Highlights
-
•
Plasma HIST1H2BK was a biomarker for predicting neoadjuvant-chemotherapy (NACT) response and prognosis.
-
•
HIST1H2BK inhibited 5-FU-induced apoptosis through upregulating A2M transcription and then activating LRP1/PI3K/Akt signaling pathway in GC cells.
-
•
HIST1H2BK-overexpression 5-FU-resistant GC cells transmitted 5-FU resistance to 5-FU-sensitive GC cells through secreted HIST1H2BK.
Keywords: HIST1H2BK, Neoadjuvant Chemotherapy, Gastric Cancer, Plasma, 5-Fluorouracil
Abstract
Background
Neoadjuvant chemotherapy (NACT) is routinely used to treat patients with advanced gastric cancer (AGC). However, the identification of reliable markers to determine which AGC patients would benefit from NACT remains challenging.
Methods
A systematic screening of plasma proteins between NACT-sensitive and NACT-resistant AGC patients was performed by a mass spectrometer (n = 6). The effect of the most differential plasma protein was validated in two independent cohorts with AGC patients undergoing NACT (ELISA cohort: n = 155; Validated cohort: n = 203). The expression of this candidate was examined in a cohort of AGC tissues using immunohistochemistry (n = 34). The mechanism of this candidate on 5-Fluorouracil (5-FU) resistance was explored by cell-biology experiments in vitro and vivo.
Results
A series of differential plasma proteins between NACT-sensitive and NACT-resistant AGC patients was identified. Among them, plasma HIST1H2BK was validated as a significant biomarker for predicting NACT response and prognosis. Moreover, HIST1H2BK was over-expression in NACT-resistant tissues compared to NACT-sensitive tissues in AGC. Mechanistically, HIST1H2BK inhibited 5-FU-induced apoptosis by upregulating A2M transcription and then activating LRP/PI3K/Akt pathway, thereby promoting 5-FU resistance in GC cells. Intriguingly, HIST1H2BK-overexpressing 5-FU-resistant GC cells propagated resistance to 5-FU-sensitive GC cells through the secretion of HIST1H2BK.
Conclusion
This study highlights significant differences in plasma protein profiles between NACT-resistant and NACT-sensitive AGC patients. Plasma HIST1H2BK emerged as an effective biomarker for achieving more accurate NACT in AGC. The mechanism of intracellular and secreted HIST1H2BK on 5-FU resistance provided a novel insight into chemoresistance in AGC.
Introduction
Gastric cancer (GC) is one of the malignant tumors with high incidence and mortality rates worldwide [1]. Recently, numerous studies have shown that neoadjuvant chemotherapy (NACT) can reduce the tumor stage and increase the rate of R0 resection without increasing surgical morbidity and mortality when compared to surgical treatment alone [2]. Although the effectiveness of NACT for advanced gastric cancer (AGC) has been recognized, only a subset of patients can benefit from it [[3], [4], [5], [6], [7]]. In light of this clinical challenge, it is imperative to identify effective biomarkers for predicting the efficacy of NACT in AGC patients. Currently, predictive markers for the response rate of NACT in AGC encompass various factors, including clinical pathological characteristics [8], genomic biomarkers [9], proteomic biomarkers [9], and radiomics parameters [[10], [11], [12]]. Since proteins serve as essential functional molecules in biological processes, this study places particular emphasis on proteomic biomarkers. The detection of proteomic biomarkers can be achieved through either tissue biopsy or liquid biopsy, with the latter offering advantages in terms of minimally invasive and convenient sampling [13,14]. Plasma proteins were the most common biomarkers of liquid biopsy and a series of differential plasma protein profiles related to chemotherapy effectiveness were performed in ovarian cancer [15], esophageal cancer [16], and locally advanced nasopharyngeal carcinoma [17]. However, a comprehensive screening of plasma proteins associated with NACT in AGC has not been systematically conducted.
HIST1H2BK was a subtype of histone proteins, that bound chromosomes, maintained chromosome stability and regulated gene transcription [18]. In previous studies, the over-expression of HIST1H2BK was associated with tumor metastasis and chemoresistance based on the transcriptome sequencing data [[19], [20], [21], [22]]. HIST1H2BK served as an oncogene that promotes the cell proliferation, migration and invasion of PANC-1 cells [23]. Moreover, HIST1H2BK over-expression was associated with the poor prognosis of low-grade glioma and lung adenocarcinoma based on the expression profiles and clinical information of The Cancer Genome Atlas Program (TCGA), Chinese Glioma Genome Atlas (CGGA) and Kaplan-Meier database [24,25]. It is intriguing to note that the presence of HIST1H2BK RNA in plasma has been identified as a diagnostic indicator for the identification of pancreatic ductal adenocarcinoma [26]. The EMT-like phenotype of A431 epithelial cancer cells was experimentally induced by inhibiting E-cadherin and stimulating EGFR, which led to a reprogramming of the proteome within extracellular vesicles in A431 epithelial cancer cells, resulting in an increased concentration of HIST1H2BK [27]. The mRNA and protein molecules derived from HIST1H2BK gene exhibit a secretion phenomenon, rendering them potentially valuable as liquid biopsy markers. In summary, previous investigations on HIST1H2BK in tumors have predominantly relied on transcriptome sequencing and basic cell experiments. Therefore, this study aims to undertake a meticulous and all-encompassing examination of HIST1H2BK.
In this study, a systematic screening of plasma proteins for predicting NACT response was conducted in AGC. Plasma HIST1H2BK was screened and validated as a predictive biomarker for NACT response, and an independent prognostic factor for overall survival (OS) and disease-free survival (DFS) in AGC patients undergoing NACT. HIST1H2BK was upregulated in NACT-resistant tissues compared to NACT-sensitive tissues in AGC. Mechanistically, HIST1H2BK inhibited apoptosis induced by 5-Fluorouracil (5-FU) by upregulating A2M transcription and subsequently activating the LRP/PI3K/Akt signaling pathway, thereby promoting resistance to 5-FU in gastric cancer (GC) cells. Interestingly, HIST1H2BK-overexpression 5-FU-resistant GC cells transmitted 5-FU resistance to 5-FU-sensitive GC cells via secreted HIST1H2BK.
Materials and methods
Study cohorts
In the MS cohort, plasmas from 3 NACT-sensitive AGC patients and 3 NACT-resistant AGC patients were analyzed to determine the presence of differentially expressed proteins. Plasmas from these patients before (pre-NACT) and after (post-NACT) NACT were obtained from the GC samples bank of the Sixth Affiliated Hospital of Sun Yat-sen University and stored at −80 °C. Beijing Genomics Institute (BGI) detected the differential plasma proteins using a Q-Exactive HF mass spectrometer (MS) and a data-independent analysis (DIA) technique. The demographic and baseline characteristics of MS cohort were presented in Table S1.
