Abstract
Background
Studies suggest that cerebrospinal fluid (CSF) levels of amyloid-β (Aβ)42 and Aβ40 present a circadian rhythm. However sustained sampling of large volumes of CSF with indwelling intrathecal catheters used in most of these studies might have affected CSF dynamics and thereby confounded the observed fluctuations in the biomarker levels.
Methods
We included 38 individuals with either normal (N = 20) or abnormal (N = 18) CSF Aβ42/Aβ40 levels at baseline. CSF and plasma were collected at two visits separated by an average of 53 days with lumbar punctures and venipunctures performed either in the morning or evening. At the first visit, sample collection was performed in the morning for 17 participants and the order was reversed for the remaining 21 participants. CSF and plasma samples were analyzed for Alzheimer’ disease (AD) biomarkers, including Aβ42, Aβ40, GFAP, NfL p-tau181, p-tau217, p-tau231 and t-tau. CSF samples were also tested using mass spectrometry for 22 synaptic and endo-lysosomal proteins.
Results
CSF Aβ42 (mean difference [MD], 0.21 ng/mL; p = 0.038), CSF Aβ40 (MD, 1.85 ng/mL; p < 0.001), plasma Aβ42 (MD, 1.65 pg/mL; p = 0.002) and plasma Aβ40 (MD, 0.01 ng/mL, p = 0.002) were increased by 4.2-17.0% in evening compared with morning samples. Further, CSF levels of 14 synaptic and endo-lysosomal proteins, including neurogranin and neuronal pentraxin-1, were increased by 4.5-13.3% in the evening samples (MDrange, 0.02-0.56 fmol/µl; p < 0.042). However, no significant differences were found between morning and evening levels for the Aβ42/Aβ40 ratio, different p-tau variants, GFAP and NfL. There were no significant interaction between sampling time and Aβ status for any of the biomarkers, except that CSF t-tau was increased (by 5.74%) in the evening samples compared to the morning samples in Aβ-positive (MD, 16.46 ng/ml; p = 0.009) but not Aβ-negative participants (MD, 1.89 ng/ml; p = 0.47). There were no significant interactions between sampling time and order in which samples were obtained.
Discussion
Our findings provide evidence for diurnal fluctuations in Aβ peptide levels, both in CSF and plasma, while CSF and plasma p-tau, GFAP and NfL were unaffected. Importantly, Aβ42/Aβ40 ratio remained unaltered, suggesting that it is more suitable for implementation in clinical workup than individual Aβ peptides. Additionally, we show that CSF levels of many synaptic and endo-lysosomal proteins presented a diurnal rhythm, implying a build-up of neuronal activity markers during the day. These results will guide the development of unified sample collection procedures to avoid effects of diurnal variation for future implementation of AD biomarkers in clinical practice and drug trials.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01503-x.
Keywords: Alzheimer’s disease, Fluid biomarkers, p-tau, Aβ, Sampling time, Diurnal variability
Introduction
There is a great need for fluid biomarkers that robustly reflect various aspects of the pathophysiology of Alzheimer’s disease (AD) to improve the diagnostic workup, monitor progression and enable effective drug-development. Currently available fluid biomarkers include amyloid-β (Aβ)42, alone or in ratio with Aβ40 and phosphorylated tau (e.g., p-tau181, p-tau217 and p-tau231) reflecting core AD-related Aβ and tau pathologies, respectively. Additional promising biomarkers of pathophysiological processes that are common for many neurodegenerative disorders are neurofilament light (NfL), a marker of axonal degeneration, as well as a marker of glial activation, glial fibrillary acidic protein (GFAP) [1]. Although cerebrospinal fluid (CSF) measures are available and have proven highly useful as diagnostic and prognostic tools for AD in research settings, clinical care and drug trials, blood testing offers several advantages (e.g., lower invasiveness, higher accessibility, cost-effectiveness) [2]. Accumulating evidence suggests that plasma Aβ42/40, different p-tau isoforms (p-tau181, p-tau231 and p-tau217), NfL and GFAP approach in performance [3–9] or even outperform [10] their corresponding CSF biomarker.
To improve biomarker performance in clinical care and trials, it is important to implement standardized sample collection and handling procedures that would minimize the effects of pre-analytical component among factors impacting biomarker variability [11–14]. One such pre-analytical factor to consider is time of the day at sample collection. Even though, published protocols for CSF sampling recommend to perform lumbar puncture (LP) at a standardized time (08.00–12.00 AM) to avoid potential diurnal variation for CSF biomarkers [15], diurnal variability in CSF and plasma concentrations of AD biomarkers is not well established. Early reports showed fluctuations of CSF Aβ with 1.6-to-4-fold change over a 36-hour period in younger non-demented participants with good general health [16]. Other studies have found smaller (5.5 to 6.7%) or no significant fluctuation in older, more clinically relevant cohorts [17, 18]. Two studies have investigated Aβ dynamics in plasma, reporting 5–9% higher levels of Aβ42 and Aβ40 in samples collected in the afternoon versus morning and larger diurnal fluctuations in younger individuals than in older individuals [19, 20]. While data on NfL and GFAP, either in CSF or plasma are lacking, some evidence suggest that CSF t-tau or p-tau levels do not follow diurnal pattern both in healthy old population [17] and in neurosurgical patients with CSF pressure monitoring [21].
Synaptic homeostasis alteration and degeneration are early pathological events common in many neurodegenerative diseases, including AD. This makes synaptic proteins that reflect synaptic dysfunction interesting early biomarkers [22]. Disruption of sleep and circadian rhythm is believed to happen with ageing and contribute to development of neurodegenerative diseases in part through synaptic dysfunction [23]. AD as well as other proteinopathies are also accompanied by aberrant function of endo-lysosomal networks [24]. Several articles reported increased levels of endo-lysosomal proteins in CSF of patients with AD while their levels seem to decrease in Parkinson’s disease [25–28]. To the best of our knowledge, no studies have assessed variations in synaptic or endo-lysosomal protein levels during the day in humans.
