Abstract
Eukaryotic cells possess multiple mechanisms of self‐destruction, including pyroptosis and necroptosis. Pyroptosis is a type of programmed cell death characterized by cellular rupture and linked to inflammation. SnoRNA, a small non‐coding RNA in the nucleolus, can dysregulate specific RNAs through 2'‐O‐methylation, contributing to tumorigenesis. Our StarBase and qRT‐PCR analysis revealed SNORD99 upregulation in endometrial cancer (EC) tissue compared to normal tissue, suggesting its role in pathogenesis. SNORD99 overexpression enhanced migration and proliferation of EC cells, while ASO‐mediated suppression reduced malignant cell spread and division. RNA‐seq and base‐comparing analysis identified GSDMD's differential expression upon SNORD99 overexpression, forming the SNORD99‐FBL RNP complex. RTL‐P experiments showed SNORD99 increased GSDMD's 2'‐O‐methylation. SNORD99 reduced GSDMD, caspase‐1, and NLRP3 protein levels, implicating its role in pyroptosis. Optical and electron microscopy confirmed enhanced pyroptosis features. In summary, SNORD99 modifies GSDMD via 2'‐O‐methylation, suppressing pyroptosis and promoting EC progression. Developing pyroptosis‐inducing drugs may offer new cancer treatment avenues.
Keywords: 2'‐O‐methylation, endometrial cancer, GSDMD, pyroptosis, small nucleolar RNA
1. INTRODUCTION
Endometrial cancer (EC) is a malignancy arising from the epithelial cells lining the uterus with increasing morbidity and mortality worldwide. 1 In 2020, EC sickened approximately 417,336 patients and became the most frequently diagnosed malignancies in women. 2 Although more than 60% of patients suffer early clinical symptoms and the survival rate over a five‐year period of early‐stage cases is about 81%, the five‐year survival rates of advanced stage cases such as IVA and IVB EC stages are only 17% and 15%, respectively. 3 The occurrence of EC may be related to the mutations of multiple proto‐oncogenes or tumour suppressor genes. Thus, the molecular mechanisms that drive the onset and development of EC is crucial for advancing the discovery of efficient approaches for timely detection, diagnosis and management.
SnoRNA, a non‐coding RNA molecule, is present in eukaryotic cells and is found throughout the nucleolus. The length of these molecules ranges from 60 to 300 nucleotides. It can form complexes with ribonucleolar nucleoproteins to create snoRNPs. These snoRNPs are involved in various cellular processes, including the processing of ribosomal RNA, regulating RNA splicing and translation and responding to oxidative stress. 4 The snoRNA possesses conserved structural components, which can be categorized into three groups: box C/D snoRNA (SNORD), box H/ACA snoRNA (SNORA) and MRP RNA. 5 Emerging evidence has revealed that SNORDs have been shown to modulate gene expression by complementary base pairing and forming snoRNP complexes with ribonucleolar proteins (RNPs), leading to 2'‐O‐methylation of rRNA, tRNA, mRNA, snRNA and other RNAs. 6 , 7 In 2022, Wen et al. established that SNORD15B promoted the development of EC by regulating the TRIM25/P53 complex. 8 Chen et al. found that SNORA70E promotes the onset and progression of ovarian cancer by pseudouridylation modification of RAP1B and PARPBP. 9 In addition, Lu et al. discovered that SNORD104 promoted EC through the regulation of PARP1 2'‐O‐methylation. 10 Furthermore, Bao et al. evidenced that the modulation of 2'‐O‐methylation modifications by SNORD89 is implicated in the pathogenesis of EC, specifically in the regulation of Bim. 11 These results suggested that SNORDs participate in the tumorigenesis and progression of endometrial carcinoma.
Pyroptosis is a type of regulated cell death that is characterized by inflammation and is induced by a group of caspases known as pyroptosis‐associated caspases. It can be triggered by pathological factors, such as infections, cell damage and malignant tumours, and is characterized by swelling and rupture of the cell membranes and the release of cellular contents. Gasdermin family proteins are key molecules participating in pyroptosis. 12 , 13 , 14 Gasdermin D (GSDMD), a 53‐kDa cytoplasmic protein, is a key substrate for inflammatory caspases (such as caspase‐1, ‐4, ‐5, and ‐11) to trigger pyroptosis. 15 , 16 When pathogens enter cells, lipopolysaccharide (LPS) is released to activate caspase‐11, ‐4, and ‐5 or caspase‐1. Upon activation, inflammatory caspases cleave GSDMD and caspase‐1 can also increase the secretion of mature IL‐1β. The structural composition of GSDMD includes a pore‐forming domain situated at the N‐terminus, a regulatory domain at the C‐terminus, and a linking region positioned at the center. 17 , 18 Following cleavage, the N‐terminal portion of GSDMD is liberated and undergoes activation. Once activated, GSDMD‐N binds with lipids present in the plasma membrane, ultimately results in the development of large pores. Cell death can occur when extracellular fluid and calcium enter cells through membrane pores, activating the release of intracellular contents, such as cytokines and danger‐associated molecular patterns (DAMPs), as well as inflammatory substances. 19
Our analysis of the StarBase (https://rnasysu.com/encori/), an open platform for analysing data include lncRNAs, miRNAs, snoRNAs, mRNAs, etc. related to 32 cancer types integrated from the TCGA project, revealed that the SNORD99 expression showed a significant increase in EC tissue compared to normal endometrial tissue. SNORD99 is a member of the box C/D snoRNAs found within the snoRNA, specifically situated at 1p35.3 and known as HBII‐420 as well. Our preliminary experiments also found that the expression levels of GSDMD showed a significant decrease after SNORD99 overexpression by Ishikawa cells, suggesting that SNORD99 may regulate the pyroptosis signalling pathway, thereby promoting EC occurrence and development. However, no research has been conducted on the regulation of the mechanism of pyroptosis by snoRNA. Thus, we conducted experiments both in laboratory settings and within living organisms for the first time to investigate the potential molecular mechanism of SNORD99 involved in pyroptosis‐related EC tumorigenesis and progression.
