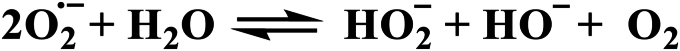Abstract
Enzymes are potent catalysts that increase biochemical reaction rates by several orders of magnitude. Flavoproteins are a class of enzymes whose classification relies on their ability to react with molecular oxygen (O2) during catalysis using ionizable active site residues. Pseudomonas aeruginosa D-arginine dehydrogenase (PaDADH) is a flavoprotein that oxidizes D-arginine for P. aeruginosa survival and biofilm formation. The crystal structure of PaDADH reveals the interaction of the glutamate 246 (E246) side chain with the substrate and at least three other active site residues, establishing a hydrogen bond network in the active site. Additionally, E246 likely ionizes to facilitate substrate binding during PaDADH catalysis. This study aimed to investigate how replacing the E246 residue with leucine affects PaDADH catalysis and its ability to react with O2 using steady-state kinetics coupled with pH profile studies. The data reveal a gain of O2 reactivity in the E246L variant, resulting in a reduced flavin semiquinone species and superoxide (O2•ˉ) during substrate oxidation. The O2•ˉ reacts with active site protons, resulting in an observed nonstoichiometric slope of 1.5 in the enzyme’s log (kcat/Km) pH profile with D-arginine. Adding superoxide dismutase results in an observed correction of the slope to 1.0. This study demonstrates how O2•ˉ can alter the slopes of limbs in the pH profiles of flavin-dependent enzymes and serves as a model for correcting nonstoichiometric slopes in elucidating reaction mechanisms of flavoproteins.
Keywords: Pseudomonas aeruginosa D-arginine dehydrogenase, pH profiles, nonstoichiometric slope, superoxide, superoxide disproportionation, reduced flavin semiquinone, rapid-reaction kinetics, O2-driven dehydrogenase activity
Flavin-dependent enzymes are a class of enzymes that often rely on ionization processes during catalysis (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15). Flavoproteins can be classified as oxidases, monooxygenases, and dehydrogenases, depending on their ability to use molecular oxygen (O2) as an electron acceptor and the products of their reactions (16, 17). Oxidases use O2 as an electron acceptor to produce H2O2. Monooxygenases insert an oxygen atom from O2 into the substrate with H2O as a product. Dehydrogenases do not react with O2, or if they do, they reduce O2 to superoxide radicals (O2•ˉ) without the production of H2O2 or H2O (18). In flavin-dependent enzymes, the spin-forbidden reaction of the triplet state O2 with the singlet state reduced flavin is overcome by successive electron transfers in a step-wise process to yield a caged O2•ˉ/flavin semiquinone radical pair (16, 17, 19, 20, 21). Studies on flavoproteins show that enzymes that react with O2, such as oxidases and monooxygenases, overcome the thermodynamically unfavorable generation of the O2•ˉ/flavin semiquinone radical pair by stabilizing the transition state of the O2•ˉ/flavin semiquinone radical pair through electrostatic catalysis using a positive charge close to the flavin C(4a) atom (16, 17, 21, 22, 23, 24).
From structural and mechanistic analysis, a structural motif comprising nonpolar residues in the active site and a positive charge, either in the protein or from the enzyme’s substrate or product, has been identified as a requirement for flavin reactivity with O2 to yield the O2•ˉ/flavin semiquinone radical pair (24). In principle, a flavoprotein can gain the ability to react with O2, provided these minimum requirements are met. The highly reactive O2•ˉ can then undergo an ionization process to acquire a proton from the enzyme or bulk solvent, yielding a hydroperoxyl radical (HO2•) with a pKa value of 4.8 (25, 26). The key players of ionization processes in several enzymes are solvent water, metal ions, and the side chains of ionizable active site residues (10, 16, 17, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36). For several decades, pH profile and mutagenesis studies have been widely employed to identify and assign pKa values to the ionizing groups during enzyme catalysis (37, 38, 39, 40, 41, 42, 43). However, the pH profiles used to identify ionizing groups and assign pKa values could be misinterpreted if an enzyme produces highly reactive species such as O2•ˉ that can react with ionized protons during catalysis. Hence, the question remains whether the formation and leakage of O2•ˉ in flavoproteins that react poorly with O2 to yield O2•ˉ can affect the pH profiles used in assessing the residues important for enzyme catalysis.
The Pseudomonas aeruginosa D-arginine dehydrogenase (PaDADH) is a flavoprotein found in the opportunistic pathogen P. aeruginosa (44). The enzyme is important for P. aeruginosa’s utilization of D-arginine as a primary source of carbon and nitrogen by oxidizing the CN bond between the Cα atom and the amino group of its substrates (45). In recent years, the increased prevalence of multidrug-resistant strains of P. aeruginosa has been largely associated with the formation of bacterial biofilms coupled with bacterial antibiotic-resistance mechanisms (46, 47, 48, 49, 50, 51, 52, 53, 54). Studies have identified that increased levels of L-arginine in P. aeruginosa reduce bacterial mobility, enhancing the formation of bacterial biofilms required for chronic infections (53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64). In P. aeruginosa, PaDADH exists in a two-enzyme conversion system with L-arginine dehydrogenase, allowing the bacterium to accumulate L-arginine from D-arginine oxidation (65). Thus, PaDADH’s activity influences P. aeruginosa to favor the sessile biofilm-forming lifestyle required for chronic infections (63, 64). More recently, the enzyme has emerged as a paradigm for flavin-containing enzymes that oxidize positively charged D-amino acids (66).
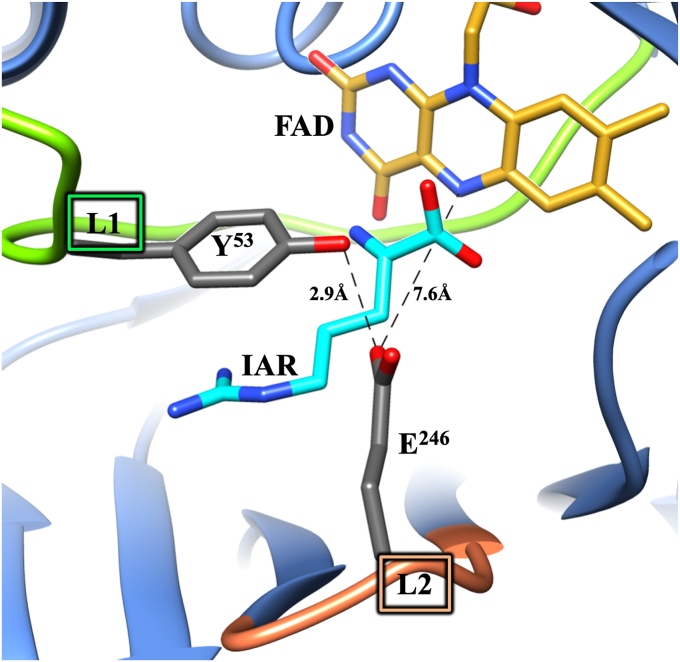
PaDADH has broad substrate specificity. The enzyme oxidizes all D-amino acids, except D-aspartate and D-glutamate, to their corresponding α-imino acids, followed by nonenzymatic hydrolysis of the α-imino acid products to yield α-keto acids and ammonia (Fig. 1) (44, 67, 68, 69). PaDADH is a strict dehydrogenase that does not react with O2 and follows a ping-pong bi-bi steady-state kinetic mechanism (44, 66). Structural analysis of the enzyme’s active site reveals only polar amino acid residues, including H48, Y53, E87, R222, E246, Y249, and R305, and a lack of nonpolar residues (69). The enzyme contains four flexible loops, L1, L2, L3, and L4 (69), with residues Y53 of loop L1 and E246 of loop L2 involved in a gating interaction that secures the substrate in the active site for catalysis (Fig. 2) (67, 69). Residue E246 of loop L2 is located 7.6 Å from the flavin N5 atom, 8.2 Å from the flavin C(4a) atom, and does not participate in flavin reduction (70).
Figure 1.
General reaction scheme of PaDADH.PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase.
Figure 2.
Gating of PaDADH active site by loop L1 and L2 residues Y53and E246. Y53 and E246 are shown in gray. All N atoms are shown in blue, and all O atoms are in red. The FAD cofactor is represented by its isoalloxazine ring with the C atoms in gold. IAR represents the iminoarginine product and is shown in cyan. The E246 residue’s hydrogen bond interaction with Y53 and distance from the flavin are shown as dashed lines. Loop L2 is shown in coral, and loop L1 is shown in green. The PDB file 3NYE was visualized and analyzed using the UCSF Chimera software (110). PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase.
In this study, the PaDADH residue E246 has been mutated to leucine to yield the glutamate 246 to leucine mutant (E246L) variant enzyme. The effects of the E246L mutation on the enzyme’s catalysis and ability to react with O2 have been investigated using rapid-reaction kinetics, steady-state kinetics, and pH profile studies. Additionally, the mutation’s effects on the slopes of the pH profile plots have been explored.
