Abstract
Background
Healthy behaviors are paramount in preventing long‐term adverse health outcomes in childhood, adolescent, and young adult (CAYA) cancer survivors. We systematically reviewed and synthesized existing literature on barriers, facilitators, and other factors associated with health behaviors in this population.
Methods
MEDLINE and PsycInfo were searched for qualitative and quantitative studies including survivors aged 16–50 years at study, a cancer diagnosis ≤25 years and ≥2 years post diagnosis. Health behaviors included physical activity, smoking, diet, alcohol consumption, sun exposure, and a combination of these behaviors (defined as health behaviors in general).
Results
Barriers, facilitators, and other factors reported in ≥2 two studies were considered relevant. Out of 4529 studies, 27 were included (n = 31,905 participants). Physical activity was the most frequently examined behavior (n = 12 studies), followed by smoking (n = 7), diet (n = 7), alcohol (n = 4), sun exposure (n = 4), and health behavior in general (n = 4). Relevant barriers to physical activity were fatigue, lack of motivation, time constraints, and current smoking. Relevant facilitators were perceived health benefits and motivation. Influence of the social environment and poor mental health were associated with more smoking, while increased energy was associated with less smoking. No relevant barriers and facilitators were identified for diet, alcohol consumption, and sun exposure. Barriers to healthy behavior in general were unmet information needs and time constraints whereas lifestyle advice, information, and discussions with a healthcare professional facilitated healthy behavior in general. Concerning other factors, women were more likely to be physically inactive, but less likely to drink alcohol and more likely to comply with sun protection recommendations than men. Higher education was associated with more physical activity, and lower education with more smoking.
Conclusion
This knowledge can be used as a starting point to develop health behavior interventions, inform lifestyle coaches, and increase awareness among healthcare providers regarding which survivors are most at risk of unhealthy behaviors.
Keywords: barriers, CAYA cancer survivors, facilitators, factors, health behaviors, healthy lifestyle, pediatric oncology, systematic review
1. INTRODUCTION
The number of childhood, adolescent, and young adult (CAYA) cancer survivors is increasing due to survival rates of up to approximately 80% in high‐income countries. 1 , 2 Unfortunately, 75% of long‐term CAYA cancer survivors experience adverse health outcomes later in life, such as cardiovascular and musculoskeletal disease, metabolic syndrome, cancer‐related fatigue, anxiety, and depression. 3 , 4 , 5 , 6 , 7 , 8 These health outcomes can lead to hospitalization, disability, reduced quality of life, and premature mortality. 9
Unhealthy behaviors—including insufficient physical activity, smoking, an unbalanced diet, excessive alcohol consumption, and unprotected sun exposure—are associated with an increased risk of developing adverse health outcomes in CAYA cancer survivors. 10 , 11 , 12 , 13 , 14 , 15 , 16 Health behavior change interventions are effective and feasible in reducing these risks. 17 , 18 , 19 , 20 , 21 Consequently, the adoption and maintenance of healthy behaviors has become paramount in the prevention of long‐term adverse health outcomes in CAYA cancer survivors.
However, CAYA cancer survivors may face specific barriers and facilitators when trying to adopt healthy behaviors. For example, physical limitations resulting from cancer treatment, chronic pain, and fatigue may hinder engagement in physical activity. 22 , 23 On the contrary, survivors might be more aware of their elevated health risks, which may increase their motivation to change their behavior. 24 Positive or negative attitudes and beliefs also play an important role in shaping survivors' willingness to adopt healthy behaviors. 25 , 26
In order to develop targeted interventions tailored to the individual needs and preferences of CAYA cancer survivors, more knowledge is needed about the barriers, facilitators, and other factors associated with their health behaviors. In addition, a comprehensive understanding of the barriers, and facilitators that influence the different health behaviors of CAYA cancer survivors can inform guidelines and help healthcare providers (HCPs) involved in survivorship care to promote healthy habits. Knowledge about other factors, such as sociodemographic, treatment‐ and clinical factors, can be used to raise awareness among CAYA cancer survivors at high risk of unhealthy lifestyle behaviors. Therefore, this systematic review aimed to synthesize the existing evidence on the relevant barriers, facilitators, and other factors associated with health behaviors in CAYA cancer survivors.
2. METHODS
2.1. Inclusion and exclusion criteria
2.1.1. Study designs
All study designs were eligible for inclusion except narrative and systematic reviews and case reports. We only included quantitative studies that reported multivariable models, as these provide more robust analyses and better control for confounding variables, increasing the reliability of the results. In addition, only English‐language studies published after 2000 were included, as earlier research largely overlooked the impact of health behavior on late adverse health outcomes.
2.1.2. Participants
To ensure inclusivity without compromising reliability and to account for differences in international definitions of age thresholds for childhood cancer, studies were eligible if ≥75% of the study population was diagnosed with cancer <25 years of age, ≥50% of the population was ≥2 years after their primary cancer diagnosis, and participants were aged 16–50 years at the time of study. Studies including participants still undergoing cancer treatment were excluded. Studies with mixed samples (e.g., survivors aged < and ≥25 years at diagnosis) were included if results allowed separation.
2.1.3. Outcomes
Outcomes were barriers, facilitators, and other factors associated with physical activity, smoking, diet, alcohol consumption, sun exposure, and a combination of health behaviors (health behavior in general). Barriers were interpreted as influencing the persistence of unhealthy behaviors or hindering/limiting healthy behaviors, whereas facilitators were interpreted as factors supporting engagement in healthier behaviors. In addition, barriers and facilitators were interpreted as potentially modifiable, for example, through lifestyle interventions. Other variables that were associated with health behavior and behavior change but were considered non‐modifiable or very difficult to modify were categorized separately as other factors, that is, sociodemographic and clinical characteristics. Factors that influence the persistence of unhealthy behaviors or hinder/limit healthy behaviors were termed “risk factors”, while factors that support engagement in healthier behaviors were termed “supportive factors”.
We distinguished between outcomes derived from qualitative and/or (semi‐)quantitative survey studies and those derived from quantitative studies, including observational or (semi)experimental studies with measures of association as outcomes. All suboutcomes, such as—in the case of smoking—smoking cessation, smoking rate, and quit attempts, were aggregated to the primary health behavior of interest, that is, smoking.
2.2. Search strategy for identification of studies
We conducted a systematic literature search in MEDLINE (Ovid until 15 April 2021 and PubMed from 15 April 2021 to 26 April 2023 for the updated search) and PsycInfo until 15 April 2021 (Appendix B). Reference lists of included studies and reviews were searched for studies not included in the electronic database searches. All authors were asked to identify any missing studies that had not been identified in the previous searches.
2.3. Study selection
After removing duplicates, two independent reviewers assessed the titles and abstracts using Rayyan (https://rayyan.ai). Studies meeting the inclusion criteria were retrieved for full‐text review. The reviewers discussed discrepancies that arose at either stage. Third party arbitration was not required. Two studies by Emmons et al. partially overlapped: Emmons et al. (2003) described the baseline data collection and intervention design of the Partnership for Health Study, and Emmons et al. (2005) described the outcomes of the intervention. 20 , 27 As both studies met the inclusion criteria, we included them in our review.
2.4. Data extraction
Standardized evidence tables (Appendix S1) were created to ensure the accuracy and consistency of data collection. These evidence tables included information on study design, participant characteristics, and outcomes. The tables were prepared by one author and checked by another author. In case of discrepancies or disagreements, the authors agreed by discussion.
2.5. Data synthesis
We have summarized the results in two separate tables: one for barriers and facilitators from qualitative and (semi‐)quantitative studies (Table 1) and one for significant barriers, facilitators, and other factors from quantitative studies (Table 2). These tables contain information about the study design, the participants and a summary of findings. Additionally, we created two overview tables (Tables 3 and 4) for all the barriers, facilitators, and other factors extracted from Tables 1 and 2. We categorized the barriers, facilitators, and other factors outlined in Tables 3 and 4 based on their content to enhance clarity and readability. Furthermore, we presented tables 3 and 4 using a three‐level color scheme. Barriers/risk factors preventing changes from unhealthy to healthy behaviors are shown in red, while facilitators/supportive factors associated with healthier behavior are shown in green. Darker colors indicate higher frequencies of specifically identified barriers, facilitators, or other factors on a 3‐point scale. Barriers, facilitators, and other factors reported in at least two studies were considered relevant and are described in the results.
TABLE 1.
Qualitative and semi‐quantitative survey studies examining barriers and facilitators to health behaviors.
| Study (design) and participants (N) | Barriers and facilitators |
|---|---|
| Physical activity | |
| Keats et al. 2007 (elicitation survey, 59 adolescent cancer survivors) |
Adopting a physically active lifestyle Barriers:
Facilitators:
|
| Arroyave et al. 2008 (cross‐sectional single‐center survey study, 118 CCS) |
Increasing exercise Barriers (as indicated by descriptive statistics):
|
| Le et al. 2017 (pilot intervention study, 19 CCS) |
Adopting a physically active lifestyle Barriers (as indicated by descriptive statistics):
Facilitators (as indicated by descriptive statistics):
|
| Dugan et al. 2021 (qualitative concept elicitation survey study, 17 CCS) |
Physical activity Barriers:
Facilitators:
|
| Marchak et al. 2023 (Cross‐sectional survey study, 27 CCS) |
Physical activity Barriers (as indicated by descriptive statistics):
|
| Diet | |
| Arroyave et al. 2008 (cross‐sectional single‐center survey study, 118 CCS) |
Eating more fruits and vegetables Barriers (as indicated by descriptive statistics):
Eating more whole grains Barriers (as indicated by descriptive statistics):
Eating more high‐calcium foods Barriers (as indicated by descriptive statistics):
Limiting high‐fat foods Barriers (as indicated by descriptive statistics):
|
| Alexander et al. 2022 (Cross‐sectional survey study, 27 young adult cancer survivors) |
Acquirement of healthy nutrition habits Facilitators (as indicated by descriptive statistics):
|
| Marchak et al. 2023 (cross‐sectional survey study, 27 CCS) |
Healthy nutrition Barriers (as indicated by descriptive statistics):
|
| Health behavior in general | |
| Mayes et al. 2016 (semi‐structured interviews, 51 CAYA cancer survivors) |
Adopting a healthy lifestyle Facilitators:
|
| Pugh et al. 2018 (individual interviews and focus groups, 13 CAYA cancer survivors) |
Health behavior change (including physical activity, diet, smoking, alcohol consumption, and sun safety) Barriers:
Facilitators:
|
| Bouwman et al. 2023 (focus groups and semi‐structured interviews, 32 CCS) |
Healthy lifestyle knowledge Barriers:
Facilitators:
Consequences Facilitators:
Environmental context and resources Barrier:
Facilitators:
Social influences Barriers:
Facilitators:
Beliefs about capabilities Facilitators:
Reinforcement Barrier:
Facilitators:
Memory, attention and decision processes Facilitators:
Skills Facilitator:
Emotion Barrier:
Behavioral regulation Facilitator:
|
Abbreviations: CAYA, childhood, adolescent, and young adult, CCS, childhood cancer survivors, LTFU, long‐term follow‐up.