Most studies on changes in CSF Aβ levels to date used frequent and sustained sampling throughout the day with an indwelling intrathecal catheter. This procedure has been shown to contribute to the rise in CSF Aβ42 and Aβ40 independent of circadian fluctuations as repeated lumbar sampling presumably drives the redistribution of CSF flow towards the lumbar space where it is collected [19, 29, 30]. To minimize the effect of sampling procedures on CSF biomarker concentrations, participants in the present study underwent two LPs, one in the morning and another one in the evening, separated by an average of 53 days and samples were analyzed for all major AD biomarkers as well as a panel of 22 synaptic and endo-lysosomal proteins. To ensure that changes in biomarker levels were not due to the sampling order, 17 participants had the first visit in the morning and the second in the evening and the order was reversed for the remaining 21 participants. In addition to CSF, we collected plasma samples on the same visit and quantified the most promising plasma AD biomarkers using currently best performing immunoassays [31, 32]. Our primary research question was whether the time at sample collection, morning or evening, affected the levels of different biomarkers (Aβ42, Aβ40, Aβ42/40, NfL, GFAP, p-tau217, p-tau181, p-tau231, t-tau and synaptic and endo-lysosomal proteins). A secondary research question was whether any of these differences were affected by the amyloid status of the participants.
Methods
Participants
Participants were enrolled at the Memory Clinic, Skåne University Hospital comprising clinical patients who underwent LP as a component of their clinical assessment, along with individuals from the longitudinal Swedish BioFINDER study. The inclusion of the participants from the BioFINDER study contributed to the relatively high numbers of asymptomatic subjects with unimpaired cognition. Participants were selected such that the numbers of Aβ + and Aβ- individuals were approximately the same. The sole inclusion criterion for participation in this study was the performance of a LP at the clinic. Exclusion criteria consisted of individuals who did not undergo the requisite two LP. All participants had two visits when LPs and venipunctures were performed approximately at the same time. We believe that any damage and CSF leakage caused by LP at the first visit would have healed after approximately one month. Therefore, study participants had second visit with LP and venipuncture on an average 53 days (range 41–65 days) after the first visit. For 17 participants the first collection was performed in the morning and the following in the evening and for the remaining 21 participants the order was reversed. Time difference between morning and evening samplings was on an average 10:30 h (range 9:45 − 11:45 h).
Plasma and CSF collection and analysis
20mL of CSF was collected in 5-mL LoBind tubes. CSF was centrifuged (2000 g, + 4 °C) for 10 min, aliquoted in 1.5 mL polypropylene tubes and stored at − 80 °C within 30–60 min of collection [15]. Blood was collected in EDTA-plasma tubes (Vacutainer K2EDTA tube, BD Diagnostics) and centrifuged (2000 g, + 4 °C) for 10 min. Resulting plasma was transferred into one 50-mL polypropylene tube, mixed and aliquoted into 1.5 mL polypropylene tubes and stored at − 80 °C within 30–60 min of collection. All samples from the same patient were measured in the same run to limit the effects of run-to-run variability on biomarker concentrations.
CSF levels of Aβ40, t-tau, NfL and GFAP were measured as part of robust prototype assay within the NeuroToolKit, on fully automated cobas® e 411 or e 601 analyzers (all Roche Diagnostics International Ltd, Rotkreuz, Switzerland) as previously described [33]. CSF Aβ42 levels were measured as part of the Roche NeuroToolKit using the in vitro diagnostic (IVD) Elecsys® assay [34]. Plasma levels of Aβ42, Aβ40, GFAP and NfL were also measured as part of the Roche NeuroToolKit using Elecsys® plasma prototype immunoassays (All Roche Diagnostics International Ltd, Rotkreuz, Switzerland) on cobas® e 411 and cobas e 601 instruments as previously described [33]. CSF and plasma p-tau231 and p-tau181 levels were measured by an in-house Simoa assay developed in the University of Gothenburg, as previously described [35, 36]. CSF and plasma p-tau-217 levels were measured using an immunoassay developed by Lilly Research laboratories on the Meso-Scale Discovery Platform as previously described [4].
CSF samples were analyzed for a panel of 18 synaptic proteins and 4 endo-lysosomal proteins (See Supplementary Table 1, Supplemental 1) using liquid chromatography with tandem mass spectrometric analysis (LC–MS/MS) as previously described [37].
Study participants were classified as amyloid negative (Aβ-) or positive (Aβ+) using CSF Aβ42/Aβ40 quantified with the Food and Drug administration (FDA)-approved Lumipulse G assay and established cut-off of 0.072 [38].
Statistical analyses
Differences in the demographic variables were evaluated with Student t-test (age, Mini Mental State Examination (MMSE) scores, estimated glomerular filtration rate (eGFR, as an indicator of kidney dysfunction) and Body Mass Index (BMI)) or Fisher’s exact test (gender, APOE ε4 carriership and diagnosis). Repeated measures two-way ANOVA including interaction effect between Aβ status and time at sampling was used to assess whether biomarker levels in Aβ + and Aβ- individuals were affected differently by time of sample collection. Similar analysis was carried out to assess interaction between order in which samples were collected (i.e., morning collection or evening collection first) and time at sampling. Multiplicity correction was applied using the Bonferroni-Dunn method except the CSF synaptic and endo-lysosomal panel where we used Benjamini–Hochberg false discovery rate (FDR). All significance were two-sided with significance level equal to 0.05. Statistical analysis was performed using Prism 9 (GraphPad Software, San Diego, California, USA).
Results
Participant demographics
The demographic and clinical data for all participants are summarized in Table 1. Out of 38 participants, 18 were Aβ-positive (Aβ+) and 20 Aβ-negative (Aβ-). There were no significant differences between Aβ + and Aβ- groups for sex, age, MMSE score, diagnosis, eGFR or BMI. There was a higher proportion of APOE ε4 carriership in Aβ + in comparison with Aβ- (70.6% vs. 15.0%, p = 0.002). Most study participants (35 out of 38) were cognitively unimpaired while 3 individuals were cognitively impaired.
Table 1.
