Abstract
Objective
To share our experience with belimumab in lupus pregnant women and to review the relevant published literature on its use in this scenario.
Methods
A prospective observational study of pregnant patients with lupus was conducted. Additionally, MEDLINE and EMBASE databases were searched, and a secondary hand search of the literature was performed. Studies were evaluated and visualised descriptively.
Results
Sixteen pregnancies of 12 lupus women were included, six (involving eight pregnancies) received belimumab throughout their illness, five of them during some period of gestation. In this group, there was one miscarriage, one elective termination and seven live foetuses (including two live twins). There was one type I intrauterine growth retardation, and a preterm pregnancy due to premature rupture of membranes (PPROM). One mild lupus flare was detected. There were no cases of pre-eclampsia, gestational diabetes mellitus or hypertension. All neonates had normal Apgar scores at birth, none needed critical care. There were no congenital anomalies. After the search, we identified 10 case reports and case series, and five registries. Among the 39 reported cases (41 pregnancies), there were 5 PPROM, 4 pre-eclampsia, and 1 eclampsia. All women made full recoveries. Nineteen new-borns had low birth weight. There were no malformations. While registries did not indicate an increased risk of birth defects or pregnancy loss, there was a higher risk of neonatal infections.
Conclusions
Belimumab may be an option for pregnant women with difficult-to-control lupus. Further research is needed to confirm the absence of association between belimumab and foetal harm.
Keywords: Systemic lupus erythematosus, pregnancy, belimumab
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by the presence of autoantibodies that cause multi-organ damage, often with severe consequences. It predominantly affects women of childbearing age. In Spain, the prevalence is estimated at 210 cases per 100,000 inhabitants according to data from the EPISER study. 1
Patients with lupus have a two-to four-fold increase in pregnancy complications. 2 In addition to experiencing disease flares during pregnancy and the puerperium, 3 there is an increased risk of complications such as miscarriage, foetal loss, preeclampsia including HELLP syndrome (haemolysis, elevated liver enzymes and thrombocytopaenia), intrauterine growth retardation and preterm delivery. This risk is particularly high when SLE has had renal manifestations, or if conception has occurred during acute disease. 4
According to European League Against Rheumatism (EULAR) recommendations, conception should occur when SLE has been in remission for at least 6 months, blood pressure is controlled and the drugs used to control disease activity are safe, using glucocorticoids at the lowest possible dose. 5 But keeping the disease under control with safe drugs for pregnancy is no easy task: not all drugs are compatible with conception, nor can they be used during pregnancy. So, treatment of SLE before and during pregnancy remains a challenge.
Belimumab is a fully humanized IgG1γ monoclonal antibody that specifically binds to B-cell activating factor (BAFF or BlyS) and inhibits B-lymphocytes differentiation into plasma cells to produce autoantibodies. Belimumab is indicated for the treatment of active autoantibody-positive SLE in patients over 5 years of age receiving standard therapy. It is the first biologic agent approved for the treatment of SLE. Although no risk of pregnancy loss or increased congenital malformations was observed in animal experiments, 6 belimumab has been classified as a class C drug for pregnancy. Both the EULAR working group and the American College of Rheumatology (ACR) considered that insufficient documentation regarding foetal safety advises substituting belimumab for another medication prior to conception, and only considering its use when there is no other drug compatible with pregnancy that can effectively control maternal disease.7,8
Our aim is to share our experience in pregnant women with SLE treated with belimumab and also review the relevant published literature on the use of belimumab during pregnancy in SLE patients.
Methods
We conducted a prospective observational study in clinical practice of pregnancies of patients diagnosed with SLE, seen in the multidisciplinary unit consisting of an Obstetrics specialist and a Rheumatology specialist, from January 2018 to August 2023. We included pregnant patients who fulfilled the EULAR/ACR 2019 SLE classification criteria, 9 excluding only those who refused to participate in the study, as well as those who have not completed their pregnancy by August 2023. Demographic data, clinical manifestations, drugs used, evolution during pregnancy and maternal and foetal outcomes were collected. The study was approved by the ISABIAL Ethics Committee (internal code: 2018-003). Patients gave informed consent to participate in the study. The study conformed to the principles of the Declaration of Helsinki.
MEDLINE and EMBASE databases were also searched until 21 July 2023, using MESH terms and free text. The strategy included synonyms for ‘systemic lupus erythematosus’, ‘pregnancy’ and ‘belimumab’. The search was limited to human studies. A secondary hand search of the literature of included studies was also performed. Studies were evaluated and visualised descriptively.
Descriptive data were summarized by median and interquartile range (IQR).
We used the STROBE checklist when writing our report ‘Considering belimumab during pregnancy: a more viable option over time’. 10
Results
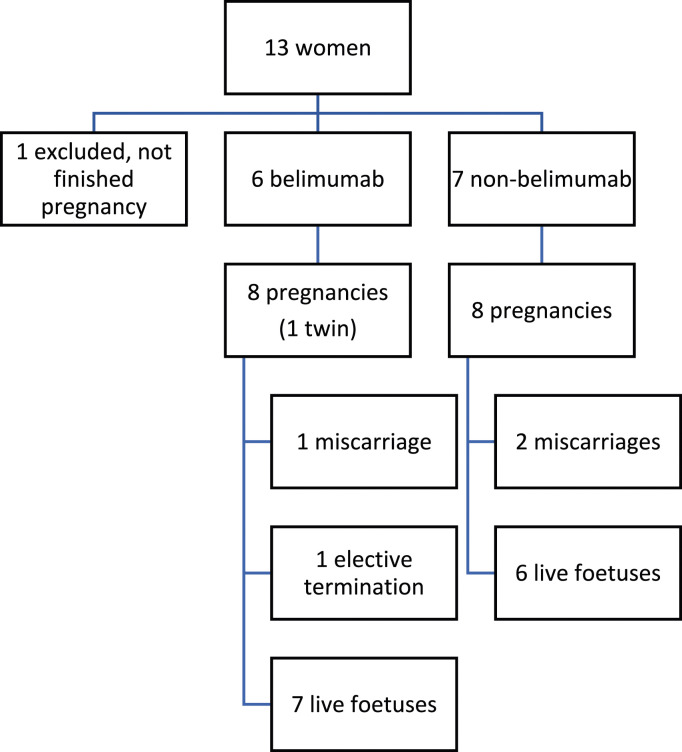
During the period January 2018 to July 2023, 17 pregnancies of 13 women diagnosed with SLE were seen in the multidisciplinary unit. All met the EULAR/ACR 2019 SLE classification criteria. All agreed to take part in the study. One woman had not completed her pregnancy by the end of the study and was therefore excluded (Figure 1). Table 1 summarises the main disease characteristics of the 12 women included. The median age of the pregnant patients was 37 years (IQR 31,25–40). Five patients had antiphospholipid antibodies and one had an overlap with mixed connective tissue disease. Seven had had lupus nephritis. All patients received aspirin and hydroxychloroquine or chloroquine during pregnancy. Only four women (5/16 pregnancies, 31,25%) received low-dose glucocorticoids (prednisone 2.5–7.5 mg/day). Three patients were prescribed low molecular weight heparin. In addition, azathioprine was also used in some patients. Six patients (Table 1, Patient 1 to 6, involving eight pregnancies) had been treated with belimumab throughout their illness, five of them during some period of pregnancy.