Note: Barriers contribute to the persistence of unhealthy behaviors, while facilitators support the transition to healthier choices.
TABLE 2.
Quantitative studies examining significant barriers, facilitators, and other factors associated with health behaviors.
| Study (design) and participants (N) | Barriers, facilitators, and other factors significantly associated with health behaviors |
|---|---|
| Physical activity (PA) | |
| Florin et al. 2007 (cross‐sectional multi‐center survey study, 2648 CCS) |
Not meeting physical activity recommendations
Inactive lifestyle
Male ALL survivor – chemo + CRT > 20 Gy (vs. male control), OR 1.9, 95% CI (1.5–2.3) |
| Cox et al. 2009 (cross‐sectional survey study, 838 CCS) |
Higher physical activity participation (as indicated by Structural Equation Modeling) In men:
In women:
|
| Ness et al. 2009 (cross‐sectional multi‐center study, 9301 CCS) |
Not meeting physical activity recommendations
Inactive lifestyle
|
| Rueegg et al. 2012 (cross‐sectional multi‐center study, 1058 CCS) |
Inactivity
No sports
|
| Rueegg et al. 2012 (cross‐sectional multi‐center study, 1038 CCS) |
Any limitations in sports
Any limitations in daily activities
|
| Slater et al. 2016 (cross‐sectional survey study, 158 CCS) | Engaging in active transportation
|
| Darabos et al. 2021 (cross‐sectional survey study, 307 CCS) | Not meeting physical activity recommendations
|
| Smoking | |
| Emmons et al. 2003 (randomized trial of a smoking cessation intervention, 796 smoking CCS) |
Higher smoking rates (β represents the increase in the odds of higher smoking rates)
Nicotine dependence
More quit attempts
Readiness to quit
|
| Emmons et al. 2005 (randomized trial of a smoking cessation intervention, 796 smoking CCS; overlap with Emmons et al. 2003) | Smoking cessation
|
| Kahalley et al. 2012 (cross‐sectional multi‐center survey, 307 CCS) | Smoking
|
| Bougas et al. 2021 (cohort study, 2887 CCS) |
Smoking
Quitting smoking
|
| Cappelli et al. 2021 (cohort study, 127 young adult cancer survivors) | Smoking
|
| Darabos et al. 2021 (cross‐sectional survey study, 307 CCS) | Smoking
|
| Cheung et al. 2022 (cross‐sectional survey study, 200 CCS) | Smoking
|
| Alcohol consumption | |
| Lown et al. 2008 (cross‐sectional survey study, 10,398 CCS) | Heavy drinking
|
| Cappelli et al. 2021 (cohort study, 127 young adult cancer survivors) | Binge drinking
|
| Darabos et al. 2021 (cross‐sectional survey study, 307 CCS) | Binge drinking
|
| Cheung et al. 2022 (cross‐sectional survey study, 200 CCS) | Alcohol consumption
|
| Diet | |
| Zhang et al. 2016 (retrospective cohort study with cross‐sectional assessment, 2570 CCS) |
High diet quality based on adjusted means Healthy Eating Index–2010 (maximum score = 100):
|
| Bhandari et al. 2021 (cross‐sectional survey study, 446 CCS) | Vitamin D deficiency
|
| Cheung et al. 2022 (cross‐sectional survey study, 200 CCS) | Adoption of a balanced diet more than ≥4 days per week
|
| Darabos et al. 2021 (cross‐sectional survey study, 307 CCS) | Not meeting fruit/vegetable intake recommendations
|
| Sun exposure | |
| Zwemer et al. 2012 (cross‐sectional survey study, 153 young adult cancer survivors) |
Low adherence to sunbathing recommendations
Low adherence recommendations during incidental sun exposure
|
| Darabos et al. 2021 (cross‐sectional survey study, 307 CCS) | Engaging in unsafe sun protective habits
|
| Cheung et al. 2022 (cross‐sectional survey study, 200 CCS) | Sunscreen use more than ≥4 days per week
|
| Fluehr et al. 2023 (cross‐sectional survey study, 94 CAYA cancer survivors) | Increased sun protection behaviors (as indicated hierarchical linear regression)
|
| Health behavior in general | |
| Klosky et al. 2012 (retrospective multi‐center survey study, 307 CAYA cancer survivors) | Poor overall behavioral health
|
Abbreviations: ALL, acute lymphocytic leukemia, BMI, body mass index, CAYA, childhood, adolescent, and young adult, CCS, childhood cancer survivors, CI, confidence interval, CNS, central nervous system, CRT, cranial radiotherapy, Gy, gray, RR, risk ratio, OR, odds ratio.
Note: This table displays only the significant study results; non‐significant results and descriptions of the models used for each included study are shown in the evidence tables (Supplementary File A). Barriers contribute to the persistence of unhealthy behaviors, while facilitators support the transition to healthier choies.
TABLE 3.
Barriers and facilitators to health behaviors.
| Health behaviors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health behavior in general | Smoking | Physical activity | Diet | Alcohol consumption | Sun exposure | |||||||
| Barriers | Facilitators | Barriers | Facilitators | Barriers | Facilitators | Barriers | Facilitators | Barriers | Facilitators | Barriers | Facilitators | |
| Information, knowledge, and skills | ||||||||||||
| Lifestyle advice and information | N = 3 [28, 29, 30] | |||||||||||
| Health promotion conversation with healthcare professional | N = 2 [28, 30] | |||||||||||
| Being able to contact healthcare professional for lifestyle choices | N = 1 [28] | |||||||||||
| Unmet information needs | N = 2 [29, 31] | |||||||||||
| Lack of knowledge and skills | N = 2 [26, 32] | |||||||||||
| Knowledge and skills | N = 1 [29] | |||||||||||
| Learning how to deal with physical limitations | N = 1 [31] | |||||||||||
| Counseling | N = 1 [20] | |||||||||||
| Self‐management and cognitive processes | ||||||||||||
| Self‐help | N = 1 [33] | |||||||||||
| Lack of self‐efficacy | N = 1 [26] | |||||||||||
| Self‐efficacy | N = 1 [29] | N = 1 [20] | ||||||||||
| Lack of confidence | N = 1 [26] | |||||||||||
| Confidence | N = 1 [29] | N = 1 [26] | ||||||||||
| Lack of motivation | N = 1 [30] | N = 4 [25, 26, 31, 34] | ||||||||||
| (Self)‐motivation | N = 2 [31, 35] | |||||||||||
| Positive reinforcement | N = 1 [30] | |||||||||||
| Conscious decision‐making | N = 1 [30] | |||||||||||
| Decisions embedded in memory | N = 1 [30] | |||||||||||
| Health and wellbeing | ||||||||||||
| Cancer‐related physical changes | N = 1 [29] | |||||||||||
| Perceived health benefits | N = 1 [31] | N = 2 [25, 26] | ||||||||||
| Weight management | N = 1 [26] | |||||||||||
| Fear of injury | N = 2 [25, 34] | |||||||||||
| Foods hurting stomach | N = 1 [36] | |||||||||||
| Perceived greater relative susceptibility to skin cancer | N = 1 [36] | |||||||||||
| Fatigue | N = 5 [25, 26, 34, 35, 37] | |||||||||||
| Stamina | N = 1 [35] | |||||||||||
| Decreased strength | N = 1 [34] | |||||||||||
| Being underweight | N = 2 [38, 39] | |||||||||||
| Being overweight | N = 2 [34, 38] | N = 1 [40] | N = 1 [41] | |||||||||
| Being obese | N = 2 [38, 39] | N = 1 [40] | ||||||||||
| Poor general health | N = 1 [42] | N = 1 [31] | N = 1 [43] | |||||||||
| Experiencing physical limitations | N = 2 [26, 34] | |||||||||||
| Having (had) cardiovascular disease | N = 1 [42] | |||||||||||
| Environmental influences | ||||||||||||
| Positive family influence | N = 1 [29] | N = 1 [31] | ||||||||||
| Negative family influence | N = 1 [29] | N = 1 [44] | N = 1 [26] | |||||||||
| Positive social environment | N = 1 [31] | N = 1 [27] | ||||||||||
| Negative influence of social environment | N = 1 [31] | N = 2 [27, 44] | N = 1 [36] | |||||||||
| Lack of peer support | N = 1 [31] | N = 1 [25] | ||||||||||
| Peer support | N = 1 [29] | N = 1 [27] | N = 1 [31] | N = 1 [33] | ||||||||
| Positive influence of social media | N = 1 [31] | |||||||||||
| Negative influence of social media | N = 1 [31] | |||||||||||
| Connectedness | N = 1 [26] | |||||||||||
| Stimulating work environment | N = 1 [30] | |||||||||||
| Sedentary profession | N = 1 [31] | |||||||||||
| Obligatory classes at school | N = 1 [31] | |||||||||||
| Doctors limiting physical activities | N = 1 [34] | |||||||||||
| Resources | ||||||||||||
| Resource unavailability | N = 1 [29] | N = 1 [36] | N = 1 [36] | |||||||||
| Distance | N = 1 [29] | N = 1 [31] | ||||||||||
| Lack of finances | N = 1 [29] | N = 2 [26, 31] | ||||||||||
| No private medical insurance | N = 1 [34] | |||||||||||
| Having enough time | N = 1 [31] | |||||||||||
| Time constraints | N = 2 [29, 30] | N = 4 [25, 26, 31, 37] | ||||||||||
| Available professional support | N = 1 [33] | N = 1 [33] | ||||||||||
| Walkability of the neighborhood | N = 1 [45] | |||||||||||
| Proximity of facilities | N = 1 [31] | |||||||||||
| Having adaptive equipment | N = 1 [31] | |||||||||||
| Digital prints/materials | N = 1 [33] | |||||||||||
| Mental health and quality of life | ||||||||||||
| Good mental health | N = 1 [32] | |||||||||||
| Poor mental health | N = 2 [27, 42] | |||||||||||
| Depression | N = 1 [38] | N = 1 [43] | ||||||||||
| Anxiety | N = 1 [43] | |||||||||||
| Suicidal behavior | N = 1 [44] | |||||||||||
| Increased energy | N = 2 [20, 26] | |||||||||||
| Poor physical quality of life | N = 1 [42] | |||||||||||
| Poor mental quality of life | N = 1 [42] | |||||||||||
| Stress | N = 1 [30] | |||||||||||
| Perceived vulnerability | N = 1 [27] | N = 1 [46] | ||||||||||
| Fear regarding future health | N = 1 [35] | |||||||||||
| Behavioral regulation | ||||||||||||
| Too much screen time | N = 1 [34] | |||||||||||
| Currently smoking | N = 3 [38, 39, 47] | |||||||||||
| Not currently smoking | N = 1 [41] | |||||||||||
| Being a past smoker | N = 1 [48] | N = 1 [38] | ||||||||||
| Drinking initiation at a young age | N = 1 [43] | |||||||||||
| Higher (baseline) exercise frequency | N = 1 [35] | N = 1 [41] | ||||||||||
| Planning | N = 1 [30] | N = 1 [45] | ||||||||||
| Not liking the taste of certain foods | N = 1 [36] | |||||||||||
Note: Numbers in brackets refer to the reference number of an included study. Barriers (red colors) contribute to the persistence of unhealthy behaviors, while facilitators (green colors) support (the transition to) healthier behaviors. The darkness of the color corresponds to the frequency of the barriers or facilitators on a 3‐point scale, with darker colors representing higher frequencies.
