Abstract
MRL-1237, [1-(4-fluorophenyl)-2-(4-imino-1,4-dihydropyridin-1-yl) methylbenzimidazole hydrochloride], is a potent and selective inhibitor of the replication of enteroviruses. To reveal the target molecule of MRL-1237 in viral replication, we selected spontaneous MRL-1237-resistant poliovirus mutants. Of 15 MRL-1237-resistant mutants obtained, 14 were cross-resistant to guanidine hydrochloride (mrgr), while 1 was susceptible (mrgs). Sequence analysis of the 2C region revealed that the 14 mrgr mutants contained a single nucleotide substitution that altered an amino acid residue from Phe-164 to Tyr. The mrgs mutant, on the other hand, contained a substitution of Ile-120 to Val. Through the construction of a cDNA-derived mutant, we confirmed that the single mutation at Phe-164 was really responsible for the reduced susceptibility to MRL-1237. MRL-1237 inhibited poliovirus-specific RNA synthesis in HeLa cells infected with a wild strain but not with an F164Y mutant. We furthermore examined the effect of mutations of the 2C region on the drug sensitivity of cDNA-derived guanidine-resistant and -dependent mutants. Two guanidine-resistant mutants were cross-resistant to MRL-1237 but remained susceptible to another benzimidazole, enviroxime. Either MRL-1237 or guanidine stimulated the viral replication of two guanidine-dependent mutants, but enviroxime did not. These results indicate that MRL-1237, like guanidine, targets the 2C protein of poliovirus for its antiviral effect.
Poliovirus is a member of the family Picornaviridae, containing a positive-sense, single-stranded RNA as a viral genome. The genome encodes a single precursor polyprotein which is eventually cleaved to four structural and seven nonstructural proteins (58). The structural capsid proteins of picornaviruses are located at the amino-terminal P1 region of the polyprotein. The remainder contains viral nonstructural proteins, including two proteases (2Apro and 3Cpro), an RNA-dependent RNA polymerase (3Dpol), and several other proteins essential for viral RNA synthesis. In poliovirus-infected cells, nonstructural proteins 2B, 2C, 3A, 3B, and 3D and their precursors are associated with a specific structure of virus-induced cytoplasmic membranous vesicles, the so-called replication complex (13, 22), and implicated in viral RNA synthesis together with viral RNA and some cellular proteins (13, 14, 17, 22, 44, 61, 64, 65, 73).
The 2C protein of picornaviruses contains conserved nucleoside triphosphate (NTP)-binding and RNA helicase motifs in its middle region (19, 28–31). Genetically manipulated mutations in the NTP-binding motif of the poliovirus 2C protein abolished viral replication and RNA synthesis (47, 70). The replication of viruses with such mutations in the NTP-binding motif was poorly complementated in trans (71). These results indicate the functional significance of the NTP-binding motif in viral replication. A recombinant poliovirus 2C (or precursor 2BC) protein fused with maltose-binding protein (MBP), MBP-2C, was expressed in Escherichia coli and was demonstrated to have both ATPase and GTPase activities (57). The ATPase activity of partially purified recombinant 2C protein of poliovirus was also detected with a baculovirus expression system (46). However, no detectable RNA helicase activity of the 2C protein has been demonstrated. The purified MBP-2C could bind to a single-strand RNA (55–57), and purified histidine-tagged 2C specifically bound to 3′-terminal sequences of negative-strand viral RNA (8).
On the other hand, recombinant poliovirus 2C and 2BC proteins expressed in human cells induced membrane rearrangement and specific vesicle formation morphologically similar to those found in poliovirus-infected cells (2, 3, 9, 16). The amino-terminal domain of the 2C protein has been identified to associate with the membrane (20, 21). Furthermore, two poliovirus mutants containing a linker insertion in the 2C protein had temperature-sensitive defects in viral replication (42, 43). Revertant viruses from the temperature-sensitive mutant showed an uncoating defect. Recently, a study with a novel inhibitor of enteroviruses (hydantoin) revealed that the 2C coding region was likely to be responsible for viral encapsidation (74). Thus, the 2C protein of poliovirus is a multifunctional protein involved in viral RNA replication, but the entire activity of 2C in the poliovirus replication cycle remains poorly understood (77).
Guanidine hydrochloride has been known to specifically inhibit the function of the 2C protein of poliovirus, providing information to elucidate the role of 2C protein in viral replication. It selectively inhibits the growth of many picornaviruses at concentrations of 0.1 to 2.0 mM. The target of this blockage is thought to be the initiation step of viral RNA synthesis (7, 15, 54). Guanidine reversibly inhibited viral RNA synthesis in the HeLa S10 in vitro translation-replication system without affecting replication complex formation and viral polyprotein processing (10–12). Guanidine-resistant and -dependent picornavirus mutants were obtained after the cultivation of guanidine-susceptible virus in the presence of guanidine (24, 45). Peptide mapping and nucleotide sequencing of guanidine-resistant and -dependent mutants revealed mutations in the 2C region (4–6, 51–53, 60, 72). From these results, Tolskaya and coworkers concluded that the “hot spot” of amino acid substitutions of guanidine-resistant and -dependent mutants is the locus of the putative NTP-binding motif (72). They speculated that guanidine affected NTP binding and/or splitting via the conformational modification of the 2C protein. Recently, Pfister and Wimmer reported that ATPase activity of the recombinant 2C protein fused to glutathione S-transferase was inhibited by guanidine hydrochloride (50). The result was the first direct evidence for the inhibitory action of guanidine on 2C function(s).
In the 1960s, some benzimidazole derivatives, such as [2-(α-hydroxybenzyl)-benzimidazole (HBB) (see Fig. 1), were shown to have selective antipicornavirus activity (25, 26). To develop safer and more potent chemotherapeutic agents against picornaviruses, attempts to evaluate newly synthesized benzimidazole derivatives have been undertaken (1, 23, 25, 26, 37, 66–68, 76). Previous reports showed that HBB inhibited viral RNA synthesis in a manner similar to guanidine, but there was little cross-resistance between these two agents (7, 67). However, the mechanism of action of other benzimidazole derivatives, including HBB, on picornavirus replication is poorly understood.
FIG. 1.
Structure of MRL-1237, enviroxime, HBB, and guanidine hydrochloride.
We designed, synthesized, and evaluated a series of benzimidazole derivatives and found that one of them, MRL-1237 (see Fig. 1), had a most potent activity against many enteroviruses. Here, we demonstrate the determinants responsible for MRL-1237 sensitivity in the 2C protein of poliovirus. Furthermore, we constructed cDNA-derived guanidine-resistant and -dependent mutants and compared the phenotypes of the mutants against MRL-1237, guanidine, and HBB. We found that mutations located in the 2C protein could be responsible for altered sensitivity against MRL-1237, HBB, or guanidine.
MATERIALS AND METHODS
Antiviral agents.
MRL-1237, HBB, and enviroxime [2-amino-1-(isopropylsulfonyl)-6-benzimidazole phenyl ketone oxime] (see Fig. 1) were synthesized at Maruishi Pharmaceutical Co., Ltd. (Osaka, Japan). Guanidine hydrochloride was purchased from Sigma Chemical Co. (St. Louis, Mo.). MRL-1237 and guanidine hydrochloride were dissolved in sterile water and diluted in culture medium. Stock solutions of HBB (50 mg/ml) and enviroxime (5 mg/ml) were prepared in dimethyl sulfoxide and diluted with culture medium so that the final concentration of dimethyl sulfoxide did not exceed 0.2%.
Cells and viruses.
HeLa cells were maintained in Eagle minimum essential medium (MEM) containing 10% fetal bovine serum (FBS). The Mahoney strain of poliovirus type 1 was used as an MRL-1237-sensitive parental virus. For the antiviral assay, Sabin 1 (F113), Sabin 2 (207-3) and Sabin 3 (F313) strains of poliovirus were used. The Nancy strain of coxsackievirus B3 (CB3) and JVB strain of coxsackievirus group B4 (CB4) were kindly provided by K. Shinohara and A. Itagaki, respectively.
