Abstract
Vehicular whole-body vibration (WBV) can have long-term adverse effects on human quality of life. Animal models can be used to study pathophysiologic effects of vibration. The goal of this study was to assess animal cooperation and well-being to determine the feasibility of a novel seated rat model for investigating the effects of WBV on biologic systems. Twenty-four male Sprague–Dawley rats were used. The experiment consisted of an acclimation phase, 2 training phases (TrP1 and TrP2), and a testing phase (TeP), including weekly radiographic imaging. During acclimation, rats were housed in pairs in standard cages without vibration. First, experimental (EG; n = 18) and control group 1 (C1; n = 3) rats were placed in a vibration apparatus without vibration, with increasing duration over 5 d during TrP1. EG rats were exposed to vertical random WBV that was increased in magnitude over 5 d during TrP2 until reaching the vibration signal used during TeP (15 min, 0.7 m·s−2 root mean square, unweighted). C1 rats were placed in the vibration apparatus but received no vibration during any phase. Control group 2 (C2; n = 3) rats remained in the home cages. Cooperation was evaluated with regard to rat-apparatus interactions and position compliance. Behavior, weight, and fecal glucocorticoid metabolite concentrations (fGCM) were used to evaluate animal well-being. We observed good cooperation and no behavioral patterns or weight loss between phases, indicating little or no animal stress. The differences in fGCM concentration between groups indicated that the EG rats had lower stress levels than the control rats in all phases except TrP1. Thus, this model elicited little or no stress in the conscious, unrestrained, seated rats.
Abbreviations and Acronyms: C1, control group 1; C2, control group 2; EG, experimental group; fGCM, fecal glucocorticoid metabolite; TeP, testing phase; TrP1, training phase one; TrP2, training phase 2; RMS, root mean square; WBV, whole-body vibration
Introduction
Vehicular whole-body vibration (WBV) exposure is part of daily life for most modern-day individuals, whether it involves vehicular operation work or commuting to work.16,21,49,50 A previous publication15 summarized studies that investigated whether occupations that expose persons to WBV caused injury, specifically to the lower back. Two other studies21,49 reviewed health disorders related to WBV and the epidemiologic and etiological aspects of low back pain in seated vibration environments, such as off-road vehicles, road vehicles, and aircraft, and found that most of these environments expose seated individuals to WBV.
Animals have been used to study the pathophysiologic effects of WBV. The vertebral anatomy and biomechanics of rats are similar to those of humans, and therefore, rats have been routinely used for studies of vertebral biomechanics.6,18,20,22,23,25,34,36,39,44,47,48,52,54 Most studies on the effects of WBV on the vertebral column involve using unrestrained rats placed on vibrating platforms, resulting in transverse distribution of vibration along the spine.10,30,31 Studies investigating the effects of sagittal vibration used animals that were either restrained in prone2,54,55 or supine33 positions or were standing upright.18
Models should be designed to represent as closely as possible the type of exposure that humans will experience. The current study considers the exposure of a seated individual to WBV. Although other positions might give some insights into the effects of WBV, exposure of a seated animal might more closely represent the seated human. Other than in studies that used subhuman primates,9,38,42,43 we are not aware of a study that used seated rats. We designed a seated model to allow the WBV to be transmitted more directly in the plane of the vertebral axis with less transverse force perpendicular to the vertebrae. The seated model also allows vibration to be applied directly to the spine without initial absorption of vibration force by the limbs.
Animal well-being and cooperation are important considerations with regard to animals used in research and could influence the practicality of the model. Factors that may affect animal well-being and cooperation include environmental stressors (such as noise, temperature, light, vibration, etc.), the apparatus that the animal is required to interact with, the position of the animal, level of consciousness, and the procedure(s) performed during the experiment. Previous studies have documented evidence of comfortable ranges for certain environmental conditions for rats; these include noise levels below 80 decibels,5,26 temperatures between 20 and 26 °C,33 and humidity between 30% and 70%.32 The goal of this study was to determine the feasibility of exposing a seated rat to WBV, based on animal cooperation (whether the rats remain seated) and well-being (whether rats experienced acute stress).
Materials and Methods
Ethical considerations.
Ethical approval was obtained from the University of Pretoria Animal Ethics Committee (AEC; approval no. 736/2020). All experimental protocols complied with the requirements of the University of Pretoria AEC.
Study sample group.
The study used the lowest number of rats needed for valid statistical analyses. A power analysis using G*Power version 3.1 indicated that the comparison of the means of repeated measurements with an effect size of 0.25, α = 0.05, and 1 – β = 0.80 would require 3 groups and 6 repeated measurements with a total sample size of 24.
The study therefore comprised 24 10-wk-old conventional male Sprague–Dawley rats (Rattus norvegicus). The rats used were bred by and obtained from the South African Vaccine Producers in Johannesburg, South Africa. With the use of random assignment, 3 rats were allocated to each of the 2 control groups (C1 and C2) and 18 were allocated to the experimental group (EG).
Animal housing.
Rats were housed in pairs at Onderstepoort Veterinary Animal Research Unit (OVARU) in the conventional rodent unit. Housing consisted of Techniplast1400U rat cages obtained from OVARU with ad libitum reverse osmosis water and rodent pellets purchased from EPOLin South Africa. The room was maintained between 22 and 24 °C with a relative humidity of 40% to 60%. The room was maintained on a 12:12-h light:dark cycle. Rats were housed on dust-free sawdust bedding with autoclaved tissues, cardboard, egg containers, and wooden sticks as enrichment as purchased by OVARU from a general pet store in Pretoria, South Africa.
Seated rat-vibration apparatus.