TABLE 4.
Sociodemographic, cancer and treatment related and other factors associated with health behavior.
| Health behaviors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health behavior in general | Smoking | Physical activity | Diet | Alcohol consumption | Sun exposure | |||||||
| Risk | Supportive | Risk | Supportive | Risk | Supportive | Risk | Supportive | Risk | Supportive | Risk | Supportive | |
| Sociodemographic factors | ||||||||||||
| Male sex | N = 1 [42] | N = 2 [43, 48] | ||||||||||
| Female sex | N = 1 [49] | N = 3 [38, 39, 47] | N = 1 [41] | N = 1 [34] | N = 1 [46] | N = 2 [34, 49] | ||||||
| Older age | N = 1 [27] | N = 1 [42] | N = 1 [38] | N = 1 [41] | N = 1 [49] | |||||||
| Younger age | N = 1 [27] | N = 1 [34] | N = 1 [43] | N = 1 [46] | ||||||||
| Being married or living with a partner | N = 1 [42] | N = 1 [45] | ||||||||||
| Having children | N = 2 [31, 39] | |||||||||||
| Lower level of education | N = 2 [27, 34] | N = 2 [39, 47] | N = 1 [43] | N = 1 [34] | ||||||||
| Higher level of education | N = 1 [42] | N = 3 [35, 38, 39] | ||||||||||
| Lower level of parent's education | N = 1 [50] | |||||||||||
| Unknown parent's education | N = 1 [50] | |||||||||||
| Non‐Hispanic White ethnicity | N = 1 [49] | N = 1 [49] | N = 1 [49] | |||||||||
| Hispanic, Black or Other Non‐Hispanic ethnicity | N = 2 [38, 47] | |||||||||||
| Hispanic or Black ethnicity | N = 1 [40] | |||||||||||
| Lower income | N = 1 [47] | N = 1 [34] | ||||||||||
| Being unable to work | N = 1 [38] | |||||||||||
| Being a student | N = 1 [38] | |||||||||||
| Cancer‐ and treatment‐related factors | ||||||||||||
| Being a survivor of lymphoma | N = 1 [50] | N = 1 [41] | ||||||||||
| Being a survivor of CNS tumor | N = 1 [42] | |||||||||||
| Solid tumor diagnosis | N = 1 [49] | |||||||||||
| Diagnosis including hematological malignancies | N = 1 [34] | |||||||||||
| Other cancer diagnosis than leukemia | N = 1 [50] | N = 1 [43] | ||||||||||
| Cancer diagnosis at a younger age | N = 1 [41] | |||||||||||
| Cancer diagnosis during late adolescence | N = 1 [43] | |||||||||||
| Higher cancer treatment intensity | N = 1 [48] | N = 1 [49] | ||||||||||
| Surgery only | N = 1 [50] | |||||||||||
| Having had surgery | N = 1 [38] | |||||||||||
| Chemotherapy | N = 1 [42] | |||||||||||
| Radiotherapy | N = 2 [38, 50] | |||||||||||
| Cranial radiotherapy | N = 1 [38] | N = 1 [43] | ||||||||||
| No cranial radiotherapy | N = 1 [44] | |||||||||||
| Chemotherapy and/or cranial radiotherapy | N = 1 [47] | |||||||||||
| Thoracic radiotherapy | N = 1 [42] | |||||||||||
| Bone marrow transplantation (vs. chemotherapy) | N = 1 [50] | N = 1 [50] | ||||||||||
| Lower abdomen radiation dose | N = 1 [41] | |||||||||||
| 4600–8999 Mg/m2 cumulative glucocorticoid dose | N = 1 [41] | |||||||||||
| Intrathecal methotrexate or cranial radiation | N = 1 [43] | |||||||||||
| Familiarity of primary care physician with cancer‐related problems | N = 1 [35] | |||||||||||
| Having had a relapse | N = 1 [49] | |||||||||||
| Having had a second cancer | N = 1 [42] | |||||||||||
| Longer time since treatment completion | N = 1 [49] | |||||||||||
| Other factors | ||||||||||||
| Amputation of lower limb | N = 1 [38] | |||||||||||
| Fair/easily burned skin type | N = 1 [51] | |||||||||||
Note: Numbers in brackets refer to the reference number of an included study. Green colors indicate a positive association with the health behavior. Red colors indicate a negative association. The darkness of the color corresponds to the frequency of the factors on a 3‐point scale, with darker colors representing higher frequencies.
3. RESULTS
After removing duplicates, 4529 abstracts were identified and screened. Next, 141 full‐text articles were assessed for inclusion in the review. Eight studies were identified by screening the reference lists of the included studies and relevant reviews, and four studies were identified by experts in the field. Finally, 27 studies met all eligibility criteria (Figure 1). Nine were qualitative or semiquantitative studies and 18 were quantitative studies. Study designs included interviews (n = 1), a combination of interviews and focus groups (n = 2), elicitation surveys (n = 2), a pilot intervention study (n = 1), randomized trials (n = 2), cohort studies (n = 3), cross‐sectional survey studies (n = 15), and a retrospective multi‐institution survey study (N = 1). Sample sizes ranged from 13 to 10,398, with a total of 31,905 participants across all included studies. All studies were conducted between 2003 and 2023. Five studies reported on more than one health behavior. 34 , 37 , 48 , 49 , 52
FIGURE 1.
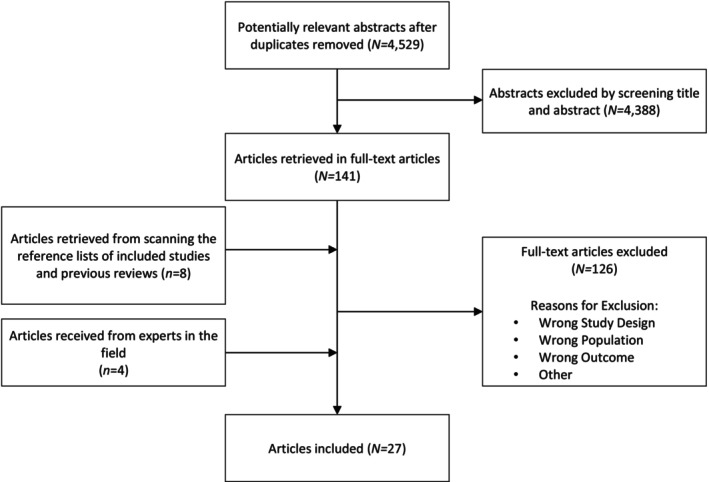
Flowchart of studies included and excluded from the systematic review.
3.1. Barriers, facilitators, and other factors associated with health behaviors (Tables 3 and 4)
3.1.1. Physical activity (n = 12 studies, n = 15,588 participants)
Twenty‐one barriers, 17 facilitators, 17 risk factors, and 10 supportive factors associated with physical activity were reported in the included studies. Barriers reported in at least two studies were fatigue (n = 5 25 , 26 , 34 , 35 , 37 ), lack of motivation (n = 4 25 , 26 , 31 , 34 ), time constraints (n = 4 25 , 26 , 31 , 37 ), being a current smoker (n = 3 38 , 39 , 47 ), a lack of knowledge and skills (n = 2 25 , 26 ); fear of injury (n = 2 25 , 34 ); lack of finances (e.g., for a gym membership; n = 2 26 , 31 ), being underweight (n = 2 38 , 39 ), being overweight (n = 2 34 , 38 ), being obese (n = 2 38 , 39 ), and experiencing physical limitations (n = 2, e.g., poor balance or lack of fitness 26 , 34 ). Facilitators for physical activity reported in at least two studies were perceived health benefits (n = 2 25 , 26 ) and (self)‐motivation (n = 2 31 , 35 ).
Relevant risk factors for physical activity were female sex (n = 3 38 , 47 ), a treatment history including radiotherapy (n = 2 38 , 50 ), having children (n = 2 31 , 39 ), lower educational level (n = 2 39 , 47 ), and being of Hispanic, Black, or other non‐Hispanic ethnicity (n = 2 38 , 47 ). In contrast, higher levels of education (n = 3 35 , 38 , 39 ) was a supportive factor for physical activity.
3.1.2. Smoking (n = 7 studies, n = 5420 participants)
Seven barriers, six facilitators, five risk factors, and 12 supportive factors were associated with smoking in the included studies. Barriers reported in at least two studies were negative influence of the social environment (n = 2, e.g., smoking in the household or a higher proportion of smokers in the social network 27 , 44 ) and poor mental health (n = 2 27 , 42 ). On the contrary, increased energy (n = 2 20 , 26 ) was identified as a facilitator in at least two studies. In terms of factors, lower educational attainment (n = 2 27 , 34 ) was a risk factors for smoking.
3.1.3. Diet (n = 7 studies, n = 3695 participants)
Six barriers (e.g., not liking the taste of certain foods), seven facilitators (e.g., peer support), two risk factors (e.g., Hispanic or Black ethnicity), and 10 supportive factors (e.g., female sex) were associated with diet in one of the included studies. No barriers, facilitators, or factors were reported in at least two studies.