Results
Rapid-reaction kinetics of the PaDADH E246L variant enzyme with D-leucine as a substrate
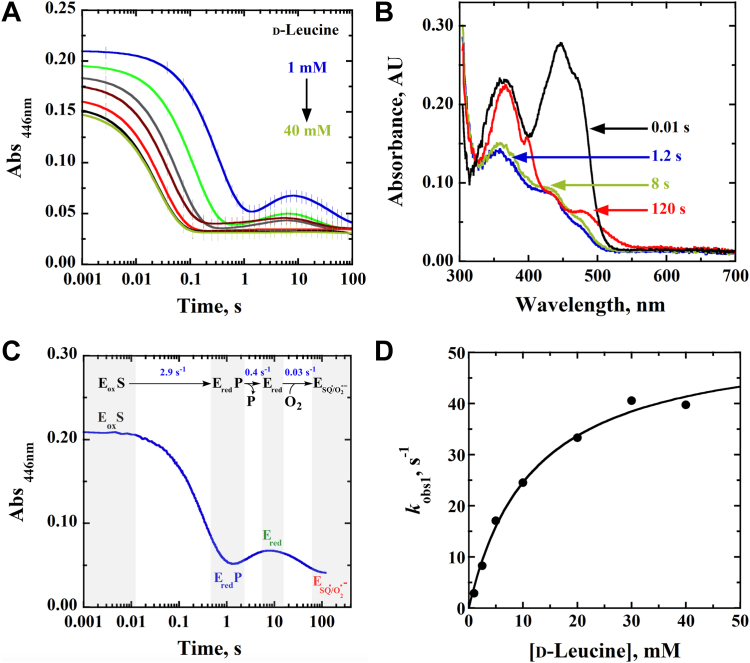
Since ∼80% of the flavin reduction of the PaDADH E246L variant enzyme with D-arginine occurs in the mixing time (2.2 ms) of the stopped-flow spectrophotometer (42, 71), the flavin reduction of the enzyme was investigated with D-leucine as the reducing substrate. The time-resolved aerobic reduction of the PaDADH E246L variant enzyme by D-leucine was investigated under pseudo-first-order conditions by monitoring the loss of the oxidized flavin’s absorbance at 446 nm. The resulting stopped-flow traces showed three distinct reaction phases at [D-leucine] ≤10 mM. As expected for flavin reduction, the absorbance at 446 nm decreased; however, there was a subsequent transient increase in the 446 nm absorbance (Fig. 3A). The stopped-flow traces were fit with triple exponentials (Equation 1).
Figure 3.
Aerobic reductive-half reaction of the PaDADH E246L variant enzyme.A, stopped-flow traces of the absorbance changes at 446 nm at different D-leucine concentrations (1–25 mM) fit with Equation 1. Each trace is the average of triplicate runs at each substrate concentration. For clarity, one out of every 100 experimental points is shown (vertical lines). Note the log time scale. B, time-resolved UV-visible absorption spectra of the various flavin species generated during the aerobic reduction of the PaDADH E246L variant enzyme with D-leucine. The reaction was monitored over 120 s (s) upon mixing 1 mM D-leucine with the E246L variant enzyme of PaDADH in the presence of atmospheric O2. The black spectrum represents the oxidized enzyme; the red spectrum represents the reduced flavin semiquinone; the green and the blue spectra represent the fully reduced flavin. C, time map for the various flavin species generated during the aerobic reductive-half reaction of the PaDADH E246L variant enzyme with 1 mM D-leucine. D, the observed rate constant for flavin reduction as a function of D-leucine concentration under aerobic conditions fit with Equation 2. The single point shown at each substrate concentration is the kobs value obtained from the fit of the average of triplicate runs with Equation 1 yielding an error of ≤5%. The assay was performed in 20 mM NaPPi, pH 10.0, using an SF-61DX2 Hi-Tech KinetAsyst high-performance stopped-flow spectrophotometer thermostated at 25 oC and equipped with a photomultiplier detector under aerobic conditions. The instrumental dead time is 2.2 ms. E246L, glutamate 246 to leucine mutant; PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase.
Analysis of the time-resolved UV-visible absorption spectra of the various flavin species generated during the aerobic reduction of the PaDADH E246L variant enzyme with D-leucine showed an oxidized flavin spectrum at 0.01 s with a λmax at 446 nm and a second peak at 367 nm. After 1.2 s, there was an observed quenching of the λ446 nm and λ367 nm peaks to yield a spectrum with a λmax at 367 nm, consistent with a reduced flavin being present. After 8 s, there was an observed slight increase in the reduced flavin absorbance between 367 nm and 500 nm, although the overall spectral characteristics of the reduced flavin at 1.2 s were maintained. After 120 s, there were three observed peaks in the flavin spectrum at 367 nm, 394 nm, and 480 nm, consistent with the formation of a reduced flavin semiquinone species (Fig. 3B).
The first phase of the time-resolved aerobic reduction of the PaDADH E246L variant with D-leucine, characterized by the bleaching of the oxidized flavin absorption at 446 nm, was assigned to flavin reduction. The next phase, characterized by the transient gain of absorbance at 446 nm, was assigned to the imino acid product release. The last phase, showing a quenching of absorbance at 446 nm, was assigned to the reaction of the reduced flavin with O2 (Fig. 3C), hinting at the formation of a caged O2•ˉ/flavin semiquinone radical pair during the aerobic reduction of the PaDADH E246L variant enzyme. The lack of a transient increase in the 446 nm absorbance at [D-leucine] ≥10 mM can be explained as a likely formation of enzyme–substrate complexes between the reduced enzyme and excess substrate following the imino acid product release, which prevents the reduced flavin from reacting with O2.
To obtain the observed rate constant (kobs1) for flavin reduction, the rate constants for the first phase at any given substrate concentration were fit with Equation 2, yielding a zero y-intercept hyperbolic dependence of the kobs1 parameter on D-leucine concentration (Fig. 3D), allowing for the determination of the limiting rate constant for flavin reduction (kred) and the apparent equilibrium constant for the dissociation of the substrate from the Michaelis complex (Kd). The resulting kinetic parameters are shown in Table 1. There was no observed dependence of the kobs2 and kobs3 parameters on [D-leucine].
Table 1.
Rapid-reaction kinetic parameters of the PaDADH E246L variant enzyme with D-leucine as substrate
| Kinetic parameter | aAerobic | bAnaerobic |
|---|---|---|
| kred (s−1) | 54 ± 3 | 35 ± 1 |
| Kd (mM) | 12 ± 2 | 12 ± 1 |
| kred/Kd (M−1s−1) | 4600 ± 400 | 2900 ± 75 |
Reductive-half reaction kinetics were measured at varying concentrations of D-leucine under aerobic conditions. Assays were performed in 20 mM NaPPi, pH 10.0, at 25 oC. The kinetic parameters’ values were obtained after fitting the kinetic data with Equation 2.
Previously reported data for the PaDADH E246L variant enzyme under anaerobic conditions (70).
O2 reactivity studies of the PaDADH E246L variant enzyme
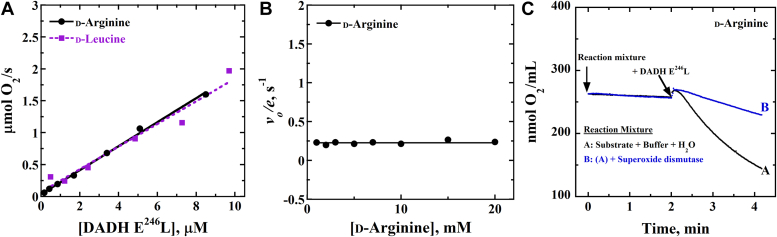
The ability of the PaDADH E246L variant enzyme to react with O2 as an electron acceptor was explored by measuring the initial velocities of the enzymatic reaction at various enzyme concentrations with 25 mM D-leucine as substrate and O2 as the electron acceptor. The data yielded a positive-sloped linear dependence of the initial velocities of enzyme activity as a function of enzyme concentration. Similar results were obtained when the assay was repeated with D-arginine as substrate (Fig. 4A).
Figure 4.
O2reactivity studies of the PaDADH E246L variant enzyme.A, plot of the initial velocity of the PaDADH E246L variant enzyme’s reactivity with O2 as a function of enzyme concentration at fixed 5 mM D-arginine in black and 25 mM D-leucine in purple
and 25 mM D-leucine in purple . B, the dependence of the rate of O2 reactivity of the PaDADH E246L variant enzyme as a function of D-arginine concentration. C, the effect of superoxide dismutase on the PaDADH E246L variant enzyme’s reaction with O2 with 5 mM D-arginine as substrate. The black trace represents the O2 reactivity without superoxide dismutase. The blue trace represents the experiment performed in the presence of superoxide dismutase. The assays were carried out in 20 mM NaPPi, pH 8.0, using a Clark-type oxygen electrode system, thermostated at 25 oC. E246L, glutamate 246 to leucine mutant; PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase.
. B, the dependence of the rate of O2 reactivity of the PaDADH E246L variant enzyme as a function of D-arginine concentration. C, the effect of superoxide dismutase on the PaDADH E246L variant enzyme’s reaction with O2 with 5 mM D-arginine as substrate. The black trace represents the O2 reactivity without superoxide dismutase. The blue trace represents the experiment performed in the presence of superoxide dismutase. The assays were carried out in 20 mM NaPPi, pH 8.0, using a Clark-type oxygen electrode system, thermostated at 25 oC. E246L, glutamate 246 to leucine mutant; PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase.
A separate experiment to investigate the dependence of the O2 reactivity on D-arginine concentration yielded a flat line with a rate constant for the oxygen-driven dehydrogenase activity† of 0.23 s−1, irrespective of the substrate concentration tested (Fig. 4B). When superoxide dismutase was introduced into the reaction assay to determine the O2 species generated during the enzyme’s turnover with O2 as the electron acceptor, there was an observed decrease in the initial velocity of the enzymatic reaction with D-arginine (Fig. 4C). Similar results were obtained when the above assays were repeated with D-leucine as substrate (data not shown).
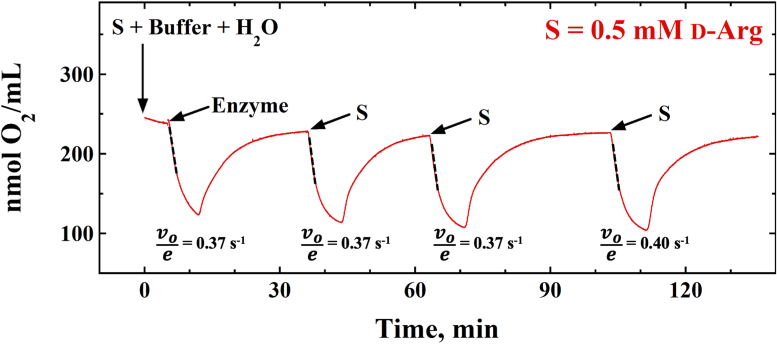
The steady-state kinetic studies of the PaDADH E246L variant enzyme reported in a previous study yielded a kcat value of 265 ± 5 s−1, a kcat/Km value of 871,000 ± 35,000 M−1s−1, and a Km value of 0.30 ± 0.02 mM with D-arginine (70). Hence, to determine the effect of the enzyme-generated O2•ˉ on PaDADH E246L turnover with D-arginine, assays of the variant enzyme with O2 as electron acceptor and 0.5 mM D-arginine as substrate were carried out under steady-state conditions. The PaDADH E246L variant underwent multiple turnover cycles spanning 2 h upon reintroduction of D-arginine into the reaction mixture (Fig. 5). For all enzyme turnover cycles, the initial rate of the oxygen-driven dehydrogenase activity was ∼0.4 s−1. Similar rates for D-arginine oxidation were observed when the experiment was repeated using 0.15 and 0.24 mM D-arginine (data not shown).