Figure 1.
Patient inclusion flowchart.
Table 1.
Disease characteristics.
| Case | Age (y) at SLE diagnosis | SLE manifestations | Treatment during disease course | Previous pregnancies | Age (y) and SLEDAI at BEL start | Treatment at start of BEL | Post-BEL outcome | Concomitant post-BEL treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 9 | Fever, oral ulcers, subacute cutaneous lupus, alopecia, asthenia, anaemia, leukopenia, polyarticular arthritis, low complements, dsDNA | PD, HCQ, AZA, CYC, MMF | Spontaneous planned pregnancy (35 years; HCQ, AZA, PD; severe lupus flare during pregnancy. Alive, term (38+ 2w), 2420g, Apgar 9-10 | 35 SLEDAI 7 | PD 60 mg/day, HCQ 400 mg, AZA 150 mg | Remission SLEDAI 0 | HCQ 200 mg, vitamin D |
| 2 | 14 | Rash malar, Raynaud, HA, myalgias, asthenia, low complements, dsDNA. | PD, HCQ, AZA, MMF, RTX | Spontaneous non-planned pregnancy (30 years), pregnancy loss at 16 w. PD, HCQ, AZA, lupus flare later | 31 SLEDAI 12 | PD 5 mg/day, HCQ 400 mg MMF 2 g | Slight low complement SLEDAI 2 | PD 5 mg/day, HCQ 200 mg, vitamin D |
| 3 | 14 | Polyarthritis, chorea, LN (type V GN), low complements, dsDNA, APA. | PD, MPA, HCQ, OA | No | 28 SLEDAI 17 | HCQ 300 mg, MPA 1440 mg, PD 2.5 mg, OA | Positive dsDNA SLEDAI 2 | HCQ 300, OA, vitamin D |
| 4 | 21 | Myositis, pancitopenia, LN (type IV GN), polyarticular arthritis, dsDNA, low complements | PD, AZA, CY-A, HCQ, CYC | Spontaneous planned pregnancy (32 years), HCQ, AZA, LDA. Alive, term (39+ 5w), vaginal delivery, 3080, Apgar 9-10 | 36 SLEDAI 10 | HCQ 400 mg, AZA 150 mg, LDA 100 mg, vitamin D | Positive dsDNA SLEDAI 2 | HCQ 400 mg, AZA 150 mg, LDA 100 mg, vitamin D |
| 5 | 14 | Polyarticular arthritis, HA, lymphopenia, rash, LN (type IV GN), vasculitis, ischaemic cerebral lesion, aseptic meningitis, dsDNA, low complements, APA | PD, CYC, MMF, RTX, AZA | No | 36 SLEDAI 10 | LDA 100 mg, HCQ 200 mg, AZA 100 mg, PD 5 mg/day | Positive dsDNA SLEDAI 2 | LDA 100, HCQ 200, AZA 50 |
| 6 | 23 | Rash, polyarticular arthritis, raynaud, oral ulcers, overlap MCTD | PD, HCQ, MTX | No | 31 SLEDAI 6 | MTX 10 mg/w, PD 5 mg, HCQ 200 mg, LDA 100 mg | Remission SLEDAI 0 | LDA 100 mg, PD 2.5 mg/day, HCQ 200 mg, vitamin D |
| 7 | 9 | Severe subacute cutaneous lupus, thrombopenia, pleuritis, dsDNA, low complements | PD, HCQ, AZA, isotretinoin | Spontaneous planned pregnancy (33 years), atebrine, HCQ, MMF, PD. Alive, term (38 w) caesarean section (non-deliver-progression), 2580 g, Apgar 9-10 | ||||
| 8 | 21 | LN (type IV), low complements, dsDNA, APA | PD, CYC, AZA | 3 pregnancy loses (1 chemical pregnancy, 1 in w 7, 1 in w 11) | ||||
| 9 | 29 | Fever, polyarthritis, transverse myelitis, lymphadenopathy, LN (type III GN). Low complements, dsDNA. | AZA, CYC, MP, PD, MMF, RTX | No | ||||
| 10 | 23 | Polyarticular arthritis, APA, dsDNA, low complements, LN (type IV GN) | MMF, HCQ, LDA, PD | No | ||||
| 11 | 42 | Fever, arthralgias, oral ulcers, dsDNA, APA | 4 pregnancies pre-LES diagnosis | |||||
| 12 | 17 | Thrombopenia, APA, dsDNA, low complements, LN (type IV GN) | MMF, HCQ, LDA, PD, CYC | 1 induced abortion at 16 years old |
APA, antiphospholipid antibodies; ART, Assisted Reproductive Technology; AZA, azathioprine; BEL, belimumab; HCQ, hydroxychloroquine; GN, glomerulonephritis; HA, haemolytic anaemia; IVIg, intravenous immunoglobulin therapy; LDA, low dose aspirin; LMWH, low molecular weight heparin; LN, lupus nephritis; MMF, mycophenolate mofetil; MPA, mycophenolic acid; MCTD, mixed connective tissue disease; MTX, methotrexate; OA, oral anticoagulants; PD, prednisone; W, weeks; Y, years.
Three pregnancies were obtained through assisted reproductive techniques, the remaining nine spontaneously. Although we instructed patients to plan their pregnancies at the right time, four of them took place without planning. Tables 2 and 3 show the pregnancy characteristics and outcomes in the belimumab (Patient 1 to 6) and non-belimumab (Patient 7 to 12) groups, respectively.
Table 2.
