; †P < 0.05 vs. 2 Hz; $P < 0.05 vs. 5 Hz, one-way repeated measures (RM) ANOVA with Holm-Sidak. D: example of AP broadening in a C-type neuron with increasing frequency of stimulation, which was not prevented by stromatoxin (ScTx); “t=” indicates time following ScTx application. Dashed line indicates membrane potential. E: ScTx increases AP duration in C-type neurons but does not prevent the frequency-dependent widening. Two-way RM ANOVA, *Pdrug = 0.049, Pfreq = 0.004, Pdrug × freq = 0.311; N = 2; n = 3.
To determine the extent that Kv2 contributes to frequency-dependent AP broadening, the duration of frequency dependence at 20 Hz was compared in the absence (control) and presence of 100 nM ScTx. As shown in the example in Fig. 5D, the increase is not eliminated by ScTx. Three cells with broad action potentials and frequency-dependent increase in duration were examined in the absence and presence of ScTx. One cell was examined at 10 Hz and grouped with those stimulated up to 20 Hz. ScTx increased the duration of the action potential but did not eliminate the increase in duration at elevated frequencies (Fig. 5E).
Kv2 Channels Do Not Modulate Presynaptic Release in the nTS
Visceral afferents whose somas contain Kv2.1 and Kv2.2 terminate in the brainstem nTS. Kv2 was not identified in their central afferent, synaptophysin-labeled, terminals yet was expressed in nTS somas (Fig. 2). To confirm the expression data, we examined synaptic transmission between sensory terminals and second-order nTS neurons before and after ScTx to block potential Kv2 channels. The TS was stimulated for 20 episodes at 0.5, 1, 5, and 10 Hz, within the frequency range that these channels limit action potential discharge (see Figs. 3 and 4), while TS-EPSC amplitudes were monitored. Block of Kv2 via bath application of ScTx (500 nM, 5 min) did not alter TS-EPSC amplitude when evoked at 0.5 Hz (control, 190.6 ± 128.5 vs. ScTx, 179.6 ± 142.7; N = 6; n = 7; paired t test, P = 0.1534) and 1 Hz (data not shown). Conversely, when the TS was stimulated at 5 and 10 Hz (example of 10 Hz in Fig. 6A), TS-EPSC amplitude across the stimulus train was significantly reduced. Quantification of the ScTx-mediated reduction at 10 Hz is presented in Fig. 6B and illustrates a main effect of ScTx to reduce TS-EPSC amplitude [two-way repeated measures (RM) ANOVA]. A similar reduction in TS-EPSC amplitude across the stimulus train during a 5-Hz stimulation occurred with ScTx (two-way RM ANOVA, PEPSC < 0.001, Pdrug = 0.017, PEPSC × drug < 0.001).
Figure 6.
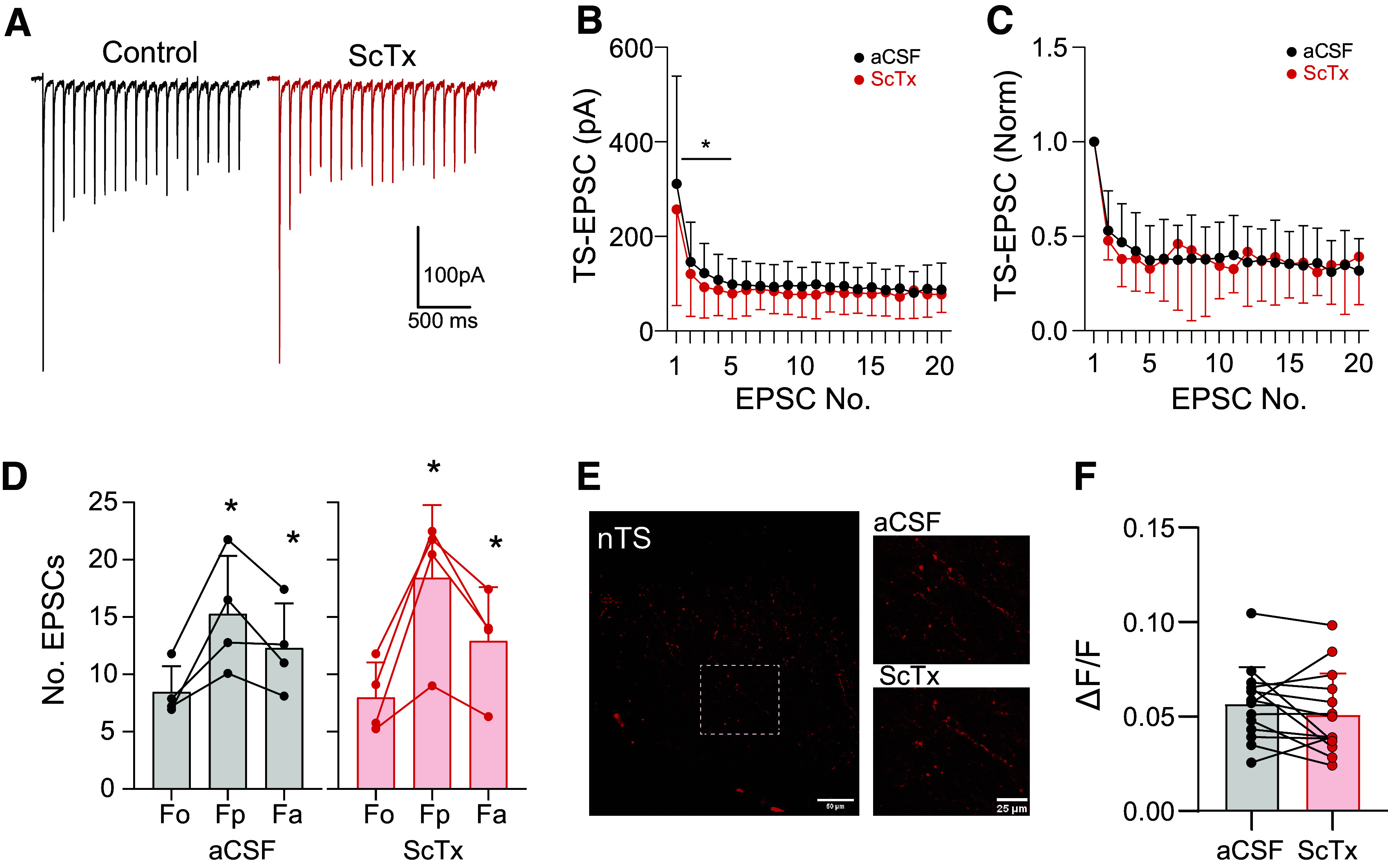
Kv2 does not modify presynaptic release in the nucleus of the solitary tract (nTS). A: in nTS slices, repetitive stimulation of the solitary tract (TS) at 10 Hz (20 events) elicited evoked excitatory postsynaptic currents (EPSCs) that accommodate over time. Block of Kv2 with stromatoxin (ScTx; 500 nM) mildly but significantly decreased EPSC amplitude. B: TS-EPSC amplitude decreased with ScTx [mean ± SD; N = 6; n = 7; two-way repeated measures (RM) ANOVA, PEPSC < 0.001, Pdrug = 0.021, PEPSC × drug = 0.012; *P < 0.05 with Holm-Sidak]. aCSF, artificial cerebrospinal fluid. C: TS-EPSCs normalized to the initial event demonstrating ScTx does not alter frequency-dependent depression (two-way RM ANOVA, PEPSC < 0.001, Pdrug = 0.931, PEPSC × drug = 0.758). D: 4 of 6 neurons exhibited asynchronous EPSCs, suggesting C-type innervation, 1 s [peak (Fp)] and 4 s [average (Fa)] following a stimulus train. ScTx did not alter aEPSCs [two-way RM ANOVA, PEPSC < 0.002, Pdrug = 0.576, PEPSC × drug = 0.166; *P < 0.05 vs. the prestimulus events (Fo)]. E: vagal afferent labeling with the Ca2+ indicator Cal-520. TS stimulation increases Ca2+ fluorescence which was not altered by ScTx. F: increase in fluorescence to TS stimulation relative to the initial baseline (ΔF/F), which was not changed by Kv2 block (P = 0.217, paired t test).