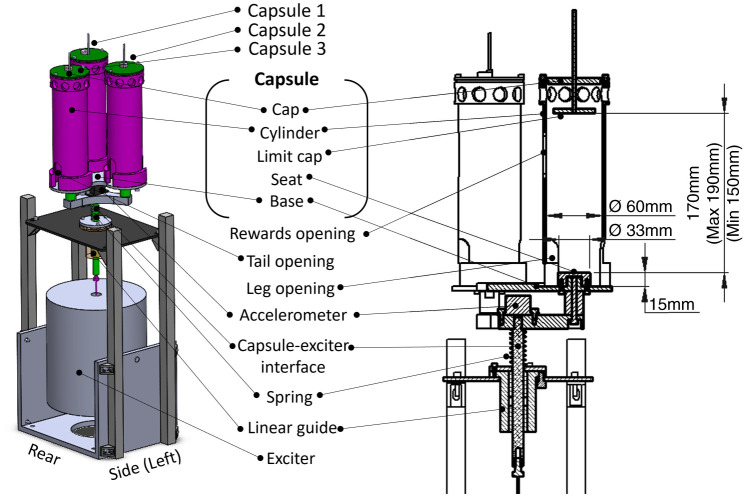
The seated rat vibration apparatus was designed to expose a seated rat to a desired vibration with a focus on animal cooperation and well-being. Figure 1 shows the apparatus used to expose a seated rat to the vibration apparatus, the individual components, and the main dimensions of the animal–apparatus interface. Rats are seated in capsules that consist of a base, cylinder, cap, and limit cap. All capsule components were manufactured from nylon polymer purchased from Maizey Plastics in South Africa. A vertical front face opening was used for offering the reward and for monitoring the rat, and 2 bottom openings accommodated the tail and legs and provided adequate ventilation. The seat had a diameter of 33 mm and a height of 15 mm. The vertically adjustable limit cap prevented the rats from standing upright. The capsules and base were suspended on a spring and constrained to move vertically by the linear guide. They were connected to an electrodynamic exciter (modal 50; MD Dynamics) via the capsule–exciter interface. The vibration signal was sent from the function generator (Helios PC/104 SBC; Diamond Systems) to the power amplifier (PA500L; LDS0) and then to the exciter. A triaxial accelerometer (CXL04GP3; MEMSIC) measured vibration in 3 translational directions. Data were recorded at a sample rate of 1 kHz on the data acquisition system (Helios PC/104 SBC; Diamond Systems).
Figure 1.
(A) Isometric view of seated rat vibration apparatus. (B) Sectioned view with the capsule’s main dimensions of importance with respect to the animal–apparatus interface.
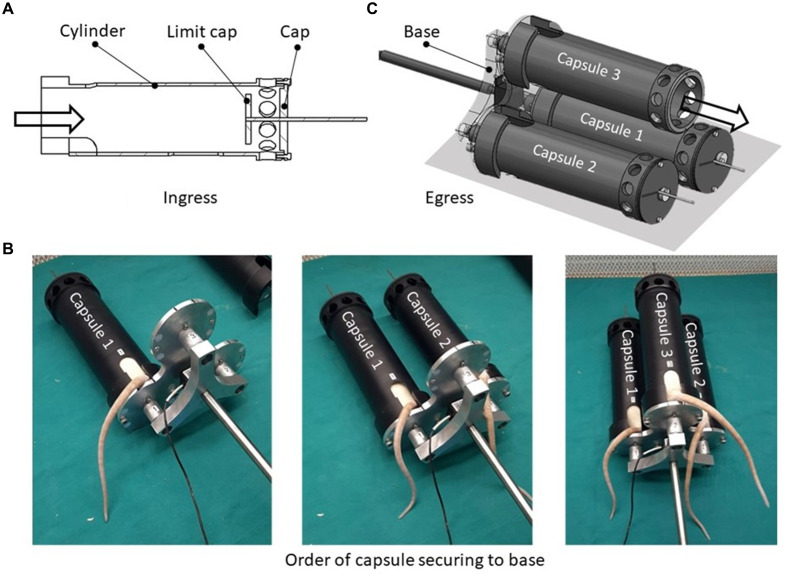
Placement into and removal of the rat from the capsules were performed horizontally. The rat was placed in the capsule from the side of the base with the cap and limit cap in place (Figure 2A). Once a rat was inside the capsule, the capsule was secured to the base while avoiding pinching the rat’s tail by holding it in the tail opening. After capsule 1 was secured, the next rat was loaded into capsule 2, followed by capsule 3. This loading process is shown in Figure 2B. Once the rats were in their respective capsules, the capsules with their shared base were connected to the exciter via the capsule–exciter interface. Each limit cap was adjusted so that it would deter rats from standing up on their hind legs yet avoid continual contact with the rat to prevent injury and/or influence effects on the rat biodynamics. Rats were removed from the capsule on the cap side, as rats are inclined to move forward (Figure 2C). Removal began with the rat in capsule 3 and ended with capsule 1. The design of the 3-capsule layout allowed access to feed and monitor for all 3 rats from the same side.
Figure 2.
(A) Capsule entry from side of base with cap and limit cap in place. (B) Images taken showing the order in which the capsules are secured to the base. (C) The cap and limit cap are removed allowing the rat to exit the capsule. The base remains in place. Exit begins with the rat in capsule 3 and ends with capsule 1.
Vibration.
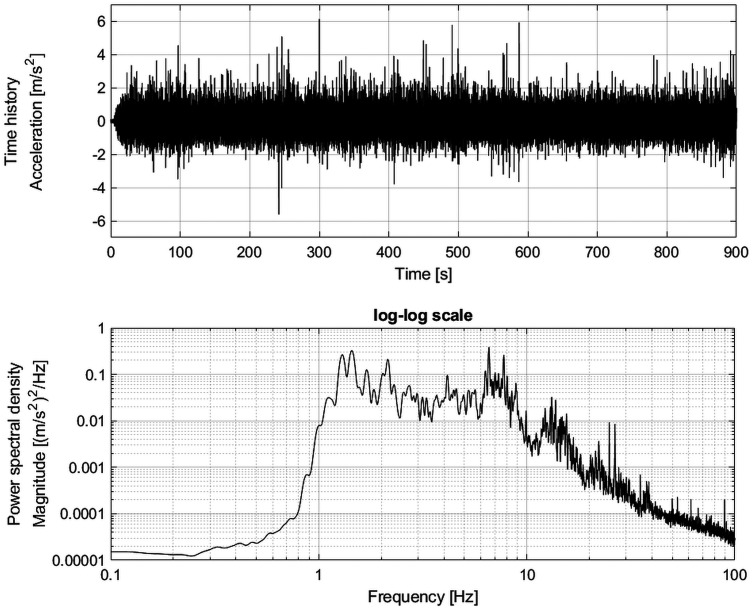
The vibration was based on vertical seat acceleration measured in a Range Rover Evoque eD4 Sports Utility Vehicle on a 4-poster test rig.13 The time history and power spectral density function of the acceleration of the capsules are shown in Figure 3. The magnitude of the vibration was quantified as the root mean square (RMS) of the unweighted acceleration time history a(t) with S number of samples (Equation 1). Another metric used for quantifying vibration severity is the fourth power vibration dose value (VDV; Equation 2). The VDV better accounts for the duration of vibration (Ts) compared with RMS14 The cumulative exposure to several acceleration time histories, N, is quantified by the cumulative VDV (VDVcum; Equation 3).