2. MATERIALS AND METHODS
2.1. Bioinformatics analysis
The expression levels of SNORD99 in specimens of normal endometrial and EC tissues were obtained from StarBase (https://rnasysu.com/encori/).
2.2. Human tissue specimens
A total of 30 samples of normal endometrial tissue and 126 samples of EC tissue were obtained from individuals who received surgical intervention at the Third Affiliated Hospital of Guangzhou Medical University. Before the surgery, all the individuals involved in the study did not receive any radiotherapy or chemotherapy treatments. The specimens were rapidly cryopreserved in liquid nitrogen and maintained at −80°C until further analysis. The study protocol was approved by the hospital's ethics committee (no. 2020066), and all patients were informed and provided written consent.
2.3. Cell culture and transfection
Ishikawa, HEC1A, HEC1B and HESC (human endometrial stromal cells) were obtained from ATCC (Manassas, VA, USA) and Jennio Biotech (Guangzhou, China). The DMEM (HyClone, Logan, UT, USA) culture medium was used for HEC1B cells, while RPMI‐1640 medium (HyClone) was utilized for the cultivation of HEC1A and Ishikawa cells. The culture medium was prepared by adding 10% FBS (Gibco, Grand Island, NY, USA) and 100 U/mL of penicillin–streptomycin (Gibco) to the media. The incubation temperature was 37°C and with 5% CO2. Transfection of plasmids and Antisense oligonucleotide (ASOs) was carried out using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). The SNORD99‐overexpressing plasmid sequence was as follows: (ACTGGTCCAGGATGAAACCT AATTTGAGTGGACATCCATG GATGAGAAATGCGGATATGG GACTGAGACCAGCTCCTAGG). The target gene sequence for ASO (Ruibo, Jiangsu, China) was ATGCGGATATGGGACTGAGA.
2.4. Cell viability experiment
After cell adherence, cells were plated in 96‐well plates at a concentration of 2000 cells per well and transfected with the appropriate agents. At designated time points (0, 24, 48 and 72 h), each well was treated with 10 μL of Cell Counting Kit‐8 (CCK‐8, Yeasen, Shanghai, China) and incubated for an additional 2 h at 37°C. The optical density (OD) of each well was detected at 450 nm using a microplate reader. The experimental procedure was carried out in triplicate.
2.5. RNA stability
Cells overexpressing or knocking down SNORD99 were seeded into 6‐well plates. When the cell growth rate reached 80%, 5 μg/mL actinomycin D (Act D, Sigma, St. Louis, MO, USA) was added to each well, and cells were collected at 0, 3 and 6 h, then lysed using TRIzol reagent and total RNA was extracted, and finally GSDMD mRNA levels were detected by qPCR.
2.6. Wound healing
Inoculate 600,000 cells into a 6‐well culture plate. The next day, using the tip of a 200 μL pipette, create two perpendicular scratches in each well. Subsequently, wash the cells in the wells three times with PBS and 2 mL of serum‐free medium was added to each well. Record the cell migration at 0 and 48 h, and analyse the wound area using ImageJ software (National Institutes of Health, Bethesda, Maryland) to determine the rate of cell migration.
2.7. Cell migration assay by transwell
A total of 50,000 cells overexpressing or knocking down SNORD99 were seeded in the upper chamber of the Transwell chamber with 200 μL serum‐free medium, then 600 μL of serum‐containing medium was added to the lower chamber. After 48 h of incubation in the incubator, the chambers were removed and washed with PBS, then the cells were fixed with paraformaldehyde for 20 min, and finally the cells were stained with crystal violet for 20 min. Cells were photographed under a microscope (Olympus, Tokyo, Japan) and counted using ImageJ.
2.8. Real‐time quantitative reverse‐transcription PCR
RNA was isolated from the cells using an RNA rapid purification kit, and random primers from the Fast Reverse Transcription Kit (Tiangen, Beijing, China) were used to generate complementary cDNA. The synthesized cDNA was utilized as a template for quantitative real‐time PCR (qRT‐PCR) with the Hieff® qPCR SYBR Green Master Mix (Yeasen, Shanghai, China). The 2−ΔCt method was employed to determine the expression level of the target gene by contrasting the Ct value of the target gene with that of a reference gene (U6).