Figure 5.
Effect of the PaDADH E246L-generated O2•ˉ on the turnover ability of the enzyme. O2 consumption and regeneration cycles were carried out with fixed 6 μM enzyme and 0.5 mM d-arginine. The steady-state regions are shown in black dotted lines and the initial rates of D-arginine oxidation for each turnover cycle is shown below as vo/e. The assay was performed in 20 mM NaPPi, pH 8.0, with O2 as the electron acceptor, using a Clark-type oxygen electrode system at 25 oC. E246L, glutamate 246 to leucine mutant; PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase.
Additionally, O2 regeneration was observed in every enzyme turnover cycle before the D-arginine substrate was reintroduced (Fig. 5). The observed oxygen regeneration can be explained by a proton-dependent O2•ˉ disproportionation to yield hydroperoxide anion HO2ˉ, hydroxide HOˉ, and O2 (Fig. 6) (72), followed by a slow re-equilibration of the solution in the O2 electrode chamber toward atmospheric oxygen. The observed nonzero O2 level after enzyme turnover with D-arginine can be explained by the O2•ˉ disproportionation reaction leading to O2 accumulation that gradually overturns the O2 consumption of the D-arginine oxidation.
Figure 6.
The disproportionationof O2•ˉ in water.
Effects of pH on the kcat/Km parameter of PaDADH E246L
Due to the O2 reactivity and formation of the flavin semiquinone species at [D-leucine] ≤10 mM during the aerobic reduction of the PaDADH E246L variant, the kinetic investigations for the steady-state pH effects focused only on the kcat/Km parameter, which reports on the enzyme’s behavior at low substrate concentrations and probes the free enzyme. Thus, to understand the effects of pH on PaDADH E246L’s substrate capture, the steady-state reactions of the enzyme were investigated with D-arginine or D-leucine as the substrate from pH 5.0 to 10.5, with phenazine methosulfate (PMS) as an artificial electron acceptor since the physiological electron acceptor for PaDADH activity is not known. The plots of the log values of the kcat/Km parameter showed an increase in the kcat/Km parameter with increasing pH, a pH-independent region above pH 9.0, and an observed pKa value for a basic group between 8.2 and 8.8 (Fig. 7 and Table 2).
Figure 7.
Effects of superoxide dismutase and pH on the kcat/Kmparameter of the PaDADH E246L variant with D-arginine or D-leucine as substrate.A, pH dependence of the kcat/Km parameter with D-arginine. B, pH dependence of the kcat/Km parameter with D-leucine. Activity assays were carried out with varying concentrations of D-arginine or D-leucine as a substrate and fixed PMS as an artificial electron acceptor at 1 mM from pH 5.0 to 10.5 in 20 mM NaPPi. Assays without superoxide dismutase are shown in black and red
and red for D-arginine at 25 oC and 12 oC, respectively, and purple
for D-arginine at 25 oC and 12 oC, respectively, and purple for D-leucine at 25 oC. The D-arginine assay with superoxide dismutase at 25 oC is shown in blue
for D-leucine at 25 oC. The D-arginine assay with superoxide dismutase at 25 oC is shown in blue . The D-arginine plots were obtained by fitting the kinetic data to Equation 3 for the kcat/Km parameter without superoxide dismutase at 12 oC and 25 oC, and Equation 4 for the kcat/Km parameter with superoxide dismutase. The D-leucine plot was obtained by fitting the kinetic data with Equation 4. The observed pKa values for the various assays are recorded in Table 2 for D-arginine and Table 3 for D-leucine. PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase; PMS, phenazine methosulfate.
. The D-arginine plots were obtained by fitting the kinetic data to Equation 3 for the kcat/Km parameter without superoxide dismutase at 12 oC and 25 oC, and Equation 4 for the kcat/Km parameter with superoxide dismutase. The D-leucine plot was obtained by fitting the kinetic data with Equation 4. The observed pKa values for the various assays are recorded in Table 2 for D-arginine and Table 3 for D-leucine. PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase; PMS, phenazine methosulfate.
Table 2.
Effects of superoxide dismutase on the pH effects on the steady-state kinetic parameters of the PaDADH E246L variant enzyme with D-arginine as substratea
|
kcat/Km | ||||
|---|---|---|---|---|
| Condition | CH | pKa | Slope | R2 |
| 25 oC | 665,000 ± 78,000 | 8.2 ± 0.1 | 1.5 ± 0.1 | 0.997 |
| 25 oC + superoxide dismutase | 263,000 ± 80,000 | 8.8 ± 0.2 | 1.1 ± 0.1 | 0.983 |
| 12 oC | 531,000 ± 64,000 | 8.5 ± 0.1 | 1.4 ± 0.1 | 0.997 |
Enzymatic activities were measured at varying concentrations of D-arginine and fixed 1 mM PMS. Reactions were carried out in 20 mM sodium pyrophosphate. The kcat/Km parameter values were obtained after fitting the kinetic data with Equation 3.
For the D-arginine substrate, the kcat/Km pH profile at 25 oC fit with Equation 3 yielded a plot with a nonstoichiometric slope of +1.5 for the increasing limb, with an observed pKa value for a basic group of ∼8.2. When the assay was repeated at 12 oC to investigate the effect of temperature on the slope of the kcat/Km pH profile plot, similar results were obtained, suggesting that temperature does not contribute to the observed slope. When the effect of the enzyme-generated O2•ˉ on the slope of the kcat/Km pH profile plot was investigated by adding 200 to 500 units of superoxide dismutase to each apparent steady-state reaction mixture at 25 oC, the kcat/Km pH profile plot fit with Equation 3 yielded a stoichiometric value of +1 (Table 2).
The effect of pH on the kcat/Km pH profile of PaDADH E246L with D-leucine at 25 oC fit with Equation 4 yielded a kinetic plot with a slope of +1 for the increasing limb, with an observed pKa value for a basic group of ∼8.7 (Table 3) and a pH-independent region above pH 9.0 (Fig. 7).
Table 3.
Effects of pH on the steady-state kinetic parameters of the PaDADH E246L variant enzyme with D-leucine as substrate at 25 oCa
| Kinetic parameter | CH | pKa | R2 |
|---|---|---|---|
| kcat/Km | 2200 ± 500 | 8.7 ± 0.1 | 0.987 |
Enzymatic activities were measured at varying concentrations of D-leucine and fixed 1 mM PMS. Reactions were carried out in 20 mM sodium pyrophosphate. The kcat/Km parameter values were obtained after fitting the kinetic data with Equation 4.
Discussion
This study aimed to investigate the effects of the E246L mutation on the ability of PaDADH to react with O2. The study also investigated the effect of the E246L mutation on the catalysis of PaDADH using steady-state kinetics coupled with pH profile studies. The data demonstrate that upon the E246L mutation, PaDADH, which is a strict dehydrogenase that does not react with O2, gains the ability to react with O2, although poorly, to yield a reduced flavin semiquinone and O2•ˉ (66, 67). Consequently, the PaDADH E246L variant turns over with O2 as an electron acceptor through an alternative dehydrogenase pathway. Following the acquired O2 reactivity, the variant enzyme yields a nonstoichiometric slope in the plot of the log (kcat/Km) parameter as a function of pH with D-arginine as substrate. Details on the gain of function and the implications on PaDADH catalysis are discussed below.
The PaDADH E246L variant enzyme reacts with O2 to form a flavin semiquinone during substrate oxidation. Evidence supporting this conclusion comes from the enzyme-monitored aerobic flavin reduction with D-leucine and oxygen (Fig. 3), showing the formation and decay of an enzyme intermediate between 1.2 and 120 s. Analysis of the spectral data of the flavin reduction revealed a flavin spectrum with a peak at 367 nm, characteristic of a flavin semiquinone species at 120 s (Fig. 3) (73, 74, 75). By comparison, this feature is not observed with the PaDADH wildtype enzyme under both aerobic and anaerobic conditions (42, 44, 66, 67, 69, 76, 77). In the same way, the flavin semiquinone species was not observed with the E246L variant enzyme under anaerobic conditions, although similar kinetic parameters as reported for the aerobic flavin reduction in this study were observed: kred = ∼50 s−1 and Kd = 12 mM (Table 1) (70). The data are consistent with the PaDADH E246L variant reacting with O2 to yield the flavin semiquinone after flavin reduction and product release, as evidenced by the observed transient increase in the λ466 nm absorbance of the reduced flavin at 8 s (Fig. 3). The presence or absence of the product in the active site alters the flavin oscillator strength, yielding different absorbance intensities of the flavin 446 nm peak upon product release (78). The observation that the flavin semiquinone was not formed at [D-leucine] ≥10 mM can be explained as a likely binding of the reduced enzyme to excess substrate. At [D-leucine] ≥10 mM, the reduced enzyme likely forms a complex with free unreacted substrate in the bulk solvent to yield the EredS complex. Such a complex renders the reduced flavin unavailable, preventing the free reduced flavin from reacting with O2 to yield the semiquinone.