Demographics and clinical characteristics of all subjects
| All subjects | Aβ + | Aβ - | P-value Aβ+ vs. Aβ − | |
|---|---|---|---|---|
| N | 38 | 18 | 20 | |
| Age (years) | 77 (5.91) | 78 (4.41) | 76 (6.86) | 0.08 |
| Gender (F/M) | 16/22 | 8/10 | 8/12 | 0.52 |
| APOE ε4 positivity, n (%) | 15 (39) | 12 (71) | 3 (15) | 0.002 |
| MMSE | 28 (2.10) | 28 (1.84) | 29 (2.25) | 0.16 |
| Diagnosis | ||||
|
Cognitively Unimpaired Cognitively Impaired |
35 3 |
16 2 |
19 1 |
0.32 |
| Kidney Function (eGFR)a | 122.29 (21.54) | 119.71 (20.52) | 124.57 (23.09) | 0.54 |
| BMIb | 26.64 (2.84) | 25.86 (2.31) | 27.29 (3.13) | 0.31 |
Data shown as mean (SD) unless specified otherwise
Abbreviations: M, male; F, female; APOE, apolipoprotein; MMSE, Mini-Mental State Examination, eGFR, estimated glomerular filtration rate; BMI, Body Mass Index
a Data was missing in 5 participants (2 Aβ + and 3 Aβ -)
b Data was missing in 3 participants (2 Aβ + and 1 Aβ-)
CSF and plasma AD biomarkers
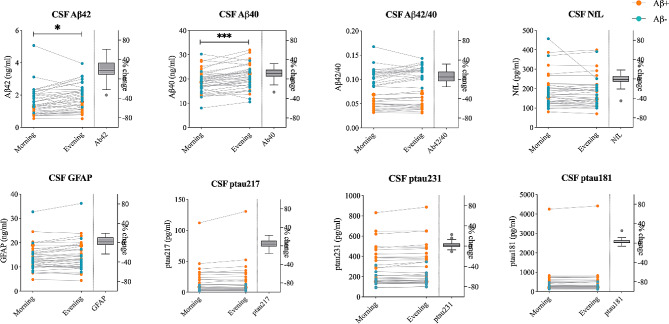
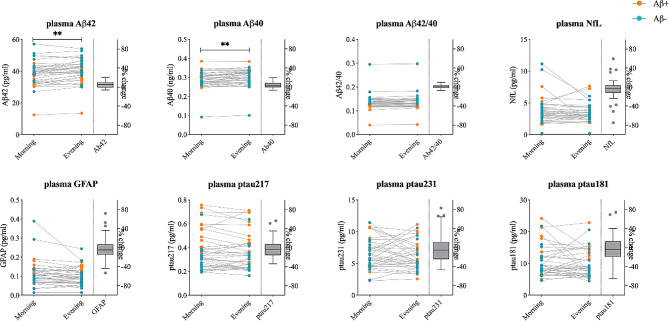
CSF concentrations of Aβ42 (mean difference [MD], 0.21 ng/mL; p = 0.038) and Aβ40 (MD, 1.85 ng/mL; p < 0.001) were increased by 17.0% (95% CI, 10-24.1) and 10.5% (95% CI, 6.5–14.4), respectively, in samples collected in the evening compared to those collected in the morning (Fig. 1; Table 2). Similarly, plasma levels of Aβ42 (MD, 1.65 pg/mL; p = 0.002) and Aβ40 (MD, 0.01 ng/mL, p = 0.002) were significantly higher in samples collected in the evening compared to those collected in the morning. However, the increases were smaller for plasma Aβ42 (4.8%; 95% CI, 2.7–6.7) and Aβ40 (4.2%; 95% CI, 2.2–6.3) compared with CSF Aβ42 and Aβ40 (Fig. 2; Table 3). In contrast, we did not find any significant differences in either the CSF Aβ42/Aβ40 ratio (5.3%; 95% CI, 1.6- 9.0; p = 0.16) or the plasma Aβ42/Aβ40 ratio (0.7%; 95% CI, -0.7 to 1.1; p = 1.0) (Figs. 1 and 2; Tables 2 and 3). We also did not find any changes in CSF and plasma levels of p-tau217, p-tau181, p-tau231, NfL and GFAP (p = 1.00) between collection in the morning and evening (Figs. 1 and 2; Tables 2 and 3).
Fig. 1.
CSF biomarkers levels in samples collected in the morning and evening. Subject specific biomarker concentration in samples collected in the morning vs. evening. Average percent changes between time points are shown in box-plots plotted with the Tukey method. Blue and orange dots represent participants with negative and positive amyloid status, respectively. Asterisks represent p-values for the main effects of sampling time from repeated measures two-way ANOVA; * p < 0.05, *** p < 0.001
Table 2.
CSF biomarker concentrations in samples collected in the morning and evening
| Biomarkers | Morning concentration | Evening concentration | Differences evening-morning | % Change | F (df1, df2) a | P-value corrected (uncorrected) b |
|---|---|---|---|---|---|---|
| Aβ42 [ng/mL] | 1.53 (0.85) | 1.74 (0.82) | 0.21 (0.35 to 0.07) | 17.02 (9.99 to 24.06) | 8.99 (1, 37) | 0.038 (0.005) |
| Aβ40 [ng/mL] | 19.08 (4.44) | 20.94 (4.86) | 1.85 (1.09 to 2.62) | 10.46 (6.50 to 14.42) | 24.10 (1, 37) | 0.0008 (0.0001) |
| Aβ42/Aβ40 ratio | 0.081 (0.03) | 0.085 (0.04) | 0.004 (0.001 to 0.008) | 5.29 (1.57 to 9.01) | 5.91 (1, 37) | 0.16 (0.02) |
| P-tau217 [pg/mL] | 14.50 (19.66) | 15.52 (22.61) | 1.02 (-0.06 to 2.10) | 4.03 (0.81 to 7.25) | 3.67 (1, 37) | 0.50 (0.06) |
| P-tau231 [pg/mL] | 269.54 (176.24) | 272.73 (184.1) | 7.84 (2.11 to 13.56) | 2.60 (0.48 to 4.72) | 7.71 (1, 36) c | 0.07 (0.009) |
| P-tau181 [pg/mL] | 466.51 (669.2) | 472.96 (687.58) | 14.83 (3.76 to 25.90) | 2.80 (0.94 to 4.66) | 7.38 (1, 36) c | 0.08 (0.01) |
| GFAP [pg/mL] | 13.76 (5.22) | 14.25 (5.92) | 0.49 (0.03 to 0.95) | 2.59 (-0.64 to 5.83) | 4.62 (1, 37) | 0.30 (0.04) |
| NfL [ng/mL] | 184.92 (84.22) | 179.58 (76.04) | -5.33 (-6.52 to 17.18) | -1.54 (-5.19 to 2.12) | 0.83 (1, 37) | 1 (0.37) |
Data shown as mean (SD) or mean (95%CI) unless specified otherwise. Abbreviations: CSF, cerebrospinal fluid; Aβ, amyloid beta; GFAP, glial fibrillary acidic protein; NfL, neurofilament light; P-tau, phosphorylated tau
a Main effects of sampling time from repeated measures two-way ANOVA
bP-values were corrected for multiple comparison using the Bonferroni-Dunn method
c Data was missing for 1 participant
Fig. 2.