In ELISA cohort, to evaluate the predictive potential of NACT response of the above differential plasma proteins, a total of 155 samples were collected before NACT from April 2013 to December 2018 in the GC samples bank of the Sixth Affiliated Hospital of Sun Yat-sen University, including 11 samples from healthy, 47 samples from NACT-sensitive AGC patients and 97 samples from NACT-resistant AGC patients. The level of the differential proteins in plasmas was determined using an Enzyme-linked Immunosorbent Assay (ELISA) Kit (FineTest) according to the manufacturer's protocol.
In the validation cohort, to further validate the predictive potential on NACT response of the candidate plasma proteins, a total of 203 samples were collected before NACT from January 2019 to July 2022 in the GC samples biobank of the Sixth Affiliated Hospital of Sun Yat-sen University, including 51 samples from NACT-sensitive AGC patients and 152 samples from NACT-resistant AGC patients.
In GC-tissues cohort, to observe the role on NACT response of the candidate proteins in GC tissues, a total of 34 samples were obtained through gastroscope before NACT and stored in the GC samples bank of the Sixth Affiliated Hospital of Sun Yat-sen University, including 6 samples from NACT-sensitive AGC patients and 28 samples from NACT-resistant AGC patients.
The AGC patients of all cohorts were confirmed by gastroscopic pathology and computerized tomography, performed neoadjuvant SOX and followed by surgery. TRG-Ryan system with 0 to 3 tumor regression grades was used to assess the histopathological response of NACT [28]. The patients with 0 to 1 were defined as NACT-sensitive patients, while those with 2 to 3 were defined as NACT-resistant patients. The treatments and follow-ups for these patients were performed according to our institutional protocols that were based on the National Comprehensive Cancer Network guide as previously described [29]. The interval between the date of diagnosis and the date of death or the last follow-up visit was defined as OS. The interval between the date of R0 resection operation and the date of local recurrence and/or metastasis was defined as DFS. The clinicopathological variables were collected from the GC database of the Sixth Affiliated Hospital of Sun Yat-sen University.
The cell experiments in vitro and vivo
The specific conditions of cell experiments in vitro and vivo were detailed in Doc S1.
Statistical analysis
All the statistical analyses were performed using SPSS 20, GraphPad Prism 8.0 or R 4.1.0. The comparisons of MS data between NACT-sensitive and NACT-resistant groups were performed using the R package of MSstats. Receiver operating characteristic (ROC) model was used to distinguish NACT-resistant patients from AGC patients undergoing NACT. Comparisons between two or more groups were performed using Student's t-test, Wilcoxon signed-rank test or Chi-squared test. The survival analyses were performed using Kaplan–Meier analysis with Log-rank test and Cox proportional-hazards regression model. The cut-off value of HIST1H2BK expression to categorize low and high expression levels was determined according to Youden's index of Receiver operating characteristic (ROC) model. IC50s were performed using a Nonlinear regression model with Dose-response-inhibition method. The correlated analyses were performed using Spearman correlation analysis. Values of P < 0.05 were considered statistically significant in all analyses.
Results
Plasma HIST1H2BK was screened and validated as a predictive biomarker for NACT response in AGC
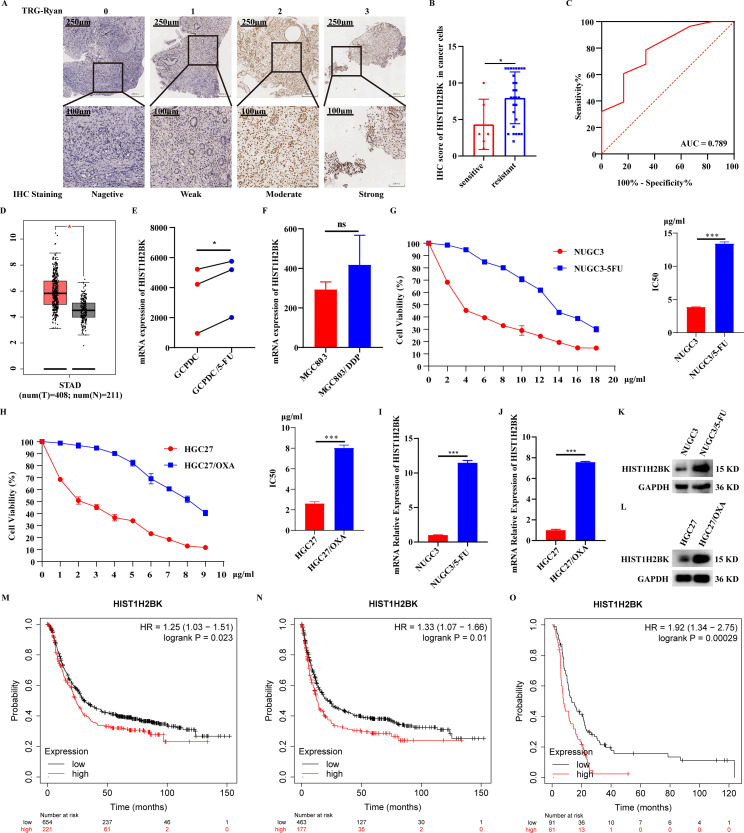
In plasmas collected prior to NACT of MS cohort, 12 proteins (HIST1H2BK cluster, SHH, CFAI, IBP4, HBD, HBB, CSTN3, CO4A2, CBPN, HBE, GRN and MMP14) were found to be differentially expressed between NACT-sensitive and NACT-resistant groups, and all of them were overexpression (Fig. 1A; Table S2). In plasmas collected after NACT, 29 proteins were identified between two groups, with 17 upregulated (H31 cluster, H4 cluster, HIST1H2BK cluster, ARPC2, ENSA, F13A, BTD, ALDOC, C1QA, UBA7, APOB, THRB, PP14A, A2MG, GUC2B, ECM1 and ABI1) and 12 downregulated (HV404, NIBA2, ADIRF, GRAN, SBP1, ENOA, RHOC, PF4V, MMP25, DOPD, MT2 and PECA1) (Fig. 1B; Table S3). HIST1H2BK histone cluster ranked highest among all differential plasma proteins between two groups before and after NACT (Fig. 1A-C).
Fig. 1.