2.9. RNA immunoprecipitation analysis
Cell scraping was employed to harvest the cells. The cells were resuspended in an equal volume of RNA immunoprecipitation (RIP) lysis buffer. After centrifugation, the supernatant was collected and incubated with magnetic beads conjugated with anti‐human FBL antibody and normal rabbit IgG (negative control) in RIP buffer, then washed twice with a magnetic rack, and the supernatant was discarded. Then, total RNA was extracted from the pellet by adding 1 mL of TRIzol reagent (Takamura, Shibuya, Japan). Chloroform (200 μL) was added to the mixture, mixed and centrifuged at 12,000 rpm for 20 min. The supernatant was removed and an equal volume of isopropanol was added to precipitate the RNA. After centrifuging again at 12,000 rpm for 20 min, the supernatant was discarded and the precipitate was washed with 75% ethanol. After the alcohol has completely evaporated, the precipitate was dissolved with DEPC water. Spectrophotometry (Thermo Fisher Scientific, Shanghai, China) was used to determine the concentration of total RNA. Then, total RNA was converted into cDNA by employing reverse transcriptase alongside random primers following the protocol provided by manufacturer (Yeasen). This cDNA served as the template for qRT‐PCR, employing the SYBR Prime X Ex‐TAQ Kit (Takara Bio Inc., Shiga, Japan). Finally, according to the method, the Ct value of the target gene and the reference gene (U6) was compared to calculate the relative expression of the gene.
2.10. Western blotting
The GSDMD antibody (20770‐1‐AP, diluted at 1:5000; Proteintech), NLRP3 (D4D8T, diluted at 1:800; Cell signal), caspase‐1 (D7F10, diluted at 1:800; Cell signal), and GAPDH (10494‐1‐AP, diluted at 1:10,000; Proteintech) were used to evaluate protein expression. To extract proteins from EC cells, PBS was used to wash the cells followed by lysis in RIPA lysis buffer supplemented with protease inhibitors. The lysates were then subjected to centrifugation at 12,000 rpm and 4°C for 20 min, and the protein concentration was measured with a BCA Protein assay Kit (P0009; Beyotime, Shanghai, China). Subsequently, protein samples were separated by electrophoresis on a 10% SDS‐polyacrylamide gel and subsequently transferred onto western blotting membranes (Amersham Hybond, Munich, Germany). The membranes were incubated in a blocking solution of 3% BSA in TBST at room temperature for 2 h, and primary antibodies specific to GSDMD, NLRP3 and caspase‐1 were added and incubated overnight at 4°C. A secondary antibody (1:10000, Proteintech) was added to the membrane on the second day and incubated at room temperature for 2 h, and GAPDH was used as the internal reference. The membranes were subjected to three washes with TBST after incubation and protein development was detected using a Ultra High Sensitivity ECL Substrate Kit (NcmECL UItra; Sliding Biotech, Shanghai, China). The protein bands were visualized using an enhanced chemical lighting system.
2.11. In vivo tumour formation experiment in nude mice
A subcutaneous tumour transmission detection kit was utilized in this experiment to induce tumour formation, female BALB/C nude mice were used in this study (n = 6/each group). The mice were maintained under conditions that were free of specific pathogens and were randomly assigned to different groups. Female mice that were 5 weeks old were subjected to subcutaneous injection of Ishikawa cells transfected with Plasmids carrying SNORD99 or no‐load control suspended in 150 of FBS‐free medium to induce tumour formation. Weekly measurements were taken using vernier callipers to monitor the growth of the tumours and determine their volume. After 4 weeks, humane euthanasia was performed on the mice, and the tumours were excised and measured for size. This study was conducted with the approval of the relevant ethical committee.
2.12. Reverse transcription at low dNTP concentrations followed by polymerase chain reaction
To detect Nm of SNORD99, RT‐PCR was conducted using a 20‐μL reaction system comprising 5 μg of total RNA, different concentrations of dNTPs (TaKaRa), and 1 μL of specific reverse transcription primers for the target gene (PF) at a temperature of 65°C for a duration of 5 min. Subsequently, after the addition of 4 μL of 5 × M‐MLV Reverse Transcriptase buffer (PROMEGA), the mixture was subjected to incubation at 50°C for a duration of 1 h, 1 μL of 0.1 M DL‐Dithiothreitol (PROMEGA), and 1 μL of 40 u/μL RNase inhibitor (PROMEGA), followed by the incubation that was carried out at 70 C for a duration of 15 min. The SYBR Prime X Ex‐TAQ Kit (Takara) was employed for qRT‐PCR, using the cDNA as template. Ultimately, the gene's expression level was assessed through the 2−ΔCt method by comparing the Ct value of the target gene in the experimental group with that in the control group at low concentrations.
2.13. EdU assay
Cells from the Ishikawa or HEC1B cell lines were cultured in 96‐well plates and treated with EdU assay kit (Thermo Fisher, USA) as per the manufacturer's instructions. Subsequently, the cells were stained and observed using a microscope (Olympus, Tokyo, Japan).
2.14. Immunofluorescence
Ishikawa/HEC‐1B cells were fixed onto 10‐mm circular glass slides with 4% paraformaldehyde for 15 min. Afterward, the cells were washed thrice using PBS and then exposed to 0.2% Triton X‐100 (w/v) in PBS for a duration of 15 min. Following three washes with PBS, the cells were subjected to a blocking procedure for 2 h using 5% bovine serum albumin (BSA). The cells were subjected to incubation with the primary antibody targeting GSDMD (20770‐1‐AP, 1:400, Proteintech, China) for an overnight duration at 4°C. Next, the cells were treated with the secondary antibody, Alexa Fluor 596‐labelled goat anti‐rabbit IgG (1:500, Beyotime, China), and incubated for 2 h. The fluorescence images were acquired using a Leica microscope.