Pregnancy characteristics and outcome in belimumab group.
| Case | Age (y) at pregnancy | Characteristics of pregnancy | BEL exposure of the neonate | Concomitant treatment during pregnancy | SLEDAI | Maternal outcome | Foetal outcome |
|---|---|---|---|---|---|---|---|
| 1 | 38 | Disease in remission 2 years Spontaneous planned pregnancy | Conception to 33rd w of gestation | HCQ 400 mg, LDA 150 mg, vitamin D | At conception: 0 At delivery: 0 3 m after delivery: 0 | Lupus in remission. No incidents BEL restart at delivery | Alive, term (38 + 3w) twins, caesarean section due to breech foetal position of 2nd foetus; 3030 g, Apgar 9-10; 2680 g. Apgar 5-9-10 |
| 2, 1st pregnancy | 34 | Disease in remission 6 m Spontaneous non-planned pregnancy | Conception to 4th w of gestation | PD 7.5 mg, HCQ 300 mg, AZA 50 mg, LDA 100 mg, vitamin D | At conception: 2 At delivery: 2 3 m after delivery: 2 | Lupus in remission. PPROM BEL restart 16 m after delivery due to LES flare | Alive, preterm (35 + 5), vaginal delivery, LBW (2220 g) Apgar 10-10 |
| 2, 2nd pregnancy | 37 | Disease in remission 3 years Spontaneous planned pregnancy | Conception to 12th w of gestation | PD 5 mg, HCQ 200 mg, LDA 100, vitamin D | At conception: 1 3 m after pregnancy interruption: 1 | Lupus in remission BEL continued | Trisomy 18 detected at 12 w, elective termination |
| 3, 1st pregnancy | 31 | Disease in remission 4 years Spontaneous planned pregnancy | Conception to miscarriage | HCQ 300 mg, LMWH, vitamin D | At conception: 2 3 m after pregnancy loss: 2 | Lupus in remission, only positive dsDNA. No incidents BEL continued | Spontaneous miscarriage at 7 w |
| 3, 2nd pregnancy | 32 | Disease in remission 5 years Spontaneous planned pregnancy | Conception to 35 + 5w of gestation | HCQ 300 mg, LMWH, vitamin D | At conception: 0 At delivery: 4 3 m after delivery: 6 | Lupus in remission, only positive dsDNA. No incidents. BEL restart at delivery | Alive, term (39 + 4w), caesarean section for non-progression; 3890 g, Apgar 8-9 |
| 4 | 37 | Disease in low activity 3 years Spontaneous non-planned pregnancy | Weekly: Conception to 12th w of gestation Every two w: 13th to 35th of gestation | HCQ 400 mg, AZA 150 mg, LDA 100 mg, vitamin D | At conception: 4 At delivery: 2 3 m after delivery: 4 | Lupus in low activity. No incidents She decided not to restart belimumab at delivery but did it 3 m later due to lupus flare |
Alive, term (39 w) vaginal delivery, 3240 g Apgar 10-10 |
| 5 | 40 | Disease in remission 4 years Programmed pregnancy, obtained through ART | None (the last belimumab, 15 m prior to pregnancy) | LDA 100 mg, HCQ 200 mg, AZA 50 mg, LMWH | At conception: 2 At delivery: 10 3 m after delivery: 8 | W 28: Mild flare (arthralgias, lymphopenia, low complements, increase in dsDNA) requiring PD 5 mg and HCQ 300 mg Moderate flare at delivery, BEL restart 3 w after delivery with PD 15 mg/day | Alive, term (38 + 4w) induction due to foetal growth arrest. Vaginal delivery, 2680 g. Apgar 10-10 |
| 6 | 33 | Disease in remission 3 years Spontaneous planned pregnancy | Weekly: Conception to 16th w of gestation Every two w: 17th to 36th w of gestation | LDA 100 mg, PD 2.5 mg, HCQ 200 mg, vitamin D | At conception: 0 At delivery: 0 3 m after delivery: 0 | Lupus in remission. No incidents Seroma in caesarean scar, topical treatment; BEL restarted 6 w after delivery | Alive, term (38 w) induction due to IUGR type I; caesarean section due to LFW, LBW (2360 g). Apgar 9-10 |
AZA, azathioprine; BEL, belimumab; HCQ = hydroxychloroquine; CTG, cardiotocography; GN, glomerulonephritis; HA, haemolytic anaemia; IVIg, intravenous immunoglobulin therapy; IUGR, Intrauterine growth restriction; LBW, low birth weight; LDA, low dose aspirin; LFW, loss of foetal wellbeing; LMWH, low molecular weight heparin; LN, lupus nephritis; M: months; MPA, mycophenolic acid; MCTD, mixed connective tissue disease; MTX, methotrexate; OA, oral anticoagulants; PD, prednisone; W: weeks; Y: years.
Table 3.
Pregnancy characteristics and outcome in non-belimumab group.
| Case | Age (y) at pregnancy | Characteristics of pregnancy | Treatment during pregnancy | SLEDAI | Maternal outcome | Foetal outcome |
|---|---|---|---|---|---|---|
| 7 | 41 | Disease in remission 2 years Spontaneous, non-programmed pregnancy | HCQ, AZA, LDA | At conception: 0 At delivery: 0 3 m after delivery: 0 | Lupus in remission. No incidents | Alive, term (38 + 6w), vaginal delivery, 2820 g, Apgar 9-10 |
| 8 | 40 | Disease in remission 6 years Programmed pregnancy, obtained through ART | CQ, LDA, PD, labetalol, alfa-metil-dopa | At conception: 0 At delivery: 0 3 m after delivery: 0 | Reoperation for postpartum haemorrhage. Seroma in caesarean scar, topical treatment Lupus in remission. No other incidents | Alive, term (38 w) programmed caesarean section, 2800 g, Apgar N/A |
| 9 | 41 | Disease in remission 3 years Programmed pregnancy, obtained through ART | HCQ, AZA, LDA, PD, Vit D | At conception: 0 At delivery: 0 3 m after delivery: 0 | Gestational diabetes Lupus in remission. No other incidents | Alive, term (39 + 3w), caesarean section due to non-deliver-progression, 2980 g, Apgar 9-10 |
| 10 | 31 | Disease in remission 4 years Spontaneous, programmed pregnancy | HCQ, LDA | At conception: 2 At delivery: 2 3 m after delivery: 6 | Lupus in remission, only positive dsDNA. Mild flare lupus 3 m after delivery | Alive, term (39 + 1w), vaginal delivery, 3780 g, Apgar 10-10 |
| 11, 1st pregnancy | 40 | Spontaneous, non-programmed pregnancy | Simvastatin, betahistina, omeprazol, ibuprofen, HCQ, LDA | At conception: 2 At end of gestation: 2 3 m after delivery: 2 | Lupus in remission, only positive dsDNA. No incidents | Spontaneous miscarriage at 12 + 2w |
| 11, 2nd pregnancy | 41 | Spontaneous, programmed pregnancy | LDA, HCQ, HBPM. | At conception: 2 At delivery: 2 3 m after delivery: 2 | Lupus in remission, only positive dsDNA. No incidents | Alive, term (38 + 3w) caesarean section due to LFW, 2440 g, Apgar 3/6/9 |
| 12, 1st pregnancy | 27 | Disease in remission 1 year Spontaneous, programmed pregnancy | HCQ, LDA | At conception: 2 At end of gestation: 2 3 m after delivery: 2 | Lupus in remission, only positive dsDNA. No incidents | Chemical pregnancy |
| 12, 2nd pregnancy | 28 | Disease in remission 2 years Spontaneous, programmed pregnancy | HCQ, LDA | At conception: 2 At delivery: 2 3 m after delivery: 2 | Lupus in remission, only positive dsDNA. No incidents | Alive, term (39 + 1w), vaginal delivery, 2875 g, Apgar 9-10 |
ART: assisted reproductive techniques. AZA, azathioprine; BEL, belimumab; CQ: chloroquine; HCQ = hydroxychloroquine; CTG, cardiotocography; GN, glomerulonephritis; HA, haemolytic anaemia; IVIg, intravenous immunoglobulin therapy; IUGR, Intrauterine growth restriction; LBW, low birth weight; LDA, low dose aspirin; LFW, loss of foetal wellbeing; LMWH, low molecular weight heparin; LN, lupus nephritis; M: months; MPA, mycophenolic acid; MCTD, mixed connective tissue disease; MTX, methotrexate; N/A: not available; OA, oral anticoagulants; PD, prednisone; Y: years; W: week.