Plasma HIST1H2BK was a predictive biomarker for neoadjuvant chemotherapy (NACT) response in advanced gastric cancer (AGC). (A-B) Volcano plots showed the differential plasma proteins between NACT-sensitive and NACT-resistant AGC patients before NACT (pre-NACT; A) and after NACT (post-NACT; B). (C) Wayne diagram showed different and overlapping differential plasma proteins between pre-NACT and post-NACT. (D-E) Comparision of plasma HIST1H2BK between NACT-sensitive and NACT-resistant patients in ELISA (D) or validated cohort (E). (F-I) Receiver operator curve (ROC) model based on plasma HIST1H2BK, CEA and CA19–9 to distinguish NACT-resistant patients from the whole AGC patients (F and H) or stage II patients (G and I) in ELISA (F-G) or validated cohort (H-I). (J-M) Kaplan–Meier curves of overall survival (OS; J, L) and disease-free survival (DFS; K, M) according to the concentrations of plasma HIST1H2BK in ELISA (J-K) or validated cohort (L-M). (N—O) Cox proportional hazard analyses on OS and DFS in AGC patients undergoing NACT. No significance (ns), P > 0.05; * P < 0.05; **P < 0.01; ***P < 0.001.
For further evaluating the predictive potential on NACT response of plasma HIST1H2BK, the plasmas of two independent cohorts with AGC patients undergoing NACT were collected. In ELISA cohort, the concentrations of plasma HIST1H2BK obtained from AGC patients were found to be higher in comparison to that from healthy individuals (Fig. 1D). Moreover, plasma HIST1H2BK obtained from NACT-resistant AGC patients were found to be elevated in comparison to that from NACT-sensitive AGC patients (Fig. 1D). Similarly, in validation cohorts, the concentrations of plasma HIST1H2BK obtained from NACT-resistant AGC patients were found to be up-regulated compared to that from NACT-sensitive AGC patients (Fig. 1E).
In ELISA cohort, using ROC model with plasma HIST1H2BK to distinguish NACT-resistant patients from AGC patients yielded an AUC value of 0.827 (Fig. 1F). Conversely, using ROC model with plasma CEA and CA19–9 yielded AUC values of 0.563 and 0.533, respectively (Fig. 1F). As it is debatable whether stage II AGC patients should undergo surgery or NACT first, a ROC analysis of plasma HIST1H2BK to distinguish NACT-resistant patients in stage II AGC patients was performed and revealed an AUC of 0.851 (Fig. 1G). Similar results have been observed in validated cohorts. Using ROC model with plasma HIST1H2BK to distinguish NACT-resistant patients from AGC patients yielded an AUC value of 0.822 (Fig. 1H). In subgroup analysis, a ROC analysis of plasma HIST1H2BK in stage II AGC patients revealed an AUC of 0.884 (Fig. 1I).
Plasma HIST1H2BK was an independent prognostic factor in AGC patients undergoing NACT
Due to the crucial role of NACT in determining the prognosis of AGC, our study aimed to explore the prognostic value of plasma HIST1H2BK as a NACT-resistant biomarker in AGC patients undergoing NACT. In ELISA cohort, the correlations between clinicopathological characteristics and plasma HIST1H2BK were shown in Table S4. In Kaplan-Meier curve analysis, the upregulation of plasma HIST1H2BK was associated with a worse outcome in AGC patients undergoing NACT (Fig. 1J-K). Further, in univariate Cox proportional hazards analysis, plasma HIST1H2BK retained its prognostic significance even after considering other variations (Fig. 1N).
In validated cohort, the correlations between clinicopathological characteristics and plasma HIST1H2BK were shown in Table S5. In Kaplan-Meier curve analysis, the higher expression of plasma HIST1H2BK was connected to a poorer outcome in AGC patients undergoing NACT. (Fig. 1L-M). Further, in univariate Cox proportional hazards analysis, plasma HIST1H2BK continued to exhibit prognostic significance even after accounting for other variations (Fig. 1O).
The overexpression of HIST1H2BK was significantly associated with chemoresistance in GC
Since plasma HIST1H2BK was differentially expressed, we further investigated HIST1H2BK over-expression in NACT-resistant tissues compared with NACT-sensitive tissues in AGC (Fig. 2A-B). ROC model based on HIST1H2BK in AGC tissues to distinguish NACT-resistant patients from AGC patients undergoing NACT yielded AUC of 0.789 (Fig. 2C). The correlations between clinicopathological characteristics and HIST1H2BK in AGC tissues were shown in Table S6. Based on the expression profiles from GEPIA database, HIST1H2BK was over-expression in tumor tissues compared with normal tissues in GC (Fig. 2D). Based on the expression profiles from GEO database, HIST1H2BK was upregulated in 5-FU-resistant patient-derived gastric cancer cell (PDGCC) compared with 5-FU-sensitive PDGCC (Fig. 2E). Moreover, HIST1H2BK was over-expression in DDP-resistant MGC803 cells compared with DDP-sensitive MGC803 cells, but there is no statistical difference (Fig. 2F). Furthermore, a 5-FU-resistant GC cell and an OXA-resistant GC cell were established (Fig. 2G-H), and HIST1H2BK was upregulated in resistant GC cells compared with sensitive GC cells (Fig. 2I-L). Based on the expression profiles and clinical information from Kaplan-Meier Plotter database, worse outcomes were observed in GC patients with high HIST1H2BK compared to those with low HIST1H2BK, including OS (Fig. 2M) and DFS (Fig. 2N). In subgroup analysis, high HIST1H2BK was associated with poor OS in GC patients undergoing chemotherapy (Fig. 2O).
Fig. 2.
The expression profile and prognosis of HIST1H2BK in GC tissues. (A) Representative immunohistochemistry (IHC) images of HIST1H2BK in AGC tissues from gastroscopic samples before NACT. (B) HIST1H2BK was over-expression in NACT-resistant tissues compared with NACT-sensitive tissues in AGC. (C) ROC model based on HIST1H2BK in AGC tissues to distinguish NACT-resistant patients from AGC patients. (D) The differential expression of HIST1H2BK between normal and tumor tissues in GC from GEPIA database. (E) The differential expression of HIST1H2BK between 5-FU-resistant and sensitive patient-derived gastric cancer cell (PDGCC) from GSE139331 data set. (F) The differential expression of HIST1H2BK between DDP-resistant and sensitive MGC803 cells from GSE192631 data set. (G-H) The validation for successful construction of 5-fluorouracil-resistant (5-FU; G) and oxaliplatin-resistant (OXA; H) GC cells. (I-L) The mRNA and protein expression of HIST1H2BK in 5-FU-resistant and OXA-resistant GC cells. (M-O) The prognosis analysis of HIST1H2BK in GC from Kaplan-Meier Plotter database. No significance (ns), P > 0.05; * P < 0.05; **P < 0.01; ***P < 0.001.
