Abstract
Sub-high temperature Daqu, a traditional solid fermenting agent used in Chinese strong-aroma Baijiu production, is abundant in diverse microorganisms, including bacteria, yeasts, molds, and actinomycetes. Among these, yeasts are pivotal for ethanol production and flavor formation. However, counting yeasts in Daqu is challenging due to interference from molds and bacteria. Antibiotics are employed to inhibit bacterial growth, but there is no practical way to suppress molds without affecting the growth of yeasts. In this study, short-chain carboxylates (C1-C6) were added to the culture medium at various pH conditions to investigate their effects on the growth of molds and yeasts. The results demonstrated distinct inhibitory effects of the short-chain carboxylates, depending on both pH and concentration. Several tested short-chain carboxylates effectively suppressed mold growth on agar plates while leaving yeast growth unaffected. This suggests a simple and feasible method for enhancing the efficiency of yeast isolation and counting in Daqu. Such an approach is valuable for studying yeasts in diverse and complex habitats.
Keywords: Sub-high temperature Daqu, short-chain carboxylates, yeast counting and isolation, Chinese strong-aroma Baijiu
Introduction
Sub-high-temperature Daqu (STD) is a saccharifying and fermenting agent derived from wheat, commonly used in producing Chinese strong-aroma Baijiu. It plays a pivotal role in the fermentation process (Yan et al. 2019a; Wang et al. 2020), facilitating the degradation of complex natural substrates such as starch and protein. Moreover, it catalyzes the transformation of these degraded products into a diverse array of compounds, including alcohols, acids, esters, aldehydes, and other flavor substances (De Vuyst and Leroy 2020), which significantly influences the quality and distinctive characteristics of Baijiu brewing (Yan et al. 2019a; Zhu et al. 2022).
STD is produced through natural inoculation, harboring a rich diversity of microorganisms, including numerous molds, yeasts, bacteria, and a smaller proportion of actinomycetes (Zou et al. 2018; Yan et al. 2019b). A recent study employing second-generation sequencing technology to investigate the fungal community structure within STD revealed that yeasts comprise approximately 60% of the total fungi population (Yang et al. 2018). A total of 420 fungal strains were isolated from 30 Daqu starter samples using the plate culture method and identified through ITS region sequencing. Among these strains, 386 (92%) were yeasts, while 34 (8%) were filamentous fungi. The dominant species identified were Saccharomyces cerevisiae, Wickerhamomyces anomalus, and Saccharomyces fibuligera, collectively accounting for 79% of the relative abundance (Zhou et al. 2022). Yeasts are the primary microorganisms that convert sugars into ethanol during fermentation and participate in producing some volatile flavor compounds (Pu et al. 2021). Consequently, numerous studies have focused on the entire yeast population and isolated yeast colonies within STD (Grangeteau et al. 2015; Li et al. 2020; Fan et al. 2021).
Effectively isolating and counting yeasts in STD presents challenges due to interfering microorganisms, especially molds (Xu et al. 2020). Supplementing antibiotics in culture media can effectively inhibit bacterial growth and circumvent the interference of bacteria (Liu et al. 2014). Using the classical plate culture method, sixteen pure yeast cultures and various yeast strains with specific functions have been successfully isolated from STD samples (Hu et al. 2020; Ma et al. 2022; Li et al. 2023). However, the number of pure yeast species obtained through classical methods is notably lower than those identified through high-throughput sequencing, It is imperative to develop methods that inhibit the growth of molds while preserving yeast growth to isolate and analyze the entire yeast population.
Previous studies have demonstrated the inhibitory effects of organic acids on the growth of molds and yeasts. Valerate, propionate, and butyrate at concentrations ranging from 0.5 to 2.5 g/l have been shown to completely suppress mold growth, while higher concentrations of acetate and lactate are required for similar inhibitory effects (Moon 1983; Higgins and Brinkhaus 1999; Dijksterhuis et al. 2019). Various organic acids, including formic acid, acetic acid, propionate, lactate, and caproic acid, have been found to inhibit yeast growth in a concentration-dependent manner. For instance, yeasts can naturally grow when the lactate concentration is below 100 mM, but growth rates decrease by 50% when lactate concentration reaches 400 mM (Hundová and Fencl 1977).