The reactivity of the PaDADH E246L variant enzyme with O2 yields O2•ˉ during catalysis. Evidence supporting this conclusion comes from the rapid-reaction kinetics with D-leucine and the steady-state enzymatic assay of the PaDADH E246L variant enzyme with O2 as the electron acceptor with D-arginine or D-leucine as substrate, with and without superoxide dismutase (Figs. 3 and 4). The plot of the initial velocity of the E246L variant enzyme as a function of time showed a decrease in O2 consumption in the presence of superoxide dismutase (Fig. 4), consistent with a superoxide dismutase–mediated conversion of the PaDADH E246L-generated O2•ˉ to O2, which overturns O2 consumption (25, 72, 75, 79, 80, 81, 82, 83, 84, 85, 86, 87). The formation of O2•ˉ is concomitant with the observed stabilization of a flavin semiquinone species (vide supra) during the aerobic reduction of PaDADH E246L to yield the O2•ˉ/flavin semiquinone radical pair. The O2•ˉ/flavin semiquinone radical pair likely arises from a one-electron reduction of O2 by the reduced flavin required to overcome the spin-forbidden reaction of the triplet state O2 with the singlet state reduced flavin during PaDADH E246L reactivity with O2, as reported for flavin-dependent enzymes (16, 17, 19, 20, 21, 75, 88).
The PaDADH E246L variant enzyme undergoes multiple turnover cycles with O2. This conclusion is supported by the steady-state assays of PaDADH E246L with D-arginine or D-leucine as substrate and O2 as electron acceptor. The positive-sloped linear dependence of the plot of the enzymatic initial velocities against enzyme concentration (Fig. 4) demonstrates that PaDADH E246L turns over with O2. Accordingly, we propose that PaDADH E246L follows an O2-driven dehydrogenase mechanism (Fig. 8). In the O2-driven dehydrogenase mechanism, a hydride transfer from the amino acid substrate reduces the enzyme-bound oxidized flavin to yield the reduced flavin and the imino acid product. The reduced flavin then reacts with O2 after product release to form the caged O2•ˉ/flavin semiquinone radical pair. The flavin semiquinone subsequently donates a hydrogen atom to O2•ˉ, producing HO2ˉ and the re-oxidized flavin. The observation that the initial rates of PaDADH E246L turnover with D-arginine and O2 remained unchanged at ∼0.4 s−1 for 2 h (Fig. 5) suggests that the PaDADH E246L-generated O2•ˉ does not compromise the enzyme’s ability to turnover. The sustained catalytic integrity of PaDADH E246L can then be explained as a likely prevention of the accumulation of the highly reactive O2•ˉ that may damage the enzyme. A likely mechanism preventing O2•ˉ accumulation is the disproportionation of O2•ˉ, which yields HO2ˉ, HOˉ, and O2 (Fig. 6) (vide supra). Moreover, the conversion of O2•ˉ to HO2ˉ following a hydrogen transfer from the flavin semiquinone during the O2-driven dehydrogenase mechanism cannot be ruled out.
Figure 8.
Proposed reaction scheme of the PaDADH E246L dehydrogenase activity with PMS or O2 as an electron acceptor during turnover. A hydride transfer from the amino acid substrate reduces the enzyme-bound oxidized flavin to yield the reduced flavin and the imino acid product. The reduced flavin is then re-oxidized through a PMS-driven or an O2-driven dehydrogenase activity. For the PMS-driven turnover, the reduced flavin is re-oxidized by PMS to yield PMSH2, restoring the enzyme to its resting state. For the O2-driven turnover, the reactivity of O2 with the reduced flavin yields the highly reactive caged O2•ˉ/flavin semiquinone radical pair. The flavin semiquinone then donates a hydrogen atom to the O2•ˉ, yielding HO2ˉ and the re-oxidized flavin in the resting state of the enzyme. E246L, glutamate 246 to leucine mutant; O2•ˉ, superoxide radicals; PMS, phenazine methosulfate.
The O2-driven dehydrogenase activity of PaDADH E246L with a rate of 0.23 s−1, irrespective of the substrate and concentration tested, can be explained as a likely saturation of the PaDADH E246L variant with the amino acid substrate during turnover with O2 as the electron acceptor. The data agree well with a recent report investigating the role of the E246 residue in PaDADH catalysis in which a small O2-driven dehydrogenase activity of ∼0.2 s−1 was reported for the E246L, E246G, and E246Q variant enzymes (70). In the same study, the kinetic parameters were investigated using PMS as an artificial electron acceptor for PaDADH since the physiological electron acceptor is unknown. The study reported a kcat value of ∼270 s−1 with PMS and a maximum rate of ∼0.2 s−1 with O2 for the E246L variant enzyme with D-arginine (70). In this way, the previous study demonstrates that the PaDADH E246L variant enzyme turns over primarily through a PMS-driven dehydrogenase mechanism, not an O2-driven dehydrogenase mechanism (Fig. 8). Hence, the O2-driven dehydrogenase activity does not affect the PMS-driven dehydrogenase activity of PaDADH E246L.
The E246L variant-generated O2•ˉ diverts protons from the active site during D-arginine oxidation. This conclusion is supported by the steady-state pH profiles of the PaDADH E246L variant with D-leucine and D-arginine in the presence and absence of superoxide dismutase (Fig. 7). The pH profile of the kcat/Km parameter with D-leucine yielded a plot with a +1 slope for the increasing limb from low to high pH. Conversely, with D-arginine, the kcat/Km pH profile yielded a nonstoichiometric slope of +1.5 that was corrected to +1 after superoxide dismutase addition. Conventionally, the slope of a kinetic parameter’s pH profile has been used as an indicator for the number of ionizable processes required to complete the catalytic processes probed by the kinetic parameter (10, 11, 12, 13, 14, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102). Thus, the observed differences in the PaDADH E246L kcat/Km pH profile slope with D-arginine and D-leucine can be explained in light of different ionization processes being essential for the catalysis of the two substrates.
With D-arginine, the slope of +1 for the log (kcat/Km) pH profile is assigned to the ionization of the substrate’s α-NH3+ during enzyme catalysis. The observed slope of +1 compared to the slope of +2 previously reported for the wildtype enzyme suggests that the unprotonated E246 residue is important for binding D-arginine. However, in a previous study investigating the role of residue E87 in PaDADH, the observation of only a single ionizable group with a slope of +1 in the PaDADH E87L variant led to the assignment of residue E87 as one of the two unprotonated groups required for D-arginine binding, with the substrate’s α-NH3+ group being the other (77). Altogether, the data portray the requirement for three groups: the substrate’s α-NH3+, E87, and E246 for the kcat/Km kinetic parameter during D-arginine oxidation. With the substrate being the commonality between all enzyme forms under discussion: wildtype, E87 variant, and E246 variant, the first ionizable group can be unequivocally assigned to the substrate’s α-NH3+ group. Thus, given that the wildtype enzyme reports only two ionizable groups for the kcat/Km pH profile, the assignment of the second group must be critically assessed. From the PaDADH crystal structure (Fig. 9), both E87 and E246 interact with the guanidinium side chain and are important for D-arginine binding and imino arginine release (70, 77). Since the guanidinium charge is delocalized, we propose that the second ionizable group reflects not one but the joint ionization of the E87 and E246 residues to ensure maximal binding of D-arginine in the wildtype enzyme. Thus, mutation of either E87 or E246 yields similar and nonadditive effects on the log (kcat/Km) pH profile as reported for His256 and Asp266 of the carbohydrate-binding domain in rat hepatic lectin-1 (103).
Figure 9.
The active site topology of PaDADH showing substrate interactions and the highly polar active site pocket. The PDB file 3NYE was visualized and analyzed using the UCSF Chimera software (110). IAR, iminoarginine product; PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase.
The correction of the log (kcat/Km) pH profile’s slope from +1.5 to +1 following superoxide dismutase addition suggests that the O2•ˉ produced by PaDADH E246L’s reaction with D-arginine and O2 is responsible for the observed nonstoichiometric slope. As the enzyme turns over with D-arginine, the highly reactive O2•ˉ generated during flavin reduction progressively accumulates in the solution due to multiple enzyme turnovers. However, during PaDADH E246L oxidation of D-arginine, the proton released from the α-NH3+ ionization is unavailable for O2•ˉ reactivity due to the H48-mediated proton relay network to the bulk solvent (Fig. 10), as previously described for p-hydroxybenzoate hydroxylase (66, 77, 104). Thus, the accumulated and highly reactive O2•ˉ most likely reacts with the E87 proton to yield HO2• during PaDADH E246L turnover with low concentrations of D-arginine, thereby favoring E87 ionization. The observed steeper and nonstoichiometric slope of +1.5 (Fig. 7) in the log (kcat/Km) pH profile, therefore, likely reflects inflated values for the kcat/Km parameter, which probes the free forms of enzymes at low substrate concentrations rather than enzyme–substrate complexes. Due to the O2•ˉ diversion of the E87 ionized protons, there is a partial detection of the E87 ionization in the log (kcat/Km) pH profile with D-arginine despite the absence of E246 (103).
Figure 10.
The proton-relay network of PaDADH. The PDB file 3NYE was visualized and analyzed using the UCSF Chimera software (110). IAR, iminoarginine product; PaDADH, Pseudomonas aeruginosa D-arginine dehydrogenase.
In contrast, the D-leucine substrate has a short and nonpolar side chain and does not require ionization of the E87 residue for binding. Thus, there is no observed effect of O2•ˉ on the log (kcat/Km) pH profile plot. The observed slope of +1 with an intrinsic pKa value of ∼8.5 and common to both D-leucine and D-arginine corresponds to the ionization of the substrate’s α-NH3+ group that initiates hydride transfer for amine oxidation (42, 77).