To differentiate the potential influence of Kv2 channels on the reduced TS-EPSC amplitude via an effect on pre- or postsynaptic mechanisms, we examined the TS-EPSC inverse of coefficient of variation (1/CV2; an indicator of changes in presynaptic release), paired-pulse ratio (PPR; EPSC2/EPSC1), and the magnitude of TS-EPSC depression throughout the stimulus relative to the initial event. 1/CV2 was not altered by Kv2 block (0.5 Hz, control, 44.5 ± 22.3 vs. ScTx, 60.1 ± 64.8; N = 6; n = 7; paired t test, P = 0.47). Within any stimulus frequency, Kv2 block with ScTx did not alter the paired pulse ratio (e.g., 10 Hz, control, 0.53 ± 0.21 vs. ScTx, 0.48 ± 0.10; N = 5; n = 6; paired t test, P = 0.5) nor the magnitude of current depression across the 20 evoked events (for 10 Hz, Fig. 6C; N = 5; n = 6; two-way RM ANOVA).
Examination of the A- and C-type ganglia somas demonstrated that Kv2 had the most prominent influence on C-type discharge. Within the nTS, the innervation of C-type fibers onto nTS neurons induces greater asynchronous glutamate release (i.e., aEPSCs) compared to that of A-type fibers (40). To examine the potential contribution of Kv2 in C-fiber afferent presynaptic terminal and thus release, we analyzed the subset of neurons that contained aEPSCs. In the six cells recorded (N = 5), four cells from four rats contained prominent aEPSCs following a 10-Hz stimulus compared to their initial events. As shown in Fig. 6D, the increase in spontaneous EPSCs during the 1 s [peak (Fp)] and 4 s [average (Fa)] following the stimulus train increased relative to before the stimulus [baseline (F0)], and these were unaltered following ScTx. Moreover, upon focusing on only those four cells possessing aEPSCs, ScTX did not alter TS-EPSC amplitude across stimulus frequencies nor their parameters (1/CV2, PPR, and magnitude of depression, P > 0.05 for all, data not shown).
Terminal neurotransmitter release requires an influx of Ca2+. To examine the Kv2 current influence on sensory afferent Ca2+ entry, we stimulated Cal-520-prelabeled sensory afferents at 10 Hz and monitored Ca2+ fluorescence in the absence and presence of ScTx (500 nM, 5 min). aCSF alone served as time control, which did not alter Ca2+ fluorescence (N = 3; n = 15). As shown in the representative example (Fig. 6E) and quantified in Fig. 6F (ΔF/F; N = 3; n = 14), block of Kv2 with ScTx did not alter the increase in Ca2+ fluorescence in response to TS stimulation.
Collectively, our electrophysiological and imaging data indicate no influence of Kv2 on presynaptic glutamate release and its underlying calcium influx and support the lack of expression in synaptophysin-labeled terminals observed via immunohistochemistry.
Kv2 Contribute to nTS Postsynaptic Properties
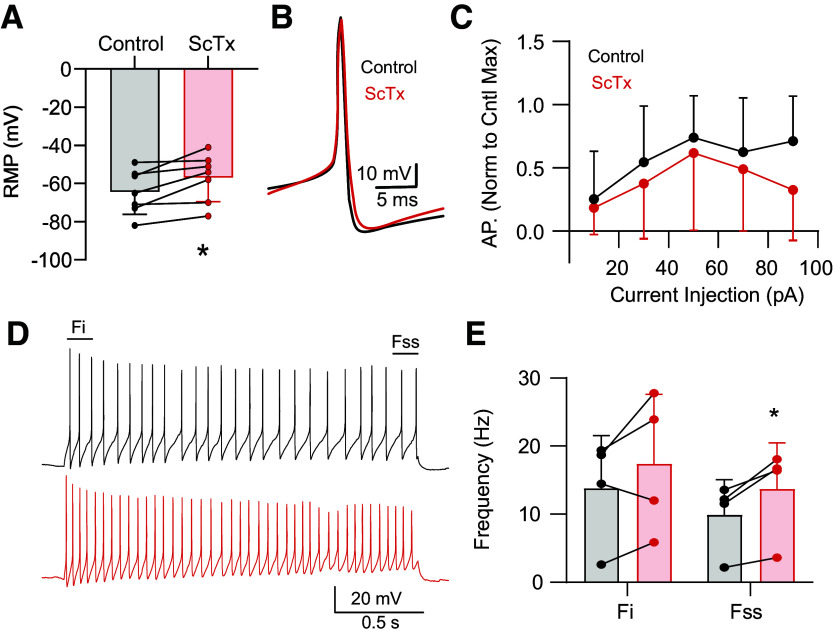
Immunohistochemistry supports robust Kv2 expression in nTS neurons (Fig. 2). In addition, block of Kv2 with ScTx did not alter presynaptic properties although EPSCs were attenuated at higher frequencies of stimulation suggesting a potential postsynaptic influence. In nTS somas, block of Kv2 depolarized membrane potential (Fig. 7A). Brief current injection (10–90 pA, 20-pA steps, 100 ms) induced APs whose discharge rate (Fig 7, B and C) and kinetics (data not shown) were not altered by block of Kv2. For instance, AP half-width was unaltered by ScTx (1.38 ± 0.71 ms vs. 1.39 ± 0.59 ms; N = 6; n = 7; paired t test, P = 0.8551). Prolonged depolarization (30 pA, 2 s) induced discharge that adapted throughout the stimulation (i.e., spike frequency adaptation). While block of Kv2 did not significantly increase the discharge rate of the initial (Fi) events, the steady-state (Fss) discharge examined during the last 200 ms was significantly increased (Fig 7, D and E). These data suggest that Kv2 modifies nTS discharge.