Plasma biomarkers levels in samples collected in the morning and evening. Subject specific biomarker concentration in samples collected in the morning vs. evening. Average percent changes between time points are shown in box-plots plotted with the Tukey method. Blue and orange dots represent participants with negative and positive amyloid status, respectively. Asterisks represent p-values for the main effects of sampling time from repeated measures two-way ANOVA; ** p < 0.01
Table 3.
Plasma biomarker concentration in samples collected in the morning and evening
| Plasma Biomarkers [units] | Morning concentration | Evening concentration | Differences evening-morning | % Change | F (df1, df2) a | P-value corrected (uncorrected) b |
|---|---|---|---|---|---|---|
| Aβ42 [pg/mL] | 38.43 (7.76) | 40.08 (7.37) | 1.65 (0.84 to 2.47) | 4.84 (2.68 to 7) | 16.81 (1, 37) | 0.002 (0.0002) |
| Aβ40 [ng/mL] | 0.29 (0.05) | 0.30 (0.05) | 0.01 (0.006 to 0.02) | 4.24 (2.15–6.32) | 16.05 (1, 37) | 0.002 (0.0003) |
| Aβ42/40 | 0.14 (0.03) | 0.14 (0.03) | 0.001 (-0.002 to 0.001) | 0.65 (-0.70 to 1.10) | 0.79 (1, 37) | 1 (0.38) |
| P-tau217 [pg/mL] | 0.37 (0.16) | 0.36 (0.16) | -0.01 (-0.01 to 0.03) | -0.56 (-7.13 to 6.01) | 0.95 (1, 37) | 1 (0.34) |
| P-tau231 [pg/mL] | 6.88 (3.05) | 6.67 (3) | -0.22 (-0.52 to 0.95) | 2.48 (-8.86 to 13.83) | 0.35 (1, 35) c | 1 (0.55) |
| P-tau181 [pg/mL] | 10.61 (4.89) | 9.94 (4.43) | -0.71 (-0.76 to 2.10) | -0.15 (-11.75 to 11.46) | 0.90 (1, 37) | 1 (0.35) |
| GFAP [pg/mL] | 0.11 (0.07) | 0.10 (0.05) | -0.01 (-0.004 to 0.02) | -1.81 (-10.03 to 6.40) | 2.04 (1, 37) | 1 (0.16) |
| NfL [ng/mL] | 3.83 (2.10) | 3.42 (1.47) | -0.41 (-0.12 to 0.94) | -3.96 (-11.75 to 3.82) | 2.46 (1, 37) | 1 (0.13) |
Data shown as mean (SD) or mean (95%CI) unless specified otherwise. Abbreviations: Aβ, amyloid beta; GFAP, glial fibrillary acidic protein; NfL, neurofilament light; P-tau, phosphorylated tau
a Main effects of sampling time from repeated measures two-way ANOVA
bP-values were corrected for multiple comparison using the Bonferroni-Dunn method
c Data was missing for 2 participants
Effects of sampling time on CSF and plasma biomarkers were not different in the Aβ+ and Aβ- groups (prange uncorrected 0.06–0.88 for interaction between sampling time and Aβ status, Supplementary Table 3, Supplementary Figs. 2–3), except CSF t-tau (p = 0.007). There was a relatively small increase (5.74% (95% CI, 2.85–8.63)) in CSF t-tau levels in evening samples compared to the morning samples in Aβ+ participants (MD, 16.46 ng/ml; p = 0.009) but not in Aβ- participants (1.20% (95%CI, -1.99-4.39); MD, 1.89 ng/ml; p = 0.47) (Supplementary Fig. 2).
Morning or evening biomarker levels were not different depending on the order in which samples were collected (morning first vs. evening first) for any of the biomarkers (prange uncorrected 0.051–0.85 for interaction between sampling time and order of sample collection, Supplementary Table 3).
Synaptic and endo-lysosomal panel
We found differences between morning and evening samples for 14 out of 22 synaptic and endo-lysosomal proteins (Table 4 and Supplementary Table 2, Supplemental 1). CSF levels of amyloid precursor protein (APP), syntaxin-1B (STX1B), neurogranin (Ng), neuronal pentraxin receptor (NPTXR), neuronal pentraxin 1 (NPTX1), β-synuclein (β-Syn), γ-synuclein (γ-Syn), 14-3-3ε, phosphatidylethanolamine-binding protein 1 (PEBP-1), cathepsin F (CTSF), GM2 activator (GM2A), neurosecretory protein VGF (VGF), secretogranin-2 (SgII) and chromogranin A (CgA) were all increased by 4.5-13.3% (95% CI, 1.7–7.2 to 4.4–22.1; p < 0.048) in samples collected in the evening compared to those collected in the morning (Supplementary Fig. 1, Supplemental 1, Table 4).
Table 4.