| (1) |
| (2) |
| (3) |
Figure 3.
Time history and power spectral density function of the unweighted capsule vibration [0.05-Hz frequency resolution, 126 df].
The vibration used in this study was applied for a duration of 15 min with a magnitude of 0.68 m·s−2, RMS and a VDV of 5.5 m·s−1.75 of the unweighted capsule acceleration (Figure 3). The vibration was weighted using the frequency weighting Wb3 to compare it to the ranges reported for different vehicle categories in another study.35 The frequency-weighted vibration magnitude of the vibration used in this study is 0.6 m·s2, RMS. Among the 14 vehicle categories considered in the aforementioned study35 weighted vertical vibration magnitude (in m·s−2, RMS) ranged as follows: cars, 0.16 to 0.78; vans (0.30 to 0.57); lorries (trucks), 0.33 to 1.04; tractors, 0.29 to 0.98; busses, 0.31 to 0.65; and dumpers (dump trucks), 0.54 to 1.29. The vibration used in the current study was near the upper end of the levels reported for cars, vans, and busses; midrange for lorries and tractors; and near the lower end for dumpers. Readers who are not familiar with the vibration concepts presented above can refer to the following.3,14
Procedure.
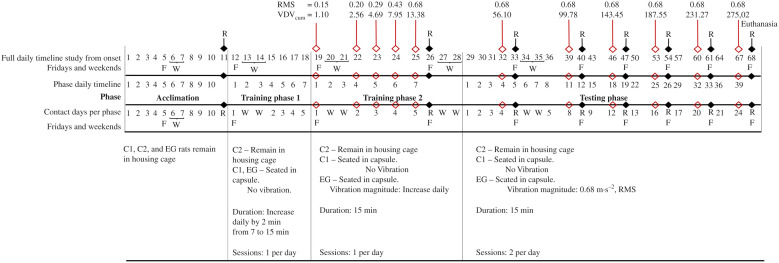
The experimental procedure consisted of a 10-d acclimation phase with radiographs taken on day 11, 2 training phases lasting a total of 14 d (with rest on the weekends), and a testing phase lasting 39 d (with radiographs but no vibration on Fridays and weekends for rest) (Figure 4). Testing began at the approximate age of 14 wk (average weight of all 3 groups of rats in the study: 338 ± 25 g). The rats were euthanized at the end of the testing phase at the age of 19 wk and 5 d by using an isoflurane overdose. The average weight of all 3 groups was 361 ± 25 g.
Figure 4.
Timeline indicating the 4 phases of the experiment. RMS, root mean square acceleration in m·s−2; VDVcum, cumulative vibration dose value in m·s−1.75; R, radiographic imaging day; F, Fridays; W, weekend (Saturday and Sunday); C1, control group 1; C2, control group 2; EG, experimental group.
During the 10-d acclimation phase, all rats remained in cages. An additional 14 d (including weekends) were used to accustom rats to the seated position in the capsule (for EG and C1 rats) with exposure to vibration for EG rats. Training was performed using a mixture of ad-lib reverse osmosis water, normal fat yogurt, and Bokomo ProNutro (a South-African powdered wheat-based porridge) as rewards. These can all be purchased at any general store in South Africa. These ingredients with a 1:1:1 ratio by volume in a bowl to produce approximately 300 mL. The mixture was offered to rats by using a 100-mL syringe.
In training period 1 (TrP1), rats were at first allowed to roam freely on a platform with the apparatus in proximity for a few minutes. They were then lured into the capsules by placing a small drop of the reward mixture at the far end inside the capsule. The bottom cap was positioned once the rats entered their respective capsules.
To familiarize the rats with being inside the capsules, they were exposed daily to the vibration apparatus by placing 3 of the EG rats at a time into capsules. The C1 rats were placed in separate, stationary capsules that were not transferred onto the vibration apparatus. This allowed the EG and C1 rats to be trained simultaneously. When rats assumed the correct position, they were positively reinforced with Cerelac (instant baby cereal), yogurt, or purity (puréed baby food) as rewards. The rats spent 7 min inside their capsule on day one. This duration was increased by 2 min each day for 5 d, resulting in a final seating time of 15 min (Figure 4). The rats were monitored continuously and rewarded each time they displayed correct seating or interfacing.
TrP2 began as soon as the rats appeared to be accustomed to being inside the capsules. TrP2 involved subjecting the EG rats to 15 min of vibration at 20% of the full signal. The vibration was subsequently increased daily over 6 d. The C1 rats also spent 15 min in their capsules but were not subjected to vibration. (Figure 4).
After the rats finished TrP2, they entered a 6-wk test phase. At this time, they were approximately 14 wk old. Rats were allocated to their groups of 3 each week based on their weights to ensure an overall similar average weight across groups. The C1 rats were placed in the static capsule on a separate table for 2 sets of 15-min sessions a day. The EG rats were exposed to 2 sets of 15-min WBV sessions a day. These 2 groups underwent the testing procedures daily for 24 d on every Monday to Thursday (39 d, including Fridays and weekends). On Fridays, rats were evaluated radiographically. The rats were closely monitored to ensure their well-being.
