Abstract
Increased kynurenine pathway metabolism has been implicated in the etiology of AIDS dementia complex (ADC). The rate-limiting enzyme for this pathway is indolamine 2,3-dioxygenase (IDO). We tested the efficacy of different strains of human immunodeficiency virus type 1 (HIV1-BaL, HIV1-JRFL, and HIV1-631) to induce IDO in cultured human monocyte-derived macrophages (MDM). A significant increase in both IDO protein and kynurenine synthesis was observed after 48 h in MDM infected with the brain-derived HIV-1 isolates, laboratory-adapted (LA) HIV1-JRFL, and primary isolate HIV1-631. In contrast, almost no kynurenine production or IDO protein was evident in MDM infected with the highly replicating macrophage-tropic LA strain HIV1-BaL. The induction of IDO and kynurenine synthesis by HIV1-JRFL and HIV1-631 declined to baseline levels by day 8 postinfection. Abundant HIV-1 replication did not reduce the ability of exogenous gamma interferon (IFN-γ) to induce IDO and kynurenine synthesis in HIV-infected MDM. The addition of anti-IFN-γ antibody to MDM infected with HIV1-JRFL resulted in an absence of detectable IDO protein after 48 h and a decrease of 64% ± 1% in supernatant kynurenine concentration. Together, these results indicate that only selected strains of HIV-1 are capable of inducing IDO synthesis and subsequent kynurenine metabolism in MDM. The induction of IDO, while apparently independent of replication capacity, appears to be mediated by a transient production of IFN-γ in MDM responding to the initial infection with selected strains of HIV-1.
A significant percentage of HIV-1 infected individuals develop cognitive/motor abnormalities (16), which are referred to collectively as AIDS-related dementia complex (ADC). In ADC the CNS pathology is characterized by neuronal cell loss, astrogliosis, infiltrating macrophages, and formation of microglial nodules and giant cells (4, 9, 15). However, the underlying cause of neuronal degeneration in ADC is unknown. Productive HIV infection in the CNS is limited to macrophages and microglial cells, with restricted infection in astrocytes and essentially no infection within neuronal cells (16). This suggests that HIV-1 infection of brain macrophages may be central to the loss of neurological function in ADC through an indirect immune system-mediated mechanism (13).
Macrophages activated with HIV-1 or HIV-1 envelope glycoprotein (gp120) contribute to the production of a number of putative neurotoxins including glutamate (8), arachidonic acid metabolites (12, 13), nitric oxide (12), platelet-activating factor (13), tumor necrosis factor alpha (12, 13, 38), and quinolinic acid (30, 36). Elevated levels of quinolinic acid, in particular, have been consistently observed in vivo in the cerebrospinal fluid and brain parenchyma of patients with ADC (1, 19, 36). The severity of neurological symptoms has been correlated with the increase in quinolinic acid levels in the brain in the simian immunodeficiency virus model of ADC (21).
Quinolinic acid is an endogenous agonist (excitotoxin) at the N-methyl-d-aspartate receptor, a subtype of the glutamate receptor in the CNS, and therefore may act as a primary mediator of neuronal dysfunction in ADC (35).
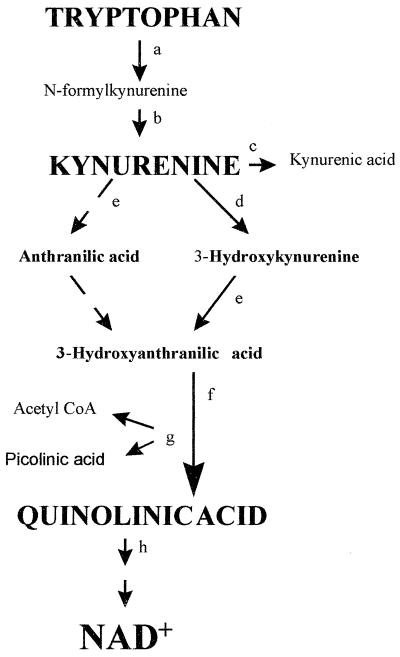
Quinolinic acid is produced de novo by the oxidative catabolism of the essential amino acid tryptophan through the kynurenine pathway (5). The first and rate-limiting enzyme for this pathway is IDO, which can be induced by the proinflammatory cytokine IFN-γ (2, 19, 37). IDO catalyzes the conversion of tryptophan to N-formylkynurenine, which can then be converted nonenzymatically to the first stable product, kynurenine (Fig. 1). An increase in IDO activity has been found in the frontal cortex of patients with ADC but not in HIV-1 infected patients without encephalopathy (35). This suggests that quinolinic acid is synthesized locally in the CNS of these patients and may be one of the factors associated with the unique pathology of ADC.