2.15. Lactate dehydrogenase analysis
The release of lactate dehydrogenase (LDH) from cells into the culture supernatant is a commonly used indicator of cell damage and death. To measure LDH levels in the culture supernatants of EC cells that were exposed to LPS for 24 h, the non‐radioactive CytoTox 96 Cytotoxicity Assay Kit (Promega) was utilized as per the manufacturer's guidelines. All infection assays were carried out in serum‐ and antibiotic‐free media, and the results were analysed using a Synergy Mx plate reader (BioTek, USA).
2.16. Scanning electron microscopy (SEM)
HEC‐1B cells were grown on 10‐mm glass coverslips, rinsed twice with PBS, and fixed in 2.5% Gluta‐specific fixative for electron microscopy (Solarbio) for 1–2 h. The fixed cells were washed with PBS buffer, post‐fixed in 1% OsO4 for 30 min, and gradually dehydrated using an ethanol series. Next, the cells were critically dried, mounted on stubs, coated with a thin layer of conductive metal (gold and palladium) and examined under an SEM microscope (Hitachi‐S‐3400).
2.17. Data analysis
Data analysis and graphing were performed using GraphPad Prism v 8.01(244) (GraphPad Software, CA, USA). The data were presented as mean ± standard error of the mean, and statistical analysis was conducted using a two‐sample t‐test. A p‐value less than 0.05 was considered statistically significant.
3. RESULTS
3.1. The expression of SNORD99 is upregulated in endometrial cancer tissues
We analysed the StarBase, and found that the expression of SNORD99 was markedly elevated in EC tissue in comparison with that in normal endometrial tissue (Figure 1A, *p < 0.05). We collected 30 normal endometrial tissue specimens and 126 EC tissue specimens, and performed qRT‐PCR on these samples. The results showed that the expression of SNORD99 in EC tissues was significantly higher than that in normal endometrial tissues (Figure 1B, *p < 0.05). Additionally, qRT‐PCR results showed that SNORD99 expression was highest in HEC‐1B cells and lowest in Ishikawa cells (Figure 1C, *p < 0.05). To further investigate, we overexpressed SNORD99 in Ishikawa cells and used SNORD99 ASO to knock down its expression in HEC‐1B cells. The transfection efficiency was confirmed by qRT‐PCR (Figure 1D,E, *p < 0.05).
FIGURE 1.

Gene expression of SNORD99 in endometrial cancer (EC) tissues and cell lines. (A) Data from the StarBase databases. (B) SNORD99 expression in EC tissues. (C) SNORD99 expression in EC cell lines and human endometrial stromal cells (n = 3). (D) Transfection efficiency after overexpression of SNORD99 (n = 3); (E) Transfection efficiency after downregulation of SNORD99 by ASO (n = 3). Values are presented as mean ± SD. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
3.2. Overexpression of SNORD99 promotes cell proliferation and migration of EC cells
After SNORD99 overexpression in Ishikawa cells, we performed cell counting, plate clone formation, wound‐healing and cell proliferation EdU‐fluorescent labelling experiments to determine the function of SNORD99 in the proliferation and migration of EC cells. The results showed that SNORD99 overexpression promoted cell proliferation (Figure 2A–C, *p < 0.05) and enhanced cell migration ability (Figure 2D,E, *p < 0.05).
FIGURE 2.

Cellular function of SNORD99 overexpression in vitro. SNORD99 overexpression (A) induced cell viability (n = 3), (B) promoted cell colony formation (n = 3), (C) induced EdU fluorescence labelling for cell proliferation and (D & E) induced cell migration (n = 3). Values are presented as mean ± SD. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
3.3. Knockdown of SNORD99 inhibits the malignant tumour characteristics of EC cells
After knocking down SNORD99 by transfection with ASO‐SNORD99 in HEC‐1B cells, we performed cell function experiments. SNORD99 knockdown inhibited cell proliferation (Figure 3A–C, *p < 0.05) and migration (Figure 3D,E, *p < 0.05) in comparison to the negative control group.
FIGURE 3.

Cellular function of knockdown SNORD99 in vitro. Downregulation of SNORD99 (A) reduced cell viability (n = 3), (B) inhibited cell colony formation (n = 3), (C) inhibited EdU fluorescence labelling for cell proliferation and (D & E) reduced cell migration (n = 3). Values are presented as mean ± SD. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
3.4. In vivo nude mouse tumorigenicity assay of SNORD99
To investigate the in vivo effects of SNORD99, tumour formation experiments were conducted in nude mice (Figure 4A). The findings indicated that Ishikawa cells overexpressing SNORD99 lead to faster tumour formation (Figure 4C, *p < 0.05) and larger tumour volumes compared to the control group (Figure 4B,D, *p < 0.05).
FIGURE 4.

Tumour formation effect of overexpressed SNORD99 in vivo. (A–C) Mice injected with SNORD99‐overexpressing cells were more tumorigenic than controls. (D) The tumour volume was larger (n = 6). Values are presented as mean ± SD. ****p ≤ 0.0001.
