Abstract
The Edmonston strain of measles virus (MV) that utilizes the human CD46 as the cellular receptor produced cytopathic effects (CPE) in all of the primate cell lines examined. In contrast, the wild-type MV strains isolated in a marmoset B-cell line B95a (the KA and Ichinose strains) replicated and produced CPE in some but not all of the primate lymphoid cell lines. To determine the mechanism underlying this difference in cell tropism, we used a recently developed recombinant vesicular stomatitis virus (VSV) containing as a reporter the green fluorescent protein gene in lieu of the VSV G protein gene (VSVΔG*). MV glycoproteins were efficiently incorporated into VSVΔG*, producing the VSV pseudotypes. VSVΔG* complemented with VSV G protein efficiently infected all of the cell lines tested. The VSV pseudotype bearing the Edmonston hemagglutinin (H) and fusion (F) protein (VSVΔG*-EdHF) infected all cell lines in which the Edmonston strain caused CPE, including the rodent cell lines to which the human CD46 gene was stably transfected. The pseudotype bearing the wild-type KA H protein and Edmonston F protein (VSVΔG*-KAHF) infected all lymphoid cell lines in which the wild-type MV strains caused CPE as efficiently as VSVΔG*-EdHF, but it did not infect any of the cell lines resistant to infection with the KA strain. The results indicate that the difference in cell tropism between these MV strains was largely determined by virus entry, in which the H proteins of respective MV strains play a decisive role.
Measles continues to be a major childhood killer and is currently estimated to cause almost 1 million deaths a year (8). Measles virus (MV) is an enveloped negative-strand RNA virus that is a member of the Morbillivirus genus in the Paramyxoviridae family. MV has two envelope glycoproteins, the hemagglutinin (H) and fusion (F) proteins, mediating receptor binding and membrane fusion, respectively (8).
MV was first isolated in tissue culture by inoculating primary human kidney cells with the blood of a child with measles (6). Since then, continuous monkey kidney cell lines (e.g., Vero) have been commonly used to isolate MV strains from clinical samples. However, several blind passages were generally required in these cell lines before virus propagation and development of cytopathic effects (CPE). Recently, Kobune et al. reported that MV strains could be isolated much more rapidly and efficiently in the Epstein-Barr virus (EBV)-transformed marmoset B-cell line B95-8 and its adherent subline B95a than in monkey kidney cell lines, and that MV strains isolated in B95a cells, but not Vero cell-adapted strains, retained pathogenicity for monkeys (12, 13). These studies suggested that MV strains grown in B95a cells may be more representative of MV circulating in humans than are MV strains selected in Vero cells. Subsequently, other human B-cell lines were also used to isolate wild-type MV strains (14, 25).
Several years ago, CD46 (also called membrane cofactor protein) was identified as the cellular receptor for the Edmonston and Halle strains of MV (4, 15, 17). The H protein was found to induce downregulation of CD46 from the surface of MV-infected cells (18). However, many recent wild-type MV strains isolated in B-cell lines were found not to grow in other CD46-positive cell lines (9, 12, 14, 24, 25, 30). Furthermore, Schneider-Schaulies et al. showed that wild-type strains can be classified into CD46-downregulating and -non-downregulating groups (24).
Two amino acid residues of the H protein (at positions 451 and 481) were shown to be critical for determining the ability of MV strains to cause hemadsorption, HeLa cell fusion, and CD46 downregulation (1, 14). More recently, using a direct binding assay with insect cells expressing the H protein, Hsu et al. demonstrated that a single amino acid change at position 481 determines the ability of the H protein to bind CD46 (9). We showed that the H gene of the wild-type MV isolates induced cell fusion in B95a cells, but not in other CD46-positive cell lines, when coexpressed with the F gene (30). All of these observations led to the proposal that the H protein of the Edmonston strain, but not of many wild-type strains isolated in B-cell lines, interacts with CD46, and that there is another cellular receptor for these wild-type MV strains (2, 3, 9, 14, 30).
On the other hand, by analyzing differences in the growth properties and nucleotide sequences of B95a-grown strains and their Vero cell-adapted strains, Takeda et al. concluded that the changes in the H protein were not important for MV adaptation to Vero cells (29). Further, Johnston et al. generated a recombinant Edmonston MV expressing wild-type WTF strain envelope proteins and showed that the recombinant virus expressing the WTF H protein spread in Vero cells although the parental WTF virus did not, suggesting that cell-specific factors other than receptor usage are also important in determining MV cell tropism (11).