FIG. 1.
The kynurenine pathway (a) IDO (EC 1.13.11.17). (b) Kynurenine formylase (EC 3.5.1.9). (c) Kynurenine aminotransferase (EC 2.6.1.7). (d) Kynurenine 3-hydroxylase (EC 1.14.13.9). (e) Kynureninase (EC 3.7.1.3). (f) 3-Hydroxyanthranilic acid oxidase (EC 1.13.11.6). (g) Picolinic acid carboxylase (EC 4.1.1.45). (h) Quinolinic acid phosphoribosyltransferase (EC 2.4.2.19). (i) poly(ADP)polymerase (EC 2.4.2.30). (j) Nicotinamide phosphoribosyltransferase (EC 2.4.2.12). CoA, coenzyme A.
HIV-1 can be isolated from the CNS of virtually all subjects with AIDS; however, only about 30% of those individuals develop dementia (17). Activated macrophages and microglia are apparently the only cells capable of catabolizing tryptophan to quinolinic acid in the CNS (20, 25). Low levels of quinolinic acid production from HIV-1-infected macrophages have been observed (30, 36); however, neither the mechanism of quinolinic acid synthesis (i.e., IDO induction) nor the effect of different viral strains on the induction of IDO has been previously investigated. Although the role of strain variability in the development of ADC is unknown, it has been suggested that conservation of key amino acids in the third hypervariable region (V3) of gp120 in brain-derived HIV-1 isolates correlates with their ability to infect brain microglia/macrophages (26, 32). This cellular tropism, which is also influenced by the expression and utilization of various types of HIV-1 coreceptors (18), may be important in the etiology of ADC.
In this study we investigated whether direct infection of MDM in culture with different HIV-1 strains could induce the first enzyme of the kynurenine pathway, IDO, leading to kynurenine synthesis. The results showed that only selected HIV-1 strains induce IDO and kynurenine pathway metabolism efficiently in MDM and that this did not appear to be related to their level of replication.
MATERIALS AND METHODS
Abbreviations used in this paper.
ADC, AIDS-related dementia complex; IDO, indolamine,2,3-dioxygenase; MDM, monocyte derived macrophages; IFN-γ, interferon gamma; HIV-1, human immunodeficiency virus type 1; CNS, central nervous system; LA, laboratory adapted; TBS, Tris-buffered saline.
Monocyte isolation.
Human peripheral blood monocytes were extracted from 400 ml of whole blood from healthy HIV-1-seronegative volunteers as previously described (23). Briefly, blood mononuclear cells were obtained by differential centrifugation in Ficoll-Hypaque (Pharmacia-AMRAD, Sydney, Australia). Monocytes were separated from other mononuclear cells by countercurrent elutriation (Beckmann centrifuge J-6M/E fitted with a JE 5.0 elutriation rotor) followed by adherence to plastic for 7 days with extensive washing to eliminate any residual T-cell contamination. Cells were plated at a density of 106 cells/ml of medium, which consisted of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 10% heat-inactivated pooled AB+ human serum, and 50 μM tryptophan (Sigma, Sydney, Australia) (RF10/10+), in a 48-well tissue culture plate (Nunc, Sydney, Australia). Monocytes were allowed to adhere for 7 days, facilitating their differentiation into mature macrophages, before being infected with various strains of HIV-1. The purity of cell cultures using this method is typically >99% MDM as determined by flow cytometry using a monoclonal antibody to CD14 and CD68.
Contamination-free culture conditions in this laboratory are ensured by the routine testing of selected cultures for the presence of endotoxin and mycoplasmas by the Limulus amoebocyte lysate chromogenic assay (Sigma) and Hoechst staining (Sigma), respectively.
HIV-1 infection and IFN-γ and anti-IFN-γ antibody treatments.
Following a complete medium exchange with RF10/10+, 7- to 9-day-old MDM (106) were either infected with various strains of HIV-1, exposed to 600 U of human recombinant IFN-γ (Sigma) per ml, or left untreated for 1, 2, 5, or 8 days. Appropriate culture supernatants were sampled and the cells were homogenized on days 1, 2, 5, and 8 posttreatment. Human antibody to IFN-γ (100 U/ml) (R&D Systems, Sydney Australia) was added to selected cultures immediately following HIV-1 infection with the neurotropic strain HIV1-JRFL and sampled after 48 h. Selected MDM on day 6 after HIV-1 (BaL, JR-FL, or 631) infection were treated with 600 U of IFN-γ per ml and sampled after 48 h.