The acquired O2 reactivity of the E246L variant yielding a caged O2•ˉ/flavin semiquinone radical pair can be explained by the modified active site topology of the E246L variant. Studies on flavoproteins demonstrate that for an enzyme to react with O2, there must be a nonpolar residue and a positive charge close to the flavin cofactor to favor the electrostatics required for O2 reactivity (18, 20, 21, 81, 83). In PaDADH, the interaction between the R222/R305 network and the α-carboxylate group renders the amino acid substrate or imino acid product with a net positive charge in the ligand-bound state (Fig. 9). Nonetheless, in the free form of the enzyme, which is the form that reacts with O2, the positive charge likely arises from either R222 or R305. However, the wildtype enzyme contains only polar amino acid residues, negating one requirement for O2 reactivity (66). Conversely, the presence of the nonpolar L246 in PaDADH E246L provides a suitable active site topology for O2 reactivity, having a positive charge and a nonpolar residue (24). Thus, the E246L variant enzyme gains the ability to react with O2. Similarly, PaDADH reactivity with O2 has been recently reported for the Y249F variant enzyme after replacing the polar tyrosine 249 residue with the nonpolar phenylalanine residue in the active site (105, 106). However, unlike this study, the Y249F variant’s O2 reactivity yielded either a flavin N5 adduct or a green 6-OH-FAD species. Nonetheless, these studies exemplify how substrates and protein residues dictate the versatility of flavin reactivity to favor specific reactions (105, 106, 107, 108).
In conclusion, this study has used steady-state and rapid-reaction kinetic approaches to investigate the effects of the E246L mutation on the ability of PaDADH to react with O2 and the role of the generated O2•ˉ in the catalysis of the PaDADH E246L variant enzyme. The study demonstrates a mutation-induced gain of PaDADH reactivity with O2. pH studies on the steady-state kinetic parameters of the variant enzyme demonstrate that the O2•ˉ generated during PaDADH E246L catalysis diverts protons from the active site during D-arginine binding, leading to an observed nonstoichiometric slope of +1.5 in the log (kcat/Km) pH profile plot. Conventionally, pH profile slopes of enzyme variants have been the paradigm for identifying ionizable residues involved in enzyme catalysis (37, 38, 39, 40, 41, 42, 43). The technique, which is widely used across the field of enzymology, relies on the identification of integer slopes in pH profile plots for the assignment of catalytic groups after site-directed mutagenesis of enzyme residues. To date, not much information exists on interpreting data from systems with nonstoichiometric pH profile slopes, thereby limiting the applications of pH profiles when elucidating enzyme catalytic mechanisms in such systems. This study provides useful information on correcting and interpreting nonstoichiometric slopes in the pH plots of enzymes that react with O2 to form O2•ˉ during catalysis (99, 101, 109). Additionally, this study demonstrates that a charge and nonpolar residue near the flavin can induce oxygen reactivity in an enzyme.
Experimental procedures
Materials
Escherichia coli strain Rosetta(DE3)pLysS was purchased from Novagen. The QIAprep Spin Miniprep Kit and the QIAquick Polymerase Chain Reaction Purification Kit were obtained from Qiagen. Pfu DNA polymerase was purchased from Stratagene, and DpnI was obtained from New England BioLabs. Oligonucleotides were purchased from Sigma Genosys for site-directed mutagenesis and sequencing of the variant genes. Bovine liver superoxide dismutase and PMS were from MilliporeSigma. D-Amino acids were obtained from Alfa-Aesar. All other reagents used were obtained at the highest purity commercially available.
Site-directed mutagenesis, protein expression, and purification
The E246L variant gene of PaDADH was engineered by mutagenic polymerase chain reaction (PCR) with the pET20b(+)/PA3863 plasmid harboring the wildtype gene (dauA) as a template. A concentration of 5% dimethyl sulfoxide was added to the PCR reaction mixture to ensure proper separation of the high GC-rich, double-stranded DNA template. Site-directed mutagenesis amplicons were purified using the QIAquick PCR Purification Kit following the manufacturer’s protocol. The purified plasmid was then subjected to endonuclease activity using DpnI at 37 oC for 2 h. The resulting plasmid was used to transform the DH5α strain of E. coli cells. The success of the mutation was confirmed by sequencing the gene using the services of Humanizing Genomics Microgen USA Corp in Maryland. The E246L variant enzyme of PaDADH was then expressed in E. coli Rosetta(DE3)pLysS and purified to homogeneity as previously described for the PaDADH wildtype enzyme in the presence of 10% (v/v) glycerol for enzyme stability and to prevent the loss of the bound FAD cofactor (44). The purified enzyme was stored at −20 oC in 20 mM Tris-Cl, pH 8.0, and 10% glycerol and was found to be active for at least 6 months.
Rapid-reaction kinetics of the PaDADH E246L variant enzyme
To establish the effect of the E246L mutation on the rapid-reaction kinetic parameters of the PaDADH E246L variant enzyme and whether the mutation resulted in an acquired reactivity of the enzyme with O2, the reductive half-reaction of the PaDADH E246L variant enzyme was carried out under aerobic conditions. Using an SF-61DX2 Hi-Tech KinetAsyst performance stopped-flow spectrophotometer, the reaction was followed in 20 mM NaPPi, pH 10.0, and 25 oC and compared to the anaerobic reductive half-reactions of the wildtype and E246L variant enzymes that were previously investigated (42, 70). Since ∼80% of flavin reduction occurs in the mixing time (2.2 ms) of the stopped-flow spectrophotometer with D-arginine as a substrate (42, 71), the flavin reduction of the E246L variant enzyme was investigated with D-leucine as the reducing substrate. Substrate solutions (1–40 mM) were loaded into syringes and mounted onto the stopped-flow spectrophotometer. The reaction was followed by observing the spectroscopic decay of the 446 nm flavin peak over time upon reacting ∼10 μM enzyme with substrate solutions under pseudo-first-order conditions in single mixing mode.
Data analysis
The time-resolved flavin reductions were fit to Equation 1, which describes a triple exponential process for flavin reduction. Here, kobs1, kobs2, and kobs3 represent the observed first-order rate constant for reducing the enzyme-bound flavin at any given substrate concentration at 446 nm. A represents the absorbance at 446 nm at any given time, B1, B2, and B3 are the amplitudes of the absorption changes, t is time, and C is the absorbance at an infinite time that accounts for the nonzero absorbance of the fully reduced enzyme-bound flavin.
| (Eq 1) |
The resulting kinetic parameters of the reductive half-reaction were determined after fitting the observed rate constants for flavin reduction at various D-leucine concentrations with Equation 2. The equation defines a hyperbolic saturation of the enzyme with the D-leucine substrate, yielding a y-intercept value of zero. The data were fit with the KaleidaGraph software (Synergy Software). Here, kobs1 represents the observed first-order rate constant for reducing the enzyme-bound flavin at any substrate concentration (S). kred is the limiting first-order rate constant for flavin reduction at saturating substrate concentrations. Kd is the apparent equilibrium constant for dissociating the enzyme–substrate complex into the free substrate and enzyme. The same data were obtained when an equation that defines a hyperbolic saturation with a finite y-intercept was used.
| (Eq 2) |
O2 reactivity studies of the PaDADH E246L variant enzyme
To investigate the effect of the E246L mutation on the ability of the PaDADH E246L variant enzyme to react with O2 under steady-state conditions, the initial velocities of the enzyme reaction were measured with D-leucine as a substrate and O2 as an electron acceptor at pH 8.5. The reduction of O2 was followed using a Clark-type oxygen electrode in a 1 ml reaction volume containing final enzyme concentrations of 0.48 μM to 9.7 μM and fixed D-leucine concentration at 25 mM in 20 mM NaPPi at 25 oC. Substrate solutions were prepared in the reaction buffer, and the pH was readjusted after the amino acid substrate was dissolved. The experiment was repeated with D-arginine as substrate at pH 8.5, in a 1 ml reaction volume containing final enzyme concentrations of 0.17 μM to 8.5 μM and fixed D-arginine concentration at 5 mM in 20 mM NaPPi at 25 oC.
In a separate experiment, the dependence of the PaDADH E246L variant enzyme’s O2 reactivity on substrate concentration was investigated at fixed 0.5 μM enzyme and varying concentrations of D-arginine (1 mM – 20 mM) or D-leucine (1 mM–10 mM).
To probe the oxygen species generated by the E246L variant O2 reaction, the enzymatic assay was carried out with 6 μM E246L variant enzyme and fixed 5 mM D-arginine or 25 mM D-leucine. The reaction was then repeated by adding 200 to 500 units of superoxide dismutase to the reaction mixture.
To investigate the effect of the PaDADH E246L-generated O2•ˉ on the enzyme’s ability to turnover with D-arginine, the steady-state kinetic properties of the variant enzyme with O2 as an electron acceptor were investigated using ∼6 μM enzyme with 0.15 mM, 0.24 mM, or 0.5 mM D-arginine. Upon reaching a plateau in the reaction cycle, D-arginine was reintroduced to the reaction mixture to yield a total of four reaction cycles.
pH effects on the steady-state kinetics of the PaDADH E246L variant enzyme
To determine the effects of pH on the steady-state kinetics of the E246L variant enzyme of PaDADH, the apparent steady-state kinetic parameters of the enzyme with D-arginine or D-leucine as a substrate and PMS as an artificial electron acceptor were obtained by monitoring the initial PMS-driven O2 consumption rates with a computer-interfaced Oxy-32 oxygen-monitoring system (Hansatech Instruments Ltd) under similar conditions. D-Arginine concentrations were between 0.1 and 80 mM, D-leucine concentrations were between 1.25 and 62.5 mM, the enzyme concentration ranged from 1.15 μM to 5.75 μM, PMS concentration was fixed at 1 mM, and the pH ranged from 5.0 to 10.5 at 25 oC. Temperature effects on the steady-state pH profiles for D-arginine were investigated by repeating the assays at 12 oC. All assays were carried out to ensure that the Km value was within the range of the substrate concentrations used at each pH. To ensure that the variant enzyme was fully saturated with PMS, the steady-state kinetic parameters were also determined at 1.5 mM PMS, yielding similar results.
Due to the observation of a nonstoichiometric slope of +1.5 for the log (kcat/Km) pH profile with D-arginine at 12 oC and 25 oC, the assay was repeated with 200 to 500 units of superoxide dismutase in each apparent steady-state reaction mixture to investigate the effect of the enzyme-O2 reactivity on the D-arginine pH profile. The data analyses and interpretation focused only on the kcat/Km pH profiles due to the observation that superoxide dismutase affected only the kcat/Km pH profiles with D-arginine.