In this study, we first examined cell tropism of the Edmonston strain and the KA strain, a CD46-non-downregulating wild-type MV isolate, in a large number of cell lines. We then used a recombinant vesicular stomatitis virus (VSV) to produce pseudotypes bearing MV envelope proteins and showed unequivocally that virus entry is a major determinant of cell tropism of the Edmonston and KA strains.
MATERIALS AND METHODS
Cells.
293T is derived from the human kidney cell line 293 and contains the simian virus 40 large T antigen (5). The other cell lines used in this study and their derivations are listed in Table 1. BJAB and BJAB-B95-8 were kindly provided by Kenzo Takada. CHO/CD46 and CHO/pME18S are CHO-derived cell lines in which the expression vector pME18S with and without the human CD46 gene, respectively, was transfected and were kindly provided by Yusuke Murakami (10). All adherent cell lines except B95a and CHO-derived cell lines were grown in Dulbecco modified Eagle medium supplemented with 7% heat-inactivated fetal bovine serum (FBS), 0.15% sodium bicarbonate, and 50 μg of gentamicin per ml. L.Neo and L.CD46.1 (32) were grown in the medium supplemented with 0.5 mg of G418 per ml. B95a, CHO-derived cell lines, and all cell lines in suspension were grown in RPMI 1640 supplemented with 10% heat-inactivated FBS, 0.15% sodium bicarbonate, and 50 μg of gentamicin per ml. CHO/CD46 and CHO/pME18S were grown in medium supplemented with 0.7 mg of hygromycin B per ml.
TABLE 1.
Susceptibility of cell lines to different strains of MV
| Cell line | Species | Derivation | CPE by MV strain
|
|
|---|---|---|---|---|
| Edmonston | KA | |||
| B95a | Marmoset | Adherent subline of B95-8 | + | + |
| Vero | Monkey | Kidney | + | − |
| HeLa | Human | Cervical carcinoma | + | − |
| BHK-21 | Hamster | Kidney | −a | − |
| RK13 | Rabbit | Kidney | −a | − |
| CHO | Hamster | Ovary | − | − |
| CHO/pME18S | Hamster | CHO transfected with the expression vector | − | − |
| CHO/CD46 | Hamster | CHO transfected with human CD46 cDNA | + | − |
| L | Mouse | Connective tissue | −a | − |
| L.Neo | Mouse | L transfected with the expression vector | −a | − |
| L.CD46.1 | Mouse | L transfected with human CD46 cDNA | + | − |
| B95-8 | Marmoset | EBV-transformed B cell | + | + |
| Raji | Human | Burkitt's lymphoma (EBV positive) | + | + |
| Daudi | Human | Burkitt's lymphoma (EBV positive) | +b | − |
| Ramos | Human | Burkitt's lymphoma (EBV negative) | + | + |
| BJAB | Human | Burkitt's lymphoma (EBV negative) | + | − |
| BJAB-B95-8 | Human | EBV-infected line of BJAB | + | + |
| MT-2 | Human | HTLV-1-transformed cord blood cell | + | + |
| C91/PL | Human | HTLV-1-transformed cord blood cell | + | + |
| Jurkat | Human | T-cell leukemia | + | − |
| MOLT-4 | Human | T-cell leukemia | + | − |
| THP-1 | Human | Monocytic leukemia | + | − |
| U-937 | Human | Histiocytic lymphoma (macrophage) | + | − |
| HL-60 | Human | Promyelocytic leukemia | + | − |
| RMA | Mouse | T-cell lymphoma | − | − |
| EL4 | Mouse | T-cell lymphoma | − | − |
The Edmonston strain of MV caused a few giant cells on these nonprimate cell lines when infected at a high MOI (>10).
CPE caused by the Edmonston strain of MV was weaker on Daudi than CPE on other human cell lines in suspension.
Viruses.
The Edmonston strain of MV was obtained from American Type Culture Collection and grown on Vero cells. The wild-type MV strains, KA and Ichinose, were isolated from patients with measles by using B95a cells and were grown on B95a cells (generous gifts of Fumio Kobune) (30). (In this report, the term “wild-type” refers to MV strains that have been isolated and propagated in marmoset or human B-cell lines and usually do not grow well in Vero cells.) The Edmonston strain was titrated on Vero cells, and the KA and Ichinose strains were titrated on B95a cells. VSVΔG*-G is the recombinant VSV derived from a full-length cDNA clone of VSV genome (Indiana serotype) in which the coding region of the G protein was replaced by the coding region of a modified version of the green fluorescent protein (GFP) gene and the G protein was expressed in trans by pCVSVG (28). VSVΔG*-G, kindly provided by Michael A. Whitt, was grown and harvested by infecting 293T cells which had been transfected with pCVSVG.