For HIV infection, MDM from two donors were exposed to the same virus inoculum of one of either two LA strains, HIV1-BaL, or HIV1-JRFL, or the brain-derived primary isolate HIV1-631 using a multiplicity of infection of 0.025 50% tissue culture infective dose per cell. All HIV strains used were propogated in peripheral blood mononuclear cells pooled from the same three donors. Virus stocks were also measured by a reverse transcriptase assay using 105 CPM of reverse transcriptase activity per ml.
The HIV1-BaL (11) and HIV1-JRFL (27) isolates were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. The primary isolate, HIV1-631, was obtained from an AIDS rapid progressor with encephalopathy (10).
p24 antigen assay.
The level of extracellular HIV p24 antigen in cell culture supernatants was determined by a commercial enzyme-linked immunosorbent assay (Coulter Electronics) as specified by the manufacturer. The concentrations of antigen were calculated, and the values were designated nonproductive (<25 pg/ml), low (<1 ng/ml), intermediate (1 to 49 ng/ml), or high (>50 ng/ml).
Kynurenine assay.
The change in kynurenine concentration in the HIV-infected culture supernatant was measured spectrophotometrically (37). Briefly 100 μl of 30% trichloroacetic acid was added to 200 μl of the culture supernatant, vortexed, and then centrifuged at 10,000 rpm for 5 min. A 125-μl volume of the supernatant was added to 125 μl of Ehrlich's reagent (100 mg of p-dimethylbenzaldehyde, 5 ml of glacial acetic acid) in a microtiter plate well (96-well format). Samples were read against a reagent blank with a 492-nm filter in a Multiskan MS (Labsystems) microplate reader. The change in kynurenine concentration was obtained by subtracting control levels (uninfected culture supernatant) from the sample value.
Western blot analysis for IDO.
Cells were homogenized in 500 μl of homogenate buffer (50 μl of Triton X-100 plus 122 mg of nicotinamide in 10 ml of phosphate-buffered saline [pH 7.4]). Then 15 μl of sample was loaded into each well of a Mini-Protean 11 10% (wt/vol) polyacrylamide slab gel (Bio-rad) in the presence of 1 mg of sodium dodecyl sulfate per ml and subjected to electrophoresis for 1 h. Electrophoretic transfer of the protein onto nitrocellulose paper was done using a Mini Trans Blot apparatus (Bio-rad) (100-V constant voltage for 15 min). The membrane was then washed with TBS buffer and placed in 4% skim milk powder–TBS for 1 h to block all nonspecific binding sites on the membrane. The membrane was removed, washed at least three times with TBS (5 min each), placed in a buffered solution of primary monoclonal IDO antibody (1:10,000 dilution) supplied by O. Takikawa (University of Wollongong) (37), and then left overnight at 4°C. The membrane was removed, washed three times with TBS, and placed in secondary antibody (biotinylated goat anti-mouse immunoglobulin G; 1:1000) for 1 h at 25°C. The membrane was washed three times with TBS and incubated for 1 h at 25°C with peroxidase-conjugated streptavidin. Finally, the membrane was washed and the IDO protein was stained using a nickel-enhanced diaminobenzedine reaction (10 to 20 min). Authentic human IDO was used as a positive control. This IDO was a recombinant protein expressed in Escherichia coli (T. Littlejohn and O. Takikawa, submitted for publication).
IDO densitometry.
The bands corresponding to IDO (from Western blots) were quantified using a Bio-Rad model GS-700 imaging densitometer and Image Tool software (University of Texas Health Sciences Center, San Antonio, Tex.).
RESULTS
HIV-1 productive infection (p24 antigen).
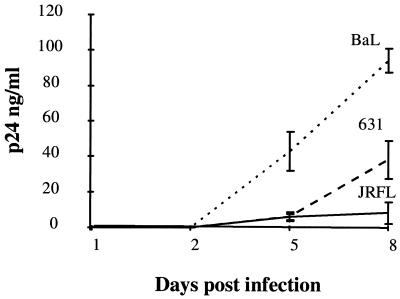
The infection and replication kinetics of the three HIV-1 strains were compared for up to 8 days postinfection by determining the level of p24 antigen in the culture supernatants. All viral strains produced readily detectable concentrations of p24 antigen by day 8 post infection. The LA macrophage-tropic HIV1-BaL showed a high level of replication by day 8 post infection in MDM from donors 1 and 2. The brain-derived primary isolate HIV1-631 showed an intermediate level of replication by day 8 postinfection in MDM from donor 2 (Fig. 2). The LA brain-derived HIV1-JRFL showed a high level of replication in donor 1 MDM, comparable to that observed for HIV1-BaL (data not shown), but an intermediate level of replication in donor 2 MDM (Fig. 2).