3.1.4. Alcohol consumption (n = 4 studies, n = 11,032 participants)
Three barriers, one facilitator, seven risk factors, and four supportive factors associated with (reducing) alcohol consumption were reported in the included studies. No barriers or facilitators were reported in at least two studies. However, in terms of factors, men had significantly higher levels of alcohol consumption than women, especially with regard to binge drinking (n = 2 43 , 48 ).
3.1.5. Sun exposure (n = 4 studies, n = 754 participants)
The included studies reported zero barriers, two facilitators, four risk factors, and three supportive factors associated with (increased) sun exposure. No barriers or facilitators were reported in at least two studies. However, women were significantly more likely than men to adhere to sun exposure recommendations (n = 3 34 , 46 , 49 ).
3.1.6. Health behavior in general (n = 4 studies, n = 403 participants)
Twelve barriers and 19 facilitators associated with health behavior in general were identified in the included studies. Barriers identified in at least two studies were unmet information needs (n = 2 29 , 30 ) and time constraints (n = 2 29 , 30 ). Lifestyle advice and information (n = 3 28 , 29 , 30 ) and having a health promotion conversation with a healthcare professional (n = 2 28 , 30 ) were identified as facilitators in at least two studies. There were no other factors associated with health behavior in general.
3.1.7. Nonsignificant results
For the quantitative studies, Tables 1, 2, 3, 4 include only the significant results. Non‐significant results are reported in Appendix S2. Across all health behavior outcomes (NB: a single study may examine multiple outcomes), the most nonsignificant results were found for age at diagnosis (n = 17 28 , 34 , 39 , 40 , 46 , 49 ), cancer diagnosis (n = 14 28 , 39 , 43 , 46 , 49 ), attained age (n = 14 20 , 28 , 34 , 39 , 40 , 44 , 45 , 46 , 49 , 51 ), cancer treatment (n = 12 28 , 38 , 39 , 40 , 41 , 46 , 49 , 51 ), sex (n = 11 20 , 34 , 39 , 44 , 48 , 49 , 51 ), race/ethnicity (n = 9 28 , 36 , 41 , 43 , 46 , 49 ), and household income (n = 7 28 , 34 , 46 ). None of the significant results identified in the included studies were outweighed by a greater number of nonsignificant results. In other words, the results described in Tables 1, 2, 3, 4 were all found to be statistically significant more often than statistically nonsignificant.
4. DISCUSSION
To our knowledge, this systematic review is the first to provide a comprehensive overview of the evidence on barriers, facilitators, and other factors associated with health behaviors in CAYA cancer survivors. Physical activity was the most commonly studied health behavior in this systematic review. The most frequently identified barriers to physical activity were fatigue, time constraints, lack of motivation, current smoking, lack of knowledge and skills, fear of injury, financial constraints, being either underweight, overweight, or obese, and experiencing physical limitations. Of note, feeling fatigued may reduce physical activity, but regular physical activity may in its turn reduce cancer‐related fatigue. 51 , 53 Female sex was the most commonly identified risk factor associated with lower levels of physical activity, followed by a treatment history including radiotherapy, having children, having less education, and being of Hispanic, black, or other non‐Hispanic ethnicity. Facilitators for physical activity included perceived health benefits and levels of motivation. Higher education was the only supportive factor associated with increased physical activity in at least two studies.
Systematic reviews in people without cancer found comparable correlates of physical activity, including sex, having knowledge/appreciation of the benefits of physical activity, (lack of) motivation, smoking, access to facilities, lack of time, lack of energy, and having underlying health problems. 54 , 55 , 56 , 57 Besides, Brown and colleagues recently synthesized evidence from eight qualitative studies of barriers and facilitators to physical activity from the perspective of childhood cancer survivors. 58 Parental influence and support were found to be major themes, possibly because parental factors were the main focus of two of the included studies. The current review adds to these findings by synthesizing evidence from both qualitative and quantitative studies, including the impact of sociodemographic, cancer and treatment‐related factors on survivors' physical activity levels.
Higher smoking rates among CAYA cancer survivors were related to lower levels of education, poor mental health, and having more peers or household members who smoke. In contrast, increased energy was associated with lower smoking rates. Men were more likely than women to have higher levels of alcohol consumption. These findings are consistent with the literature on smoking and alcohol consumption in the general population 59 , 60 , 61 and highlight the importance of sociodemographic factors such as sex and educational level in identifying those at risk of unhealthy behaviors. Furthermore, as smoking and mental health are linked through the withdrawal effect of tobacco, HCPs can explain to smokers that the decrease in nicotine levels after smoking a cigarette leads to withdrawal symptoms such as poor concentration, insomnia, feelings of tension, restlessness, low mood, and anxiety. 60 One strategy that can be used to support smoking cessation is cognitive behavioral therapy (CBT), which helps people understand the relationships between their thoughts, feelings, behaviors, and physical experiences. 60 CBT can also be used for improving other health behaviors.
This review also found that women were more likely than men to adhere to sun exposure recommendations, with the exception of occasional sun exposure. This is consistent with research in the general population suggesting that men are more likely to perceive the inconvenience and cost of sunscreen and sun‐protective clothing as barriers to their sun‐protective behavior. 62 In addition, men tend to perceive skin damage from sun exposure as less severe than women do. 62
We did not find any barriers, facilitators, or other factors associated with a healthy diet that were identified by two or more studies. In the general population, systematic reviews found that social environment plays an important role in dietary health behavior, along with automaticity, self‐regulation, motivational regulation, subjective norm, and relationships with sedentary behavior. 63 , 64 However, the evidence is suggestive at best, because of the widespread use of cross‐sectional designs in the studies included in the reviews. More research is therefore needed to understand the barriers and facilitators associated with a healthy diet among both the general population and, particularly, CAYA cancer survivors.
4.1. Health behavior interventions and identification of survivors most at risk
The barriers and facilitators identified in this review can be used as a starting point for developing health behavior interventions that meet the needs and preferences of individual CAYA cancer survivors and support them in adopting healthier lifestyles. For example, a targeted health behavior intervention can help survivors to manage their clinical symptoms of fatigue and time constraints, and increase their motivation by addressing their individual preferences and needs and by emphasizing the benefits of healthy lifestyles. A recent systematic review and meta‐analysis on healthy lifestyle interventions found that current health behavior interventions are primarily exercise‐based without significant effects on physical outcomes such as physical fitness, fatigue, and body mass index. 65 Therefore, a different, more holistic and individualized approach to health behavior interventions is warranted. 66 Overall, further clinical trials are needed to increase the body of research on effective health behavior interventions for CAYA cancer survivors. Such interventions should build on the accumulated evidence on barriers and facilitators and address strategies to overcome fatigue, increase and sustain motivation over time, and include aspects of time management techniques. Moreover, engaging key stakeholders such as survivors, HCPs, and policymakers at the initial stages of intervention development increases the likelihood of creating interventions that are not only delivered on time and within budget but also deemed acceptable and feasible. 67
The insights in relevant risk and supportive factors associated with health behaviors can help to increase awareness among HCPs regarding which survivors are most at risk of certain unhealthy behaviors. However, the consistent lack of statistical significance observed for risk and supportive factors related to cancer history and treatment, such as age at diagnosis, cancer diagnosis, and cancer treatment, highlights the possibility that these specific factors may not be of substantial importance in relation to health behaviors in CAYA cancer survivors. In other words, this review indicates that the primary results identified are not inherently specific to CAYA cancer survivors. Consequently, HCPs and lifestyle coaches may need to broaden their focus beyond medical history when assisting CAYA cancer survivors to improve their health behaviors and adopt new habits. For instance, other individual characteristics such as sex and educational attainment should be taken into account.
4.2. The importance of knowledge dissemination
This review highlights the importance of increasing knowledge about healthy behaviors in general among CAYA cancer survivors through health behavior advice, information dissemination, and health promotion discussions with HCPs. These findings align with a recent qualitative study of HCPs, which highlighted the critical role of education and training of HCPs in effectively guiding CAYA cancer survivors toward healthy behaviors. 68 Survivorship care clinics should prioritize the integration of health behavior support services such as lifestyle coaching and ensure that HCPs are adequately equipped with the necessary knowledge and skills to support survivors in adopting and maintaining healthy behaviors. In addition, a systematic review of 17 randomized controlled trials among all types of patients showed that using deliberate communication strategies when providing information can improve patient outcomes more effectively than not using such strategies. 69 Therefore, when HCPs aim to encourage survivors to engage in specific health behaviors, they may particularly benefit from using explicit persuasive information strategies.
4.3. Strengths and limitations
The methodology used in this review had several strengths. First, we followed a rigorous and transparent approach, including a comprehensive search strategy and the involvement of two independent reviewers in the screening of studies and data extraction. We included both quantitative and qualitative studies to enrich the scope and depth of our review. However, this review brings together very different study designs and methodologies such as studies reporting on survivors' perceived influences on behavior and cohort studies reporting on risk factors. The results should therefore be interpreted with caution and used as a starting point to develop health behavior interventions and identify survivors most at risk of unhealthy behaviors. Furthermore, our strict inclusion criteria limited the age range of participants to 16–50 years. As a result, we might have missed relevant findings from studies that included participants outside this age range and AYA cancer survivors with an adult cancer diagnosis. This may somewhat limit the generalizability of our conclusions. Nevertheless, our findings are still valuable for understanding the barriers and facilitators that may promote healthy behaviors in CAYA cancer survivors.
5. CONCLUSION
Our comprehensive review examined different aspects of health behavior, including physical activity, smoking, diet, alcohol consumption, sun exposure, and health behavior in general. Barriers, such as fatigue, unmet information needs, time constraints, lack of motivation, social influences, poor mental health, and facilitators, such as the need for lifestyle advice and health promotion discussions with HCPs, highlight the importance of targeted interventions. The identification of other factors associated with health behavior outcomes, including (among others) sex and educational attainment, highlights the need to consider individual context and sociodemographic characteristics. Overall, our findings can be used as a starting point for the development of more targeted and effective health behavior change interventions to promote healthy behaviors in CAYA cancer survivors, to support them in adapting these behaviors, and to inform lifestyle coaches. Knowledge of other factors can be used to raise awareness among HCPs of which survivors are most at risk of unhealthy behaviors.
