Highlights
-
•
We herein present a case of chronic progressive autoimmune GFAP astrocytopathy.
-
•
Symmetrical high-intensity signals on FLAIR were observed in the white matter of the temporal and occipital lobes, lateral cerebral ventricle walls, hippocampus, amygdala, and occipital cortex, with extensive Gd enhancement in radial perivascular lesions and the ependyma in the choroid plexus.
-
•
Improvements were achieved by 4 courses of IVMP and one of IVIg.
Keywords: Autoimmune GFAP astrocytopathy, Chronic progressive meningoencephalomyelitis, Extensive perivascular gadolinium enhancement, Intravenous methylprednisolone, Intravenous immunoglobulin
Dear Editor,
Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy (GFAP-A) is a rare autoimmune meningoencephalomyelitis characterized by the appearance of anti-GFAP antibodies in serum or cerebrospinal fluid (CSF). GFAP-A has an acute or subacute onset with a wide range of symptoms, including headache, encephalopathy, optic papillitis, myelitis, postural tremors, and cerebellar ataxia [1]. Magnetic resonance imaging (MRI) of patients with GFAP-A shows typical radial perivascular gadolinium (Gd) enhancement, which leads to a distinct diagnosis [[2], [3], [4]]. However, a few cases show a chronic progressive onset and prognosis [5]. We herein describe a 54-year-old woman who developed chronic progressive meningoencephalomyelitis with widespread symmetrical MRI lesions showing extensive Gd enhancement. Based on brain biopsy findings and the presence of an anti-GFAPα antibody in CSF, the patient was diagnosed with GFAP-A. Improvements were achieved by intravenous methylprednisolone (IVMP) and intravenous immunoglobulin (IVIg).
1. Case report
The patient was a 54-year-old woman who developed gait ataxia, headache, and back pain 4 months before admission. Four days before admission, she developed memory loss, forgot how to use switches and her phone, was unable to find her way to the bathroom. Neurological examinations at admission revealed disorientation for time and place, memory disturbance, dyscalculia, and ideomotor apraxia. The mini-mental state examination (MMSE) score was 19 points and the frontal assessment battery (FAB) was 6 points. Dysesthesia in the second to fifth fingers of both hands, exaggerated knee and Achilles reflexes, and a positive Babinski reflex were detected in both lower extremities. An ataxic gait and positive Romberg's and Mann's signs were noted.
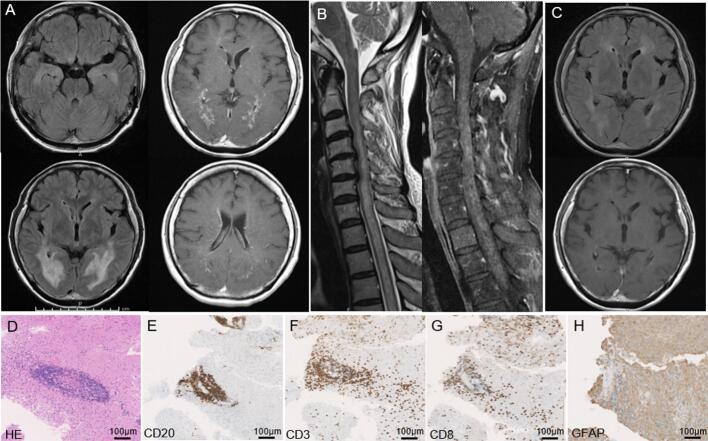
Brain MRI revealed symmetrical high-intensity signals on T2-weighted images (T2WI) and fluid-attenuated inversion recovery (FLAIR) for lesions in the white matter of the temporal and occipital lobes, marginal lateral cerebral ventricle areas, hippocampus, amygdala, and occipital cortex (Fig. 1A). Gd contrast showed extensive radial perivascular enhancement from enhanced ventricle walls, the ependyma in the choroid plexus, pons, and meninges on T1-weighted images (T1WI; Fig. 1A). Spinal cord MRI revealed a long cord lesion in the central gray matter from C3 to Th7 with a hazy pattern of Gd enhancement (Fig. 1B).
Fig. 1.
Brain MRI and biopsy histological findings.
(A) Brain MRI before treatment. High-intensity signals on FLAIR were observed in the white matter of the temporal and occipital lobes, hippocampus, amygdala, and occipital cortex in the left top and bottom: A Gd contrast-enhanced radial perivascular lesion from enhanced ventricle walls, the ependyma in the choroid plexus, and meninges in the right top and bottom.
(B) Spinal cord MRI revealed a long cord lesion in the central gray matter with a hazy pattern of Gd enhancement (Fig. 1B).
(C) Head MRI after treatment. FLAIR image in the upper panel. Gd enhancement was not observed on T1WI in the lower panel.