FIG. 2.
p24 antigen production in HIV-infected MDM. MDM (7 days old) were infected with one of three strains of HIV-1, i.e., HIV1-BaL, HIV1-631, and HIV1-JRFL. Supernatants were sampled for p24 antigen production. Results are shown for donor 2 only.
IDO induction and supernatant kynurenine concentration in HIV-1-infected macrophage cultures.
Using Western blot analysis, we assayed for IDO protein in the cell homogenate of macrophages from two different donors infected with different HIV-1 strains. MDM from donor 1 were infected with HIV1-BaL and HIV1-JRFL only, while MDM from donor 2 were infected with HIV1-BaL, HIV1-JRFL, and HIV1-631. Samples were taken on days 1, 2, 5, and 8 postinfection. IFN-γ a potent inducer of IDO in MDM (2, 19), was added to selected cultures as a positive control.
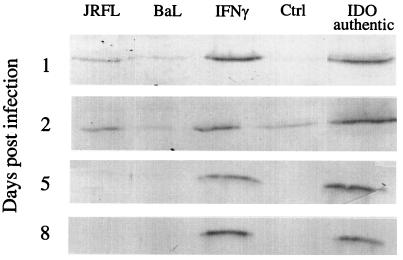
An increase in antibody staining for IDO protein was observed on day 1 postinfection for MDM infected with brain-derived LA HIV1-JRFL or treated with IFNγ (Fig. 3). On day 2 postinfection, a marked increase in IDO staining intensity was detected in MDM either treated with IFN-γ or infected with the brain-derived LA HIV1-JRFL or the brain-derived primary isolate HIV1-631 (Fig. 3 and 4). MDM infected with the highly replicating macrophage-tropic LA HIV1-BaL showed only a slight increase in detectable IDO above baseline on day 2 postinfection (Fig. 3 and 4). All HIV-1-mediated induction of IDO declined to baseline levels by day 8 postinfection.
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western immunoblot analyses for IDO in HIV-1-infected and IFN-γ-treated MDM. MDM in culture from donor 1 were either left uninfected (Ctrl) or infected with HIV1-BaL or HIV1-JRFL and cultured for the indicated periods or treated with IFN-γ (600 U/ml) for 48 h. Cultures were homogenized and analysed for IDO as indicated in Materials and Methods.
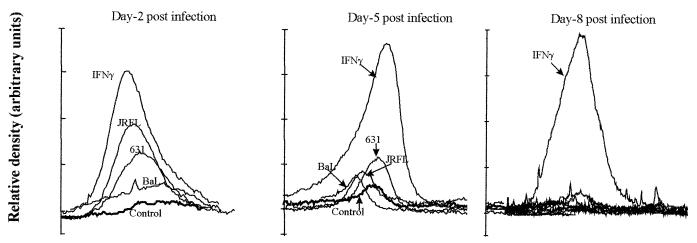
FIG. 4.
Differential induction of IDO by various strains of HIV-1. Relative density tracings of sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western immunoblot analyses for IDO in HIV-1-infected and IFN-γ-treated MDM are shown. MDM in culture from donor 1 were either left untreated (Control), infected with one of three different strains of HIV (HIV1-BaL, HIV1-631, and HIV1-JRFL) and cultured for the indicated periods, or treated with IFN-γ (600 U/ml) for 48 h. Cultures were homogenized and analyzed for IDO as indicated in Materials and Methods.
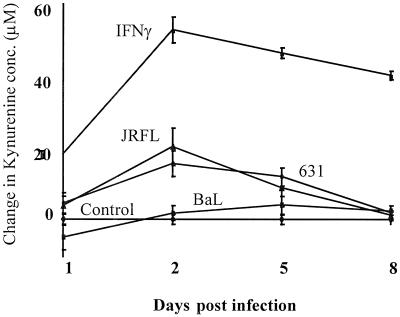
To clarify how the induction of IDO by HIV may affect tryptophan metabolism in these cultured MDM, the level of kynurenine, a major metabolite, was measured in the supernatant of these macrophage cell cultures. Supernatant kynurenine levels increased markedly by day 2 postinfection in cultures either exposed to IFN-γ or infected with the brain-derived LA strain HIV1-JRFL and the brain-derived primary isolate HIV1-631. However, little or no increase in the kynurenine level was observed in the supernatant of cells infected with LA macrophage tropic HIV1-BaL (Fig. 5). At maximal IDO induction (day 2 post infection, Fig. 4), the level of IDO induction by the different viral strains correlated precisely with the pattern of kynurenine concentration measured in the culture supernatant, HIV1-JRFL > HIV1-631 > HIV1-BaL, where the induction of IDO by HIV1-BaL was only marginally above baseline (Fig. 4).
