Abstract
The long-term efficacy of combination antiretroviral therapy may relate to augmentation of anti-human immunodeficiency virus type 1 (HIV-1) CD8+ T-cell responses. We found that prolonged treatment of late-stage HIV-1-infected patients with a protease inhibitor and two nucleoside reverse transcriptase inhibitors failed to restore sustained, high levels of HIV-1-specific, HLA class I-restricted, cytotoxic-T-lymphocyte precursors and gamma interferon (IFN-γ) production by CD8+ T cells. In some patients, particularly those initiating three-drug combination therapy simultaneously rather than sequentially, there were early, transient increases in the frequency of anti-HIV-1 CD8+ T cells that correlated with decreases in HIV-1 RNA and increases in T-cell counts. In the other patients, HIV-1-specific T-cell functions either failed to increase or declined from baseline during triple-drug therapy, even though some of these patients showed suppression of plasma HIV-1 RNA. These effects of combination therapy were not unique to HIV-1 specific T-cell responses, since similar effects were noted for CD8+ T cells specific for the cytomegalovirus pp65 matrix protein. The level and breadth of CD8+ cell reactivity to HLA A*02 HIV-1 epitopes, as determined by IFN-γ production and HLA tetramer staining after combination therapy, were related to the corresponding responses prior to treatment. There was, however, a stable, residual population of potentially immunocompetent HIV-1-specific T cells remaining after therapy, as shown by tetramer staining of CD8+ CD45RO+ cells. These results indicate that new strategies will be needed to target residual, immunocompetent HIV-1-specific CD8+ T cells to enhance the effectiveness of antiretroviral therapy in patients with advanced immunodeficiency.
Combination antiretroviral therapy with two nucleoside reverse transcriptase (RT) inhibitors and a potent protease inhibitor can produce sustained suppression of human immunodeficiency virus type 1 (HIV-1) RNA in blood to fewer than 50 copies per μl (16, 17). The success of triple combination therapy has led to the hypothesis that HIV-1 could eventually be eliminated from infected individuals. The finding of persistent, latent HIV-1 in patients despite virus suppression with therapy (6, 10, 11, 13, 53, 54), however, indicates that such treatment strategies alone may not be sufficient for control or elimination of viral infection.
The ability to generate high levels and a broad specificity of anti-HIV-1 cytotoxic T lymphocytes (CTL) is considered to be a critical component of the host immune response to HIV-1 (2, 5, 23, 24, 29, 43, 44, 52). As for other chronic infectious diseases, it is possible that the long-term efficacy of combination antiretroviral therapy will require augmentation of host immune responses such as anti-HIV-1 CTL reactivity. Recent studies, however, have yielded contradictory evidence on the impact of combination antiretroviral therapy on anti-HIV-1 CTL reactivity. Several reports have shown that the numbers of anti-HIV-1 CTL precursors (CTLp), as measured in vitro in a 2-week, limiting dilution assay, increase with suppressive antiretroviral therapy in acute (8) and chronically infected patients (21, 37). In contrast, others have reported that levels of circulating, CD8+ CD38+ T cells that bind HLA A2 tetrameric HIV-1 Gag p17 and RT peptide complexes decrease after initiation of antiretroviral therapy in patients with advanced immunodeficiency (15, 21, 30, 31). In addition, none of these reports have addressed the breadth of the HIV-1-specific, CD8+ T-cell reactivity that recovers with virus suppression.
Significant differences in the CTLp and tetramer binding assays may account for the discrepant findings (33). The CTLp assay is dependent on cell growth in vitro in response to antigen stimulation, which is subject to many experimental variables. In contrast, tetramer staining is a direct measure of the number of CD8+ T cells specific for CTL epitopes that does not require in vitro cell growth, although it is not a direct measure of CD8+ T-cell function. Single cell production of gamma interferon (IFN-γ) has recently been reported to be a sensitive assay for both the quantity and function of antiviral CTL (25, 33). The antiviral and immunomodulatory effects of IFN-γ are important in host control of viral infections (9). This measure of immune reactivity does not depend on the capacity of the CD8+ T cells to replicate and become active cytotoxic cells during prolonged in vitro culture. IFN-γ production is also a result of both antigen-specific T-cell binding and consequent immune reactivity rather than just a measure of HLA class I tetramer binding to T cells.
We have therefore performed a detailed, longitudinal assessment of the effects of prolonged treatment with a simultaneous or sequential combination of a protease inhibitor (indinavir [IDV]) and two nucleoside RT inhibitors (zidovudine [ZDV] and lamivudine [3TC]), on multiple parameters of HIV-1-specific, CD8+ T-cell function in patients enrolled in the Merck 035 trial (16, 17). Our results show that combination antiretroviral therapy did not lead to sustained recovery of high levels of CTLp and IFN-γ-producing CD8+ T cells specific for HIV-1 Gag, Pol, and Env proteins. Thus, it may be necessary to use additional therapeutic approaches to augment HIV-1-specific CD8+ cells in patients with advanced immunodeficiency on suppressive antiretroviral therapy.
MATERIALS AND METHODS
Patients.
Of 27 HIV-1-seropositive adults who participated in the Pittsburgh portion of the Merck 035 trial (17), 14 were enrolled in this immunology substudy. Each patient gave written, informed consent approved by the University of Pittsburgh Institutional Review Board. Patients in the Merck 035 trial received at least 6 months of prior ZDV treatment; prior therapy with 3TC or a protease inhibitor was not allowed. Samples were also available from several years before the trial from 3 of the 14 patients who were previously enrolled in the Pittsburgh portion of the Multicenter AIDS Cohort Study (MACS), a longitudinal investigation of the natural history of HIV-1 infection. The 14 study participants were randomized to one of three treatment regimens as previously described (45). Seven patients (group A) received 800 mg of IDV (Crixivan; Merck, West Point, Pa.) every 8 h plus 200 mg of ZDV (Retrovir; Glaxo-Wellcome, Research Triangle Park, N.C.) every 8 h and 150 mg of 3TC (Epivir; Glaxo-Wellcome) every 12 h concurrently; three patients (group B) received 200 mg of ZDV every 8 h and 150 mg of 3TC every 12 h, and four patients (group C) received 800 mg of IDV every 8 h. Matching placebos for these drugs were administered to subjects in the latter two groups.
The duration of the original clinical trial was to be 52 weeks, but because of the preliminary finding of greater antiviral activity of the triple-drug regimen, the study was amended during the trial to reduce the randomized, blinded treatment to at least 24 weeks, followed by open label triple-drug therapy for all study participants.
Six of the seven group A patients remained in this immunology substudy for its 148-week duration, while one (A1155) discontinued the substudy after 48 weeks but remained in the Merck 035 trial. One group B patient (B1171) and one group C patient (C1180) discontinued from the substudy due to virologic failure (a 2- to 3-log10 rebound in viral RNA load) after 100 weeks.
Viral serology and load.
Determination of HIV-1 antibody was done by enzyme immunoassay and immunoblotting. All of the patients were confirmed positive for prior cytomegalovirus (CMV) infection by an immunofluorescence immunoglobulin G antibody assay of serum (Immunopathology Laboratory, University of Pittsburgh Medical Center). Serum samples were assayed for HIV-1 RNA by the ultrasensitive quantitative RT-PCR assay (Amplicor; Roche) (16, 41). Data are presented as copies of HIV-1 RNA per ml of serum, with the lower limit of detection being 50 copies of RNA per ml of serum.
T-cell phenotyping.
T-cell subsets were quantified by flow cytometric analysis (Profile II; Coulter, Miami, Fla.) after staining with monoclonal antibodies (MAb) specific for CD3, CD4, and CD8 T cells (Becton-Dickinson, Mountain View, Calif.). Peripheral blood mononuclear cells (PBMC) were assessed for the proportions of CD8+ and CD4+ memory (CD45RO+) and naive (CD45RA+) subsets by three-color fluorescence using MAb conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), and phycoerythrin-cyanin 5.1 (PECy5); antibody combinations were CD45RO/CD45RA/CD8 or /CD4, HLA-DR/CD38/CD8 or /CD4, and CD4/CD28/CD8 or /CD4. Three-color analyses were performed on an Elite ESP flow cytometer (Coulter).
CTLp assay.