AUTHOR CONTRIBUTIONS
Ismay A. E. de Beijer: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); project administration (equal); visualization (lead); writing – original draft (lead). Eline Bouwman: Conceptualization (equal); formal analysis (equal); methodology (equal); writing – review and editing (lead). Renée L. Mulder: Methodology (equal); supervision (equal); writing – review and editing (equal). Philippa Steensma: Methodology (equal). Morven C. Brown: Conceptualization (equal); funding acquisition (supporting); methodology (equal); writing – review and editing (equal). Vera Araújo‐Soares: Conceptualization (equal); funding acquisition (supporting); methodology (equal); writing – review and editing (equal). Magdalena Balcerek: Methodology (equal); writing – review and editing (equal). Edit Bardi: Methodology (equal); writing – review and editing (equal). Jeanette Falck‐Winther: Funding acquisition (equal); methodology (equal); writing – review and editing (equal). Line Elmerdahl Frederiksen: Methodology (equal); writing – review and editing (equal). Marloes van Gorp: Methodology (equal); writing – review and editing (equal). Sara Oberti: Methodology (equal); writing – review and editing (equal). Rebecca J. van Kalsbeek: Methodology (equal); writing – review and editing (equal). Tomas Kepak: Funding acquisition (equal); methodology (equal); writing – review and editing (equal). Katerina Kepakova: Methodology (equal); writing – review and editing (equal). Hannah Gsell: Methodology (equal); writing – review and editing (equal). Anita Kiesinger: Methodology (equal); writing – review and editing (equal). Raphaële van Litsenburg: Methodology (equal); writing – review and editing (equal). Luzius Mader: Methodology (equal); writing – review and editing (equal). Gisela Michel: Funding acquisition (equal); methodology (equal); writing – review and editing (equal). Monica Muraca: Funding acquisition (equal); methodology (equal); writing – review and editing (equal). Selina R. van den Oever: Methodology (equal); writing – review and editing (equal). Helena J. H. van der Pal: Funding acquisition (equal); methodology (equal); writing – review and editing (equal). Katharina Roser: Methodology (equal); writing – review and editing (equal). Roderick Skinner: Funding acquisition (equal); methodology (equal); writing – review and editing (equal). Iridi Stolman: Methodology (equal); writing – review and editing (equal). Anne Uyttebroeck: Funding acquisition (equal); methodology (equal); writing – review and editing (equal). Leontien C. M. Kremer: Conceptualization (supporting); funding acquisition (lead); methodology (equal); supervision (equal); writing – review and editing (equal). Jacqueline Loonen: Conceptualization (lead); funding acquisition (equal); methodology (lead); supervision (lead); writing – review and editing (lead). Elvira C. van Dalen: Conceptualization (lead); methodology (lead); supervision (lead); writing – review and editing (lead). Saskia M. F. Pluijm: Conceptualization (lead); formal analysis (lead); funding acquisition (lead); methodology (lead); supervision (lead); writing – original draft (equal); writing – review and editing (lead).
FUNDING INFORMATION
This work was supported by the European Union's Horizon 2020 Framework Program (Grant Number 824982). The funder had no role in study design, data col‐lection, data analysis, data interpretation, or in writing the report. The material presented and views expressed here are the responsibility of the author(s) only. The EU Commission takes no responsibility for any use made of the information set out.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting information
Appendix S1.
Appendix S2.
ACKNOWLEDGMENTS
We would like to thank Rikie Deurenberg, who developed the search strategies FOR OVID‐MedLine and PsycInfo, and all the PanCareFollowUp partner institutes and coauthors who have been involved in this study. The PanCareFollowUp Consortium, established in 2018, is a unique and multidisciplinary collaboration between 14 project partners from 10 European countries, including patient experts (https://pancarefollowup.eu). The aim of the consortium is to improve the quality of life for survivors of childhood, adolescent, and young adult (CAYA) cancer by bringing evidence‐based, person‐centered care to clinical practice. The PanCareFollowUp Consortium has developed and evaluated two interventions, including a person‐centered and guideline‐based model of survivorship care (Care Intervention) and an eHealth lifestyle coaching model (Lifestyle intervention). After the project, Replication Manuals that contain the instructions and tools required for implementation of the PanCareFollowUp interventions will be freely distributed.
APPENDIX A. Members PanCare FollowUp Consortium
A.1.
Leontien C.M. Kremer, Princess Máxima Center for Paediatric Oncology, Hei delberglaan 25, 3584 CS, Utrecht, the Netherlands; Department of Paediatrics, Emma Children's Hospital, Amsterdam UMC, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands; Faculty of Medicine, Utrecht University and Utrecht Medical Center, Universiteitsweg 98, 3584 CG Utrecht, the Netherlands.
Helena J.H. van der Pal, Princess Máxima Center for Paediatric Oncology, Heidelberglaan 25, 3584 CS, Utrecht, the Netherlands; PanCare, Jacobus Bellamylaan 16, 1401 AZ Bussum, the Netherlands.
Renée L. Mulder, Princess Máxima Center for Paediatric Oncology, Heidelberg laan 25, 3584 CS, Utrecht, the Netherlands.
Saskia M.F. Pluijm, Princess Máxima Center for Paediatric Oncology, Heidelberglaan 25, 3584 CS, Utrecht, the Netherlands. Rebecca J. van Kalsbeek, Princess Máxima Center for Paediatric Oncology, Heidelberglaan 25, 3584 CS, Utrecht, the Netherlands.
Selina R. van den Oever, Princess Máxima Center for Paediatric Oncology, Heidelberglaan 25, 3584 CS, Utrecht, the Netherlands.
Lars Hjorth, Lund University, Skane University Hospital, Department of Clinical Sciences Lund, Paediatrics, Lasarettsgatan 40, 221 85 Lund, Sweden.
Cecilia Follin, Lund University, Skane University Hospital, Department of Clini cal Sciences Lund, Oncology, Lasarettsgatan 40, 221 85 Lund, Sweden.
Lill Eriksson, Lund University, Skane University Hospital, Department of Clinical Sciences Lund, Oncology, Lasarettsgatan 40, 221 85 Lund, Sweden.
Thomas Relander, Lund University, Skane University Hospital, Department of Clinical Sciences Lund, Oncology, Lasarettsgatan 40, 221 85 Lund, Sweden.
Jacob Engellau, Lund University, Skane University Hospital, Department of Clinical Sciences Lund, Oncology, Lasarettsgatan 40, 221 85 Lund, Sweden.
Karin Fjordén, Lund University, Skane University Hospital, Department of Clini cal Sciences Lund, Oncology, Lasarettsgatan 40, 221 85 Lund, Sweden.
Karolina Bogefors, Lund University, Skane University Hospital, Department of Clinical Sciences Lund, Oncology, Lasarettsgatan 40, 221 85 Lund, Sweden.
Anna S. Holmqvist, Lund University, Skane University Hospital, Department of Clinical Sciences Lund, Paediatrics, Lasarettsgatan 40, 221 85 Lund, Sweden.
Riccardo Haupt, Epidemiology and Biostatistics Unit and DOPO clinic, IRCCS Istituto Giannina Gaslini, Via G. Gaslini, 5, 16147 Genoa, Italy.
Monica Muraca, Epidemiology and Biostatistics Unit and DOPO clinic, IRCCS Istituto Giannina Gaslini, Via G. Gaslini, 5, 16147 Genoa, Italy.
Brigitte Nicolas, Epidemiology and Biostatistics Unit and DOPO clinic, IRCCS Istituto Giannina Gaslini, Via G. Gaslini, 5, 16147 Genoa, Italy.
Francesca Bagnasco, Epidemiology and Biostatistics Unit and DOPO clinic, IRCCS Istituto Giannina Gaslini, Via G. Gaslini, 5, 16147 Genoa, Italy.
Marina Benvenuto, Epidemiology and Biostatistics Unit and DOPO clinic, IRCCS Istituto Giannina Gaslini, Via G. Gaslini, 5, 16147 Genoa, Italy.
Anna Aulicino, Epidemiology and Biostatistics Unit and DOPO clinic, IRCCS Istituto Giannina Gaslini, Via G. Gaslini, 5, 16147 Genoa, Italy.
Luca Laudisi, Epidemiology and Biostatistics Unit and DOPO clinic, IRCCS Istituto Giannina Gaslini, Via G. Gaslini, 5–16147 Genoa, Italy.
Vera Araújo‐Soares, Center for Preventive Medicine and Digital Health, Department for Prevention, Medical Faculty Mannheim, Heidelberg University, Röntgenstraße 7 D‐68167, Mannheim, Germany.
Tomas Kepak, International Clinical Research Center, St. Anne's University Hospital Brno, Pekařská 53, Brno 656 91, Czech Republic (FNUSA‐ICRC). Katerina Kepakova, International Clinical Research Center, at St. Anne's University Hospital, Masaryk University, Pekařská 53, Brno 656 91, Czech Republic (FNUSA‐ICRC).
Hana Hrstkova, International Clinical Research Center, St. Anne's University Hospital Brno, Pekařská 53, Brno 656 91, Czech Republic (FNUSA‐ICRC).
Viera Bajciova, International Clinical Research Center, St. Anne's University Hospital Brno, Pekařská 53, Brno 656 91, Czech Republic (FNUSA‐ICRC).
Marta Holikova, International Clinical Research Center, St. Anne's University Hospital Brno, Pekařská 53, Brno 656 91, Czech Republic (FNUSA‐ICRC). Lucie Strublova, International Clinical Research Center, St. Anne's University Hospital Brno, Pekařská 53, Brno 656 91, Czech Republic (FNUSA‐ICRC).
Anne Uyttebroeck, Department of Oncology, Paediatric Oncology, KU Leuven, Department of Paediatric Haematology and Oncology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Marleen Renard, Department of Paediatric Haematology and Oncology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Sandra Jacobs, Department of Oncology, Paediatric Oncology, KU Leuven, Department of Paediatric Haematology and Oncology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium. Heidi Segers, Department of Oncology, Paediatric Oncology, KU Leuven, Department of Paediatric Haematology and Oncology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium. Monique van Helvoirt, Department of Paediatric Haematology and Oncology, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Jeanette F. Winther, Childhood Cancer Research Group, Danish Cancer Society Research Center, Strandboulevarden 49, 2100 Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health, Aarhus University and Aarhus University Hospital, Palle Juul‐Jensens Boulevard 82, 8200 Aarhus, Denmark.
Luzius Mader, Childhood Cancer Research Group, Danish Cancer Society Research Center, Strandboulevarden 49, 2100 Copenhagen, Denmark; Institute of Social and Preventive Medicine, University of Bern, Mittelstrasse 43, 3012 Bern, Switzerland.
Line E. Frederiksen, Childhood Cancer Research Group, Danish Cancer Society Research Center, Strandboulevarden 49, 2100 Copenhagen, Denmark.
Elisabeth A. W. Andersen, Statistics and Data Analysis, Danish Cancer Society Research Center, Strandboulevarden 49, 2100 Copenhagen, Denmark.