FIG. 5.
Differential increases in the supernatant kynurenine concentration of HIV-infected and IFN-γ-treated MDM in culture. MDM in culture from three different donors were either infected with one of three strains of HIV (HIV1-BaL, HIV1-631 or HIV1-JRFL) or treated with IFN-γ (600 U/ml). The change in kynurenine concentration in the culture supernatant was measured on days 1, 2, 5, and 8 postinfection. A complete medium exchange was made to remaining cultures on days 2 and 5, with addition of IFN-γ to appropriate wells.
Effect of viral replication on IDO induction by exogenous IFN-γ.
IDO induction and kynurenine production in HIV-1 infected MDM decreased to control levels by day 8 postinfection, a time when viral replication was most abundant (Fig. 2). To investigate whether viral replication was affecting the mechanism through which cytokines induce IDO, 7-day-old MDM were infected with the three strains of HIV-1. On day 6 postinfection, 600 U of IFN-γ per ml was added to the HIV-1-infected cultures, and 48 h later samples were taken for Western blot analysis of IDO and measurement of the supernatant kynurenine concentration as described above. IDO was strongly induced by IFN-γ in all HIV-1-infected cultures (data not shown), similar to treatment with IFN-γ alone. The kynurenine concentration in the supernatant of these cultures was also markedly increased (HIV1-BaL plus IFN-γ, 52 ± 2 μM; HIV1-JRFL plus IFN-γ, 55 ± 5 μM), similar to that observed for IFN-γ treatment alone (Fig. 5). These findings indicated that the replication of HIV-1 itself does not affect the induction of IDO by exogenous IFN-γ.
Effect of anti-IFN-γ antibody on kynurenine metabolism in HIV1-JRFL-infected macrophages.
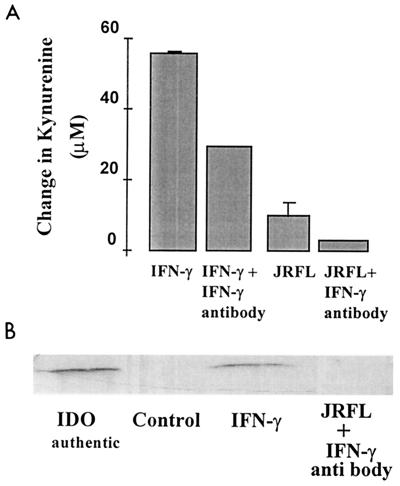
To investigate the mechanism of IDO induction (leading to kynurenine synthesis), we added a human antibody to IFN-γ (100 U/ml) to cells infected with HIV1-JRFL on day 9 in culture and assayed for both the supernatant kynurenine concentration and IDO protein level after 48 h. Figure 6 shows that the supernatant kynurenine concentration was reduced by greater than 60% in the presence of anti-IFN-γ antibody (Fig. 6A). This was consistent with a complete lack of detectable IDO protein in the cell homogenate (Fig. 6B). The presence of an isotype control for this antibody did not significantly affect kynurenine production in HIV1-JRFL-infected cells (data not shown).
FIG. 6.
Effect of anti-IFN-γ antibody treatment on supernatant kynurenine concentration and IDO induction in HIV1-JRFL-infected MDM. An antibody to human IFN-γ (100 U/ml) was added to MDM immediately following infection with HIV1-JRFL. On day 2 postinfection, samples were taken for analysis of the change in supernatant kynurenine concentration (A) and Western blot analysis of IDO protein in the cell homogenate (B).
DISCUSSION
In this study we have shown for the first time that IDO, the rate-limiting enzyme for oxidative tryptophan catabolism, can be induced by HIV-1 in human MDM. Infection with the brain-derived isolates LA HIV1-JRFL and the primary isolate HIV1-631 but not the macrophage-tropic LA HIV1-BaL induced MDM to produce considerable amounts of IDO and its metabolic product, kynurenine. Addition of an antibody to IFN-γ on the day of infection resulted in undetectable levels of IDO protein in the cell homogenate and a large reduction in kynurenine concentration in the culture supernatant.
