Summary
The model of M2-like tumor-associated macrophages (TAMs) is an increasingly attractive model for the study of TAMs. However, the detailed process of M2-like TAMs polarization induced by lactic acid or conditioned medium from Lewis cells (LCM) and the identification of M2-like TAMs is not yet available. In this protocol, we present the detailed methods to induce M2-like TAMs polarization and verify its functionality in order to better carry out related research.
For complete details on the use and execution of this protocol, please refer to Fang et al.1
Subject areas: Cell culture, Cell isolation, Cancer, Immunology
Graphical abstract

Highlights
-
•
Instructions for collecting and culturing BMDM cells
-
•
Steps for polarizing BMDMs into TAMs by Lewis cell conditional medium
-
•
Assessment of polarization of BMDMs into M2-like TAMs
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The model of M2-like tumor-associated macrophages (TAMs) is an increasingly attractive model for the study of TAMs. However, the detailed process of M2-like TAMs polarization induced by lactic acid or conditioned medium from Lewis cells (LCM) and the identification of M2-like TAMs is not yet available. In this protocol, we present the detailed methods to induce M2-like TAMs polarization and verify its functionality in order to better carry out related research.
Before you begin
The protocol below describes two approaches of polarizing bone marrow derived macrophages (BMDMs) into M2-like TAMs by treatment with conditioned medium from Lewis cells (LCM) or with 25 mM lactic acid. Additionally, the phenotype and functionality of M2-like TAMs also be identified here. Besides, it is worth noting that this protocol can also be used to collect and culture BMDMs.
Institutional permissions
All animals were acclimated under standard laboratory conditions (ventilated room, 25 ± 1°C, 60 ± 5% humidity, 12 h light/dark cycle) and had free access to standard water and food. This study was approved by the Tongji University Institutional Animal Use and Care Committee (approval number: TJBB03723101).
Preparation of mice
The best age for mice is 6–8 weeks old, and the maximum age of mice should not exceed 12 weeks. We have preliminarily investigated the cell number of bone marrow-derived macrophages (BMDMs) in 8-, 10- and 12-week-old mice, and found that the cell number of BMDMs in 12-week-old mice was significantly less than that in 8- to 10-week-old mice. Based on our experience, approximately 30 million cells can be extracted from one mouse.
Preparation of regents and equipment
Timing: 30 min
-
1.
Add 10% FBS (heat activated), 1% penicillin/streptomycin and M-CSF (20 ng/mL) to DMEM with high glucose and keep it at 4°C before bone marrow cells collection.2
-
2.
Add 10% FBS (heat inactivated), 1% penicillin/streptomycin to RPMI 1640 and keep it at 4°C before use.
-
3.
Prepare 150 mm Petri dishes (3 dishes per mouse’s bone marrow cells), cold PBS (50 mL per mouse), 50 mL centrifugal tubes, 20 mL syringe with 23G needles and 40 μm cell strainer.
-
4.
Autoclave surgical tools, including one ophthalmic scissor and two forceps.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Arginase-1 rabbit (1:1,000) | CST | Cat#93668S |
| Anti-beta actin rabbit (1:1,000) | Abcam | Cat#ab8227 |
| Anti-Phospho-Jak1 (Tyr1034/1035) rabbit (1:1,000) | CST | Cat#3331S; |
| Anti-Jak1 rabbit (1:1,000) | CST | Cat#3332S |