PBMC were prepared from cryopreserved cells for use as effectors in anti-HIV-1 CTLp frequency and bulk lysis assays (19). Assay results from cryopreserved PBMC are similar to those using fresh PBMC (19). There is also minimal variation in cytotoxic activity between replicates and split samples and in fresh PBMC obtained within several months from the same, untreated individuals (19). A median of 10 samples (range, 8 to 13) obtained at baseline through up to 148 weeks of the trial were tested from each patient. PBMC were stimulated with psoralen-treated, UV light-irradiated, autologous, Epstein-Barr virus-transformed B-lymphocyte cell lines (B-LCL) that had been infected overnight with vaccinia virus (VV) containing the combined Gag-Pol and Env coding sequences from the BH10 and HXB2 strains of HIV-1 LAI (VVgpe) (Therion Biologics, Cambridge, Mass.). This results in a consistent expression of the HIV-1 vector in >70% of the B-LCL (19). The precursor frequencies were determined by limiting-dilution assay of PBMC seeded in complete medium containing 15% fetal calf serum (FCS). PBMC were seeded at 0 (medium control) and at 250, 500, 1,000, 3,000, 6,000, 12,000, and 16,000 cells per well in 24 replicate wells of 96-well round-bottom microtiter plates. To each well were added 2.5 × 104 gamma-irradiated allogeneic PBMC from one or two HIV-1-seronegative normal donors as feeder cells, 100 U of recombinant interleukin-2 (rIL2; Chiron, Emeryville, Calif.), and stimulator cells (1,600 stimulator cells per well) from VV-GPE-infected, inactivated B-LCL. The cells were cultured for 14 days at 37°C in a 5% CO2 atmosphere, with fresh complete medium containing 15% FCS and rIL2 added every 5 days. On day 14, the cells in culture were divided, transferred to two new wells, and adjusted to 100 μl with complete medium containing 15% FCS. The numbers and viability of the cells were monitored by trypan blue dye exclusion. Cytotoxicity was measured against 51Cr-labeled, autologous B-LCL (104) infected with recombinant VVgag, VVpol, VVenv, or the NYCBH strain of VV as a control (VVvac). The fraction of nonresponding wells was the number of wells in which the 51Cr release did not exceed the mean spontaneous release plus 10% of the incorporated 51Cr (total 51Cr release − spontaneous 51Cr release) divided by the number of wells assayed.
The precursor frequency was estimated by the maximum-likelihood method with a statistical program provide by S. Kalams (Boston, Mass.). CTLp activity was expressed as the net precursor frequency per 106 PBMC, i.e., the number of CTLp/106 PBMC specific for HIV-1 antigen minus the number of CTLp/106 PBMC specific for non-HIV-1-expressing target cells. The mean (± standard error [SE]) number of CTLp in the VVvac control was 65 (±9) (n = 158).
For bulk lysis assays, PBMC were stimulated as in the precursor frequency assay and assessed against the same targets at three effector/target cell ratios (40:1, 20:1, and 10:1). For each determination, the value for the lysis of targets infected with the VV control was subtracted from the value for the HIV-1 protein-expressing targets. Data were calculated as lytic units per 107 cells derived from an exponential regression analysis of the multiple effector/target ratios (4), since these were highly correlated with the percent lysis for the individual effector/target cell ratios (43).
Only data from the CTLp assays are shown because of the quantitative nature of CTLp determinations and the similar patterns of CTL lytic activity delineated by both the CTLp and bulk lysis methods (19, 43). We have found that lytic activity was mediated by purified CD8+ T cells and not by CD4+ T cells and was not seen against HLA class I mismatched targets, indicating that the anti-HIV-1 CTLp response was mediated by HLA class I-restricted, CD8+ T cells (19, 43).
Single cell IFN-γ assay.
A single cell-based enzyme immunoassay (ELISPOT) was done to enumerate the number of IFN-γ producing cells (19a) by a modification of the methods of Tanguay and Killion (47) and Lalvani et al. (25). A median of 11 (range, 7 to 13) cryopreserved samples were available from 11 of the 14 patients in this assay. Nitrocellulose membranes in 96-microwell polyvinylidene difluoride-backed plates (Millipore, Bedford, Mass.) were coated overnight at 4°C with 50 μl of anti-IFN-γ MAb (10 mg/ml, 1-DIK; Mabtech, Stockholm, Sweden) per well. The antibody-coated plates were then washed four times with phosphate-buffered saline (PBS; Biowhittaker, Walkersville, Md.) and treated with 180 μl of RPMI medium (Life Technologies, Grand Island, N.Y.) per well containing 10% human serum (Sigma, St. Louis, Mo.) for 1 h at 37°C. The responder cells for this assay were either PBMC or, when sufficient cells were available, CD8+ cells enriched by negative selection of PBMC with antibody-coated magnetic beads (anti-CD4, anti-CD19, and anti-CD16 MAb; Dynal, Lake Success, N.Y.) to remove CD4+ T cells, B cells, and natural killer cells, respectively. We have found that CD8+ T cells are the predominant cell type producing IFN-γ in PBMC used in our assay (19a). A total of 105 to 106 of these PBMC or CD8+ cells (98% pure) were stimulated with 104 to 105 VVgpe-infected, inactivated B-LCL as in the CTLp assay, in 200 μl of AIM V Medium (Life Technologies) and incubated overnight at 37°C in 5% CO2. B-LCL infected with a VV expressing CMV pp65 matrix phosphoprotein (VVcmv; a gift from S. Riddell, University of Washington), known to be a major target antigen for CD8+ CTL (27), were used for comparative stimulation of IFN-γ-producing cells by a non-HIV-1 antigen. In certain experiments, PBMC or CD8+ cells were incubated overnight at 37°C in 5% CO2 with HLA A*02-associated, HIV-1 peptides (10 μg/ml) in nitrocellulose membrane 96-well plates. These peptides were Gag p1777–85 SLYNTVATL (49), Gag p24151–159 TLNAWVKVV (36), Pol RT476–484 ILKEPVHGV (50), and Env gp120192–199 KLTSCNTSV (3). The plates were washed four times with PBS containing 0.05% Tween 20 (Sigma), and 2 μl of the secondary antibody (biotin-conjugated anti-IFN-γ MAb 7-B6-1; Mabtech) per ml was added in 100 μl to each well; the plates were then incubated for 2 h at 37°C in CO2. The plates were washed four times with PBS containing 0.05% Tween 20 and treated with avidin-bound, biotinylated horseradish peroxidase H (Vectastain Elite Kit; Vector Laboratories, Burlingame, Calif.) for 1 h at room temperature. The plates were then washed three times with PBS containing 0.05% Tween 20 and three times with PBS, followed by a 5-min incubation with 100 μl of 3-amino-9-ethylcarbazole (Sigma) per well. The reaction was stopped with running tap water. The red-brown spots, representing single CD8+ T cells producing IFN-γ, were counted with a dissecting microscope. PBMC or CD8+ T cells stimulated with the phorbol ester, phorbol 12-myristate 13-acetate (1 ng/ml), and the calcium ionophore, ionomycin (1 μM/ml) (PMA-ionomycin; Sigma), were the positive control. PBMC or CD8+ T cells were stimulated with B-LCL infected with the VVvac as the negative control for cells stimulated with VV–HIV-1 protein-expressing B-LCL and with medium alone as the negative control for the peptide-stimulated cells. The number of antigen-specific, CD8+ T-cell-producing IFN-γ was calculated by subtracting the values for the cells stimulated with control VVvac-infected B-LCL from the cells stimulated with the B-LCL infected with either VVgag, VVpol, VVenv, or VVcmv or by subtracting the number of spot-forming cells in the medium control from the peptide-stimulated cells. The mean (± the SE) numbers of spot-forming cells in the medium controls and in the VVvac control were 15 (±4) (n = 97) and 100 (±14) (n = 130), respectively.
Staining with peptide-HLA tetramers.