HIV infection of the CNS is present in most subjects with AIDS; however, only a subset of these patients develop the neurological symptoms associated with ADC (16). This suggests that not all strains of HIV-1 are able to induce the production of neurotoxins, which have been associated with the neuropathology of ADC (28). Secretion of a number of potential neurotoxins from HIV-1-infected macrophages and microglia (6, 16, 32, 36, 38), including the excitotoxin quinolinic acid (6, 30), has been reported. Quinolinic acid is increased in the CNS of HIV-1-infected patients with dementia but not in patients without neurological symptoms (36).
The development of clinical dementia (32) and the production of quinolinic acid in HIV-1-infected macrophages (6) differ according to the viral strain. Quinolinic acid synthesis is regulated by IDO, the rate-limiting enzyme of the kynurenine pathway (Fig. 1). We have shown, for the first time, that HIV-1 infection results in a significant induction of IDO protein in MDM. Consistent with previous reports regarding increased quinolinic acid production (6), not all strains of HIV-1 were able to induce IDO. The brain derived viruses LA HIV1-JRFL and primary isolate HIV1-631 were able to markedly induce IDO, resulting in a significant increase in kynurenine secretion into the culture supernatant. This may be relevant clinically, since increasing kynurenine pathway metabolism in MDM has been shown consistently to result in elevated levels of quinolinic acid in extracellular fluid (20, 34).
Interestingly, little or no induction of IDO or kynurenine production was observed in cells infected with the highly replicating LA macrophage-tropic strain (HIV1-BaL).
From the data above, it appears that the induction of IDO may be related to the viral tropism (i.e., neurotropism); however, a much larger panel of different viral strains must be examined to confirm this hypothesis. These results also suggest that IDO induction in vitro correlates with the viral characteristics associated with in vivo strain adaptation rather than with the level of viral replication (6).
It was noted that both induction of IDO and kynurenine secretion decreased to baseline levels by day 8 postinfection (Fig. 3 and 4), a time when HIV-1 replication was highest (Fig. 2). However, addition of exogenous IFN-γ to HIV-1-infected cultures during this time (i.e., day 7 to 8 postinfection) resulted in induction of IDO to the same degree as in uninfected IFN-γ-treated cells. This indicates that productive HIV-1 replication does not affect the induction of IDO if IFN-γ is present in the extracellular fluid. Therefore, the mechanism of IDO induction may involve endogenous production of IFN-γ by MDM in the initial stages of HIV-1 infection, which may then be down regulated by productive viral replication (29).
It is well known that IFN-γ is a potent inducer of IDO and kynurenine pathway metabolism in macrophages (2, 33, 34, 39). T cells and NK cells are considered the primary source of IFN-γ during an immune response (14). Recently, other cell types, including monocyte/macrophages, have been shown to be able to synthesize IFN-γ (3, 14). Therefore, we investigated the possibility that endogenous IFN-γ production may mediate the induction of IDO in these HIV-1-infected MDM. Addition of a human antibody to IFN-γ simultaneously with infection (HIV1-JRFL) resulted in an absence of detectable IDO protein on day 2 postinfection (Fig. 6B), the time point when IDO induction is greatest (Fig. 3 and 4).
These results suggest that infection of MDM with selected HIV-1 strains (such as HIV1-JRFL and HIV1-631) stimulates the production of endogenous IFN-γ, resulting in immune system activation, IDO induction, and subsequent increased flux through the kynurenine pathway. However, this phenomenon was transitory, declining to baseline by day 8 postinfection (Fig. 3 and 4), while HIV-1 replication continued to increase (Fig. 2). In agreement with this result, it has recently been reported that acute HIV-1 infection downregulates IFN-γ expression in activated cells by a DNA methyltransferase-dependent process (29).
In conclusion, we observed that both LA and primary brain-derived strains of HIV-1, but not the macrophage-tropic isolate HIV1-BaL, effectively induce IDO, the rate-limiting enzyme of kynurenine pathway metabolism. Evidence presented above indicates that this induction is mediated through a transient synthesis of endogenous IFN-γ by HIV-infected MDM. Although IDO induction was associated with brain-derived strains of HIV-1 in this study, further investigations using a wider panel of diverse HIV-1 strains are required to clarify the association between viral tropism and IFN-γ and IDO induction.
These results suggest that therapeutic strategies targeted at regulating IFN-γ production may be an alternative approach to managing patients at risk of ADC, in addition to strategies already suggested for dealing with individual downstream excitotoxins such as quinolinic acid (22, 24).
ACKNOWLEDGMENTS
We thank Z. Miklowska for the donation of IFN-γ antibody and Beena Devenapalli, Shan Li, and Mohomed Alali for technical advice and assistance.