HIST1H2BK promoted the 5-FU resistance and proliferation of GC cells
In this study, we focused on the role of HIST1H2BK on 5-FU resistance in GC cells. The construction and validation of stable-transfected GC cells with HIST1H2BK cDNA or shRNA was shown in Fig. S1. In vitro, with the upregulation of HIST1H2BK, the IC50 of 5-FU (Fig. 3A-B) and cell proliferation (Fig. 3E-F) in NUGC3 and MKN45 cells were increased. On the contrary, with the downregulation of HIST1H2BK, the IC50 of 5-FU (Fig. 3C-D) and cell proliferation (Fig. 3G-H) in NUGC3/5-FU and HGC27 cells were decreased. After the upregulation or downregulation of HIST1H2BK, there was no difference in the mortality of GC cells (Fig. 3I-L). However, under the treatment of 5-FU, the mortality of NUGC3 and MKN45 cells was increased with the upregulation of HIST1H2BK, while the mortality of NUGC3/5-FU and HGC27 cells was reduced with the downregulation of HIST1H2BK (Fig. 3I-L).
Fig. 3.
HIST1H2BK promoted the 5-FU resistance and proliferation of GC. (A-D) The role of HIST1H2BK on the half-maximal inhibitory concentrations (IC50) of 5-FU in NUGC3 (A), MKN45 (B), NUGC3/5-FU (C), and HGC27 (D). (E-H) The role of HIST1H2BK on cell proliferation in NUGC3 (E), MKN45 (F), NUGC3/5-FU (G), and HGC27 (H) under the treatment of 5-FU (NUGC3, 1.5 μg/ml; MKN45,0.5 μg/ml; NUGC3/5-FU, 4 μg/ml; HGC27, 0.5 μg/ml) or not. (I-L) The role of HIST1H2BK on cell mortality in NUGC3 (I), MKN45 (J), NUGC3/5-FU (K), HGC27 (L) under the treatment of 5-FU or not. (M-O) The role of HIST1H2BK on the 5-FU resistance and proliferation of MKN45 cells in nude mice under the treatment of 5-FU or not. (P) Representative IHC images of HIST1H2BK, ki67 and cleaved-caspase3 in tumor tissues. No significance (ns), P > 0.05; * P < 0.05; **P < 0.01; ***P < 0.001.
In vivo, HIST1H2BK promoted tumor growth based on tumor volume and weight, regardless of 5-FU treatment (Fig. 3M-O). Ki-67 analysis revealed that HIST1H2BK promoted the proliferation of tumor cells in vivo (Fig. 3P). Although HIST1H2BK itself did not regulate tumor cell apoptosis as determined by c-caspase3 analysis, HIST1H2BK inhibited 5-FU-induced tumor cell apoptosis (Fig. 3P).
HIST1H2BK inhibited 5-FU-induced apoptosis through the activation of A2M transcription and LRP1/PI3K/Akt signaling pathway to promote the 5-FU resistance of GC cells
RNAseq-based heat map analysis revealed differential genes, with A2M ranking highest among these distinct genes (Fig. 4A). The mRNA expression of A2M was influenced by HIST1H2BK expression (Fig. 4B-C), and then produced the change of A2M protein level in GC cell lines (Fig. 4D). Dual-Luciferase Reporter assays confirmed that HIST1H2BK regulated the transcriptional activation of A2M (Fig. 4E-F). Moreover, the mRNA and protein expression of A2M was upregulated in 5-FU-resistant NUGC3 cells compared to wild-type NUGC3 cells (Fig. 4G-H). Furthermore, the silencing of A2M increased 5-FU-induced cell mortality that was inhibited by the upregulation of HIST1H2BK, while A2M overexpression reversed the effect of HIST1H2BK downregulation on 5-FU-induced cell mortality (Fig. 4I-Q).
Fig. 4.
HIST1H2BK inhibited 5-FU-induced apoptosis through the activation of A2M transcription and Akt signaling pathway. (A) Heat map analysis showed differential genes between NUGC3-vector and NUGC3-HIST1H2BK cells incubated in 1.5 μg/ml 5-FU for 48 h. (B-C) Quantitative real-time polymerase chain reactions were performed to detect the changes of A2M mRNA expression in the indicated cell lines. (D) Western blot was performed to detect the changes of A2M protein expression in the indicated cell lines. (E-F) Dual-luciferase reporter assays were performed to analyze the activity of the pGL3-A2M in the indicated cell lines. (G-H) A2M was upregulated in 5-FU-resistant NUGC3 cells compared to wild-type NUGC3 cells. (I) Western blot was performed to analyze the changes of A2M protein expression in the indicated cell lines. (J-Q) Annexin V-APC/7-AAD Apoptosis Kit were used to assess cell mortality in the indicated cell lines under the treatment of 5-FU.
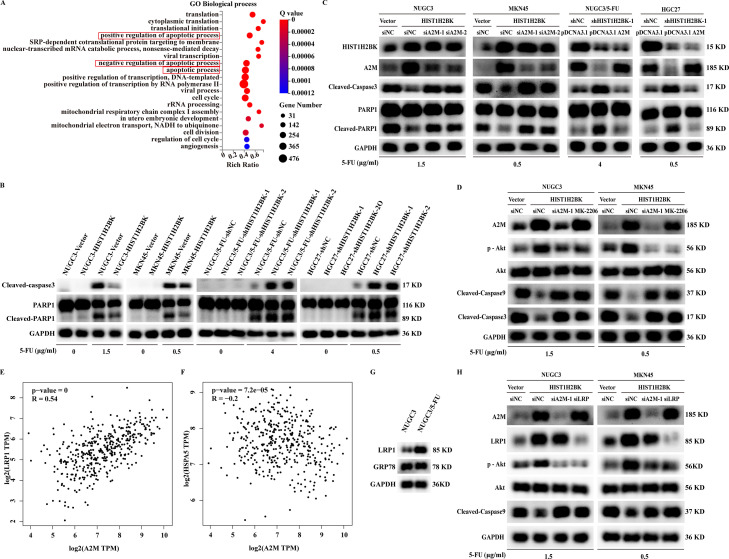
RNAseq-based GO analysis revealed that HIST1H2BK was significantly associated with three apoptosis-related biological processes (Fig. 5A). In validated experiments, HIST1H2BK did not directly modulate the expression of the main functional proteins of apoptosis, including cleaved-caspase3 and cleaved-PARP1 (Fig. 5B). However, HIST1H2BK inhibited the 5-FU-induced cleaved-caspase3 and cleaved-PARP1 (Fig. 5B). Since A2M was verified as a key target for HIST1H2BK in the above experiments, we investigated the role of A2M for HIST1H2BK on 5-FU-induced apoptosis. A2M silencing increased 5-FU-induced apoptosis that was inhibited by HIST1H2BK upregulation (Fig. 5C). In contrast, A2M overexpression reversed the effect of HIST1H2BK downregulation on 5-FU-induced apoptosis (Fig. 5C). Moreover, we evaluated that A2M inhibited 5-FU-induced apoptosis through activating the phosphorylation of Akt (Fig. 5D). Since LRP1 and GRP78 was the main receptors of A2M to activate PI3K/Akt signaling pathway [[30], [31], [32], [33]], we assessed that A2M was positively associated with LRP1 but passively associated with GRP78 in GC using GEPIA database (Fig. 5E-F). Moreover, LRP1 was over-expressed in 5-FU-resistant GC cells compared with wily-type GC cells, while GRP78 was not different (Fig. 5G). Therefore, we guessed and verified that A2M was bound to LRP1 and then activated PI3K/Akt signaling pathway (Fig. 5H).