| Anti-Phospho-Stat6 (Tyr641) rabbit (1:1,000) | CST | Cat#56554 |
| Anti-Stat6 rabbit (1:1,000) | CST | Cat#5397 |
| Anti-iNOS rabbit (1:1,000) | CST | Cat#13120S |
| Anti-mouse CD16/CD32 | BD Biosciences | Cat#553141 |
| FITC rat anti-mouse CD45 | BD Biosciences | Cat#553080 |
| APC rat anti-mouse F4/80 | BD Biosciences | Cat#566787 |
| PerCP-Cy5.5 rat anti-CD11b | BD Biosciences | Cat#550993 |
| BV510 rat anti-mouse CD45 (clone: 30-F11) | BD Biosciences | RRID:AB_2734134 |
| FITC rat anti-CD11b (clone: M1/70) | BD Biosciences | RRID:AB_396679 |
| PE rat anti-mouse F4/80 (clone: T45-2342) | BD Biosciences | RRID:AB_2687527 |
| PE-Cy7 rat anti-mouse CD86 (clone: GL1) | BD Biosciences | RRID:AB_1727518 |
| Alexa Fluor 647 rat anti-mouse CD206 (clone: MR5D3) | BD Biosciences | RRID:AB_2739133 |
| Fixable viability stain 780 | BD Biosciences | RRID:AB_2869673 |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM (high glucose) | Gibco | Cat#11965092 |
| RPIM 1640 | Gibco | Cat#2732541 |
| Heat-inactivated FBS | Gibco | Cat#10100147C |
| FBS | Gibco | Cat#10091148 |
| Penicillin streptomycin | Gibco | Cat#15140163 |
| PBS | Servicebio | Cat#G4202 |
| Lactic acid | Sigma | Cat#1003418151 |
| Recombinant mouse M-CSF | R&D | Cat#416-ML |
| Cell dissociation buffer enzyme-free PBS based | Gibco | Cat#15400054 |
| Recombinant mouse IL-4 | R&D | Cat#404-ML-010 |
| LPS | InvivoGen | Cat#13I06-MM |
| MACS tissue storage solution | Miltenyi | Cat#130-100-008 |
| Bovine serum albumin | Sigma | Cat#B2064-100G |
| EDTA | Invitrogen | Cat#2085657 |
| Permeabilization buffer | Thermo Scientific | Cat#00-8333 |
| 4× Laemmli sample buffer | Bio-Rad | Cat#1610747 |
| RIPA buffer | Thermo Scientific | Cat#89901 |
| PhosSTOP | Roche | Cat#04906845001 |
| EDTA-free protease inhibitor cocktail | Roche | Cat#11873580001 |
| 2-mercaptoethanol | Roche | Cat#M3148 |
| Trizma base | Sigma | Cat#T1503 |
| Glycine | Bio-Rad | Cat#1610718 |
| SuperSignal chemiluminescent substrate | Thermo Scientific | Cat#34580 |
| SuperSignal maximum sensitivity substrate | Thermo Scientific | Cat#34095 |
| Blotting-grade blocker non-fat dry milk | Bio-Rad | Cat#1706404 |
| Trans-Blot Turbo 5× transfer buffer | Bio-Rad | Cat#10026938 |
| SYBR green | Applied Biosystems | Cat#4309155 |
| HEPES | Gibco | Cat#15630080 |
| 2-mercaptoethanol | Gibco | Cat#21985–023 |
| IL-2 | BioLegend | Cat#575404 |
| Non-essential amino acids MEM | Gibco | Cat#11140050 |
| Anti-CD3 | Bio X Cell | Cat#BE0001-1 |
| Anti-CD28 | Bio X Cell | Cat#BE0015-1 |
| Critical commercial assays | ||
| Tumor dissociation kit, mouse | Miltenyi | Cat#130-096-730 |
| Cytofix/Cytoperm fixation/permeabilization kit | BD Biosciences | Cat#554656 |
| TGX FastCast acrylamide kit, 10% | Bio-Rad | Cat#1610173 |
| Pierce BCA protein assay kit | Thermo Fisher Scientific | Cat#23227 |
| Mouse CD8+T cell isolation kit | STEMCELL | Cat#19853 |
| HiScript II Q select RT SuperMix | Vazyme | Cat#R233 |
| Experimental models: Cell lines | ||
| Lewis lung cancer cells | KeyGEN BioTECH | Cat#KG070 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL6/J, 6–12 weeks, female | Shanghai Model Organisms | N/A |
| Oligonucleotides | ||
| Primer: Arg1 forward: CCACAGTCTGGCAGTTGGAAG | This paper | N/A |
| Primer: Arg1 reverse: GGTTGTCAGGGGAGTGTTGATG | This paper | N/A |
| Primer: Vegf forward: CCACGACAGA AGGAGAGCAGAAGTCC |
This paper | N/A |