Radiographic imaging.
Evidence that WBV can affect the material properties of the spine (specifically the lumbar spine).11,50 Therefore, we included radiographic imaging to determine whether we had produced a realistic animal model for investigating the effects of WBV on the spine. Rats were anesthetized with sevoflurane for imaging. Imaging was performed weekly; rodents have a faster metabolic aging cycle than humans and could potentially show spinal effects from WBV faster than humans would.1,40
Animal cooperation and well-being.
Cooperation was evaluated based on how the rats interacted with the vibration apparatus and their willingness to remain in the desired position. The desired seating position was reinforced using food rewards (by Bokomo ProNutro and yogurt). Good cooperation was defined as remaining seated, with continual contact between the seat and the rat’s ischial surface. Contact between the seat and the hind legs (such as standing or crouching) was considered to be poor cooperation and was not rewarded. The seating position is important to ensure that the vibration is applied directly to the vertical plane of the spine and not absorbed by the limbs, thereby allowing the model to study possible effects of WBV on the spine.
Well-being was monitored using behavioral patterns, weight, and fecal glucocorticoid metabolite (fGCM) concentrations. Behavioral signs of stress included piloerection, excessive grooming, abnormal breathing, and porphyrin staining.17,37 Rats were weighed on arrival and once a week thereafter on Fridays.
Quantifying fGCM using enzyme immunoassay (EIA) techniques is often used to monitor responses to stressors in various animals.7,24,29,53 Fecal samples were collected in microfuge tubes twice daily (once in the morning during the daily weighing [AM] and once during afternoon rounds [PM]), Samples were collected and frozen at −10 to −20 °C immediately after defecation. Fecal samples were collected from individual rats while they were being handled.
For processing, samples were lyophilized, pulverized, and sifted using a mesh strainer.10 Then, 0.05 g of the fecal powder was extracted with 80% ethanol in water (3 mL) and measured for immunoreactive fCGM concentrations using a 5α-pregnane-3β,11β,21-triol-20-one EIA.28,45,46
Data and statistical analyses.
Weekly weights were compared with the previous week, with significant weight loss (10%) considered indicative of stress.8,27 Each rat served as its own baseline.
The fGCM concentrations were tested for normal distribution and equal variance using the Shapiro-Wilk and Levene tests, respectively. Normal distribution was obtained for all subsets, and subsequent analyses were conducted using parametric tests. The data indicated equal variance across groups of animals and phases. A repeated measures ANOVA test was used to test for differences between the 3 groups for each phase. If the ANOVA tests indicated that significant differences existed among groups, a Bonferroni post hoc test was used to identify the significant differences. Statistical analyses were conducted using IBM SPSS Statistics, Version 26.0 (IBM, Armonk, NY).
Results
Animal cooperation.
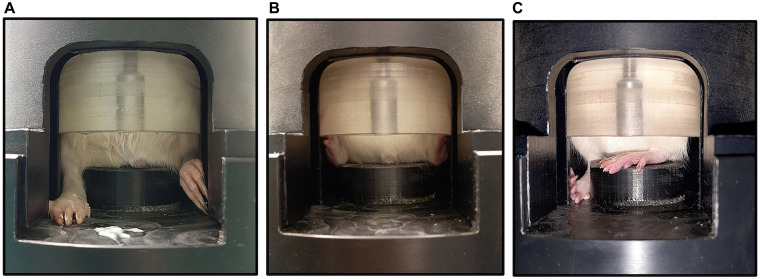
Rats acclimated almost immediately during each phase of training and experimentation and showed no behavioral signs of stress or discomfort. When the capsules were placed in front of them, they voluntarily entered with some yogurt as a lure initially and without lures later on. Rats left the capsules voluntarily during each phase after the capsule was placed on a table next to their cage after each session. All rats remained seated during all phases. The 60-mm inner diameter insert accommodated the rats for the duration of the study. Figure 5 shows observed hind leg positions. Suboptimal positioning did not appear to result in weight bearing and only lasted a couple of seconds. The positioning of the limit cap encouraged continued good seating.
Figure 5.
Hind leg positions. (A, B) Optimal hind leg positions, with low probability that hind legs are weight bearing. (C) Suboptimal hind leg position, with the possibility that hind legs are weight bearing.
Animal well-being.
No stress-related behavior was observed, and healthy weight gain occurred (Table 1). Table 2 provides the descriptive statistics for fGCM concentrations of all 3 groups; concentrations were higher than the baseline over the acclimation and TrP phases and fell over time during the experimental phase.
Table 1.
Average weekly weight in grams of the 3 groups of rats for each phase
| Phases and weeks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acclim | TrP1 | TrP2 | TeP | ||||||||
| Group | n | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 |
| C1 | 3 | 293.5 ± 11.5 | 310.6 ± 13.5 (+6.0 ± 1.0) | 324.1 ± 13.3 (+4.3 ± 1.3) | 342.4 ± 13.7 (+5.7 ± 0.5) | 342.4 ± 8.3 (+0.0 ± 1.6) | 341.4 ± 12.5 (−0.3 ± 2.4) | 349.4 ± 11.9 (+2.4 ± 2.6) | 350.9 ± 12.5 (+0.4 ± 0.2) | 360.1 ± 9.6 (+2.6 ± 1.3) | 366.4 ± 15.4 (+1.7 ± 2.1) |
| C2 | 3 | 300.8 ± 23.5 | 317.9 ± 22.8 (+5.7 ± 13.5) | 335.3 ± 19.4 (+5.7 ± 1.3) | 148.6 ± 18.5 (+4.0 ± 0.8) | 350.6 ± 17.9 (+0.7 ± 0.5) | 348.8 ± 16.4 (−0.5 ± 0.5) | 353.7 ± 17.1 (+1.4 ± 0.7) | 361.4 ± 15.2 (+2.2 ± 0.7) | 365.0 ± 12.8 (+1.0 ± 0.7) | 370.4 ± 12.9 (+1.5 ± 0.6) |
| EG | 18 | 296.8 ± 28.4 | 314.4 ± 27.4 (+6.1 ± 1.6) | 326.9 ± 26.0 (+4.1 ± 1.4) | 335.9 ± 26.0 (+2.8 ± 1.7) | 334.9 ± 26.2 (−0.3 ± 0.9) | 335.2 ± 25.6 (+0.1 ± 1.2) | 341.6 ± 25.8 (+2.0 ± 1.9) | 346.8 ± 26.5 (+1.5 ± 1.4) | 354.1 ± 26.5 (+2.1 ± 1.0) | 358.3 ± 26.5 (+1.2 ± 0.9) |
Percentage weight gain (+) or weight loss (−) per phase is presented in parenthesis. Weight and percentage weight gain is presented as mean ± one SD. C1, control group 1; C2, control group 2; EG, experimental group; n, sample size; Acclim, acclimatization phase; TrP1, training phase 1; TrP2, training phase 2; TeP, testing phase; W, week.
