Abstract
This literature review explores the role of dietary oxalate in the development of chronic inflammatory kidney disease in middle-aged and older individuals. The authors pose the following questions: Is oxalate produced endogenously? If food sources contribute to chronic kidney disease and inflammation, what are those foods? What role do cultural food preparation and cooking play in denaturing food oxalates?
The concentration of oxalates found within the body at any particular time is not limited to edible plants; normal human metabolic processes of breaking down ascorbic acid may create up to 30 mg of oxalate daily. Research supports urolithiasis as a common urologic disease in industrialized societies. Approximately 80% of kidney stones are composed of calcium oxalate, resulting in hyperoxaluria. Exogenous (originating outside the cell or organism) oxalate sources include ascorbic acid, amino acids, and glyoxal metabolism. Additional research estimates the daily endogenous (produced within the cell or organism) production of oxalate to be 10–25 mg. Suboptimal colonization of oxalate-degrading bacteria and malabsorptive disease are also contributing factors to the development of chronic kidney disease. Oxalate transcellular processes, though poorly understood, rely on multifunctional anion exchangers, and are currently being investigated.
A review of research showed that normal human metabolic processes, including the breakdown of ascorbic acid, account for 35-55% of circulating oxalates and can create ≤30 mg of circulating serum oxalate daily. Glyoxylic acid accounts for 50-70% of circulating urinary oxalate in compromised individuals with liver glycation, bacterial insufficiencies, malabsorption, and anion exchange challenges.
For persons with a family history of kidney stones, consumption of foods high in oxalates may be consumed in moderation, provided there is adequate calcium intake in the diet to decrease the absorption of oxalates from the meal ingested.
Introduction
Public interest in dietary oxalates and their role in developing chronic inflammatory conditions, including kidney disease in middle-aged and older individuals, led the authors to review oxalate research. The authors reviewed and compared statistical information from >80 studies and medical texts published between 1962 to 2023 on kidney function, disease, and dietary oxalate sources. After excluding research dated by more than ten years, redundant and full-text–inaccessible articles, the authors pose the following questions:
Is Oxalate Produced Endogenously?
Nutrition is closely associated with the risk of oxalate stone events.1 Urolithiasis is a common urologic disease in industrialized societies, with kidney stones being the most prevalent form of kidney inflammation.2 Urinary oxalate is derived from two major sources: the diet and endogenous synthesis (produced within the cell or organism).3 Hyperoxaluria is excessive urinary excretion of oxalate. Primary hyperoxaluria is characterized by recurrent kidney and bladder stones (≈12% of the population) caused by overproduction of oxalates filtered through the kidneys and excreted as waste in urine. Notably, oxalate levels are not limited to plants ingested. Interestingly, normal human metabolic processes,4 including the breakdown of ascorbic acid, account for 35-55% of circulating oxalates and can create ≤30mg of serum oxalate daily.5 Glyoxylic acid accounts for 50-70% of urinary oxalate. Within our body, oxalate and calcium bind together in the intestine and are excreted in the stool.6 Kidney stones usually comprise calcium salts (75-85%) and uric acid (5-8%).7 Approximately 80% of kidney stones contain calcium oxalate, resulting in hyperoxaluria.8-10
What Causes Hypercalciuria?
Restriction of dietary calcium can reduce the urinary excretion of calcium but severe dietary restriction or deficiencies conversely cause hyperoxaluria and a progressive loss of bone mineral components. Urinary calcium excretion is influenced by nutrients other than calcium such as sodium, potassium, protein, and refined carbohydrates. Up to 40% of the daily excretion of oxalate in the urine is from dietary sources but oxalate absorption in the intestine depends linearly on the concomitant dietary intake of calcium and is influenced by the bacterial degradation involving several bacterial species of intestinal flora.11 Excess calcium in the urine can result from elevated calcium absorption via the intestines or bones into the blood. Chronic hypercalciuria may result in impaired renal function, nephrocalcinosis, and chronic kidney disease. Impaired renal calcium regulation subsequently results in elevated levels of oxalate in the urine.12
Malabsorption Conditions in Kidney Stone Development
Studies from 2020-2023, show patients with kidney stone disease, and particularly calcium oxalate nephrolithiasis, exhibit dysbiosis in their fecal and urinary microbiota compared to controls.13 Malabsorption conditions and unabsorbed fatty acids that bind calcium in the digestive tract also increase oxalate absorption, creating elevated urine/serum oxalate levels.14 Malabsorption due to conditions such as Crohn’s disease, ulcerative colitis, celiac disease, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), small intestinal bacterial overgrowth (SIBO), disordered eating, and bariatric surgery increases the risk for urolithiasis. Historical studies have demonstrated that the prevalence of symptomatic nephrolithiasis is higher in patients with IBD, typically in patients who underwent extensive small bowel resection or in those with persistent severe small bowel inflammation.15 Low-level and chronic endothelial inflammation and urinary tract complications concerning IBD have been neglected in the literature.16
Exogenous (originating outside the cell or organism) oxalate sources include ascorbic acid, tyrosine, tryptophan, phenylalanine, hydroxyproline, and glyoxal metabolism.17 Suboptimal colonization of oxalate-degrading bacteria Oxalobacter formigenes, due to malnutrition conditions and antibiotic use, contribute to the endogenous production of oxalate.18 Several short-chain fatty acids (SCFAs)-producing gut bacteria and metabolic pathways associated with SCFA production- were considerably lower in the gut microbiota among kidney stone patients.19 Significant associated risk is reduced by 70% when Oxalobacter formigenes is in the GI tract.20-23 O. formigenes is an anaerobic bacterium relying solely on oxalate for growth which is crucial for oxalate-degradation in the intestinal tract and hence plays a critical role in preventing renal toxicity. In Western societies, 30-40% of the population have Oxalobacter formigenes GI tract colonization. In contrast, kidney stone patients are found to have half this amount. The association between gut microbiota and kidney stones does not solely depend on the presence of the genus Oxalobacter formigenes. A more integrated approach using multiple omics platforms is needed to better understand the pathogenesis of kidney stones.24 Oxalate transcellular processes, though poorly understood, rely on multifunctional anion exchangers which are under investigation.25,26
Dietary Minerals in Kidney Stone Development
Contradictory results exist in the literature regarding the impact of trace elements on the pathogenesis of calcium oxalate stones. A negative association was found between the exposure to the 13 trace elements and the prevalence of kidney stones.27-29 For individuals who produce calcium oxalate stones, adequate dietary calcium consumption is vital to maintain bone calcium stores (800-1200mg/day). Absorption of soluble oxalate with calcium (200-1800 mg/day) showed a linear inverse relationship in oxalate absorption. Potassium intake is inversely related to the risk of kidney stones. A low normal potassium and high sodium ratio can be found in stone formers. Nutrition texts encourage a diet high in potassium from low oxalate fruits and vegetables. Notably, research does not support calcium restriction to prevent kidney stones. The calcium-sensing receptor (CaSR) is a protein-coupled receptor widely expressed in the kidneys and the vascular system that regulates the renal management of calcium.30 In contrast, uric acid stones are a product of purine metabolism from foods such as organ and processed meats, some fish, and poultry. Uric acid stones are the only stones amenable to dissolution therapy by urine alkalinization to a pH of 6-6.5. Sodium bicarbonate increases urinary monosodium urate and calcium and should not be used as a supplement for stones containing uric acid. Exposure to cadmium, trimethyltin, oxalic acid, and ethylene glycol ethers has been shown to predispose individuals to kidney stones.31,32
