Abstract
Fibrosis, the morphologic end-result of a plethora of chronic conditions and the scorch for organ function, has been thoroughly investigated. One aspect of its development and progression, namely the permissive role of vascular endothelium, has been overshadowed by studies into (myo)fibroblasts and TGF-β; thus, it is the subject of the present review. It has been established that tensile forces of the extracellular matrix acting on cells are a prerequisite for mechanochemical coupling, leading to liberation of TGF-β and formation of myofibroblasts. Increased tensile forces are prompted by elevated vascular permeability in response to diverse stressors, resulting in the exudation of fibronectin, fibrinogen/fibrin, and other proteins, all stiffening the extracellular matrix. These processes lead to the development of endothelial cells dysfunction, endothelial-to-mesenchymal transition, premature senescence of endothelial cells, perturbation of blood flow, and gradual obliteration of microvasculature, leaving behind “string” vessels. The resulting microvascular rarefaction is not only a constant companion of fibrosis but also an adjunct mechanism of its progression. The deepening knowledge of the above chain of pathogenetic events involving endothelial cells, namely increased permeability–stiffening of the matrix–endothelial dysfunction–microvascular rarefaction–tissue fibrosis, may provide a roadmap for therapeutic interventions deemed to curtail and reverse fibrosis.
Keywords: endothelial cell dysfunction, endothelial-mesenchymal transition, extracellular matrix, premature cell senescence, vascular permeability
INTRODUCTION
Tissue fibrosis is a morphologic term to designate the state of disproportionally high accumulation of stiffened matrix proteins and broadening of the interstitium. This can be accomplished by an excessive deposition of matrix proteins, their relatively insufficient decomposition, or both. Fibrosis deforms and distorts tissue architecture and, in so doing, impairs many organ functions. Widening of interstitial spaces and companion dropout of vasculature also result in the elongation of distances for oxygen diffusion from capillary beds to the abounding tissues, thus igniting hypoxic reprogramming of affected cells.
With the existing surfeit of reviews dedicated to various aspects of fibrosis (1–6), I shall avoid reduplication of the previous work, rather attempting to elaborate on the questions: “What is the contribution of vascular endothelial cells?” and “What are the mechanisms of their participation?”
The quintessential role in fibrogenesis is played by TGF-β and myofibroblasts. Notably, both “villains” are normally latent or repressed, respectively, and their prior activation is needed for the subsequent unveiling of the program of fibrogenesis. This key point is frequently lost in studies of TGF-β and myofibroblasts, yet both could be triggered to activation via a common hemodynamic mechanism. The microvascular contribution to this process is both necessary and sufficient, as will be discussed at length here. I shall focus on the microvascular endothelium and its contribution to organ fibrosis by discussing dynamics and mechanisms of the following chain of events: stress-induced increase in vascular endothelial (VE) permeability; exudation of proteins; extracellular matrix stiffening, allowing liberation of TGF-β and formation of myofibroblasts; endothelial-mesenchymal transition (Endo-MT), endothelial dysfunction and premature senescence with profibrogenic secretome of the dysfunctional endothelium; vascular rarefaction; and fibrosis. At the end of this discussion, I shall venture to briefly outline the potential strategies to prevent and revert microvascular rarefaction and how those might affect the progression of fibrosis. Although the bulk of my arguments will be confined to kidney fibrosis, the proposed chain of events is relevant to fibrogenesis in other organs.