Table 2.
fGCM concentrations (in µg/g dry weight) of the 3 groups of rats for each phase
| Group | n | Acclim | n | TrP1 | n | TrP2 | n | TeP |
|---|---|---|---|---|---|---|---|---|
| C1 | 12 | 12.23 ± 2.69 | 6 | 14.2 ± 4.52 | 9 | 14.24 ± 4.59 | 33 | 9.15 ± 3.12 |
| C2 | 12 | 9.02 ± 2.08 | 9 | 10.32 ± 3.59 | 14 | 10.95 ± 3.90 | 33 | 7.69 ± 2.92 |
| EG | 74 | 10.11 ± 3.39 | 41 | 11.09 ± 3.93 | 72 | 11.27 ± 3.76 | 228 | 7.63 ± 3.39 |
Values are mean ± SD. C1/ C1, control group 1; C2, control group 2; EG, experimental group; n, sample size; Acclim, acclimatization phase; TrP1, training phase 1; TrP2, training phase 2; TeP, testing phase.
Results of the repeated measures ANOVA indicated significant differences among the 3 groups during all phases except for TrP1 (Table 3). Post hoc Bonferroni tests (Table 4) showed that during the acclimation phase, C1 rats had significantly higher fGCM concentrations than C2 rats (P = 0.01) and had marginally higher concentrations during TeP (P = 0.06). C1 rats also had significantly higher fGCM concentrations than EG rats during the acclimation (P = 0.02) and TeP (P = 0.03) phases and marginally higher levels during TrP2 (P = 0.06). No significant differences were detected between EG and C2 rats for any phase.
Table 3.
Repeated measures ANOVA test output results determining whether differences exist in the fGCM concentrations between groups for each phase
| Phase | Mauchly test of sphericity | Test for difference between groups | ||
|---|---|---|---|---|
| df | P value | F statistic | P value | |
| Acclim | 2 | 0.18 | 5.54 | 0.01* |
| TrP1 | 2 | 0.79 | 2.70 | 0.12 |
| TrP2 | 2 | 0.72 | 5.13 | 0.02* |
| TeP | 2 | 0.57 | 11.11 | <0.01* |
Acclim, acclimatization phase; TrP1, training phase 1; TrP2, training phase 2; TeP, testing phase. *, P < 0.05, significant difference.
Table 4.
Bonferroni post hoc tests comparing differences between fGCM concentrations of each group during each phase
| Phase | Comparison between groups | Bonferroni P value |
|---|---|---|
| Acclim | C1 compared with C2 | 0.01* |
| C1 compared with EG | 0. 02* | |
| C2 compared with EG | 0.69 | |
| TrP1 | C1 compared with C2 | 0.39 |
| C1 compared with EG | 0.21 | |
| C2 compared with EG | 1.00 | |
| TrP2 | C1 compared with C2 | 0.15 |
| C1 compared with EG | 0.06† | |
| C2 compared with EG | 1.00 | |
| TeP | C1 compared with C2 | 0.06† |
| C1 compared with EG | 0.03* | |
| C2 compared with EG | 1.00 |
Acclim, acclimatization phase; TrP1, training phase 1; TrP2, training phase 2; TeP, testing phase; C1, control group 1; C2, control group 2; EG, experimental group. *, P < 0.05, significant difference; †, P = 0.05–0.1, marginally significant difference.
Radiographic imaging.
The rats underwent successful weekly imaging, followed by a weekend rest period. The fGCM concentrations indicated that even the imaging protocol did not adversely affect any of the groups.
Discussion
The seated rat model aims to investigate the biologic effects of WBV by better representing a seated individual; in comparison, previous studies used either free-roaming animals on vibrating platforms or animals that were restrained in a prone position.2,12,18,30,31,33,54,55 These positions may be less representative of the seated individual than our seated rat model. Transmission of vibration along the spine can be affected by the limbs or the orientation of the spine relative to the direction of vibration. A previous study25 investigated the effects of WBV direction, frequency, and application regimen on lumbar spine properties in ovariectomized rats. Their results indicated that the spine can be affected differently based on the direction and frequency of vibration. To apply the vibration along the long axis of the spine, some studies have used anesthetized or euthanized rats that are held in a prone position with restraints under the shoulders and above the hips.2,19,54,55 In contrast, our seated rat model does not require restraint and avoids its potential influence on the biodynamic response or well-being of the rat. Anesthetized and euthanized rats were probably used to maintain the correct position during the vibration. However, the effects of anesthetics on the physiologic interactions of bone are not well known and anesthesia could therefore potentially influence results, especially in studies over longer periods. Furthermore, the use of anesthetics may decrease muscle tone, potentially altering biomechanics and natural responses of the musculoskeletal system. Testing conscious animals allows the simulation of a natural biomechanical response to WBV and eliminates possible anesthetic effects. Testing conscious animals provides a better simulation of natural biomechanical responses to WBV.