| Primer: Vegf reverse: CGTTACAGCAGCCTGCACAGCG | This paper | N/A |
| Primer:β-actin forward: GATGCTCCCGGGCTGATT | This paper | N/A |
| Primer:β-actin reverse: GGGGTACTTCAGGGTCAGGA | This paper | N/A |
| Primer: IL-10 forward: GAGAGCTGCAGGGCCCTTTGC | This paper | N/A |
| Primer: IL-10 reverse: CTCCCTGGTTT CTCTTCCCAAGACC |
This paper | N/A |
| Primer: Ym1 forward: GCCACTGAGGTCTGGGATGC | This paper | N/A |
| Primer: Ym1 reverse: TCCTTGAGCCACTGAGCCTTC | This paper | N/A |
| Primer: Mrc1 forward:TCTTTTACGAGAAGTTGGGGTCAG | This paper | N/A |
| Primer: Mrc1 reverse: ATCATTCCGTTCACCAGAGGG | This paper | N/A |
| Software and algorithms | ||
| Prism 9 | GraphPad | https://www.graphpad.com/features |
| FlowJo | BD Biosciences | https://www.flowjo.com/ |
| Excel | Microsoft | https://www.microsoft.com/zh-cn/microsoft-365/excel |
| Other | ||
| Trans-Blot Turbo transfer starter system, midi PVDF | Bio-Rad | Cat#17001919 |
| Petri dish 150 × 15 mm style | Falcon | Cat#351058 |
| 6 well cell culture plate | Corning | Cat#3471 |
| 12 well cell culture plate | Corning | Cat#3512 |
| 25 cm2 cell culture flask | Corning | Cat#430168 |
| 24 well transwell plate | Corning | Cat#3421 |
| Cell lifter | Corning | Cat#3008 |
| 15 mL centrifuge tubes | Corning | Cat#430791 |
| 50 mL centrifuge tubes | Corning | Cat#430291 |
| Countness II | Thermo Scientific | Cat#2185A18083484 |
| gentleMACS octo dissociator with heaters | Miltenyi Biotec | Cat#130-096-427 |
| PowerPac basic power supply | Bio-Rad | Cat#1645050 |
| SpectraMax i3× | Molecular Devices | N/A |
| RNeasy mini kit | QIAGEN | Cat#74106 |
Materials and equipment
Complete Lewis Cell Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM (high glucose) | N/A | 45 mL |
| FBS | 10% | 5 mL |
| Penicillin streptomycin | 1% | 500 μL |
| Total | N/A | 50 mL |
Storage conditions: 4°C for 1 month.
Bone marrow culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM (high glucose) | N/A | 45 mL |
| Heat-Inactivated FBS | 10% | 5 mL |
| Penicillin streptomycin | 1% | 500 μL |
| M-CSF | 20 ng/mL | 1000 ng |
| Total | N/A | 50 mL |
Storage conditions: 4°C for 1 week.
T cell culture Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| RPMI-1640 | N/A | 45 mL |
| FBS | 10% | 5 mL |
| Penicillin streptomycin | 1% | 500 μL |
| HEPES (1 M) | N/A | 500 μL |
| 2-mercaptoethanol (1000×) | N/A | 50 μL |
| Non-essential amino acids MEM (100×) | N/A | 500 μL |
| IL-2 | 100 units/mL | 5000 units |
| Total | N/A | 50 mL |
Storage conditions: 4°C for 1 week.
Protein lysis Buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 10×RIPA | N/A | 10 μL |
| 10×PhosSTOP | 10% | 10 μL |
| 25×Protease inhibitor cocktail | 1% | 4 μL |
| ddH2O | 20 mmol/L | 76 μL |
| Total | N/A | 100 μL |
Storage conditions: 4°C for 1 day.
Step-by-step method details
Collection of bone marrow cells from mice
Timing: 1 h
In this step, we collect the bone marrow cells from mice and culture until they become mature. Before beginning, tweezers and scissors should be sterilized thoroughly to avoid contamination.
-
1.
Euthanize mouse according to institutionally approved regulations and then place mouse on the operating platform.
-
2.
Immobilize mouse limbs in the plate and keep the mice belly up, soak with 70% ethanol.
-
3.
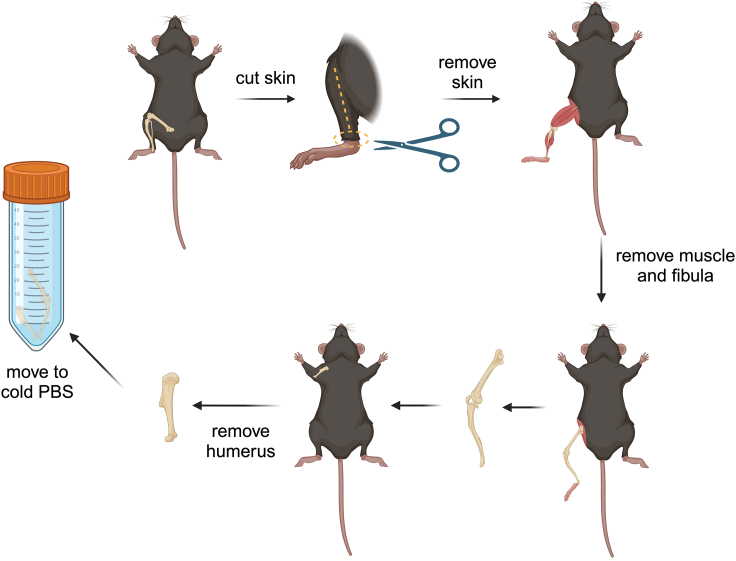
Incise skin around the Achilles, and the skin along the lower limb, remove skin and expose the lower limb (Figure 1).