In the belimumab group, there were seven live foetuses from six mothers (including two live twins). Two patients had two pregnancies during this period. There was one miscarriage (defined as a spontaneous pregnancy loss occurring before 24 weeks gestation), and one elective termination of pregnancy after a trisomy 18 was detected in the foetus (second pregnancy in Case 2). Of the remaining six pregnancies, five went to term, three of them by caesarean section. One foetus had type I intrauterine growth retardation (defined as a foetus with an estimated foetal weight below the 10th percentile for gestational age, Case 6), so the pregnancy was ended at 38 weeks. Subsequent development of the baby has occurred normally. There was a preterm pregnancy (at 35 + 5weeks, Case 2) due to premature rupture of membranes (PPROM, defined as rupture of membranes before 37 weeks’ gestation), delivering a low-birth-weight baby girl vaginally, with no other complications. She had discontinued belimumab after a positive pregnancy test (in the fourth week of gestation). We only detected a mild lupus flare during pregnancy (change in SLE Disease Activity Index 2000 – SLEDAI-2K – score from two to eight due to hypocomplementemia and mild arthritis) in Case 5 (the patient did not receive belimumab during pregnancy). She was treated by increasing the dose of hydroxychloroquine and adding 5 mg/day of prednisone. After delivery, lupus activity worsened, requiring an increase in prednisone and restart of belimumab. There were no cases of pre-eclampsia, gestational diabetes mellitus or hypertension. All neonates had normal Apgar scores at birth, and none of them needed critical care. There were no congenital anomalies. All women were able to breastfeed their infants.
In the non-belimumab group, there were six live foetuses from six mothers. Two patients had two pregnancies during this period. There were two miscarriages. The remaining six pregnancies went to term, three of them by caesarean section. One patient suffered a postpartum haemorrhage that needed reoperation. There was a gestational diabetes mellitus. There were no cases of pre-eclampsia, hypertension or any flare during pregnancy. All but one neonate had normal Apgar scores at birth, and none required critical care. There were no intrauterine growth retardation, preterm pregnancies, PPROM, low birth weight babies or congenital anomalies. Three of the six women opted for artificial breastfeeding.
After searching MEDLINE and EMBASE databases, 10 case reports and case series (Table 4) and five registries (Table 5) were identified (the paper of Juliao et al 27 is included in Petri et al, 23 so we only comment on the last one). All studies were published between 2014 and 2023.
Table 4.
Case series.
| Reference, year of publication | Case | Age (y) | BEL exposure of the neonate | Concomitant medication during pregnancy | Maternal outcome | Foetal outcome |
|---|---|---|---|---|---|---|
| Danve et al, 2014 11 | 1 (1st pregnancy) | 38 | Conception to 32nd week | PSL + HCQ + BEL + LWMH | No incidents | Alive, term. Mild Ebstein’s anomaly |
| Kumthekar a et al, 2017 12 | 1 (2nd pregnancy) | 40 | Miscarriage because of aneuploidy | |||
| 1 (3rd pregnancy) | 41 | Conception to 33rd week | PSL + HCQ + BEL + LWMH | No incidents | Alive, term. Extrarenal pelvis, which is considered a normal anatomic variant | |
| Emmi G et al. 2016 13 | 2 | 24 | Conception to 24th week | PSL + HCQ + BEL | Alive, term, caesarean section | |
| Bitter H et al 2018, 14 2023, 15 | 3 | 29 | Conception to 26th week | AZA + HCQ + BEL | No incidents | Alive, term (40 weeks); reduced T-cell subsets at birth |
| Chehab G et al 2019 16 | 4 | 32 | Conception to early pregnancy (not specified) | PSL 5 mg/day + HCQ + AZA + LDA + LMWH | PPROM | Alive, preterm (32 weeks). Twins, both with umbilical hernias |
| Gandino IJ et al. 2021 17 | 5 | 27 | Conception to 12th week | PSL + HCQ | No incidents | Alive, term (39 + 6weeks), caesarean section due to oligohydramnios and lack of adequate progression |