(D to H) Brain biopsy findings.
Perivascular lymphocytic infiltration (hematoxylin and eosin staining) (D) with CD20- (E) and CD3-positivive lymphocytes (F). Infiltration of CD8-positive lymphocytes into the brain parenchyma (G). Patchy disappearance of GFAP immunoreactivity in perivascular infiltration sites (H).
Blood tests showed hyponatremia (130 mEq/L) and hypokalemia (2.97 mEq/L). CSF examination revealed an increased cell count (67/μL; monocytes 97%) and protein level (247 mg/dL), a normal glucose level (57 mg/dL), and oligoclonal band positivity. Soluble interleukin-2 receptor (417 IU/L) and β2 microglobulin (6.4 mg/L) levels in CSF were higher than those in serum (260 IU/L and 1.1 mg/L, respectively). Physical examinations, blood tests, whole body CT and 18F-fluoro-deoxy-glucose positron emission tomography CT did not indicate the presence of malignant tumor. The anti-GFAPα antibody was positive in CSF. Brain biopsy from the right occipital lesion showed CD3-positive lymphocytes around blood vessels and the parenchyma and CD20-positive lymphocytes around blood vessels. CD8-positive lymphocytes and macrophages were observed in the parenchyma (Fig. 1D-G) [6,7]. GFAP immunoreactivity was absent in the perivascular lymphocytic infiltrate area (Fig. 1H).
The patient received 4 courses of IVMP at a dose of 1 g/day for three consecutive days and 1 course of IVIg at a dose of 400 mg/kg/day for five consecutive days. Headache and back pain immediately improved after IVMP, and cognitive disturbance recovered after the addition of IVIg. On the 50th hospital day, the CSF cell count (30/μL) and protein level (39 mg/dL) recovered. The MMSE score normalized to 29 points. Other clinical symptoms and gait disturbance improved. Cerebral and spinal cord lesions and periventricular contrast enhancement disappeared on MRI (Fig. 1C).
2. Discussion
Between 71 and to 86% of GFAP-A cases show an acute or subacute onset (< 8 weeks), while 27 to 82% have prodromal symptoms [2,5,6]. The present case was a rare chronic progressive type and had no prodromal symptoms or complications with other autoimmune diseases or malignant tumors. Two chronic GFAP-A cases showed drug-resistant epilepsy and had no typical MRI findings [6]. In one case, a 48-year-old female presented with optic disc swelling, seizures, cognitive decline, and an upper motor neuron disorder for >3 months with radial perivascular enhancement and long cord lesions [8]. In the other case, a male patient in his 60s presented with 6 months of progressive ataxia, proximal myoclonus, and bulbar symptoms with CSF pleocytosis in addition to elevated protein levels and positive anti-GFAP antibodies. Although no abnormal brain or spinal cord MRI findings were detected, methylprednisolone did not attenuate neurological symptoms and the patient died [9].
The present case showed typical symptoms, such as headache, back pain, gait ataxia, spastic paresis, sensory disturbance, and cognitive impairment. Seizure, optic papillitis, involuntary movement, and peripheral neuropathy were not observed. Consistent with these symptoms, T2WI and FLAIR showed bilateral symmetrical widespread white matter and cortex lesions and a long spinal cord lesion. Gd contrast revealed extensive radial perivascular enhancement from enhanced ventricle walls and a strongly enhanced ependyma in the choroid plexus, meninges, symmetrical cortical and white matter lesions, and long spinal lesions on T1WI. Although only 32–56% of GFAP-A patients show radial perivascular emphasis [1,2,5], this MRI finding is not exclusive to GFAP-A and has been reported rarely in other brain diseases [10]. Viral CNS infections, other form of autoimmune encephalitis, neuromyelitis optica, malignant lymphoma, traumatic brain injury, vascular dementia and astrocytoma were suggested for differential diagnosis [10]. In the present case, brain biopsy findings were consistent with GFAP-A. The perivascular infiltration of lymphocytes and anti-GFAP antibodies affects vascular permeability, astrocyte vascular foot processes, and ependymal cells. These mechanisms may be associated with radial perivascular enhancement, which indicates the disruption of the blood-brain barrier, leading to vasogenic edema corresponding to high-intensity signals on T2WI and FLAIR.
Since GFAP-A responds well to immunotherapy, steroid therapy is selected as the first-line treatment [7]. IVIg, plasma exchange, and other immunosuppressants are recommended for refractory cases [5]. Chronic cases may be less responsive to immunotherapy [9]. Although the period from onset to the start of treatment was 4 months in the present case, the combination of IVMP and IVIg contributed to recovery. Although ataxic gait, headache, back pain, and cognitive impairment were severe, immunotherapy markedly attenuated these symptoms, ADL, and MRI findings. Therefore, intense immunotherapy is plausible for chronic cases of GFAP-A.