Figure 7.
Kv2 contributes to nucleus of the solitary tract (nTS) activity. A: depolarization of resting membrane potential (RMP) by stromatoxin (ScTx). N = 6; n = 7. *P < 0.05, paired t test. B and C: example of similar action potential (AP) kinetics during control and ScTX (B) and the number of APs during brief depolarization (C; 100 ms; N = 6; n = 7). D: example of AP discharge during a 2-s, 30-pA depolarizing step. The initial frequency of events was not altered, yet the steady-state frequency at the end increased during ScTx. E: quantification of initial (Fi) and steady-state (Fss) AP frequency during control and ScTX. N = 4; n = 4. *P < 0.05, paired t test.
DISCUSSION
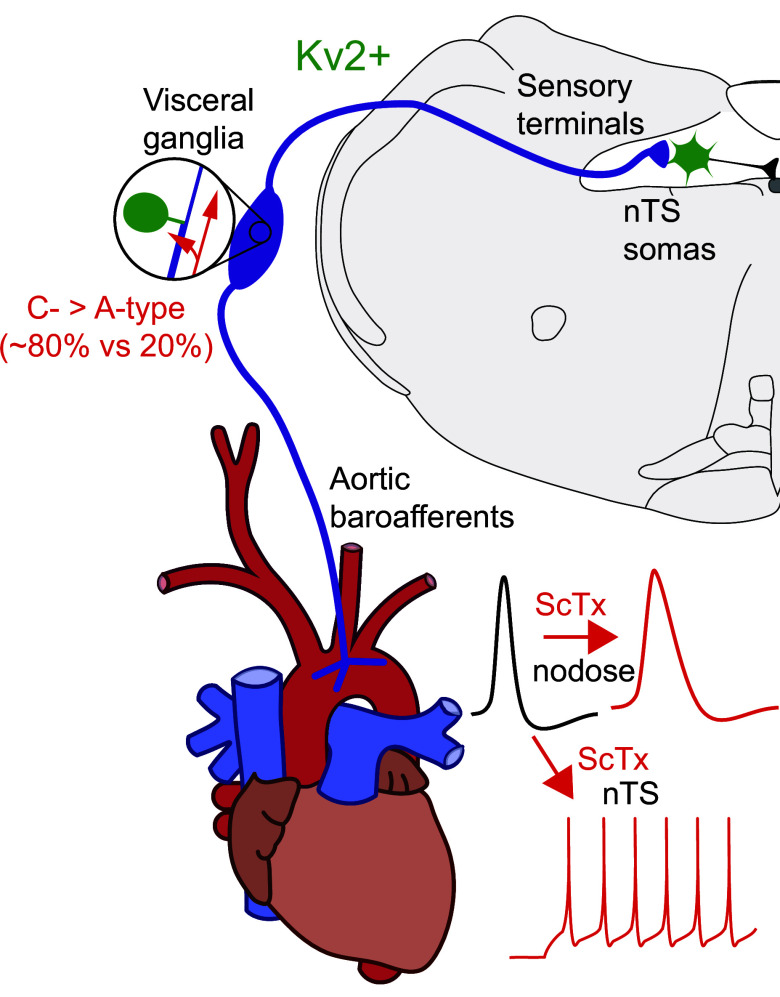
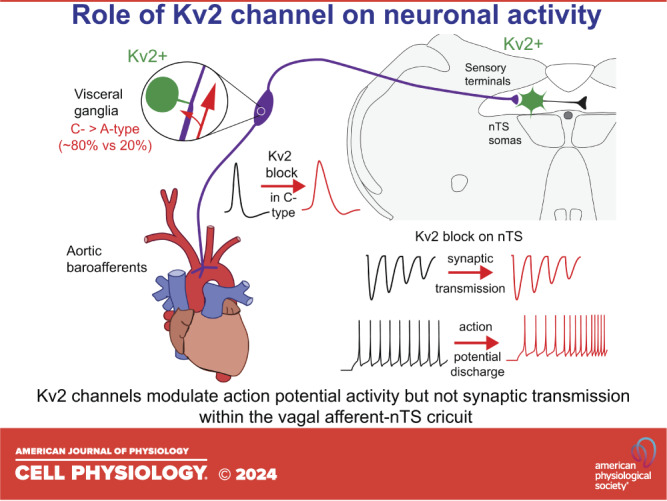
We present several major findings in the current study (Fig. 8): 1) Kv2 channels are responsible for a delayed rectifying current present in the soma and initial axonal segment of both A- and C-type nodose vagal sensory neurons; 2) Kv2 current contributes to repolarization of action potentials of C-type but not A-type neurons; 3) Kv2 is not expressed in peripheral terminals of vagal sensory afferents innervating the aortic arch; 4) Kv2 is not expressed in central vagal terminals within the nTS and therefore does not contribute to glutamate release; and 5) Kv2 influences membrane potential and discharge of second-order nTS neurons. Together, we show Kv2 channels modulate action potential discharge within the ascending vagal afferent arc to likely contribute to reflex integration.
Figure 8.
Schematic summarizing functional location of Kv2 channels in the vagal reflex afferent arc. Kv2 (green) was observed in neurons within the nodose ganglia (comprised of ∼80% C-type neurons) and nucleus of the solitary tract (nTS). C-type nodose neurons increased action potential width following Kv2 block with stromatoxin (ScTx; top traces), whereas Kv2 block augments nTS discharge rate (bottom traces).