| Kao JH, et al, 2021 18 | 6 | 38 | 3rd trimester | LDA + HCQ + PSL + AZA + BEL | No incidents | Alive, term |
| 7 | 32 | 3rd trimester | LDA + HCQ + PSL + BEL | PPROM, APH | Alive, preterm (31 + 4weeks), LBW (1594 g), and RDS | |
| 8 | 48 | 1st and 2nd trimester | LDA + HCQ + PSL+ AZA + LMWH + BEL | No incidents | Alive, preterm (35 + 3weeks) IUGR and LBW | |
| 9 | 38 | 3rd trimester | LDA + HCQ + PSL + AZA + BEL | APH | Alive, preterm (32 + 4weeks), LBW (2216 g), and RDS | |
| 10 | 31 | 1st, 2nd and 3rd trimester | LDA + HCQ + PSL + AZA + LMWH + IVIg + BEL | PPROM | Alive, preterm (33 + 1week), LBW (1774 g) | |
| 11 | 31 | 1st and 3rd trimester | LDA + HCQ + PSL + BEL | Gestational diabetes mellitus | Alive, term (38 + 2weeks) | |
| 12 | 41 | 2 nd trimester | LDA + HCQ + PSL + AZA + IVIg + BEL | No incidents | Miscarriage (18 weeks) | |
| 13 | 48 | 3 rd trimester | LDA + HCQ + PSL + AZA + LMWH + BEL | No incidents | Miscarriage (15 + 4weeks) | |
| 14 | 46 | 1st, 2nd and 3rd trimester | LDA + HCQ + PSL + AZA + LMWH + IVIg + BEL | Preeclampsia, placenta accreta | Alive, preterm (34 + 4weeks), RDS | |
| 15 | 37 | 3 rd trimester | LDA + HCQ + PSL + AZA + SASP + BEL | No incidents | - Twin A: Alive, term (37 + 1week), LBW (2266 g), RDS, omphalitis - Twin B: Alive, term (37 + 1week), LBW (1870 g) |
|
| 16 | 31 | 3 rd trimester | LDA + HCQ + PSL + AZA + LMWH + BEL | PPROM | Alive, preterm (36 + 6weeks), LBW (2424 g) | |
| 17 | 43 | 2 nd , and 3 rd trimester | LDA + HCQ + PSL + AZA + BEL | No incidents | - Twin A: Alive, preterm (31 + 6weeks), LBW (1250 g), RDS - Twin B: Alive, preterm (31 + 6) weeks, LBW (1402 g), RDS, and neonatal jaundice |
|
| 18 | 37 | 3rd trimester | LDA + HCQ + PSL + AZA + IVIg + BEL | No incidents | Alive, preterm (36 + 5weeks), LBW (2282 g) | |
| Crisafulli F et al, 2021 19 | 19 – 31 (aggregated data) | 32 [range 24-41] | 2: Stopped before conception 7: Stopped at positive pregnancy test 2: Conception to 11th week 1: Conception to 22ndweek 1: Conception to 24th week | 12 PD; 1 IV PSL; 10 HCQ; 1 chloroquine; 5 AZA; 5 calcineurin inhibitors; 10 LDA; 9 LMWH. | 3 flares; 1 preeclampsia, 1 eclampsia, 1 PPROM, 1 gestational diabetes | 1 late miscarriage, 12 live birth (4 preterm), 2 IUGR, 5 SGA, 1 perinatal death. No malformations |
| Lai Y et al, 2022 20 | 32 | 34 | Conception to 4th week | PD + immunosuppressants + sildenafil | HTN, LN, preeclampsia | Alive, preterm (28 weeks), caesarean section, LBW (950 g) |
| 33 | 32 | 13 to 18th week | PD + HCQ + CsA | HTN, LN, preeclampsia | Alive, preterm, caesarean section (33 + 5weeks) LBW (1800 g) | |
| 34 | 34 | 9th week to end | PD + HCQ + TAC + LDA + propylthiouracil | Mild thrombopenia | Alive, term (37 + 3weeks), caesarean section due to LFW | |
| 35 | 37 | 23rd week to end | PD + HCQ + TAC + LDA + LMWH | No incidents | Alive, term (39 weeks) | |
| Wei SR, et al, 2023 21 | 36 | 37 | 14 to 36th week | PD + HCQ + TAC + alfacalcidol | No incidents | Alive, term (37 + 6weeks), LBW (2420 g) |
| 37 | 25 | 12 to 35th week | PSL + HCQ + CsA | No incidents | Alive, term (37 + 6weeks) | |
| Nakai T et al, 2023 22 | 38 | 27 | Conception to 5th week | PSL + BEL + TAC | Pregnancy hypertension | Alive, term (37 + 1week), SGA, LBW (1876 g) |
| 39 | 32 | Conception to 7th week | PSL + BEL + TAC + HCQ | No incidents | Missed abortion |
APH; antepartum haemorrhage; AZA, azathioprine; BEL, belimumab; CsA, cyclosporine A; HCQ, hydroxychloroquine; HTN, secondary hypertension; IVIg, intravenous immunoglobulin therapy; LBW, low birth weight; LDA, low dose aspirin; LFW, loss of foetal wellbeing; LMWH, low molecular weight heparin; LN, lupus nephritis; PAH, pulmonary arterial hypertension; PD, prednisone; PSL, prednisolone; PPROM, premature rupture of membrane; RDS, respiratory distress syndrome; SASP, sulfasalazine; SLE, systemic lupus erythematosus; SGA, small for gestational age; TAC, tacrolimus; Y: years.
Table 5.
Published registries.