For the D-arginine substrate, the log values of the kcat/Km parameters under varying conditions of temperature and superoxide dismutase were plotted by fitting the log values of the kcat/Km parameters with Equation 3. The equation describes a curve that increases with increasing pH with a slope of S and a pH-independent limiting value (CH) at high pH.
The plot of the kcat/Km parameter for the D-leucine substrate was made by fitting the log values with Equation 4, which describes a curve that increases with increasing pH with a slope of +1 and a pH-independent limiting value (CH) at high pH.
| (Eq 3) |
| (Eq 4) |
Data availability
All data are contained within the manuscript.
Conflict of interest
The authors declare no conflicts of interest with the contents of this article.
Acknowledgments
We acknowledge the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311, for the Molecular graphics and analyses performed with UCSF Chimera. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
J. A. Q. and G. G. writing–review & editing; J. A. Q., K. E. W., and D. O. writing–original draft; J. A. Q., K. E. W., C. S., and D. O. investigation; J. A. Q. and G. G. formal analysis; J. A. Q. and G. G. conceptualization; G. G. supervision; G. G. project administration; G. G. funding acquisition.
Funding and additional information
This work was partly supported by Georgia State University’s Department of Chemistry (J. A. Q.), a Georgia Alabama Louis Stokes Alliance for Minority Participation Fellowship at GSU (K. E. W.), and a Molecular Basis of Disease Fellowship from Georgia State University (C. S. & D. O.).
Reviewed by members of the JBC Editorial Board. Edited by Joan B. Broderick
Footnotes
Enzymes that react with O2 to yield H2O2 are classified as oxidases while those that react with O2 to yield H2O are classified as monooxygenases (17, 18). In the case of the PaDADH E246L variant enzyme, neither H2O2 nor H2O is produced during turnover with O2 as an electron acceptor, instead, O2•ˉ is produced. Hence, the enzymatic activity of the PaDADH E246L variant enzyme with O2 yielding O2•ˉ has been labeled as an O2-driven dehydrogenase activity.
References
- 1.Matte A., Goldie H., Sweet R.M., Delbaere L.T.J. Crystal structure of Escherichia coli phosphoenolpyruvate carboxykinase: a new structural family with the P-loop nucleoside triphosphate hydrolase fold. J. Mol. Biol. 1996;256:126–143. doi: 10.1006/jmbi.1996.0072. [DOI] [PubMed] [Google Scholar]
- 2.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997;74:1–20. doi: 10.1016/s0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 3.An H., Tu C., Ren K., Laipis P.J., Silverman D.N. Proton transfer within the active-site cavity of carbonic anhydrase III. Biochim. Biophys. Acta. 2002;1599:21–27. doi: 10.1016/s0167-4838(02)00374-6. [DOI] [PubMed] [Google Scholar]
- 4.Taoka S., Tu C., Kistler K.A., Silverman D.N. Comparison of intra- and intermolecular proton transfer in human carbonic anhydrase II. J. Biol. Chem. 1994;269:17988–17992. [PubMed] [Google Scholar]
- 5.Kiefer L.L., Fierke C.A. Functional Characterization of human carbonic anhydrase II variants with altered Zinc binding sites. Biochemistry. 1994;33:15233–15240. doi: 10.1021/bi00255a003. [DOI] [PubMed] [Google Scholar]
- 6.Gudiksen K.L., Urbach A.R., Gitlin I., Yang J., Vazquez J.A., Costello C.E., et al. Influence of the Zn(II) cofactor on the refolding of bovine carbonic anhydrase after denaturation with sodium dodecyl sulfate. Anal. Chem. 2004;76:7151–7161. doi: 10.1021/ac0488560. [DOI] [PubMed] [Google Scholar]
- 7.Tu C., N.Silverman D., Forsman C., Harald Jonsson B., Lindskog S. Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. Biochemistry. 2002;28:7913–7918. doi: 10.1021/bi00445a054. [DOI] [PubMed] [Google Scholar]
- 8.Smitherman C., Rungsrisuriyachai K., Germann M.W., Gadda G. Identification of the catalytic base for alcohol activation in choline oxidase. Biochemistry. 2015;54:413–421. doi: 10.1021/bi500982y. [DOI] [PubMed] [Google Scholar]
- 9.Wongnate T., Sucharitakul J., Chaiyen P. Identification of a catalytic base for sugar oxidation in the pyranose 2-oxidase reaction. ChemBioChem. 2011;12:2577–2586. doi: 10.1002/cbic.201100564. [DOI] [PubMed] [Google Scholar]
- 10.Pennati A., Gadda G. Involvement of ionizable groups in catalysis of human liver glycolate oxidase. J. Biol. Chem. 2009;284:31214–31222. doi: 10.1074/jbc.M109.040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J., Konigsberg W.H. Two-metal-Ion catalysis: inhibition of DNA polymerase activity by a Third Divalent metal ion. Front. Mol. Biosci. 2022;9:1–10. doi: 10.3389/fmolb.2022.824794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan R., Roderick S.L., Huang B., Cook P.F. Roles of Histidines 154 and 189 and aspartate 139 in the active site of serine Acetyltransferase from Haemophilus influenzae. Biochemistry. 2008;47:6322–6328. doi: 10.1021/bi800075c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craggs P.D., Mouilleron S., Rejzek M., De Chiara C., Young R.J., Field R.A., et al. The mechanism of Acetyl transfer catalyzed by Mycobacterium tuberculosis GlmU. Biochemistry. 2018;57:3387–3401. doi: 10.1021/acs.biochem.8b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quaye O., Cowins S., Gadda G. Contribution of flavin covalent linkage with histidine 99 to the reaction catalyzed by choline oxidase. J. Biol. Chem. 2009;284:16990–16997. doi: 10.1074/jbc.M109.003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.E.Alber B.M., Colangelo C., Dong J., M.V.Stålhandske C.T., Baird T., Tu C., et al. Kinetic and spectroscopic Characterization of the Gamma-carbonic anhydrase from the Methanoarchaeon Methanosarcina thermophila. Biochemistry. 1999;38:13119–13128. doi: 10.1021/bi9828876. [DOI] [PubMed] [Google Scholar]
- 16.Massey V. The reactivity of oxygen with flavoproteins. Int. Congr. Ser. 2002;1233:3–11. [Google Scholar]
- 17.Massey V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem. 1994;269:22459–22462. [PubMed] [Google Scholar]
- 18.Romero E., Gómez Castellanos J.R., Gadda G., Fraaije M.W., Mattevi A. Same substrate, Many reactions: oxygen activation in flavoenzymes. Chem. Rev. 2018;118:1742–1769. doi: 10.1021/acs.chemrev.7b00650. [DOI] [PubMed] [Google Scholar]
- 19.Malmstrom B.G. Enzymology of oxygen. Annu. Rev. Biochem. 1982;51:21–59. doi: 10.1146/annurev.bi.51.070182.000321. [DOI] [PubMed] [Google Scholar]
- 20.Mattevi A. To be or not to be an oxidase: challenging the oxygen reactivity of flavoenzymes. Trends Biochem. Sci. 2006;31:276–283. doi: 10.1016/j.tibs.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Chaiyen P., Fraaije M.W., Mattevi A. The enigmatic reaction of flavins with oxygen. Trends Biochem. Sci. 2012;37:373–380. doi: 10.1016/j.tibs.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Klinman J.P. How do enzymes activate oxygen without inactivating themselves? Acc. Chem. Res. 2007;40:325–333. doi: 10.1021/ar6000507. [DOI] [PubMed] [Google Scholar]
- 23.Du Y., Xu J. Engineered bifunctional proteins for targeted cancer therapy: prospects and challenges. Adv. Mater. 2021;33:1–32. doi: 10.1002/adma.202103114. [DOI] [PubMed] [Google Scholar]
- 24.Gadda G. Oxygen activation in flavoprotein oxidases: the importance of being positive. Biochemistry. 2012;51:2662–2669. doi: 10.1021/bi300227d. [DOI] [PubMed] [Google Scholar]
- 25.Hayyan M., Hashim M.A., Alnashef I.M. Superoxide ion: generation and chemical implications. Chem. Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 26.Bielski B.H.J., Cabelli D.E., Arudi R.L., Ross A.B. Reactivity of HO2/O2− radicals in Aqueous solution. J. Phys. Chem. Ref. Data. 1985;14:1041–1100. [Google Scholar]
- 27.Agarwal P.K. A Biophysical Perspective on enzyme catalysis. Biochemistry. 2019;58:438–449. doi: 10.1021/acs.biochem.8b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadda G. Cofactor assisted enzymatic catalysis. Arch. Biochem. Biophys. 2014;544:1. doi: 10.1016/j.abb.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Bruice T.C. A view at the millennium: the efficiency of enzymatic catalysis. Acc. Chem. Res. 2002;35:139–148. doi: 10.1021/ar0001665. [DOI] [PubMed] [Google Scholar]
- 30.Nakamoto H., Bardwell J.C.A. Catalysis of disulfide bond formation and isomerization in the Escherichia coli periplasm. Biochim. Biophys. Acta. 2004;1694:111–119. doi: 10.1016/j.bbamcr.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Kunugi S., Hirohara H., Ise N. pH and temperature dependences of Thermolysin catalysis catalytic role of Zinc-Coordinated water. Eur. J. Biochem. 1982;124:157–163. doi: 10.1111/j.1432-1033.1982.tb05919.x. [DOI] [PubMed] [Google Scholar]