While it is well-established that adding organic acids to the culture medium can regulate the growth of molds and yeasts, there is a lack of studies investigating whether organic acids exhibit differential effects on the growth of molds and yeasts at varying pH values and concentrations. This study aims to fill this gap by investigating the effects of short-chain carboxylates (C1-C6) on the growth of yeasts and molds in sub-high temperature Daqu. The goal is to identify a culture condition that favors yeast growth while inhibiting mold growth, thus enabling efficient isolation and counting of yeasts from mixed cultures. To our knowledge, this is the first study to examine the inhibitory effects of various short-chain carboxylates on molds and yeasts in sub-high temperature Daqu.
Experimental
Materials and Methods
Sample collection
Sampling was conducted at a strong-flavor Baijiu production factory in Yibin, Sichuan Province, China. Daqu intended for production was gathered from ten fermentation workshops, with 0.5 kg samples collected from each workshop on November 3, 2022. These samples comprised a mixture of small particles and powder. After thoroughly mixing and milling, the samples were sifted through a 60-mesh screen and transferred to sterile bags. The bags were sealed, frozen at –20°C, and shipped to Sichuan University of Science and Engineering, Yibin, China, on dry ice for further analysis.
Yeast culture media
Five types of basal media were used in this study. Malt extract agar (MEA) (Heard and Fleet 1986) was prepared by dissolving 20 g malt extract, 10 g glucose, 5 g peptone, and 20 g agar in 1 l of distilled water. For potato dextrose agar (PDA) (Madbouly et al. 2020), 15 g of potato extract, 20 g of glucose, and 20 g of agar were dissolved in 1 l of distilled water. For rose Bengal agar (RBA) (Echevarría and Bello 2023), 5 g peptone, 10 g glucose, 1 g potassium dihydrogen phosphate, 0.5 g magnesium sulfate (MgSO4 · 7H2O), 100 ml of a 1/3,000 aqueous solution of rose Bengal, 20 g agar were dissolved in 1 l of distilled water. For Wallerstein laboratory nutrient agar (WL) (Li et al. 2010), 4 g yeast extract, 5 g tryptone, 50 g glucose, 0.425 g potassium chloride, 0.125 g calcium chloride, 0.125 g magnesium sulfate (MgSO4 · 7H2O), 0.55 g potassium dihydrogen phosphate, 0.0025 g ferric chloride, 0.0025 g manganese sulfate (MnSO4 · H2O), 0.022 g bromocresol green, 20 g agar were dissolved in 1 l of distilled water. For yeast-extract peptone dextrose agar (YPD) (Gerard et al. 2023),10 g yeast extract, 20 g peptone, 20 g glucose, and 20 g agar were dissolved in 1 l of distilled water. When the sterilized culture medium was cooled to approximately 50°C, antibiotics chloramphenicol was added to all culture medium to the final concentration of 50 μg/ml.
Microbial culture
To select an appropriate culture medium for studying the effects of short-chain carboxylates on yeast and mold growth in Daqu, microbial suspensions of STD with three serial dilutions (10–2, 10–3, and 10–4) were plated onto five basal media: MEA, PDA, RBA, WL, and YPD. According to the screening results, MEA was chosen as a basal medium in this study. The short-chain carboxylate was supplemented to MEA with final concentrations of 0.05 M, 0.1 M, and 0.2 M. The pH value of the medium was adjusted within appropriate ranges: formate (4.2 to 5.2), acetate (4.8 to 5.8), propionate, butyrate, valerate, and caproate (5.4 to 6.4), pyruvate (2.6 to 4.0), and lactate (3.0 to 4.4), by adding 1 M HCl or 1 M NaOH. To compare the inhibitory effects of short-chain carboxylates on the growth of molds and yeasts in STD, the MEA medium was supplemented with formate, acetate, propionate, butyrate, valerate, caproate, lactate, and pyruvate at a final concentration of 0.05 M.
Additionally, MEA was supplemented with butyrate, valerate, and caproate at final concentrations of 0.01 M, 0.02 M, 0.03 M, and 0.04 M to investigate their effects on mold and yeast growth at low concentrations. Considering that the pH of the control group, consisting of MEA culture without short-chain carboxylates, was 5.7, the pH of the culture medium with different shortchain carboxylates was adjusted to the same pH value.
To validate their inhibitory effects in five different types of yeast medium, acetate, butyrate, and valerate were added at final concentrations of 0.05 M, 0.03 M, and 0.02 M. The pH value was adjusted to 5.0 for the media containing acetate and to 5.7 for the media containing butyrate or valerate. The pH value of control groups is the natural pH of these media without any adjustment.