-
4.
Cut the Achilles tendon and remove the fibula, then cut the muscle around the tibia and expose the knee joint (Figure 1).
-
5.
Cut and remove the major muscle around the knee joint and remove muscle along femur.
-
6.
Expose the caput femoris and cut the lower limb (Figure 1).
-
7.
Cut the Achilles and put the lower limb in the cold PBS for the collection of bone marrow.
-
8.
Remove the humerus following the same procedure.
CRITICAL: Try to remove fur and muscle from the tibia and femur of mouse as much as possible to prevent contamination, please refer to the section of problem 3 for details.
Note: Ensure that the following steps are carried out on a clean bench for cell cultivation or in a biological safety cabinet to avoid contamination.
-
9.
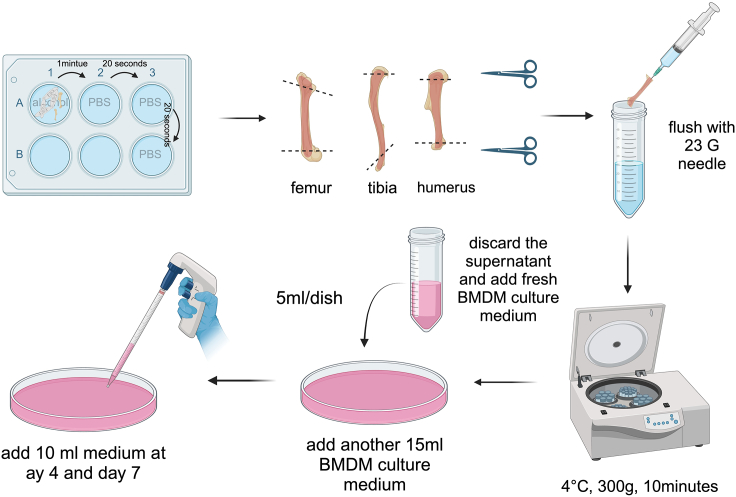
Place the lower limb in the 6-well plate and soak with 70% ethanol for 1 min, and then wash three times with PBS (Figure 2).
-
10.
Cut at knee joint and remove the epiphyses of the femur and tibia so that the needle can insert into the bone marrow cavity (Figure 2).
-
11.
Insert the 23G needle into the bone marrow cavity for flushing until the color of the bone marrow cavity turns white.
-
12.
Collect the flushing solution into 50 mL centrifuge tubes (Figure 2).
-
13.
Centrifuge the bone marrow suspension at 4°C, 300 × g for 10 min (Figure 2).
-
14.
Discard the supernatant and resuspend the cell precipitation with 15 mL BMDM culture medium, transfer suspension into 150 mm Petri dish (5 mL/dish) and add 15 mL BMDM culture medium per dish. Then, culture at 37°C in a 5% CO2 incubator (Figure 2).
-
15.
Add fresh BMDM culture medium (10 mL) into each 150 mm dish on Day 4 and Day 6, respectively (Figure 2).
Note: On Day 7, most BMDMs adhere to the bottom of the culture dish and stretch their antenna instead of being round.
-
16.Discard the supernatant and wash the cells three times with sterile PBS (5 mL PBS/dish).
-
a.Add 6 mL trypsin per dish and incubate at 37°C for 8 min.
-
b.After 10 min, add 6 mL BMDM culture medium per dish to stop the digestion and use cell lifter to collect cells.
-
c.Transfer suspension into 50 mL tubes and centrifuge at 4°C, 300 × g for 10 min.
-
a.
CRITICAL: Ensure that the direction of BMDMs scraping is the same at every time to avoid damage to cells after scraping in different directions. The flushing time for the bone marrow cavity should not be too long to prevent a decrease in cell viability. Generally, the flushing time for the femur and tibia is less than 5 min, and that for the humerus is less than 3 min.
-
17.