- 32.Finnegan S., Yuan H., Wang Y.F., Orville A.M., Weber I.T., Gadda G. Structural and kinetic studies on the Ser101Ala variant of choline oxidase: catalysis by compromise. Arch. Biochem. Biophys. 2010;501:207–213. doi: 10.1016/j.abb.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Gadda G., Fan F., Hoang J.V. On the contribution of the positively charged headgroup of choline to substrate binding and catalysis in the reaction catalyzed by choline oxidase. Arch. Biochem. Biophys. 2006;451:182–187. doi: 10.1016/j.abb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Quaye J.A., Gadda G. The Pseudomonas aeruginosa PAO1 metallo flavoprotein D-2-hydroxyglutarate dehydrogenase requires Zn2+ for substrate orientation and activation. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quaye J.A., Gadda G. Uncovering Zn2+ as a cofactor of FAD-dependent Pseudomonas aeruginosa PAO1 D-2-hydroxyglutarate dehydrogenase. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.103007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quaye J.A., Gadda G. Kinetic and Bioinformatic Characterization of D-2-hydroxyglutarate dehydrogenase from Pseudomonas aeruginosa PAO1. Biochemistry. 2020;59:4833–4844. doi: 10.1021/acs.biochem.0c00832. [DOI] [PubMed] [Google Scholar]
- 37.Gadda G. pH and deuterium kinetic isotope effects studies on the oxidation of choline to betaine-aldehyde catalyzed by choline oxidase. Biochim. Biophys. Acta. 2003;1650:4–9. doi: 10.1016/s1570-9639(03)00188-2. [DOI] [PubMed] [Google Scholar]
- 38.Frenkel-Mullerad H., Avnir D. Sol-gel materials as efficient enzyme protectors: preserving the activity of phosphatases under extreme pH conditions. J. Am. Chem. Soc. 2005;127:8077–8081. doi: 10.1021/ja0507719. [DOI] [PubMed] [Google Scholar]
- 39.Pennati A., Zanetti G., Aliverti A., Gadda G. Effect of salt and pH on the reductive half-reaction of Mycobacterium tuberculosis FprA with NADPH. Biochemistry. 2008;47:3418–31425. doi: 10.1021/bi702250h. [DOI] [PubMed] [Google Scholar]
- 40.Gadda G., Choe D.Y., Fitzpatrick P.F. Use of pH and kinetic isotope effects to dissect the effects of substrate size on binding and catalysis by nitroalkane oxidase. Arch. Biochem. Biophys. 2000;381:138–144. doi: 10.1006/abbi.2000.2009. [DOI] [PubMed] [Google Scholar]
- 41.da Silva L.C.A., Honorato T.L., Cavalcante R.S., Franco T.T., Rodrigues S. Effect of pH and temperature on enzyme activity of Chitosanase produced under Solid stated Fermentation by Trichoderma spp. Indian J. Microbiol. 2012;52:60–65. doi: 10.1007/s12088-011-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan H., Xin Y., Hamelberg D., Gadda G. Insights on the mechanism of amine oxidation catalyzed by D-arginine dehydrogenase through pH and kinetic isotope effects. J. Am. Chem. Soc. 2011;133:18957–18965. doi: 10.1021/ja2082729. [DOI] [PubMed] [Google Scholar]
- 43.Purich D.L. Factors influencing enzyme activity. Enzyme Kinet. Catal. Contrl. 2010;1:379–484. [Google Scholar]
- 44.Yuan H., Fu G., Brooks P.T., Weber I., Gadda G. Steady-state kinetic mechanism and reductive half-reaction of D-arginine dehydrogenase from Pseudomonas aeruginosa. Biochemistry. 2010;49:9542–9550. doi: 10.1021/bi101420w. [DOI] [PubMed] [Google Scholar]
- 45.Quaye J.A. Vol. 238. Scholarlyworks @ Georgia State University; Atlanta, GA: 2022. pp. 1–387. (Biochemical Characterization of Selected Flavin-dependent Metabolic Enzymes of Pseudomonas aeruginosa PAO1 toward Alternative Therapeutic Development). [Google Scholar]
- 46.Blair J.M.A., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 47.Ma L., Conover M., Lu H., Parsek M.R., Bayles K., Wozniak D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009;5:1–11. doi: 10.1371/journal.ppat.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morello E., Saussereau E., Maura D., Huerre M., Touqui L. Pulmonary Bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westwater C., Kasman L.M., Schofield D.A., Werner P.A., Dolan J.W., Schmidt M.G., et al. Use of genetically engineered phage to deliver antimicrobial agents to bacteria: an alternative therapy for treatment of bacterial infections. Antimicrob. Agents Chemother. 2003;47:1301–1307. doi: 10.1128/AAC.47.4.1301-1307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spoering A.L., Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 2001;183:6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thi M.T.T., Wibowo D., Rehm B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020;21:1–25. doi: 10.3390/ijms21228671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chegini Z., Khoshbayan A., Taati Moghadam M., Farahani I., Jazireian P., Shariati A. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: a review. Ann. Clin. Microb. Antimicrob. 2020;19:1–17. doi: 10.1186/s12941-020-00389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Botelho J., Grosso F., Peixe L. Antibiotic resistance in Pseudomonas aeruginosa – mechanisms, epidemiology and evolution. Drug Resist. Updat. 2019;44:26–47. doi: 10.1016/j.drup.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Turner K.H., Everett J., Trivedi U., Rumbaugh K.P., Whiteley M. Requirements for Pseudomonas aeruginosa Acute burn and chronic Surgical wound infection. PLoS Genet. 2014;10:1–12. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glucksam-Galnoy Y., Sananes R., Silberstein N., Krief P., Kravchenko V.V., Meijler M.M., et al. The bacterial quorum-sensing Signal molecule N -3-Oxo-Dodecanoyl-l-Homoserine Lactone Reciprocally Modulates Pro- and Anti-Inflammatory Cytokines in Activated Macrophages. J. Immun. 2013;191:337–344. doi: 10.4049/jimmunol.1300368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y., Chen S., Ren Y., Cheng J., Liu Q.H. A broadband proximity-coupled dual-polarized microstrip antenna with L-shape backed cavity for X-band applications. AEU Int. J. Electron. Commun. 2015;69:1226–1232. [Google Scholar]
- 58.Juan C., Peña C., Oliver A. Host and pathogen Biomarkers for Severe Pseudomonas aeruginosa infections. J. Infect. Dis. 2017;215:S44–S51. doi: 10.1093/infdis/jiw299. [DOI] [PubMed] [Google Scholar]
- 59.Secor P.R., Michaels L.A., Ratjen A., Jennings L.K., Singh P.K. Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 2018;115:10780–10785. doi: 10.1073/pnas.1806005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schick A., Kassen R., Greenberg E.P. Rapid diversification of Pseudomonas aeruginosa in cystic fibrosis lung-like conditions. Proc. Natl. Acad. Sci. U. S. A. 2018;115:10714–10719. doi: 10.1073/pnas.1721270115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balasubramanian D., Schneper L., Kumari H., Mathee K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013;41:1–20. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J., Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernier S.P., Ha D.G., Khan W., Merritt J.H., O’Toole G.A. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res. Microbiol. 2011;162:680–688. doi: 10.1016/j.resmic.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Everett J., Turner K., Cai Q., Gordon V., Whiteley M., Rumbaugh K. Arginine is a critical substrate for the pathogenesis of Pseudomonas aeruginosa in burn wound infections. mBio. 2017;8:e02160–e02216. doi: 10.1128/mBio.02160-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li C., Lu C.-D. Arginine Racemization by Coupled Catabolic and Anabolic Dehydrogenases. National Academy of Sciences; Washington, DC: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ouedraogo D., Ball J., Iyer A., Reis R.A.G., Vodovoz M., Gadda G. Amine oxidation by D-arginine dehydrogenase in Pseudomonas aeruginosa. Arch. Biochem. Biophys. 2017;632:192–201. doi: 10.1016/j.abb.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 67.Ouedraogo D., Souffrant M., Vasquez S., Hamelberg D., Gadda G. Importance of loop L1 Dynamics for substrate capture and catalysis in Pseudomonas aeruginosa D-arginine dehydrogenase. Biochemistry. 2017;56:2477–2487. doi: 10.1021/acs.biochem.7b00098. [DOI] [PubMed] [Google Scholar]
- 68.Li C., Lu C.D. Arginine racemization by coupled catabolic and anabolic dehydrogenases. Proc. Natl. Acad. Sci. U. S. A. 2009;106:906–911. doi: 10.1073/pnas.0808269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu G., Yuan H., Li C., Lu C.D., Gadda G., Weber I.T. Conformational changes and substrate recognition in Pseudomonas aeruginosa D-arginine dehydrogenase. Biochemistry. 2010;49:8535–8545. doi: 10.1021/bi1005865. [DOI] [PubMed] [Google Scholar]
- 70.Quaye J.A., Ouedraogo D., Gadda G. Targeted mutation of a non-catalytic gating residue increases the rate of Pseudomonas aeruginosa D-arginine dehydrogenase catalytic turnover. J. Agric. Food Chem. 2023;71:17343–17352. doi: 10.1021/acs.jafc.3c05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gannavaram S., Sirin S., Sherman W., Gadda G. Mechanistic and computational studies of the reductive half-reaction of tyrosine to phenylalanine active site variants of d -arginine dehydrogenase. Biochemistry. 2014;53:6574–6583. doi: 10.1021/bi500917q. [DOI] [PubMed] [Google Scholar]
- 72.Hang Chin D., Chiericato G., Jr., J.Nanni E., Jr., T.Sawyer D. Proton-induced disproportionation of superoxide ion in aprotic media. J. Am. Chem. Soc. 2002;104:1296–1299. [Google Scholar]
- 73.Duan H.D., Khan S.A., Miller A.F. Photogeneration and reactivity of flavin anionic semiquinone in a bifurcating electron transfer flavoprotein. Biochim. Biophys. Acta Bioenerg. 2021;1862:148415–148427. doi: 10.1016/j.bbabio.2021.148415. [DOI] [PubMed] [Google Scholar]
- 74.Beinert H. Spectral characteristics of flavins at the semiquinoid oxidation level. J. Am. Chem. Soc. 1956;78:5323–5328. [Google Scholar]