Funding
None.
CRediT authorship contribution statement
Hironori Oka: Writing – review & editing. Takumi Nakamura: Writing – review & editing. Kunihiko Ishizawa: Resources. Masakuni Amari: Resources. Takeshi Kawarabayashi: Resources. Koichi Okamoto: Resources. Masamitsu Takatama: Resources. Satoshi Nakata: Resources. Yuhei Yoshimoto: Resources. Ayako Yamazaki: Resources. Hideaki Yokoo: Resources. Akio Kimura: Resources. Takayoshi Shimohata: Resources. Yoshio Ikeda: Resources. Mikio Shoji: Writing – review & editing.
Declaration of competing interest
None.
References
- 1.Fang B., McKeon A., Hinson S.R., Kryzer T.J., Pittock S.J., Aksamit A.J., Lennon V.A. Autoimmune glial fibrillary acidic protein Astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 2016;73(11):1297–1307. doi: 10.1001/jamaneurol.2016.2549. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan E.P., Hinson S.R., Lennon V.A., Fang B., Aksamit A.J., Morris P.P., Basal E., Honorat J.A., Alfugham N.B., Linnoila J.J., Weinshenker B.G., Pittock S.J., McKeon A. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann. Neurol. 2017;81(2):298–309. doi: 10.1002/ana.24881. [DOI] [PubMed] [Google Scholar]
- 3.Kimura A., Takekoshi A., Yoshikura N., Hayashi Y., Shimohata T. Clinical characteristics of autoimmune GFAP astrocytopathy. J. Neuroimmunol. 2019;332:91–98. doi: 10.1016/j.jneuroim.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Kimura A., Takekoshi A., Shimohata T. Characteristics of movement disorders in patients with autoimmune GFAP astrocytopathy. Brain Sci. 2022;12(4) doi: 10.3390/brainsci12040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gravier-Dumonceau A., Ameli R., Rogemond V., Ruiz A., Joubert B., Muniz-Castrillo S., Vogrig A., Picard G., Ambati A., Benaiteau M., Rulquin F., Ciron J., Deiva K., de Broucker T., Kremer L., Kerschen P., Sellal F., Bouldoires B., Genet R., Biberon J., Bigot A., Duval F., Issa N., Rusu E.C., Goudot M., Dutray A., Devoize J.L., Hopes L., Kaminsky A.L., Philbert M., Chanson E., Leblanc A., Morvan E., Andriuta D., Diraison P., Mirebeau G., Derollez C., Bourg V., Bodard Q., Fort C., Grigorashvili-Coin I., Rieul G., Molinier-Tiganas D., Bonnan M., Tchoumi T., Honnorat J., Marignier R. Glial fibrillary acidic protein autoimmunity: a French cohort study. Neurology. 2022;98(6):e653–e668. doi: 10.1212/WNL.0000000000013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iorio R., Damato V., Evoli A., Gessi M., Gaudino S., Di Lazzaro V., Spagni G., Sluijs J.A., Hol E.M. Clinical and immunological characteristics of the spectrum of GFAP autoimmunity: a case series of 22 patients. J. Neurol. Neurosurg. Psychiatry. 2018;89(2):138–146. doi: 10.1136/jnnp-2017-316583. [DOI] [PubMed] [Google Scholar]
- 7.Long Y., Liang J., Xu H., Huang Q., Yang J., Gao C., Qiu W., Lin S., Chen X. Autoimmune glial fibrillary acidic protein astrocytopathy in Chinese patients: a retrospective study. Eur. J. Neurol. 2018;25(3):477–483. doi: 10.1111/ene.13531. [DOI] [PubMed] [Google Scholar]
- 8.Kong Y., McKeon A., Koh O.S.Q., Chiew Y.R., Purohit B., Chin C.F., Zekeridou A., Ng A.S.L. Teaching NeuroImages: linear radial periventricular enhancement in glial fibrillary acidic protein astrocytopathy. Neurology. 2021;96(19):e2454–e2455. doi: 10.1212/WNL.0000000000011496. [DOI] [PubMed] [Google Scholar]
- 9.A C. Novo, Perez B. Venegas. Autoimmune glial fibrillary acidic protein astrocytopathy presented as ataxia, myoclonus and bulbar syndrome: a case report and review of the literature. BMJ Neurol. Open. 2021;3(2) doi: 10.1136/bmjno-2021-000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickel J., Chung H.Y., Kirchhof K., Boeckler D., Merkelbach S., Kuzman P., Mueller W.C., Geis C., Gunther A. Encephalitis with radial perivascular emphasis: not necessarily associated with GFAP antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(2) doi: 10.1212/NXI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]