Fig. 5.
HIST1H2BK inhibited 5-FU-induced apoptosis through the activation of A2M transcription and LRP1/PI3K/Akt signaling pathway. (A) GO Analysis showed that HIST1H2BK was significantly associated with the biological process of apoptosis. (B) Western blot was performed to detect the expression of cleaved-caspase3 and cleaved-PARP1 in the indicated cell lines under the treatment of 5-FU or not. (C) HIST1H2BK inhibited 5-FU-induced apoptosis through upregulating A2M expression. (D) HIST1H2BK-induced A2M inhibited 5-FU-induced apoptosis through activating PI3K/Akt signaling pathway. (E-F) The correlation analysis of A2M and LRP1 (or GRP78) in GC using GEPIA database. (G) The differential expression of LRP1 and GRP78 respectively between 5-FU-resistant and wily-type GC cells. (H) HIST1H2BK-induced A2M inhibited 5-FU-induced apoptosis through activating LRP1/PI3K/Akt signaling pathway. No significance (ns), P > 0.05; * P < 0.05; **P < 0.01; ***P < 0.001.
HIST1H2BK-overexpression 5-FU resistant GC cells induced the increasing 5-FU resistance of wild-type GC cells by the transmission of secreted HIST1H2BK
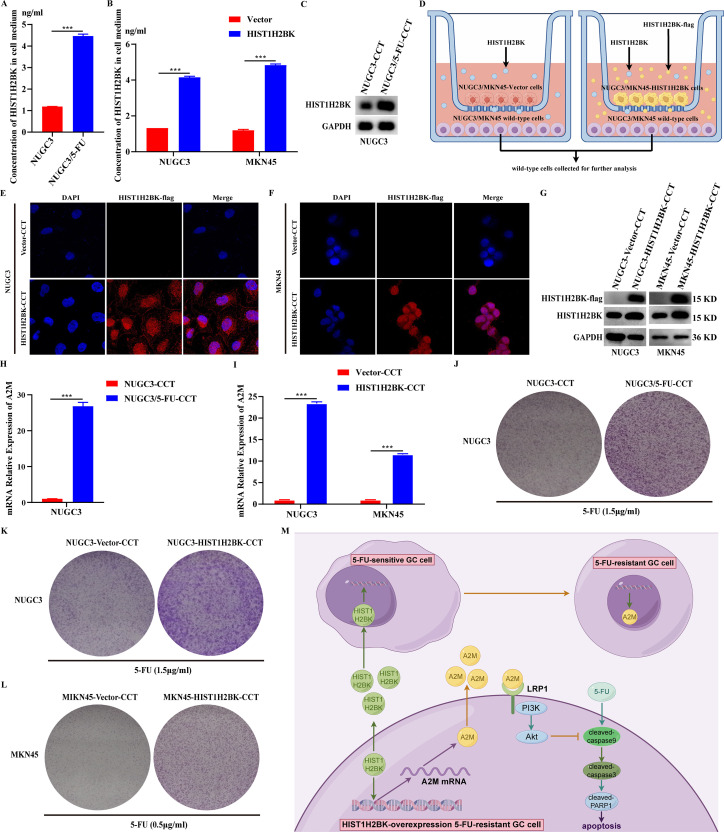
Previous research has shown that HIST1H2BK is a secreted protein [26,27], and the current study confirms that it is a 5-FU-resistant protein. The goal of our study is to find out how secreted HIST1H2BK affects the transfer of 5-FU resistance from 5-FU-resistant cells to 5-FU-sensitive cells. The concentration of secreted HIST1H2BK in the conditioned medium of 5-FU-resistant NUGC3 (NUGC3/5-FU) cells exhibited an upregulation compared to that of wild-type NUGC3 cells (Fig. 6A). The concentration of secreted HIST1H2BK in the conditioned medium of HIST1H2BK-overexpression GC cells exhibited an upregulation compared to that of GC cells with control vector (Fig. 6B). The expression level of HIST1H2BK in the NUGC3 cells, which was co-cultured with NUGC3/5-FU cells, exhibited a significant increase (Fig. 6C). Secreted HIST1H2BK, which originated from HIST1H2BK-overexpression GC cells, was genetically modified to include a FLAG tag. This modification allowed for the detection of secreted HIST1H2BK using an anti-FLAG antibody in the non-transfected wild-type GC cells that were co-cultured with HIST1H2BK-overexpression GC cells (Fig. 6D-F). The expression level of HIST1H2BK in the wild-type GC cells, which were co-cultured with HIST1H2BK-overexpression GC cells, exhibited a significant increase (Fig. 6G). To ascertain the biological activity of the secreted HIST1H2BK on 5-FU resistance in wild-type cells, we observed that NUGC3 cells co-cultured with NUGC3/5-FU cells, compared to those co-cultured with NUGC3 wild-type cells, exhibited higher levels of A2M expression (Fig. 6H). The wild-type GC cells co-cultured with HIST1H2BK-overexpression GC cells, compared to those co-cultured with GC cells with control vector, exhibited higher levels of A2M expression (Fig. 6I). Colony growth assay showed that NUGC3 cells co-cultured with NUGC3/5-FU cells exhibited lower cell mortality with the treatment of 5-FU (Fig. 6J). The wild-type GC cells co-cultured with HIST1H2BK-overexpression GC cells exhibited lower cell mortality with the treatment of 5-FU (Fig. 6K-L).
Fig. 6.