Enumeration
Diluent saline peptone (SPO) was prepared by dissolving 8.5 g sodium chloride, 0.3 g disodium hydrogen phosphate (Na2HPO4 · 12H2O), and 1 g peptone in 1 l of distilled water. The pH of SPO was adjusted to 5.6 by adding 1 M HCl or 1 M NaOH. Samples (10 g) were mixed with 90 ml of SPO, soaked at 4°C for 30 minutes, and homogenized with a Vortex-Genie2 (Scientific Industries, Inc., USA) at “10” speed for 2 minutes, duplicate counting plates were prepared using 10–2, 10–3, and 10–4 serial dilutions. For spread plating, 0.1 ml of the dilution was spread on the surface of a dry plate. Agar plates were incubated at 30°C for 48 hours, 60 hours, or 72 hours until colonies appeared. Subsequently, the colonies were counted and calculated as colony-forming units (CFU) per gram of Daqu sample. In cases where plates were covered with molds that the naked eye could not directly count, the plates were inverted on a strong light source, a photograph was taken, and yeast counting was performed.
Data analysis
The yeast counting numbers from three independent experiments are presented as means ± standard error of the mean (SEM). Statistical significance was determined using a two-sided unpaired t-test, with a significance threshold set at p < 0.05. All statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, USA, www.graphpad.com).
Results and Discussion
MEA is used as a yeast culture medium
Microbial suspensions of STD with three dilutions were plated onto five commonly used yeast culture media types, including MEA, PDA, RBA, WL, and YPD. When the dilution factor is 100, many yeast colonies grow on plates, making yeast counting difficult. Moreover, excessive mold growth interferes with yeast counting (Fig. 1A). When the dilution factor is increased to 1,000, a few hundred yeast colonies can be counted, and mold interference is attenuated (Fig. 1A). More importantly, the number of yeast colonies in this range meets the yeast isolation and counting criteria, which typically range from 30 to 300 CFU (Deak et al. 1998). Only a few yeast colonies can be counted when the microbial suspension is diluted 10,000 times, which falls far below the counting and culture criteria (Fig. 1A). Therefore, a dilution factor of 1,000 was used in subsequent experiments.
Fig. 1.

The microbial growth of STD in different yeast culture media.
A) representative agar plates;
B) yeast colony counting numbers at 1,000 times dilution of Daqu. MEA – malt extract agar; PDA – potato dextrose agar;
RBA – rose Bengal agar; WL – Wallerstein laboratory nutrient agar; YPD – yeast extract peptone dextrose agar.
10–2 – 100 times dilution;
10–3 – 1,000 times dilution; 10–4 – 10,000 times dilution.
ns – no statistically significant difference;
* – statistically significant difference, p < 0.05;
** – statistically significant difference, p < 0.01.
MEA showed the highest number of yeast colonies among the five tested yeast culture media, followed by PDA, RBA, WL, and YPD (Fig. 1B). There was no statistically significant difference in the number of yeast colonies between MEA and PDA. The primary nutrients in MEA are derived from malt juice, which aligns well with the raw materials used to prepare STDs. Therefore, MEA was chosen as the medium to explore the effects of short-chain carboxylates on mold and yeast growth.
The effects of formate and propionate on the growth of yeasts and molds
In this study, the MEA medium was supplemented with short-chain carboxylates at concentrations of 0.05 M, 0.1 M, and 0.2 M, with the pH of the medium adjusted within appropriate ranges. The pH was adjusted within the range of 4.2 to 5.2 for the medium supplemented with formate. Both the pH value and formate concentration were observed to affect the growth of molds and yeasts. At pH below 4.8, minimal biomass was observed on agar plates (Fig. 2A). Molds and yeasts began to grow at pH 4.8, with increased biomass observed at pH 5.0 and 5.2. Agar plates with higher pH values exhibited a more significant number of yeasts. When the final concentration of formate added in the medium was 0.05 M, the number of yeast colonies at pH 4.8, 5.0, and 5.2 was (2.17 ± 0.51) × 105 CFU/g Daqu, (8.43 ± 0.21) × 105 CFU/g Daqu, and (12.17 ± 0.21) × 105 CFU/g Daqu, respectively (Fig. 2C). Furthermore, formate inhibited the growth of molds and yeasts in a concentration-dependent manner. For agar plates at pH 5.2 and formate concentrations of 0.05 M, 0.1 M, and 0.2 M, the number of yeast colonies was (12.17 ±0.21) × 105 CFU/g Daqu, (8.37 ± 0.87) × 105 CFU/g Daqu, and (2.30 ± 0.26) × 105 CFU/g Daqu, respectively (Fig. 2C).