This study was supported by grants from the R. L. Cooper Medical Research Foundation. R. S. Grant is supported by a Dora Lush postgraduate scholarship from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Achim C L, Heyes M P, Wiley C A. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J Clin Investig. 1993;91:2769–2775. doi: 10.1172/JCI116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberati-Giani D, Ricciardi-Castagnoli P, Kohler C, Cesura A M. Regulation of the Kynurenine pathway by IFN-γ in murine cloned macrophages and microglial cells. Adv Exp Med Biol. 1996;398:171–175. doi: 10.1007/978-1-4613-0381-7_28. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste E N. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg B M, Gelbard H A, Epstein L G. HIV-1 infection of the developing nervous system: Central role of astrocytes in pathogenesis. Virus Res. 1994;32:253–267. doi: 10.1016/0168-1702(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 5.Botting N P. Chemistry and neurochemistry of the kynurenine pathway of tryptophan metabolism. Chem Soc Rev. 1995;1995:401–412. [Google Scholar]
- 6.Brew B J, Corbeil J, Pemberton L, Evans L, Saito K, Penny R, Cooper D A, Heyes M P. Quinolinic acid production is related to macrophage tropic isolates. J Neurovirol. 1995;1:369–374. doi: 10.3109/13550289509111026. [DOI] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Nottet H S L M, Schmidtmayerova H, Dubrovsky L, Flanagan C R, Mullins M E, Lipton S A, Gendelman H E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: Implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyer E B, Lipton S A. The coat protein gp120 of HIV-1 inhibits astrocyte uptake of exitatory amino acids via macrophage arachidonic acid. Eur J Neurosci. 1995;7:2502–2507. doi: 10.1111/j.1460-9568.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 9.Epstein L G, Gendelman H E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenic mechanisms. Neurol Prog. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- 10.Fear W R, Kesson A M, Naif H, Lynch G W, Cunningham A L. Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte-derived macrophages, and placental macrophages. J Virol. 1998;72:1334–1344. doi: 10.1128/jvi.72.2.1334-1344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gartner S P, Markovits D M, Markovitz M H, Kaplan M H, Gallo R C, Papovic M. Virus isolation and identification of HTLV-III/LAV producing cells in brain tissue from a patient with AIDS. Science. 1986;233:215–219. [Google Scholar]
- 12.Gendelman H E, Lipton S A, Tardieu M, Bukrinsky M I, Nottet H S L M. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994;56:389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- 13.Genis P, Jett M, Berton E W, Boyle T, Gelbard H A, Dzenko K, Keane R W, Resnick L, Mizrachi Y, Volsky D J, Epsteine L G, Gendelman H E. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: Implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gessani S, Belardelli F. IFNγ expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998;9:117–123. doi: 10.1016/s1359-6101(98)00007-0. [DOI] [PubMed] [Google Scholar]
- 15.Giulian D, Yu J, Li X, Tom D, Li J, Wendt E, Lin S, Schwarcz R, Noonan C. Study of receptor mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J Neurosci. 1996;16:3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 17.Griffin D E. Cytokines in the brain during viral infection: clues to HIV-associated dementia. J Clin Investig. 1997;100:2948–2951. doi: 10.1172/JCI119847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Markey C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are coreceptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 19.Heyes M P, Brew B, Martin A, Price R W, Salazar A M, Sidtis J J, Yergey J A, Mouradian M, Sadler A E, Keilip J, Rubinow D, Markey S P. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 20.Heyes M P, Chen C Y, Major E O, Saito K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J. 1997;326:351–356. doi: 10.1042/bj3260351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyes M P, Jordan E K, Lee K, Saito K, Frank J A, Snoy P J, Markey S P, Gravell M. Relationship of neurological status in macaques infected with the simian immunodeficiency virus to cerebrospinal fluid quinolinic acid and kynurenic acid. Brain Res. 1992;570:237–250. doi: 10.1016/0006-8993(92)90587-y. [DOI] [PubMed] [Google Scholar]