Plasmids.
cDNA clones of the Edmonston H and F genes were obtained from M. A. Billeter (23) and subcloned into the expression vector pCXN2 (19). They were designated pCXN2H and pCXN2F. A cDNA clone of the KA H gene was obtained by reverse transcriptase-PCR and cloned into pCXN2, yielding pCXN2KAH (30). pCVSVG is the expression plasmid in which cDNA encoding the VSV G protein was cloned into the expression vector pCAGGS (19).
Virus growth in cell lines.
Each cell line (2.5 × 105 cells) was infected with the Edmonston (titrated on Vero cells) or KA (titrated on B95a cells) strain at a multiplicity of infection (MOI) of 0.25. After 1 h of infection, cells were washed with phosphate-buffered saline three times, replenished with 1 ml of fresh medium, and incubated in 24-well plate at 37°C in a 5% CO2 incubator. Cells and medium were recovered at various times after infection and then treated by one cycle of freezing-thawing and low-speed centrifugation. The suspensions containing the Edmonston strain were titrated on Vero cells, and those containing the KA strains were titrated on B95a cells.
Preparation of pseudotype viruses.
293T cells were transfected with pCVSVG, pCXN2H plus pCXN2F, pCXN2KAH plus pCXN2F, or pCXN2 by using Lipofectamine (GIBCO/BRL). Thirty-two hours after transfection, cells were infected with VSVΔG*-G at an MOI of 1 (titrated on 293T cells) for 1 h at 37°C. They were then washed with Dulbecco modified Eagle medium without FBS seven times, and culture medium was added. After 21 h of incubation at 37°C in a CO2 incubator, culture fluid and scraped cell debris were collected, treated by one cycle of freezing-thawing, and sonicated. The suspensions containing pseudotype viruses were clarified by low-speed centrifugation and stored at −80°C. They were designated VSVΔG*-G, VSVΔG*-EdHF, VSVΔG*-KAHF, and VSVΔG*. When VSVΔG*-EdHF was prepared, culture medium was supplemented with the fusion block peptide (Z-d-Phe-Phe-Gly) (21) from 5 h after lipofection to immediately before infection with VSVΔG*-G, in order to prevent 293T cells from fusing each other upon transfection with pCXN2H plus pCXN2F.
Titration of pseudotype viruses in various cell lines.
For adherent cell lines, 2 × 104 cells (5 × 104 cells for B95a) in 100 μl of fresh culture medium were sedimented in the well of 96-well flat-bottom plate. After overnight incubation, 50 μl of serially diluted virus stock was added to each well, followed by incubation at 37°C in a CO2 incubator. At 24 h after infection, infectious units of pseudotype virus stocks were determined by counting the number of GFP-expressing cells under a fluorescence microscope. Since infected cells may divide during 24 h of incubation, a doublet of GFP-expressing cells, when observed, was counted as 1 infectious unit. For cell lines in suspension, 5 × 104 cells in 100 μl of fresh culture medium were infected in a similar manner. At 24 h after infection, cells in each well were treated by pipetting to break cell clumps and left to settle for 30 to 90 min; then GFP-expressing cells were counted. Since single infected cells may produce GFP-expressing progeny cells during 24 h of incubation, we may overestimate infectious units for cell lines in suspension, depending on the doubling time of the cell lines. This may become a problem when we compare infectious units between different cell lines. However, as long as we compare infectious units of four pseudotype viruses within a single cell line, the proportional difference in infectivity is not affected. The same preparations of virus stocks were used for all titrations, and each cell line was simultaneously prepared for the titration of four types of pseudotype viruses.
Electron microscopy.
Virus samples were prepared as described above except that only culture supernatant containing pseudotype viruses (not cell debris) was used. They were partially purified by centrifugation through 20% sucrose and labeled with serum from a patient with subacute sclerosing panencephalitis (SSPE) (31) and protein G conjugated with gold. Virus samples were negatively stained with uranyl formate and examined in an electron microscope.
RESULTS
Cell tropism of the wild-type KA strain of MV.