To assess the association between frequency of cells producing IFN-γ and binding the tetramers (1), we performed a parallel experiment using HLA A*0201 tetramer refolded around Gag p1777–85 SLYNTVATL and HLA A*0201 tetramer refolded around Pol RT476–484 ILKEPVHGV to stain cells from the same samples that were tested by the ELISPOT assay. PBMC from 6 of the 14 patients who were HLA-A*0201 (A1160, A1166, A1169, B1178, and C1164) and HLA-A*0212 (C1180), as typed by high-resolution, reference-strand-mediated conformation analysis (51), were stained and analyzed for tetramer-positive cells according to a protocol obtained from the NIH Tetramer Synthesis Facility. Briefly, approximately 106 freeze-thawed cells were surface stained with FITC-conjugated MAb against CD45 RO (Coulter), PE-conjugated HLA-A2 tetramers for Gag p1777–85 or Pol RT476–484 (NIH Tetramer Synthesis Facility), and PECy5-conjugated MAb against CD8 (Coulter). As a negative control, a similar number of cells were stained with FITC- or PECy5-conjugated isotype-matching MAb and PE-conjugated avidin (Coulter). After incubation and washing, cells were fixed with 1% paraformaldehyde and analyzed in an EPICS XL-MCL flow cytometer (Coulter) within 24 h. Approximately 20,000 events were collected within a CD8+ lymphocyte gate. The data were calculated as the percentage of tetramer-staining cells per CD8+ cell and reported in this study as the number of tetramer-positive cells per 106 CD8+ cells to allow direct comparisons with other immunologic parameters. We determined a threshold level of tetramer-positive cells of >200/106 (0.02%) CD8+ cells based on the mean (±3 SD) staining of CD8+ T cells of HLA A*02 HIV-1-seronegative donors.
Statistical analysis.
Comparisons of the different cumulative parameters were done by the paired and unpaired Student's t test. Data among different groups were assessed by analysis of variance (ANOVA) with the Scheffe multiple comparison test and then analyzed for associations between different parameters by the Pearson correlation coefficient test.
RESULTS
Suppression of HIV-1 load and changes in T-cell numbers with triple-combination antiretroviral therapy.
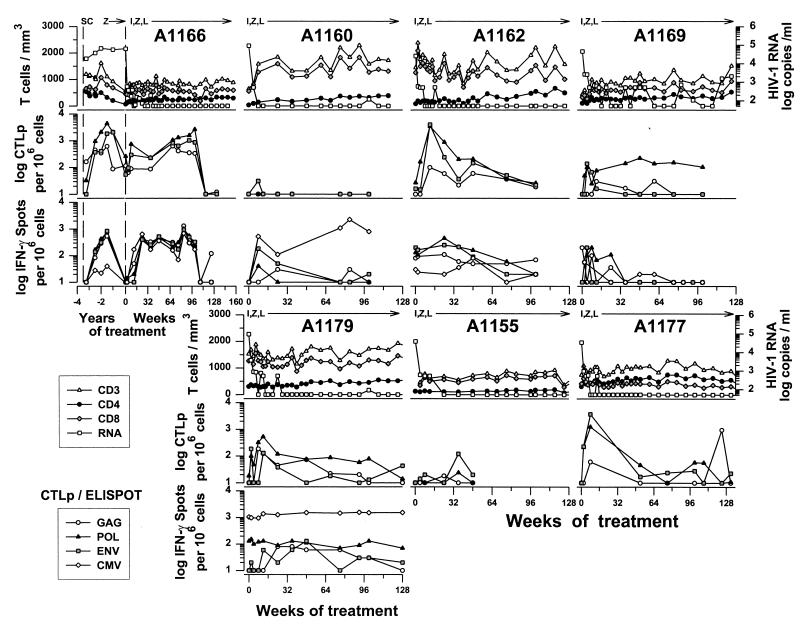
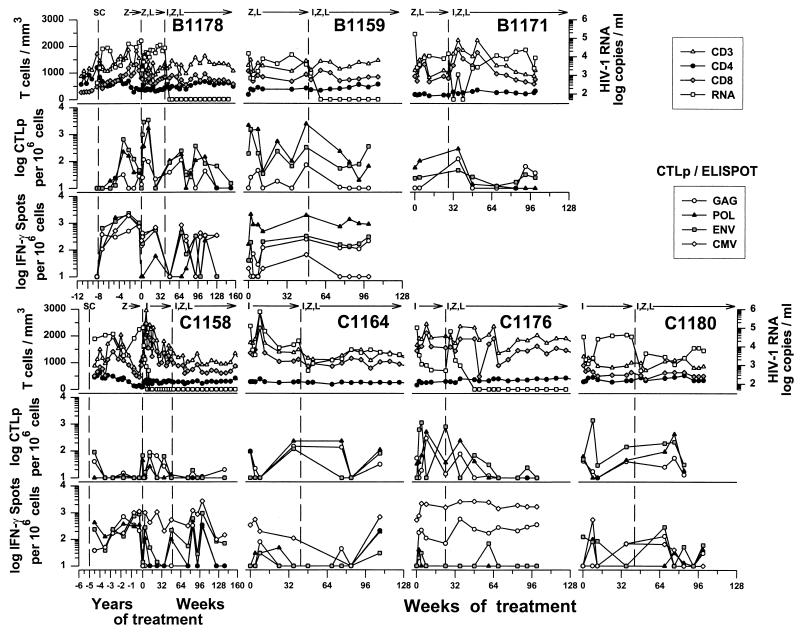
The baseline characteristics prior to study entry were similar among the 14 patients in the three treatment groups in this immunology study (P > 0.05 for T-cell numbers and HIV-1 RNA levels) (Table 1). By 14 weeks, all of the 7 patients who received the triple-drug combination from the study onset (group A) had <500 copies of HIV-1 RNA per ml of serum (Fig. 1). HIV-1 RNA further decreased to <50 copies/ml by 24 weeks in several patients (A1155, A1160, A1162, and A1177). Virus was still intermittently detectable, however, at low copy numbers in samples from each of these patients during the trial. Only one patient in group A (A1169; Fig. 1) had a breakthrough of virus above 500 copies/ml late in the trial. The numbers of circulating CD4+ T cells increased in all of these patients during the 2 years of combination therapy (Fig. 1) (45). In contrast, as previously reported (16, 17), there was less effect on viral load and CD4+ T cell numbers in patients who first received ZDV-3TC or IDV (groups B and C; Fig. 2) for 24 to 42 weeks before being switched to open-label, triple combination therapy. Patients B1159, B1178, C1158, and C1176 had <50 copies of HIV-1 RNA, whereas there was much less suppression of virus in the other three patients, after the switch to the triple-drug regimen. The four patients with <50 copies of HIV-1 RNA had increases in CD4+ T-cell numbers after the switch to open-label, triple therapy. There were no significant changes in the numbers of CD8+ T cells in the three groups throughout the course of treatment (Fig. 1 and 2).
TABLE 1.
Baseline characteristics of patients in the immunology substudy of the Merck 035 triala
| Group | No. of patients (sex) | Serum HIV-1 RNA (copies/ml) | T cells/mm3
|
|
|---|---|---|---|---|
| CD4+ | CD8+ | |||
| A | 7 (M) | 54,535 ± 10,089 | 147 ± 42 | 711 ± 177 |
| B | 3 (M) | 83,996 ± 43,766 | 241 ± 60 | 907 ± 81 |
| C | 3 (M), 1 (F) | 89,001 ± 19,814 | 205 ± 46 | 1,197 ± 124 |
Values are means ± the SE. P was not significant for all comparisons, as determined by one-way ANOVA.
FIG. 1.
Effect of treatment with IDV (I), ZDV (Z), and 3TC (L) initiated simultaneously for seven individually numbered group A patients on serum HIV-1 RNA and T-cell numbers (top rows), anti-HIV-1 CTLp (middle rows), and IFN-γ-producing CD8+ cells (bottom rows). Data on IFN-γ production were not available from patients A1155 and A1177. Longitudinal data are given for MACS participant A1166 from seroconversion (SC) through the Merck 035 drug trial.
FIG. 2.
Effect of treatment with IDV (I), ZDV (Z), and 3TC (L) given sequentially after treatment with ZDV-3TC (group B) or IDV (group C) for three individually numbered group B patients and four group C patients on serum HIV-1 RNA and T-cell numbers (top rows), anti-HIV-1 CTLp (middle rows), and IFN-γ producing CD8+ cells (bottom rows). Data on IFN-γ production were not available from patient B1171. Longitudinal data are given for MACS participants B1178 and C1158 from seroconversion (SC) through the Merck 035 drug trial.