CSF synaptic and endo-lysosomal biomarkers with increased levels in samples collected in the evening vs. morning
| Biomarkers | Morning concentration (fmol/µl) | Evening concentration (fmol/µl) | Differences evening-morning | % Change | F (df1, df2)a | P-value corrected (uncorrected) b |
|---|---|---|---|---|---|---|
| Synaptic panel | ||||||
| Amyloid Precursor Protein | 3.80 (1.32) | 4.04 (1.41) | 0.24 (0.12 to 0.37) | 7.06 (4.02 to 10.10) | 15.46 (1, 37) | 0.005 (0.0004) |
| Syntaxin-1B | 0.31 (0.10) | 0.34 (0.11) | 0.02 (0.01 to 0.03) | 7.10 (3.38 to 10.81) | 13.77 (1, 37) | 0.005 (0.0007) |
| Neuronal pentraxin receptor | 1.22 (0.55) | 1.35 (0.60) | 0.12 (0.05 to 0.19) | 12.15 (6.15 to 18.15) | 13.58 (1, 37) | 0.005 (0.0007) |
| Neuronal pentraxin-1 | ||||||
|
ETVLQQK LTPGEVYNLATCSTK |
0.67 (0.24) 1.52 (0.46) |
0.71 (0.26) 1.61 (0.49) |
0.04 (0.01 to 0.07) 0.09 (0.04 to 0.14) |
7.66 (3.28 to 12.04) 6.24 (3.02 to 9.47) |
8.38 (1, 37) 11.92 (1, 37) |
0.020 (0.006) 0.008 (0.001) |
| Neurogranin | 0.60 (0.28) | 0.66 (0.31) | 0.06 (0.02 to 0.10) | 11.59 (6.64 to 16.54) | 10.56 (1, 37) | 0.012 (0.003) |
| β-synuclein | 0.28 (0.10) | 0.30 (0.12) | 0.03 (0.04 to 0.007) | 9.59 (3.90 to 15.28) | 7.91 (1, 37) | 0.020 (0.008) |
| γ-synuclein | 0.60 (0.21) | 0.64 (0.20) | 0.13 (0.001 to 0.26) | 8.50 (3.31 to 13.70) | 5.82 (1, 37) | 0.042 (0.021) |
| PEBP- 1 | 7.91 (2.43) | 8.23 (2.57) | 0.32 (0.10 to 0.54) | 4.45 (1.71 to 7.20) | 8.96 (1, 37) | 0.020 (0.005) |
| 14-3-3ε | 0.43 (0.14) | 0.45 (0.16) | 0.03 (0.01 to 0.05) | 6.62 (2.29 to 10.96) | 8.05 (1, 37) | 0.020 (0.007) |
| Chromogranin-A | 4.04 (2.82) | 4.38 (2.99) | 0.34 (0.10 to 0.57) | 9.38 (4.97 to 13.78) | 8.2 (1, 37) | 0.020 (0.007) |
| Secretogranin-2 | 4.10 (1.55) | 4.36 (1.74) | 0.26 (0.06 to 0.47) | 7.06 (2.65–11.47) | 6.75 (1, 37) | 0.030 (0.013) |
| Neurosecretory protein VGF | ||||||
|
NSEPQDEGELFQGVDPR AYQGVAAPFPK |
3.77 (2.00) 7.52 (4.02) |
3.98 (2.13) 8.08 (4.49) |
0.21 (0.03 to 0.38) 0.56 (0.14 to 0.98) |
6.38 (1.89 to 10.88) 8.45 (3.47 to 13.43) |
5.84 (1, 37) 7.41 (1, 37) |
0.041 (0.021) 0.023 (0.010) |
| Endo-lysosomal panel | ||||||
| Ganglioside GM2 activator | ||||||
|
EVAGLWIK ESVLSSSGK |
3.91 (1.45) 5.38 (1.95) |
4.11 (1.49) 5.60 (2.01) |
0.20 (0.10 to 0.30) 0.23 (0.11 to 0.35) |
5.95 (2.94 to 8.96) 4.70 (2.27 to 7.13) |
15.87 (1, 37) 14.15 (1, 37) |
0.005 (0.0003) 0.005 (0.0006) |
| Cathepsin F | 0.45 (0.13) | 0.49 (0.13) | 0.04 (0.01 to 0.07) | 13.29 (4.44 to 22.14) | 7.97 (1, 37) | 0.020 (0.008) |
Data shown as mean (SD) or mean (95%CI) unless specified otherwise. Data for all synaptic and endo-lysosomal proteins is shown in Supplementary Table 2. Abbreviations: CSF cerebrospinal fluid
a Main effects of sampling time from repeated measures two-way ANOVA
bP-values were corrected for multiple comparison using the FDR method
Effects of sampling time were not different in the Aβ + and Aβ- groups (prange uncorrected 0.06–0.88, for interaction between sampling time and Aβ status) for any of the proteins from the MS panel. In addition, morning or evening protein levels were not different depending on the order in which samples were collected (morning first vs. evening first) (prange uncorrected 0.09–0.85, for interaction between sampling time and order of sample collection).
Discussion
In this study, we show higher levels of Aβ42 and Aβ40 in samples collected in the evening compared to those collected in the morning. The increases were modest and consistent in both CSF and plasma. Importantly, no changes were observed in the Aβ42/Aβ40 ratio, or any other tested AD biomarker (i.e., p-tau217, p-tau231, p-tau181, NfL and GFAP) either in CSF or plasma. Additionally, 14 out of 22 synaptic and endo-lysosomal proteins were also increased in CSF in the evening in comparison to the morning samples.
Although there have been handful of studies on diurnal variation in the CSF levels of AD biomarkers, results have been inconsistent. Some have pointed to fluctuations in biomarker concentrations during the day [16, 17, 30], whereas other have not found any significant changes [18, 21]. The conflicting results are possibly caused by the small sample size in several of the studies, cohort specific differences as well as differences in the CSF sampling methods and assays used for Aβ quantification. Many reports have highlighted that frequent sampling and extraction of large volumes of CSF via indwelling catheter leads to increased levels of CSF Aβ [17, 19, 29, 39–41] possibly by promoting the transfer from the interstitial fluid to CSF [17] and by redistribution of fluid towards the lumbar space [29, 41]. To mitigate these sampling-related effect, CSF in the present study was collected at two separate LPs with an average interval of 53 days allowing sufficient time for tissue damage caused by the LP to heal. Of note, no interaction effects were seen between sampling time (i.e., morning and evening) and order in which samples were collected for any biomarker indicating that the differences in levels we report were not due to samples being collected at a later date. Our results support those that suggest a circadian rhythm for Aβ42 and Aβ40, with higher levels in the evening. The increases in CSF were modest with 17% and 10.5% for Aβ42 and Aβ40, respectively, (Table 2) and in a similar range (3.8–15%) to some studies that have included elderly subjects as well as patients with AD [17, 29]. The increases in plasma Aβ42 and Aβ40 were lower than in CSF (4.8% and 4.2% respectively; Table 3) and in a similar range (2-9%) as in previous reports [19, 20]. The smaller changes in the evening of Aβ in plasma could be partly due to the contribution of peripheral sources of Aβ that are less affected by circadian rhythms. The differences in Aβ42 and Aβ40 levels in the morning and evening samples were not influenced by brain Aβ status, which is important since 47% of our sample had abnormal Aβ-status (Table 1). These findings are in line with previous reports indicating that in elderly individuals day/night variability in Aβ42 and Aβ40 levels, did not vary between Aβ + and Aβ- groups [19, 39]. At the same time, we show that CSF and plasma Aβ42/Aβ40 ratios remained unaltered, suggesting that increased production or decreased clearance of Aβ peptides during daytime similarly affect the CSF and plasma levels of the Aβ42 and Aβ40.