Selection of spontaneous MRL-1237-resistant mutants.
The parental Mahoney virus (100 PFU) was inoculated to a HeLa cell monolayer (12.5 cm2) in the presence of 50 μM MRL-1237. The monolayer was incubated at 35.5°C until the cultures exhibited extensive cytopathic effects (CPEs). After freeze-thawing and removal of the cell debris by centrifugation, 0.1 ml of each supernatant was used to infect fresh HeLa cells again in the presence of 50 μM MRL-1237. The culture supernatants were further applied to second-round plaque purifications in the presence of 50 μM MRL-1237. Ten independent plaque-purified mutants were isolated. Five mutants (MFR10-1 to MFR10-5) were also isolated in the presence of 10 μM MRL-1237 as described above without using 10 μM MRL-1237 for selection during viral passages and plaque purifications.
Determination of drug susceptibility.
HeLa cells (4 × 105 cells) were infected with the virus at a multiplicity of infection of 0.005. After adsorption for 1 h, the cell suspension (0.1 ml/well) was dispensed into 96-well microtiter plates containing twofold serial dilutions of an appropriate drug in triplicate and incubated at 35.5°C. Three days after infection, antiviral activity was measured by a cell protection assay with WST-1 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) (39). Briefly, 20 μl of WST-1 dye solution per well (6 mM WST-1 and 0.4 mM 1-methoxy PMS [Wako Chemicals] in phosphate-buffered saline) was added to the microtiter plates and incubated at 35.5°C for 3 h. The absorbance at 450 nm with a reference wavelength of 620 nm was measured by a microplate reader (Multiskan Bichromatic; Labsystems, Helsinki, Finland). The 50% effective concentration (EC50) of each compound was evaluated as the quantity of drug required to inhibit 50% of virus-induced cell killing. The cytotoxicity of the drug was determined as described above without inoculation of the virus and expressed as the 50% cytotoxic concentration (CC50), i.e., the concentration required to reduce the viability of uninfected cells by 50%. The effect of each drug on viral replication was also examined by the plaque reduction assay with or without the drug. Briefly, samples of a 10-fold serial dilution of the virus were inoculated to the HeLa cell monolayer in triplicate. After adsorption for 1 h, the cells were overlaid with MEM containing 2% FBS and 0.9% Noble agar in the presence or absence of the drug. The monolayers were incubated at 35.5°C for 72 h. The viable cells were subsequently stained with 1.0 ml of MEM containing 0.01% neutral red.
Sequencing of the 2C region.
To identify mutations in the 2C region, RNA sequences of the MRL-1237-resistant viruses were determined. Viruses were grown on the HeLa cell monolayer until exhibiting CPE. After removal of the cell debris by centrifugation, viral RNA was isolated from the culture supernatant by phenol-sodium dodecyl sulfate extraction. cDNA was prepared using Moloney murine leukemia virus reverse transcriptase (Perkin-Elmer Cetus, Norwalk, Conn.) and a 1,161-bp DNA fragment (position 4053 to 5213 for the Mahoney strain) containing the entire 2C region was amplified by PCR by using the antisense 2CR-1 primer (5′-CTC TCA CCT CCT GGG AGT CAA CTG C-3′) and the sense 2CF-1 primer (5′-ATG CTT CAC CAT GGC AGT GGC TTA-3′). The amplified DNA fragment was purified with the QIAquick PCR purification kit (Qiagen GmbH, Hilden, Germany) and sequenced by the dideoxy termination method using a 373A sequencer system (Applied Biosystems, Inc., Foster City, Mo.).
Construction of infectious mutant clones.
To confirm that amino acid substitutions in the 2C region were sufficient to confer altered drug sensitivity, the mutations, which had been identified in spontaneous mutants isolated in this study (or previously reported), were introduced into an infectious cDNA clone of poliovirus type 1. An infectious clone, pVMT7(1)pDS306(T) (38), derived from a type 1 Mahoney strain, was kindly provided by A. Nomoto.
A 3.1-kb NheI/BglII fragment (positions 2473 to 5607) of pVMT7(1)pDS306(T) was subcloned into the NheI/BglII site of a pKF3 vector (Takara Shuzo Co., Kyoto, Japan) to create a pKFpVM-NB clone. To generate a pVMT7(1)2C-F164Y clone, a 230-bp BamHI/NsiI fragment (bp 4,603 to 4,832) of pKFpVM-NB was replaced with a corresponding PCR-amplified DNA fragment derived from a MRL-1237-resistant MFR50-1 mutant.
To obtain pVMT7(1)2C-N179A and pVMT7(1)2C-N179G clones, a DNA fragment was PCR amplified from 5 ng of pVM1(T7)pDS306(T) as a template DNA by using the primers 5′-CCC CCG GAT CCA TCA CAC TTC GAC GGA TAC AAA CAA CAG GGA GTG GTG ATT ATG GAC GAC CTG G(G/T)T CAA AAC CCA GAT GG-3′ and 5′-GGC TAA TGC ATC ACT GTG TGC CAC AGT GGG-3′ (mutations are underlined, and G/T designates a mixture of two bases). The 246-bp amplified fragment was digested with BamHI and NsiI, and the 230-bp fragment was cloned into the BamHI/NsiI site of the pKFpVM-NB. Among mutated pKFpVM-NB clones, N179A (GCT, bp 4658 to 4660) and N179G (GGT, bp 4658 to 4660) were screened by sequencing. For pVMT7(1)2C-M187L/A233S and pVMT7(1)2C-M187L/A233T, a DNA fragment was amplified by using the primers 5′-CCC CCG GAT CCA TCA CAC TTC GAC GGA TAC AAA CAA CAG GGA GTG GTG ATT ATG GAC GAC CTG AAT CAA AAC CCA GAT GGT GCG GAC TTG AAG CTG TTC TGT-3′ and 5′-GGC TAA TGC ATC ACT GTG TG(A/T) CAC AGT GGG-3′. The amplified fragment was digested with BamHI and NsiI and cloned into pKFpVM-NB. Among mutated pKFpVM-NB clones, M187L/A233S (TTG, 4682 bp to 4684; CCA, 4820 bp to 4822) and M187L/A233T (TTG, bp 4682 to 4684; ACA, bp 4820 to 4822) were screened. A pVMT7(1)2C-I120V clone was constructed by the replacement of 647 bp of the AflII/BamHI fragment (bp 3956 to 4602) with the PCR-amplified DNA fragment derived from an MFR10-1 mutant.
The entire 2C region of each pKFpVM-NB mutant was sequenced to confirm the presence of the defined mutations and no additional mutation. Thereafter, a 3.1-kb NheI/BglII fragment of pVMT7(1)pDS306(T) was replaced with the corresponding fragments prepared from each pKFpVM-NB mutant clone.
In vitro transcription and RNA transfection.
Each mutant cDNA clone was linearized with AatI and transcribed in vitro with T7 polymerase (RiboMAX Large Scale RNA Production System; Promega, Madison, Wis.). The template DNA was digested with DNase I, and the transcribed RNA was purified by isopropanol precipitation. Purified RNAs (2 μg) were transfected into HeLa cells (5 × 105 cells) using Lipofectin (Gibco BRL Life Technologies, Gaithersburg, Md.) according to the manufacturer's instructions (27). The serum-free medium (Opti-MEM; Gibco BRL Life Technologies) was replaced with MEM at 12 h after transfection, and cells were subsequently maintained for 48 h. For the pVMT7(1)2C-M187L/A233S or pVMT7(1)2C-M187L/A233T clone, the transfectant was cultured in serum-free medium containing 2 mM of guanidine hydrochloride immediately after transfection and maintained in the presence of guanidine. After exhibiting CPE, the virus stock was obtained by freeze-thawing, and the virus titer of transfectants was determined by the plaque assay. The resultant viruses prepared from cells transfected with pVMT7(1)pDS306(T), pVMT7(1)2C-F164Y, pVMT7(1)2C-I120V, pVMT7(1)2C-N179A, pVMT7(1)2C-N179G, pVMT7(1)2C-M187L/A233S, and pVMT7(1)2C-M187L/A233T were designated PV1(M), PV1(M)2C-F164Y, PV1(M)2C-I120V, PV1(M)2C-N179A, PV1(M)2C-N179G, PV1(M)2C-M187L/A233S, and PV1(M)2C-M187L/A233T, respectively.