| Reference | Objective | Methods | Results | Foetal outcome | Conclusions |
|---|---|---|---|---|---|
| Petri M, et al, 2023 23 | To describe available data on birth defects and pregnancy loss in women with SLE exposed to belimumab | Data on birth defects and pregnancy loss (miscarriage or stillbirth) with belimumab exposures prior to or during pregnancy were collected from 18 belimumab clinical trials, the belimumab pregnancy registry (BPR, GSK study BEL114256; NCT01532310) and postmarketing/spontaneous reports from the argus database (which included searches of the US FDA adverse event reporting System and EV databases) up to 8 March 2020 | A total of 586 pregnancy reports were identified 319 pregnancies with known outcomes excluding elective terminations were analysed: 126 were identified in clinical trials (n = 110 belimumab, n = 16 placebo); 56 (n = 48 prospective, n = 8 retrospective) were identified in the BPR; and 137 were postmarketing/spontaneous reports 223 ended in live births |
Birth defects were identified
− 4/72 (5.6%) in belimumab-exposed pregnancies and 0/9 placebo-exposed pregnancies across 18 clinical trials − 10/46 (21.7%) belimumab-exposed pregnancies in the BPR prospective cohort (enrolled prior to pregnancy outcome) − 0/4 belimumab-exposed pregnancies in the BPR retrospective cohort (enrolled after pregnancy outcome) − 1/92 (1.1%) in belimumab-exposed pregnancies from postmarketing/spontaneous reports There was no consistent pattern of birth defects across datasets Pregnancy loss occurred in −31.8% (35/110) of belimumab-exposed women and 43.8% (7/16) of placebo-exposed women in clinical trials −4.2% (2/48) of women in the BPR prospective cohort − 50% (4/8) in the BPR retrospective cohort −31.4% (43/137) of belimumab-exposed women from postmarketing/spontaneous reports |
Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use |
| Ghalandari N, et al, 2022 24 | To evaluate the number and nature of reported CMs after intrauterine exposure to non-TNF inhibitor biologics compared to CZP. | A search was performed in the EV database among pregnancy-related ADR reports (all reports until 11 March 2021) | There were 93 reports of pregnancies with belimumab | Of the 93 reports of pregnancies with belimumab, 17 (18.27%) involved CMs. Compared to CZP, belimumab had a statistically significantly higher crude and adjusted OR of CMs. The most reported CM were cardiac or neurological disorders, but no specific patterns were observed. It must be considered that previous reports have shown a risk ratio for CMs in neonates born to SLE patients comparable to the result of this study | No special safety signal was identified regarding the occurrence of CMs after exposure to belimumab |
| Ghalandari N, et al. 2023 25 | To compare the reported foetal outcomes in SLE patients who stopped scheduled belimumab within the first trimester (group A) and those who continued (group B) | All belimumab-exposed pregnancy-related reports were extracted from the EV database until March 11th, 2021 | 87 pregnancies with 90 foetal outcomes were included (including three twin pregnancies) -Group A, n = 68 -Group B, n = 20 |
-Exposure to concomitant medications aimed for management of SLE (corticosteroids, NSAID, azathioprine, MTX and mycophenolate mofetil, were higher in group A -Reporting rates of preterm birth and low birth weight were higher – though not statistically different – in group A. -No statistical difference in foetal death was observed between groups |
The positive results are supportive for the continuation of belimumab during pregnancy |
| Dernoncourt A, et al, 2023 26 | To detect pharmacovigilance signals for foetal and neonatal adverse drug reactions (ADRs) to biologics taken by pregnant women with autoimmune diseases | A disproportionality analysis of VigiBase pharmacovigilance was performed The frequency of all identified ADRs for biologics of interest was compared with that of all other reports for all other drugs and quoted as the reporting odds ratio (ROR) [95% confidence interval] |
The total number of individual case safety reports with belimumab was 112 | After the exclusion of reports with steroids, the ROR was significant for neonatal infections with belimumab (28.49 [5.75–141.25]) The RORs for musculoskeletal malformations was not elevated with belimumab |
The reporting odds ratios were not elevated for musculoskeletal malformations but were significant for neonatal infections |
CMs, congenital malformations; CZP, certolizumab pegol; EV database, EudraVigilance database; TNF, tumour necrosis factor.
Discussion
We report on 16 females with SLE in which there were eight pregnancies with belimumab exposure. Based on the available literature, after careful assessment and counselling of the women, we jointly decided to maintain the treatment already started with belimumab in these cases, considering that the benefits of SLE treatment outweighed the risks to the foetus. Each was assessed individually, and the duration of treatment was tailored to the patient’s needs: one discontinued belimumab at week four upon confirmation of pregnancy (with good control thereafter with azathioprine), one ended in miscarriage at week 7, and four maintained treatment with belimumab until the third trimester (week 33–36). Second pregnancy of Case 2 ended in elective termination at week 12 after a trisomy 18 was detected in the foetus. Maternal IgG antibodies are actively transported across the placenta to confer immunity to the foetus while his/her humoural response is inefficient. Placental transport occurs in a linear fashion, with minimal transport in the first trimester, and maximal transport during the third trimester. Transplacental passage of belimumab has been shown to occur in humans , 15 although it probably does not start until 15 weeks of gestation, as with other biologics whose structure is an immunoglobulin, 28 so it is very unlikely to be related to trisomy. Regarding obstetric morbidity, six of the 12 pregnancies were delivered by caesarean section (three in each group), there were no cases of pre-eclampsia, only one preterm delivery at 35 + 5weeks (the first pregnancy in Case 2, who had discontinued belimumab at the time of conception), resulting in vaginal delivery of an otherwise healthy low birth weight girl with normal subsequent development, and one foetal growth retardation (in Case 6), requiring termination of the pregnancy at 38 weeks of gestation, resulting in a caesarean delivery of an otherwise healthy low birth weight girl with no subsequent developmental problems. This complication rate is within the expected range in women with SLE, as it is 2-4 times higher than in the general population. 2 More than one third of pregnancies in women with SLE are delivered by caesarean section, intrauterine growth retardation is detected in 5.6%, and 4%–5% of new-borns are small for gestational age (4). PPROM, present in 3% of pregnancies in the general population, 29 reaches 12.9% of pregnancies in women with SLE. 30 All women in the belimumab group were able to breastfeed. Belimumab is considered a low-risk drug during breastfeeding.31,32 One published case demonstrated that exposure to belimumab through breast milk was safe for infants. 33 The 10 case reports previously published involve 39 patient cases (which included 41 pregnancies, three of them twins), with belimumab exposure ranging from a few weeks to the entire pregnancy (Table 4). The median age of the pregnant patients was 33,75 years (range 24–48 years). 20 pregnancies ended at full term, 16 preterm and five miscarried. The main maternal complications were five cases of PPROM, four cases of pre-eclampsia and one eclampsia. All women recovered satisfactorily. As for the newborns, 19 had low birth weight. No malformations were detected, although one mild Ebstein’s anomaly was reported.
Our results are in line with the study published by Ghalandari et al 25 using data from the EudraVigilance (EV) database, a pharmacovigilance database launched in 2001, used for collecting spontaneous reports of suspected adverse drug reactions (ADRs) to medications that have been authorized by the European Medicines Agency (EMA). 34 After excluding three missing cases, they analyse 87 pregnancies in patients treated with belimumab, comparing the reported foetal outcomes in SLE patients who stopped scheduled belimumab within the first trimester and those who continued during the first trimester or thereafter. There were no statistical differences in foetal death between groups. No cases of LBW were observed if belimumab was continued, compared to 9 cases (24.3%) if belimumab was stopped during early pregnancy (Table 5). The authors attributed this difference to possible better disease control in patients who continued belimumab therapy, requiring 30% less glucocorticoids.
In a recent report, Petri et al 23 summarised data on birth defects and pregnancy losses from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR, NCT01532310) and post-marketing/spontaneous reports in women with SLE exposed to belimumab before or during pregnancy (Table 5). Given the limitations of the data, the authors acknowledge that they cannot provide informed recommendations on the use of belimumab during pregnancy. However, the study did not reveal an increased risk of birth defects or pregnancy loss among women treated with belimumab.