Discard the supernatant and add BMDM culture medium into the cell precipitate. Then, resuspend the cells and adjust the number of cells to a concentration of 1 × 106/mL.
-
18.
Aspirate the cell suspension into a culture plate for the next experiment.
Figure 1.
Illustration describing steps 1–8
Figure 2.
Illustration describing steps 10–18
Polarization of BMDMs into M2-like TAMs
The following two sections will describe alternative methods for polarizing BMDMs into M2-like TAMs.
Polarization of BMDMs into M2-like TAMs by Lewis cell conditional medium
Timing: 20 min for hands-on and 48 h for incubation (for step 19)
Timing: 20 min for hands-on and 24 h for polarization (for steps 20c and 20d)
In this step, we collect the conditioned medium from Lewis cell (LCM) and induce BMDM polarize into M2-like TAMs by LCM.
-
19.Preparation of Lewis cell conditional medium (LCM).
-
a.Culture Lewis cells in complete DMEM (Lewis culture medium), and the cells will be appropriate for collecting supernatant after undergoing two or three generations.
-
b.Plate 6×106 Lewis cells in the T25 culture flask with 9 mL Lewis culture medium.3Note: The needed volume of Lewis cell conditional medium (LCM) depends on the number of BMDMs that need to be polarized into TAMs. Generally, 5 mL LCM was used per 10 million BMDMs.
-
c.After culturing for 48 h, the supernatant collect and centrifuge at 4°C, 300 g for 5 min.
-
d.The supernatant was sterile filtered using a 22 μm filter with a 10 mL syringe barrel and collected in a new 15 mL tube.
-
e.LCM can be prepared according to the above steps within 7 days of cultivating BMDMs or prepared in advance and stored at −20°C for subsequent experiments if not used immediately.
 CRITICAL: Maintain most Lewis cells in an adherent state when collecting the supernatant.
CRITICAL: Maintain most Lewis cells in an adherent state when collecting the supernatant.
-
a.
-
20.Polarization of BMDMs into M2-like TAMs.
-
a.Plate BMDMs in a 12-well plate (1 × 106 cells/well) or 24-well plate (5 × 105 cells/well) and culture overnight.
-
b.Prepare complete BMDM culture medium containing LCM at a 1:1 ratio. For instance, 500 μL of complete BMDM culture medium was mixed with 500 μL of LCM per well in a 12-well plate.
-
c.After 24 h, discard the supernatant of BMDMs, and then add the prepared culture medium containing LCM. Generally, 1 mL of mixed medium per well was added to a 12- well plate, and 500 μL of mixed medium per well was added to a 24-well plate.
-
d.Continue to culture BMDMs for 24 h, then perform subsequent experiments.
-
a.
Polarization of BMDMs into M2-like TAMs by lactic acid
Timing: 20 min for hands-on and 24 h for polarization (for steps 23 and 24)
In this step, we induce the polarization of M2-like TAMs by lactic acid.
-
21.
Plate BMDMs in a 12-well plate (1 × 106 cells/well) or 24-well plate (5 × 105 cells/well) with complete BMDM culture medium.
-
22.
Prepare complete BMDM medium with 25 mM lactic acid: calculate the quantity of lactic acid that you need and dilute it in BMDM culture medium.
-
23.
After 24 h, discard the supernatant of BMDMs, and add fresh complete BMDM culture medium containing 25 mM lactic acid.4
-
24.
Continue to culture BMDMs for 24 h and perform subsequent experiments.
Assessment of the polarization of M2-like TAMs
Timing: 3 h (for step 25)
Timing: 2 days (for step 26)
Timing: 4 h (for step 27)
The polarization of BMDMs into M2-like TAMs can be assayed for gene transcription by RT-qPCR, and protein expression by western blot and FACS.
-
25.RT-qPCR assay:
-
a.Extract RNA for RT-qPCR detection. Briefly, add 350 μL RLT buffer to each well of a 24 well plate, and the detailed steps are performed according to the instructions of the RNeasy mini Kit.
-
b.Finally, dissolve the extracted RNA in 20 μL of RNA-free water. Generally, 1–2 μg RNA can be extracted from 5×105 cells. The RNA solution can be kept at −80°C until RT-qPCR detection.
-
c.Detect RNA concentration by a NanoDrop spectrophotometer and use 1000 ng cDNA for reverse transcription. Use HiScript III RT supermix to complete reverse transcription.