3.5. SNORD99 inhibits the expression of pyroptosis‐related proteins in EC cells
The RNA sequencing analysis of SNORD99 overexpression revealed that differential expression of 223 genes, with 118 genes upregulated and 105 downregulated (Figure 5A,B, *p < 0.05). Through gene complementarity pairing between the bioinformatics prediction (NCBI database) and the sequence base of SNORD99, we identified a potential binding site between GSDMD and SNORD99 (Figure 5C). As GSDMD is a key protein involved in pyroptosis, we performed overexpression or knockdown of SNORD99 in EC cells to evaluate its effect on pyroptosis. Western blot and immunofluorescence assays were conducted to determine differences in the expression of pyroptosis‐related genes. The results of western blot experiments showed that the overexpression of SNORD99 decreased the expression of GSDMD, caspase‐1 and NLRP3 (Figure 5D), while immunofluorescence experiments revealed that SNORD99 overexpression inhibit GSDMD expression (Figure 5E), while ASO transfection to knockdown SNORD99 promoted GSDMD expression (Figure 5F). The mRNA level of GSDMD is decreased by overexpression of SNORD99 (Figure 5G, *p < 0.05). On the contrary, knocking down SNORD99 increased the mRNA expression level of GSDMD (Figure 5H, *p < 0.05). The RNA stability of GSDMD does not exhibit significant correlation with either overexpression or knockdown of SNORD99 (Figure 5I,J, *p > 0.05).
FIGURE 5.

SNORD99 overexpression inhibits the expression level of pyroptotic proteins in endometrial cancer (EC) cells. (A) Volcano plots of differentially expressed genes exist in RNA‐seq (n = 223). (B) Heat map of differentially expressed genes of pyroptosis exists in RNA‐Seq. (C) Base pairing between SNORD99 and GSDMD. (D) Expression levels of GSDMD, caspase‐1 and NLRP3 proteins in Ishikawa and HEC‐1B cells after overexpression or knockdown of SNORD99. (E) After overexpression of SNORD99, the fluorescence intensity of GSDMD protein was weakened. (F) After the knockdown of SNORD99, the fluorescence intensity of GSDMD protein was enhanced. (G) GSDMD mRNA levels after overexpression of SNORD99 (n = 3). (H) GSDMD mRNA levels after knocking down SNORD99 (n = 3). (I) GSDMD mRNA stability after overexpression of SNORD99 (n = 3). (J) GSDMD mRNA stability after knocking down SNORD99 (n = 3). Values are presented as mean ± SD. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
3.6. SNORD99 regulates GSDMD expression through 2'‐O‐methylation
To establish whether SNORD99 regulates the expression of GSDMD by affecting the level of 2'‐O‐methylation modification, we first confirmed that both SNORD99 and GSDMD can bind to 2‐O‐methyltransferase FBL, we conducted RIP experiments after overexpression or knockdown of SNORD99. The results indicate that overexpression of SNORD99 leads to an increase in the binding ability of GSDMD mRNA to the SNORD99‐FBL complex, whereas knockdown of SNORD99 results in a decrease in the binding ability of GSDMD mRNA to the SNORD99‐FBL complex (Figure 6A,B, *p < 0.05). Then, we conducted RTL‐P experiments, with specific experimental methods based on existing literature. 20 The experimental principle of RTL‐P is based on the premise that low dNTP concentrations would terminate cDNA synthesis by reverse transcriptase when encountering 2'‐O‐methylated nucleotides (Figure 6C). The results from RTL‐P revealed that SNORD99 overexpression led to a significant decrease in the quantity of the GSDMD product under low dNTP concentrations compared to the control group, indicating an increase in the level of 2'‐O‐methylation (Figure 6D, *p < 0.05). These results suggest that SNORD99 might exert its regulatory effect on GSDMD expression through 2'‐O‐methylation modification. Our results showed that FBL downregulation in SNORD99 overexpression Ishikawa cells reversed the oncogenic role of SNORD99, which was validated by CCK8, wound healing, EdU assays (Figure 6E,H,J, *p < 0.05), and the mRNA expression level of GSDMD is also increased (Figure 6G, *p < 0.05). After further downregulating the expression of GSDMD in HEC‐1B cells with ASO‐mediated knockdown of SNORD99, we observed enhanced proliferation and migration capabilities of EC cells (Figure 6I,K, *p < 0.05), accompanied by a corresponding decrease in GSDMD protein expression levels (Figure 6F, *p < 0.05).
FIGURE 6.