Ghalandari N, et al 24 performed a search in the EV database among pregnancy-related ADR reports (all reports until 11 March 2021). They compare the number and nature of congenital malformations (CMs) reported after intrauterine exposure to non-tumour necrosis factor inhibitor biologics, with certolizumab pegol (CZP) (chosen as the safest biologic in pregnancy). Seventeen (18.27%) of the 93 pregnancy reports with belimumab, involved CMs (Table 5), a statistically significantly higher crude OR (2.55 [1.44, 4.49]) compared to CZP. Although the most reported CM were cardiac or neurological disorders, no specific patterns were observed. It should be noted that belimumab is only indicated in SLE, where previous reports have shown a hazard ratio of 2.63 (95% CI 1.93–3.58) for CM, 35 which is comparable to the result of this study, while CZP is indicated for the treatment of chronic inflammatory arthritis, where no increased risk of CM has been described. As a comment, there is probably an error in this article, the correct number of reports of pregnancies with belimumab is surely 90, a conclusion reached after reading the article by the same group discussed above. 25
To detect pharmacovigilance signals for foetal and neonatal ADRs to biologics taken by pregnant women with autoimmune diseases, Dernoncourt et al 26 performed a disproportionality analysis of data collected from VigiBase database (the world’s largest pharmacovigilance database, curated by the World Health Organization) from 1968 to June 1, 2021. The ADRs selected were stillbirth, premature birth, low birth weight, small for gestational age and congenital malformations. The total number of individual case safety reports with belimumab was 112. The reporting odds ratios were not elevated for musculoskeletal malformations but were significant for neonatal infections.
To date, all case studies, case series and analyses of data from clinical trials and registries confirm the absence of risk of foetal malformations following foetal exposure to belimumab. Recently, the British Society for Rheumatology (BSR) released its reproductive health guideline 33 and concluded that, although there remains insufficient evidence to be confident that it is compatible with pregnancy, any exposure during pregnancy is unlikely to be harmful, so, it may be considered to manage severe maternal disease in pregnancy if no other pregnancy-compatible drugs are suitable. However, one concern remains unanswered: how belimumab may affect the immune status of children exposed in utero. In one published case, it has been shown a reduced T-cell subset at birth compared with non-exposed neonates, though values still were well within adult reference values. Whether it resulted from maternal use of azathioprine or from the combination with belimumab cannot be answered. The child’s B cells were in the normal range already 4 months after delivery and the vaccination response was normal. 14 Data collected from VigiBase database by Dernoncourt et al 26 demonstrated an unexpected increase in reporting odds ratios for neonatal infections exposed to intrauterous belimumab. It is important to bear in mind that disproportionality studies do not prove the existence of an association between drug exposure and an effect; in fact, they generate pharmacovigilance signals that must then be confirmed in pharmacoepidemiologic studies. Although our study did not include long-term follow-up of the children, we retrospectively asked the mothers about their children’s development, and in no case did they report any problems. Long-term follow-up studies of children prenatally exposed to belimumab are needed to confirm the absence of medium and long-term problems, as has been done with biologics in patients with inflammatory bowel disease. 36
Strengths and limitations
The limitations of this study are those of a case series, coupled with the small number of patients. However, we believe that the prospective methodology of data collection increases its value. Likewise, we consider that the literature review carried out provides useful information for clinicians.
Given the lack of evidence of harm to the foetus from the use of belimumab during pregnancy and the growing experience of benefit derived from maintaining treatment for as long as necessary depending on the mother’s clinical situation, the question is whether it is reasonable at this stage to maintain such a high level of alarm, which may lead to belimumab withdrawal in situations where continuation of treatment would have been most beneficial. The results of our study may support the continuation of belimumab during pregnancy, provided there is a risk of flare, in cases that have not responded adequately to other treatments.
Conclusion
Belimumab contributes greatly to maintaining stable lupus disease, and its discontinuation could lead to a lupus flare. A lupus flare during pregnancy is a major contributor to increased adverse pregnancy outcomes in patients with lupus, and continuation of belimumab may help maintain remission during pregnancy.
We present 5 cases (involving seven pregnancies) of women with SLE who were treated with belimumab during pregnancy, and a review of relevant published literature. In our case series, we observed that belimumab was well tolerated in pregnant patients with SLE and helped maintain disease control. There were no safety alerts for either mothers or newborns.
Based on these results, belimumab could be considered as a viable option for pregnant women with difficult-to-control SLE. We believe that our study makes a significant contribution to the literature by expanding the treatment options for pregnant women with SLE.
Further research is needed to confirm the absence of an association between belimumab and foetal harm, in particular how belimumab may affect the immune status of infants exposed in utero.
Acknowledgements
Special thanks to the Rheumatology section and Obstetrics and Gynecology service of “Hospital General Universitario Dr Balmis”, as well as to all the patients who altruistically made this study possible.
Authors’ contributions: P.V.C. contributed to the conception/design of the work, acquisition, analysis, and interpretation of data for the work, and drafting the manuscript. She approved the version to be published and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. R.C.A. contributed to the analysis and interpretation of data for the work and drafting the manuscript. She approved the version to be published and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. S.G.S. contributed to the analysis and interpretation of data for the work and drafting the manuscript. She approved the version to be published and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. S.C.A. contributed to the analysis and interpretation of data for the work and drafting the manuscript. She approved the version to be published and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. E.P.P. contributed to the acquisition, analysis, and interpretation of data for the work, and drafting the manuscript. She approved the version to be published and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PVC received grants or contracts from GSK, Abbvie, Roche, Novartis, Lilly, AstraZeneca, Pfizer; consulting fees from GSK, AstraZeneca, Pfizer; honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbvie, GSK, Galapagos, Lilly, Amgen; Support for attending meetings and/or travel from Galapagos, Abbvie.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Patient consent: Obtained.
Ethical statement
Ethical approval
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the ISABIAL Ethics Committee (internal code: 2018-003).