Kv2 Is Expressed in the Nodose Ganglia and nTS
Members of the Kv2 family, Kv2.1 and Kv2.2, have been shown to possess overlapping and distinct expression and may form heteromultimers (33). Kv2.1 has been described in the soma, dendrites, and initial segments of axons of central neurons (41, 42). Kv2.2 has also been described in the soma and dendrites (38) yet also in the initial segment of the axon (34). In the nodose cells that have no dendrites, we found Kv2.1 and Kv2.2 immunoreactivity in the soma where it is strongly represented in the internal organelles and more weakly at the surface membrane. Kv2.1 and 2.2 were expressed in somas ranging from 10 to 44 µm in diameter, as well as their initial segments. Kv2.1 in the nodose neurons does not appear in the well-defined clusters described in central neurons (43). In addition, Kv2 does not appear in the peripheral vagal branches of the aortic arch or in their central processes within the brainstem solitary tract. Kv2.1 location is dependent on the phosphorylation state of the channel with dephosphorylation leading to the dissolution of the clustered channels, a more uniform redistribution in the membrane (12), and a shift in the activation threshold for Kv2.1 in the hyperpolarizing direction (44). Several procedures, including anesthetics, lead to dephosphorylation (45, 46). While Kv2.1 clustering was more apparent in nTS than nodose neurons, it is unlikely that such expression differences were due to anesthetic alone as similar results were observed under several protocols. Recent studies report that the active form of the Kv2.1 channel is the dispersed form (47). In addition, when Kv2.1 is coexpressed with Kv2.2 in the same cell, the channels do not form clusters (48). We did not thoroughly examine Kv2.2 location throughout the vagal afferent-nTS arc, and future studies will be required to differentiate between the members of this family.
Stromatoxin (ScTx) was used to isolate the functional contribution of Kv2 within the vagal afferent-nTS arc. The ScTx-sensitive K+ current examined in the present study had similar kinetic characteristics consistent with those of Kv2 in other studies (32, 35, 37, 49–54), strongly indicating the current examined belongs to the Kv2 family. While we did not attempt to assess relative contributions of Kv2.1 and Kv2.2 to the total Kv2 current in the present study, we show that Kv2 is expressed and functional in vagal afferent somas.
Kv2 Channels Limit AP Broadening Primarily in C-Type Sensory Neurons
In examining the functional role of Kv2 in nodose neurons, we investigated the response to a brief depolarization that elicits a single action potential from the resting membrane potential as a surrogate for physiological activity in vivo where the soma generally remains quiescent at resting potentials in the range of −50 to −60 mV. Under physiological conditions, these neurons discharge only when action potentials invade from the periphery. Thus it is unlikely that the membrane potential, except during the action potential, falls in a range to substantially activate Kv2 channels. In the present study, the application of ScTx or the inclusion of anti-Kv2.1 antibodies in the recording pipette solution broadened the action potential of C-type but not A-type neurons (Fig. 8). This is consistent with the short-duration action potentials of A-type and the slow time constant of Kv2 activation. The magnitude of the increase of the C-type action potentials in the presence of ScTx or anti-Kv2.1 is dependent on the initial duration of the action potential and consistent with an increased contribution of Kv2.1 to repolarization at longer durations. A minor limitation of these studies is the use of ScTx, which cannot distinguish between Kv2.1 and Kv2.2, yet this is mitigated by the use of anti-Kv2.1 and observation of a similar action potential broadening as ScTx.
The natural frequency of invading peripheral activity is generally low for C-type neurons (≤20 Hz) but higher for A-type neurons (≤60 Hz). Most visceral sensory C-receptors discharge in the frequency range of 10 Hz or less in response to physiological stimuli (1, 5–8). As the frequency is maintained or elevated, a frequency-dependent increase in the duration of C- but not A-type vagal sensory neurons has been described (39) and confirmed in the present study. A role for K+ channels in the frequency-dependent increase in duration including Kv3 in dorsal root ganglion (DRG) cells (55) and 4-AP-sensitive transient A-type K+ channels in supraoptic neurons (56) has been proposed. A report implicating Kv2.1 in the frequency-dependent broadening of the action potentials comes from the invertebrate preparation of Aplysia bag cell neurons. With their long-duration action potentials, a frequency-dependent broadening of the action potential has been attributed to partial inactivation of the Aplysia homolog of the mammalian Kv2.1 (57, 58). In the present study, frequencies above 1 Hz produced cumulative inactivation of Kv2.1 current, which would be expected to lead to broadening of the action potential of C-type neurons in which the initial duration of the action potential was sufficiently long for Kv2.1 to make a significant contribution to the total potassium current. However, in the present study, while ScTx block of Kv2 current led to a prolonged action potential at all frequencies in C-type neurons, it did not eliminate the additional frequency-dependent increase. One explanation is an additional but necessary role for resulting calcium influx (56, 59, 60), which we did not control in the present study. Another is that the K+ channel type involved in frequency-dependent duration is cell-type specific.
Kv2 Channels Do Not Participate in Glutamate Release at the Afferent nTS Synapse
Kv2 containing vagal nodose sensory afferents terminate in the brainstem nTS. Kv2 channels in other central synapses modulate membrane potential and neurotransmitter release (14). While Kv2 channels contribute to the discharge of vagal afferent somas, the role of these channels in their central terminals appears limited. Specifically, block of Kv2 with ScTx did not alter TS-EPSC amplitude at frequencies between 0.5 and 1 Hz, similar to our observation in nodose neurons. By contrast, between 5- and 10-Hz TS afferent stimulation, ScTx generally decreased TS-EPSC amplitude across a stimulus train. Nevertheless, these reductions were not accompanied by changes in indicators of presynaptic release, including the magnitude of depression from the initial event, the paired-pulse ratio, or the inverse of the coefficient of amplitude variation (23, 24, 61). Given the broadening of the AP observed in C-type fibers, it was anticipated that block of Kv2 would increase or at least maintain glutamate release, the primary neurotransmitter released from vagal afferents, through augmented calcium influx. However, Kv2 channels did not alter EPSC parameters whether they originated from A-type or C-type fibers, the latter identified by the presence of asynchronous EPSCs (15, 40). To confirm the lack of presynaptic influence, we examined terminal calcium fluorescence in response to afferent (TS) stimulation, which was also unaltered by block of Kv2. Together, these results indicate the reduction in TS-EPSC amplitude may be due to synaptic or extrasynaptic mechanisms (62) at the second-order neuron and are consistent with our immunohistochemical observations that Kv2 is not located in synaptophysin-labeled terminals. Interestingly, prolonged synaptic activation produces dephosphorylation of Kv2.1 to shift its activation range in the hyperpolarizing direction (63). As a result, Kv2 activation may provide an intrinsic limitation on neuronal activity. Given the prominent expression of Kv2 in the postsynaptic nTS neurons, additional studies will be needed to decipher their complete role.