SNORD99 regulates the 2'‐O‐methylation level of GSDMD. (A, B) FBL can bind GSDMD mRNA and SNORD99 (n = 3). (C) Principle demonstration diagram of RTL‐P experiment. (D) The 2'‐O‐methylation level of GSDMD mRNA in Ishikawa cells overexpressing SNORD99 (n = 3). (E) Cell proliferation of knocking down FBL after over‐expression of SNORD99 (n = 3). (J) EdU fluorescence labelling for cell proliferation of knocking down FBL after over‐expression of SNORD99 (n = 3). (F) Level of GSDMD protein after downregulating the expression level of SNORD99 before knocking down GSDMD (n = 3). (G) mRNA level of GSDMD after overexpression of SNORD99 and subsequent knockdown of FBL (n = 3). (H) Cell migration of knocking down GSDMD after downregulation of SNORD99 (n = 3). (I) Cell migration of knocking down FBL after over‐expression of SNORD99 (n = 3). (K) EdU fluorescence labelling for cell proliferation of knocking down GSDMD after downregulation of SNORD99 (n = 3). Values are presented as the mean ± SD. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
3.7. SNORD99 participates in the process of pyroptosis
LDH is a well‐known marker of cell damage, and it is released from the cell when pyroptosis occurs, along with cytokines, through the cell pores. After the Ishikawa cells overexpressing SNORD99 were treated with 20 or 40 μg/mL LPS for 24 h, the LDH level in the supernatant of the cell culture medium was significantly lower than that of the control group (Figure 7A, *p < 0.05). In contrast, the knockdown of SNORD99 expression in HEC‐1B cells led to a significant increase in LDH level in the supernatant compared to the negative control group (Figure 7B, *p < 0.05). Under the light microscope, HEC‐1B cells transfected with ASO SNORD99 without LPS treatment had a normal appearance, including filamentous protrusions. After treatment with 40 μg/mL LPS for 2 h, the cells became larger and slightly swollen, and the processes were shorter. After 4 h of LPS treatment, the cell processes were much shortened and the cell bodies were significantly swollen (Figure 7C). While knocking down the expression of GSDMD on the HEC‐1B cells that have knocked down the expression of SNORD99, it can be observed under the light microscope that the characteristic changes of cell pyroptosis are reversed. In contrast, there was no relevant performance in the control group (Figure 7D). Next, the detailed surface morphology of HEC‐1B cells was evaluated by SEM. After the cells were transfected with ASO to knock down the expression of SNORD99 and treated with 20 μg/mL LPS for 24 h, many pits or pores of different sizes were observed on the cell surface, the cell shape collapsed and the protrusions shrank (Figure 7E). Conversely, no such manifestations were detected in the negative control group.
FIGURE 7.

Effects of overexpression or knockdown of SNORD99 on cell morphology. (A) Lactate dehydrogenase (LDH) levels of Ishikawa cells overexpressing SNORD99 after treatment with different concentrations of lipopolysaccharide (LPS) (n = 3). (B) LDH levels of SNORD99‐knockdown HEC‐1B cells treated with different concentrations of LPS (n = 3). (C) The morphological changes of HEC‐1B cells knocked down by SNORD99 after treatment with the same concentration of LPS at different time periods. (D) The HEC‐1B cells that knocked down SNORD99 and then the expression of GSDMD showed changes in cell morphology at different time periods after treatment with the same concentration of LPS. (E) The cell morphology changes observed by SEM after the HEC‐1B cells knocking down SNORD99 were treated with the same concentration of LPS. Values are presented as the mean ± SD. *p ≤ 0.05.
4. DISCUSSION
In an effort to identify potential biomarkers for EC diagnosis and treatment, we conducted an extensive search for various snoRNAs using the specific database. Our analysis revealed that SNORD99 expression was considerably higher in EC tissues when compared to normal endometrial tissues, highlighting its potential as a valuable diagnostic and therapeutic marker for this disease.
To better understand the role of SNORD99 in EC cells, we performed a series of experiments including both in vitro cell studies and subcutaneous tumorigenesis assays in nude mice. By introducing a SNORD99 overexpression plasmid into Ishikawa cells, we noted a marked rise in cell growth, coupled with a stimulation of cell migration. However, transfection of ASO in HEC‐1B cells to knock down the expression level of SNORD99 reversed the results of our cell experiments. Furthermore, the subcutaneous tumour formation experiments conducted in nude mice provided additional evidence to support our findings, as we observed a significant increase in tumour growth following SNORD99 overexpression, validating its role in promoting tumour growth in vivo. These results suggest that SNORD99 may play a role in promoting the initiation and progression of EC. Despite our researches indicating the potential involvement of SNORD99 in EC, the precise mechanism of its action still remains unclear and requires further investigation.
2'‐O‐methylation is the partial methylation of the 2'‐OH in nucleotide ribose, which is the main RNA modification mediated by snoRNA and associated with various cancers. An interesting example of the potential involvement of snoRNAs in cancer is the long non‐coding RNA ZFAS1, ZFAS1 has been demonstrated to enhance 2'‐O‐methylation modification mediated by snoRNA through Nop58 recruitment, and has been linked to the progression and prognostication of colorectal cancer. 21 The 3′‐terminus of Mir‐21‐5p can be modified by adding a methyl group to the 2'‐O position, which has been shown to impact the stability of the methyltransferase HENMT1 and inhibit apoptosis in lung cancer cells. 22 Recent studies have shown that snoRNAs have the ability to modulate the expression of target genes, affecting both mRNA and protein levels, through modifications such as 2'‐O‐methylation. 23 , 24 , 25 In general, 2'‐O‐methylation can increase mRNA stability. However, our research indicates that the overexpression or knockdown of SNORD99 does not affect the stability of GSDMD mRNA. Nonetheless, other studies have suggested that 2'‐O‐methylation modification may impact the spatial structure and flexibility of the RNA chain, disrupting the interaction between the ribosome and the codon–anticodon helix. This disruption can lead to errors in ribosomal recognition and proofreading of homologous aminoacyl tRNA, ultimately resulting in decreased targeted gene expression and reduced protein synthesis. 11 Based on the results, we believe that SNORD99 can influence the expression of GSDMD mRNA at the transcriptional level. According to the results of western blot and immunofluorescence, GSDMD protein expression decreased following SNORD99 overexpression, indicating that SNORD99 can affect GSDMD protein expression at the translational level. RIP results demonstrated that SNORD99 can form a complex with the 2'‐O‐methyltransferase FBL, which then binds to GSDMD mRNA. RTL‐P results showed that the 2'‐O‐methylation level of GSDMD mRNA increased after SNORD99 overexpression. These findings indicate that SNORD99 can modify GSDMD mRNA through 2'‐O‐methylation. Therefore, we hypothesize that SNORD99 modifies GSDMD through 2'‐O‐methylation, thereby promoting EC occurrence and development. However, due to technical and conditional limitations, we still lack methods to specifically detect the 2'‐O‐methylation sites of GSDMD, further research is needed in the future.