Role of Hormones in Kidney Stone Development
A 2023 study found kidney stone prevalence to be 2-3 times higher in women with Polycystic Ovary Syndrome (PCOS) compared to healthy controls.33 Researchers in 2021 concluded there are five entirely different mechanisms for kidney stone formation, and sex hormones may be key players in the development of nephrolithiasis.34 Menopausal hormonal changes, primarily in estradiol availability, often lead to metabolic syndrome, tissue friability in the urinary tract, and increased antibiotic use for urinary tract infections (UTIs).35 Metabolic syndrome is the leading co-morbidity (25.2% among U.S. seniors [>65 years]) among patients with kidney disease.36 Metabolic syndrome corresponds with type 2 diabetes and increased fructose consumption, with decreased fiber consumption, all consistent with an industrialized diet.37 In women, obesity has a larger effect on the risk of developing urolithiasis. A large cohort study of 90 000 women suggested that physical activity (regardless of its intensity) may prevent kidney stone development (KSD), as evidenced by the association of physical activity with a lower risk of KSD in postmenopausal women.38 A 2023 meta-analysis involving 13 233 patients explored the impact of body mass index (BMI) on the size and composition of urinary stones. Factors such as overweight and obesity were found to increase the risk of uric acid stones in both genders, and researchers concluded that this evidence supported a positive association between BMI and uric acid and calcium oxalate stone formation.39,40
The role of serum testosterone levels in male renal stone formation remains controversial; however, research from 2022 found serum testosterone levels were significantly inversely associated with the prevalence of kidney stones in men over 40 years of age.41 Data suggested an important role of enhanced androgen signaling in human nephrolithiasis. The important role of sex hormones, androgen receptors (ARs), and miRNA/CSF-1 in the occurrence and recurrence of calcium oxalate (CaOx) renal urolithiasis and CSF-1 signaling in human nephrolithiasis pathogenesis is under investigation.42 Males have higher risks (<12%) for developing urolithiasis than females (<7%) until women enter menopause. Exposure to ethylene glycol, heavy metals,43 chemotherapy drugs, non-steroidal anti-inflammatory drugs (NSAIDs), and antacids increases inflammatory kidney risks.44,45 Hypertension, hypothyroidism, diabetes, and obesity (common in metabolic syndrome) increase kidney stone risk.46
Despite the above discussion, one should note that kidney diseases can occur without overconsumption of oxalate foods or the development of stones.
If food sources contribute to chronic kidney disease and inflammation, what are those foods?
Industrialized societies are associated with increased inflammatory disease risks due to the consumption of highly refined and processed foods which have been linked to metabolic syndrome, diabetes, and malabsorption conditions. Soft drinks are associated with a greater risk of stone events, whereas caffeine and citrus fruit juice are not.47,48 Societies utilizing traditional dietary approaches consume more fiber and whole grains, which include arabinoxylan, inulin, β-glucan, pectin, bran, and resistant starches, and have lower incidences of conditions associated with KSD.49,50 These individual dietary fiber components meaningfully play an essential role in improving human health. Dietary fiber and whole grains contain a unique blend of bioactive components, including resistant starches, vitamins, minerals, phytochemicals, and antioxidants.51 Nutritional habits play a relevant role in the genesis and recurrence of kidney stones disease. Dietary manipulation is a fundamental tool for the medical management of nephrolithiasis.52 Available scientific evidence agrees on the harmful effects of high meat/animal protein intake and low calcium diets, whereas a high content of fruits and vegetables associated with a balanced intake of low-fat dairy products carries the lowest risk for KSD.53
Oxalate is a common component of many foods of plant origin, including nuts, fruits, mushrooms, vegetables, grains, and legumes, and is typically present as a salt of oxalic acid. It is important to note that eating whole natural foods produces the physiologic advantage of metabolic checks and balances.54 A whole food dietary model consistent with traditional diets contains a complex array of nutrients, co-factors, enzymes, and major and trace minerals with microbiome diversity.55 According to nutrition texts and research, oxalate bioavailability, the portion of food-derived oxalate that is absorbed from the gastrointestinal tract (GIT), is estimated to range from 2-15% for different foods.56 Oxalate bioavailability decreases with food ingestion due to interactions between oxalate and co-ingested food components, thus lowering the content of the soluble form of oxalic acid remaining.57 In food, oxalic acid is typically found as either sodium or potassium oxalate, which are water-soluble, or as insoluble calcium oxalate.58 According to current nutrition texts, dietary calcium reduces oxalate absorption and has a greater impact on the urinary oxalate than diet itself. The bioavailability of foods and urine oxalate are affected by oxalate salt forms, calcium present, food processing, preparation, and cooking methods. The amount of soluble oxalate and calcium in food is an important determinant of oxalate bioavailability. Oxalate degradation by oxalatede-grading bacteria within the GIT is another key factor that could affect oxalate absorption. Previous studies investigating the association between intake of vitamin B6 and risk of stones found conflicting results. In univariate and multivariate analyses, there was no association between intake of vitamin B6 and incidence of stone formation.59 Nutrition texts do not recommend the reduction of protein intake to lower the risk of stone formation for individuals in the normal to moderate stages;60 increased protein does not lead to increased oxalate synthesis due to pyridoxine (B6) acting as a co-factor converting glyoxylate to glycine.61,62 Research in 2023 concluded that a high intake of riboflavin is independently inversely associated with kidney stones, especially in the male population. However, no association was found between the dietary intake of thiamine and KSD.63 Studies exploring the association of vitamin D intake with kidney stones were reviewed in 2017. However, no statistically significant association between vitamin D intake and risk of stone formation was observed.64
Research on rice bran in 1984 showed reduced oxalate absorption when calcium-rich foods were present in the diet.65 Evidence of stone formation decreased among patients treated with rice bran for ≥3 years.66 Oxalates do not occur in foods as single anti-nutrients. Oxalates exist along with vitamins, co-factors, minerals, and organic acids citrate and malate, which control acidity.67 Research published in 2016 on hydroxycitrate (HCA, a substance isolated from Malabar tamarind) showed, under certain conditions, effective inhibition of calcium oxalate crystal growth and dissolved crystals.68,69 Additionally, consuming a fiber-rich diet has been shown to reduce kidney inflammation and stone formation.70
This systematic review and meta-analysis were performed in 2022 to evaluate the association of caffeine intake with the risk of kidney stone formation. Higher caffeine intake may be associated with a lower incidence of kidney stones.71-73 Data from 18 436 participants in a 2021 study demonstrated no significant association between coffee consumption and renal function, or the risk of chronic kidney disease.74 Research published in 2022 on coffee consumption in predominately White populations reported a decrease in the incidence of kidney stones among women compared to men. Notably, the protective effects of coffee itself were found to be greater than that of caffeine alone.75 Caffeine increases urinary excretion of calcium, sodium, and magnesium, in addition to a diuretic action with consumption >300-360 mg (≈4 cups of coffee). Additionally, tea was found to exhibit protective effects against stone formation through the accompanying water intake, its caffeine content, and antioxidant properties.76-78 The authors of this review hypothesize, that the association between reduced stone formation and coffee may relate more to the acid content, and not the caffeine.