Inhibition of poliovirus-specific RNA synthesis.
To examine the effect of the drug on viral RNA synthesis in poliovirus-infected cells, we used the Northern ELISA kit (Boehringer GmbH, Mannheim, Germany) for the detection and semiquantification of viral specific RNA, with minor modifications.
For the assay, a digoxigenin (DIG)-labeled poliovirus-specific DNA probe was prepared by PCR. The 1,161-bp DNA fragment was amplified with 2CR-1 and 2CF-1 primers as described above without using a PCR DIG-labeling mixture (which includes DIG-11-UTP [Boehringer GmbH] instead of the dioxynucleoside triphosphate mixture. Samples (multiplicity of infection = 5) of wild-type and mutant viruses were inoculated to the HeLa cell monolayer (12.5 cm2). After adsorption for 1 h, MEM (with 2% FBS) with or without appropriate concentrations of drug was added (1 ml/well), and subsequently the mixture was incubated at 35.5°C for 5 h. The cells were harvested by trypsinization, and total RNA was extracted with guanidium thiocyanate by the standard procedure (59). After RNA quantification, the extracted RNA was biotin labeled according to the recommendations included with the Northern ELISA kit. The biotin-labeled RNA (100 ng) was quantified, diluted, and hybridized with a heat-denatured DIG-labeled poliovirus-specific DNA probe (100 ng) in 130 μl of hybridization mixture for 3 h at 50°C. The DNA-RNA hybrid (100 μl) was transferred to a streptavidin-coated microtiter plate and incubated for 5 min at 50°C. After each well was washed, DIG-labeled DNA probe, hybridized to biotin-labeled RNA immobilized onto the plate, was reacted with a horseradish peroxidase-labeled anti-DIG antibody. After washing each well, 100 μl of peroxidase substrate (3,3′,5,5′-tetramethylbenzidine) solution per well was added and incubated for 15 min at room temperature. Then, absorbance at 450 nm with a reference wavelength of 620 nm was measured by a Multiskan Bichromatic microtiter plate reader. Viral RNA production was evaluated as the mean of absorbance values from three independently prepared biotin-labeled RNA samples. Total RNA extracted from mock-infected HeLa cells was similarly biotinylated and hybridized with a DIG-labeled poliovirus-specific DNA probe as a negative control.
RESULTS
Antiviral activity of MRL-1237.
The structures of MRL-1237, HBB, enviroxime, and guanidine hydrochloride are presented in Fig. 1. The activity of MRL-1237 against prototype human enteroviruses was compared with that of other anti-picornaviral compounds. As shown in Table 1, MRL-1237 inhibited the replication of all enteroviruses tested, at concentrations ranging from 10 to 200 μM, without exhibiting significant cytotoxicity. Coxsackie B viruses were most sensitive to MRL-1237, and the selectivity indexes were nearly 1,000. MRL-1237 was consistently more effective against enteroviruses than both guanidine hydrochloride and HBB. Among polioviruses, a type 2 strain was most sensitive, and type 1 strains were least sensitive to HBB, as previously described (26, 66). Such an antiviral profile of HBB was similar to that of MRL-1237. Enviroxime exhibited potent and broad-spectrum activity against the enteroviruses tested (18, 76). The selectivity index value of enviroxime against the polioviruses was more than 10 times higher than that of MRL-1237.
TABLE 1.
Antiviral activity of MRL-1237, guanidine hydrochloride, HBB, and enviroxime against enteroviruses
| Virus strain | Antiviral activity (μM)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MRL-1237
|
Guanidine hydrochloride
|
HBB
|
Enviroxime
|
|||||
| EC50 | SI | EC50 | SI | EC50 | SI | EC50 | SI | |
| Mahoney | 11 | 51 | 720 | 24 | >200 | <1.9 | 0.30 | 170 |
| Sabin 1 | 9.5 | 59 | 340 | 50 | >200 | <1.9 | 0.31 | 160 |
| Sabin 2 | 2.9 | 190 | 320 | 53 | 26 | 15 | 0.11 | 450 |
| Sabin 3 | 7.0 | 80 | 390 | 44 | 170 | 2.2 | 0.17 | 290 |
| CB3 Nancy | 0.58 | 970 | 2,200 | 7.7 | 70 | 5.4 | 0.26 | 190 |
| CB4 JVB | 0.55 | 1,000 | 2,100 | 8.1 | 66 | 5.8 | 0.14 | 360 |
CC50 values (μM) were as follows: MRL-1237, 560; guanidine hydrochloride, 17,000; HBB, 380; and enviroxime, 50. SI, selective index, measured as the ratio CC50:EC50.
Isolation and characterization of spontaneous MRL-1237-resistant mutants.
After two passages and subsequent second-round plaque purifications in the presence of MRL-1237, 15 independent MRL-1237-resistant mutants were cloned. The MRL-1237-resistant mutants could be grouped into two categories (Table 2). Of the 15 mutants, 14 were highly resistant to MRL-1237 and cross-resistant to guanidine hydrochloride (mrgr). The EC50 values of MRL-1237 against the mrgr mutants were approximately 40 times higher than those against the parental Mahoney strain (Table 2). On the other hand, the MFR10-1 mutant was only twice as resistant to MRL-1237 and remained susceptible to guanidine (mrgs). Due to the cross-resistance of mrgr variants to guanidine hydrochloride, we determined the nucleotide sequence of the entire 2C region of these mutants. Of the 15 mutants, all of them contained a single nucleotide substitution that altered one amino acid in the 2C protein (Table 2). Fourteen mrgr mutants contained the same substitution, Phe-164 to Tyr (UUC to UAC; the substitution is underlined). Another mutant, MFR 10-1, contained an amino acid substitution of Ile-120 to Val (AUU to GUU).
TABLE 2.
Drug susceptibility of spontaneous and cDNA-derived mutant viruses
| Virus strain or mutant | Origin | Mutation(s) located in 2C
|
EC50 (mM)
|
|||
|---|---|---|---|---|---|---|
| RNA or cDNA | Amino acid | MRL-1237 | Guanidine | Enviroxime | ||
| Mahoney | Wild type | —a | — | 11 | 720 | 0.30 |
| mrgr mutants | Spontaneous | UUC→UAC | Phe-164→Tyr | >400 | 3,300 ± 100b | NDc |
| MFR10-1 | Spontaneous | AUU→GUU | Ile-120→Val | 26 | 750 | ND |
| PV1(M) | cDNA derived | — | — | 9.5 | 600 | 0.34 |
| PV1(M)2C-F164Y | cDNA derived | TTC→TAC | Phe-164→Tyr | >400 | 3,400 | 0.26 |
| PV1(M)2C-I120V | cDNA derived | ATT→GTT | Ile-120→Val | 17 | 700 | 0.25 |
| PV1(M)2C-N179A | cDNA derived | AAT→GCT | Asn-179→Ala | 160 | >8,000 | 0.26 |
| PV1(M)2C-N179G | cDNA derived | AAT→GGT | Asn-179→Gly | >400 | >8,000 | 0.33 |
—, parental virus.
Mean EC50 value of 15 mutants ± standard error.
ND, not determined.
Construction and characterization of infectious mutant clones.