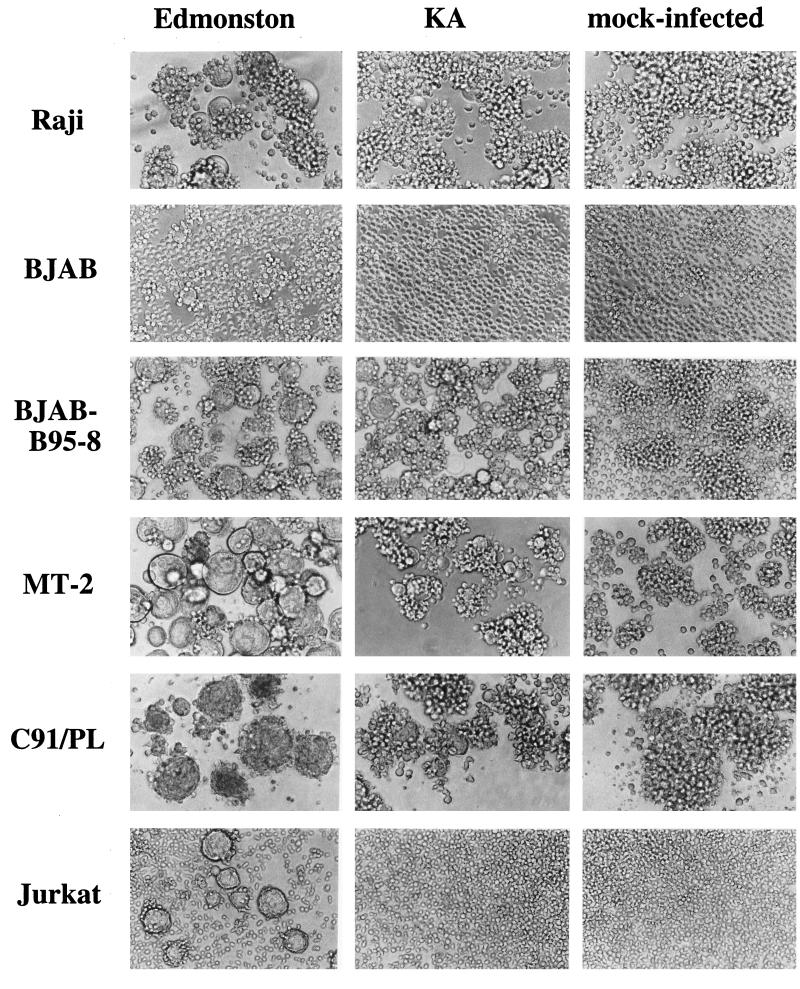
To examine the difference in cell tropism between the Edmonston strain and the wild-type KA strain of MV, we infected various cell lines and observed the development of CPE (Fig. 1 and Table 1). The Edmonston strain caused CPE in all human and monkey cell lines tested. CPE was weaker in Daudi cells than in other human cell lines, probably because the F protein is not effectively processed in Daudi cells (7). Interestingly, even the rodent and rabbit cell lines developed focal small syncytia when infected at a high MOI (>10). On the other hand, the KA strain produced CPE only in B95a, B95-8, Raji, Ramos, BJAB-B95-8, MT-2, and C91/PL cells, which are all lymphoid cell lines (Fig. 1).
FIG. 1.
Development of CPE on human lymphoid cell lines infected with MV. Each cell line was infected with the Edmonston or KA strain of MV at an MOI of 0.25, or mock infected, and then incubated in a CO2 incubator at 37°C. Cells were observed 24 h after infection. Longer incubation (up to 96 h) did not affect the presence or absence of CPE.
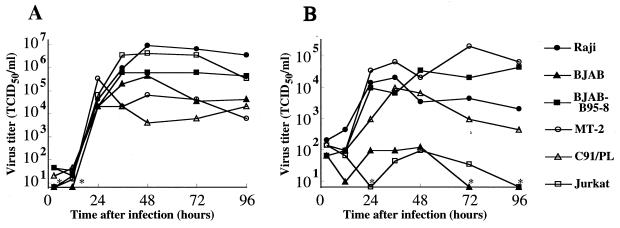
To ascertain that CPE correctly reflected the infectivities of the MV strains, virus replication was examined in representative cell lines. The Edmonston strain grew well in all cell lines examined, in which virus titer reached a high level at 24 h after infection and was maintained for 3 days thereafter (Fig. 2A). The KA strain also grew in four of the cell lines tested but not well in two cell lines that did not produce CPE (BJAB and Jurkat) (Fig. 2B). Thus, the development of CPE paralleled virus replication in these cell lines.
FIG. 2.
MV growth on human lymphoid cell lines. Each cell line was infected with the Edmonston (A) or KA (B) strain of MV at an MOI of 0.25. Virus production was examined 3, 12, 24, 36, 48, 72, and 96 h after infection. At the points indicated by ∗, the virus titer was under the detection limit (0.8 × 10 50% tissue culture infective doses [TCID50]/ml) of the assay.