-
d.The total reaction volume for qPCR is 10 μL, which contains 5 μL SYBR green, 10 ng cDNA, 10 nM primer and DEPC water. The reaction process is performed according to the reagent Kit or manufacturer’s instructions.PCR reaction master mix
Reagent Amount SYBR green 5 μL cDNA 10 ng Forward primer 10 nM Reverse primer 10 nM DEPC water Trim Total 10 μL PCR cycling conditionsStep Temperature Duration Cycles UDG activation 50°C 2 min Hold Dual-Lock DNA Polymerase 95°C 2 min Hold Denature 95°C 15 s 40 Anneal/extend 60°C 1 min Dissociation curve conditionsStep Ramp rate Temperature Time 1 1.6 °C/s 95°C 15 s 2 1.6 °C/s 60°C 1 min 3 0.15 °C/s 95°C 15 s -
e.To verify the polarization of M2-like TAMs, some M2-signature genes in macrophages (IL-4 stimulation) can also be used to validate the polarization of M2-like TAMs due to their similar phenotypes, such as Arginase 1 (Arg1), Vegf, Il-10 and Mcr1. Like IL-4 stimulation, the expression of most M2-signature genes increased in TAMs induced by 25 mM lactic acid or LCM (Figure 3).
-
a.
-
26.Western blot analysis:
-
a.To extract protein for western blot, add 40 μL protein lysis buffer to each well (12-well plate) and shake the plate at 4°C for 15 min.
-
b.Use cell lifter to scratch the cells and collect the cells lysis buffer in 1.5 mL EP tube.
-
c.Centrifuge the lysis buffer at 4°C, 3000 g for 15 min. Collect the supernatant in a new 1.5 mL EP tube for subsequent detection, or store at −80°C for western blot analysis.
-
d.Use Pierce BCA Protein Assay Kit for protein quantification, approximate 40–60 μg of protein was extracted from 1×106 cells.
-
e.Mix the protein with 4× Laemmli sample buffer containing 10% 2-Mercaptoethanol. Heat the protein mixture to 95°C for 5 min, frozen on ice for 5 min, and centrifuge at 4°C, 3000 g for 30 s.
-
f.For 10% TGX FastCast Acrylamide, add 15–20 μg of protein to each electrophoresis lane, and set the PowerPac Basic Supply to 110 V for 90 min.
-
g.Use Trans-Blot Turbo to complete transmembrane. The PVDF membrane should be activated by methanol in advance.
-
h.Block the PVDF membrane with nonfat dry milk for 1 h, discard the milk and incubate membrane with primary antibody at 4°C overnight.
-
i.Recycle primary antibody and wash the membrane with TBST three times, incubate the membrane with secondary antibody at room temperature for 1 h, remove the antibody, and wash the membrane with TBST.
-
j.Use Chemiluminescent Substrate to observe the protein bands.
-
k.To verify the polarization of M2-like TAMs, the expression of Arg1, p-JAK and p-STAT6 that are biomarker of M2 macrophages can be detected in TAMs. In addition, the expression of M1-signature gene iNOS in macrophages (LPS stimulation) can also be detected to further confirm the polarization of TAMs. As expected, the expression of Arg1, p-JAK and p-STAT6 increased in TAMs. Conversely, the expression of iNOS decreased in TAMs (Figure 3).
-
a.
-
27.FACS assay:
-
a.Add cell dissociation buffer (200 μL per well for 6-well plate) and incubate at 37°C for 10 min.
-
b.Add complete BMDM culture medium and collect the cells with a cell lifter. Then, centrifuge the samples at 350 × g, 4°C for 5 min.
-
c.Suspend cells in 1 mL stain buffer (500 mL PBS+2 mL EDTA+5 g BSA), add FcBlock antibody (0.5 μL/tube) and incubate at 4°C for 30 min, centrifuge at 350 × g, 4°C for 5 min.
-
d.Add CD45 (1.25 μL/tube), CD11b (1 μL/tube), F4/80 (2.5 μL/tube), CD86 (0.5 μL/tube) antibody and incubate at 4°C for 30 min, centrifuge at 350 × g, 4°C for 5 min.
-
e.Add 200 μL permeabilization buffer, suspend cells and incubate at 4°C for 30 min, centrifuge at 350 × g, 4°C for 5 min, discard the supernatant.
-
f.Add 1 mL wash buffer, centrifuge at 350 × g, 4°C for 5 min, repeat this step and discard the supernatant.
-
g.Add CD206(1 μL/tube) antibody, suspend the cells and incubate at 4°C for 30 min, centrifuge at 350 × g, 4°C for 5 min.