Effect of triple-combination antiretroviral therapy on the numbers of CTLp specific for HIV-1 proteins.
We examined CD8+ CTLp function specific for the three major HIV-1 structural proteins with a conventional limiting-dilution assay (19). The results show that several of the group A patients, who received the triple combination from the study onset, had increases in CTLp, particularly to Pol and Env, at consecutive times, peaking at weeks 8 to 12 (A1162, A1166, A1169, A1177, and A1179; Fig. 1). Changes in HIV-1-specific, bulk CTL lysis were comparable to that with CTLp (data not shown). Patients A1155 and A1160 had very low numbers of anti-HIV-1 CTLp (Fig. 1) and bulk CTL lysis (data not shown). Only two of these seven patients, however, maintained elevated CTLp activity throughout most of the study (A1166, anti-Gag, -Pol, and -Env; A1169, anti-Pol), with eventual decline to baseline levels after 2 years. Although there was a gap in CTLp data available from patient A1177 from weeks 8 to 52, results from the bulk lysis assay at all times before week 12 and at weeks 12, 24, and 36 showed that high levels of CTL activity against Gag, Pol, and Env were maintained through 36 weeks, with a concurrent decrease in both bulk lysis and CTLp responses at 52 weeks (data not shown).
The seven patients in groups B and C received ZDV-3TC (group B) or IDV alone (group C) for a median of 45 weeks (range, 24 to 50 weeks) before being switched to the triple-drug combination. After initiation of the three-drug regimen, group B and C patients had heterogeneous and variable changes in the numbers of CTLp. Three patients (B1178, C1176, and C1180) had transient increases in anti-Pol and anti-Gag CTLp during triple-drug therapy (Fig. 2), as with the group A patients. These patients also had transient increases in CTLp responses during the double-combination or single-combination drug treatment phase of the trial (Fig. 2). Although there was a gap in data available for CTLp from weeks 8 through 36 for patient B1171, results from the bulk CTL lysis experiments during this time indicated that there was no CTL response to any HIV-1 protein (data not shown).
Effect of combination antiretroviral therapy on the number of IFN-γ-producing CD8+ T cells in response to HIV-1 and CMV proteins.
We quantified the number of IFN-γ-producing, CD8+ T cells specific for the three major HIV-1 structural proteins and to the CMV pp65 matrix protein by a method recently developed in our laboratory (19a). The study was done on a subset of 11 of the 14 patients who had sufficient numbers of cryopreserved PBMC for testing. Group A patients A1166, A1162, and A1179 had early, persistent elevations in IFN-γ-producing, CD8+ cells specific for at least one HIV-1 protein that decreased to, or below, baseline levels by approximately 2 years of triple-drug therapy (Fig. 1). Patients A1160 and A1169 had lower and fewer persistent elevations in IFN-γ-producing cells in response to the HIV-1 proteins. The number of IFN-γ-producing CD8+ T cells in the group A patients also correlated with the levels of CTLp specific for Gag, Pol, and Env (Table 2).
TABLE 2.
Correlations between CD8+ T-cell reactivities to HIV-1 proteins and various T-cell and viral parameters in group A patients on combination antiretroviral therapy
| Test combination
|
Duration (wk) | Antigen | Parameter
|
|||
|---|---|---|---|---|---|---|
| Test 1 | Test 2 | na | rb | P | ||
| CTLp | IFN-γ | 0–128 | Gag | 45 | 0.48 | <0.01 |
| Pol | 45 | 0.45 | <0.01 | |||
| Env | 45 | 0.61 | <0.01 | |||
| HIV-1 RNA | 0–12 | Gag | 25 | −0.41 | <0.05 | |
| Pol | 25 | −0.47 | <0.05 | |||
| Env | 25 | −0.52 | <0.05 | |||
| IFN-γ | CD4+ T cells | 0–12 | Gag | 17 | −0.18 | NSc |
| Pol | 17 | 0.68 | <0.05 | |||
| Env | 17 | −0.27 | NS | |||
| CMV | 12 | 0.87 | <0.01 | |||
| CD8+ T cells | 0–12 | Gag | 17 | −0.13 | NS | |
| Pol | 17 | 0.61 | <0.05 | |||
| Env | 17 | 0.36 | NS | |||
| CMV | 12 | 0.38 | NS | |||
n, number of determinations.
Pearson correlation coefficient.
NS, not significant (P > 0.05).
Sequential initiation of triple-drug therapy was not associated with consistent recovery of HIV-1-specific, CD8+-cell IFN-γ production in four of the six group B and C patients either before or after receiving the triple-drug regimen (i.e., B1178, C1158, C1164, and C1180) (Fig. 2). Patients B1159 and C1176, however, had a high, sustained number of IFN-γ-producing cells specific for at least one 1 HIV-1 protein during the pre-open-label portion of the drug trial and after the three-drug combination therapy (Fig. 2). In further contrast to the group A patients, there were no significant correlations between the numbers of IFN-γ-producing CD8+ cells and the CTLp levels in the group B and C patients (data not shown).
The changes in IFN-γ production by CD8+ T cells during combination antiretroviral therapy were not unique for HIV-1 proteins. That is, the numbers of IFN-γ-producing, CD8+ cells specific for the CMV pp65 matrix antigen were comparable to the IFN-γ responses to at least one HIV-1 protein during triple-drug treatment in most of the group A patients (Fig. 1) and the group B and C patients (Fig. 2).
Relation of HIV-1- and CMV-specific, CD8+ T-cell responses to viral load and T-cell counts during triple combination antiretroviral therapy.
We investigated whether anti-HIV-1 CTLp and IFN-γ reactivity after triple-drug therapy was related to changes in HIV-1 RNA levels and T-cell counts. We found that increases in CTLp correlated with decreases in HIV-1 plasma load during the first 12 weeks of simultaneous triple drug treatment in the group A patients (Table 2). A significant although progressively weaker inverse correlation with HIV-1 RNA levels was maintained for 48 weeks for all three CTLp HIV-1-specific activities and for 132 weeks of followup for anti-Gag and anti-Pol CTLp in these patients (data not shown). There was no significant correlation between the CTLp levels and the number of T-cell subsets in group A patients or with viral load and T-cell subsets in group B and C patients during combination therapy (data not shown).
The only significant correlation for IFN-γ production was the response to Pol and the number of CD4+ and CD8+ T cells and the response to CMV with the CD8+ T-cell counts during the first 12 weeks of combination therapy in the group A patients (Table 2). These correlations were progressively weaker at later time points (data not shown).
Effect of combination antiretroviral therapy on reactivity of CD8+ T cells to HIV-1 peptides assessed by single-cell IFN-γ production.
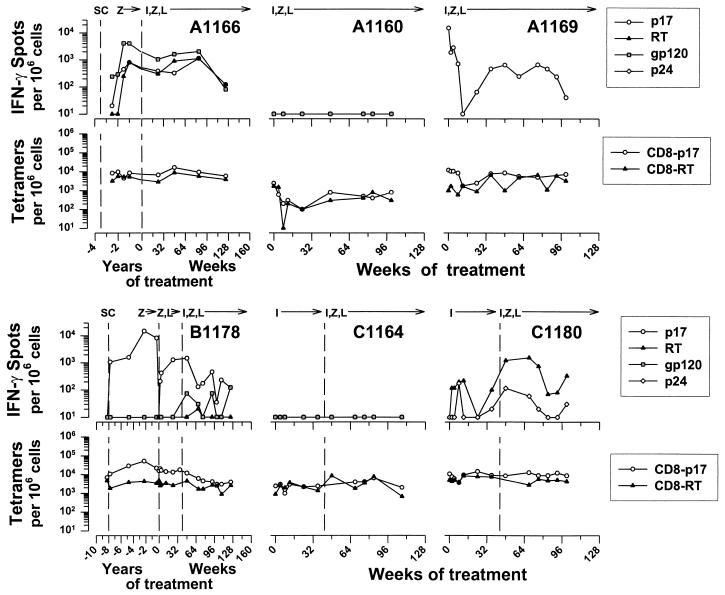
We next examined the breadth of the IFN-γ response of CD8+ T cells using HIV-1 peptides representing known HLA A*02 CTL epitopes in the subset of six HLA A*02 patients in this cohort. Some but not all of these peptides were recognized by CD8+ T cells during the combined antiretroviral therapy. Thus, high, persistent numbers of IFN-γ-producing, CD8+ cells were induced during triple-drug therapy by Gag p1777–85 in three of five patients tested (A1166, A1169, and B1178), by Pol RT476–484 in two of five patients (A1166 and C1180), by Env gp120192–199 in two of three patients (A1166 and B1178), and by Gag p24151–159 in one of the three patients (C1180) (Fig. 3).