In previous studies, wild-type lymphotropic MV strains have been reported to grow in such B-cell lines as B95a, B95-8, Raji, and BJAB, as well as in mitogen-stimulated human peripheral blood mononuclear cells (9, 12, 24, 25, 30). Although the KA strain did not grow well in BJAB, it caused CPE in BJAB-B95-8, an EBV-infected line of BJAB. The KA strain also caused CPE in an EBV-negative B-cell line Ramos and in T-cell lines MT-2 and C91/PL. Flow cytometry analysis indicated that cell surface markers of MT-2 and C91/PL cells were CD19− CD3− CD4+ CD8− CD25+. These cells were also strongly stained by serum from a patient with human T-cell leukemia virus type 1 (HTLV-1)-associated myelopathy, confirming that they are HTLV-1-infected cell lines. To determine whether the observed tropism was peculiar to the KA strain, another wild-type strain isolated in B95a cells, Ichinose, was also used to infect the cell lines examined in Fig. 1, and exactly the same tropism was observed (data not shown).
Incorporation of MV envelope proteins into VSV pseudotypes.
To determine whether virus entry or subsequent intracellular replication is responsible for the difference in cell tropism between the Edmonston and wild-type MV strains, we used the recently developed recombinant VSVΔG*, which contains the GFP gene instead of the VSV G protein gene and thus is not infectious unless the envelope proteins mediating receptor binding and membrane fusion are provided in trans (28). VSVΔG* complemented with the VSV G gene (VSVΔG*-G) can infect the majority of cell lines (28). To confirm the incorporation of MV envelope proteins into VSV particles, 293T cells were transfected with the KA H protein and the Edmonston F protein, followed by infection with VSVΔG*-G, producing VSVΔG*-KAHF. VSVΔG*-G and VSVΔG*-KAHF were partially purified by centrifugation and stained with SSPE serum containing high levels of anti-MV antibodies and protein G conjugated with gold, followed by negative staining. Electron microscopic analysis revealed that VSVΔG*-G and VSVΔG*-KAHF virions had almost the same sizes, presumably reflecting the similar composition of nucleocapsids, and that the majority of VSVΔG*-KAHF virions, but no VSVΔG*-G virions, were labeled with SSPE serum (Fig. 3). Thus, MV envelope proteins were successfully incorporated into VSV particles, consistent with a previous report (26).
FIG. 3.
Electron microscopy of VSV pseudotype particles. VSVΔG* complemented with the VSV G protein (A) or with the KA H protein and the Edmonston F protein (B) was prepared and partially purified by centrifugation. Viruses were stained with SSPE serum and protein G conjugated with gold, followed by negative staining. Bar, 100 nm.
Susceptibility of cell lines to VSV pseudotypes.
We prepared four types of pseudotype viruses: VSVΔG*-G, VSVΔG*-EdHF bearing the Edmonston H and F proteins, VSVΔG*-KAHF, and VSVΔG* bearing no envelope protein. The Edmonston F gene was used to generate both VSVΔG*-EdHF and VSVΔG*-KAHF, and thus any difference in infectivity between these two pseudotypes should be due to the H protein on their envelopes. VSVΔG* was prepared without supplying envelope proteins, so that the cell's susceptibility to VSVΔG* will reflect virus entry independent of VSV or MV envelope proteins.
Figure 4A shows the susceptibility of adherent cell lines to pseudotype viruses. VSVΔG*-G infected all of the cell lines tested, with titers ranging from 106.0 to 108.7 infectious units/ml (as measured by counting the number of GFP-expressing cells). On B95a, Vero, and HeLa cells, VSVΔG*-EdHF had higher infectivity titers than VSVΔG* by over 3 logs, whereas these two pseudotype viruses showed similar levels of titers on BHK21 and RK13 cells. CHO/CD46 and L.CD46.1, the rodent cell lines to which the human CD46 gene was stably transfected, were more susceptible to VSVΔG*-EdHF than their parental cell lines to which only the control plasmid was transfected. The VSVΔG*-KAHF titer was over 4 logs higher than the VSVΔG* titer on B95a cells but not significantly higher on the other adherent cell lines tested. Thus, each adherent cell line showed susceptibility to VSVΔG*-EdHF and VSVΔG*-KAHF, in a manner consistent with its susceptibility to the Edmonston strain and KA strain.
FIG. 4.
Infectivities of pseudotype viruses for adherent cell lines (A) and cell lines in suspension (B). Each cell line was infected with the four pseudotype viruses (VSVΔG*-G, VSVΔG*-EdHF, VSVΔG*-KAHF, and VSVΔG*), and infectivity titers were measured by counting the number of GFP-expressing cells.