Our qualitative observations with regard to animal cooperation indicate that the dimensions of the capsule and seat appear to be adequate to promote a good seating position of the rat. Rats are naturally comfortable in tight dark environments41 and quickly acclimated to the capsules. The limit cap promoted good hind leg positioning and seating posture. The positive reinforcement with food also assisted in having rats maintain the correct seated position. Less reinforcement was required as the study progressed. Rats could be prompted to adopt the optimal hind leg position by gently moving the stray leg to the side of the seat. In a previous study,18 rats stood upright on their hind legs on a vibrating platform to provide vibration along the long-axis of the spine. This study evaluated the transmission of vibration between the vibrating platform and the lumbar spine (L5 region) of 2 rats. Results indicated that the frequency at L5 was similar to that of the platform, with 64% and 77% of the magnitude transmitted for a 45 and 90 Hz sinusoidal vibration, respectively. This shows the possible dampening effect of the legs and implies that the legs were weight bearing, similar to our suboptimal hind leg positions (Figure 5).
Our seated animal model did not seem to cause signs of acute stress, indicating animal well-being. The fGCM concentrations were significantly different among groups for all phases except for TrP1. In all phases where differences were observed between groups, the descriptive data and post hoc tests show that C1 rats had significantly higher fGCM concentrations than did the other 2 groups. However, no significant differences were found between EG and C2 rats for any of the phases. This provides statistical support that the testing method we used did not cause adverse psychologic or physiologic stress in the experimental group. Weight steadily increased and subsequently stabilized, indicating a low-stress environment.4,27 The rats showed no notable changes in behavioral patterns, further indicating a low-stress environment.
Our experimental design began with a 5 d vibration introduction regimen at 20% of the eventual test target; this approach seemingly avoided potential stress and promoted cooperation. The magnitude of the vibration was increased logarithmically. We used a logarithmic increase rather than a linear increase to initially expose the rats to smaller increases, giving them a longer period to become familiar with the vibration before experiencing larger increases later in the study. A different vibration introduction regimen might be required for vibrations with larger magnitudes and similar spectra or with similar magnitudes but different spectra. The former would require a smaller initial percentage and/or a longer period for acclimation. However, a previous study with conscious free-standing mice10 used peak acceleration from 4.9 to 19.6 m·s.−2 of the 90 Hz sinusoidal vibration during the first week of the experiment. The study did not report any observation indicating stress of the mice or differences in weight between the exposed and the control groups. Similarly, another study51 reported that conscious free-standing rats become accustomed to sinusoidal WBV of 13.3 m·s−2 at 15 Hz and 53.3 m·s−2 at 30 Hz (peak acceleration) within 2 to 3 d without any signs of stress such as weight loss or lack of grooming. Other studies that used conscious rats in a standing position did not mention any observation indicating that the sinusoidal WBV caused stress in the rats.12,18,30,31 The sinusoidal WBV used in these studies had a much larger amplitude than the WBV used in the current study. A vibration introduction regimen is most likely only required when using conscious animals.
Our novel seated rat model for spinal exposure to vibration was found to be feasible with respect to animal cooperation and well-being. The model uses conscious and unrestrained rats, thereby maintaining the natural biomechanical properties of the spine and providing a better representation of a seated individual. The model can be used to study the effects of WBV on individuals in a seated position (for example, driving posture). Future studies are needed to investigate the characteristics (magnitude, frequency, duration) of vibration that does not cause an unacceptable amount of stress. Future investigation will also be needed to assess the biofidelity of the rat model with regard to emulating the effects of WBV on the human spine.
Conflict of Interest
The authors have no competing interest to declare.
Funding
This work was supported by the National Research Foundation of South Africa (grant nos. 121753 and 120410).
References
- 1.Agoston DV. How to translate time? The temporal aspect of human and rodent biology. Front Neurol. 2017;8:92. doi: 10.3389/fneur.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baig HA, Guarino BB, Lipschutz D, Winkelstein BA. 2013. Whole body vibration induces forepaw and hind paw behavioral sensitivity in the rat J Orthop Res 31 1739–1744 10.1002/jor.22432 [DOI] [PubMed] [Google Scholar]
- 3.British Standards Institution. British standard guide to measurement and evaluation of human exposure to whole-body mechanical vibration and repeated shock. London (UK): BSI; [Google Scholar]
- 4.Brower M, Grace M, Kotz CM, Koya V. 2015. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources Lab Anim Res 31 166–173 10.5625/lar.2015.31.4.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelhano-Carlos MJ, Baumans V. 2009. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats Lab Anim 43 311–327 10.1258/la.2009.0080098 [DOI] [PubMed] [Google Scholar]
- 6.Chung SL, Leung KS, Cheung WH. 2014. Low-magnitude high-frequency vibration enhances gene expression related to callus formation, mineralization and remodeling during osteoporotic fracture healing in rats J Orthop Res 32 1572–1579 10.1002/jor.22715. [DOI] [PubMed] [Google Scholar]