Fig. 2.

The effect of formate or propionate on microbial growth of STD.
A) representative agar plates at different pHs and supplemented with different amounts of formate;
B) representative agar plates at different pHs and supplemented with different amounts of propionate;
C) yeast colony counting numbers from the agar plates at different pHs and supplemented with different amounts of formate;
D) yeast colony counting numbers from the agar plates at different pHs and supplemented with different amounts of propionate.
For the medium supplemented with propionate, the pH was adjusted within a range of 5.4 to 6.4. Molds and yeasts began to grow at pH 5.4, with increased biomass observed at pH 6.0, 6.2, and 6.4 (Fig. 2B). When the final concentration of propionate added in the medium was 0.05 M, the number of yeast colonies at pH 5.6, 5.8, and 6.0 was (8.53 ± 0.55) × 105 CFU/g Daqu, (12.23 ± 0.47) × 105 CFU/g Daqu, and (18.73 ± 0.75) × 105 CFU/g Daqu, respectively (Fig. 2D). Consistent with the observations from agar plates supplemented with formate, higher propionate concentrations resulted in higher cellular toxicity to the yeasts. For agar plates at pH 6.0 and propionate concentrations of 0.05 M, 0.1 M, and 0.2 M, the number of yeast colonies was (18.73 ± 0.75) × 105 CFU/g Daqu, (11.77 ± 0.76) × 105 CFU/g Daqu, and (6.17 ± 0.40) × 105 CFU/g Daqu, respectively (Fig. 2D).
The effects of acetate and butyrate on the growth of yeasts and molds
It has been reported that acetate and butyrate affect the growth of yeast and mold (Perna et al. 2018; Dijksterhuis et al. 2019; Rodrigues and Pais 2000). Molds predominated in the microflora that grew on agar plates supplemented with acetate when the pH was higher than 5.4 (Fig. 3A). In contrast, lower pH values favored the growth of yeast colonies and strongly inhibited mold growth. Only yeast colonies were observed when the pH of agar plates reached 4.8 and 5.0 (Fig. 3A). A high concentration of acetate suppressed the growth of yeasts. When agar plates were at pH 5.0, and the concentrations of acetate in the medium were 0.05 M, 0.1 M, and 0.2 M, the number of yeast colonies was (15.70 ± 0.20) x 105 CFU/g Daqu (10.90 ± 0.85) x 105 CFU/g Daqu, and (7.50 ± 0.44) x 105 CFU/g Daqu, respectively (Fig. 3C).
Fig. 3.

The effect of acetate or butyrate on microbial growth of STD.
A) representative agar plates at different pHs and supplemented with different amounts of acetate;
B) representative agar plates at different pHs and supplemented with different amounts of butyrate;
C) yeast colony counting numbers from the agar plates at different pHs and supplemented with different amounts of acetate;
D) yeast colony counting numbers from the agar plates at different pHs and supplemented with different amounts of butyrate.
Butyrate showed stronger cellular toxicity compared to acetate. In a pH range of 5.4 to 6.4, no yeast colonies or mold were observed when the butyrate was added at a concentration of 0.2 M (Fig. 3B). Yeasts and molds began to grow when the pH value of the medium was 5.8, and the final concentration of butyrate in the medium was 0.05 M. For agar plates supplemented with butyrate, higher pH values favored microbial growth. When the final concentration of butyrate added to the medium was 0.05 M, the number of yeast colonies at pH 5.8, 6.0, and 6.2 was (12.83 ± 0.21) × 105 CFU/g Daqu, (15.53 ± 1.32) × 105 CFU/g Daqu, and (16.17 ± 0.61) × 105 CFU/g Daqu, respectively (Fig. 3D).
Acetate and butyrate exhibited different effects on microbial growth in STD. At pH 5.8, a large number of molds grew on agar plates supplemented with acetate. At the same time, only yeast colonies were observed on agar plates supplemented with butyrate, indicating that besides their distinct cellular toxicity, short-chain carboxylates may regulate the growth of yeasts and molds through altering metabolic pathways. The growth pattern of yeasts and molds could be modulated so that molds were suppressed, and yeasts were unaffected, facilitating the study of yeasts in a mixed culture.