The susceptibility of cell lines in suspension to pseudotype viruses was also examined (Fig. 4B). All human cell lines tested were susceptible to VSVΔG*-EdHF, while neither of the mouse cell lines was. VSVΔG*-KAHF titers were 2 to 3 logs higher than VSVΔG* titers on B95-8, Raji, Ramos, BJAB-B95-8, and MT-2. VSVΔG* showed a high infectivity titer on C91/PL, making it impossible to evaluate virus entry into this cell line dependent on the MV envelope proteins. Again, each cell line in suspension showed susceptibility to VSVΔG*-EdHF and VSVΔG*-KAHF, in a manner consistent with its susceptibility to the Edmonston and KA strains.
DISCUSSION
In this study, we first showed the clear difference in cell tropism between the Edmonston strain and the wild-type KA strain, using a large number of various cell lines. The Edmonston strain caused CPE in all human and monkey cell lines tested and in rodent cell lines expressing human CD46, whereas the KA strain caused CPE only in a restricted number of human and marmoset lymphoid cell lines. It was also confirmed in several cell lines that the development of CPE paralleled virus replication. Thus, the KA strain neither produced CPE nor replicated well in BJAB and Jurkat cells. Another wild-type strain (Ichinose) isolated in B95a cells showed the same tropism as the KA strain.
Since both marmoset B95-8 cells and human Raji cells, which are highly susceptible to wild-type MV strains, have been infected with EBV, the relationship between EBV infection and susceptibility to wild-type MV strains has been discussed elsewhere (3). In fact, we showed that the KA and Ichinose strains caused CPE in BJAB-B95-8, an EBV-infected line of BJAB cells, whereas they did not in BJAB. On the other hand, these wild-type strains caused CPE in Ramos, an EBV-negative B-cell line. Furthermore, although our wild-type MV strains did not grow in BJAB, another group used this cell line to isolate and propagate the wild-type MV strains (24, 25). These results suggest that EBV infection per se is not a prerequisite for the cell's susceptibility to wild-type MV. EBV infection may, however, make the cells more susceptible to wild-type MV by upregulating certain proteins that serve either as the cellular receptor or as certain host factors.
We also showed that the KA and Ichinose strains caused CPE in two human T-cell lines, MT-2 and C91/PL, which are HTLV-1-transformed cell lines derived from human cord blood mononuclear cells (16, 20). Though MT-2 and C91/PL cells were originally identified as T cells, they did not express CD3 but were still HTLV-1 positive. We found that wild-type MV strains propagated on these cell lines did not infect Vero cells, indicating that they retained the wild-type phenotype (data not shown). Although we have previously reported that the KA and Ichinose strains produced CPE in phytohemagglutinin-stimulated human peripheral blood mononuclear cells (30), this is the first description of T-cell lines susceptible to wild-type MV strains.
Which step of virus growth determines the difference in cell tropism between MV strains? To evaluate virus entry, we prepared VSV pseudotypes bearing MV envelope proteins. It had been reported that the H and F proteins of MV were efficiently incorporated into VSV virions (26). In this study, we used a recombinant VSV (VSVΔG*) lacking the G protein gene. Thus, the pseudotype viruses enter cells using envelope proteins provided in trans, and then subsequent intracellular steps progress as part of the VSV replication cycle. As a result, the difference in the infectivity between pseudotype viruses reflects the difference in the efficiency of entry using the envelope proteins provided. The infectivity titration of pseudotype viruses on various cell lines showed that the difference in cell tropism between the Edmonston and KA strains could be explained by the efficiency of virus entry, in which the H proteins of the involved MV strains play a decisive role.
This pseudotype virus system contains two factors which may cause a false infectivity. First, VSVΔG*-G used to prepare pseudotypes may contaminate virus stocks, producing a background infectivity. Second, virus entry independent of VSV or MV envelope glycoproteins may occur. To evaluate these two factors, we included VSVΔG* in our experiments. The infectivity titers of VSVΔG* on most cell lines were negligible, indicating that neither residual VSVΔG*-G in pseudotype virus stocks nor envelope protein-independent virus entry was significant in our system. VSVΔG* showed a high infectivity titer on C91/PL cells, which was presumably caused by the second factor described above. We hypothesize that VSVΔG* bears the HTLV-1 receptor molecule derived from 293T cells, which might facilitate the interaction of the pseudotype with HTLV-1 envelope proteins expressed on C91/PL cells, although we have not explored this further. We have previously reported that the H gene of wild-type MV strains induced cell fusion in B95a cells, but not in other CD46-positive human and monkey cell lines, when transfected together with the F gene (30). The pseudotype virus system used in this study has advantages over the transfection experiment, as it can quantitatively determine the entry of cell-free virus, rather than cell-cell fusion, in adherent cells as well as in cells in suspension.