To confirm that the mutations of 2C were directly responsible for resistance to MRL-1237, we introduced nucleotide substitutions into an infectious cDNA clone derived from the Mahoney strain of poliovirus type 1. In addition, we constructed other infectious mutant clones, which were expected to have guanidine-resistant and -dependent phenotypes. The mutants with a single amino acid substitution at Asn-179 of 2C have been demonstrated to exhibit a guanidine-resistant phenotype (5, 51–53, 72). The amino acid substitution of Ala-233, together with the Met-187–to–Leu substitution, was reported to generate a guanidine-dependent or -resistant phenotype (5, 72). According to these reports, we constructed four more cDNA-derived mutants containing the substitutions Asn-179–to–Ala, Asn-179–to–Gly, Met-187–to–Leu/Ala-233–to–Ser (double mutation), and Met-187–to–Leu/Ala-233–to–Thr (double mutation).
After transfection of each transcribed RNA into HeLa cells, viruses derived from pVMT7(1)pDS306(T), pVMT7(1)2C-F164Y, pVMT7(1)2C-I120V, pVMT7(1)2C-N179A, and pVMT7(1)2C-N179G could be recovered in the absence of drug. Sixty hours after transfection of transcribed RNA derived from pVMT7(1)2C-M187L/A233S or pVMT7(1)2C-M187L/A233T, no virus was detected when the transfectant was cultured in drug-free medium. Two guanidine-dependent viruses could be recovered when the HeLa cells were maintained in the presence of 2 mM guanidine hydrochloride after transfection.
We examined the drug susceptibility of cDNA-derived mutants to MRL-1237, guanidine hydrochloride, and enviroxime. We were unable to compare the EC50 values of the mutants for HBB, because HBB originally showed little effectiveness even against the wild poliovirus type 1 strains (Table 1). As shown in Table 2, the EC50 value of PV1(M)2C-F164Y for MRL-1237 was more than 40-fold higher than that of the parental PV1(M) virus. The F164Y mutant was cross-resistant to guanidine hydrochloride but remained susceptible to enviroxime. The EC50 value of PV1(M)2C-I120V to MRL-1237 was slightly increased compared with that of the parental virus, and the mutant remained susceptible to guanidine hydrochloride and to enviroxime. The EC50 values of both PV1(M)2C-N179A and PV1(M)2C-N179G for guanidine hydrochloride were more than 10 times higher than that of the parental virus, and the mutants were cross-resistant to MRL-1237 but not to enviroxime.
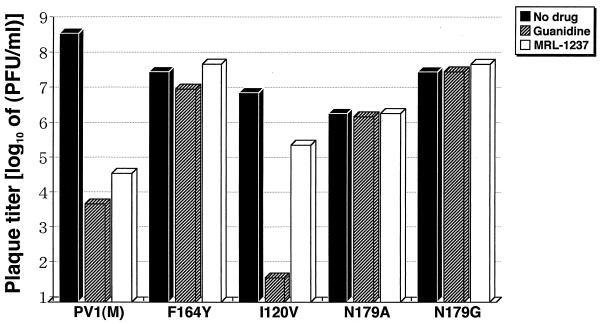
MRL-1237 at a 50 μM concentration reduced the plaque titer of PV1(M) virus more than 4.0 log10-fold compared with that of the untreated control (Fig. 2). On the other hand, PV1(M)2C-F164Y virus produced almost the same number of plaques in the presence of MRL-1237 as did the controls without the compounds. PV1(M)2C-F164Y was relatively tolerant of 2 mM guanidine compared with PV1(M). The results indicate that amino acid residue 164 within the 2C protein is a major determinant for reduced sensitivity to MRL-1237 and also contributes to the generation of a guanidine-resistant phenotype. To rule out the possibility of mutants emerging with additional mutations during the plaque assay, we have determined sequences in the 2C region of several mutants picked up from the plaques. No additional mutations were found (data not shown).
FIG. 2.
Drug susceptibility of cDNA-derived MRL-1237- and guanidine-resistant mutants. The effect of 2C mutations on drug susceptibility was examined with MRL-1237 and guanidine hydrochloride by plaque-reduction assay. A wild-type [PV1(M)] and drug-resistant [PV1(M)2C-F164Y, PV1(M)2C-I120V, PV1(M)2C-N179A, or PV1(M)2C-N179G] mutants derived from cDNA were inoculated to HeLa cells. After adsorption, HeLa cell monolayers were incubated for 72 h without drug (solid bars), with 2 mM guanidine (striped bars), or with 50 μM of MRL-1237 (open bars).
As shown in Fig. 2, PV1(M)2C-I120V virus was reduced in titer by 1.5 log10 PFU/ml in the presence of 50 μM MRL-1237. The amount of the plaque reduction of I120V was less than that of PV1(M). However, the plaque size of I120V treated with MRL-1237 was reduced from that observed in the absence of drug (data not shown). In general, the drug-resistant phenotypes of cDNA-derived mutants were identical to those of the corresponding spontaneous mutants.
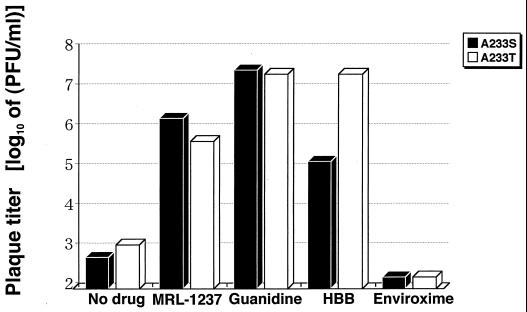
We next examined drug-dependent phenotypes of guanidine-dependent, site-directed mutants, PV1(M)2C-M187L/A233S and PV1(M)2C-M187L/A233T, in the presence or absence of drug (Fig. 3). The plaque titers of both M187L/A233S and M187L/A233T were increased by more than 4.0 log10-fold in the presence of 2 mM guanidine hydrochloride compared with those of the untreated controls. The incubation of M187L/A233T resulted in a 4.3 log10-fold-higher titer in the presence of 200 μM HBB than in its absence. The M187L/A233S virus was less sensitive to HBB than M187L/A233T at the 200 μM concentration. The guanidine-dependent mutants (M187L/A233S and M187L/A233T) displayed more than 2.6 log10-fold-higher titers in the presence of 50 μM MRL-1237 than in its absence. However, the stimulative effect of MRL-1237 was less than that found for the guanidine-dependent mutants treated with guanidine hydrochloride or HBB (Fig. 3), and the sizes of plaques induced by MRL-1237 were significantly smaller than those induced by guanidine hydrochloride or HBB (data not shown). On the other hand, no stimulative activity of enviroxime for viral replication against both guanidine-dependent mutants was observed at a 30 μM concentration (Fig. 3). We could not examine the effect of enviroxime at more than 30 μM due to cytotoxicity. The guanidine-dependent mutants exhibited no drug-dependent phenotypes for enviroxime at concentrations ranging from 0.1 to 30 μM (data not shown).
FIG. 3.
Effect of guanidine hydrochloride and benzimidazoles on the replication of cDNA-derived guanidine-dependent mutants. Drug-dependent phenotypes of PV1(M)2C-M187L/A233S (solid bars) and PV1(M)2C-M187L/A233T (open bars) were examined in the plaque assay. After viral adsorption, the cell monolayers were incubated for 72 h in the presence or absence of the drug. Final concentrations of MRL-1237, guanidine hydrochloride, HBB, and enviroxime were 50, 2,000, 200, and 30 μM, respectively.
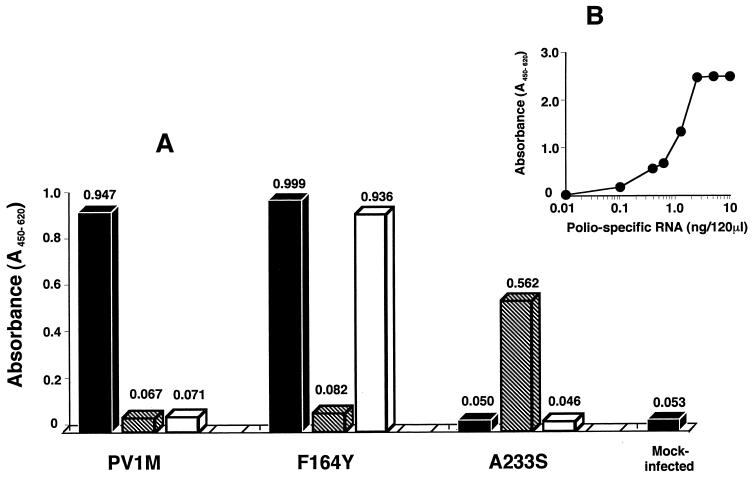
Inhibition of poliovirus-specific RNA synthesis.