The effects of lactate and pyruvate on the growth of yeasts and molds
Lactate and pyruvate are common organic acids produced during microbial fermentation. Previous studies showed that they affect the growth of molds and yeasts (Narendranath et al. 2001; Zhang et al. 2018). Molds appeared on all agar plates supplemented with lactate, indicating that lactate cannot effectively inhibit mold growth (Fig. 4A). At low pH values, a higher lactate concentration inhibited yeasts’ growth. When agar plates were at pH 3.0, and the concentrations of lactate in the medium were 0.05 M, 0.1 M, and 0.2 M, the number of yeast colonies was (2.67 ± 0.42) × 105 CFU/g Daqu, (0.60 ± 0.10) × 105 CFU/g Daqu, and (0.03 ± 0.06) × 105 CFU/g Daqu, respectively. However, a higher lactate concentration did not have stronger inhibitory effects on yeast growth when agar plates were at pH 3.4 (Fig. 4C).
Fig. 4.


The effect of lactate or pyruvate on microbial growth of STD.
A) representative agar plates at different pHs and supplemented with different amounts of lactate;
B) representative agar plates at different pHs and supplemented with different amounts of pyruvate.
C) yeast colony counting numbers from the agar plates at different pHs and supplemented with different amounts of lactate;
D) yeast colony counting numbers from the agar plates at different pHs and supplemented with different amounts of pyruvate.
Little biomass was found on agar plates supplemented with pyruvate when the pH of the medium was adjusted to 2.6 or 2.8. The number of yeast colonies started to increase at pH 3.0, but molds predominated in microbial growth at higher pH values (Fig. 4B). Consistent with what has been found in other shortchain carboxylates, pyruvate inhibited the growth of yeast in a concentration-dependent manner. As the concentration of pyruvate increased, the number of yeast colonies decreased. When agar plates were at pH 3.2 and the concentrations of pyruvate in the medium were 0.05 M, 0.1 M, and 0.2 M, the number of yeast colonies was (15.53 ± 0.81) × 105 CFU/g Daqu, (9.53 ± 0.38) × 105 CFU/g Daqu, and (7.73 ± 0.47) × 105 CFU/g Daqu, respectively (Fig. 4D). Interestingly, molds and yeasts grew at very low pH levels when agar plates were supplemented with lactate or pyruvate, in contrast to agar plates supplemented with other shortchain carboxylates where no molds or yeasts grew when the pH was below 4.2.
Short-chain carboxylates have different inhibitory effects on fungal growth
The MEA medium was supplemented with formate, acetate, propionate, butyrate, valerate, caproate, lactate, and pyruvate at a final concentration of 0.05 M to compare the inhibitory effects of short-chain carboxylates on the growth of yeasts and molds in STD. Given that the natural pH of MEA culture without short-chain carboxylates is 5.7, the pH of the culture medium with different short-chain carboxylates was adjusted to 5.7. Many molds grew on agar plates supplemented with formate, acetate, lactate, and pyruvate (Fig. 5A). Isolated yeast colonies were found on agar plates supplemented with propionate or butyrate. No yeast colonies or molds were found on agar plates supplemented with valerate or caproate, suggesting stronger inhibitory effects on microbial growth than other short-chain carboxylates (Fig. 5A). Notably, butyrate differentially suppressed the growth of molds and yeasts. Molds did not grow on agar plates supplemented with butyrate, while the number of yeast colonies reached (9.97 ± 0.45) × 105 CFU/g Daqu, much lower than that in the control (Fig. 5B).
Fig. 5.


Short-chain carboxylic acids have different inhibitory effects on microbial growth.
A) representative agar plates at pH 5.7 and supplemented with formate, acetate, propionate, butyrate, valerate, caproate, lactate, and pyruvate in a final concentration of 0.05 M and control without supplementing short chain carboxylic acid;
B) yeast colony counting numbers from the agar plates at pH 5.7 and supplemented with various short-chain carboxylic acids and control.
C) representative agar plates at pH 5.7 and supplemented with different amounts of butyrate, valerate, and caproate;
D) yeast colony counting numbers from the agar plates at pH 5.7 and supplemented with different amounts of butyrate, valerate, caproate.