- 22.Heyes M P, Saito K, Lackner A, Wiley C A, Achim C L, Markey S P. Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques. FASEB J. 1998;12:881–896. doi: 10.1096/fasebj.12.10.881. [DOI] [PubMed] [Google Scholar]
- 23.Kazazi F, Mathijs J M, Foley P, Cunningham A L. Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J Gen Virol. 1989;70:2661–2672. doi: 10.1099/0022-1317-70-10-2661. [DOI] [PubMed] [Google Scholar]
- 24.Kerr S J, Armati P J, Pemberton L A, Smythe G, Tattam B, Brew B J. Kynurenine pathway inhibition reduces neurotoxicity of HIV-1-infected macrophages. Neurology. 1997;49:1671–1681. doi: 10.1212/wnl.49.6.1671. [DOI] [PubMed] [Google Scholar]
- 25.Kohler C, Eriksson L G, Okuno E, Schwarcz R. Localization of quinolinic acid metabolizing enzymes in the rat brain. Immunohistochemical studies using antibodies to 3-hydroxyanthranilic acid oxygenase and quinolinic acid phosphoribosyltransferase. Neuroscience. 1988;27:49–76. doi: 10.1016/0306-4522(88)90219-9. [DOI] [PubMed] [Google Scholar]
- 26.Korber B T M, Kunstman K J, Patterson B K, Furtado M, McEvilly M M, Levy R, Wolinsky S M. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyanagi Y S, Miles R T, Mitsuyasu J E, Merrill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 28.Lipton S A. Neuronal injury associated with HIV-1: approaches to treatment. Annu Rev Pharmacol Toxicol. 1998;38:159–177. doi: 10.1146/annurev.pharmtox.38.1.159. [DOI] [PubMed] [Google Scholar]
- 29.Mikovits J A, Young H A, Vertino P, Issa J P J, Pitha P M, Turcoskicorrales S, Taub D D, Petrow C L, Baylin S B, Ruscetti F W. Infection with human immunodeficiency virus type 1 upregulates dna methyltransferase, resulting in de novo methylation of the gamma interferon (IFN-γ) promoter and subsequent downregulation of IFN-γ production. Mol Cell Biol. 1998;18:5166–5177. doi: 10.1128/mcb.18.9.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nottet H S L M, Flanagan C R, Gelbard H A, Gendelman H E, Reinhard J F. The regulation of quinolinic acid in human immunodeficiency virus-infected monocytes. J Neurovirol. 1996;2:111–117. doi: 10.3109/13550289609146544. [DOI] [PubMed] [Google Scholar]
- 31.Nottet H S L M, Jett M, Flanagan C R, Zhai Q, Persidsky Y, Rizzino A, Bernton E W, Genis P, Baldwin T, Schwartz J, LaBenz C, Gendelman H E. A regulatory role for astrocytes in HIV-1 encephalitis. An over expression of ecosanoids, platelet-activating factor, and tumour necrosis factor-a by activated HIV-1-infected monocytes is attenuated by primary human astrocytes. J Immunol. 1995;154:3567–3581. [PubMed] [Google Scholar]
- 32.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito K, Markey S P, Heyes M P. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience. 1992;51:25–39. doi: 10.1016/0306-4522(92)90467-g. [DOI] [PubMed] [Google Scholar]
- 34.Saito K, Seishima M, Noma A, Markey S P, Heyes M P. Cytokine and drug modulation of kynurenine pathway metabolism by blood mononuclear cells. Adv Exp Med Biol. 1996;398:161–165. doi: 10.1007/978-1-4613-0381-7_26. [DOI] [PubMed] [Google Scholar]
- 35.Sardar A M, Reynolds G P. Frontal cortex indolamine-2,3-dioxygenase activity is increased in HIV-1-associated dementia. Neurosci Lett. 1995;187:9–12. doi: 10.1016/0304-3940(95)11324-p. [DOI] [PubMed] [Google Scholar]
- 36.Sei S, Saito K, Stewart S K, Crowley J S, Brouwers P, Kleiner D E, Katz D A, Pizzo P A, Heyes M P. Increased human immunodeficiency virus (HIV) type 1 DNA content and quinolinic acid concentration in brain tissues from patients with HIV encephalopathy. J Infect Dis. 1995;172:638–647. doi: 10.1093/infdis/172.3.638. [DOI] [PubMed] [Google Scholar]
- 37.Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-γ action: characterization of indoleamine 2,3-dioxygenase in cultured human cells by interferon-g and evaluation of the enzyme mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988;263:2041–2048. [PubMed] [Google Scholar]
- 38.Talley A K, Dewhurst S, Perry S W, Dollard S C, Gummuluru S, Fine S M, New D, Epstein L G, Gendelman H E, Gelbard H A. Tumor necrosis factor alpha-induced apoptosis in human neuronal cells: protection by the antioxidant N-acetylcysteine and the genes bcl-2 and crmA. Mol Cell Biol. 1995;15:2359–2366. doi: 10.1128/mcb.15.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner-Felmeyer G, Werner E, Fuchs D, Hausen A, Reibnegger G, Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in-vitro. Biochim Biophys Acta. 1989;1012:140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]