However, our pseudotype assay may not perfectly reflect MV entry for the following reasons. First, the amount of MV envelope proteins on VSV pseudotype virus may be lower than that on MV virion, which will make the pseudotype bearing MV envelope proteins enter only highly susceptible cells. Second, VSV pseudotype viruses do not have quasispecies as regards envelope proteins because they are expressed from the plasmids. This may explain why rodent cells developed focal small syncytia when infected with the Edmonston strain at a high MOI, but VSVΔG*-EdHF showed no infectious titers on them.
Recently, Takeda et al. reported that changes in the polymerase and accessory proteins, not in envelope proteins, were responsible for the growth difference between a B95a-grown MV strain and its Vero-adapted strain (29). Johnston et al. generated a recombinant Edmonston MV expressing envelope proteins of the wild-type WTF strain and showed that the recombinant virus expressing the WTF H protein spread in Vero cells although the parental WTF virus did not (11). These studies indicate that cellular factors other than virus entry play an important role in determining MV cell tropism. How are these studies reconciled with our results? One interpretation is that although Vero cells do not have the authentic receptor for wild-type MV strains, they may still enter Vero cells with a low efficiency and thereafter replicate and produce CPE to a certain extent. The adaptation to the intracellular environment may increase such viral growth in Vero cells, thus affecting MV cell tropism. However, our results clearly showed that there exist large differences in the efficiencies of virus entry between susceptible and nonsusceptible cell lines, accounting for the major part of the tropism. Furthermore, Takeda et al. (29), reported that the Vero-adapted virus had a serine-to-glycine change at position 546 of the H protein, which may also allow this virus to efficiently infect Vero cells (22, 27). In the study by Johnston et al. (11), the WTF strain did not spread in Vero cells, but transfection of the WTF H protein gene and the Edmonston F gene caused cell fusion in Vero cells, suggesting that the WTF strain is able to fuse with Vero cells at a higher efficiency than the KA and Ichinose strains. Thus, even in these studies, virus entry seems to be a major determinant of MV cell tropism, although other cell-specific factors and/or other viral proteins (e.g., polymerase) apparently influence subsequent viral replication, contributing to tropism.
In summary, we newly identified several cell lines, including T-cell lines, that can support the efficient replication of wild-type MV strains. We successfully generated the VSV pseudotype expressing MV envelope proteins in the absence of the VSV G protein. Using this pseudotype virus system, we showed that the infectivity of a MV strain for the cell was perfectly correlated with the efficiency of entry into that cell by the VSV pseudotype bearing the corresponding MV H protein, indicating that virus entry is a major determinant of cell tropism of MV strains. We are now using this VSV pseudotype system to identify the molecule that enables wild-type MV strains to enter cells.
ACKNOWLEDGMENTS
We thank M. A. Whitt for allowing us to use the VSVΔG*-GFP system. We also thank F. Kobune, K. Takada, and Y. Murakami for providing the wild-type MV strains, BJAB and BJAB-B95-8 cell lines, and CHO-derived cell lines, respectively.
This work was supported by grants from the Ministry of Education, Science and Culture of Japan and from the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan.
REFERENCES
- 1.Bartz R, Brinckmann U, Dunster L M, Rima B, ter Meulen V, Schneider-Schaulies J. Mapping amino acids of the measles virus hemagglutinin responsible for receptor (CD46) downregulation. Virology. 1996;224:334–337. doi: 10.1006/viro.1996.0538. [DOI] [PubMed] [Google Scholar]
- 2.Bartz R, Firsching R, Rima B, ter Meulen V, Schneider-Schaulies J. Differential receptor usage by measles virus strains. J Gen Virol. 1998;79:1015–1025. doi: 10.1099/0022-1317-79-5-1015. [DOI] [PubMed] [Google Scholar]
- 3.Buckland R, Wild T F. Is CD46 the receptor for measles virus? Virus Res. 1997;48:1–9. doi: 10.1016/s0168-1702(96)01421-9. [DOI] [PubMed] [Google Scholar]
- 4.Dorig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 5.DuBridge R B, Tang P, Hsia H C, Leong P-M, Miller J H, Calos M P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enders J F, Peebles T C. Propagation in tissue cultures of cytopathic agents from patients with measles. Proc Soc Exp Biol Med. 1954;86:277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- 7.Fujinami R S, Oldstone M B A. Failure to cleave measles virus fusion protein in lymphoid cells: a possible mechanism for viral persistence in lymphocytes. J Exp Med. 1981;154:1489–1499. doi: 10.1084/jem.154.5.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1267–1312. [Google Scholar]