Considering their concentration-dependent inhibitory effect on fungal growth, lower amounts of butyrate, valerate, and caproate were added to the MEA medium. For agar plates supplemented with butyrate at a final concentration of 0.03 M, the number of yeast colonies reached (20.07 ± 0.60) × 105 CFU/g Daqu, which is higher than that in the control (Fig. 5B, 5C, and 5D). For agar plates supplemented with valerate at a final concentration of 0.01 M, the number of yeast colonies was (20.67 ± 0.35) × 105 CFU/g Daqu (Fig. 5B, 5C, and 5D), and decreased at higher concentrations of valerate. Even though lower amounts of caproate (0.01 M) were added to the medium, no microbial growth was found on agar plates, indicating the potent toxicity of caproate to the microbes in STD (Fig. 5C and 5D).
Acetate, butyrate, and valerate differentially suppress mold and yeast growth in yeast culture medium
Based on the above results, acetate, butyrate, and valerate were shown to suppress mold and yeast growth differentially, and isolated yeast colonies can be counted on agar plates supplemented with these short-chain carboxylates. To validate their inhibitory effects in five different types of yeast medium, acetate, butyrate, and valerate were added at concentrations of 0.05 M, 0.03 M, and 0.02 M. The results showed that all three shortchain carboxylates effectively inhibited mold growth on agar plates compared to the control group (Fig. 6A). The agar plates supplemented with 0.03 M butyrate showed a larger number of yeasts than the control group in all five yeast culture media (Fig. 6B), indicating that it is possible to find a condition that suppresses mold growth and favors yeast growth.
Fig. 6.
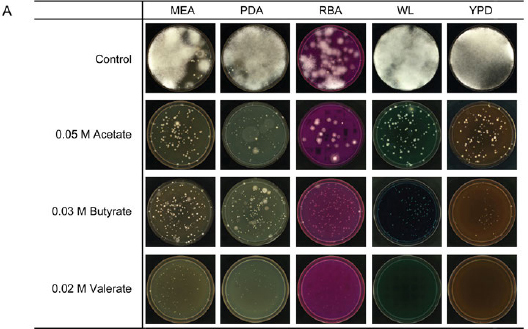

The inhibitory effects of acetate, butyrate, and valerate on microbial growth in different yeast culture media.
A) representative agar plates supplemented with acetate in a final concentration of 0.05 M at pH 5.0, with 0.03 M butyrate or 0.02 M valerate at pH 5.7.
B) yeast colony counting numbers from the agar plates supplemented with acetate in a final concentration of 0.05 M at pH 5.0, with 0.03 M butyrate or 0.02 M valerate at pH 5.7.
The numbers in the table are the yeast colony counting numbers divided by 105; “ / ” indicates no colony.
Conclusions
In this report, we investigated the effects of shortchain carboxylates on the growth of molds and yeasts in sub-high temperature Daqu. We observed that the inhibition of yeast and mold growth on agar plates by short-chain carboxylates depends on the culture medium’s pH value and the concentration of shortchain carboxylates. Notably, adding specific short-chain carboxylates to yeast culture media resulted in differential mold and yeast growth regulation. This finding suggests that manipulating short-chain carboxylate concentrations could be a simple and feasible strategy for improving yeast counting and isolation from mixed cultures. It is important to note that different Daqu starters contain diverse microorganism compositions. Therefore, an optimization process is required to determine the optimal conditions that effectively suppress mold growth while preserving yeast growth.
Acknowledgments
This study was supported by an open fund project of Liquor Making Biotechnology and Application of Key Laboratory of Sichuan Province (Grant No. NJ2022-07).
Footnotes
Author contributions
Zhiqiang Ren: conceptualization, methodology, supervision, funding acquisition, writing-original draft preparation, writing review and editing; Juan Xie: data curation, writing-original draft preparation; Tuoxian Tang: visualization, formal analysis, writingreview and editing; Zhiguo Huang: supervision, resources, funding acquisition.
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Contributor Information
Zhiqiang Ren, Email: renzhiqiang@suse.edu.cn.
Zhiguo Huang, Email: hzguo@suse.edu.cn.