Wild edible herbs, commonly used since ancient times, can represent a good alternative to improve the daily human intake of minerals. A 2021 study looked at four edible herbs used traditionally in Mediterranean areas. Rumex acetosa, Picris hieracioides, Cichorium intybus, and Plantago coronopus were analyzed for their Na, K, Ca, Mg, Cu, Mn, Fe, and Zn content. Nitrate and oxalate contents were also evaluated. Domesticated plants had higher oxalate and nitrate content, especially in R. acetosa versus wild plants.79
Traditional Chinese Medicine (TCM) is an efficient approach for the treatment of kidney stones with specialized medicinal herbs. The action of medicinal herbs was poorly understood due to their complex components and the inhibitory effect of the constituents on stone formation. A 2022 study found that soluble extracts from 20 kinds of herbs show almost 100% inhibition. The authors found limited studies reporting oxalate toxicity associated with two Chinese herbs.80 A 2015 study showed that Chinese medicinal herbs contain significantly different amounts of oxalate, even if from the same family. Using medicinal herbs with high oxalate content for susceptible individuals might increase the risk of urolithiasis.81-84
Curcumin, an extract from the herb turmeric, was found to inhibit the formation of kidney stones by decreasing urea, creatine, uric acid, and inorganic phosphorus compounds. Reverse transcription polymerase chain reaction (RT-PCR) detection and immunohistochemical results showed osteopontin (OPN) in the kidney was significantly reduced after curcumin use.85
A 2015 review of dietary treatments for urolithiasis found a diet low in oxalate and containing average calcium intake (800-1200 mg/day for adults) decreased urinary excretion of oxalate, and a diet high in oxalates and low in calcium increased urinary oxalate output.86 Numerous studies have indicated reactive oxygen species (ROS), and oxidative stress (OS) are crucial pathogenic factors in stone formation. Dietary polyphenols are reported to exhibit protective action against stone formation.87,88
What Role Does Cultural Food Preparation Play in Denaturing Oxalates Present in Foods?
In conducting the research for this paper, the authors found the initial data on oxalate levels in food was older than 20 years and contained numerous statistical errors. Statistical data sources included the Harvard T.H. Chan School of Public Health oxalate chart (2004), Food Standards Australia New Zealand oxalate levels in foods data, and USDA agricultural data on oxalate content. Research on food preparation methods included oxalate content following boiling, stir-frying, soaking, fermentation, juicing, and raw.89-92
Cultivation in calcium-rich soils and combining high oxalate foods with animal protein for oxalate mitigation is supported by the reviews of gastronomy traditions.93-96 Chemically, oxalic acid is characterized as a dicarboxylic organic acid with low molecular weight and high acidity, with chelating and reducing abilities. In plants, it plays a relevant role in many biological processes such as calcium homeostasis; pH regulation; plant growth, development, and protection; photosynthesis; and detoxification of heavy metals.97 Traditional cooking processes (soaking,98 fermenting,99,100 sprouting,101 blanching, boiling, and wok-frying) reduce oxalate levels.102,104
Combining food provides protective compounds that reduce the epithelial damage that affects renal function. A 2020 study found wine (a fermented liquid) containing polyphenols exert protective effects on renal cells.116 Plasma markers of chronic inflammation were significantly reduced in chronic kidney disease patients during the combined consumption of white wine and olive oil, suggesting a possible anti-inflammatory effect.106 Research on vinegar in 2022, found that it reduced renal calcium oxalate (CaOx) stones by regulating gut microbiota and increasing blood acetate level to restore renal tight junction and reduce CaOx crystal adhesion.107,108 Furthermore, individuals consuming vinegar daily compared to those without consumption have higher citrate and lower calcium excretion in urine, two critical molecules responsible for CaOx kidney stones.109-111 Fermented cocoa beans contain various chemicals that contribute to their bioactivity and nutritional properties. The bioactive phytochemicals include methylxanthines, polyphenols, biogenic amines, melanoidins, isoprostanoids, and oxalates. These phytochemicals of cocoa are related to various in vivo and in vitro biological activities and fermentation influences bioactive compounds in cocoa beans, depending on the variety cultivated.112
Glycine, a simple proteinogenic amino acid, has been found to reduce urine oxalate levels.113 Foods high in glycine also contain oxalate and or calcium such as bone broth, gelatin, poultry skin, seafood, meat, legumes, dairy, spinach, dried seaweed, watercress, asparagus, and cabbage. Oxalate levels increase with some forms of cooking, such as deep-frying high starch foods (e.g., russet potatoes used in potato chips and fries),114 and juicing raw parsley, spinach, chard, celery, and beet greens and roots increased oxalate levels due to concentration.115,116 Cooking spinach increases oxalate levels when boiled, but decreases when blanched. The authors postulate that blanching decreases oxalate by removing the oxalate-rich liquid, along with the effects of freezing.117,118 Absorbed levels of soluble oxalate depend on many factors; each variety of spinach has a different oxalate content. Agricultural research has identified >300 spinach cultivars with oxalate levels ranging from 647.2 – 1286.9 mg/100g by raw weight (200% variability by cultivar), among which eight spinach varieties contain >780 mg/100g of oxalate. However, nutritional research data does not always account for inconsistencies in oxalate content due to the common practice of using averaged agricultural data vs distinct cultivar data.119
Research recognizes that dietary inclusion of spinach, mushrooms, strawberries, raspberries, beets, rhubarb, tea, nuts, wheat bran, chocolate, sorrel, sesame, carambola, amaranth, almonds, and soybean (all high oxalate foods) may contribute ≈40-50% of urinary oxalate in individuals with compromised liver glycation, malnutrition, metabolic syndrome, bacterial insufficiencies, fat/mineral malabsorption, and anion exchange challenges.120,121 Interestingly, researchers found that consumption of ripe fruits lowers both oxalate content and dietary absorption of oxalate.122
Clinical Tools
Presently no consensus exists about the effects of dietary oxalate on stone formation, even in clients with hyperoxaluria.123 Examining research and management protocols revealed a range of interventions for clients with kidney inflammation.124 Protocols include reducing highly refined processed foods, excess phosphorus, sugar (fructose), and purines (if uric acid is a concern), and increasing water intake with unsweetened citrus, cranberry, and black cherry juices to balance urine pH level. Additionally, walking to improve kidney circulation and blood sugar control and increased fiber intake through consumption of whole natural food sources and fermented foods including supplementation of multi-strain probiotics is recommended.