- 9.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P A, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwata K, Seya T, Ariga H, Nagasawa S. Expression of a hybrid complement regulatory protein, membrane cofactor protein decay accelerating factor on Chinese hamster ovary: comparison of its regulatory effect with those of decay accelerating factor and membrane cofactor protein. J Immunol. 1994;152:3436–3444. [PubMed] [Google Scholar]
- 11.Johnston I C D, ter Meulen V, Schneider-Schaulies J, Schneider-Schaulies S. A recombinant measles vaccine virus expressing wild-type glycoproteins: consequences for viral spread and cell tropism. J Virol. 1999;73:6903–6915. doi: 10.1128/jvi.73.8.6903-6915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobune F, Sakata H, Sugiura A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990;64:700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobune F, Takahashi H, Terao K, Ohkawa T, Ami Y, Suzaki Y, Nagata N, Sakata H, Yamanouchi K, Kai C. Nonhuman primate models of measles. Lab Anim Sci. 1996;46:315–320. [PubMed] [Google Scholar]
- 14.Lecouturier V, Fayolle J, Caballero M, Carabana J, Celma M L, Fernandez-Munoz R, Wild T F, Buckland R. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J Virol. 1996;70:4200–4204. doi: 10.1128/jvi.70.7.4200-4204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manchester M, Liszewski M K, Atkinson J P, Oldstone M B A. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 17.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naniche D, Wild T F, Rabourdin-Combe C, Gerlier D. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J Gen Virol. 1993;74:1073–1079. doi: 10.1099/0022-1317-74-6-1073. [DOI] [PubMed] [Google Scholar]
- 19.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants by a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 20.Popovic M, Sarin P S, Robert-Gurroff M, Kalyanaraman V S, Mann D, Minowada J, Gallo R C. Isolation and transmission of human retrovirus (human T-cell leukemia virus) Science. 1983;219:856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- 21.Richardson C D, Scheid A, Choppin P W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980;105:205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- 22.Rima B K, Earle J A P, Baczko K, ter Meulen V, Liebert U G, Carstens C, Carabana J, Caballero M, Celma M L, Fernandez-Munoz R. Sequence divergence of measles virus haemagglutinin during natural evolution and adaptation to cell culture. J Gen Virol. 1997;78:97–106. doi: 10.1099/0022-1317-78-1-97. [DOI] [PubMed] [Google Scholar]
- 23.Schmid, A., C. R., and M. A. Billeter. 1987. A procedure for selective full length cDNA cloning of specific RNA species. Nucleic Acids Res. 15:3987–3996. [DOI] [PMC free article] [PubMed]
- 24.Schneider-Schaulies J, Dunster L M, Kobune F, Rima B, ter Meulen V. Differential downregulation of CD46 by measles virus strains. J Virol. 1995;69:7257–7259. doi: 10.1128/jvi.69.11.7257-7259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider-Schaulies J, Schnorr J-J, Brinckmann U, Dunster L M, Baczko K, Liebert U G, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnell M J, Buonocore L, Kretzschmar E, Johnson E, Rose J K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci USA. 1996;93:11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibahara K, Hotta H, Katayama Y, Homma M. Increased binding activity of measles virus to monkey red blood cells after long-term passage in Vero cell cultures. J Gen Virol. 1994;75:3511–3516. doi: 10.1099/0022-1317-75-12-3511. [DOI] [PubMed] [Google Scholar]
- 28.Takada A, Robinson C, Goto H, Sanchez A, Murti K G, Whitt M A, Kawaoka Y. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci USA. 1997;94:14764–14769. doi: 10.1073/pnas.94.26.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda M, Kato A, Kobune F, Sakata H, Li Y, Shioda T, Sakai Y, Asakawa M, Nagai Y. Measles virus attenuation associated with transcriptional impediment and a few amino acid changes in the polymerase and accessory proteins. J Virol. 1998;72:8690–8696. doi: 10.1128/jvi.72.11.8690-8696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka K, Xie M, Yanagi Y. The hemagglutinin of recent measles virus isolates induces cell fusion in a marmoset cell line, but not in other CD46-positive human and monkey cell lines, when expressed together with the F protein. Arch Virol. 1998;143:213–225. doi: 10.1007/s007050050281. [DOI] [PubMed] [Google Scholar]
- 31.Yanagi Y, Cubitt B A, Oldstone M B A. Measles virus inhibits mitogen-induced T cell proliferation but does not directly perturb the T cell activation process inside the cell. Virology. 1992;187:280–289. doi: 10.1016/0042-6822(92)90316-h. [DOI] [PubMed] [Google Scholar]
- 32.Yanagi Y, Hu H-L, Seya T, Yoshikura H. Measles virus infects mouse fibroblast cell lines, but its multiplication is severely restricted in the absence of CD46. Arch Virol. 1994;138:39–53. doi: 10.1007/BF01310037. [DOI] [PubMed] [Google Scholar]