Literature
- De Vuyst L, Leroy F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes FEMS Microbiol Rev 2020 NaN44(4):432. doi: 10.1093/femsre/fuaa014. . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Deak T, Beuchat LR, Guerzoni ME, Lillie A, Peter G, Rohm H, Schnürer F, Tabajdi PV, Westphal S. A collaborative study on media for the enumeration of yeasts in foods Int J Food Microbiol 1998 NaN43(1–2):91. doi: 10.1016/S0168-1605(98)00102-0. . . ; ( ) –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Dijksterhuis J, Meijer M, van Doorn T, Houbraken J, Bruinenberg P. The preservative propionic acid differentially affects survival of conidia and germ tubes of feed spoilage fungi Int J Food Microbiol 2019 NaN306:108258. doi: 10.1016/j.ijfoodmicro.2019.108258. . . ; : . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Echevarría L, Bello F. Mycoflora of well water in the dairy farms of the town of Hatillo Puerto Pico PSM Microbiol 2023;8(1):9. . . ; ( ): –. . [Google Scholar]
- Fan G, Liu P, Chang X, Yin H, Cheng L, Teng C, Gong Y, Li X. Isolation and identification of a high-yield ethyl caproate-producing yeast from Daqu and optimization of its fermentation Front Microbiol 2021 NaN12:663744. doi: 10.3389/fmicb.2021.663744. . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard LM, Corrado MB, Davies CV, Soldá CA, Dalzotto MG, Esteche S. Isolation and identification of native yeasts from the spontaneous fermentation of grape musts Arch Microbiol 2023 NaN205(9):302. doi: 10.1007/s00203-023-03646-1. . . ; ( ): . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Grangeteau C, Gerhards D, Rousseaux S, von Wallbrunn C, Alexandre H, Guilloux-Benatier M. Diversity of yeast strains of the genus Hanseniaspora in the winery environment: What is their involvement in grape must fermentation? Food Microbiol 2015 NaN50:70. doi: 10.1016/j.fm.2015.03.009. . ; : –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Heard GM, Fleet GH. Evaluation of selective media for enumeration of yeasts during wine fermentation J. Appl. Microbiol 1986;60(6):477. doi: 10.1111/j.1365-2672.1986.tb01086.x. . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Higgins C, Brinkhaus F. Efficacy of several organic acids against molds J Appl Poult Res 1999 NaN8(4):480. doi: 10.1093/japr/8.4.480. . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Hu X, Wang K, Chen M, Fan J, Han S, Hou J, Chi L, Liu Y, Wei T. Profiling the composition and metabolic activities of microbial community in fermented grain for the Chinese strong-flavor Baijiu production by using the metatranscriptome, high-throughput 16S rRNA and ITS gene sequencings Food Res Int 2020 NaN138:109765. doi: 10.1016/j.foodres.2020.109765. . . ; (Pt_A): . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Hundová Z, Fencl Z. Toxic effects of fatty acids on yeast cells: dependence of inhibitory effects on fatty acid concentration Biotechnol Bioeng 1977 NaN19(11):1623. doi: 10.1002/bit.260191103. . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Li H, Liu S, Liu Y, Hui M, Pan C. Functional microorganisms in Baijiu Daqu: Research progress and fortification strategy for application Front Microbiol 2023 NaN14:1119675. doi: 10.3389/fmicb.2023.1119675. . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS, Cheng C, Li Z, Chen JY, Yan B, Han BZ, Reeves M. Yeast species associated with wine grapes in China Int J Food Microbiol 2010 NaN138(1–2):85. doi: 10.1016/j.ijfoodmicro.2010.01.009. . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Li W, Fan G, Fu Z, Wang W, Xu Y, Teng C, Zhang C, Yang R, Sun B, Li X. Effects of fortification of Daqu with various yeasts on microbial community structure and flavor metabolism Food Res Int 2020 NaN129:108837. doi: 10.1016/j.foodres.2019.108837. . . ; : . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Liu Y, Tang TX, Pei XQ, Zhang C, Wu ZL. Identification of ketone reductase ChKRED20 from the genome of Chryseobacterium sp. CA49 for highly efficient anti-Prelog reduction of 3,5-bis(trifluoromethyl)acetophenone J Mol Catal B: Enzym 2014 NaN102:1. doi: 10.1016/j.molcatb.2014.01.009. . . ; : –. . https://doi.org/ [DOI] [Google Scholar]