Health and wellness coaches, along with licensed healthcare professionals, advise clients on meaningful lifestyle changes. The coach approaches kidney inflammation from a whole health perspective, aiding the client in assessing areas of health on a scale of 1-10. The client and coach explore the importance, readiness, and safety of agreed-upon goals. The coach assists the client in exploring the feasibility of dietary and lifestyle options, obstacles, and success measures in reaching goals.125-127
Conclusion
The role of oxalate in affecting kidney health is complex and poorly understood. When writing this review, the authors were reminded of an important research principle that states; “association does not prove causation.” The most reasonable approach to understanding the complexities of human physiology is a holistic view that acknowledges nature’s intrinsic and intricate systems of checks and balances designed to restore and maintain the health of living organisms.
Outdated research contributes to confusion about soluble and insoluble oxalate content in raw and cooked foods. The current data comes from agricultural research but creates an opportunity for misinterpretation due to the influence of cultivars, climate, and location of specific foods on oxalate levels. Traditional cultivation, harvesting, preparation, and combination of foods can reduce excessive risks, with seasonal dietary practices and a synergistic combination of co-factors that bind and remove oxalates from the body. Specific co-factors that bind or inhibit oxalate content in foods (calcium, acetic and malic acid, potassium, magnesium, vitamin C, fatty acids, fiber, and probiotics) lower the risks of urolithiasis and overall inflammatory effects on kidneys. Age, gender (hormone production), and overall health play the most central roles in developing kidney inflammation/stones with overall health impacted by hydration and processed food consumption, especially those high in sugar (fructose).
For individuals with a family history of urolithiasis, oxalate foods may be eaten in moderation, provided multi-strain probiotic and absorbable calcium intake are adequate to decrease absorption of oxalates. A holistic dietary approach that includes adequate hydration, diversity of whole unrefined foods, cooked and fermented fruits and vegetables, with lifestyle coaching, can be effective strategies to reduce the uptake of oxalates in predisposed individuals.
Figure 1.
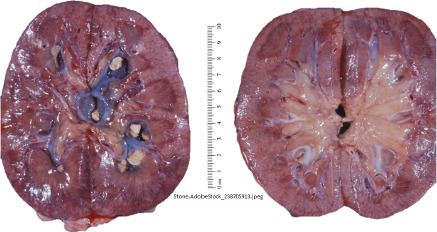
Kidney Stone
Figure 2.

Oxalate Metabolisum
Figure 3.

Oxalate content of food
Figure 4.
Flow images
Figure 5.

Wheel of Wellness and Longevity
References
- 1.Noonan SC, Savage GP. Oxalate content of foods and its effect on humans. Asia Pac J Clin Nutr. 1999;8(1):64-74. doi:10.1046/j.1440-6047.1999.00038.x [PubMed] [Google Scholar]
- 2.Alelign T, Petros B. Kidney Stone Disease: An Update on Current Concepts. Adv Urol. 2018;2018:3068365. doi:10.1155/2018/3068365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell T, Kumar P, Reddy T, et al. Dietary oxalate and kidney stone formation. Am J Physiol Renal Physiol. 2019;316(3):F409-F413. doi:10.1152/ajprenal.00373.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ermer T, Eckardt KU, Aronson PS, Knauf F. Oxalate, inflammasome, and progression of kidney disease. Curr Opin Nephrol Hypertens. 2016;25(4):363-371. doi:10.1097/MNH.0000000000000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knight J, Madduma-Liyanage K, Mobley JA, Assimos DG, Holmes RP. Ascorbic acid intake and oxalate synthesis. Urolithiasis. 2016;44(4):289-297. doi:10.1007/s00240-016-0868-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stones K. John Hopkins Medicine. Accessed February 12, 2024. https://www.hopkinsmedicine.org/health/conditions-and-diseases/kidney-stones
- 7.Pizzorno J, Murray MT, Joiner-Bey H. The Clinician’s Handbook of Natural Medicine. 3rd ed. Elsevier, Inc; 2016. [Google Scholar]
- 8.Huang Y, Zhang YH, Chi ZP, et al. The Handling of Oxalate in the Body and the Origin of Oxalate in Calcium Oxalate Stones. Urol Int. 2020;104(3-4):167-176. doi:10.1159/000504417 [DOI] [PubMed] [Google Scholar]
- 9.Chai W, Liebman M. Effect of different cooking methods on vegetable oxalate content. J Agric Food Chem. 2005;53(8):3027-3030. doi:10.1021/jf048128d [DOI] [PubMed] [Google Scholar]
- 10.Martin-Higueras C, Ludwig-Portugall I, Hoppe B, Kurts C. Targeting kidney inflammation as a new therapy for primary hyperoxaluria? Nephrol Dial Transplant. 2019;34(6):908-914. doi:10.1093/ndt/gfy239 [DOI] [PubMed] [Google Scholar]
- 11.Trinchieri A. Diet and renal stone formation. Minerva Med. 2013;104(1):41-54. [PubMed] [Google Scholar]
- 12.Yilmaz MI, Carrero JJ, Axelsson J, Lindholm B, Stenvinkel P. Low-grade inflammation in chronic kidney disease patients before the start of renal replacement therapy: sources and consequences. Clin Nephrol. 2007;68(1):1-9. doi:10.5414/CNP68001 [DOI] [PubMed] [Google Scholar]
- 13.Ticinesi A, Nouvenne A, Chiussi G, Castaldo G, Guerra A, Meschi T. Calcium Oxalate Nephrolithiasis and Gut Microbiota: Not just a Gut-Kidney Axis. A Nutritional Perspective. Nutrients. 2020;12(2):548. doi:10.3390/nu12020548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maisto M, Schiano E, Luccheo G, et al. Efficacy of a Multicomponent Nutraceutical Formulation for the Prevention and Treatment of Urinary Tract Stones. Int J Mol Sci. 2023;24(9):8316. doi:10.3390/ijms24098316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambruzs JM, Larsen CP. Renal Manifestations of Inflammatory Bowel Disease. Rheum Dis Clin North Am. 2018;44(4):699-714. doi:10.1016/j.rdc.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Pollok R, Goldsmith D. Renal and Urological Disorders Associated With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2023;29(8):1306-1316. doi:10.1093/ibd/izac140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Zhang YH, Chi ZP, et al. The Handling of Oxalate in the Body and the Origin of Oxalate in Calcium Oxalate Stones. Urol Int. 2020;104(3-4):167-176. doi:10.1159/000504417 [DOI] [PubMed] [Google Scholar]