To determine the effect of MRL-1237 and guanidine on the viral RNA synthesis of wild [PV1(M)], guanidine-resistant [PV1(M)2C-F164Y], and guanidine-dependent [PV1(M)2C-M187L/A233S] viruses in infected cells, we used the Northern ELISA. Optical density at 450 nm (OD450) ranging from 0.1 to 2.0 could be quantitatively determined with dilutions of a positive control poliovirus RNA (Fig. 4B).
FIG. 4.
Effect of MRL-1237 and guanidine on poliovirus-specific RNA synthesis. Poliovirus-specific RNA synthesis in virus-infected HeLa cells was determined by the Northern ELISA assay described in Materials and Methods. (A) The HeLa cell monolayers were inoculated with a wild-type PV1(M) virus, with an MRL-1237-resistant PV1(M)2C-F164Y virus, or with a guanidine-dependent PV1(M)2C-M187L/A233S virus. The infected cells were incubated in MEM without drug (solid bar), with 2 mM guanidine (striped bar), or with 50 μM MRL-1237 (open bar) for 5 h. The extracted RNAs were biotinylated, and 100 ng of biotinylated RNA per 130 μl was hybridized with 100 ng of a DIG-labeled poliovirus-specific DNA probe. Finally, the biotinylated poliovirus-specific RNA was visualized by the peroxidase-tetramethylbenzidine system. The viral RNA production is presented as the mean OD450–620 (the absorbance at 450 nm with a reference wavelength of 620 nm) values of three individual RNA samples. Total RNA from mock-infected cells was also prepared as a negative control. (B) Poliovirus RNA was transcribed from a pVMT7(1)pDS306(T) clone and biotinylated as a positive control. The OD450–620 values were determined with dilutions of the biotinylated poliovirus RNA.
As shown in Fig. 4A, MRL-1237 at 50 μM inhibited the production of viral RNA at least 20-fold in PV1(M)-infected cells. In contrast, MRL-1237 did not inhibit viral RNA synthesis in cells infected with a PV1(M)2C-F164Y virus. Guanidine reduced viral RNA synthesis in PV1(M)-infected cells and remained to inhibit viral RNA synthesis in F164Y-infected cells at the concentration of 2 mM. Guanidine stimulated viral RNA synthesis of a guanidine-dependent mutant, PV1(M)2C-M187L/A233S, but MRL-1237 exhibited little, if any, effect in this assay system. The stimulative effect of MRL-1237 on viral RNA synthesis of the M187/A233 mutant was significantly less than that of guanidine, like the effect on virus production in the plaque assay shown in Fig. 3.
DISCUSSION
To elucidate the role of picornavirus proteins in viral replication, specific inhibitors of the proteins and their resistant and dependent mutants have been studied (32–36, 40, 41, 48, 49, 62). In this study, we demonstrated that a novel antienteroviral agent, MRL-1237, targeted the nonstructural protein, 2C, of poliovirus, and a particular amino acid substitution (Phe-164–to–Tyr) within the protein was directly responsible for altered sensitivity to MRL-1237. We used the Mahoney strain of poliovirus type 1 for the analysis of MRL-1237-resistant mutants because almost all genetic studies of guanidine-resistant and -dependent mutants have used this strain (5, 6, 51–53, 72).
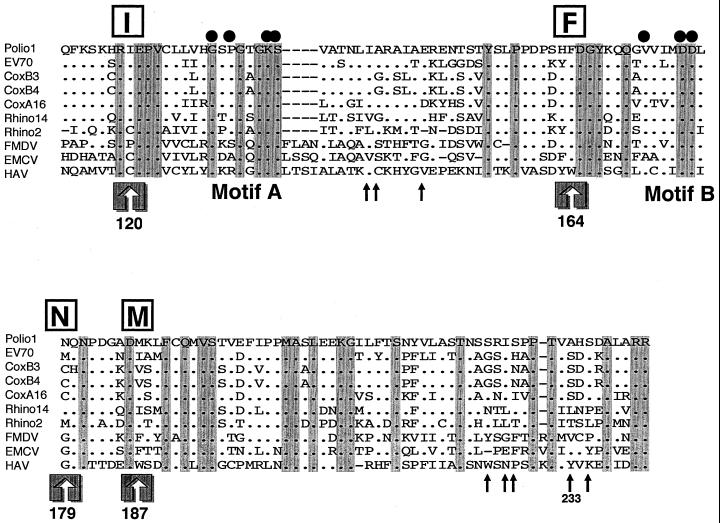
Previous reports indicated that poliovirus mutants with a guanidine-resistant or -dependent phenotype usually contained one or more nucleotide substitutions within 2C. As summarized in detail by Tolskaya and coworkers (72), guanidine mutants could be categorized into two major groups. One mutant group, class N, contained an amino acid substitution at Asn-179 to Ala or Gly. The mutation at Asn-179 to Ala or Gly needs two nucleotide changes, from AAU to GCU, or to GGU, respectively (substitutions are underlined). Another group, class M, had an amino acid change at Met-187 to Leu (from AUG to UUG) usually coupled with at least one additional mutation. The difficulty in obtaining guanidine-resistant or -dependent mutants with a single nucleotide substitution suggests that a point mutation is insufficient to alter the phenotype. Alternatively, such a single nucleotide change may be lethal to the growth of the virus (6, 72).
In contrast to a number of typical class N and class M mutants, only two nontypical guanidine-resistant mutants have been isolated (5, 51). One of them, gr1, had an amino acid change at Phe-164 to Tyr (5). The gr1 virus was resistant to 1 mM guanidine at 35°C but did not grow well in the absence of guanidine at 41°C. Interestingly, the gr1 mutation is identical to that of the major MRL-1237-resistant mutants (mrgr) isolated in this study. Among spontaneous MRL-1237-resistant mutants, most were of the gr1 type (Table 2). The spontaneous mrgr mutants and a corresponding cDNA-derived mutant [PV1(M)2C-F164Y] were markedly resistant to MRL-1237 and cross-resistant to guanidine. In addition, we have followed up the guanidine-dependent trait of F164Y at 41°C (data not shown) as previously observed with gr1 (69). We easily isolated mrgr mutants in the presence of 50 μM MRL-1237. This is reasonable because only one nucleotide change (UUC to UAC) was sufficient to confer the MRL-1237-resistant phenotype. For guanidine mutants, however, it is difficult to answer why most of the guanidine mutants needed multiple mutations to confer the phenotype.
As shown in Fig. 5, a major determinant for MRL-1237 resistance, a Phe-164 residue, is located between the so-called Walker A motif (GXXXXGKS/T) and Walker B motif (DD/E) found in putative ATP- and GTP-utilizing enzymes (75). The motifs are highly conserved among picornaviruses (GXXGXGKS and DD) and are thought to play a significant role in viral replication because the introduction of defined mutations into the motifs was lethal (Fig. 5) (47, 70). Crystal structures of DNA and RNA helicases (63, 78) suggest that the conserved motifs of the proteins share a catalytic pocket for NTP-binding and/or NTPase activity. The Walker A motif is responsible for binding the triphosphate tail of NTP, and the B motif is involved in the binding of Mg2+, which is required for NTP hydrolysis. The mutations conferring altered MRL-1237 or guanidine susceptibility were located in close proximity to the loci of NTP-binding motifs. In the case of guanidine mutants, the major determinants of the phenotypes, Asn-179 and Met-187, were hypervariable residues among picornaviruses (Fig. 5). On the other hand, an FDGY motif containing the Phe-164 residue is highly conserved among picornaviruses (WDGY is conserved only in hepatitis A virus). This suggests a significant role for the FDGY motif in viral RNA replication of picornaviruses. In fact, we have confirmed that the replacement of Phe-164 by Ala abolished viral replication of poliovirus by site-directed mutagenesis (data not shown). The FDGY motif is buried in a polar amino acid cluster (XXFDGYXXX, polar amino acids are underlined and Gly has an intermediate property), but Phe-164 is the only nonpolar amino acid in the cluster. It may be important to the MRL-1237-resistant phenotype for the mutation to introduce a hydroxyl moiety into the phenyl ring of phenylalanine, increasing the hydrophilicity of the cluster.