- Ma S, Shang Z, Chen J, Shen Y, Li Z, Huang D, Luo H. Differences in structure, volatile metabolites, and functions of microbial communities in Nongxiangxing daqu from different production areas LWT 2022 NaN166:113784. doi: 10.1016/j.lwt.2022.113784. . . ; : . https://doi.org/ [DOI] [Google Scholar]
- Madbouly AK, Abo Elyousr KAM, Ismail IM. Biocontrol of Monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts Biol Control 2020 NaN144:104239. doi: 10.1016/j.biocontrol.2020.104239. . . ; : . https://doi.org/ [DOI] [Google Scholar]
- Moon NJ. Inhibition of the growth of acid tolerant yeasts by acetate, lactate and propionate and their synergistic mixtures J Appl Bacteriol 1983 NaN55(3):453. doi: 10.1111/j.1365-2672.1983.tb01685.x. . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Narendranath NV, Thomas KC, Ingledew WM. Acetic acid and lactic acid inhibition of growth of Saccharomyces Cerevisiae by different mechanisms J Am Soc Brew Chem 2001;59(4):187. doi: 10.1094/ASBCJ-59-0187. . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Perna MDSC, Bastos RG, Ceccato-Antonini SR. Single and combined effects of acetic acid, furfural, and sugars on the growth of the pentose-fermenting yeast Meyerozyma guilliermondii 3 Biotech 2018 NaN8(2):119. doi: 10.1007/s13205-018-1143-0. . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S, Zhang Y, Lu N, Shi C, Yan S. Yeasts from Chinese strong flavour Daqu samples: Isolation and evaluation of their potential for fortified Daqu production AMB Express 2021 NaN11(1):176. doi: 10.1186/s13568-021-01337-y. . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G, Pais C. The influence of acetic and other weak carboxylic acids on growth and cellular death of the yeast Yarrowia lipolytica Food Technol Biotechnol 2000 NaN38(1):27. . . ; ( ): –. . [Google Scholar]
- Wang B, Wu Q, Xu Y, Sun B. Synergistic effect of multiple saccharifying enzymes on alcoholic fermentation for Chinese baijiu production Appl Environ Microbiol 2020 NaN86(8):2024015. doi: 10.1128/AEM.00013-20. . . ; ( ) :e00013-20. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Chen P, Liu T, Ren D, Dong N, Cui W, He P, Bi Y, Lv N, Ntakatsane M. A novel sensitive visual count card for detection of hygiene bio-indicator-molds and yeasts in contaminated food LWT 2020 NaN117:108687. doi: 10.1016/j.lwt.2019.108687. . . ; : . https://doi.org/ [DOI] [Google Scholar]
- Yan S, Tong Q, Guang J. Yeast dynamics and changes in volatile compounds during the fermentation of the traditional Chinese strong-flavor Daqu LWT 2019a NaN106:57. doi: 10.1016/j.lwt.2019.02.058. . . ; : –. . https://doi.org/ [DOI] [Google Scholar]
- Yan S, Xiangsong C, Jiaquan G. Bacterial and fungal diversity in the traditional Chinese strong flavour liquor Daqu J. Inst. Brew 2019b;125(4):443. doi: 10.1002/jib.574. . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Yang JG, Dou X, Ma YY. Diversity and dynamic succession of microorganisms during Daqu preparation for Luzhou-flavour liquor using second-generation sequencing technology J Inst Brew 2018;124(4):498. doi: 10.1002/jib.528. . . ; ( ): –. . https://doi.org/ [DOI] [Google Scholar]
- Zhang X, St Leger RJ, Fang W. Stress-induced pyruvate accumulation contributes to cross protection in a fungus Environ Microbiol 2018 NaN20(3):1158. doi: 10.1111/1462-2920.14058. . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Zhou Q, Ma K, Song Y, Wang Z, Fu Z, Wang Y, Zhang X, Cui M, Tang N, Xing X. Exploring the diversity of the fungal community in Chinese traditional Baijiu daqu starters made at low-, medium- and high-temperatures LWT 2022 NaN162:113408. doi: 10.1016/j.lwt.2022.113408. . . ; : . https://doi.org/ [DOI] [Google Scholar]
- Zhu M, Zheng J, Xie J, Zhao D, Qiao ZW, Huang D, Luo HB. Effects of environmental factors on the microbial community changes during medium-high temperature Daqu manufacturing Food Res Int 2022 NaN153:110955. doi: 10.1016/j.foodres.2022.110955. . . ; : . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Zou W, Zhao C, Luo H. Diversity and function of microbial community in Chinese strong-flavor baijiu ecosystem: A review Front Microbiol 2018 NaN9:671. doi: 10.3389/fmicb.2018.00671. . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]