- 18.Liebman M, Al-Wahsh IA. Probiotics and other key determinants of dietary oxalate absorption. Adv Nutr. 2011;2(3):254-260. doi:10.3945/an.111.000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Jin X, Hong HG, et al. The relationship between gut microbiota and short chain fatty acids in the renal calcium oxalate stones disease. FASEB J. 2020;34(8):11200-11214. doi:10.1096/fj.202000786R [DOI] [PubMed] [Google Scholar]
- 20.Daniel SL, Moradi L, Paiste H, et al. Forty Years of Oxalobacter formigenes, a Gutsy Oxalate-Degrading Specialist. Appl Environ Microbiol. 2021;87(18):e0054421. doi:10.1128/AEM.00544-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamberlain CA, Hatch M, Garrett TJ. Oxalobacter formigenes produces metabolites and lipids undetectable in oxalotrophic Bifidobacterium animalis. Metabolomics. 2020;16(12):122. doi:10.1007/s11306-020-01747-2 [DOI] [PubMed] [Google Scholar]
- 22.Tavasoli S, Alebouyeh M, Naji M, et al. Association of intestinal oxalate-degrading bacteria with recurrent calcium kidney stone formation and hyperoxaluria: a case-control study. BJU Int. 2020;125(1):133-143. doi:10.1111/bju.14840 [DOI] [PubMed] [Google Scholar]
- 23.Morrow K, Raymond JL. Krause and Mahan’s Food and the Nutrition Care Process. 16th ed. Elsevier; 2023. [Google Scholar]
- 24.Liu M, Zhang Y, Wu J, Gao M, Zhu Z, Chen H. Causal relationship between kidney stones and gut microbiota contributes to the gut-kidney axis: a two-sample Mendelian randomization study. Front Microbiol. 2023;14:1204311. doi:10.3389/fmicb.2023.1204311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozenfeld J, Tal O, Kladnitsky O, et al. The pendrin anion exchanger gene is transcriptionally regulated by uroguanylin: a novel enterorenal link. Am J Physiol Renal Physiol. 2012;302(5):F614-F624. doi:10.1152/ajprenal.00189.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Biotechnology Information. PubChem Pathway Summary for Pathway R-HSA-427601, Multifunctional anion exchangers, Source: Reactome. Published 2019. Accessed February 12, 2024. https://pubchem.ncbi.nlm.nih.gov/pathway/Reactome:R-HSA-427601
- 27.Wang X, Zhang J, Ma Z, et al. Association and interactions between mixed exposure to trace elements and the prevalence of kidney stones: a study of NHANES 2017-2018. Front Public Health. 2023;11:1251637. doi:10.3389/fpubh.2023.1251637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu W, Wang C, Wu J, et al. Dietary copper intake and the prevalence of kidney stones among adult in the United States: A propensity score matching study. Front Public Health. 2022;10:973887. doi:10.3389/fpubh.2022.973887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negri AL. The role of zinc in urinary stone disease. Int Urol Nephrol. 2018;50(5):879-883. doi:10.1007/s11255-017-1784-7 [DOI] [PubMed] [Google Scholar]
- 30.Stamatelou K, Goldfarb DS. Epidemiology of Kidney Stones. Healthcare (Basel). 2023;11(3):424. doi:10.3390/healthcare11030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang X, Li N, Kang L, et al. Chronic low level trimethyltin exposure and the risk of developing nephrolithiasis. Occup Environ Med. 2013;70(8):561-567. doi:10.1136/oemed-2012-101261 [DOI] [PubMed] [Google Scholar]
- 32.Stamatelou K, Goldfarb DS. Epidemiology of Kidney Stones. Healthcare (Basel). 2023;11(3):424. doi:10.3390/healthcare11030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rostami Dovom M, Rahmati M, Amanollahi Soudmand S, Ziaeefar P, Azizi F, Ramezani Tehrani F. The Hidden Link between Polycystic Ovary Syndrome and Kidney Stones: Finding from the Tehran Lipid and Glucose Study (TLGS). Diagnostics (Basel). 2023;13(17):2814. doi:10.3390/diagnostics13172814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Zhang Y, Zhang J, Deng Q, Liang H. Recent advances on the mechanisms of kidney stone formation (Review). [Review]. Int J Mol Med. 2021;48(2):149. doi:10.3892/ijmm.2021.4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill AJ, Basourakos SP, Lewicki P, et al. Incidence of Kidney Stones in the United States: The Continuous National Health and Nutrition Examination Survey. J Urol. 2022;207(4):851-856. doi:10.1097/JU.0000000000002331 [DOI] [PubMed] [Google Scholar]
- 36.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2):12. doi:10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson RJ, Perez-Pozo SE, Lillo JL, et al. Fructose increases risk for kidney stones: potential role in metabolic syndrome and heat stress. BMC Nephrol. 2018;19(1):315. doi:10.1186/s12882-018-1105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen MD, Chi T, Shara NM, et al. Activity, energy intake, obesity, and the risk of incident kidney stones in postmenopausal women: a report from the Women’s Health Initiative. J Am Soc Nephrol. 2014;25(2):362-369. doi:10.1681/ASN.2013050548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, Tan J, Geng E, et al. Impact of body mass index on size and composition of urinary stones: a systematic review and meta-analysis. Int Braz J Urol. 2023;49(3):281-298. doi:10.1590/s1677-5538.ibju.2022.0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W, Zhao Z, Chou F, et al. Loss of the androgen receptor suppresses intrarenal calcium oxalate crystals deposition via altering macrophage recruitment/M2 polarization with change of the miR-185-5p/CSF-1 signals. Cell Death Dis. 2019;10(4):275. doi:10.1038/s41419-019-1358-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang F, Li Y, Cui Y, et al. Relationship Between Serum Testosterone Levels and Kidney Stones Prevalence in Men. Front Endocrinol (Lausanne). 2022;13:863675. doi:10.3389/fendo.2022.863675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elshal AM, Shamshoun H, Awadalla A, et al. Hormonal and molecular characterization of calcium oxalate stone formers predicting occurrence and recurrence. Urolithiasis. 2023;51(1):76. doi:10.1007/s00240-023-01440-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beer MH, Porter RS, Jones TV. The Merck Manual. 18th ed. Wiley–Blackwell; 2006. [Google Scholar]
- 44.Pizzorno J, Murray MT, Joiner-Bey H. The Clinician’s Handbook of Natural Medicine. 3rd ed. Elsevier, Inc; 2016. [Google Scholar]
- 45.Donald V, Thomas CL, eds. Taber’s Cyclopedic Medical Dictionary. 20th ed. 2005:1876-1877. [Google Scholar]