HIST1H2BK-overexpression 5-FU resistant GC cells induced the increasing 5-FU resistance of wild-type GC cells by the transmission of secreted HIST1H2BK. (A-B) The concentration of secreted HIST1H2BK in conditioned medium culturing the indicated cell lines. (C) Western blot was performed to detect the expression of HIST1H2BK in NUGC3 cells co-culturing with wild-type or 5-FU-resistant NUGC3 (NUGC3/5-FU) cells. (D) Schematic diagram of the co-culture between wild-type and HIST1H2BK-overexpression GC cells. (E-F) Immunofluorescence Assays was performed to detect secreted HIST1H2BK-flag in the wild cells that were co-cultured with HIST1H2BK-overexpression GC cells. Vector-CCT means NUGC3 cells (or MKN45 cells) co-culturing with NUGC3-vector cells (or MKN45-vector cells). HIST1H2BK-CCT means NUGC3 cells (or MKN45 cells) co-culturing with NUGC3-HIST1H2BK cells (or MKN45-HIST1H2BK cells). (G) Western blot was performed to detect the expression of HIST1H2BK-flag and HIST1H2BK in the indicated cell lines. (H-I) QRT-PCR was performed to detect A2M mRNA expression in the indicated cell lines. (J-L) Colony formation assay was performed to detect the cell survival of the indicated cell lines under the treatment of 5-FU. (M) Schematic diagram of the mechanism of HIST1H2BK on 5-FU resistance in GC. No significance (ns), P > 0.05; * P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Differential plasma protein detection is considered a suitable approach for predicting NACT response in esophageal cancer and nasopharyngeal carcinoma [16,17]. This study indicated significant differences in plasma protein profiles between NACT-resistant and NACT-sensitive patients with AGC, suggesting that differential plasma protein detection may be a feasible method for predicting NACT response in AGC. Among these differential proteins, HIST1H2BK histone cluster exhibited the most significant difference. HIST1H2BK histone cluster included HIST1H2BK, HIST1H2BE, H2BFS, HIST1H2BN, HIST1H2BH, HIST1H2BD, HIST1H2BL, HIST1H2BF, HIST1H2BM. In this study, we focused on the role of HIST1H2BK in predicting NACT response. Upregulated plasma HIST1H2BK showed good diagnostic performances for predicting NACT resistance in two independent cohorts with AUCs of 0.827 and 0.822 respectively. There have been debates regarding the optimal treatment sequence between surgery and NACT for stage II AGC patients. Therefore, predicting NACT response in stage II AGC patients holds great clinical significance. Subgroup analysis in stage II AGC patients demonstrated the good predictive ability of plasma HISHT1H2BK in two independent cohorts with AUCs of 0.851 and 0.884 respectively. These results highlighted the effectiveness of using differential plasma protein detection to predict NACT response in AGC. Plasma-based testing is non-invasive, and the ELISA method used in this study for detecting plasma proteins is suitable for clinical batch testing.
The basic function of histones is to bind to DNA, stabilize chromosomes, and regulate gene transcription [34]. Previous research has primarily focused on histone modifications rather than the histone proteins themselves [35]. However, recent studies have shown that some histone subtypes differ only by a few amino acids, but these subtle differences may confer unique functions [36,37]. In this study, a group of histone subtypes potentially related to the NACT resistance of AGC was identified through proteomic screening of plasmas. In previous studies, HIST1H2BK was over-expression in the doxorubicin-resistant breast cancer cell line MDA-MB-231 based on the transcriptome sequencing data [22]. Here, experiments conducted both in vivo and in vitro have confirmed that histone HIST1H2BK plays a role in promoting 5-FU resistance in GC cells. Mechanistically, we found that HIST1H2BK mediates 5-FU resistance in GC cells by promoting the transcription of A2M. Liu et al. reported that knockout of A2M enhances the sensitivity of acute myeloid leukemia cells to cytarabine [38]. However, the mechanisms underlying A2M-mediated chemoresistance remain unexplored. A2M always binds to LRP1 or GRP78 and then performs its biological functions [30,31]. LRP1 and GRP78 decreased chemotherapy-induced apoptosis and promoted chemoresistance through the activation of PI3K/Akt signaling pathway [32,33]. Therefore, we assessed that A2M was positively associated with LRP1 but passively associated with GRP78 in GC using GEPIA database. Moreover, LRP1 was over-expression in 5-FU-resistant GC cells compared with wily-type GC cells, while GRP78 was not different. Furthermore, we guessed and verified that A2M was bound to LRP1 and then activated PI3K/Akt signaling pathway (Fig. 6M).
GC is characterized by significant tumor heterogeneity [39,40]. Consequently, tumor cells from the same individual's GC tissue can often be classified into distinct subpopulations, with some subpopulations exhibiting greater resistance to chemotherapy, while others are more sensitive [41,42]. Of particular concern is the finding that chemo-resistant subpopulations of GC cells can induce the chemoresistance of chemo-sensitive subpopulations [43,44]. In this study, we found that HIST1H2BK-overexpressing 5-FU-resistant GC cells can transmit the secreted HIST1H2BK proteins into 5-FU-sensitive GC cells, facilitating the transmission of 5-FU resistance (Fig. 6M). Most eukaryotic proteins are secreted through conventional endoplasmic reticulum (ER)–Golgi, non-conventional or exosomal transport pathway. Previous studies have reported the presence of a significant amount of HIST1H2BK proteins in exosomes derived from tumor cells in an epithelial-to-mesenchymal transition state [27]. Since proteins in exosomes were packaged by an exosomal membrane, they failed to be detected by ELISA directly without specific instruments and kits. However, we detected a significant amount of HIST1H2BK proteins in extracellular fluids and serums using ELISA directly in our study, which suggested that HIST1H2BK can be secreted through conventional endoplasmic reticulum (ER)–Golgi or non-conventional pathway. The concrete molecular mechanism of chemo-resistant transmission through secreted HIST1H2BK needs to be further explored.
Despite employing various experimental methods at the cellular, animal, and patient tissue levels to investigate the role and mechanism of HIST1H2BK in NACT for AGC, there are still some limitations. Firstly, the predictive efficacy of a single marker is always limited. By using proteomic screening, we identified a series of plasma proteins related to drug resistance. A combination of several significantly different plasma proteins might provide higher diagnostic efficacy for predicting the response of NACT in AGC. Secondly, the specific mechanism by which HIST1H2BK regulates A2M transcription and transfers resistance between cells is still not well understood and requires further in-depth investigation.
Conclusions
In conclusion, a systematic screening of differential plasma proteins between NACT-sensitive and NACT-resistant patients with AGC was performed and beneficial to identify validated markers for predicting NACT response. Plasma HIST1H2BK was a biomarker for predicting NACT response and prognosis in AGC patients undergoing NACT. HIST1H2BK inhibited 5-FU-induced apoptosis by upregulating A2M transcription and then activating Akt signaling pathway, thereby promoting 5-FU resistance in GC cells. Interestingly, HIST1H2BK-overexpression 5-FU-resistant GC cells transmitted 5-FU resistance to 5-FU-sensitive GC cells via secreted HIST1H2BK.