Kv2 Is Functional within nTS Second-Order Neurons
In contrast to the central terminals, Kv2 was identified immunohistologically and functionally in nTS second-order neurons (Fig. 8). ScTx induced depolarization of the resting membrane potential, in contrast to that observed in nodose neurons. Moreover, this effect occurred at potentials more hyperpolarized than the activation threshold for these channels in our pathway. This paradoxical depolarization may have occurred due to one or more mechanisms, including persistent fluctuations in membrane potential due to spontaneous synaptic glutamatergic events or the modulation of secondary messengers that may lower the activation threshold through dephosphorylation (64–68). Interestingly, glutamate exposure results in declustering of Kv2.1 and a hyperpolarizing shift in its activation (46). nTS neurons are under a persistent barrage of spontaneous and evoked EPSCs from the periphery, internal network, and other central circuits, thus increasing the likelihood of Kv2 activation. In addition, Kv2.1 has been shown to cluster additional proteins such as the L-type Cav 1.2 channel, which may also promote depolarization (69, 70). However, the Kv2 channels mediating calcium influx are proposed to be nonconducting (71), and thus this mechanism may be less likely. Block of Kv2 with ScTx did not alter the kinetics of the nTS action potential, which is consistent with the activation time of Kv2 and the short duration of the action potential, as in nodose A-type neurons. In addition, ScTx did not alter the number of action potentials generated in response to brief (100 ms) depolarization. However, when the neurons were exposed to a prolonged 2-s depolarization, a period sufficient for activation of Kv2, ScTx increased the steady-state frequency, consistent with the role of Kv2 in limiting excitability (46, 72).
What Role Does Kv2 Play in Afferent Activity?
Altogether, we show that Kv2 plays a significant role in blunting neuronal activity at the level of the nodose and nTS soma but not in controlling glutamate release. What role does Kv2.1/Kv2.2 play in normal function in the sensory afferent where it appears restricted to the soma and initial segment? Several physiological possibilities may exist and will require further study. One role of the soma may be as a monitor of the activity of its sensory terminals. For instance, for C-type neurons, Kv2 is essential for limiting action potential duration. An increase in discharge frequency of the sensory terminals delivered to the soma may lead to an increase in the duration of the somal action potential and subsequent increased calcium influx providing a signal to the neuron that may be used in, for instance, activity-dependent gene regulation (73). An increase in calcium may also release chemical substances from the soma that may activate adjacent nodose neurons (74). Whatever alters the functional expression of Kv2.1 (e.g., development, disease, or injury; Refs. 75, 76) or alters its kinetic properties (e.g., associated subunits) could alter action potential duration or discharge rate and by extension increase calcium influx.
Another role may exist for the expression of Kv2 in the soma and initial segment. Pseudo-unipolar nodose neurons have a single process emerging from the soma, which diverges to peripheral and central branches (77, 78). At this T-junction, it has been reported in nodose and dorsal root ganglia neurons there are differences in the length, diameter, and myelination status of the initial process and peripheral and central branches (78–80). Our immunohistochemistry demonstrated that Kv2 was located at the initial segment of nodose neurons, yet the extent it is expressed and functional at the T-junction requires further study. The potential existence of Kv2 at the T-junction may play critical roles in controlling excitability and action potential backpropagation (81). Consistent with this idea, slowly activating voltage-dependent K+ currents have been suggested to be important in low-pass filtering of activity at the T-junction of DRG neurons (80). Peripheral generated vagal afferent discharge does not require entry into the soma for its continuation to the nTS; however, the T-junction may present another opportunity in which high-frequency afferent viscerosensory activity is filtered at the level of the nodose as it projects to the nTS synapse.
While Kv2 does not appear to influence glutamate release, its role in tempering prolonged discharge at the level of the second-order neuron is likely critical for the integration of one or more viscerosensory signals and preventing overexcitation to limit, for instance, depressor responses to baroreflex activation.
In summary, we demonstrate the delayed rectifier Kv2 family is expressed and functionally important in controlling neuronal excitability of the primary and secondary neurons within the afferent vagal reflex arc (Fig. 8). Its expression likely contributes to modulation of several functions, including gene expression, discharge fidelity, and physiological responses to activation of numerous vagal reflexes.
DATA AVAILABILITY
All data are presented within the text and figures and available upon request.
GRANTS
The study was supported in part by National Heart, Lung, and Blood Institute Grants HL061436 (to D.L.K.) and HL128454 (to D.D.K.) and American Heart Association Grant 836140 (to L.L.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.L.S., D.D.K., and D.L.K. conceived and designed research; A.R.-N., L.L.S., P.A.G., H.A.D., D.D.K., and D.L.K. performed experiments; A.R.-N., L.L.S., P.A.G., H.A.D., D.D.K., and D.L.K. analyzed data; A.R.-N., L.L.S., D.D.K., and D.L.K. interpreted results of experiments; L.L.S., D.D.K., and D.L.K. prepared figures; D.D.K. and D.L.K. drafted manuscript; L.L.S., P.A.G., H.A.D., D.D.K., and D.L.K. edited and revised manuscript; A.R.-N., L.L.S., P.A.G., H.A.D., D.D.K., and D.L.K. approved final version of manuscript.