FIG. 5.
Amino acid alignment of the NTP-binding motif of the 2C proteins of picornaviruses. The sequence data were obtained from the GenBank sequence database. The names of virus species are abbreviated as follows and the GenBank accession number is shown in parentheses: polio1, Mahoney strain of poliovirus type 1 (J02281); EV70, J670/71 strain of enterovirus 70 (D00820); coxB3, Nancy strain of coxsackievirus B3 (M16560); coxB4, JVB strain of coxsackievirus B4 (X05690); coxA16, G-10 strain of coxsackievirus A16 (V05876); Rhino14, 1059 strain of human rhinovirus type 14 (K02121); Rhino2, HGP strain of human rhinovirus type 14 (X00429); FMDV, Argentine/61 strain of foot-and-mouth disease virus A10 (K02990); EMCV, R strain of encephalomyocarditis virus (M81861); HAV, LA strain of human hepatitis A virus (K02990). Amino acids are numbered from the amino terminus of the 2C protein of poliovirus type 1. The consensus A and B motifs of the NTP-binding proteins are indicated. Solid circles indicate amino acids critical for the viral replication of poliovirus, as previously demonstrated by site-directed mutagenesis (47, 70). Highly conserved amino acid residues among 10 picornavirus strains are shaded in gray. Major phenotypic determinants for drug resistance or dependence (Ile-120, Phe-164, Asn-179, and Met-187) are indicated as I, F, N, and M (boxed) and by large arrows. The minor determinants responsible for guanidine phenotypes are indicated by small arrows (72).
Site-directed mutagenesis confirmed that the single amino acid substitution of Val for Ile-120 could confer the MRL-1237-resistant phenotype. However, the effect of an Ile-120–to–Val mutation on MRL-1237 susceptibility was relatively moderate. Ile-120 is located in the vicinity of the consensus A motif (Fig. 5). The I120V mutant remained susceptible to guanidine. No guanidine mutants with the mutation at Ile-120 have been described. The results indicate that Ile-120 is a specific determinant responsible only for the intermediate MRL-1237-resistant phenotype.
The cDNA-derived class N guanidine-resistant mutants, N179A and N179G, were cross-resistant to MRL-1237; likewise, an MRL-1237-resistant mutant, F164Y, was cross-resistant to guanidine. Moreover, class M mutants of poliovirus, M187L/A233S and M187L/A233T, exhibited both guanidine- and MRL-1237-dependent phenotypes. The reciprocal cross-resistant phenotype for guanidine and MRL-1237 strongly suggests that the antiviral mechanism of action of MRL-1237 resemblances that of guanidine, interfering in the function of the 2C protein. In contrast with virus replication, such cross-resistant and -dependent phenotypes were not apparent in the RNA synthesis assay (Fig. 4). The discrepancy between virus replication and RNA synthesis may be due to the sensitivity of each assay system. The RNA synthesis of the F164Y mutant was inhibited but detectable even in the presence of 2 mM guanidine, but that of the wild-type virus was completely suppressed by guanidine (Fig. 4).
It is noteworthy that another benzimidazole derivative, HBB, also supported the full viral replication of two guanidine-dependent mutants. HBB is known to be one of the prototype antipicornavirus benzimidazoles, and its antiviral activity has been well characterized (7, 22–26, 68). Recently, the HBB-dependent phenotype of echovirus type 9 mutants mapped to the 2C protein (32). In this study, dependence not only on guanidine but also on MRL-1237 and HBB was found in two guanidine-dependent mutants (M187L/A233S and M187L/A233T) (Fig. 4). These results clearly demonstrate that the nonstructural protein 2C is a target molecule of HBB, like guanidine and MRL-1237. In contrast to MRL-1237 and HBB, enviroxime did not alter the drug susceptibility of the 2C mutants of poliovirus. Heinz and Vance isolated enviroxime-resistant mutants of poliovirus and human rhinovirus and found that mutations conferring enviroxime-resistant phenotypes were located within the 3A coding region (35, 36). As shown in Fig. 1, MRL-1237, HBB, and enviroxime contain the same benzimidazole nucleus. For enviroxime, all three substitutions in positions 1, 2, and 6 of the benzimidazole nucleus were believed to be essential for its antiviral activity (76). On the other hand, neither MRL-1237 nor HBB contains a substitution at position 6 in the nucleus. Thus, enviroxime is likely to possess different characteristics from other benzimidazole derivatives, as discussed previously (76).
In summary, MRL-1237 is a potent inhibitor of poliovirus replication targeting 2C protein. Elucidation of the exact mechanism of action of MRL-1237 will provide important information on the function of the 2C protein in viral replication.
ACKNOWLEDGMENTS
We thank Harumi Chiba and Akio Nomoto for providing a cDNA clone of poliovirus, Katsuaki Shinohara for providing coxsackievirus B3, and Asao Itagaki for providing coxsackievirus B4. We thank Shigeru Morikawa and Yoshiharu Matsuura for critical reading of the manuscript and helpful discussion. We also thank Junko Wada for technical assistance.
This work was supported in part by Grants-in-Aid from the Ministry of Health and Welfare and the Ministry of Education, Science, and Culture, Japan.