Fundings
This study was supported by grants from Research Fund of the Sixth Affiliated Hospital of Sun Yat-sen University (Grant No. P20200217202309876), National Natural Science Foundation of China (Grant Nos 81772594, 81802322, and 81902949), Natural Science Foundation of Guangdong Province in China (Grant Nos 2020A1515011362 and 2022A1515010262) and National Key Clinical Discipline.
Ethics approval and consent to participate
The clinical study was approved by the Institutional Ethical Review Board of the Sun Yat-Sen University Cancer Center (Permit number: 2023ZSLYEC-343). Informed consent was obtained from all subjects involved in the study. The animal study was approved by the Institutional Ethical Review Boards of the Sun Yat-Sen University Cancer Center (Permit number: IACUC-2022060901).
Data availability
All the data and material in this study can be requested from the corresponding author reasonably.
CRediT authorship contribution statement
Zijian Chen: Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. Xiaocheng Tang: Formal analysis, Investigation, Visualization, Writing – review & editing. Weiyao Li: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft. Tuoyang Li: Investigation, Validation. Jintuan Huang: Conceptualization, Funding acquisition, Methodology, Project administration. Yingming Jiang: Conceptualization, Methodology, Project administration, Software, Visualization. Jun Qiu: Investigation, Project administration. Zhenze Huang: Investigation, Resources. Rongchang Tan: Investigation, Resources. Xiang Ji: Investigation. Li Lv: Investigation. Zuli Yang: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. Hao Chen: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We extend our thanks to the patients and their families and acknowledge the GC samples bank of the Sixth Affiliated Hospital for providing access to blood and tissue materials.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102017.
Contributor Information
Zuli Yang, Email: yangzuli@mail.sysu.edu.cn.
Hao Chen, Email: chenhao29@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wang F.H., Zhang X.T., Li Y.F., Tang L., Qu X.J., Ying J.E., et al. The chinese society of clinical oncology (csco): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. (Lond) 2021;41:747–795. doi: 10.1002/cac2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ychou M., Boige V., Pignon J.P., Conroy T., Bouche O., Lebreton G., et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., CJHVd Velde, Nicolson M., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X., Liang H., Li Z., Xue Y., Wang Y., Zhou Z., et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081–1092. doi: 10.1016/S1470-2045(21)00297-7. [DOI] [PubMed] [Google Scholar]
- 6.Kang Y.-K., Yook J.H., Park Y.-K., Lee J.S., Kim Y.-W. PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant s-1 versus surgery and adjuvant s-1 for resectable advanced gastric cancer. J. Clin. Oncol. 2021;39:2903–2913. doi: 10.1200/JCO.20.02914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terazawa T., Matsuyama J., Goto M., Kawabata R., Endo S., Imano M., et al. A phase II study of perioperative capecitabine plus oxaliplatin therapy for clinical ss/se n1-3 m0 gastric cancer (ogsg 1601) Oncologist. 2020;25:119–e208. doi: 10.1634/theoncologist.2019-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang X.W., Xiao W.S., Lei H., Huag Q.C., Dong Y.L., Wang F., et al. Risk model and factors for prediction of response to neoadjuvant chemotherapy in patients with advanced gastric cancer-a two-center cohort study. BMC Cancer. 2023;23:41. doi: 10.1186/s12885-023-10513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurczyk K., Nowak N., Carlson R., Skoczylas T., Wallner G. Pre-therapeutic molecular biomarkers of pathological response to neoadjuvant chemotherapy in gastric and esophago-gastric junction adenocarcinoma: a systematic review and meta-analysis. Adv. Med. Sci. 2023;68:138–146. doi: 10.1016/j.advms.2023.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Cui Y., Wei K., Li Z., Li D., Song R., et al. Deep learning predicts resistance to neoadjuvant chemotherapy for locally advanced gastric cancer: a multicenter study. Gastric Cancer. 2022;25:1050–1059. doi: 10.1007/s10120-022-01328-3. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y., Zhang J., Li Z., Wei K., Lei Y., Ren J., et al. A CT-based deep learning radiomics nomogram for predicting the response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer: a multicenter cohort study. EClinicalMedicine. 2022;46 doi: 10.1016/j.eclinm.2022.101348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwee R.M., Kwee T.C. Role of imaging in predicting response to neoadjuvant chemotherapy in gastric cancer. World J. Gastroenterol. 2014;20:1650–1656. doi: 10.3748/wjg.v20.i7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lengyel C.G., Hussain S., Trapani D., El Bairi K., Altuna S.C., Seeber A., et al. The emerging role of liquid biopsy in gastric cancer. J. Clin. Med. 2021;10 doi: 10.3390/jcm10102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landegren U., Hammond M. Cancer diagnostics based on plasma protein biomarkers: hard times but great expectations. Mol. Oncol. 2021;15:1715–1726. doi: 10.1002/1878-0261.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z., Qin K., Zhang W., Yang B., Zhao C., Zhang X., et al. Postoperative recurrence of epithelial ovarian cancer patients and chemoresistance related protein analyses. J. Ovar. Res. 2019;12:29. doi: 10.1186/s13048-019-0499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo N., Minas G., Synowsky S.A., Dunne M.R., Ahmed H., McShane R., et al. Identification of plasma proteins associated with oesophageal cancer chemotherapeutic treatment outcomes using SWATH-MS. J. Proteom. 2022;266 doi: 10.1016/j.jprot.2022.104684. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S.Q., Pan S.M., Lai S.Z., Situ H.J., Liu J., Dai W.J., et al. Novel plasma proteomic biomarkers for early identification of induction chemotherapy beneficiaries in locoregionally advanced nasopharyngeal carcinoma. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.889516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh R., Bassett E., Chakravarti A. Replication-dependent histone isoforms: a new source of complexity in chromatin structure and function. Nucl. Acids Res. 2018;46:8665–8678. doi: 10.1093/nar/gky768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J.C., Ha Y.J., Park I.J., Kim C.W., Yoon Y.S., Lee J.L., et al. Tumor immune microenvironment of primary colorectal adenocarcinomas metastasizing to the liver or lungs. J. Surg. Oncol. 2021;124:1136–1145. doi: 10.1002/jso.26631. [DOI] [PubMed] [Google Scholar]