- 46.Kever J. Researchers propose new treatment to prevent kidney stones: Modifier appears to dissolve crystals of the most common kidney stone. University of Houston. Published 2016. Accessed February 12, 2024. https://www.uh.edu/news-events/stories/2016/July/08082016New-Treatment-for-Kidney-Stones.php#:~:text=The%20work%20offers%20the%20first,also%20explain%20how%20it%20works [Google Scholar]
- 47.Wołyniec W, Szwarc A, Kasprowicz K, et al. Impact of hydration with beverages containing free sugars or xylitol on metabolic and acute kidney injury markers after physical exercise. Front Physiol. 2022;13:841056. doi:10.3389/ fphys.2022.841056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferraro PM, Bargagli M. Dietetic and lifestyle recommendations for stone formers [published correction appears in Arch Esp Urol. 2021 Oct;74(8):725-726]. Consejos dietéticos y de estilo de vida en pacientes con litiasis urinarias [published correction appears in Arch Esp Urol. 2021 Oct;74(8):725-726]. Arch Esp Urol. 2021;74(1):112-122. [PubMed] [Google Scholar]
- 49.Liu Y, Jin X, Ma Y, et al. Short-Chain Fatty Acids Reduced Renal Calcium Oxalate Stones by Regulating the Expression of Intestinal Oxalate Transporter SLC26A6. mSystems. 2021;6(6):e0104521. doi:10.1128/mSystems.01045-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin X, Jian Z, Chen X, et al. Short Chain Fatty Acids Prevent Glyoxylate-Induced Calcium Oxalate Stones by GPR43-Dependent Immunomodulatory Mechanism. Front Immunol. 2021;12:729382. doi:10.3389/fimmu.2021.729382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2(12):1266-1289. doi:10.3390/nu2121266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferraro PM, Bargagli M, Trinchieri A, Gambaro G. Risk of Kidney Stones: Influence of Dietary Factors, Dietary Patterns, and Vegetarian-Vegan Diets. Nutrients. 2020;12(3):779. doi:10.3390/nu12030779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferraro PM, Bargagli M, Trinchieri A, Gambaro G. Risk of Kidney Stones: Influence of Dietary Factors, Dietary Patterns, and Vegetarian-Vegan Diets. Nutrients. 2020;12(3):779. doi:10.3390/nu12030779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petroski W, Minich DM. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients. 2020;12(10):2929. Published 2020 Sep 24. doi:10.3390/nu12102929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parizadeh M, Arrieta MC. The global human gut microbiome: genes, lifestyles, and diet. Trends Mol Med. 2023;29(10):789-801. doi:10.1016/j.molmed.2023.07.002 [DOI] [PubMed] [Google Scholar]
- 56.Gundogdu A, Nalbantoglu OU. The role of the Mediterranean diet in modulating the gut microbiome: A review of current evidence. Nutrition. 2023;114:112118. doi:10.1016/j.nut.2023.112118 [DOI] [PubMed] [Google Scholar]
- 57.Liebman M, Al-Wahsh IA. Probiotics and other key determinants of dietary oxalate absorption. Adv Nutr. 2011;2(3):254-260. doi:10.3945/an.111.000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liebman M, Al-Wahsh IA. Probiotics and other key determinants of dietary oxalate absorption. Adv Nutr. 2011;2(3):254-260. doi:10.3945/an.111.000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Vitamin B6 intake and the risk of incident kidney stones. Urolithiasis. 2018;46(3):265-270. doi:10.1007/s00240-017-0999-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barghouthy Y, Corrales M, Somani B. The Relationship between Modern Fad Diets and Kidney Stone Disease: A Systematic Review of Literature. Nutrients. 2021;13(12):4270. doi:10.3390/nu13124270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fargue S, Rumsby G, Danpure CJ. Multiple mechanisms of action of pyridoxine in primary hyperoxaluria type 1. Biochim Biophys Acta. 2013;1832(10):1776-1783. doi:10.1016/j.bbadis.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 62.Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Vitamin B6 intake and the risk of incident kidney stones. Urolithiasis. 2018;46(3):265-270. doi:10.1007/s00240-017-0999-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di XP, Gao XS, Xiang LY, Wei X. The association of dietary intake of riboflavin and thiamine with kidney stone: a cross-sectional survey of NHANES 2007. BMC Public Health. 2023;23(1):964. Published. 2018;2023(May):26. doi:10.1186/s12889-023-15817-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferraro PM, Taylor EN, Gambaro G, Curhan GC, Vitamin D. Vitamin D Intake and the Risk of Incident Kidney Stones. J Urol. 2017;197(2):405-410. doi:10.1016/j.juro.2016.08.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ala-Opas M, Elomaa I, Porkka L, Alfthan O. Unprocessed bran and intermittent thiazide therapy in prevention of recurrent urinary calcium stones. Scand J Urol Nephrol. 1987;21(4):311-314. doi:10.3109/00365598709180789 [DOI] [PubMed] [Google Scholar]
- 66.Ohkawa T, Ebisuno S, Kitagawa M, Morimoto S, Miyazaki Y, Yasukawa S. Rice bran treatment for patients with hypercalciuric stones: experimental and clinical studies. J Urol. 1984;132(6):1140-1145. doi:10.1016/S0022-5347(17)50065-8 [DOI] [PubMed] [Google Scholar]
- 67.Etienne A, Génard M, Lobit P, Mbeguié-A-Mbéguié D, Bugaud C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J Exp Bot. 2013;64(6):1451-1469. doi:10.1093/jxb/ert035 [DOI] [PubMed] [Google Scholar]
- 68.Kever J. Researchers propose new treatment to prevent kidney stones: Modifier appears to dissolve crystals of the most common kidney stone. University of Houston. Published 2016. Accessed February 12, 2024. https://www.uh.edu/news-events/stories/2016/July/08082016New-Treatment-for-Kidney-Stones.php#:~:text=The%20work%20offers%20the%20first,also%20explain%20how%20it%20works [Google Scholar]
- 69.Kim D, Rimer JD, Asplin JR. Hydroxycitrate: a potential new therapy for calcium urolithiasis. Urolithiasis. 2019;47(4):311-320. doi:10.1007/s00240-019-01125-1 [DOI] [PubMed] [Google Scholar]
- 70.Dreher ML. Dietary Fiber in Health and Disease. Nutrition and Health. In: Fiber-Rich Diets in Chronic Kidney Disease. Humana Press;. 2018, doi:10.1007/978-3-319-50557-2_15. [Google Scholar]
- 71.Zhao J, Huang Y, Yu X. Caffeine intake and the risk of incident kidney stones: a systematic review and meta-analysis. Int Urol Nephrol. 2022;54(10):2457-2466. doi:10.1007/s11255-022-03295-1 [DOI] [PubMed] [Google Scholar]