REFERENCES
- 1.Akihama S, Okude M, Sato K, Iwabuchi S. Inhibitory effect of 1,2-bis(2-benzimidazolyl) 1,2-ethanediol derivatives on poliovirus. Nature. 1968;217:562–563. doi: 10.1038/217562a0. [DOI] [PubMed] [Google Scholar]
- 2.Aldabe R, Barco A, Carrasco L. Membrane permeabilization by poliovirus proteins 2B and 2BC. J Biol Chem. 1996;271:23134–23137. doi: 10.1074/jbc.271.38.23134. [DOI] [PubMed] [Google Scholar]
- 3.Aldabe R, Carrasco L. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem Biophys Res Commun. 1995;206:64–76. doi: 10.1006/bbrc.1995.1010. [DOI] [PubMed] [Google Scholar]
- 4.Anderson-Sillman K, Bartal S, Tershak D R. Guanidine-resistant poliovirus mutants produce modified 37-kilodalton proteins. J Virol. 1984;50:922–928. doi: 10.1128/jvi.50.3.922-928.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltera R F, Jr, Tershak D R. Guanidine-resistant mutants of poliovirus have distinct mutations in peptide 2C. J Virol. 1989;63:4441–4444. doi: 10.1128/jvi.63.10.4441-4444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baltera R F, Jr, Tershak D R. Role of amino acid residue 187 of poliovirus polypeptide 2C in determining the guanidine trait. Intervirology. 1992;33:165–172. doi: 10.1159/000150246. [DOI] [PubMed] [Google Scholar]
- 7.Baltimore D, Eggers H J, Franklin R M, Tamm I. Poliovirus-induced RNA polymerase and the effects of virus-specific inhibitors on its production. Proc Natl Acad Sci USA. 1963;49:843–849. doi: 10.1073/pnas.49.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee R, Echeverri A, Dasgupta A. Poliovirus-encoded 2C polypeptide specifically binds to the 3′-terminal sequences of viral negative-strand RNA. J Virol. 1997;71:9570–9578. doi: 10.1128/jvi.71.12.9570-9578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barco A, Carrasco L. A human virus protein, poliovirus protein 2BC, induces membrane proliferation and blocks the exocytic pathway in the yeast Saccharomyces cerevisiae. EMBO J. 1995;14:3349–3364. doi: 10.1002/j.1460-2075.1995.tb07341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton D J, Flanegan J B. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol. 1993;67:822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barton D J, Black E P, Flanegan J B. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J Virol. 1995;69:5516–5527. doi: 10.1128/jvi.69.9.5516-5527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton D J, Flanegan J B. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J Virol. 1997;71:8482–8489. doi: 10.1128/jvi.71.11.8482-8489.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bienz K, Egger D, Pfister T, Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66:2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bienz K, Egger D, Troxler M, Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990;64:1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caliguiri L A, Tamm I. Action of guanidine on the replication of poliovirus RNA. Virology. 1968;35:408–417. doi: 10.1016/0042-6822(68)90219-5. [DOI] [PubMed] [Google Scholar]
- 16.Cho M W, Teterina N, Egger D, Bienz K, Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology. 1994;202:129–145. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- 17.Cuconati A, Xiang W, Lahser F, Pfister T, Wimmer E. A protein linkage map of the P2 nonstructural proteins of poliovirus. J Virol. 1998;72:1297–1307. doi: 10.1128/jvi.72.2.1297-1307.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLong D C, Reed S E. Inhibition of rhinovirus replication in organ culture by a potential antiviral drug. J Infect Dis. 1980;141:87–91. doi: 10.1093/infdis/141.1.87. [DOI] [PubMed] [Google Scholar]
- 19.Dever T E, Glynias M J, Merrick W C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci USA. 1987;84:1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Echeverri A C, Dasgupta A. Amino terminal regions of poliovirus 2C protein mediate membrane binding. Virology. 1995;208:540–553. doi: 10.1006/viro.1995.1185. [DOI] [PubMed] [Google Scholar]
- 21.Echeverri A C, Banerjee R, Dasgupta A. Amino-terminal region of poliovirus 2C protein is sufficient for membrane binding. Virus Res. 1998;54:217–223. doi: 10.1016/s0168-1702(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 22.Egger D, Pasamontes L, Bolten R, Boyko V, Beinz K. Reversible dissociation of the poliovirus replication complex: functions and interactions of its components in viral RNA synthesis. J Virol. 1996;70:8675–8683. doi: 10.1128/jvi.70.12.8675-8683.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggers H J. Successful treatment of enterovirus-infected mice by 2-(α-hydroxybenzyl)-benzimidazole and guanidine. J Exp Med. 1976;143:1367–1381. doi: 10.1084/jem.143.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggers H J, Reich E, Tamm I. The drug-requiring phase in the growth of drug-dependent enteroviruses. Proc Natl Acad Sci USA. 1963;50:183–190. doi: 10.1073/pnas.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggers H J, Tamm I. On the mechanism of selective inhibition of enterovirus multiplication by 2-(α-hydroxybenzyl)-benzimidazole. Virology. 1962;18:426–438. [Google Scholar]
- 26.Eggers H J, Tamm I. Spectrum and characteristics of the virus inhibitory action of 2-(α-hydroxybenzyl)-benzimidazole. J Exp Med. 1961;113:657–682. doi: 10.1084/jem.113.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felgner P L, Gadek T R, Holm M, Chan H W, Wenz M, Northrop J P, Ringolg G M, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorbalenya A E, Blinov V M, Donchenko A P, Koonin E V. An NTP-binding motif is the most conserved sequence in a highly diverged monophyletic group of proteins involved in positive strand RNA viral replication. J Mol Evol. 1989;28:256–268. doi: 10.1007/BF02102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. A conserved NTP-motif in putative helicases. Nature. 1988;333:22. doi: 10.1038/333022a0. [DOI] [PubMed] [Google Scholar]
- 30.Gorbalenya A E, Koonin E V. Viral proteins containing the purine NTP-binding sequence pattern. Nucleic Acids Res. 1989;17:8413–8440. doi: 10.1093/nar/17.21.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorbalenya A E, Koonin E V, Wolf Y I. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 1990;262:145–148. doi: 10.1016/0014-5793(90)80175-i. [DOI] [PubMed] [Google Scholar]
- 32.Hadaschik D, Klein M, Zimmermann H, Eggers H J, Nelsen-Salz B. Dependence of echovirus 9 on the enterovirus RNA replication inhibitor 2-(α-hydroxybenzyl)-benzimidazole maps to nonstructural protein 2C. J Virol. 1999;73:10536–10539. doi: 10.1128/jvi.73.12.10536-10539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadfield A T, Oliveira M A, Kim K H, Minor I, Kremer M J, Heinz B A, Shepard D, Pevear D C, Rueckert R R, Rossmann M G. Structural studies on human rhinovirus 14 drug-resistant compensation mutants. J Mol Biol. 1995;253:61–73. doi: 10.1006/jmbi.1995.0536. [DOI] [PubMed] [Google Scholar]
- 34.Heinz B A, Rueckert R R, Shepard D A, Dutko F J, McKinlay M A, Fancher M, Rossmann M G, Badger J, Smith T J. Genetic and molecular analyses of spontaneous mutants of human rhinovirus 14 that are resistant to an antiviral compound. J Virol. 1989;63:2476–2485. doi: 10.1128/jvi.63.6.2476-2485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinz B A, Vance L M. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J Virol. 1995;69:4189–4197. doi: 10.1128/jvi.69.7.4189-4197.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinz B A, Vance L M. Sequence determinants of 3A-mediated resistance to enviroxime in rhinoviruses and enteroviruses. J Virol. 1996;70:4854–4857. doi: 10.1128/jvi.70.7.4854-4857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrmann E C, Jr, Herrmann J A, Delong D C. Comparison of the antiviral effects of substituted benzimidazoles and guanidine in vitro and in vivo. Antivir Res. 1981;1:301–314. [Google Scholar]
- 38.Iizuka N, Kohara M, Hagino-Yamagishi K, Abe S, Komatsu T, Tago K, Arita M, Nomoto A. Construction of less neurovirulent polioviruses by introducing deletions into the 5′ noncoding sequence of the genome. J Virol. 1989;63:5354–5363. doi: 10.1128/jvi.63.12.5354-5363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishiyama M, Shiga M, Sasamoto K, Mizoguchi M, He P. A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem Pharm Bull. 1993;41:1118–1122. [Google Scholar]