MAIN PROTAGONISTS OF FIBROGENESIS
The key cell type orchestrating the fibrotic transformation of diverse tissues is a myofibroblast—a cell rarely encountered under physiological conditions but abundant upon exposure to a plethora of stressors. Myofibroblasts are indispensable for the development and progression of fibrosis. These cells are characterized by overexpression of markers, neither of which is specific, such as α-smooth muscle actin (α-SMA), matrix metalloprotease 2 (MMP-2), collagen 1A2, SNAIL 1 Slug, and TGF-β2. The latter is critical to fibrogenesis. Emergence of these matrix-synthesizing and replicating cells occurs via reprogramming of resident fibroblasts, pericytes, and, to a lesser degree, epithelial or endothelial cells (ECs). Controversy surrounds the proportional contribution of each of these cell types to formation of myofibroblasts, most likely due to the lack of highly specific markers (1). One of their precursor cell types–fibroblasts—shows distinct heterogeneity between different organs and within the same organ (7). Not all fibroblasts could become committed to fibrogenesis: in separate studies of mesothelial and dermal fibroblasts by Rinkevich et al. (8, 9), their heterogeneity has been confirmed and only a single subset was found to be fibrogenic. Henderson et al. (6) have scholarly recounted most recent discoveries made using single cell or nucleus RNA sequencing, all confirming the heterogeneity of fibroblasts. In the kidney, Asada et al. (10) have demonstrated that transdifferentiation of erythropoietin-producing fibroblasts is primarily responsible for fibrosis of this organ. Those fibroblasts capable of transforming into myofibroblasts develop de novo stress fibers and exert contractile force in vivo (11), both considered to be the most reliable markers of myofibroblasts. Those cytoskeletal reorganizations are prerequisite for achieving mechanochemical coupling between the extracellular matrix (ECM) stiffness and TGF-β liberation from a latent form, culminating in paratensile signaling toward generation of myofibroblasts (12).
INCREASED VASCULAR PERMEABILITY AS A PREREQUISITE FOR STIFFENING EXTRACELLULAR MATRIX
What could be the impulse serving to transform “physiological” stiffness of ECM in a specific organ to the “pathological” one to ignite mechanochemical coupling? Most stressors produce an initial hyperemic response capable of unleashing a cascade of de novo synthesis and deposition of matrix proteins, stiffening of ECM, myofibroblasts formation, and subsequent tissue deformation and microvascular dropout. During the hyperemic response, leukocytic infiltration of injured cells and tissues is short-lived and assists in the clearance of cell debris (13). In the process, vascular permeability increases to allow the exudation of circulating fibrinogen, fibronectin, and other circulating matrix proteins to the interstitium, thus leading to a temporary increase in tissue stiffness and liberation of TGF-β from the latent form (14, 15) to initiate “wound healing” response. The process is readily reversible upon cessation of noxious stimulation. The incidental involvement of an array of proinflammatory and lipid mediators and neuroimmune circuits involved in the initiation and resolution phases has been well documented and recently comprehensively reviewed (16–18); thus, its further discussion is unwarranted here. With protracted stress or perturbed mechanisms of resolution, the process evades reversibility and acquires self-perpetuating trajectory for reasons described below in this section. This scenario abuts issues related to vascular permeability and responses of tissue fibroblasts to increased ECM stiffness. In fact, in vitro studies using the application of exogenous TGF-β instead of activating the endogenous one have oversimplified the pathogenesis of the fibrotic process. In vivo, however, liberation of TGF-β1 from the latent state (so-called “straitjacket” that keeps it inactive) requires mechanical forces exerted via α(v) integrins to the RGD sequence within the prodomain bound to matrix proteins to change the conformation of latency-associated protein (14, 15).
As mentioned earlier, vascular response to noxious stimuli, such as mechanical, chemical, ischemic, and infectious, invariably consists of hyperemia. When protracted, these stressors induce innate immune response, microthrombi, and endothelial dysfunction, all eventuating in sluggishness of blood flow, promoting obliteration of microvessels, stress-induced premature senescence (SIPS) of EC, release of senescence-associated secretory products (SASPs), and fibrogenic signaling.
Recently, the mechanisms and causality of increased vascular permeability have been advanced to address the question: What are the causes of the loss of endothelial barrier function? Our current view distinguishes three major routes of increased vascular permeability: 1) paracellular, 2) degraded glycocalyx layer, and 3) transcellular (Fig. 1) (19) Remarkably, all three appear to be interrelated. The leakiness of the paracellular path is regulated by the vascular endothelial (VE)-cadherin, the main component of adherens junctions, and tight junctions represented by members of junction-associated molecular family, occludin, and endothelial cell-selective adhesion molecule. As shown in Fig. 1, the paracellular permeability pathway is influenced by histamine, bradykinin, thrombin, an early phase of VEGF response, platelet-activating factor, and several cytokines and chemokines, all of which impair junctional stability via acting upon VE-cadherin tyrosine phosphorylation (reviewed in Ref. 19). Notably, VE-cadherin influences tight junctions (20) and forms complexes with VEGF receptor-2 (21), thus orchestrating the patency of both the paracellular and transcellular permeability paths.