Our results with higher APP levels in the evening in comparison to the morning suggest that circadian rhythm and synaptic activity might affect brain and CSF Aβ levels through modulation of APP expression, release and/or metabolism (Table 4). Aβ42 and Aβ40 are produced by the cleavage of APP and increased synaptic activity promotes the amyloidogenic processing of APP [42] leading to increased interstitial Aβ levels [43]. Interestingly, it has been shown in mice that interstitial fluid levels of Aβ correlate with time spent awake and change in response to activation of orexin which is known to regulate wakefulness under physiological conditions and follow a diurnal fluctuation [44, 45].
In agreement with earlier data, we did not find any significant fluctuations over the day for CSF or plasma p-tau [18, 21]. Furthermore, we show for the first time, that there are no differences in CSF and plasma NfL and GFAP concentrations between samples collected in the morning and evening. Collectively, these results suggest that during daytime there is a specific increase in the CSF and plasma levels of Aβ proteins rather than a general build-up of AD biomarkers.
Higher neuronal activity and increased synaptic strength during wakefulness compared to sleep have been reported in mice and rats [46–48]. High synaptic activity is associated with increased production of synaptic proteins, especially proteins that regulate the secretory pathways [49]. Taken together these findings may explain the higher levels of synaptic proteins in evening samples in comparison to the morning seen in our study (See Supplementary Table 2, Supplemental 1, Table 4). It remains unclear why only some synaptic and endo-lysosomal proteins were selectively affected in our study. Future investigations should explore the underlying mechanisms behind these findings.
The strength of the current study is that we assessed a wide range of the established and candidate CSF and plasma AD biomarkers measured using state-of-the art assays. However, this study has limitations. The sample size was relatively small and determined based on previous studies examining the effects of diurnal variability on Aβ levels (no power calculations were performed). The difference in biomarker levels between the morning and evening samples, in plasma in particular, were also small with intra-individual variability potentially influencing these results. Future work in larger cohorts accounting for the effects of intra-individual variability in biomarker concentration are warranted. These studies should also assess the impact of diurnal variability on diagnostic performance of AD biomarkers.
Conclusions
In summary, we demonstrate that Aβ42 and Aβ40 levels in CSF and plasma have diurnal fluctuations with higher levels in the evening. Previous data have indicated that Aβ42/Aβ40 ratio is less affected than Aβ42 alone by different AD non-specific factors and pathologies (e.g., pre-analytical sample handling, inter-individual variability in Aβ levels, subcortical injury) [50]. Here, we also show that CSF and plasma Aβ42/Aβ40 levels are not influenced by the timing of the sample collection further supporting the use of Aβ42/Aβ40 ratio over Aβ42 alone in the diagnostic workup of AD. While the CSF and plasma levels of p-tau variants, NfL and GFAP did not exhibit diurnal variability, CSF levels of many synaptic and endo-lysosomal proteins were increased in samples collected in the evening. These results suggest an increase and build-up of markers associated with neuronal activity during wakefulness. In addition, our data highlight the need to consider the effects of circadian rhythms on the CSF (and potentially plasma) levels of synaptic and endo-lysosomal proteins that are considered as candidate biomarkers of AD. Overall, the findings of the present study support the standardization of sample collection protocols for AD biomarker determination, with sampling at a specific time interval during the day.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Supplementary Table 1, Supplementary Table 2, Supplementary Fig. 1, Supplementary Table 3, Supplementary Fig. 2 and Supplementary Fig. 3.
Abbreviations
- AD
Alzheimer’s Disease
- Aβ
Amyloid-β
- APP
Amyloid precursor protein
- BMI
Body mass index
- β-syn
Beta-synuclein
- CTSF
Cathepsin F
- CSF
Cerebrospinal fluid
- CgA
Chromogranin A
- FDA
Food and Drug administration
- FDR
False discovery rate
- eGFR
Estimated glomerular filtration rate
- GFAP
Glial fibrillary acidic protein
- GM2A
GM2 activator
- IVD
In-vitro diagnostic
- LC-MS/MS
Liquid chromatography with tandem mass spectrometry
- LP
Lumbar puncture
- MD
Mean difference
- MS
Mass spectrometry
- MMSE
Mini-Mental State Examination
- NfL
Neurofilament light
- Ng
Neurogranin:
- NPTX1
Neuronal pentraxin 1
- NPTXR
Neuronal pentraxin receptor
- PEBP-1
Phosphatidylethanolamine-binding protein 1
- p-tau
Phosphorylated- tau
- SgII
Secretogranin II
- STX1B
Syntaxin-1B
- VGF
Neurosecretory protein VGF
Author contributions
AOD analyzed and interpreted the data, drafted, and revised the manuscript. ES designed the study and helped acquire the data and revised the manuscript for content. NJA helped acquire the data and revised the manuscript for content. JN helped acquire the data and revised the manuscript for content. CQR helped acquire the data and revised the manuscript for content. AJ helped acquire the data and revised the manuscript for content. WSB helped acquire the data and revised the manuscript for content. ABW helped acquire the data and revised the manuscript for content. HZ helped acquire the data and revised the manuscript for content. KB helped acquire the data and revised the manuscript for content. SJ designed the study, helped acquire the data, supervised the analysis and interpretation of the data, and critically reviewed the manuscript. OH designed the study, helped acquire the data, supervised the analysis and interpretation of data and critically reviewed the manuscript.
Funding
Open access funding provided by Lund University. Work at Lund University was supported by the Swedish Research Council (2022 − 00775), ERA PerMed (ERAPERMED2021-184), the Knut and Alice Wallenberg foundation (2017 − 0383), the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation (AF-980907), the Swedish Brain Foundation (FO2021-0293), The Parkinson foundation of Sweden (1412/22), the Cure Alzheimer’s fund, the Konung Gustaf V: s och Drottning Victorias Frimurarestiftelse, the Skåne University Hospital Foundation (2020-O000028), Regionalt Forskningsstöd (2022 − 1259) and the Swedish federal government under the ALF agreement (2022-Projekt0080). Work at the University of Gothenburg was supported by grants from the Swedish Research Council (#2022 − 01018, #2019–02397, #2017 − 00915 and #2022 − 00732), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, and Swedish State Support for Clinical Research (#ALFGBG-71320 and #ALFGBG-965240). HZ is a Wallenberg Scholar. KB is supported by the Swedish Alzheimer Foundation (#AF-930351, #AF-939721 and #AF-968270), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), and the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495).