FIG. 3.
Effect of treatment with IDV, ZDV, and 3TC given simultaneously (patients A1166, A1160, and A1169) or sequentially (patients B1178, C1164, and C1180) on the number of IFN-γ-producing CD8+ cells reactive to HLA A*02 HIV-1 peptides and the number of HLA A*02 tetramer-HIV-1 peptide staining CD8+ cells.
The ability of the CD8+ T cells to produce IFN-γ to these CTL epitopes was not directly associated with the suppression of virus. This was demonstrated by the lack of any detectable IFN-γ response to the A*02 peptides in patient A1160 (Fig. 3), who had prolonged suppression of HIV-1 (Fig. 1), and the relatively robust IFN-γ activity in C1180 (Fig. 3), whose virus was not completely suppressed (Fig. 1).
The number of CD8+ T cells producing IFN-γ in response to Gag p1777–85 peptide was higher than that for the Pol RT476–484 peptide in these patients (Gag p1777–85 = 1,085 ± 419; Pol RT476–484 = 165 ± 48; n = 52; P = 0.03). IFN-γ reactivity to the Gag p1777–85 peptide, but not to the Pol RT476–484 peptide, correlated with the IFN-γ and CTLp responses to the corresponding HIV-1 proteins (Table 3). There were insufficient data for such comparisons of different types of T-cell reactivity to the other HIV-1 peptides.
TABLE 3.
Correlations between CD8+ T-cell reactivities to HIV-1 peptides in six HLA A*02 group A, B, and C patients during combination antiretroviral therapy
| Test combination
|
Parameter
|
|||
|---|---|---|---|---|
| Test 1 | Test 2 | na | rb | P |
| IFN-γ for Gag p1777–85 | IFN-γ for VVgag p55 | 53 | 0.50 | <0.01 |
| CTLp for VVgag p55 | 44 | 0.33 | <0.05 | |
| Tetramer for Gag p1777–85 | 50 | 0.76 | <0.01 | |
| IFN-γ for Pol RT476–484 | IFN-γ for VVpol | 50 | 0.27 | NSc |
| CTLp for VVpol | 41 | 0.24 | NS | |
| Tetramer for Pol RT476–484 | 51 | 0.48 | <0.01 | |
| Tetramer for Gag p1777–85 | CTLp for VVgag p55 | 52 | 0.67 | <0.01 |
| IFN-γ for VVgag p55 | 59 | 0.39 | <0.01 | |
| Tetramer for Pol RT476–484 | CTLp for VVpol | 51 | 0.31 | <0.05 |
| IFN-γ for VVpol | 61 | 0.09 | NS | |
n, number of determinations.
Pearson correlation coefficient.
NS, not significant (P > 0.05).
Specificity of CD8+ T cells for HIV-1 peptides during combination antiretroviral therapy as assessed by HLA tetramer staining.
We compared CD8+ T-cell CTLp and IFN-γ responses to the number of CD8+ T cells expressing T-cell receptors for HIV-1 Gag p1777–85 and Pol RT476–484 by staining with HLA A*02 tetramers in the subset of six HLA A*02 patients. The levels of Gag p1777–85 tetramer staining in the six patients were higher than those for Pol RT476–484 (mean ± the SE = 8,236 ± 947 and 3,402 ± 280 per 106 CD8+ T cells, respectively; n = 69; P < 10−5). These levels were greater than the number of IFN-γ-producing, CD8+ cells induced by the corresponding peptides (IFN-γ for Gag p1777–85 = 1,085 ± 419 and for Pol RT476–484 = 165 ± 48; n = 52; P < 10−8 for both compared to the tetramer levels). The number of CD8+ cells producing IFN-γ in response to the Gag p1777–85 and Pol RT476–484 peptides, however, correlated with those staining with the matching tetramers (Table 3). Similar associations were noted for the Gag p1777–85 and RT476–484 tetramers and the number of CTLp and IFN-γ producing, CD8+ cells stimulated by p55 Gag and Pol.
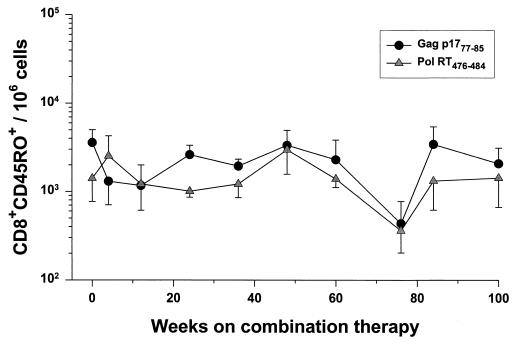
The median of the baseline levels before triple-drug treatment for the six patients was 8,900 (range, 2,400 to 16,300) per 106 CD8+ cells for the Gag tetramer and 3,300 (range, 1,000 to 7,500) per 106 CD8+ cells for the Pol tetramer. The number of CD8+ cells staining with the p17 tetramer initially declined and then stabilized in the first 8 to 16 weeks of triple combination therapy in patients A1160, A1169, and B1178 and were relatively stable from the onset of the trial in patients A1166, C1164, and C1180 (Fig. 3). This was also reflected in the relatively stable number of CD8+ CD45RO+ cells staining with the tetramers (Fig. 4). Notably, patient A1160 had the lowest numbers of tetramer-positive cells; these levels were just above the level of detection (200 per 106 cells, or 0.02%) after the initial decline.
FIG. 4.
Relationship of the number of CD8+ CD45RO+ cells binding HLA A*02 tetramers with the duration of triple-combination therapy (mean ± the SE). The data represent a median of four (range, two to six) determinations at each time point for the six patients from Fig. 3.
Longitudinal anti-HIV-1 CD8+ T-cell responses from seroconversion through combination antiretroviral therapy.
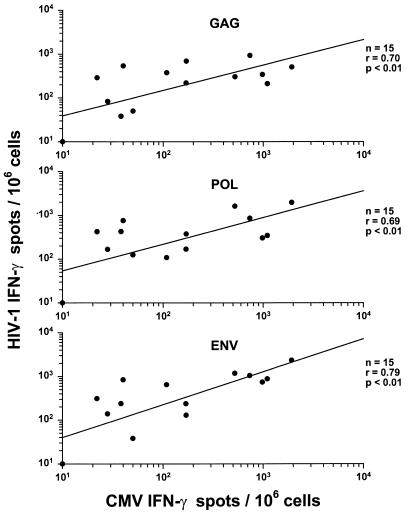
Three of the patients receiving either the triple-drug therapy simultaneously at baseline (A1166) or the three-drug combination sequentially (B1178 and C1158) were also part of the MACS. This provided an unusual opportunity to assess anti-HIV-1 CD8+ T-cell responses in relation to the complete course of HIV-1 infection from seroconversion through different courses of antiretroviral therapy. None of the HIV-1- or CMV-specific T-cell functional parameters correlated with changes in T-cell numbers or HIV-1 RNA load during the MACS study (data not shown). However, there were strong correlations between the number of anti-HIV-1 and anti-CMV IFN-γ-producing CD8+ cells in these three MACS participants prior to the Merck 035 trial (Fig. 5).
FIG. 5.
Correlations between the IFN-γ responses to CMV and HIV-1 Gag, Pol, and Env in the three MACS participants (A1166, B1178, and C1158) after seroconversion and prior to entry into the Merck 035 trial.