REFERENCES
- 1. Fidone SJ, Sato A. A study of chemoreceptor and baroreceptor A and C-fibres in the cat carotid nerve. J Physiol 205: 527–548, 1969. doi: 10.1113/jphysiol.1969.sp008981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Landgren S. On the excitation mechanism of the carotid baroceptors. Acta Physiol Scand 26: 1–34, 1952. doi: 10.1111/j.1748-1716.1952.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 3. Widdicombe JG. Pulmonary and respiratory tract receptors. J Exp Biol 100: 41–57, 1982. doi: 10.1242/jeb.100.1.41. [DOI] [PubMed] [Google Scholar]
- 4. Yao T, Thoren P. Characteristics of brachiocephalic and carotid sinus baroreceptors with non-medullated afferents in rabbit. Acta Physiol Scand 117: 1–8, 1983. doi: 10.1111/j.1748-1716.1983.tb07172.x. [DOI] [PubMed] [Google Scholar]
- 5. Coleridge HM, Coleridge JC, Schultz HD. Characteristics of C fibre baroreceptors in the carotid sinus of dogs. J Physiol 394: 291–313, 1987. doi: 10.1113/jphysiol.1987.sp016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coleridge JC, Coleridge HM, Schelegle ES, Green JF. Acute inhalation of ozone stimulates bronchial C-fibers and rapidly adapting receptors in dogs. J Appl Physiol (1985) 74: 2345–2352, 1993. doi: 10.1152/jappl.1993.74.5.2345. [DOI] [PubMed] [Google Scholar]
- 7. Kaufman MP, Coleridge HM, Coleridge JC, Baker DG. Bradykinin stimulates afferent vagal C-fibers in intrapulmonary airways of dogs. J Appl Physiol Respir Environ Exerc Physiol 48: 511–517, 1980. doi: 10.1152/jappl.1980.48.3.511. [DOI] [PubMed] [Google Scholar]
- 8. Thoren P, Saum WR, Brown AM. Characteristics of rat aortic baroreceptors with nonmedullated afferent nerve fibers. Circ Res 40: 231–237, 1977. doi: 10.1161/01.res.40.3.231. [DOI] [PubMed] [Google Scholar]
- 9. Schild JH, Kunze DL. Differential distribution of voltage-gated channels in myelinated and unmyelinated baroreceptor afferents. Auton Neurosci 172: 4–12, 2012. doi: 10.1016/j.autneu.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 10. Glazebrook PA, Ramirez AN, Schild JH, Shieh CC, Doan T, Wible BA, Kunze DL. Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons. J Physiol 541: 467–482, 2002. doi: 10.1113/jphysiol.2001.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramirez-Navarro A, Glazebrook PA, Kane-Sutton M, Padro C, Kline DD, Kunze DL. Kv1.3 channels regulate synaptic transmission in the nucleus of solitary tract. J Neurophysiol 105: 2772–2780, 2011. doi: 10.1152/jn.00494.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci 7: 711–718, 2004. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 13. Johnston J, Forsythe ID, Kopp-Scheinpflug C. Going native: voltage-gated potassium channels controlling neuronal excitability. J Physiol 588: 3187–3200, 2010. doi: 10.1113/jphysiol.2010.191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feinshreiber L, Singer-Lahat D, Ashery U, Lotan I. Voltage-gated potassium channel as a facilitator of exocytosis. Ann N Y Acad Sci 1152: 87–92, 2009. doi: 10.1111/j.1749-6632.2008.03997.x. [DOI] [PubMed] [Google Scholar]
- 15. Kline DD, Buniel MCF, Glazebrook P, Peng Y-JJ, Ramirez-Navarro A, Prabhakar NR, Kunze DL. Kv1.1 deletion augments the afferent hypoxic chemosensory pathway and respiration. J Neurosci 25: 3389–3399, 2005. doi: 10.1523/JNEUROSCI.4556-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kunze DL, Krauhs JM, Orlea CJ. Direct action of norepinephrine on aortic baroreceptors of rat adventitia. Am J Physiol Heart Circ Physiol 247: H811–H816, 1984. doi: 10.1152/ajpheart.1984.247.5.H811. [DOI] [PubMed] [Google Scholar]
- 17. Langen KR, Dantzler HA, de Barcellos-Filho PG, Kline DD. Hypoxia augments TRPM3-mediated calcium influx in vagal sensory neurons. Auton Neurosci 247: 103095, 2023. doi: 10.1016/j.autneu.2023.103095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dantzler HA, Kline DD. Exaggerated potassium current reduction by oxytocin in visceral sensory neurons following chronic intermittent hypoxia. Auton Neurosci 230: 102758, 2020. doi: 10.1016/j.autneu.2020.102758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doan TN, Kunze DL. Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J Physiol 514: 125–138, 1999. doi: 10.1111/j.1469-7793.1999.125af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bocksteins E, Raes AL, Van de Vijver G, Bruyns T, Van Bogaert PP, Snyders DJ. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am J Physiol Cell Physiol 296: C1271–C1278, 2009. doi: 10.1152/ajpcell.00088.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Escoubas P, Diochot S, Celerier ML, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol 62: 48–57, 2002. doi: 10.1124/mol.62.1.48. [DOI] [PubMed] [Google Scholar]
- 22. Shiau YS, Huang PT, Liou HH, Liaw YC, Shiau YY, Lou KL. Structural basis of binding and inhibition of novel tarantula toxins in mammalian voltage-dependent potassium channels. Chem Res Toxicol 16: 1217–1225, 2003. doi: 10.1021/tx0341097. [DOI] [PubMed] [Google Scholar]
- 23. Lima-Silveira L, Hasser EM, Kline DD. Cardiovascular deconditioning increases GABA signaling in the nucleus tractus solitarii. J Neurophysiol 128: 28–39, 2022. doi: 10.1152/jn.00102.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lima-Silveira L, Martinez D, Hasser EM, Kline DD. Mechanisms underlying neuroplasticity in the nucleus tractus solitarii following hindlimb unloading in rats. Neuroscience 449: 214–227, 2020. doi: 10.1016/j.neuroscience.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 26. Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol 164: 105–111, 2008. doi: 10.1016/j.resp.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klemic KG, Durand DM, Jones SW. Activation kinetics of the delayed rectifier potassium current of bullfrog sympathetic neurons. J Neurophysiol 79: 2345–2357, 1998. doi: 10.1152/jn.1998.79.5.2345. [DOI] [PubMed] [Google Scholar]
- 28. Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol 58: 253–262, 2000. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- 29. Stansfeld CE, Wallis DI. Properties of visceral primary afferent neurons in the nodose ganglion of the rabbit. J Neurophysiol 54: 245–260, 1985. doi: 10.1152/jn.1985.54.2.245. [DOI] [PubMed] [Google Scholar]