- 72.Yuan S, Larsson SC. Coffee and Caffeine Consumption and Risk of Kidney Stones: A Mendelian Randomization Study. Am J Kidney Dis. 2022;79(1):9-14.e1. doi:10.1053/j.ajkd.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 73.Ferraro PM, Curhan GC. More Good News: Coffee Prevents Kidney Stones. Am J Kidney Dis. 2022;79(1):3-4. doi:10.1053/j.ajkd.2021.07.002 [DOI] [PubMed] [Google Scholar]
- 74.Mazidi M, Mikhailidis DP, Dehghan A, et al. The association between coffee and caffeine consumption and renal function: insight from individual-level data, Mendelian randomization, and meta-analysis. Arch Med Sci. 2021;18(4):900-911. doi:10.5114/aoms/144905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geng J, Qiu Y, Kang Z, et al. The association between caffeine intake and risk of kidney stones: A population-based study. Front Nutr. 2022;9:935820. doi:10.3389/fnut.2022.935820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barghouthy Y, Corrales M, Doizi S, Somani BK, Traxer O. Tea and coffee consumption and pathophysiology related to kidney stone formation: a systematic review. World J Urol. 2021;39(7):2417-2426. doi:10.1007/s00345-020-03466-8 [DOI] [PubMed] [Google Scholar]
- 77.Siener R, Hesse A. Effect of Black Tea Consumption on Urinary Risk Factors for Kidney Stone Formation. Nutrients. 2021;13(12):4434. doi:10.3390/nu13124434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rode J, Bazin D, Dessombz A, et al. Daily Green Tea Infusions in Hypercalciuric Renal Stone Patients: No Evidence for Increased Stone Risk Factors or Oxalate-Dependent Stones. Nutrients. 2019;11(2):256. doi:10.3390/nu11020256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ceccanti C, Brizzi A, Landi M, Incrocci L, Pardossi A, Guidi L. Evaluation of Major Minerals and Trace Elements in Wild and Domesticated Edible Herbs Traditionally Used in the Mediterranean Area. Biol Trace Elem Res. 2021;199(9):3553-3561. doi:10.1007/s12011-020-02467-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li S, Macaringue EGJ, Zhou D, Shi P, Tang W, Gong J. Discovering inhibitor molecules for pathological crystallization of CaOx kidney stones from natural extracts of medical herbs. J Ethnopharmacol. 2022;284:114733. doi:10.1016/j.jep.2021.114733 [DOI] [PubMed] [Google Scholar]
- 81.Huang J, Huang C, Liebman M. Oxalate contents of commonly used Chinese medicinal herbs. J Tradit Chin Med. 2015;35(5):594-599. doi:10.1016/S0254-6272(15)30145-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Assimos DG. Re: Oxalate Contents of Commonly Used Chinese Medicinal Herbs. J Urol. 2016;196(1):137-138. doi:10.1016/j.juro.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 83.Emiliani E, Jara A, Kanashiro AK. Phytotherapy and Herbal Medicines for Kidney Stones. Curr Drug Targets. 2021;22(1):22-30. doi:10.2174/1389450121666200929115555 [DOI] [PubMed] [Google Scholar]
- 84.George S, McCann N. Expert Digs Deep Into Research of Kidney Stones Causes and Treatments. Children’s Hospital of Philadelphia. Published 2020. Accessed February 12, 2024. https://www.research.chop.edu/cornerstone-blog/expert-digs-deep-into-research-of-kidney-stones-causes-and-treatments [Google Scholar]
- 85.Huang JJ, Yao XP, Zhang P, et al. Curcumin alleviated oxidation stress injury by mediating osteopontin in nephrolithiasis rats. Acta Cir Bras. 2023;38:e380223. doi:10.1590/acb380223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prezioso D, Strazzullo P, Lotti T, et al. Dietary treatment of urinary risk factors for renal stone formation. A review of CLU Working Group [published correction appears in Arch Ital Urol Androl. 2016. Mar;88(1):76. [DOI] [PubMed] [Google Scholar]
- 87.Hong SY, Qin BL. The Protective Role of Dietary Polyphenols in Urolithiasis: Insights into Antioxidant Effects and Mechanisms of Action. Nutrients. 2023;15(17):3753. doi:10.3390/nu15173753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dang YKT, Nguyen HVH. Effects of Maturity at Harvest and Fermentation Conditions on Bioactive Compounds of Cocoa Beans. Plant Foods Hum Nutr. 2019;74(1):54-60. doi:10.1007/s11130-018-0700-3 [DOI] [PubMed] [Google Scholar]
- 89.Petroski W, Minich DM. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients. 2020;12(10):2929. doi:10.3390/nu12102929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.López-Martínez LX, Leyva-López N, Gutiérrez-Grijalva EP, Heredia J B. Effect of cooking and germination on bioactive compounds in pulses and their health benefits. Journal of Functional Foods. 2017;38(Part B):624-634. doi:10.1016/j.jff.2017.03.002 [Google Scholar]
- 91.Savage G, Vanhanen L. Oxalate Contents of Raw, Boiled, Wok-Fried and Pesto and Juice Made from Fat Hen (Chenopodium album) Leaves. Foods. 2018;8(1):2. doi:10.3390/foods8010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park KY, Jeong JK, Lee YE, Daily JW, III. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J Med Food. 2014;17(1):6-20. doi:10.1089/jmf.2013.3083 [DOI] [PubMed] [Google Scholar]
- 93.White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets--iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182(1):49-84. doi:10.1111/j.1469-8137.2008.02738.x [DOI] [PubMed] [Google Scholar]
- 94.Quinteros A, Farré R, Lagarda MJ. Effect of cooking on oxalate content of pulses using an enzymatic procedure. Int J Food Sci Nutr. 2003;54(5):373-377. doi:10.1080/09637480310001595270 [DOI] [PubMed] [Google Scholar]
- 95.Dhaliwal SS, Sharma V, Shukla AK, et al. Biofortification-A Frontier Novel Approach to Enrich Micronutrients in Field Crops to Encounter the Nutritional Security. Molecules. 2022;27(4):1340. doi:10.3390/molecules27041340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kihara J, Bolo P, Kinyua M, Rurinda J, Piikki K. Micronutrient deficiencies in African soils and the human nutritional nexus: opportunities with staple crops. Environ Geochem Health. 2020;42(9):3015-3033. doi:10.1007/s10653-019-00499-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salgado N, Silva MA, Figueira ME, Costa HS, Albuquerque TG. Oxalate in Foods: Extraction Conditions, Analytical Methods, Occurrence, and Health Implications. Foods. 2023;12(17):3201. doi:10.3390/foods12173201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brigide P, de Toledo NMV, López-Nicolás R, Ros G, Frontela Saseta C, de Carvalho RV. Fe and Zn in vitro bioavailability in relation to antinutritional factors in biofortified beans subjected to different processes. Food Funct. 2019;10(8):4802-4810. doi:10.1039/C9FO00199A [DOI] [PubMed] [Google Scholar]