Open access funding provided by Lund University.
Data availability
Anonymized data from the study will be shared upon request from a qualified academic investigator.
Declarations
Ethics approval and consent to participate
The study was approved by the Regional Ethics Committee in Lund, Sweden. Written informed consent was obtained from all participants.
Competing interests
CQ-R is a full‑time employee of Roche Diagnostics International Ltd. AJ is a full‑time employee of Roche Diagnostics GmbH. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant and at advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. OH has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. The Roche NeuroToolKit is a panel of exploratory prototype assays designed to robustly evaluate biomarkers associated with key pathologic events characteristic of AD and other neurological disorders, used for research purposes only and not approved for clinical use. Elecsys β-amyloid(1–42) CSF is approved for clinical use. COBAS and ELECSYS are trademarks of Roche. All other product names and trademarks are the property of their respective owners. The rest of authors do not report any disclosures.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna Orduña Dolado, Email: anna.orduna_dolado@med.lu.se.
Oskar Hansson, Email: Oskar.Hansson@med.lu.se.
References
- 1.Hansson O. Biomarkers for neurodegenerative diseases. Nature Medicine 2021 27:6. 2021;27:954–63. https://www.nature.com/articles/s41591-021-01382-x. [DOI] [PubMed]
- 2.Zetterberg H, Blennow K. Moving fluid biomarkers for Alzheimer’s disease from research tools to routine clinical diagnostics. Mol Neurodegener. 2021;16:1–7. https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-021-00430-x. [DOI] [PMC free article] [PubMed]
- 3.Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 2020. 2020;26:3. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 4.Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al. Discriminative accuracy of plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative disorders. JAMA. 2020;324:772–81. doi: 10.1001/jama.2020.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton NJ, Pascoal TA, Karikari TK, Benedet AL, Lantero-Rodriguez J, Brinkmalm G, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021;141:709–24. doi: 10.1007/s00401-021-02275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson-Carlgren N, Janelidze S, Palmqvist S, Cullen N, Svenningsson AL, Strandberg O, et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain. 2020;143:3234–41. doi: 10.1093/brain/awaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nat 2018. 2018;554:7691. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 8.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between Longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer Disease. JAMA Neurol. 2019;76:791–9. doi: 10.1001/jamaneurol.2019.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmqvist S, Janelidze S, Stomrud E, Zetterberg H, Karl J, Zink K, et al. Performance of fully automated plasma assays as screening tests for Alzheimer Disease–related β-Amyloid status. JAMA Neurol. 2019;76:1060–9. doi: 10.1001/jamaneurol.2019.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedet AL, Milà-Alomà M, Vrillon A, Ashton NJ, Pascoal TA, Lussier F, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer Disease Continuum. JAMA Neurol. 2021;78:1471–83. doi: 10.1001/jamaneurol.2021.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattsson-Carlgren N, Palmqvist S, Blennow K, Hansson O. Increasing the reproducibility of fluid biomarker studies in neurodegenerative studies. Nature Communications 2020 11:1. 2020;11:1–11. https://www.nature.com/articles/s41467-020-19957-6. [DOI] [PMC free article] [PubMed]
- 12.Hansson O, Mikulskis A, Fagan AM, Teunissen C, Zetterberg H, Vanderstichele H, et al. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: a review. Alzheimer’s Dement. 2018;14:1313–33. doi: 10.1016/j.jalz.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Verberk IMW, Misdorp EO, Koelewijn J, Ball AJ, Blennow K, Dage JL, et al. Characterization of pre-analytical sample handling effects on a panel of Alzheimer’s disease–related blood-based biomarkers: results from the standardization of Alzheimer’s blood biomarkers (SABB) working group. Alzheimer’s Dement. 2022;18:1484–97. doi: 10.1002/alz.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashton NJ, Suárez-Calvet M, Karikari TK, Lantero-Rodriguez J, Snellman A, Sauer M, et al. Effects of pre-analytical procedures on blood biomarkers for Alzheimer’s pathophysiology, glial activation, and neurodegeneration. Alzheimer’s Dementia: Diagnosis Assess Disease Monit. 2021;13:e12168. doi: 10.1002/dad2.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature Reviews Neurology 2010 6:3. 2010;6:131–44. https://www.nature.com/articles/nrneurol.2010.4. [DOI] [PubMed]
- 16.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-β levels. Neurology. 2007;68:666–9. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- 17.Slats D, Claassen JAHR, Spies PE, Borm G, Besse KTC, van Aalst W, et al. Hourly variability of cerebrospinal fluid biomarkers in Alzheimer’s disease subjects and healthy older volunteers. Neurobiol Aging. 2011;33:e8311–9. doi: 10.1016/j.neurobiolaging.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Moghekar A, Goh J, Li M, Albert M, O’Brien RJ. Cerebrospinal fluid Aβ and tau level fluctuation in an older clinical cohort. Arch Neurol. 2012;69:246–50. doi: 10.1001/archneurol.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Potter R, Sigurdson W, Kasten T, Connors R, Morris JC, et al. β-Amyloid dynamics in Human plasma. Arch Neurol. 2012;69:1591–7. doi: 10.1001/archneurol.2012.18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rózga M, Bittner T, Batrla R, Karl J. Preanalytical sample handling recommendations for Alzheimer’s disease plasma biomarkers. Alzheimer’s Dementia: Diagnosis Assess Disease Monit. 2019;11:291. doi: 10.1016/j.dadm.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cicognola C, Chiasserini D, Eusebi P, Andreasson U, Vanderstichele H, Zetterberg H et al. No diurnal variation of classical and candidate biomarkers of Alzheimer’s disease in CSF. Mol Neurodegener. 2016;11. [DOI] [PMC free article] [PubMed]