Patient A1166 had a progressive increase in numbers of CTLp and IFN-γ-producing CD8+ cells specific for Gag, Pol, and Env and for IFN-γ production to CMV, from seroconversion to approximately 2 years after infection, associated with a consistently high level of HIV-1 RNA (Fig. 1). There was a similar increase in IFN-γ-producing cells in response to the p17, RT, and gp120 peptides, whereas the number of tetramer-positive cells was relatively high and stable during this time (Fig. 3). By 4.4 years after infection, when the patient began this drug treatment trial, the numbers of CD8+ T cells specific for HIV-1 proteins and CMV had declined severalfold. After placement on triple-drug therapy, the viral load decreased to <500 copies by 4 weeks. The number of anti-HIV-1 and anti-CMV CD8+ cells increased concurrently in 24 weeks of combination antiretroviral therapy to numbers similar to the peak pretreatment level. These T-cell functions eventually decreased, however, to undetectable levels by 114 weeks of triple-drug therapy, while the virus remained suppressed.
Patient B1178 had a more prolonged delay in developing CTLp specific for HIV-1 proteins after seroconversion (Fig. 2). In contrast, there was a robust IFN-γ response by 0.8 years after seroconversion to all three HIV-1 proteins and CMV (Fig. 2), as well as to the p17 peptide (400 spots/106 CD8+ cells), with concurrently higher levels of p17 tetramer-positive cells (10,700/106 CD8+ cells, or 1.07%) (Fig. 3). These T-cell parameters declined by 8 years after infection. There were no CD8+ IFN-γ responses to the p24, RT, or Env peptides, although there were low numbers of Pol RT476–484 tetramer-positive cells maintained for years after seroconversion. There was a sharp, transient rise in anti-HIV-1 and CMV CD8+ T-cell reactivity in the 40 weeks of the dual-drug therapy, during which time the viral load rebounded to high, baseline levels. T-cell reactivity to HIV-1 proteins and CMV recovered after the switch to triple-drug therapy, with suppression of the viral load, but most of these immune parameters decreased by 132 weeks.
Patient C1158 developed CTLp against Gag and Env soon after seroconversion but subsequently had very low or undetectable numbers of CTLp precursors in the 5 years prior to entry into this drug therapy trial (Fig. 2). Interestingly, there were high levels of IFN-γ-producing CD8+ cells early after seroconversion to all three HIV-1 proteins and CMV that declined only by the start of this drug trial. This patient also maintained a high viral load during the 5 years of infection before the treatment trial, except for a transient decline during ZDV monotherapy. This participant showed an excellent virologic response to treatment with IDV alone, with suppression by 12 weeks in the trial. There were variable, fluctuating levels of CD8+ T-cell responses to HIV-1 proteins and CMV antigen during the pre-open-label period and during the triple-drug treatment, even though the virus load remained suppressed.
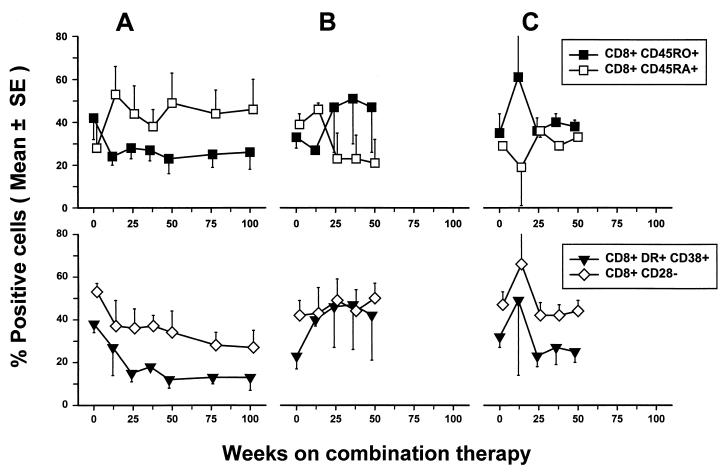
Alteration in T-cell lineage and activation phenotypes by combination drug therapy.
We next determined whether anti-HIV-1 CD8+ cell reactivity was related to changes in CD8+ memory and activated T cells. The percentage of CD8+ CD45RO+ memory T cells decreased and that of CD8+ CD45RA+ naive T cells increased during the first several months of treatment in the group A patients and then stabilized (Fig. 6A). A similar pattern was noted for CD4+ CD45RO+ memory T cells and CD4+ CD45RA+ naive T cells in these patients (data not shown). Group A patients also showed decreases in activated CD8+ CD38+ HLA-DR+ cells that correlated with decreases in HIV-1 RNA (r = 0.63, P = 0.006), increases in CD4+ cells (r = −0.65, P < 0.001), and decreases in CD8+ CD28− cells (r = 0.55, P = 0.001).
FIG. 6.
Changes in T-cell phenotypes in late-stage HIV-1-infected patients during triple combination therapy (mean ± the SE) (n = 5, group A; n = 3, group B; n = 4, group C [patients]).
Group B and C patients showed changes in T-cell subpopulations when switched to triple-drug therapy that were different from those in group A. Thus, there was a rise in memory and activated CD8+ T cells and a concurrent decrease in naive CD8+ T cells in group B patients after the start of combination antiretroviral therapy (Fig. 6B). There was little change from the baseline level in the memory and naive CD8+ T cells in group C patients, whereas there were decreases in activated CD8+ T cells during triple-drug therapy (Fig. 6C). This may be related to the heterogeneous effects of the triple combination antiretroviral therapy on their viral load, i.e., one of three group B patients and two of four group C patients tested did not show complete suppression of HIV-1 RNA (Fig. 2).
We found no correlation of anti-HIV-1 CTLp or IFN-γ-producing cell numbers with the percentages of these CD8+ T-cell subpopulations (data not shown). It should be noted, however, that the HIV-1-stimulated, cultured cells used in the CTLp assessments were >80% CD8+ CD38+ HLA-DR+ and CD8+ CD28− regardless of the patients' treatment group or levels of anti-HIV-1 CTLp and IFN-γ-producing cells. Moreover, there was no difference in the growth of the cells in the CTLp cultures among the three groups of patients throughout the 2 years of followup (e.g., mean increases [± the SE] in cell numbers from an initial 25,000 cells per culture were 10.9 [±1.3]-, 16.8 [±0.7]-, and 14.8 [±4.1]-fold in groups A, B, and C, respectively, during the first 36 weeks of treatment; P > 0.05).
DISCUSSION
This study provides evidence that triple-drug antiretroviral therapy (IDV plus 3TC plus ZDV) fails to produce a sustained increase in anti-HIV-1 CD8+ T-cell functions in HIV-1-infected patients with advanced immunodeficiency. There were two basic patterns of HIV-1-specific T-cell reactivity after combination antiretroviral therapy in the 14 patients in our study. One was an early rise from very low pretreatment levels in anti-HIV-1 CTLp and IFNγ-producing, CD8+ cells specific for HIV-1 Gag, Pol, and Env during the triple-drug therapy. This response, however, declined to baseline levels in most of these patients by 2 years. This pattern is similar to the temporary enhancing effect of combination therapy on anti-HIV-1 CTLp in two late-stage patients recently described by Kalams et al. (21). The second pattern of anti-HIV-1 CD8+ T-cell responses that we observed was a failure of CTLp and IFN-γ reactivity to increase above the low baseline levels throughout the 2 years of triple-drug therapy. It is possible that we missed brief, temporary rises of anti-HIV-1 T-cell activity in these patients during the time interval between test samples. However, Pontesilli et al. (37) have also noted that some late-stage patients on several different combination therapies for 1 year do not recover anti-HIV-1 CTLp reactivity.
Several factors could be related to the changes in anti-HIV-1 CD8+ T-cell reactivity after combination antiretroviral therapy. Group A patients, who initiated the triple-drug combination from the study onset, had a strong correlation early in treatment between an increase in anti-HIV-1 CTLp to all three HIV-1 proteins and decreases in viral load. Interestingly, an increase in the number of IFN-γ-producing CD8+ cells specific for Pol in the group A patients correlated with an increase in the number of CD4+ and CD8+ T cells but not with a decrease in viral load. These correlations were not evident in group B and C patients, who initiated treatment with the three drugs sequentially. The data suggest that CTLp and IFN-γ CD8+ cell functions, while related, are not identical. Thus, the ability of CD8+ T cells to produce IFN-γ in response to HIV-1 antigens may be a better correlate of recovery of T-cell immunity during combination therapy than the number of anti-HIV-1 CTLp. The data also suggest that sequential initiation of the three drugs, which is known to promote antiviral drug resistance (16), has less restorative effect on functional, anti-HIV-1 CD8+ cell reactivity than does initiating the three drugs simultaneously.