ORCID iDs
Paloma Vela-Casasempere https://orcid.org/0000-0001-8566-5172
Rocío Caño Alameda https://orcid.org/0000-0002-2803-4347
Silvia Gómez Sabater https://orcid.org/0000-0002-2833-565X
Silvia Cortell Aznar https://orcid.org/0000-0003-3527-3327
References
- 1.Cortés Verdú R, Pego-Reigosa JM, Seoane-Mato D, et al. Prevalence of systemic lupus erythematosus in Spain: higher than previously reported in other countries? Rheumatology 2020; 59(9): 2556–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clowse ME, Jamison M, Myers E, et al. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol 2008; 199(2): 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molad Y, Borkowski T, Monselise A, et al. Maternal and fetal outcome of lupus pregnancy: a prospective study of 29 pregnancies. Lupus 2005; 14: 145–151. [DOI] [PubMed] [Google Scholar]
- 4.Wagner SJ, Craici I, Reed D, et al. Maternal and foetal outcomes in pregnant patients with active lupus nephritis. Lupus 2009; 18: 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017; 76: 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auyeung-Kim DJ, Devalaraja MN, Migone TS, et al. Developmental and peri-postnatal study in cynomolgus monkeys with belimumab, a monoclonal antibody directed against B-lymphocyte stimulator. Reprod Toxicol 2009; 28: 443–455. [DOI] [PubMed] [Google Scholar]
- 7.Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016; 75: 795–810. [DOI] [PubMed] [Google Scholar]
- 8.Sammaritano LR, Bermas BL, Chakravarty EE, et al. American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020; 72: 529–556. [DOI] [PubMed] [Google Scholar]
- 9.Aringer M, Costenbader K, Daikh D, et al. European League against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 10.STROBE Checklist: cohort, case-control, and cross-sectional studies (combined). https://www.strobe-statement.org/ [accessed 24 July 2023].
- 11.Danve A, Perry L, Deodhar A. Use of belimumab throughout pregnancy to treat active systemic lupus erythematosus: a case report. Semin Arthritis Rheum 2014; 44: 195–197. [DOI] [PubMed] [Google Scholar]
- 12.Kumthekar A, Danve A, Deodhar A. Use of belimumab throughout 2 consecutive pregnancies in a patient with systemic lupus erythematosus. J Rheumatol 2017; 44: 1416–1417. [DOI] [PubMed] [Google Scholar]
- 13.Emmi G, Silvestri E, Squatrito D, et al. Favorable pregnancy outcome in a patient with systemic lupus erythematosus treated with belimumab: a confirmation report. Semin Arthritis Rheum 2016; 45: e26–e27. [DOI] [PubMed] [Google Scholar]
- 14.Bitter H, Bendvold AN, Østensen ME. Lymphocyte changes and vaccination response in a child exposed to belimumab during pregnancy. Ann Rheum Dis 2018; 77: 1692–1693. [DOI] [PubMed] [Google Scholar]
- 15.Bitter H, Warren DJ, Bolstad N, et al. Transplacental passage of belimumab during pregnancy and follow-up of a child exposed in utero. Ann Rheum Dis 2023; 82: 577–579. [DOI] [PubMed] [Google Scholar]
- 16.Chehab G, Krüssel J, Fehm T, et al. Successful conception in a 34-year-old lupus patient following spontaneous pregnancy after autotransplantation of cryopreserved ovarian tissue. Lupus 2019; 28: 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandino IJ, Lutgen S, Basta MC, et al. Exposure to belimumab in the first trimester of pregnancy in a young woman with systemic lupus erythematosus. Reumatol Clin (Engl Ed) 2021; 17: 428–429. [DOI] [PubMed] [Google Scholar]
- 18.Kao JH, Lan TY, Lu CH, et al. Pregnancy outcomes in patients treated with belimumab: report from real-world experience. Semin Arthritis Rheum 2021; 51: 963–968. [DOI] [PubMed] [Google Scholar]
- 19.Crisafulli F, Gerardi MC, Moschetti L, et al. Pregnancy in SLE patients treated with belimumab: experience from 3 Italian centers. Ann Rheum Dis 2021; 80: 600–601. [Google Scholar]
- 20.Lai Y, Li B, Huang J, et al. Different pregnancy outcomes in patients with systemic lupus erythematosus treated with belimumab. Lupus 2023; 32: 149–154. [DOI] [PubMed] [Google Scholar]
- 21.Wei SR, Zhu ZZ, Xu J, et al. Favorable pregnancy outcomes in two patients with systemic lupus erythematosus treated with belimumab. Int J Rheum Dis 2023; 26: 154–156. [DOI] [PubMed] [Google Scholar]
- 22.Nakai T, Ikeda Y, Yamaguchi K, et al. A case report of two systemic lupus erythematosus pregnancies with early placental exposure to belimumab: case report with review. Mod Rheumatol Case Rep 2023; 7: 82–86. [DOI] [PubMed] [Google Scholar]
- 23.Petri M, Landy H, Clowse MEB, et al. Belimumab use during pregnancy: a summary of birth defects and pregnancy loss from belimumab clinical trials, a pregnancy registry and postmarketing reports. Ann Rheum Dis 2023; 82: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghalandari N, Crijns HJMJ, Bergman JEH, et al. Reported congenital malformations after exposure to non-tumour necrosis factor inhibitor biologics: a retrospective comparative study in EudraVigilance. Br J Clin Pharmacol 2022; 88: 5378–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghalandari N, Crijns HJ, Dolhain RJ, et al. Dilemma of belimumab therapy (dis)continuation during pregnancy: results of a retrospective study in eudravigilance. Lupus 2023; 32: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dernoncourt A, Liabeuf S, Bennis Y, et al. Fetal and neonatal adverse drug reactions associated with biologics taken during pregnancy by women with autoimmune diseases: insights from an analysis of the world health organization pharmacovigilance database (VigiBase®). BioDrugs 2023; 37: 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juliao P, Wurst K, Pimenta JM, et al. Belimumab use during pregnancy: interim results of the belimumab pregnancy registry. Birth Defects Res 2023; 115: 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol 2009; 104: 228–233. [DOI] [PubMed] [Google Scholar]
- 29.Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol 2003; 101: 178–193. [DOI] [PubMed] [Google Scholar]
- 30.Dos Santos FC, Ignacchiti ML, Rodrigues B, et al. Premature rupture of membranes - a cause of foetal complications among lupus: a cohort study, systematic review and meta-analysis. Lupus 2021; 30: 2042–2053. [DOI] [PubMed] [Google Scholar]
- 31.https://www.e-lactancia.org/breastfeeding/belimumab/product/ (accessed 24 July 2023).
- 32.Saito J, Yakuwa N, Ishizuka T, et al. Belimumab concentrations in maternal serum and breast milk during breastfeeding and the safety assessment of the infant: a case study. Breastfeed Med 2020; 15: 475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell MD, Dey M, Flint J, et al. British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology 2023; 62: e48–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Medicines Agency . Screening for adverse reactions in EudraVigilance, https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/eudravigilance-electronic-reporting (accessed 24 July 2023).
- 35.Bundhun PK, Soogund MZ, Huang F. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: a meta-analysis of studies published between years 2001-2016. J Autoimmun 2017; 79: 17–27. [DOI] [PubMed] [Google Scholar]
- 36.Mahadevan U, Long MD, Kane SV, et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology 2021; 160: 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]