- 30. Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Methods 164: 75–85, 2007. doi: 10.1016/j.jneumeth.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kline DD, Wang S, Kunze DL. TrpV1 channels contribute to spontaneous glutamate release in nucleus tractus solitarius (nTS) following chronic intermittent hypoxia (CIH). J Neurophysiol 121: 881–892, 2019. doi: 10.1152/jn.00536.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shi G, Kleinklaus AK, Marrion NV, Trimmer JS. Properties of Kv2.1 K+ channels expressed in transfected mammalian cells. J Biol Chem 269: 23204–23211, 1994. doi: 10.1016/S0021-9258(17)31640-X. [DOI] [PubMed] [Google Scholar]
- 33. Kihira Y, Hermanstyne TO, Misonou H. Formation of heteromeric Kv2 channels in mammalian brain neurons. J Biol Chem 285: 15048–15055, 2010. doi: 10.1074/jbc.M109.074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnston J, Griffin SJ, Baker C, Skrzypiec A, Chernova T, Forsythe ID. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons. J Physiol 586: 3493–3509, 2008. doi: 10.1113/jphysiol.2008.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kerschensteiner D, Stocker M. Heteromeric assembly of Kv2.1 with Kv9.3: effect on the state dependence of inactivation. Biophys J 77: 248–257, 1999. doi: 10.1016/S0006-3495(99)76886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klemic KG, Shieh CC, Kirsch GE, Jones SW. Inactivation of Kv2.1 potassium channels. Biophys J 74: 1779–1789, 1998. doi: 10.1016/S0006-3495(98)77888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kramer JW, Post MA, Brown AM, Kirsch GE. Modulation of potassium channel gating by coexpression of Kv2.1 with regulatory Kv5.1 or Kv6.1 α-subunits. Am J Physiol Cell Physiol 274: C1501–C1510, 1998. doi: 10.1152/ajpcell.1998.274.6.C1501. [DOI] [PubMed] [Google Scholar]
- 38. Guan D, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. J Physiol 581: 941–960, 2007. doi: 10.1113/jphysiol.2007.128454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li BY, Feng B, Tsu HY, Schild JH. Unmyelinated visceral afferents exhibit frequency dependent action potential broadening while myelinated visceral afferents do not. Neurosci Lett 421: 62–66, 2007. doi: 10.1016/j.neulet.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 40. Peters JH, McDougall SJ, Fawley JA, Andresen MC. TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLoS One 6: e25015, 2011. doi: 10.1371/journal.pone.0025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trimmer JS. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc Natl Acad Sci U S A 88: 10764–10768, 1991. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sarmiere PD, Weigle CM, Tamkun MM. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci 9: 112, 2008. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scannevin RH, Murakoshi H, Rhodes KJ, Trimmer JS. Identification of a cytoplasmic domain important in the polarized expression and clustering of the Kv2.1 K+ channel. J Cell Biol 135: 1619–1632, 1996. doi: 10.1083/jcb.135.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol 52: 821–828, 1997. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- 45. Kulkarni RS, Zorn LJ, Anantharam V, Bayley H, Treistman SN. Inhibitory effects of ketamine and halothane on recombinant potassium channels from mammalian brain. Anesthesiology 84: 900–909, 1996. doi: 10.1097/00000542-199604000-00018. [DOI] [PubMed] [Google Scholar]
- 46. Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated K+ channel critical to dynamic control of neuronal excitability. Neurotoxicology 26: 743–752, 2005. doi: 10.1016/j.neuro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 47. Johnson B, Leek AN, Tamkun MM. Kv2 channels create endoplasmic reticulum/plasma membrane junctions: a brief history of Kv2 channel subcellular localization. Channels (Austin) 13: 88–101, 2019. doi: 10.1080/19336950.2019.1568824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jenkins PM, McIntyre JC, Zhang L, Anantharam A, Vesely ED, Arendt KL, Carruthers CJ, Kerppola TK, Iniguez-Lluhi JA, Holz RW, Sutton MA, Martens JR. Subunit-dependent axonal trafficking of distinct alpha heteromeric potassium channel complexes. J Neurosci 31: 13224–13235, 2011. doi: 10.1523/JNEUROSCI.0976-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murakoshi H, Trimmer JS. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci 19: 1728–1735, 1999. doi: 10.1523/JNEUROSCI.19-05-01728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salinas M, de Weille J, Guillemare E, Lazdunski M, Hugnot JP. Modes of regulation of shab K+ channel activity by the Kv8.1 subunit. J Biol Chem 272: 8774–8780, 1997. doi: 10.1074/jbc.272.13.8774. [DOI] [PubMed] [Google Scholar]
- 51. Wood MJ, Korn SJ. Two mechanisms of K(+)-dependent potentiation in Kv2.1 potassium channels. Biophys J 79: 2535–2546, 2000. doi: 10.1016/S0006-3495(00)76494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chiara MD, Monje F, Castellano A, Lopez-Barneo J. A small domain in the N terminus of the regulatory alpha-subunit Kv2. 3 modulates Kv2.1 potassium channel gating. J Neurosci 19: 6865–6873, 1999. doi: 10.1523/JNEUROSCI.19-16-06865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kirsch GE, Drewe JA. Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. J Gen Physiol 102: 797–816, 1993. doi: 10.1085/jgp.102.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ju M, Stevens L, Leadbitter E, Wray D. The roles of N- and C-terminal determinants in the activation of the Kv2.1 potassium channel. J Biol Chem 278: 12769–12778, 2003. doi: 10.1074/jbc.M212973200. [DOI] [PubMed] [Google Scholar]
- 55. Liu PW, Blair NT, Bean BP. Action potential broadening in capsaicin-sensitive DRG neurons from frequency-dependent reduction of Kv3 current. J Neurosci 37: 9705–9714, 2017. doi: 10.1523/JNEUROSCI.1703-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hlubek MD, Cobbett P. Differential effects of K(+) channel blockers on frequency-dependent action potential broadening in supraoptic neurons. Brain Res Bull 53: 203–209, 2000. doi: 10.1016/s0361-9230(00)00335-x. [DOI] [PubMed] [Google Scholar]