- 22.Nilsson J, Cousins KAQ, Gobom J, Portelius E, Chen-Plotkin A, Shaw LM, et al. Cerebrospinal fluid biomarker panel of synaptic dysfunction in Alzheimer’s disease and other neurodegenerative disorders. Alzheimer’s Dement. 2022 doi: 10.1002/alz.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Sci (1979). 2016;354:1004–8. [DOI] [PMC free article] [PubMed]
- 24.Whyte LS, Lau AA, Hemsley KM, Hopwood JJ, Sargeant TJ. Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer’s disease? J Neurochem. 2017;140:703–17. 10.1111/jnc.13935. [DOI] [PubMed]
- 25.Sjödin S, Brinkmalm G, Öhrfelt A, Parnetti L, Paciotti S, Hansson O et al. Endo-lysosomal proteins and ubiquitin CSF concentrations in Alzheimer’s and Parkinson’s disease. Alzheimer’s Research & Therapy 2019 11:1. 2019;11:1–16. https://alzres.biomedcentral.com/articles/10.1186/s13195-019-0533-9. [DOI] [PMC free article] [PubMed]
- 26.Lerche S, Sjödin S, Brinkmalm A, Blennow K, Wurster I, Roeben B, et al. CSF Protein Level of Neurotransmitter Secretion, synaptic plasticity, and autophagy in PD and DLB. Mov Disord. 2021;36:2595–604. doi: 10.1002/mds.28704. [DOI] [PubMed] [Google Scholar]
- 27.Heywood WE, Galimberti D, Bliss E, Sirka E, Paterson RW, Magdalinou NK et al. Identification of novel CSF biomarkers for neurodegeneration and their validation by a high-throughput multiplexed targeted proteomic assay. Mol Neurodegener. 2015;10. [DOI] [PMC free article] [PubMed]
- 28.Armstrong A, Mattsson N, Appelqvist H, Janefjord C, Sandin L, Agholme L, et al. Lysosomal network proteins as potential novel CSF biomarkers for Alzheimer’s disease. Neuromolecular Med. 2014;16:150–60. doi: 10.1007/s12017-013-8269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Llano DA, Ellis T, Leblond D, Bhathena A, Jhee SS, et al. Effect of human cerebrospinal fluid sampling frequency on amyloid-β levels. Alzheimer’s Dement. 2012;8:295–303. doi: 10.1016/j.jalz.2011.05.900. [DOI] [PubMed] [Google Scholar]
- 30.Lucey BP, Gonzales C, Das U, Li J, Siemers ER, Slemmon JR, et al. An integrated multi-study analysis of intra-subject variability in cerebrospinal fluid amyloid-β concentrations collected by lumbar puncture and indwelling lumbar catheter. Alzheimers Res Ther. 2015;7:1–17. doi: 10.1186/s13195-015-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janelidze S, Bali D, Ashton NJ, Barthélemy NR, Vanbrabant J, Stoops E, et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer’s disease. Brain. 2012;139:16–7. doi: 10.1093/brain/awac333/6695388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janelidze S, Teunissen CE, Zetterberg H, Allué JA, Sarasa L, Eichenlaub U, et al. Head-to-Head comparison of 8 plasma Amyloid-β 42/40 assays in Alzheimer Disease. JAMA Neurol. 2021;78:1375–82. doi: 10.1001/jamaneurol.2021.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmqvist S, Stomrud E, Cullen N, Janelidze S, Manuilova E, Jethwa A, et al. An accurate fully automated panel of plasma biomarkers for Alzheimer’s disease. Alzheimer’s Dement. 2023;19:1204–15. doi: 10.1002/alz.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12:517–26. doi: 10.1016/j.jalz.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–33. doi: 10.1016/S1474-4422(20)30071-5. [DOI] [PubMed] [Google Scholar]
- 36.Ashton NJ, Benedet AL, Pascoal TA, Karikari TK, Lantero-Rodriguez J, Brum WS, et al. Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer’s disease. EBioMedicine. 2022;76:103836. doi: 10.1016/j.ebiom.2022.103836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsson J, Gobom J, Sjödin S, Brinkmalm G, Ashton NJ, Svensson J et al. Cerebrospinal fluid biomarker panel for synaptic dysfunction in Alzheimer’s disease. Alzheimer’s Dementia: Diagnosis Assess Disease Monit. 2021;13. /pmc/articles/PMC8087978/. [DOI] [PMC free article] [PubMed]
- 38.Gobom J, Parnetti L, Rosa-Neto P, Vyhnalek M, Gauthier S, Cataldi S, et al. Validation of the LUMIPULSE automated immunoassay for the measurement of core AD biomarkers in cerebrospinal fluid. Clin Chem Lab Med. 2021;60:207–19. doi: 10.1515/cclm-2021-0651. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju Y, El, et al. Effects of age and amyloid deposition on Aβ Dynamics in the Human Central Nervous System. Arch Neurol. 2012;69:51–8. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucey BP, Fagan AM, Holtzman DM, Morris JC, Bateman RJ. Diurnal oscillation of CSF Aβ and other AD biomarkers. Mol Neurodegener. 2017;12:1–3. doi: 10.1186/s13024-017-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucey BP, Mawuenyega KG, Patterson BW, Elbert DL, Ovod V, Kasten T, et al. Associations between β-Amyloid kinetics and the β-Amyloid diurnal pattern in the Central Nervous System. JAMA Neurol. 2017;74:207–15. doi: 10.1001/jamaneurol.2016.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, et al. APP Processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/S0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 43.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid Amyloid-β levels in vivo. Neuron. 2005;48:913–22. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 44.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science (1979). 2009;326:1005–7. https://www.science.org/doi/full/10.1126/science.1180962. [DOI] [PMC free article] [PubMed]
- 45.Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light–dark cycle and sleep–wake activities. Eur J Neurosci. 2001;14:1075–81. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 46.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of Extracellular Glutamate in the rat cerebral cortex across Sleep and Waking States. J Neurosci. 2009;29:620–9. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, Huganir RL. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science (1979). 2017;355:511–5. https://www.science.org/doi/10.1126/science.aai8355. [DOI] [PMC free article] [PubMed]
- 48.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 49.Gürth CM, Dankovich TM, Rizzoli SO, D’Este E. Synaptic activity and strength are reflected by changes in the post-synaptic secretory pathway. Sci Rep 2020. 2020;10:1. doi: 10.1038/s41598-020-77260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res Ther. 2019;11:1–15. doi: 10.1186/s13195-019-0485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary Table 1, Supplementary Table 2, Supplementary Fig. 1, Supplementary Table 3, Supplementary Fig. 2 and Supplementary Fig. 3.
Data Availability Statement
Anonymized data from the study will be shared upon request from a qualified academic investigator.