The results indicate that the early rise in anti-HIV-1 CD8+ T-cell responses in the subgroup of our patients represents a true, albeit transient, enhancement of CD8+ cell function. It does not appear to be simply a result of redistribution of preexisting populations of these CD8+ memory cells from extravascular spaces during therapy (35). That is, although we observed a decrease in the numbers of memory, CD8+ CD45RO+ cells and activated, CD8+ CD38+ HLA DR+ and CD8+ CD28− cells in many of these patients, which include anti-HIV-1 CTL effectors (12, 18), this did not correlate with changes in the number of CD8+ CTLp or IFN-γ-producing cells. Alternatively, the enhancement in anti-HIV-1 CTLp could have been due to a selective increase in the growth potential of circulating CD8+ T cells in response to HIV-1 antigen-presenting cells during combination therapy. This was not the case, however, as there was a similar, temporary augmentation in HIV-1-specific, IFN-γ-producing CD8+ T cells in these patients that is not dependent on cell replication. Moreover, there was no difference in outgrowth of CD8+ cells in CTLp cultures from patients with or without temporal increases in this anti-HIV-1 CD8+ T-cell function.
The longitudinal data in the three MACS participants from seroconversion suggest that persons who develop the strongest and most persistent CD8+ T-cell reactivity to HIV-1 after infection have the best recovery of such immunologic functions after combination antiretroviral therapy. Both MACS participants with persistently high numbers of CTLp and IFN-γ-producing CD8+ cells in response to the three HIV-1 proteins prior to the drug trial (A1166 and B1178) recovered similar levels of this T-cell reactivity for a prolonged period after receiving the triple-drug combination. The third patient (C1158) failed to develop consistently high numbers of CTLp specific for any of the HIV-1 proteins before or after treatment. He did, however, maintain relatively high numbers of IFN-γ-producing CD8+ cells specific for HIV-1 during the MACS study that also reached high levels after triple-drug treatment.
An important finding of this study is that changes in CD8+ T-cell reactivity were not restricted to anti-HIV-1 responses. The results from the three MACS patients were most illustrative of this where there were concurrent increases and eventual declines in both HIV-1-specific and CMV-specific, IFN-γ-producing CD8+ cells after seroconversion and during the drug trial. We have observed a similar pattern of slow increase of anti-HIV-1 CD8+ CTL after seroconversion, together with a persistently high HIV-1 load in about 75% of MACS subjects tested to date (43). However, the concurrent rise and fall in anti-HIV-1 and anti-CMV CD8+ T-cell reactivity during the natural progression of HIV-1 infection was unexpected. It could be a response to an increased burden of CMV antigen with progressive immunosuppression, although major elevations in systemic CMV load only appear late in HIV-1 infection (39). Alternatively, it is possible that the increases in the number of anti-CMV CD8+ T cells are due to a bystander effect where these cells are signaled to expand by cytokines produced in response to other, possibly HIV-1-specific stimuli (48). The present study suggests that these antiviral CD8+ memory T cells are under similar homeostatic control during both the natural progression of HIV-1 infection and after highly viral suppressive, triple-drug therapy.
We found a limited breadth of CD8+ T-cell responses to four HLA A*02 peptides representing known CTL epitopes in patients receiving combination therapy. The IFN-γ response to the Gag p1777–85 peptide, one of the most widely recognized HLA A*02 HIV-1 epitopes (49), was present throughout treatment in three patients but was completely absent in the other two patients tested. Likewise, T-cell IFN-γ reactivity to HLA A*02 epitope Pol RT476–484 was found in only two of five patients after combination therapy. The patients also had heterogenous IFN-γ responses to the Gag p24151–159 and Env gp120192–199 HLA A*02 epitopes. This restriction in T-cell specificity to certain HIV-1 peptides could be related to the persistence of oligoclonal CD8+ T-cell repertoire perturbations after treatment (14). In support of this, we found that a selective T-cell reactivity to these known HLA A*02 epitopes was present throughout the natural history of HIV-1 infection prior to initiation of triple-drug therapy in the two MACS HLA A*02 patients. Dalod and colleagues (7) have also recently shown that restricted CD8+ T cell responses to HIV-1 peptides persist during natural HIV-1 infection. Our data suggest that dominance of T cell epitopes during natural HIV-1 infection is not broadened during combination therapy.
We postulate that the late decline in numbers of functional, HIV-1 specific, CD8+ T cells during triple combination drug therapy may be a normal homeostatic response to the persistent reductions in HIV-1 antigen. Similar low levels of anti-HIV-1 CTLp have been found in some long-term nonprogressors with a very low viral load (23, 38, 43). This finding is distinct from the loss of anti-HIV-1 CD8+ T cells that occurs in progressive HIV-1 infection associated with a high viral burden and low CD4+ T-cell numbers, as shown in our three MACS subjects and in other studies (23, 38, 43). Different mechanisms may underlie this loss in progressive disease, including T-cell clonal deletion and anergy (28), that are only partially reversed during triple-combination drug therapy.
A second basis for the loss of functional anti-HIV-1 CD8+ T cells during treatment with the three-drug combination may be the lack of recovery of anti-HIV-1 CD4+ T-cell responses, as we have noted in these same trials (45). This notion is supported by reports that long-term maintenance of high levels of other types of antiviral CD8+ CTL requires CD4+ T-cell help (26, 42). High CD4+ T-cell reactivity to HIV-1 has also been related to lower HIV-1 load in long-term nonprogressors and in patients with early HIV-1 infection who are treated with combination therapies (20, 22, 46).
The intensity of the T-cell response was also determined by staining the CD8+ cells with HLA A*02 Gag p1777–85 and Pol RT476–484 tetramers. The numbers of CD8+ cells binding the Gag tetramer were higher than for the Pol tetramer, as previously reported (30). Our data show that the tetramer staining and single-cell IFN-γ responses to these peptides were correlated but were not identical parameters of immunity. Of interest is that there was persistent reactivity of CD8+ T cells to these two tetramers in the two MACS participants in the years prior to the trial. These data contrast with a recent report that lower levels of tetramer-binding CD8+ cells are associated with disease progression in HIV-1-infected persons not receiving combination therapy (32). As noted in other studies (15, 30, 31), we found an early decrease in tetramer staining in some patients during combination therapy. However, like the two patients recently studied by Kalams et al. (21), most of the patients on triple-drug therapy in our study maintained relatively stable levels of tetramer-positive CD8+ cells, including CD45RO+ memory T cells. This population of CD8+ CD45RO+ cells may be an important, residual source of memory T cells specific for HIV-1 in patients on combination therapy.
It is known that HIV-1 can break through combination therapy resulting in viral load rebound (16) and that latent virus can persist in resting CD4+ T cells and be reactivated (6, 10, 11, 53). Our study and others (34, 40) suggest that strategies to increase T-cell function will be required as adjunct therapy to restore anti-HIV-1 immunity and better control HIV-1 infection. In this regard, we have found that HIV-1-specific, CD8+ T cells can be induced in vitro by multiple, repetitive stimulations with IL-12 and dendritic cells loaded with HIV-1 proteins (55; Z. Fan, X. Huang, and C. R. Rinaldo, Jr., unpublished results). This induction is possible with samples from patients who have not responded to stimulation with our conventional, VV–HIV-1 and B-LCL system (Fan et al., unpublished). This, together with our present finding of residual CD8+ T cells specific for HIV-1 immunodominant peptides, suggests that competent T cells specific for HIV-1 are still present after prolonged combination therapy. Such cells offer a target for immunomodulation in vivo that could improve the host's capacity to control HIV-1 infection and enhance the effectiveness of antiretroviral therapy.
ACKNOWLEDGMENTS
We thank A. Zeevi and R. Kaslow for portions of the HLA typing; J. Liebmann, A. Bazmi, E. Molina, S. McQuiston, B. Colleton, H. Li, M. Tseng, W. Jiang, Q. Al-Shboul, and E. Ramirez for technical assistance; and T. Silvestre, W. Buchanan, L. Johnson, N. Mantz, J. Stewart, A. Sparks, and R. Kudray for clinical assistance.