Unlike apoptosis, pyroptosis is a type of inflammation‐dependent programmed cell death that acts as the body's self‐protection mechanism to remove pathogens from the body. 26 , 27 Recent studies suggest that pyroptosis may also be closely related to the proliferation and migration of different cancer cells. It can promote the inflammatory cell death of cancer cells and inhibit their proliferation and migration. 28 Normally, GSDMD is in a state of self‐inhibition. However, when cleaved by pyroptosis‐related caspase enzymes, GSDMD releases the N‐terminal fragment, which forms a hole in the cell membrane, causing the cell to swell and rupture, 15 , 16 , 29 , 30 which indicates that GSDMD is involved in pyroptosis. Western blot and immunofluorescence experiments showed that the overexpression of SNORD99 decreased the expression of GSDMD and the pyroptosis‐related protein caspase‐1 and NLRP3, whereas the knockdown of SNORD99 increased GSDMD expression. Therefore, we propose that SNORD99 may affect pyroptosis by reducing the expression level of the GSDMD protein. Moreover, changes in cell phenotype were observed under an optical microscope. Finally, we observed changes in cell phenotype by scanning electron microscopy. Under the optical microscope, we found that the negative control HEC‐1B cells still had clear cell protrusions after their treatment with LPS. The cell protrusions were not shortened and disappeared over time, and the cells did not swell significantly. However, after the HEC‐1B cells with knocked‐down SNORD99 expression were treated with LPS, the cell protrusions were significantly shortened or even disappeared over time, and the cell bodies would also be significantly swollen. Through scanning electron microscopy, we observed that these cells with knocked‐down SNORD99 expression became shorter and disappeared after treatment with LPS, and there was a large number of pores in the cell membrane. Therefore, SNORD99 overexpression may inhibit the process of pyroptosis by targeting and regulating GSDMD, representing a new target for the treatment of cancer.
5. CONCLUSIONS
SNORD99 can downregulate the expression of GSDMD protein by increasing the 2'‐O‐methylation modification level of GSDMD mRNA. Further, it inhibits the process of pyroptosis to facilitate the initiation and progression of EC. Our present findings indicated that snoRNA may be involved in the regulation of GSDMD expression and cell pyroptosis (Figure 8), which provides a new direction in EC prevention and therapy. Therefore, SNORD99 may become a new therapeutic target for EC.
FIGURE 8.
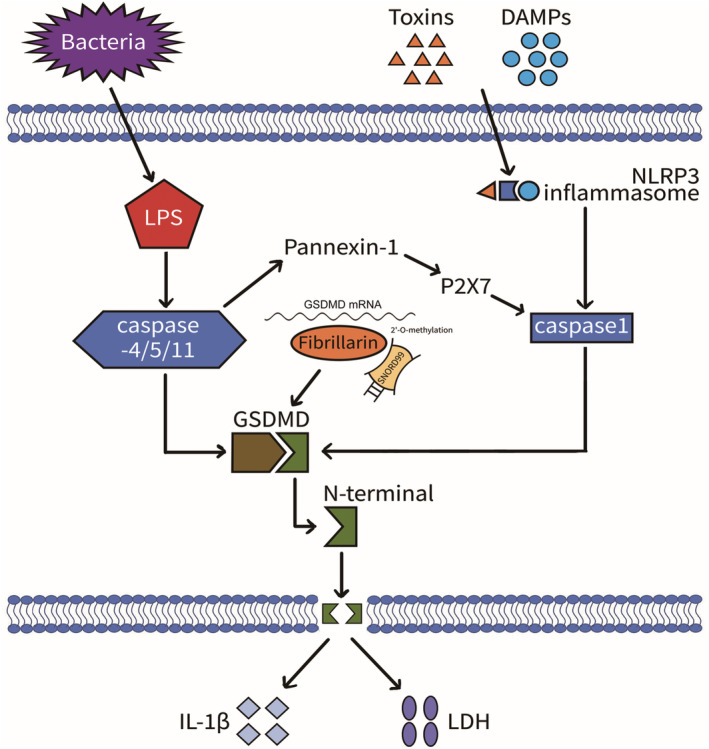
Mechanism diagram of SNORD99 involvement in cell pyroptosis.
AUTHOR CONTRIBUTIONS
Jing‐yuan Xian: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (supporting); investigation (lead); methodology (lead); project administration (lead); resources (equal); software (lead); supervision (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Wu Wu: Conceptualization (equal); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (supporting); methodology (supporting); project administration (supporting); resources (supporting); software (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Xi Chen: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (supporting); methodology (supporting); project administration (supporting); resources (supporting); software (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Hai‐juan Bao: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (supporting); methodology (supporting); project administration (supporting); resources (supporting); software (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Song Zhang: Writing – review and editing (equal). Xiu‐Jie Sheng: Writing – review and editing (equal). Shuo Chen: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (lead); investigation (supporting); methodology (supporting); project administration (supporting); resources (lead); software (supporting); supervision (lead); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting).