-
h.Wash the cells three times with wash buffer.
-
i.Suspend cells in 300 μL stain buffer and detect by flow cytometry.
-
j.To verify the polarization of M2-like TAMs, the expression of CD206 that is biomarker of M2 macrophages, and the expression of CD86 that is a biomarker of M1 macrophages can be detected in TAMs. As shown in Figure 3, compare with M1-signature CD86, the expression of CD206 relatively increased in TAMs.
-
a.
Figure 3.
The validation of TAMs polarization
(A and B) The expression of M2-signature genes in TAMs were detected by RT-qPCR. The expression of Arg1, Vegf and IL-10 increased in TAMs stimulate with LCM (A) or 25 mM lactic acid (B).
(C) The expression of M2-signature genes in macrophages stimulate with IL-4 were detected by RT-qPCR. After stimulated with IL-4 (20 ng/mL), the expression of Arg1, Vegf and Mcr1 increased in macrophages.
(D) The expression of M1-signature genes iNOS in macrophages stimulated with LPS (100 ng/mL) was detected by RT-qPCR. Compared with the expression of M2-signature genes, the expression of iNOS significantly increased in macrophages.
(E) The expression of M1- and M2-signature proteins in TAMs were detected by FACS. Compared with the expression of CD86, the expression of CD206 was obviously increased in TAMs.
(F and G) The expression of M2-signature proteins in TAMs and macrophages stimulated with IL-4 were detected by western blot. The expression of Arg1, p-JAK and p-STAT6 significantly increased in TAMs and IL-4 induced macrophages.
(H) The expression of M1-signature iNOS in macrophages stimulated with LPS was detected by western blot. After LPS stimulation, the expression of iNOS obviously increased in macrophages. (∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
Validation the function of M2-like TAMs
Timing: 1 h (for step 28)
Timing: 4 h (for step 29)
According to previous literature,5 it has been confirmed that M2-like TAMs can inhibit T cell migration. Therefore, we subsequently verify the function of M2-like TAMs, which also aimed to better distinguish them from IL4 induced M2 like macrophages, as the gene expression profiles of these two macrophages are similar.
-
28.T cell culture and activation.
-
a.Euthanize mouse according to institutionally approved regulations and then place mouse on the operating platform.
-
b.Remove the spleen of mouse to the sterilized PBS.Note: Ensure that the following steps are carried out on a clean bench for cell cultivation or in a biological safety cabinet to avoid contamination.
-
c.Remove the spleen to a sterilized 1.5 mL EP tube, add 200 μL PBS and grid the spleen with pestle.
-
d.Filtrate the spleen suspension with 40 μm cell strainer to a 15 mL centrifuge tube and add 20 μL Fc block to sample.
-
e.Remove the sample to a 5 mL polystyrene round-bottom tube, add 50 μm Isolation Cocktail to the sample, mix and incubate at room temperature for 10 min.
-
f.Vortex RapidSpheres 30 s and add 125 μL to the sample, mix and incubate at room temperature for 5 min.
-
g.Add CD8+T cell culture medium to 2.5 mL, mix by gently pipetting up and down 2–3 times.
-
h.Place the tube into the magnet and incubate at room temperature for 2.5 min.
-
i.Pick up the magnet and invert the magnet and tube, pouring the CD8+T cell suspension to a new tube.
-
j.Count CD8+ T cells and plate in a 24-well plate (1 × 106/well) coated with 5 mg/mL anti-CD3 and 2 mg/mL anti-CD28, culture 48 h at 37°C.
-
a.
-
29.T cell migration assay:
-
a.T cell migration assay was performed by using 24-well 6.5 mm transwell with 5 μm pore polycarbonate membrane insert.
-
b.Prepare BMDMs and LCM according to the methods of above steps.
-
c.Digest and collect the cultured BMDMs (refer to the steps of collection of BMDMs), count the cells and plate in the bottom chamber (5 × 105 cells/well) and culture overnight.
-
d.Prepare complete BMDM culture medium containing LCM at a 1:1 ratio. For instance, 250 μL of complete BMDM culture medium was mixed with 250 μL of LCM per well in a 24-well plate.
-
e.Discard the supernatant of BMDMs, and then add the prepared culture medium containing LCM. Containing to culture BMDMs for 24 h.
-
f.Add activated CD8+ T cells in the top chamber (1 × 106/200 μL) and coculture at 37°C in a 5% atmosphere for 4 h (Figure 4).