Gisela Michel, University of Lucerne, Faculty of Health Sciences and Medicine, Alpenquai 4, 6005 Lucerne, Switzerland.
Stefan Boes, University of Lucerne, Faculty of Health Sciences and Medicine, Alpenquai 4, 6005 Lucerne, Switzerland.
Katharina Roser, University of Lucerne, Faculty of Health Sciences and Medicine, Alpenquai 4, 6005 Lucerne, Switzerland.
Jacqueline Loonen, Radboud University Medical Center, Radboud Institute for Health Sciences, Department of Hematology, Geert Grooteplein Zuid 10, 6525 GA, Nijmegen, the Netherlands.
Rosella Hermens, Radboud University Medical Center, Radboud Institute for Health Sciences, Scientifc Institute for Quality of Healthcare (IQ Healthcare), Geert Grooteplein 21, 6525 EZ, Nijmegen, the Netherlands.
Irene Göttgens, Radboud University Medical Center, Radboud Institute for Health Sciences, Department of Primary and Community Care, Geert Grooteplein 21, 6525 EZ, Nijmegen, the Netherlands.
Eline Bouwman, Radboud University Medical Center, Radboud Institute for Health Sciences, Department of Hematology, Geert Grooteplein Zuid 10, 6525 GA, Nijmegen, the Netherlands.
Iridi Stollman, Radboud University Medical Center, Radboud Institute for Health Sciences, Department of Hematology, Geert Grooteplein Zuid 10, 6525 GA, Nijmegen, the Netherlands. Adriaan Penson, Radboud University Medical Center, Radboud Institute for Health Sciences, Department of Hematology, Geert Grooteplein Zuid 10, 6525 GA, Nijmegen, the Netherlands.
Dionne Breij, Radboud University Medical Center, Radboud Institute for Health Sciences, Department of Hematology, Geert Grooteplein Zuid 10, 6525 GA, Nijmegen, the Netherlands.
Roderick Skinner, Newcastle University Centre for Cancer, Wolfson Childhood Cancer Research Centre, Herschel Building, Brewery Lane, Newcastle upon Tyne, NE1 7RU, United Kingdom; Great North Children's Hospital, Royal Victoria Infrmary, Queen Victoria Road, Newcastle upon Tyne, NE1 4 LP, United Kingdom; Translational and Clinical Research Institute, Wolfson Childhood Cancer Research Centre, Herschel Building, Brewery Lane, Newcastle upon Tyne, NE1 7RU, United Kingdom. Morven C. Brown, Population Health Sciences Institute, Newcastle University, Sir James Spence Institute, Royal Victoria Infrmary, Queen Victoria Road, Newcastle upon Tyne, NE1 4LP, United Kingdom; Newcastle University Centre for Cancer, Wolfson Childhood Cancer Research Centre, Herschel Building, Brewery Lane, Newcastle upon Tyne, NE1 7RU, United Kingdom.
Samira Essiaf, European Society for Paediatric Oncology, c/o BLSI, Clos Chapelle‐aux‐Champs 30, Bte 1.30.30, BE‐1200 Brussels, Belgium.
Anne Blondeel, European Society for Paediatric Oncology, c/o BLSI, Clos Chapelle‐aux‐Champs 30, Bte 1.30.30, BE‐1200 Brussels, Belgium.
William Sciberras, European Society for Paediatric Oncology, c/o BLSI, Clos Chapelle‐aux‐Champs 30, Bte 1.30.30, BE‐1200 Brussels, Belgium.
Joke Korevaar, Netherlands Institute for Health Services Research (Nivel), P.O. Box 1568, 3500 BN Utrecht, the Netherlands.
Mieke Rijken, Netherlands Institute for Health Services Research (Nivel), P.O. Box 1568, 3500 BN Utrecht, the Netherlands; University of Eastern Finland, Department of Health and Social Management, P.O. Box 1627, FI‐70211 Kuopio, Finland.
Anita Kienesberger, Childhood Cancer International Europe, Servitengasse 5/16, 1090 Vienna, Austria.
Jaap den Hartogh, Princess Máxima Center for Paediatric Oncology, Heidelberglaan 25, 3584 CS, Utrecht, the Netherlands.
Hannah Gsell, Childhood Cancer International Europe, Servitengasse 5/16, 1090 Vienna, Austria. Carina Schneider, Childhood Cancer International Europe, Servitengasse 5/16, 1090 Vienna, Austria. Edit Bardi, PanCare, Jacobus Bellamylaan 16, 1401 AZ Bussum, the Netherlands.
Jeroen te Dorsthorst, PanCare, Jacobus Bellamylaan 16, 1401 AZ Bussum, the Netherlands.
APPENDIX B. Search strategy for barriers, facilitators, factors and effectiveness of eHealth lifestyle interventions
B.1.
Search Strategy for OVID‐Medline and PsycInfo (adapted for PubMed update):
| 1 | “cochrane review e‐health facilitators and barriers”.ti. |
| 2 | exp nervous system neoplasms |
| 3 | leukemias/ |
| 4 | (leukemia or leukemi* or leukaemi*).tw,id. |
| 5 | (aml or anll or lymphoma or lymphom* or hodgkin* or T‐cell or B‐cell or non‐hodgkin or sarcoma or sarcom* or Ewing* or osteosarcom* or wilms* or nephroblastom* or neuroblastom* or rhabdomyosarcom* or teratom* or hepatom* or hepatoblastom* or PNET or medulloblastom* or PNET* or (neuroectodermal adj2 tumors adj2 primitive) or retinoblastoma or retinoblastom* or meningiom* or gliom*).tw,id. |
| 6 | exp neoplasms/ |
| 7 | ((brain adj tumo?r*) or (brain adj neoplasm?) or (central adj nervous adj system adj neoplasm?) or (central adj nervous adj system adj tumo?r?) or (central adj nervous adj system adj cancer?) or (brain adj cancer*) or (brain adj neoplasm*) or (intracranial adj neoplasm*) or (leukemia adj lymphocytic adj acute*)).tw,id. |
| 8 | or/2‐7 |
| 9 | “P variant neurocognief”.ti. |
| 10 | (neoplasm* or hemato?oncolog* or (hemato adj oncological)).tw,id. |
| 11 | malignan*.tw,id. |
| 12 | (tumour* or tumor*).tw,id. |
| 13 | cancer*.tw,id. |
| 14 | carcinoma*.tw,id. |
| 15 | leuk?emia*.tw,id. |
| 16 | oncolog*.tw,id. |
| 17 | or/10‐16 |
| 18 | “P variant breed”.ti. |
| 19 | ((p?ediatric adj3 oncolog*) or (child* adj3 (cancer? or tumo?r? or neoplasm?))).tw,id. |
| 20 | 8 or 17 or 19 =pediatric oncology |
| 21 | ((late? adj3 effect*) or (long adj3 term) or long?term or (later adj3 side effect*)).id,tw. |
| 22 | survivors/ or symbolic interactionism/ |
| 23 | (surviv* or survivor? or survival?).ti,id. |
| 24 | aftercare/ or “continuum of care”/ or exp maintenance therapy/ or exp outpatient treatment/ or partial hospitalization/ or posttreatment followup/ |
| 25 | or/21‐24 = survivors late effects |
| 26 | exp lifestyle/ or exp health behaviour/ |
| 27 | (lifestyle? or (life adj2 style?)).tw,id. |
| 28 | health promotion/ |
| 29 | client education/ or exp health education/ |
| 30 | weight control/ or exp exercise/ or food intake/ or “obesity (attitudes toward)”/ or weight gain/ or weight loss/ |
| 31 | sedentary behaviour/ |
| 32 | tobacco smoking/ or smoking cessation/ |
| 33 | exp alcohol drinking patterns/ or drinking behaviour/ or exp alcoholism/ |
| 34 | exercise/ or physical activity/ |
| 35 | exp sports/ or swimming/ |
| 36 | treatment compliance/ |
| 37 | ((smoking adj3 cessat*) or nutrition or diet* or self‐care or (dietary adj3 chang*) or (weight adj3 control*) or (stimulat* adj3 physical)).tw,id. |
| 38 | ((body adj2 weight adj3 maintena*) or exercis* or walking or training or smoking or (physical adj3 exercis*) or diet or alcohol or eating).tw,id. |
| 39 | ((weight adj3 loss) or overweight or obesit* or (dietary adj3 intake)).tw,id. |
| 40 | (fruit or vegetable? or nutrition or smoking or alcohol or self?help or self‐care or (self adj help) or (self adj care)).tw,id. |
| 41 | self‐management/ |
| 42 | exp motor performance |
| 43 | ((behavio?r or life?style or (life adj style)) adj3 (change or intervent* or counsel*)).tw,id. |
| 44 | diets/ or exp food/ |
| 45 | exp cognitive behaviour therapy/ |
| 46 | acceptance.mp. and commitment therapy.tw,id. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh] |
| 47 | mindfulness/ or mindfulness‐based interventions/ |
| 48 | exp relaxation therapy/ |
| 49 | motivational interviewing/ or exp behaviour change/ |
| 50 | counseling/ |
| 51 | exp behaviour therapy/ |
| 52 | (CBT or (cognitive adj3 behavi??r adj3 therap*) or (motivational adj3 interview*) or (commitment adj therap*) or (behavi??r adj3 interventi*)).tw,id. |
| 53 | or/26‐52 = life style |
| 54 | 20 and 25 and 53 |
| 55 | ((need? adj3 demand*) or (need? adj3 assess*) or tailor* or (patient adj3 cent*) or personali* or facilitat* or barrier*).tw. |
| 56 | ((need? adj3 demand*) or (need? adj3 assess*) or tailor* or (patient adj3 cent*) or personali* or facilitat* or barrier*).id. |
| 57 | health care services/ or exp electronic health services/ or exp health care delivery/ or exp hospital programs/ or long term care/ or exp mental health services/ or exp health care seeking behaviour/ or health service needs/ or exp managed care/ |
| 58 | (attitude? or need? or prefer*).ti,id. |
| 59 | attitudes/ or health attitudes/ |
| 60 | treatment compliance/ or treatment barriers/ |
| 61 | or/55‐60=facilitators and barriers |
| 62 | 54 and 61 |
| 63 | “onderdeel ehealth”.ti. |
| 64 | internet/ or blog/ or electronic collaboration/ or exp electronic communication/ or online experiments/ or online therapy/ or exp social media/ or exp telemedicine/ or exp websites/ or exp wireless technologies/ or internet usage/ |
| 65 | telerehabilitation/ or rehabilitation counseling/ or videoconferencing/ |
| 66 | telemetry/ |
| 67 | (telemetr* or (rehabilitation adj3 (remote or virtual or tele)) or telemedicine).tw,id. |
| 68 | (mhealth or ehealth or telehealth or telemedicine or (mobile adj health) or telerehabilitation).tw,id. ( |
| 69 | (web?based or online).tw,id. |
| 70 | (online adj3 (coach* or support* or platform or environ*)).tw,id. |
| 71 | (web adj3 (coach* or support* or platform or environ*)).tw,id. |
| 72 | (online adj3 (support* or self*)).tw,id. |
| 73 | (internet adj3 (support or self*)).tw,id. |
| 74 | or/64‐73 =eHealth |
| 75 | 54 |
| 76 | limit 75 to (all journals and english language) |
| 77 | 20 and 25 and 53 and 61 = barriers, facilitators and factors |
| 78 | english.la. |
| 79 | 20 and 25 and 74 |
| 80 | 20 and 25 and 53 and 61 |
| 81 | 80 and 78 |
| 82 | limit 81 to all journals |
| 83 | 20 and 25 and 53 and 74 = effectiveness of eHealth lifestyle interventions. |
| 84 | 83 and 78 |
| 85 | limit 84 to all journals |
Abbreviations: Id = article identifier, Ti = title, Tw = text word, La = language.
de Beijer IAE, Bouwman E, Mulder RL, et al. Barriers, facilitators, and other factors associated with health behaviors in childhood, adolescent, and young adult cancer survivors: A systematic review. Cancer Med. 2024;13:e7361. doi: 10.1002/cam4.7361
Ismay A E de Beijer and Eline Bouwman shared first authorship.