- 20.Di Benedetto M., Toullec A., Buteau-Lozano H., Abdelkarim M., Vacher S., Velasco G., et al. MDA-MB-231 breast cancer cells overexpressing single VEGF isoforms display distinct colonisation characteristics. Br J. Cancer. 2015;113:773–785. doi: 10.1038/bjc.2015.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campone M., Campion L., Roche H., Gouraud W., Charbonnel C., Magrangeas F., et al. Prediction of metastatic relapse in node-positive breast cancer: establishment of a clinicogenomic model after FEC100 adjuvant regimen. Breast Cancer Res. Treat. 2008;109:491–501. doi: 10.1007/s10549-007-9673-x. [DOI] [PubMed] [Google Scholar]
- 22.Han J., Lim W., You D., Jeong Y., Kim S., Lee J.E., et al. Chemoresistance in the human triple-negative breast cancer cell line mda-mb-231 induced by doxorubicin gradient is associated with epigenetic alterations in histone deacetylase. J. Oncol. 2019;2019 doi: 10.1155/2019/1345026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H., Li H., Zhao T., Khan A.A., Pan R., Wang S., et al. TSPAN1-elevated FAM110A promotes pancreatic cancer progression by transcriptionally regulating HIST1H2BK. J. Cancer. 2022;13:906–917. doi: 10.7150/jca.66404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W., Xu Z., Zhou J., Xing S., Li Z., Gao X., et al. High levels of HIST1H2BK in low-grade glioma predicts poor prognosis: a study using CGGA and TCGA data. Front. Oncol. 2020;10:627. doi: 10.3389/fonc.2020.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Z., Lu J., Wu D., Zuo R., Li Y., Huang H., et al. Poly(ADP-ribose) glycohydrolase silencing-mediated H2B expression inhibits benzo(a)pyrene-induced carcinogenesis. Environ. Toxicol. 2021;36:291–297. doi: 10.1002/tox.23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu S., Li Y., Liao Z., Wang Z., Wang Z., Li Y., et al. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut. 2020;69:540–550. doi: 10.1136/gutjnl-2019-318860. [DOI] [PubMed] [Google Scholar]
- 27.Garnier D., Magnus N., Meehan B., Kislinger T., Rak J. Qualitative changes in the proteome of extracellular vesicles accompanying cancer cell transition to mesenchymal state. Exp. Cell Res. 2013;319:2747–2757. doi: 10.1016/j.yexcr.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Chen X., Leng W., Zhou Y., Yu Y., Meng W., Cao P., et al. Pathological response and safety of FOLFOXIRI for neoadjuvant treatment of high-risk relapsed locally advanced colon cancer: study protocol for a single-arm, open-label phase II trial. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-062659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J., Li T., Chen H., Jiang Y., Zhao Y., Huang J., et al. ADAMTS10 inhibits aggressiveness via JAK/STAT/c-MYC pathway and reprograms macrophage to create an anti-malignant microenvironment in gastric cancer. Gastric Cancer. 2022;25:1002–1016. doi: 10.1007/s10120-022-01319-4. [DOI] [PubMed] [Google Scholar]
- 30.Gunner C.B., Azmoon P., Mantuano E., Das L., Zampieri C., Pizzo S.V., et al. An antibody that targets cell-surface glucose-regulated protein-78 inhibits expression of inflammatory cytokines and plasminogen activator inhibitors by macrophages. J. Cell Biochem. 2023;124:743–752. doi: 10.1002/jcb.30401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantuano E., Mukandala G., Li X., Campana W.M., Gonias S.L. Molecular dissection of the human alpha2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J. Biol. Chem. 2008;283:19904–19911. doi: 10.1074/jbc.M801762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y., Zheng Z., Liu C., Li W., Zhao L., Nie G., et al. Inhibiting DNA methylation alleviates cisplatin-induced hearing loss by decreasing oxidative stress-induced mitochondria-dependent apoptosis via the LRP1-PI3K/AKT pathway. Acta Pharm. Sin B. 2022;12:1305–1321. doi: 10.1016/j.apsb.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray M.J., Mhawech-Fauceglia P., Yoo E., Yang W., Wu E., Lee A.S., et al. AKT inhibition mitigates GRP78 (glucose-regulated protein) expression and contribution to chemoresistance in endometrial cancers. Int. J. Cancer. 2013;133:21–30. doi: 10.1002/ijc.27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marzluff W.F., Koreski K.P. Birth and death of histone mRNAs. Trends Genet. 2017;33:745–759. doi: 10.1016/j.tig.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrence M., Daujat S., Schneider R. Lateral thinking: how histone modifications regulate gene expression. Trends Genet. 2016;32:42–56. doi: 10.1016/j.tig.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Biterge B., Schneider R. Histone variants: key players of chromatin. Cell Tissue Res. 2014;356:457–466. doi: 10.1007/s00441-014-1862-4. [DOI] [PubMed] [Google Scholar]
- 37.Amatori S., Tavolaro S., Gambardella S. The dark side of histones: genomic organization and role of oncohistones in cancer. Clin Epigen. 2021;13:71. doi: 10.1186/s13148-021-01057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Wang G., Zhang J., Chen X., Xu H., Heng G., et al. CD9, a potential leukemia stem cell marker, regulates drug resistance and leukemia development in acute myeloid leukemia. Stem Cell Res. Ther. 2021;12:86. doi: 10.1186/s13287-021-02155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeoh K.G., Tan P. Mapping the genomic diaspora of gastric cancer. Nat. Rev. Cancer. 2022;22:71–84. doi: 10.1038/s41568-021-00412-7. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y., Kawazoe A., Lordick F., Janjigian Y.Y., Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat. Rev. Clin. Oncol. 2021;18:473–487. doi: 10.1038/s41571-021-00492-2. [DOI] [PubMed] [Google Scholar]
- 41.Carrasco-Garcia E., Garcia-Puga M., Arevalo S. Towards precision medicine: linking genetic and cellular heterogeneity in gastric cancer. Ther. Adv. Med. Oncol. 2018;10:1–15. doi: 10.1177/1758835918794628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao J.P., Xu W., Liu W.T., Yan M., Zhu Z.G. Tumor heterogeneity of gastric cancer: from the perspective of tumor-initiating cell. World J. Gastroenterol. 2018;24:2567–2581. doi: 10.3748/wjg.v24.i24.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J., Wei Q., Koay E.J., Liu Y., Ning B., Bernard P.W., et al. Chemoresistance transmission via exosome-mediated EphA2 transfer in pancreatic cancer. Theranostics. 2018;8:5986–5994. doi: 10.7150/thno.26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D., Zhao C., Xu F., Zhang A., Jin M., Zhang K., et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics. 2021;11:2860–2875. doi: 10.7150/thno.51797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data and material in this study can be requested from the corresponding author reasonably.