- 75.Su D., Kabir M.P., Orozco-Gonzalez Y., Gozem S., Gadda G. Fluorescence properties of flavin semiquinone radicals in nitronate monooxygenase. ChemBioChem. 2019;20:1646–1652. doi: 10.1002/cbic.201900016. [DOI] [PubMed] [Google Scholar]
- 76.Ball J.A. Vol. 1. Scholarly works @ Georgia State University; Atlanta, GA: 2018. pp. 1–213. (Dissertation: Mechanistic Studies on D-Arginine Dehydrogenase and Functional Annotation of a Novel NADH:quinone Oxidoreductase (PA1024)). [Google Scholar]
- 77.Ball J., Bui Q.V.V., Gannavaram S., Gadda G. Importance of glutamate 87 and the substrate α-amine for the reaction catalyzed by D-arginine dehydrogenase. Arch. Biochem. Biophys. 2015;568:56–63. doi: 10.1016/j.abb.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 78.Dratch B.D., Orozco-Gonzalez Y., Gadda G., Gozem S. Ionic Atmosphere effect on the absorption spectrum of a flavoprotein: a reminder to consider solution ions. J. Phys. Chem. Lett. 2021;12:8384–8396. doi: 10.1021/acs.jpclett.1c02173. [DOI] [PubMed] [Google Scholar]
- 79.Reis R.A.G., Li H., Johnson M., Sobrado P. New frontiers in flavin-dependent monooxygenases. Arch. Biochem. Biophys. 2021;699 doi: 10.1016/j.abb.2021.108765. [DOI] [PubMed] [Google Scholar]
- 80.Gadda G., Francis K. Nitronate monooxygenase, a model for anionic flavin semiquinone intermediates in oxidative catalysis. Arch. Biochem. Biophys. 2010;493:53–61. doi: 10.1016/j.abb.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 81.Frederick R.E., Mayfield J.A., Dubois J.L. Regulated O2 activation in flavin-dependent monooxygenases. J. Am. Chem. Soc. 2011;133:12338–12341. doi: 10.1021/ja203397s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Francis K., Nishino S.F., Spain J.C., Gadda G. A novel activity for fungal nitronate monooxygenase: detoxification of the metabolic inhibitor propionate-3-nitronate. Arch. Biochem. Biophys. 2012;521:84–89. doi: 10.1016/j.abb.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 83.Franceschini S., Fedkenheuer M., Vogelaar N.J., Robinson H.H., Sobrado P., Mattevi A. Structural insight into the mechanism of oxygen activation and substrate selectivity of flavin-dependent N-hydroxylating monooxygenases. Biochemistry. 2012;51:7043–7045. doi: 10.1021/bi301072w. [DOI] [PubMed] [Google Scholar]
- 84.Su D., Aguillon C., Gadda G. Characterization of conserved active site residues in class I nitronate monooxygenase. Arch. Biochem. Biophys. 2019;672:108085. doi: 10.1016/j.abb.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 85.Giegel D., Jr C.H.W., Giegel A., Williams C.H. L-Lactate 2-monooxygenase from Mycobacterium smegmatis. Cloning from Mycobacterium. J. Biol. Chem. 1990;265:6626–6632. [PubMed] [Google Scholar]
- 86.Francis K., Gadda G. Kinetic evidence for an anion binding pocket in the active site of nitronate monooxygenase. Bioorg. Chem. 2009;37:167–172. doi: 10.1016/j.bioorg.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 87.Smitherman C., Gadda G. Evidence for a transient Peroxynitro acid in the reaction catalyzed by nitronate monooxygenase with propionate 3-nitronate. Biochemistry. 2013;52:2694–2704. doi: 10.1021/bi400030d. [DOI] [PubMed] [Google Scholar]
- 88.Eberlein G., Bruice T.C. The chemistry of a 1,5-diblocked flavin. 2. Proton and electron transfer steps in the reaction of dihydroflavins with oxygen. J. Am. Chem. Soc. 1983;105:6685–6697. [Google Scholar]
- 89.Pozzi M.H., Gawandi V., Fitzpatrick P.F. pH dependence of a mammalian Polyamine oxidase: insights into substrate Specificity and the role of Lysine 315. Biochemistry. 2009;48:1508–1516. doi: 10.1021/bi802227m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castro C., Smidansky E.D., Arnold J.J., Maksimchuk K.R., Moustafa I., Uchida A., et al. Nucleic acid polymerases employ a general acid for nucleotidyl transfer. Nat. Struct. Mol. Biol. 2009;16:212–218. doi: 10.1038/nsmb.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castro C., Smidansky E., Maksimchuk K.R., Arnold J.J., Korneeva V.S., Götte M., et al. Two proton transfers in the transition state for nucleotidyl transfer catalyzed by RNA- and DNA-dependent RNA and DNA polymerases. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4267–4287. doi: 10.1073/pnas.0608952104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vashishtha A.K., West A.H., Cook P.F. Probing the Chemical mechanism of Saccharopine Reductase from Saccharomyces cerevisiae using site-directed mutagenesis. Arch. Biochem. Biophys. 2015;584:98–106. doi: 10.1016/j.abb.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raber M.L., Arnett S.O., Townsend C.A. A conserved Tyrosyl–Glutamyl catalytic Dyad in Evolutionarily Linked enzymes: carbapenam Synthetase and β-Lactam Synthetase. Biochemistry. 2009;48:4959–4971. doi: 10.1021/bi900432n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrews L.D., Fenn T.D., Herschlag D. Ground state Destabilization by anionic Nucleophiles contributes to the activity of Phosphoryl transfer enzymes. PLoS Biol. 2013;11:1–18. doi: 10.1371/journal.pbio.1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ekanayake D.K., Andi B., Bobyk K.D., West A.H., Cook P.F. Glutamates 78 and 122 in the active site of Saccharopine dehydrogenase contribute to reactant binding and Modulate the Basicity of the acid-base catalysts. J. Biol. Chem. 2010;285:20756–20768. doi: 10.1074/jbc.M110.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thibodeaux C.J., Liu H.W. Mechanistic studies of 1-Aminocyclopropane-1-carboxylate Deaminase (ACCD): characterization of an Unusual PLP-dependent reaction. Biochemistry. 2011;50:1950–1962. doi: 10.1021/bi101927s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodríguez S.B., Stitt B.L., Ash D.E. Cysteine 351 is an essential Nucleophile in catalysis by Porphyromonas gingivalis Peptidylarginine Deiminase. Arch. Biochem. Biophys. 2010;504:190–196. doi: 10.1016/j.abb.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wayne Schultz L., Quirk D.J., Raines R.T. His⋯Asp catalytic Dyad of Ribonuclease A: structure and function of the wild-type, D121N, and D121A enzymes. Biochemistry. 1998;37:8886–8898. doi: 10.1021/bi972766q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He R., Cristobal J.R., Gong N.J., Richard J.P. Hydride transfer catalyzed by glycerol phosphate dehydrogenase: recruitment of an acidic amino acid side chain to Rescue a damaged enzyme. Biochemistry. 2020;59:4856–4863. doi: 10.1021/acs.biochem.0c00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dissanayake T., Swails J.M., Harris M.E., Roitberg A.E., York D.M. Interpretation of pH-activity profiles for acid-base catalysis from molecular Simulations. Biochemistry. 2015;54:1307–1313. doi: 10.1021/bi5012833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barry K.P., Taylor E.A. Characterizing the Promiscuity of LigAB, a Lignin Catabolite Degrading Extradiol Dioxygenase from Sphingomonas paucimobilis SYK-6. Biochemistry. 2013;52:6724–6736. doi: 10.1021/bi400665t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salvi F., Rodriguez I., Hamelberg D., Gadda G. Role of F357 as an oxygen gate in the oxidative half-reaction of choline oxidase. Biochemistry. 2016;55:1473–1484. doi: 10.1021/acs.biochem.5b01356. [DOI] [PubMed] [Google Scholar]
- 103.Wragg S., Drickamer K. Identification of amino acid residues that determine pH dependence of ligand binding to the Asialoglycoprotein Receptor during Endocytosis. J. Biol. Chem. 1999;274:35400–35406. doi: 10.1074/jbc.274.50.35400. [DOI] [PubMed] [Google Scholar]
- 104.Palfey B.A., Moran G.R., Entsch B., Ballou D.P., Massey V. Substrate recognition by “password” in p-hydroxybenzoate hydroxylase. Biochemistry. 1999;38:1153–1158. doi: 10.1021/bi9826613. [DOI] [PubMed] [Google Scholar]
- 105.Iyer A., Reis R.A.G., Agniswamy J., Weber I.T., Gadda G. Discovery of a new flavin N5-adduct in a tyrosine to phenylalanine variant of D-Arginine dehydrogenase. Arch. Biochem. Biophys. 2022;715:1–8. doi: 10.1016/j.abb.2021.109100. [DOI] [PubMed] [Google Scholar]
- 106.Iyer A., Reis R.A.G., Gannavaram S., Momin M., Spring-Connell A.M., Orozco-Gonzalez Y., et al. A single-point mutation in D-arginine dehydrogenase Unlocks a transient Conformational state resulting in altered cofactor reactivity. Biochemistry. 2021;60:711–724. doi: 10.1021/acs.biochem.1c00054. [DOI] [PubMed] [Google Scholar]
- 107.Romero E., Gadda G. Alcohol oxidation by flavoenzymes. Biomol. Concepts. 2014;5:299–318. doi: 10.1515/bmc-2014-0016. [DOI] [PubMed] [Google Scholar]
- 108.Piano V., Palfey B.A., Mattevi A. Flavins as covalent catalysts: new mechanisms Emerge. Trends Biochem. Sci. 2017;42:457–469. doi: 10.1016/j.tibs.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Mráček T., Holzerová E., Drahota Z., Kovářová N., Vrbacký M., Ješina P., et al. ROS generation and multiple forms of mammalian mitochondrial glycerol-3-phosphate dehydrogenase. Biochim. Biophys. Acta. 2014;1837:98–111. doi: 10.1016/j.bbabio.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 110.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., et al. UCSF Chimera - a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the manuscript.