Figure 1.
Guardians of endothelial barrier function (A) and mediators leading to its impairment (B). Paracellular permeability is guarded by adherence and tight junctions (dark blue), transcellular permeability depends on the vesicular and vesiculo-vacuolar organelles (green), and glycocalyx layer (yellow) normally protecting the above permeability paths is degraded by diverse sheddases. The inset in the upper panel displays caveolin-1/GFP mobilization to form elongated tubular structures in response to VEGF. B: briefly summarizes the mediators affecting permeability of each path. MMP, matrix metalloproteases; ADAM, a disintegrin and metalloproteinase; LPS, lipopolysaccharide; VEGF, vascular endothelial growth factor; PAF, platelet-activating factor; VVO, vesiculo-vacuolar organelles.
Endothelial glycocalyx, covering the entire cell surface to overpass fenestrae, caveolae, and cell-cell junctions, provides a size- and charge-dependent molecular sieve to guard the barrier function (22–25). Of note, the distinct components of glycocalyx are so tightly intertwined that the enzymatic degradation of either hyaluronan, proteoglycan, or heparan sulfate produces a “domino” effect leading to the collapse of glycocalyx. Given the fact that such sheddases are activated in a broad range of cardiorenal, metabolic, infectious, and oncologic diseases, the degradation of this protective layer is pervasive (26). It would not be an exaggeration, then, to conclude that the loss of integrity of endothelial glycocalyx is necessary, though not sufficient, for the actuation of paracellular and transcellular permeability paths.
Under stress conditions that activate endothelial cells, early increase in permeability lasts for ∼15 min and is followed by many hours of increased microvascular permeability, at least as modeled using thermal injury (27). Curry’s group (28) has measured cytosolic calcium transients and changes in permeability in mesenteric microvasculature and detected near-instantaneous elevation in cytosolic calcium, closely followed by the increase in permeability in response to ATP, later extended to other agonists, like bradykinin, histamine, VEGF, and ionomycin. It is, thus, safe to conclude that activation of endothelial cells leading to elevation of cytosolic calcium concentration represents a dominant mechanism for igniting an increase in microvascular permeability. The latter, in turn, is responsible for the subendothelial escape of plasma macromolecules, like fibronectin and fibrinogen (turning into fibrin), that could participate in the initial phase of matrix protein accumulation, encourage the formation of focal adhesions and stress fibers in adjacent interstitial fibroblasts and pericytes, and, unless halted, promote their transformation into myofibroblasts responsible for the protracted synthesis of matrix proteins. These account for the increased matrix stiffness, activation of TGF-β, vascular drop-out, and induction of the hypoxic program (Fig. 2).
Figure 2.
A roadmap of stress-induced pathogenetic steps culminating in vascular rarefaction. The ensuing vascular rarefaction negatively affects resolution, while enhancing the self-perpetuating matrix deposition, myofibroblasts formation, and further microvascular obliteration. VEGF, vascular endothelial growth factor; ET-1, endothelin-1; PAF, platelet-activating factor; EC, endothelial cells; ECM, extracellular matrix; TG-2, transglutaminase-2; EST, endostatin.
To monitor endothelium exposed to the excess production of VEGF, thus emulating hypoxic conditions, we used cultured endothelial cells expressing caveolin-1-GFP protein and performed three-dimensional (3-D) reconstruction of confocal fluorescence microscopy images prior to and following application of VEGF-165 (29). Data obtained demonstrated a rapid, within 10 min, formation of vesiculo-vacuolar organelles spanning what appears to be the entire endothelial cell (the inset in Fig. 1). This gain in transcellular communication was confirmed using transmission electron microscopy (increased numbers of internalized cytoplasmic vesicles, presumably caveolar in origin, groups of high-density caveolar vesicles forming tubulovesicular structures in the perinuclear area) and an electrical measurement of the impedance of confluent monolayers [decreased resistance with the time course similar to the confocal microscopy data on the formation of vesiculo-vacuolar organelles (29)].