- 7.Crossey B, Chimimba C, du Plessis C, Hall G, Ganswindt A. 2020. Using faecal glucocorticoid metabolite analyses to elucidate stressors of African wild dogs Lycaon pictus from South Africa Wildl Biol 2020 e1–e10 10.2981/wlb.00646 [DOI] [Google Scholar]
- 8.Dietze S, Lees KR, Fink H, Brosda J, Voigt JP. Food deprivation, body weight loss and anxiety-related behavior in rats. Animals (Basel) 2016;6:4. doi: 10.3390/ani6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards RG, Lafferty JF, Knapp CF. 1976. Experimental and analytical determinations of the mechanical impedance response of animals to vertical vibration J Biomech 9 55–61 10.1016/0021-9290(76)90140-8 [DOI] [PubMed] [Google Scholar]
- 10.Fieß M, Heistermann M, Hodges JK. 1999. Patterns of urinary and fecal steroid excretion during the ovarian cycle and pregnancy in the African elephant (Loxodonta africana) Gen Comp Endocrinol 115 76–89 10.1006/gcen.1999.7287 [DOI] [PubMed] [Google Scholar]
- 11.Francis TB, Benzel EC. Biomechanics of motion preservation techniques, p 1581–1586. In: Steinmetz MP, Benzel EC. editors. Benzel’s spine surgery: techniques, complication avoidance, and management. Philadelphia (PA) Elsevier [Google Scholar]
- 12.Gnyubkin V, Guignandon A, Laroche N, Vanden-Bossche A, Malaval L, Vico L. 2016. High-acceleration whole body vibration stimulates cortical bone accrual and increases bone mineral content in growing mice J Biomech 49 1899–1908 10.1016/j.jbiomech.2016.04.031 [DOI] [PubMed] [Google Scholar]
- 13.Gräbe RP, Kat C-J, Van Staden PJ, Els PS. Difference thresholds for a vehicle on a 4-poster test rig. Appl Ergon. 2020;87:103115. doi: 10.1016/j.apergo.2020.103115. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MJ. Handbook of human vibration. London: Academic Press; 1990. [Google Scholar]
- 15.Griffin MJ. An introduction to whole-body vibration, p 27–42Handbook of human vibration. London: Academic Press [Google Scholar]
- 16.Griffin MJ. Vibration and human responses, p 1–25Handbook of human vibration. London: Academic Press [Google Scholar]
- 17.Harkin A, Connor TJ, O’Donnell JM, Kelly JP. 2002. Physiological and behavioral responses to stress: What does a rat find stressful? Lab Anim (NY) 31 42–50 [DOI] [PubMed] [Google Scholar]
- 18.Holguin N, Uzer G, Chiang FP, Rubin C, Judex S. 2011. Brief daily exposure to low-intensity vibration mitigates the degradation of the intervertebral disc in a frequency-specific manner J Appl Physiol 111 1846–1853 10.1152/japplphysiol.00846.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holsgrove TP, Zeeman ME, Welch WC, Winkelstein BA. Pain after whole-body vibration exposure is frequency dependent and independent of the resonant frequency: Lessons from an in vivo rat model. J Biomech Eng. 2020;142:061005. doi: 10.1115/1.4044547. [DOI] [PubMed] [Google Scholar]
- 20.Jaumard NV, Leung J, Gokhale AJ, Guarino BB, Welch WC, Winkelstein BA. 2015. Relevant anatomic and morphological measurements of the rat spine: Considerations for rodent models of human spine trauma Spine 40 e1084–e1092 10.1097/BRS.0000000000001021 [DOI] [PubMed] [Google Scholar]
- 21.Johanning E. 2015. Whole-body vibration-related health disorders in occupational medicine – An international comparison Ergonomics 58 1239–1252 10.1080/00140139.2015.1005170. [DOI] [PubMed] [Google Scholar]
- 22.Keller BV, Davis ML, Thompson WR, Dahners LE, Weinhold PS. 2013. Varying whole body vibration amplitude differentially affects tendon and ligament structural and material properties J Biomech 46 1496–1500 10.1016/j.jbiomech.2013.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr GJ, McCann MR, Branch JK, Ratneswaran A, Pest MA, Holdsworth DW, Beier F, Dixon SJ, Seguin CA. 2017. C57BL/6 mice are resistant to joint degeneration induced by whole-body vibration Osteoarthritis Cartilage 25 421–425 10.1016/j.joca.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 24.Khonmee J, Vorawattanatham N, Pinyopummin A, Thitaram C, Somgird C, Punyapornwithaya V, Brown JL. Assessment of faecal glucocorticoid metabolite excretion in captive female fishing cats (Prionailurus viverinus) in Thailand. Conserv Physiol. 2016;4:cow021. doi: 10.1093/conphys/cow021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komrakova M, Stuermer EK, Tezval M, Stuermer KM, Dullin C, Schmelz U, Doell C, et al. 2017. Evaluation of twelve vibration regimes applied to improve spine properties in ovariectomized rats Bone Rep 7 172–180 10.1016/j.bonr.2014.12.001 [DOI] [Google Scholar]
- 26.Lauer AM, May BJ, Hao ZJ, Watson J. 2009. Analysis of environmental sound levels in modern rodent housing rooms Lab Anim (NY) 38 154–160 10.1038/laban0509-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Nam H, Kim J, Cho H, Jang Y, Lee E, Choi E, Jin DI, Moon H. 2012. Body weight changes of laboratory animals during transportation Asian-Australas J Anim Sci 25 286–290 10.5713/ajas.2011.11227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepschy M, Touma C, Hruby R, Palme R. 2007. Non-invasive measurement of adrenocortical activity in male and female rats Lab Anim 41 372–387 10.1258/002367707781282730 [DOI] [PubMed] [Google Scholar]
- 29.Ludwig C, Wachter B, Silinski-Mehr S, Ganswindt A, Bertschinger H, Hofer H, Dehnhard M. 2013. Characterisation and validation of an enzyme-immunoassay for the non-invasive assessment of faecal glucocorticoid metabolites in cheetahs (Acinonyx jubatus) Gen Comp Endocrinol 180 15–23 10.1016/j.ygcen.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 30.McCann MR, Veras MA, Yeung C, Lalli G, Patel P, Leitch KM, Holdsworth DW, Dixon SJ, Seguin CA. 2017. Whole-body vibration of mice induces progressive degeneration of intervertebral discs associated with increased expression of Il1beta and multiple matrix degrading enzymes Osteoarthritis Cartilage 25 779–789 10.1016/j.joca.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 31.McCann MR, Yeung C, Pest MA, Ratneswaran A, Pollmann SI, Holdsworth DW, Beier F, Dixon SJ, Seguin CA. 2017. Whole-body vibration of mice induces articular cartilage degeneration with minimal changes in subchondral bone Osteoarthritis Cartilage 25 770–778 10.1016/j.joca.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 32.National Research Council Environment, housing, and management, p 41–104Guide for the care and use of laboratory animals. Washington (DC) The National Academies Press [Google Scholar]