-
g.Collect the cells in the bottom chambers (T cells were suspend in the supernatant) and count the number of CD8+ T cells.
-
h.Compared with IL-4 induced M2 macrophages and BMDMs cocultured groups, the number of CD8+ T cells that migrated into the lower chambers were significantly reduced in TAMs cocultured group, which verified the function of TAMs and distinguished it better from IL-4-induced M2 macrophages (Figure 4).
-
a.
Figure 4.
TAMs inhibit the migration of CD8+ T cells
(A) Flow chart of co-cultivation of TAMs induced by LCM and CD8+ T cells.
(B) The number of CD8+ T cells that migrated into the lower chambers in BMDMs, LCM induced TAMs and IL-4 induced M2 macrophages. (∗∗p < 0.01).
Expected outcomes
Approximately 30 million BMDMs can be extracted from an 8-week-old mouse. After stimulation with 25 mM lactic acid or LCM (Figure 3), the expression of TAMs-related genes was increased, as shown by RT-qPCR or western blot detection. For T cell migration assay (Figure 4), the number of CD8+ T cells that migrated into the lower chambers was significantly reduced in TAMs cocultured groups compared with IL-4-induced macrophages and BMDMs cocultured groups.
Limitations
This article only elucidates the experimental steps of TAMs induced by mouse lung adenocarcinoma Lewis cells. The steps of TAMs polarization induced by other tumor cell lines need to be explored based on the characteristics of each tumor cell line.
Troubleshooting
Problem 1
BMDMs with poor viability were treated with DMEM containing LCM or 25 mM lactic acid.
Potential solution
In our experimental study, BMDMs were treated with RPMI 1640 medium containing LCM or 25 mM lactic acid, and the vitality of the cells was relatively low. When BMDMs were treated with DMEM with high glucose containing LCM or 25 mM lactic acid, the cells had favorable vitality. Of course, specific experiments need to be conducted based on the individual features of tumor cells.
Problem 2
Which day is it better to collect LCM for BMDMs treatment?
Potential solution
In our study, LCM collected at 48 h showed the best effect on TAMs polarization.
Problem 3
How to remove fur and muscles clearly from the tibia and femur of mouse?
Potential solution
To remove fur, mice can be sprayed with 70% alcohol to moisten the fur and make it easier to remove. For removing muscles, it is best to follow the direction in which the muscles adhere to the bones (Figure 1).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Qiang Li: Liqressh1962@163.com.
Technical contact
Further information and requests for technical will be fulfilled by technical contact, Xia Fang: dilay_110@126.com.
Materials availability
This protocol does not include unique materials.
Data and code availability
This protocol does not include datasets.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81970073, 82170090, and 82103412) and the Shanghai Medical Innovation Research Special Program of Shanghai Science and Technology Innovation Action Plan (22Y11901100).
Figures 1, 2, and 4 were created by BioRender (https://www.biorender.com/).
Author contributions
X.F., Y.W., H.Q., P.Z., and M.S. performed the experiments; X.F., Y.W., F.W., and Q.L. designed the experiments and wrote the protocol.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Feilong Wang, Email: wang_feilong@tongji.edu.cn.
Qiang Li, Email: liqressh1962@163.com.
References
- 1.Fang X., Zhao P., Gao S., Liu D., Zhang S., Shan M., Wang Y., Herrmann J., Li Q., Wang F. Lactate induces tumor-associated macrophage polarization independent of mitochondrial pyruvate carrier-mediated metabolism. Int. J. Biol. Macromol. 2023;237 doi: 10.1016/j.ijbiomac.2023.123810. [DOI] [PubMed] [Google Scholar]
- 2.Ran L., Zhang S., Wang G., Zhao P., Sun J., Zhou J., Gan H., Jeon R., Li Q., Herrmann J., Wang F. Mitochondrial pyruvate carrier-mediated metabolism is dispensable for the classical activation of macrophages. Nat. Metab. 2023;5:804–820. doi: 10.1038/s42255-023-00800-3. [DOI] [PubMed] [Google Scholar]
- 3.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M., et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D., Tang Z., Huang H., Zhou G., Cui C., Weng Y., Liu W., Kim S., Lee S., Perez-Neut M., et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y.J., Li G.N., Li X.J., Wei L.X., Fu M.J., Cheng Z.L., Yang Z., Zhu G.Q., Wang X.D., Zhang C., et al. Targeting IRG1 reverses the immunosuppressive function of tumor-associated macrophages and enhances cancer immunotherapy. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adg0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This protocol does not include datasets.