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (Grant no. 82272985 to Shuo Chen; 82103139 to Song Zhang), and the Project for Key Medicine Discipline Construction of Guangzhou Municipality (no. 2021‐2023‐17).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
Not applicable.
Xian J‐y, Wu W, Chen X, et al. SNORD99 promotes endometrial cancer development by inhibiting GSDMD‐mediated pyroptosis through 2'‐O‐methylation modification. J Cell Mol Med. 2024;28:e18500. doi: 10.1111/jcmm.18500
Contributor Information
Xiu‐Jie Sheng, Email: 1815260097@qq.com.
Shuo Chen, Email: chenshuo077003@163.com.
DATA AVAILABILITY STATEMENT
The data generated or analysed during this study are included in this published article and its supplementary information files, Supplementary information is available at Journal of Cellular and Molecular Medicine website.
REFERENCES
- 1. Henley SJ, Ward EM, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126(10):2225‐2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 4. Esteller M. Non‐coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861‐874. [DOI] [PubMed] [Google Scholar]
- 5. Samarsky DA, Fournier MJ, Singer RH, Bertrand E. The snoRNA box C/D motif directs nucleolar targeting and also couples snoRNA synthesis and localization. EMBO J. 1998;17(13):3747‐3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liang J, Wen J, Huang Z, Chen XP, Zhang BX, Chu L. Small nucleolar RNAs: insight into their function in cancer. Front Oncol. 2019;9:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lui L, Lowe T. Small nucleolar RNAs and RNA‐guided post‐transcriptional modification. Essays Biochem. 2013;54:53‐77. [DOI] [PubMed] [Google Scholar]
- 8. Wen JT, Chen X, Liu X, et al. Small nucleolar RNA and C/D box 15B regulate the TRIM25/P53 complex to promote the development of endometrial cancer. J Oncol. 2022;2022:7762708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen S, Li QH, Chen X, et al. SNORA70E promotes the occurrence and development of ovarian cancer through pseudouridylation modification of RAP1B and alternative splicing of PARPBP. J Cell Mol Med. 2022;26(20):5150‐5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu B, Chen X, Liu X, et al. C/D box small nucleolar RNA SNORD104 promotes endometrial cancer by regulating the 2'‐O‐methylation of PARP1. J Transl Med. 2022;20(1):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bao HJ, Chen X, Liu X, et al. Box C/D snoRNA SNORD89 influences the occurrence and development of endometrial cancer through 2'‐O‐methylation modification of Bim. Cell Death Dis. 2022;8(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan Y, Chen Q, Li X, et al. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Qin X, Liang J, Ge P. Induction of pyroptosis: a promising strategy for cancer treatment. Front Oncol. 2021;11:635774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660‐665. [DOI] [PubMed] [Google Scholar]
- 16. Kayagaki N, Stowe IB, Lee BL, et al. Caspase‐11 cleaves gasdermin D for non‐canonical inflammasome signalling. Nature. 2015;526(7575):666‐671. [DOI] [PubMed] [Google Scholar]
- 17. Ding J, Wang K, Liu W, et al. Pore‐forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111‐116. [DOI] [PubMed] [Google Scholar]
- 18. Liu X, Zhang Z, Ruan J, et al. Inflammasome‐activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis MA, Fairgrieve MR, den Hartigh A, et al. Calpain drives pyroptotic vimentin cleavage, intermediate filament loss, and cell rupture that mediates immunostimulation. Proc Natl Acad Sci USA. 2019;116(11):5061‐5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong ZW, Shao P, Diao LT, Zhou H, Yu CH, Qu LH. RTL‐P: a sensitive approach for detecting sites of 2'‐O‐methylation in RNA molecules. Nucleic Acids Res. 2012;40(20):e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu H, Qin W, Lu S, et al. Long noncoding RNA ZFAS1 promoting small nucleolar RNA‐mediated 2'‐O‐methylation via NOP58 recruitment in colorectal cancer. Mol Cancer. 2020;19(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang H, Jiao Z, Rong W, et al. 3'‐Terminal 2'‐O‐methylation of lung cancer miR‐21‐5p enhances its stability and association with Argonaute 2. Nucleic Acids Res. 2020;48(13):7027‐7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elliott BA, Ho HT, Ranganathan SV, et al. Modification of messenger RNA by 2'‐O‐methylation regulates gene expression in vivo. Nat Commun. 2019;10(1):3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi J, Indrisiunaite G, DeMirci H, et al. 2'‐O‐methylation in mRNA disrupts tRNA decoding during translation elongation. Nat Struct Mol Biol. 2018;25(3):208‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoernes TP, Heimdörfer D, Köstner D, et al. Eukaryotic translation elongation is modulated by single natural nucleotide derivatives in the coding sequences of mRNAs. Genes. 2019;10(2):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miao EA, Leaf IA, Treuting PM, et al. Caspase‐1‐induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21(11):678‐695. [DOI] [PubMed] [Google Scholar]
- 28. Fang Y, Tian S, Pan Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. [DOI] [PubMed] [Google Scholar]
- 29. Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143‐157. [DOI] [PubMed] [Google Scholar]
- 30. Rathkey JK, Zhao J, Liu Z, et al. Chemical disruption of the pyroptotic pore‐forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3(26):eaat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated or analysed during this study are included in this published article and its supplementary information files, Supplementary information is available at Journal of Cellular and Molecular Medicine website.