Although single-cell transcriptional profiling has established organ specificity and phenotypic heterogeneity of endothelial cells, including highly specific renal endothelial cells (30–33), the abovementioned stress responses are conserved and qualitatively quasiinvariable in different organs, like the lung and kidney (5, 6, 34).
Hence, the increase in permeability could be accomplished via intercellular clefts (paracellular path) and transcellular vesicular and vesiculo-vacuolar organelles. It occurs in such a way that the former pathway is considered to be more relevant to the acute stressors (35), whereas the transcellular pathway is predominantly operational under the conditions of protracted stress accompanied by hypoxia and induction of VEGF signaling, although exceptions are well known with regard to long-acting IL-6 and TNF-α. Degradation of the glycocalyx layer in both acute and chronic conditions is permissive for the actuation of other permeability paths. In parallel with it, such conditions are deemed to promote endothelial cell dysfunction (ECD), as discussed below.
ENDOTHELIAL CELL DYSFUNCTION
ECD is a common gateway toward Endo-MT, profibrogenic secretome, premature cell senescence, and microvascular rarefaction. ECD is defined as a temporal or permanent defect in permeability, regulation of coagulation, vasomotion, antioxidant defense, and leukocyte traffic, among others (36). The clinical spectrum of ECD is remarkably broad and includes the majority of acute and chronic cardiorenal, metabolic, and some infectious diseases and cancers. All those entities have been well studied and comprehensively reviewed (36). One of the most recent additions to this list is the bourgeoning evidence that small vessel disease of the cerebral microvessels (37–39) is causatively implicated in a host of genetic and sporadic cases of cognitive impairment (up to 50% of dementias worldwide) and ischemic and hemorrhagic strokes. This newest aspect of cerebral ECD further confirms the role of impaired microcirculation in the pathogenesis of disease.
Blood flow patterns coordinate endothelial cell behavior. Although laminar shear stress increases, among others, the endothelial level of sirtuin 1 (SIRT1), a master regulator of stress response and energy metabolism, it is contrasted by oscillatory (turbulent) shear stress having the opposite effect on SIRT1, thus reducing deacetylation and inactivating endothelial nitric oxide synthase (eNOS) (40). Chien’s laboratory has demonstrated that oscillatory shear stress, by facilitating binding of c-Jun to the promoter region of microRNA-21 (miR-21), induces its expression, in turn inhibiting the translation of peroxisome proliferator-activated receptor-α and leading to enhanced expression of vascular cell adhesion molecule 1 (VCAM-1) and monocyte chemotactic protein 1 (MCP-1) (41). In addition, the proinflammatory phenotype of endothelial cells subjected to oscillatory shear stress is mediated via upregulation of miR-663, which facilitates monocyte adhesion (42). Furthermore, oscillatory shear stress has been implicated in the downregulation of Krüppel-like factors 2 and 4 (43), nuclear like factor-2, antioxidative peroxiredoxins (44), and induction of mitochondrial superoxide production via activation of c-Jun NH2-terminal kinases and NADPH oxidase and upregulation of TWIST (45, 46). Endothelium in disturbed flow also exhibit upregulation of nuclear k-light-chain enhancer of activated B cells, activator protein-1, YAP/TAZ/TEAD, and hypoxia-inducible factor 1α (47). These multipronged effects of oscillatory shear stress, most recently exhaustively reviewed by Davis et al. (48), have a comparable time course and could be responsible for developing endothelial cell activation and eventually dysfunction.
Our earlier studies using gene microarray analysis of cultured EC treated with an inhibitor of nitric oxide synthase have revealed upregulation of collagen XVIII and its antiangiogenic fragment endostatin, as further confirmed in vivo in mice chronically treated with an NOS inhibitor (49). Enhanced generation of endostatin in these animals leads to the development of Endo-MT and eventual rarefaction of renal microvasculature, thus further compounding vascular and parenchymal pathology. EC exposed to diverse stressors respond with lysosomal dysfunction, leakage of cathepsins to the cytoplasm, and degradation of SIRT1. SIRT1 depletion, in turn, leads to the downregulation of MMP-14 and accounts for one of the pathways for accumulation of ECM (50).