- 57. Kaczmarek LK, Strumwasser F. The expression of long lasting afterdischarge by isolated Aplysia bag cell neurons. J Neurosci 1: 626–634, 1981. doi: 10.1523/JNEUROSCI.01-06-00626.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang Y, McKay SE, Bewley B, Kaczmarek LK. Repetitive firing triggers clustering of Kv2.1 potassium channels in Aplysia neurons. J Biol Chem 283: 10632–10641, 2008. doi: 10.1074/jbc.M800253200. [DOI] [PubMed] [Google Scholar]
- 59. Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A 88: 380–384, 1991. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aldrich RW, Jr, Getting PA, Thompson SH. Mechanism of frequency-dependent broadening of molluscan neurone soma spikes. J Physiol 291: 531–544, 1979. doi: 10.1113/jphysiol.1979.sp012829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Huijstee AN, Kessels HW. Variance analysis as a tool to predict the mechanism underlying synaptic plasticity. J Neurosci Methods 331: 108526, 2020. doi: 10.1016/j.jneumeth.2019.108526. [DOI] [PubMed] [Google Scholar]
- 62. Muennich EA, Fyffe RE. Focal aggregation of voltage-gated, Kv2.1 subunit-containing, potassium channels at synaptic sites in rat spinal motoneurones. J Physiol 554: 673–685, 2004. doi: 10.1113/jphysiol.2003.056192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siddoway B, Hou H, Yang J, Sun L, Yang H, Wang GY, Xia H. Potassium channel Kv2.1 is regulated through protein phosphatase-1 in response to increases in synaptic activity. Neurosci Lett 583: 142–147, 2014. doi: 10.1016/j.neulet.2014.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang CY, Huang AQ, Zhou MH, Mei YA. GDF15 regulates Kv2.1-mediated outward K+ current through the Akt/mTOR signalling pathway in rat cerebellar granule cells. Biochem J 460: 35–47, 2014. doi: 10.1042/BJ20140155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Song MY, Hong C, Bae SH, So I, Park KS. Dynamic modulation of the kv2.1 channel by SRC-dependent tyrosine phosphorylation. J Proteome Res 11: 1018–1026, 2012. doi: 10.1021/pr200770v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tiran Z, Peretz A, Attali B, Elson A. Phosphorylation-dependent regulation of Kv2.1 channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase epsilon. J Biol Chem 278: 17509–17514, 2003. doi: 10.1074/jbc.M212766200. [DOI] [PubMed] [Google Scholar]
- 67. Sobko A, Peretz A, Attali B. Constitutive activation of delayed-rectifier potassium channels by a src family tyrosine kinase in Schwann cells. EMBO J 17: 4723–4734, 1998. doi: 10.1093/emboj/17.16.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li Z, Dong W, Zhang X, Lu JM, Mei YA, Hu C. Protein kinase C controls the excitability of cortical pyramidal neurons by regulating Kv2.2 channel activity. Neurosci Bull 38: 135–148, 2022. doi: 10.1007/s12264-021-00773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Dwyer SC, Palacio S, Matsumoto C, Guarina L, Klug NR, Tajada S, Rosati B, McKinnon D, Trimmer JS, Santana LF. Kv2.1 channels play opposing roles in regulating membrane potential, Ca(2+) channel function, and myogenic tone in arterial smooth muscle. Proc Natl Acad Sci U S A 117: 3858–3866, 2020. doi: 10.1073/pnas.1917879117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vierra NC, Trimmer JS. Ion channel partnerships: odd and not-so-odd couples controlling neuronal ion channel function. Int J Mol Sci 23: 19–31, 2022. doi: 10.3390/ijms23041953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vierra NC, O'Dwyer SC, Matsumoto C, Santana LF, Trimmer JS. Regulation of neuronal excitation-transcription coupling by Kv2.1-induced clustering of somatic L-type Ca(2+) channels at ER-PM junctions. Proc Natl Acad Sci U S A 118: e2110094118, 2021. doi: 10.1073/pnas.2110094118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol 522: 19–31, 2000. doi: 10.1111/j.1469-7793.2000.t01-2-00019.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Park D, Dunlap K. Dynamic regulation of calcium influx by G-proteins, action potential waveform, and neuronal firing frequency. J Neurosci 18: 6757–6766, 1998. doi: 10.1523/JNEUROSCI.18-17-06757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oh EJ, Weinreich D. Chemical communication between vagal afferent somata in nodose Ganglia of the rat and the Guinea pig in vitro. J Neurophysiol 87: 2801–2807, 2002. doi: 10.1152/jn.2002.87.6.2801. [DOI] [PubMed] [Google Scholar]
- 75. Jędrychowska J, Korzh V. Kv2.1 voltage-gated potassium channels in developmental perspective. Dev Dyn 248: 1180–1194, 2019. doi: 10.1002/dvdy.114. [DOI] [PubMed] [Google Scholar]
- 76. Tsantoulas C, Zhu L, Yip P, Grist J, Michael GJ, McMahon SB. Kv2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input. Exp Neurol 251: 115–126, 2014. doi: 10.1016/j.expneurol.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ottaviani MM, Macefield VG. Structure and functions of the vagus nerve in mammals. Compr Physiol 12: 3989–4037, 2022. doi: 10.1002/cphy.c210042. [DOI] [PubMed] [Google Scholar]
- 78. Jaffe RA, Sampson SR. Analysis of passive and active electrophysiologic properties of neurons in mammalian nodose ganglia maintained in vitro. J Neurophysiol 39: 802–815, 1976. doi: 10.1152/jn.1976.39.4.802. [DOI] [PubMed] [Google Scholar]
- 79. Ranson SW, Foley JO, Alpert CD. Observations on the structure of the vagus nerve. Am J Anat 53: 289–315, 1933. doi: 10.1002/aja.1000530206. [DOI] [Google Scholar]
- 80. Sundt D, Gamper N, Jaffe DB. Spike propagation through the dorsal root ganglia in an unmyelinated sensory neuron: a modeling study. J Neurophysiol 114: 3140–3153, 2015. doi: 10.1152/jn.00226.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ducreux C, Reynaud JC, Puizillout JJ. Spike conduction properties of T-shaped C neurons in the rabbit nodose ganglion. Pflugers Arch 424: 238–244, 1993. doi: 10.1007/BF00384348. [DOI] [PubMed] [Google Scholar]
- 82. Krauhs JM. Structure of rat aortic baroreceptors and their relationship to connective tissue. J Neurocytol 8: 401–414, 1979. doi: 10.1007/BF01214800. [DOI] [PubMed] [Google Scholar]
- 83. Sobko A, Peretz A, Shirihai O, Etkin S, Cherepanova V, Dagan D, Attali B. Heteromultimeric delayed-rectifier K+ channels in Schwann cells: developmental expression and role in cell proliferation. J Neurosci 18: 10398–10408, 1998. doi: 10.1523/JNEUROSCI.18-24-10398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented within the text and figures and available upon request.