- 33.Nickerson JL, Drazic M. 1966. Internal body movement along three axes resulting from externally applied sinusoidal forces. AMRL-TR-66-102 AMRL-TR Aerospace Medical Research Laboratories 1 1–12 [DOI] [PubMed] [Google Scholar]
- 34.O’Connell GD, Vresilovic EJ, Elliott DM. 2007. Comparison of animals used in disc research to human lumbar disc geometry Spine 32 328–333 10.1097/01.brs.0000253961.40910.c1 [DOI] [PubMed] [Google Scholar]
- 35.Paddan GS, Griffin MJ. 2002. Effect of seating on exposures to whole-body vibration in vehicles J Sound Vibrat 253 215–241 10.1006/jsvi.2001.4257 [DOI] [Google Scholar]
- 36.Pasqualini M, Lavet C, Elbadaoui M, Vanden-Bossche A, Laroche N, Gnyubkin V, Vico L. 2013. Skeletal site-specific effects of whole body vibration in mature rats: From deleterious to beneficial frequency-dependent effects Bone 55 69–77 10.1016/j.bone.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 37.Perrot-Sinal TS, Gregus A, Boudreau D, Kalynchuk LE. 2004. Sex and repeated restraint stress interact to affect cat odor-induced defensive behavior in adult rats Brain Res 1027 161–172 10.1016/j.brainres.2004.08.056 [DOI] [PubMed] [Google Scholar]
- 38.Quandieu P, Pellieux L. 1982. Study in situ et in vivo of the acceleration of lumbar vertebrae of a primate exposed to vibration in the Z-axis J Biomech 15 985–1006 10.1016/0021-9290(82)90016-1 [DOI] [PubMed] [Google Scholar]
- 39.Sehmisch S, Galal R, Kolios L, Tezval M, Dullin C, Zimmer S, Stuermer KM, Stuermer EK. 2009. Effects of low-magnitude, high-frequency mechanical stimulation in the rat osteopenia model Osteoporos Int 20 1999–2008 10.1007/s00198-009-0892-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sengupta P. 2013. The laboratory rat: relating its age with human’s Int J Prev Med 4 624–630 [PMC free article] [PubMed] [Google Scholar]
- 41.Sharp P, Villano JS. The laboratory rat. Boca Raton (FL): CRC Press; 2012. Important biological features. [Google Scholar]
- 42.Slonim AR. 1985. Comparative biodynamic response of two primate species to the same vibrational environment Aviat Space Environ Med 56 945–955 [PubMed] [Google Scholar]
- 43.Smith SD, Kazarian LE. 1994. The effects of acceleration on the mechanical impedance response of a primate model exposed to sinusoidal vibration Ann Biomed Eng 22 78–87 10.1007/BF02368224 [DOI] [PubMed] [Google Scholar]
- 44.Stuermer EK, Komrakova M, Sehmisch S, Tezval M, Dullin C, Schaefer N, Hallecker J, Stuermer KM. 2014. Whole body vibration during fracture healing intensifies the effects of estradiol and raloxifene in estrogen-deficient rats Bone 64 187–194 10.1016/j.bone.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 45.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: A noninvasive technique to monitor stress hormones Horm Behav 45 10–22 10.1016/j.yhbeh.2003.07.002 [DOI] [PubMed] [Google Scholar]
- 46.Touma C, Sachser N, Möstl E, Palme R. 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice Gen Comp Endocrinol 130 267–278 10.1016/S0016-6480(02)00620-2 [DOI] [PubMed] [Google Scholar]
- 47.Wehrle E, Wehner T, Heilmann A, Bindl R, Claes L, Jakob F, Amling M, Ignatius A. 2014. Distinct frequency dependent effects of whole-body vibration on non-fractured bone and fracture healing in mice J Orthop Res 32 1006–1013 10.1002/jor.22629 [DOI] [PubMed] [Google Scholar]
- 48.Wei RL, Jung BC, Manzano W, Sehgal V, Klempner SJ, Lee SP, Ramsinghani NS, Lall C. 2016. Bone mineral density loss in thoracic and lumbar vertebrae following radiation for abdominal cancers Radiother Oncol 118 430–436 10.1016/j.radonc.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 49.Wilder DG, Pope MH. 1996. Epidemiological and aetiological aspects of low back pain in vibration environments - An update Clin Biomech (Bristol, Avon) 11 61–73 10.1016/0268-0033(95)00039-9 [DOI] [PubMed] [Google Scholar]
- 50.Wilder DG, Woodworth BB, Frymoyer JW, Pope MH. 1982. Vibration and the human spine Spine 7 243–254 10.1097/00007632-198205000-00008 [DOI] [PubMed] [Google Scholar]
- 51.Wirth F, Schempf G, Stein G, Wellmann K, Manthou M, Scholl C, Sidorenko M, et al. 2013. Whole-body vibration improves functional recovery in spinal cord injured rats J Neurotrauma 30 453–468 10.1089/neu.2012.2653 [DOI] [PubMed] [Google Scholar]
- 52.Xie P, Tang Z, Qing F, Chen X, Zhu X, Fan Y, Yang X, Zhang X. 2016. Bone mineral density, microarchitectural and mechanical alterations of osteoporotic rat bone under long-term whole-body vibration therapy J Mech Behav Biomed Mater 53 341–349 10.1016/j.jmbbm.2015.08.040 [DOI] [PubMed] [Google Scholar]
- 53.Young C, Ganswindt A, McFarland R, de Villiers C, van Heerden J, Ganswindt S, Barrett L, Henzi SP. 2017. Faecal glucocorticoid metabolite monitoring as a measure of physiological stress in captive and wild vervet monkeys Gen Comp Endocrinol 253 53–59 10.1016/j.ygcen.2017.08.025 [DOI] [PubMed] [Google Scholar]
- 54.Zeeman ME, Kartha S, Jaumard NV, Baig HA, Stablow AM, Lee J, Guarino BB, Winkelstein BA. 2015. Whole-body vibration at thoracic resonance induces sustained pain and widespread cervical neuroinflammation in the rat Clin Orthop Relat Res 473 2936–2947 10.1007/s11999-015-4315-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeeman ME, Kartha S, Winkelstein BA. 2016. Whole-body vibration induces pain and lumbar spinal inflammation responses in the rat that vary with the vibration profile J Orthop Res 34 1439–1446 10.1002/jor.23243 [DOI] [PubMed] [Google Scholar]