This project was supported by National Institutes of Health grants R01-AI41870, U01-AI37984, U01-AI35041, and T32-AI07487 and by a grant from the Merck Research Laboratories.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Borrow P, Lewick H, Hahn B, Shaw G, Oldstone M. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brander C, Pichler W J, Corradin G. Identification of HIV protein-derived cytotoxic T lymphocyte (CTL) epitopes for their possible use as synthetic vaccine. Clin Exp Immunol. 1995;101:107–113. doi: 10.1111/j.1365-2249.1995.tb02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant J, Day R, Whiteside T L, Herberman R B. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods. 1992;146:91–103. doi: 10.1016/0022-1759(92)90052-u. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active ART. Proc Nat Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalod M, Dupuis M, Deschemin J C, Sicard D, Salmon D, Delfraissy J F, Venet A, Sinet M, Guillet J G. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8(+) responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–16. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalod M, Harzic M, Pellegrin I, Dumon B, Hoen B, Sereni D, Deschemin J C, Levy J P, Venet A, Gomard E. Evolution of cytotoxic T lymphocyte responses to human immunodeficiency virus type 1 in patients with symptomatic primary infection receiving antiretroviral triple therapy. J Infect Dis. 1998;178:61–69. doi: 10.1086/515587. [DOI] [PubMed] [Google Scholar]
- 9.De Maeyer E, De Maeyer-Guignard J. Interferons. In: Thomson A, editor. The cytokine handbook. New York, N.Y: Academic Press; 1998. pp. 491–516. [Google Scholar]
- 10.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 11.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active ART. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino S, Dalod M, Olive D, Guillet J G, Gomard E. Predominant involvement of CD8+ CD28− lymphocytes in human immunodeficiency virus-specific cytotoxic activity. J Virol. 1996;70:2022–2026. doi: 10.1128/jvi.70.3.2022-2026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furtado M R, Callaway D S, Phair J P, Kunstman K J, Stanton J L, Macken C A, Perelson A S, Wolinsky S M. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. New Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 14.Gorochov G, Neumann A U, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debre P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 15.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 16.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Jonas L, Meibohm A, Holder D, Schleif W A, Condra J H, Emini E A, Isaacs R, Chodakewitz J A, Richman D D. Simultaneous vs. sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. JAMA. 1998;280:35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior ART. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 18.Ho H, Hultin L E, Mitsuyasu R T, Matud J L, Hausner M A, Bockstoce D, Chou C C, O'Rourke S, Taylor J M, Giorgi J V. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 19.Huang X L, Fan Z, Liebmann J, Rinaldo C. Detection of human immunodeficiency virus type 1-specific memory cytotoxic T lymphocytes in freshly donated and frozen-thawed peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 1995;2:678–684. doi: 10.1128/cdli.2.6.678-684.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Huang X-L, Fan Z, Kalinyak C, Mellors J W, Rinaldo C R., Jr CD8+ T-cell gamma interferon production specific for human immunodeficiency virus type 1 (HIV-1) in HIV-1-infected subjects. Clin Diagn Lab Immunol. 2000;7:279–287. doi: 10.1128/cdli.7.2.279-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalams S A, Walker B D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koup R, Safrit J, Cao Y, Andrews C, McLeod G, Borkowsky W, Farthing C, Ho D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin-Taylor E, Pande H, Forman S J, Tanamachi B, Li C R, Zaia J A, Greenberg P D, Riddell S R. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103–110. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 28.McMichael A J, Phillips R E. Escape of human immunodeficiency virus from immune control. Annu Rev Immunol. 1997;15:271–296. doi: 10.1146/annurev.immunol.15.1.271. [DOI] [PubMed] [Google Scholar]
- 29.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 30.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 31.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogg G S, Kostense S, Klein M R, Jurriaans S, Hamann D, McMichael A J, Miedema F. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J Virol. 1999;73:9153–9160. doi: 10.1128/jvi.73.11.9153-9160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogg G S, McMichael A J. Quantitation of antigen-specific CD8+ T-cell responses. Immunol Lett. 1999;66:77–80. doi: 10.1016/s0165-2478(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donohoe S M, Demoitie M-A, Kakimoto W M, Ketas T, Clas B, Heymann J J, Zhang L, Cao Y, Hurley A, Moore J P, Ho D D, Markowitz M. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Investig. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pakker N G, Notermans D W, de Boer R J, Roos M T, de Wolf F, Hill A, Leonard J M, Danner S A, Miedema F, Schellekens P T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 36.Parker K C, Bednarek M A, Hull L K, Utz U, Cunningham B, Zweerink H J, Biddison W E, Coligan J E. Sequence motifs important for peptide binding to the human MHC class I molecule, HLA-A2. J Immunol. 1992;149:3580–3587. [PubMed] [Google Scholar]
- 37.Pontesilli O, Kerkhof-Garde S, Notermans D W, Foudraine N A, Roos M T, Klein M R, Danner S A, Lange J M, Miedema F. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 38.Pontesilli O, Klein M R, Kerkhof-Garde S R, Pakker N G, deWolf F, Schuitemaker H, Miedema F. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant Gag-specific response is associated with nonprogressive infection. J Infect Dis. 1998;178:1008–1018. doi: 10.1086/515659. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen L, Morris S, Zipeto D, Fessel J, Wolitz R, Dowling A, Merigan T C. Quantitation of human cytomegalovirus DNA from peripheral blood cells of human immunodeficiency virus-infected patients could predict cytomegalovirus retinitis. J Infect Dis. 1995;171:177–182. doi: 10.1093/infdis/171.1.177. [DOI] [PubMed] [Google Scholar]
- 40.Richman D. The challenge of immune control of immunodeficiency virus. J Clin Investig. 1999;104:677–678. doi: 10.1172/JCI8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richman D, Crowe S, Harvey K. HIV viral load monitoring. Adv Exp Med Biol. 1999;458:199–212. doi: 10.1007/978-1-4615-4743-3_19. [DOI] [PubMed] [Google Scholar]
- 42.Riddell S R, Greenberg P D. Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol. 1995;113:545–586. doi: 10.1146/annurev.iy.13.040195.002553. [DOI] [PubMed] [Google Scholar]
- 43.Rinaldo C R, Jr, Gupta P, Huang X, Fan Z, Mullins J I, Gange S, Farzadegan H, Shankarappa R, Muñoz A, Margolick J B. Anti-HIV-1 memory cytotoxic T lymphocyte responses associated with changes in CD4+ T cell numbers in progression of HIV-1 infection. AIDS Res Hum Retrovir. 1998;14:1423–1433. doi: 10.1089/aid.1998.14.1423. [DOI] [PubMed] [Google Scholar]
- 44.Rinaldo C, Huang X L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinaldo C R, Jr, Liebmann J M, Huang X-L, Fan Z, Al-Shboul Q, McMahon D K, Day R D, Riddler S A, Mellors J W. Prolonged suppression of human immunodeficiency virus 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J Infect Dis. 1999;179:329–336. doi: 10.1086/314599. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 47.Tanguay S, Killion J J. Direct comparison of ELISPOT and ELISA-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res. 1994;13:259–263. [PubMed] [Google Scholar]
- 48.Tough D F, Sprent J. Viruses and T cell turnover: evidence for bystander proliferation. Immunol Rev. 1996;150:129–142. doi: 10.1111/j.1600-065x.1996.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 49.Tsomides T J, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsomides T J, Walker B D, Eisen H N. An optimal viral peptide recognized by CD8+ T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci USA. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner S, Ellexson M E, Hickman H D, Sidebottom D A, Fernandez-Vina M, Confer D L, Hildebrand W H. Sequence-based typing provides a new look at HLA-C diversity. J Immunol. 1998;161:1406–1413. [PubMed] [Google Scholar]
- 52.Wolinsky S M, Korber B T, Neumann M A U, Daniels M, Kunstman K J, Whetsel A J, Furtado M R, Cao Y, Ho D D, Safrit J T, Koup R A. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 53.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 55.Zheng L, Huang X, Fan Z, Borowski L, Wilson C C, Rinaldo C R., Jr Delivery of liposome-encapsulated HIV type 1 proteins to human dendritic cells for stimulation of HIV type 1-specific memory cytotoxic T lymphocyte responses. AIDS Res Hum Retrovir. 1999;15:1011–1020. doi: 10.1089/088922299310520. [DOI] [PubMed] [Google Scholar]