Jacqueline Loonen, Elvira C van Dalen, and Saskia M F Pluijm shared last authorship.
Contributor Information
Ismay A. E. de Beijer, Email: i.a.e.debeijer-3@prinsesmaximacentrum.nl.
the PanCareFollowUp Consortium:
Leontien C. M. Kremer, Helena J. H. van der Pal, Renée L. Mulder, Saskia M. F. Pluijm, Rebecca J. van Kalsbeek, Selina R. van den Oever, Lars Hjorth, Cecilia Follin, Lill Eriksson, Thomas Relander, Jacob Engellau, Karin Fjordén, Karolina Bogefors, Anna S. Holmqvist, Riccardo Haupt, Monica Muraca, Brigitte Nicolas, Francesca Bagnasco, Marina Benvenuto, Anna Aulicino, Luca Laudisi, Vera Araújo‐Soares, Tomas Kepak, Katerina Kepakova, Hana Hrstkova, Viera Bajciova, Marta Holikova, Lucie Strublova, Anne Uyttebroeck, Marleen Renard, Sandra Jacobs, Heidi Segers, Monique van Helvoirt, Jeanette F. Winther, Luzius Mader, Line E. Frederiksen, Elisabeth A. W. Andersen, Gisela Michel, Stefan Boes, Katharina Roser, Jacqueline Loonen, Rosella Hermens, Irene Göttgens, Eline Bouwman, Iridi Stollman, Adriaan Penson, Dionne Breij, Roderick Skinner, Morven C. Brown, Samira Essiaf, Anne Blondeel, William Sciberras, Joke Korevaar, Mieke Rijken, Anita Kienesberger, Jaap den Hartogh, Hannah Gsell, Carina Schneider, Edit Bardi, and Jeroen te Dorsthorst
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Gatta G, Zigon G, Capocaccia R, et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer. 2009;45(6):992‐1005. doi: 10.1016/j.ejca.2008.11.042 [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute . Adolescents and Young Adults with Cancer. Accessed 9/6/2023. https://www.cancer.gov/types/aya
- 3. Leerink JM, De Baat EC, Feijen EAM, et al. Cardiac disease in childhood cancer survivors. JACC CardioOncol. 2020;2(3):363‐378. doi: 10.1016/j.jaccao.2020.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. den Hoed MAH, Klap BC, te Winkel ML, et al. Bone mineral density after childhood cancer in 346 long‐term adult survivors of childhood cancer. Osteoporos Int. 2015;26(2):521‐529. doi: 10.1007/s00198-014-z [DOI] [PubMed] [Google Scholar]
- 5. Friedman DN, Tonorezos ES, Cohen P. Diabetes and metabolic syndrome in survivors of childhood cancer. Horm Res Paediatr. 2019;91(2):118‐127. doi: 10.1159/000495698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turcotte LM, Liu Q, Yasui Y, et al. Chemotherapy and risk of subsequent malignant neoplasms in the childhood cancer survivor study cohort. J Clin Oncol. 2019;37(34):3310‐3319. doi: 10.1200/JCO.19.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang TT, Hudson MM, Stokes DC, Krasin MJ, Spunt SL, Ness KK. Pulmonary outcomes in survivors of childhood cancer. Chest. 2011;140(4):881‐901. doi: 10.1378/chest.10-2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christen S, Roser K, Mulder RL, et al. Recommendations for the surveillance of cancer‐related fatigue in childhood, adolescent, and young adult cancer survivors: a report from the international late effects of childhood cancer guideline harmonization group. J Cancer Surviv. 2020;14(6):923‐938. doi: 10.1007/s11764-020-00904-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu NL, Hingorani S, Cushing‐Haugen KL, Lee SJ, Chow EJ. Late kidney morbidity and mortality in hematopoietic cell transplant survivors. Transplant Cell Ther. 2021;27(5):434.e1‐434.e6. doi: 10.1016/j.jtct.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer—a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):170‐181. doi: 10.1158/1055-9965.EPI-09-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(32):3643‐3650. doi: 10.1200/JCO.2014.56.7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Travis LB. Lung cancer following chemotherapy and radiotherapy for hodgkin's disease. Cancer Spectr Knowl Environ. 2002;94(3):182‐192. doi: 10.1093/jnci/94.3.182 [DOI] [PubMed] [Google Scholar]
- 13. Smith WA, Li C, Nottage KA, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the st. Jude lifetime cohort study: metabolic syndrome in childhood cancer. Cancer. 2014;120(17):2742‐2750. doi: 10.1002/cncr.28670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schindera C, Zürcher SJ, Jung R, et al. Physical fitness and modifiable cardiovascular disease risk factors in survivors of childhood cancer: a report from the SURfit study. Cancer. 2021;127(10):1690‐1698. doi: 10.1002/cncr.33351 [DOI] [PubMed] [Google Scholar]
- 15. Watson M, Holman DM, Maguire‐Eisen M. Ultraviolet radiation exposure and its impact on skin cancer risk. Semin Oncol Nurs. 2016;32(3):241‐254. doi: 10.1016/j.soncn.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tonorezos ES, Robien K, Eshelman‐Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24(2):313‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mays D, Black JD, Mosher RB, Heinly A, Shad AT, Tercyak KP. Efficacy of the survivor health and resilience education (share) program to improve bone health behaviors among adolescent survivors of childhood cancer. Ann Behav Med. 2011;42(1):91‐98. doi: 10.1007/s12160-011-9261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mays D, Black JD, Mosher RB, Shad AT, Tercyak KP. Improving short‐term sun safety practices among adolescent survivors of childhood cancer: a randomized controlled efficacy trial. J Cancer Surviv. 2011;5(3):247‐254. doi: 10.1007/s11764-011-0177-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hudson MM, Tyc VL, Srivastava DK, et al. Multi‐component behavioral intervention to promote health protective behaviors in childhood cancer survivors: the protect study. Med Pediatr Oncol. 2002;39(1):2‐11. doi: 10.1002/mpo.10071 [DOI] [PubMed] [Google Scholar]
- 20. Emmons KM, Puleo E, Park E, et al. Peer‐delivered smoking counseling for childhood cancer survivors increases rate of cessation: the partnership for health study. J Clin Oncol. 2005;23(27):6516‐6523. doi: 10.1200/JCO.2005.07.048 [DOI] [PubMed] [Google Scholar]
- 21. Emmons KM, Puleo E, Mertens A, Gritz ER, Diller L, Li FP. Long‐term smoking cessation outcomes among childhood cancer survivors in the partnership for health study. J Clin Oncol. 2009;27(1):52‐60. doi: 10.1200/JCO.2007.13.0880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rueegg CS, Gianinazzi ME, Michel G, et al. Do childhood cancer survivors with physical performance limitations reach healthy activity levels?: performance limitations & physical activity. Pediatr Blood Cancer. 2013;60(10):1714‐1720. doi: 10.1002/pbc.24595 [DOI] [PubMed] [Google Scholar]
- 23. Ness KK, Hudson MM, Ginsberg JP, et al. Physical performance limitations in the childhood cancer survivor study cohort. J Clin Oncol. 2009;27(14):2382‐2389. doi: 10.1200/JCO.2008.21.1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cox CL, Zhu L, Finnegan L, et al. Survivor profiles predict health behavior intent: the childhood cancer survivor study: profiling survivors for risk‐based care. Psychooncology. 2012;21(5):469‐478. doi: 10.1002/pon.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le A, Mitchell HR, Zheng DJ, et al. A home‐based physical activity intervention using activity trackers in survivors of childhood cancer: a pilot study. Pediatr Blood Cancer. 2017;64(2):387‐394. doi: 10.1002/pbc.26235 [DOI] [PubMed] [Google Scholar]
- 26. Keats MR, Culos‐Reed SN, Courneya KS, McBride M. Understanding physical activity in adolescent cancer survivors: an application of the theory of planned behavior. Psychooncology. 2007;16(5):448‐457. doi: 10.1002/pon.1075 [DOI] [PubMed] [Google Scholar]
- 27. Emmons KM, Butterfield RM, Puleo E, et al. Smoking among participants in the childhood cancer survivors cohort: the Partnership for Health Study. J Clin Oncol. 2003;21(2):189‐196. doi: 10.1200/jco.2003.06.130 [DOI] [PubMed] [Google Scholar]
- 28. Mayes J, Brown MC, Davies N, Skinner R. Health promotion and information provision during long‐term follow‐up for childhood cancer survivors: a service evaluation. Pediatr Hematol Oncol. 2016;33(6):359‐370. doi: 10.1080/08880018.2016.1225325 [DOI] [PubMed] [Google Scholar]
- 29. Pugh G, Hough R, Gravestock H, Haddrell JB, Beeken RJ, Fisher A. The lifestyle information and intervention preferences of teenage and young adult cancer survivors. Cancer Nurs. 2018;41(5):389‐398. doi: 10.1097/ncc.0000000000000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouwman E, Pluijm SMF, Stollman I, et al. Perceived barriers and facilitators to health behaviors in European childhood cancer survivors: a qualitative PanCareFollowUp study. Cancer Med. 2023;12(11):12749‐12764. doi: 10.1002/cam4.5911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dugan KF, Hidde MC, Chard CA, Graham DJ, Withycombe JS, Leach HJ. Exploring social ecological determinants of physical activity among adult survivors of childhood cancer. J Adolesc Young Adult Oncol. 2020;10:316‐325. doi: 10.1089/jayao.2019.0169 [DOI] [PubMed] [Google Scholar]
- 32. Klosky JL, Howell CR, Li Z, et al. Risky health behavior among adolescents in the childhood cancer survivor study cohort. J Pediatr Psychol. 2012;37(6):634‐646. doi: 10.1093/jpepsy/jss046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alexander GK, Bashore L, Brooks V. Improving food literacy and access among young adult cancer survivors. Cancer Nurs. 2021;45:161‐166. doi: 10.1097/ncc.0000000000000992 [DOI] [PubMed] [Google Scholar]