- 99.Wadamori Y, Vanhanen L, Savage GP. Effect of Kimchi Fermentation on Oxalate Levels in Silver Beet (Beta vulgaris var. cicla). Foods. 2014;3(2):269-278. Published 2014 Apr 23. doi:10.3390/foods3020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park KY, Jeong JK, Lee YE, Daily JW, III. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J Med Food. 2014;17(1):6-20. doi:10.1089/jmf.2013.3083 [DOI] [PubMed] [Google Scholar]
- 101.Suma PF, Urooj A. Influence of germination on bioaccessible iron and calcium in pearl millet (Pennisetum typhoideum). J Food Sci Technol. 2014;51(5):976-981. doi:10.1007/s13197-011-0585-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi L, Arntfield SD, Nickerson M. Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res Int. 2018;107:660-668. doi:10.1016/j.foodres.2018.02.056 [DOI] [PubMed] [Google Scholar]
- 103.Chai W, Liebman M. Effect of different cooking methods on vegetable oxalate content. J Agric Food Chem. 2005;53(8):3027-3030. doi:10.1021/jf048128d [DOI] [PubMed] [Google Scholar]
- 104.Makinde FM, Akinoso R. Comparison between the nutritional quality of flour obtained from raw, roasted and fermented sesame (Sesamum indicum L.) seed grown in Nigeria. Acta Sci Pol Technol Aliment. 2014;13(3):309-319. doi:10.17306/J.AFS.2014.3.9 [DOI] [PubMed] [Google Scholar]
- 105.Gerardi G, Casali CI, Cavia-Saiz M, et al. Bioavailable wine pomace attenuates oxalate-induced type II epithelial mesenchymal transition and preserve the differentiated phenotype of renal MDCK cells. Heliyon. 2020;6(11):e05396. doi:10.1016/j.heliyon.2020.e05396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Migliori M, Panichi V, de la Torre R, et al. Anti-inflammatory effect of white wine in CKD patients and healthy volunteers. Blood Purif. 2015;39(1-3):218-223. doi:10.1159/000371570 [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Jin X, Ma Y, Sun Q, Li H, Wang K. Vinegar reduced renal calcium oxalate stones by regulating acetate metabolism in gut microbiota and crystal adhesion in rats. Int Urol Nephrol. 2022;54(10):2485-2495. doi:10.1007/s11255-022-03259-5 [DOI] [PubMed] [Google Scholar]
- 108.Gerardi G, Casali CI, Cavia-Saiz M, et al. Bioavailable wine pomace attenuates oxalate-induced type II epithelial mesenchymal transition and preserve the differentiated phenotype of renal MDCK cells. Heliyon. 2020;6(11):e05396. doi:10.1016/j.heliyon.2020.e05396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu W, Liu Y, Lan Y, et al. Dietary vinegar prevents kidney stone recurrence via epigenetic regulations. EBioMedicine. 2019;45:231-250. doi:10.1016/j.ebiom.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.The 173 high oxalate foods and products: by content of oxalate. Accessed February 13, 2024. https://docs.google.com/spreadsheets/d/1p4YNGC5ybKyt8Kr1ovG_YVTYf1Hn3Z8lyP-f7-icuBg/edit?pli=1#gid=0
- 111.Garland V, Herlitz L, Regunathan-Shenk R. Diet-induced oxalate nephropathy from excessive nut and seed consumption. BMJ Case Rep. 2020;13(11):e237212. doi:10.1136/bcr-2020-237212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cortez D, Quispe-Sanchez L, Mestanza M, et al. Changes in bioactive compounds during fermentation of cocoa (Theobroma cacao) harvested in Amazonas-Peru. Curr Res Food Sci. 2023;6:100494. doi:10.1016/j.crfs.2023.100494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lan Y, Zhu W, Duan X, et al. Glycine suppresses kidney calcium oxalate crystal depositions via regulating urinary excretions of oxalate and citrate. J Cell Physiol. 2021;236(10):6824-6835. doi:10.1002/jcp.30370 [DOI] [PubMed] [Google Scholar]
- 114.Bong WC, Savage G. Oxalate content of raw, wok-fried, and juice made from bitter gourd fruits. Food Sci Nutr. 2018;6(8):2015-2019. doi:10.1002/fsn3.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vanhanen L, Savage G. Comparison of oxalate contents and recovery from two green juices prepared using a masticating juicer or a high speed blender. NFS J. 2015;1:20-23. doi:10.1016/j.nfs.2015.07.002 [Google Scholar]
- 116.Getting JE, Gregoire JR, Phul A, Kasten MJ. Oxalate nephropathy due to ‘juicing’: case report and review. Am J Med. 2013;126(9):768-772. doi:10.1016/j.amjmed.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 117.Lubem TR, Omenka AJ, Mchihi MM, Atsar FS. Effect of blanching time on oxalate and phytate content of non-conventional local vegetables in Benue State, Nigeria Tyohemba. FUW Trends in Science & Technology Journal. 2019;4(2):413-415. [Google Scholar]
- 118.Radek M, Savage GP. Oxalates in some Indian green leafy vegetables. Int J Food Sci Nutr. 2008;59(3):246-260. doi:10.1080/09637480701791176 [DOI] [PubMed] [Google Scholar]
- 119.Durham S. Making Spinach with Low Oxalate Levels. US Department of Agriculture. Published 2017. Accessed February 13, 2024. https://agresearchmag.ars.usda.gov/2017/jan/spinach/
- 120.Massey LK. Dietary influences on urinary oxalate and risk of kidney stones. Front Biosci. 2003;8(6):s584-s594. doi:10.2741/1082 [DOI] [PubMed] [Google Scholar]
- 121.Liu M, Nazzal L. Enteric hyperoxaluria: role of microbiota and antibiotics. Curr Opin Nephrol Hypertens. 2019;28(4):352-359. doi:10.1097/MNH.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 122.Ramírez-Rodríguez Y, Martínez-Huélamo M, Pedraza-Chaverri J, Ramírez V, Martínez-Tagüeña N, Trujillo J. Ethnobotanical, nutritional and medicinal properties of Mexican drylands Cactaceae Fruits: recent findings and research opportunities. Food Chem. 2020;312:126073. doi:10.1016/j.foodchem.2019.126073 [DOI] [PubMed] [Google Scholar]
- 123.Rakel D, ed. Integrative Medicine. 3rd ed. Saunders; 2012:544-548. [Google Scholar]
- 124.Stones K. Kidney Care UK. Accessed: February 13, 2024. https://www.kidneycareuk.org/about-kidney-health/conditions/kidney-stones/
- 125.To Affirm or Not Affirm. 2BWELL. Accessed Februaty 13, 2024. https://2bwell.solutions/to-affirm-or-not-to-affirm
- 126.Guptha LS. To Affirm or Not Affirm? Psychology Today. Published 2017. Accessed February 13, 2024. https://www.psychologytoday.com/us/blog/embodied-wellness/201704/affirm-or-not-affirm
- 127.Kidney Health Coach. American Kidney Fund. Accessed February 13, 2024. https://www.kidneyfund.org/get-involved/kidney-health-coach