- 40.Kraus W, Zimmermann H, Eggers H J, Nelsen-Salz B. Rhodamine resistance and dependence of echovirus 12: a possible consequence of capsid flexibility. J Virol. 1997;71:1697–1702. doi: 10.1128/jvi.71.2.1697-1702.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraus W, Zimmermann H, Zimmermann A, Eggers H J, Nelsen-Salz B. Infectious cDNA clones of echovirus 12 and a variant resistant against the uncoating inhibitor rhodamine differ in seven amino acids. J Virol. 1995;69:5853–5858. doi: 10.1128/jvi.69.9.5853-5858.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J P, Baltimore D. An intragenic revertant of a poliovirus 2C mutant has an uncoating defect. J Virol. 1990;64:1102–1107. doi: 10.1128/jvi.64.3.1102-1107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J P, Baltimore D. Isolation of poliovirus 2C mutants defective in viral RNA synthesis. J Virol. 1988;62:4016–4021. doi: 10.1128/jvi.62.11.4016-4021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride A E, Schlegel A, Kirkegaard K. Human protein Sam68 relocalization and interaction with poliovirus RNA polymerase in infected cells. Proc Natl Acad Sci USA. 1996;93:2296–2301. doi: 10.1073/pnas.93.6.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melnick J L, Crowther D, Barrera-Oro J. Rapid development of drug-resistant mutants of poliovirus. Science. 1961;134:557. doi: 10.1126/science.134.3478.557. [DOI] [PubMed] [Google Scholar]
- 46.Mirzayan C, Wimmer E. Biochemical studies on poliovirus polypeptide 2C: evidence for ATPase activity. Virology. 1994;199:176–187. doi: 10.1006/viro.1994.1110. [DOI] [PubMed] [Google Scholar]
- 47.Mirzayan C, Wimmer E. Genetic analysis of an NTP-binding motif in poliovirus polypeptide 2C. Virology. 1992;189:547–555. doi: 10.1016/0042-6822(92)90578-d. [DOI] [PubMed] [Google Scholar]
- 48.Mosser A G, Rueckert R R. WIN 51711-dependent mutants of poliovirus type 3: evidence that virions decay after release from cells unless drug is present. J Virol. 1993;67:1246–1254. doi: 10.1128/jvi.67.3.1246-1254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosser A G, Sgro J Y, Rueckert R R. Distribution of drug resistance mutations in type 3 poliovirus identifies three regions involved in uncoating functions. J Virol. 1994;68:8193–8201. doi: 10.1128/jvi.68.12.8193-8201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfister T, Wimmer E. Characterization of the nucleoside triphosphatase activity of poliovirus protein 2C reveals a mechanism by which guanidine inhibits poliovirus replication. J Biol Chem. 1999;274:6992–7001. doi: 10.1074/jbc.274.11.6992. [DOI] [PubMed] [Google Scholar]
- 51.Pincus S E, Diamond D C, Emini E A, Wimmer E. Guanidine-selected mutants of poliovirus: mapping of point mutations to polypeptide 2C. J Virol. 1986;57:638–646. doi: 10.1128/jvi.57.2.638-646.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pincus S E, Rohl H, Wimmer E. Guanidine-dependent mutants of poliovirus: identification of three classes with different growth requirements. Virology. 1987;157:83–88. doi: 10.1016/0042-6822(87)90316-3. [DOI] [PubMed] [Google Scholar]
- 53.Pincus S E, Wimmer E. Production of guanidine-resistant and -dependent poliovirus mutants from cloned cDNA: mutations in polypeptide 2C are directly responsible for altered guanidine sensitivity. J Virol. 1986;60:793–796. doi: 10.1128/jvi.60.2.793-796.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rightsel W A, Dice J R, McAlpine R J, Timm E A, McLean I W, Jr, Dixon J D, Schabel F M., Jr Antiviral effect of guanidine. Science. 1961;134:558–559. doi: 10.1126/science.134.3478.558. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez P L, Carrasco L. Nonradioactive Northwestern analysis using biotinylated riboprobes. BioTechniques. 1994;17:45–48. [PubMed] [Google Scholar]
- 56.Rodriguez P L, Carrasco L. Poliovirus protein 2C contains two regions involved in RNA binding activity. J Biol Chem. 1995;270:10105–10112. doi: 10.1074/jbc.270.17.10105. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez P L, Carrasco L. Poliovirus protein 2C has ATPase and GTPase activities. J Biol Chem. 1993;268:8105–8110. [PubMed] [Google Scholar]
- 58.Rueckert R R, Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984;50:957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 60.Saunders K, King A M Q. Guanidine-resistant mutants of aphthovirus induce the synthesis of an altered nonstructural polypeptide, p34. J Virol. 1982;42:389–394. doi: 10.1128/jvi.42.2.389-394.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schlegel A, Giddings T H, Jr, Ladinsky M S, Kirkegaard K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J Virol. 1996;70:6576–6588. doi: 10.1128/jvi.70.10.6576-6588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shepard D A, Heinz B A, Rueckert R R. WIN 52035-2 inhibits both attachment and eclipse of human rhinovirus 14. J Virol. 1993;67:2245–2254. doi: 10.1128/jvi.67.4.2245-2254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Subramanya H S, Bird L E, Brannigan J A, Wigley D B. Crystal structure of a DExx box DNA helicase. Nature. 1996;384:379–383. doi: 10.1038/384379a0. [DOI] [PubMed] [Google Scholar]
- 64.Takeda N, Kuhn R J, Yang C-F, Takegami T, Wimmer E. Initiation of poliovirus plus-strand RNA synthesis in membrane complex of infected HeLa cells. J Virol. 1986;60:43–53. doi: 10.1128/jvi.60.1.43-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takegami T, Selmer B L, Anderson C W, Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983;128:33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- 66.Tamm I, Bablanian R, Nemes M M, Shunk C H, Robinson F M, Folkers K. Relationship between structure of benzimidazole derivatives and selective virus inhibitory activity. J Exp Med. 1961;113:625–655. doi: 10.1084/jem.113.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamm I, Eggers H J. Differences in the selective virus inhibitory action of 2-(α-hydroxybenzyl)-benzimidazole and guanidine HCl. Virology. 1962;18:439–447. doi: 10.1016/0042-6822(62)90034-x. [DOI] [PubMed] [Google Scholar]
- 68.Tamm I, Eggers J H, Bablanian R, Wagner A F, Folkers K. Structural requirements of selective inhibition of enteroviruses by 2-(α-hydroxybenzyl)-benzimidazole and related compounds. Nature. 1969;223:785–788. doi: 10.1038/223785a0. [DOI] [PubMed] [Google Scholar]
- 69.Tershak D R. Effect of temperature on growth of guanidine-resistant mutants of poliovirus. Can J Microbiol. 1985;31:1166–1168. doi: 10.1139/m85-220. [DOI] [PubMed] [Google Scholar]
- 70.Teterina N L, Kean K M, Gorbalenya A E, Agol V I, Girard M. Analysis of the functional significance of amino acid residues in the putative NTP-binding pattern of the poliovirus 2C protein. J Gen Virol. 1992;73:1977–1986. doi: 10.1099/0022-1317-73-8-1977. [DOI] [PubMed] [Google Scholar]
- 71.Teterina N L, Zhou W D, Cho M W, Ehrenfeld E. Inefficient complementation activity of poliovirus 2C and 3D proteins for rescue of lethal mutations. J Virol. 1995;69:4245–4254. doi: 10.1128/jvi.69.7.4245-4254.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tolskaya E A, Romanova L I, Kolesnikova M S, Gmyl A P, Gorbalenya A E, Agol V I. Genetic studies on the poliovirus 2C protein, an NTPase. A plausible mechanism of guanidine effect on the 2C function and evidence for the importance of 2C oligomerization. J Mol Biol. 1994;236:1310–1323. doi: 10.1016/0022-2836(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 73.Troxler M, Egger D, Pfister T, Bienz K. Intracellular localization of poliovirus RNA by in situ hybridization at the ultrastructure level using single-stranded riboprobes. Virology. 1992;191:687–697. doi: 10.1016/0042-6822(92)90244-j. [DOI] [PubMed] [Google Scholar]
- 74.Vance L M, Moscufo N, Chow M, Heinz B A. Poliovirus 2C region functions during encapsidation of viral RNA. J Virol. 1997;71:8759–8765. doi: 10.1128/jvi.71.11.8759-8765.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wikel J H, Paget C J, DeLong D C, Nelson J D, Wu C Y E, Paschal J W, Dinner A, Templeton R J, Chaney M O, Jones N D, Chamberlin J W. Synthesis of syn and anti isomers of 6-[[(hydroxyamino)phenyl]methyl]-1-[(1-methylethyl)sulfonyl]-1 H-benzimidazole-2-amine. Inhibitors of rhinovirus multiplication. J Med Chem. 1980;23:368–372. doi: 10.1021/jm00178a004. [DOI] [PubMed] [Google Scholar]
- 77.Wimmer E, Hellen C U, Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 78.Yao N, Hesson T, Cable M, Hong Z, Kwong A D, Le H V, Weber P C. Structure of the hepatitis C virus RNA helicase domain. Nat Struct Biol. 1997;4:463–477. doi: 10.1038/nsb0697-463. [DOI] [PubMed] [Google Scholar]