- 34. Cheung Y, Yang L, Ma JC, et al. Health behavior practices and expectations for a local cancer survivorship programme: a cross‐sectional study of survivors of childhood cancer in Hong Kong. Hong Kong Med J. 2022;28(1):33‐44. doi: 10.12809/hkmj209112 [DOI] [PubMed] [Google Scholar]
- 35. Cox CL, Montgomery M, Oeffinger KC, et al. Promoting physical activity in childhood cancer survivors. Cancer. 2009;115(3):642‐654. doi: 10.1002/cncr.24043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fluehr M, Kwok G, Stapleton JL, Masterson M, Devine KA. Factors associated with sun protection behaviors among childhood cancer survivors. Pediatr Hematol Oncol. 2023;45(3):e323‐e327. doi: 10.1097/mph.0000000000002618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arroyave WD, Clipp EC, Miller PE, et al. Childhood cancer survivors' perceived barriers to improving exercise and dietary behaviors. Oncol Nurs Forum. 2008;35(1):121‐130. [DOI] [PubMed] [Google Scholar]
- 38. Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer. Cancer. 2009;115(9):1984‐1994. doi: 10.1002/cncr.24209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rueegg CS, Von der Weid NX, Rebholz CE, et al. Daily physical activities and sports in adult survivors of childhood cancer and healthy controls: a population‐based questionnaire survey. PLoS One. 2012;7(4):e34930. doi: 10.1371/journal.pone.0034930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhandari R, Teh JB, Herrera C, et al. Prevalence and risk factors for vitamin D deficiency in long‐term childhood cancer survivors. Pediatr Blood Cancer. 2021;68(7):e29048. doi: 10.1002/pbc.29048 [DOI] [PubMed] [Google Scholar]
- 41. Zhang FF, Ojha RP, Krull KR, et al. Adult survivors of childhood cancer have poor adherence to dietary guidelines. J Nutr. 2016;146(12):2497‐2505. doi: 10.3945/jn.116.238261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bougas N, Fresneau B, Pinto S, et al. Smoking and cannabis use among childhood cancer survivors: results of the French childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1965‐1973. doi: 10.1158/1055-9965.epi-21-0193 [DOI] [PubMed] [Google Scholar]
- 43. Lown EA, Goldsby R, Mertens AC, et al. Alcohol consumption patterns and risk factors among childhood cancer survivors compared to siblings and general population peers. Addiction. 2008;103(7):1139‐1148. doi: 10.1111/j.1360-0443.2008.02242.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kahalley LS, Robinson LA, Tyc VL, et al. Risk factors for smoking among adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2011;58(3):428‐434. doi: 10.1002/pbc.23139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Slater ME, Kelly AS, Sadak KT, Ross JA. Active transportation in adult survivors of childhood cancer and neighborhood controls. J Cancer Surviv. 2015;10(1):11‐20. doi: 10.1007/s11764-015-0447-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zwemer EK, Mahler HI, Werchniak AE, Recklitis CJ. Sun exposure in young adult cancer survivors on and off the beach: results from project REACH. J Cancer Surviv. 2011;6(1):63‐71. doi: 10.1007/s11764-011-0201-y [DOI] [PubMed] [Google Scholar]
- 47. Florin TA, Fryer GE, Miyoshi T, et al. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1356‐1363. doi: 10.1158/1055-9965.epi-07-0048 [DOI] [PubMed] [Google Scholar]
- 48. Cappelli C, Miller KA, Ritt‐Olson A, Pentz MA, Salahpour S, Milam JE. Binge drinking, tobacco, and marijuana use among young adult childhood cancer survivors: a longitudinal study. J Pediatr Oncol Nurs. 2021;38:285‐294. doi: 10.1177/10434542211011036 [DOI] [PubMed] [Google Scholar]
- 49. Darabos K, Barakat LP, Schapira M, Hill‐Kayser C, Schwartz LA. Association of Demographic and Cancer‐Specific Factors on health behavior recommendations specific to cancer prevention and control among adolescent and young adult survivors of childhood cancer. J Adolesc Young Adult Oncol. 2020;10:619‐628. doi: 10.1089/jayao.2020.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rueegg CS, Michel G, Wengenroth L, von der Weid NX, Bergstraesser E, Kuehni CE. Physical performance limitations in adolescent and adult survivors of childhood cancer and their siblings. PLoS One. 2012;7(10):e47944. doi: 10.1371/journal.pone.0047944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Whitsett SF, Guðmundsdóttir M, Davies B, McCarthy P, Friedman DN. Chemotherapy‐related fatigue in childhood cancer: correlates, consequences, and coping strategies. J Pediatr Oncol. 2008;25(2):86‐96. doi: 10.1177/1043454208315546 [DOI] [PubMed] [Google Scholar]
- 52. Marchak JG, Kegler MC, Meacham LR, Mertens AC, Effinger KE. Cancer‐related barriers to health behaviors among adolescent and young adult survivors of pediatric cancer and their families. J Adolesc Young Adult Oncol. 2022;12(1):118‐122. doi: 10.1089/jayao.2021.0169 [DOI] [PubMed] [Google Scholar]
- 53. Lucıa A, Earnest CP, Pérez M. Cancer–related fatigue: can exercise physiology assist oncologists? Lancet Oncol. 2003;4(10):616‐625. doi: 10.1016/s1470-2045(03)01221-x [DOI] [PubMed] [Google Scholar]
- 54. Brunton G, Harden A, Rees R, et al. Children and Physical Activity: a Systematic Review of Barriers and Facilitators. 2003. In: Database of Abstracts of Reviews of Effects (DARE): Quality‐Assessed Reviews [Internet]. Centre for Reviews and Dissemination (UK); 1995. Available from: http://eppi.ioe.ac.uk/cms/Portals/0/PDF%20reviews%20and%20summaries/Children_PA.pdf?ver=2006‐03‐02‐124608‐950 [Google Scholar]
- 55. Burke SM, Brunet J, Wurz A, Butler C, Utley A. Cycling through cancer: exploring childhood cancer Survivors' experiences of well‐ and ill‐being. Adapt Phys Act Q. 2017;34(4):345‐361. doi: 10.1123/apaq.2016-0011 [DOI] [PubMed] [Google Scholar]
- 56. Mbabazi J, Kanmodi KK, Kunonga E, Tolchard B, Nnyanzi LA. Barriers and facilitators of physical activity. J Health Allied Sci NU. 2022;13:019‐027. doi: 10.1055/s-0042-1753561 [DOI] [Google Scholar]
- 57. Rosselli M, Ermini E, Tosi B, et al. Gender differences in barriers to physical activity among adolescents. Nutr Metab Cardiovasc Dis. 2020;30(9):1582‐1589. doi: 10.1016/j.numecd.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 58. Brown MC, Podmore M, Araújo‐Soares V, Skinner R, Sharp L. Childhood cancer survivors' perceptions of the barriers and facilitators to physical activity: a systematic review and thematic synthesis of qualitative evidence using the theoretical domains framework. Health. Psychol Rev. 2022;17:1‐24. doi: 10.1080/17437199.2022.2032795 [DOI] [PubMed] [Google Scholar]
- 59. World Health Organization. Global status report on alcohol and health 2018 . Published September 27, 2018. https://www.who.int/publications/i/item/9789241565639
- 60. Taylor GMJ, Baker AL, Fox N, Kessler DS, Aveyard P, Munafò MR. Addressing concerns about smoking cessation and mental health: theoretical review and practical guide for healthcare professionals. BJPsych Advances. 2021;27(2):85‐95. doi: 10.1192/bja.2020.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. WHO global report on trends in prevalence of tobacco use 2000–2025, third edition. www.who.int.https://www.who.int/publications/i/item/who‐global‐report‐on‐trends‐in‐prevalence‐of‐tobacco‐use‐2000‐2025‐third‐edition
- 62. Holman DM, Berkowitz Z, Guy GP Jr, Hawkins NA, Saraiya M, Watson M. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73(1):83.e1‐92.e1. doi: 10.1016/j.jaad.2015.02.1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sleddens EF, Kroeze W, Kohl LF, et al. Determinants of dietary behavior among youth: an umbrella review. Int J Behav Nutr Phys Act. 2015;12(1):7. doi: 10.1186/s12966-015-0164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sleddens EFC, Kroeze W, Kohl LFM, et al. Correlates of dietary behavior in adults: an umbrella review. Nutr Rev. 2015;73(8):477‐499. doi: 10.1093/nutrit/nuv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kang KA, Han SJ, Chun J, et al. Healthy lifestyle interventions for childhood and adolescent cancer survivors: a systematic review and meta‐analysis. Child health. Nurs Res. 2023;29(2):111‐127. doi: 10.4094/chnr.2023.29.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sainsbury K, Walburn J, Foster L, et al. Improving photoprotection in adults with xeroderma pigmentosum: personalisation and tailoring in the “XPAND” intervention. Health Psychol Behav Med. 2020;8(1):543‐572. doi: 10.1080/21642850.2020.1840379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Araújo‐Soares V, Hankonen N, Presseau J, Rodrigues A, Sniehotta FF. Developing behavior change interventions for self‐Management in Chronic Illness. Eur Psychol. 2019;24(1):7‐25. doi: 10.1027/1016-9040/a000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bouwman E, Pluijm SMF, Stollman I, et al. Healthcare professionals' perceived barriers and facilitators of health behavior support provision: a qualitative study. Cancer Med. 2022;12:7414‐7426. doi: 10.1002/cam4.5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lie HC, Juvet LK, Street RL, et al. Effects of Physicians' information giving on patient outcomes: a systematic review. J Gen Intern Med. 2021;37(3):651‐663. doi: 10.1007/s11606-021-07044-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Appendix S2.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