Although the secretory products of intact EC contribute to the maintenance of the surrounding parenchyma, the dysfunctional endothelium secretes a host of profibrogenic factors that activate resident fibroblasts, further worsen endothelial dysfunction, and promote Endo-MT and microvascular rarefaction (50). The strategy we have elected to identify relevant fibrogenic secretomic products was as follows. Vasko et al. (51) used genetically engineered mice with a deletion of the exon 4 (encoding the deacetylase domain) in endothelial SIRT1 (Sirt1endo−/−) and have demonstrated that those mice not only develop vasculopathy and premature EC senescence but also tubulointerstitial fibrosis at an early age. One of the mechanistic links presented is the observation that endothelial SIRT1-deficient mice have lower expression of the master matrix metalloproteinase, MMP-14. Lower MMP-14 leads to impaired degradation of deposited matrix proteins as a result of reduced matrilytic activity. The similar downregulation of MMP-14 in SIRT1 deficiency has been reported by Potente et al. (52). On the contrary, TGF-β1-induced extracellular matrix expression is attenuated by activation of SIRT1, supporting the idea of SIRT1 suppressing TGF-β/Smad3 signaling and protecting against development of fibrosis (53). On the other hand, Xavier et al. (54) have genetically engineered mice with partial deletion of TGF-β receptor 2 in endothelial cells (TGF-βRIIendo+/−), which resisted development of fibrosis. Having demonstrated that mice with the limited expression of TGF-β receptor 2 only in the endothelial cells are protected against fibrosis (54), whereas mice deficient in SIRT-1 only in endothelial cells are prone to developing fibrosis (51), we argued that the endothelium represented the only distinction between two species. Therefore, we contrasted the secretomes of microvascular endothelial cells isolated from these mice and subjected to TGF-β (55, 56). We used unbiased, nontargeted mass spectroscopy (MS/MS) of secretomes in the conditioned medium after cells were exposed to a vehicle or TGF-β for 48 h (55). Of the total 332 nonredundant proteins, which belong to diverse categories, those secreted only by Sirt1endo−/− vis-a-vis TGF-βRIIendo+/− were considered to be of special interest, since they could theoretically be responsible for the pro- and antifibrogenic phenotype of the respective mice. We also observed that SIRT1endo−/− mice showed a decline in the endothelial glycocalyx, already at a basal state, due to a higher level of shedding of syndecan-4, the main proteoglycan of the endothelial glycocalyx (56).
Notably, endothelial SIRT-1 deficiency is a model of ECD. Therefore, features of the fibrogenic secretome in endothelial SIRT1 deficiency and ECD overlap, its juxtacrine and paracrine signaling involving components of Wnt, Notch, and glycocalyx, as has been summarized earlier (40). In addition, profibrogenic secretome of the dysfunctional endothelium promotes cross linking by transglutaminase-2 of matrix proteins (57).
VASCULAR RAREFACTION—THE UBIQUITOUS HALLMARK OF FIBROSIS
ECD is a precursor of microvascular rarefaction, which has been reported in a wide range of diseases, such as hypertensive disorders (58), pressure-overload heart hypertrophy (59), diabetes mellitus (60), preeclampsia, chronic heart failure, essential hypertension, and chronic renal failure (61, 62). The density of skin microvessels is reduced with aging (63) and in obesity and metabolic syndrome (64). In Zucker rats and db/db mice, capillary rarefaction in the heart, skeletal muscle, brain, and pancreas is detectable early in life; vascular dropout in muscle and pancreas is especially detrimental for glucose homeostasis. On the other hand, the list of anti-angiogenic mediators has grown to include, in addition to angiostatin and endostatin (65), other endogenous anti-angiogenic substances, such as alphastatin, anti-thrombin III, canstatin, interferon-beta, 2-methoxyestradiol, pigment epithelium-derived factor, platelet factor 4, tetrahydrocortisol, thrombospondin-1, tissue inhibitor of metalloproteinase 2 (TIMP-2), tumstatin, and matrikines, exemplified by collagen IV-derived arresten, pentastatin, collagen XVIII-derived endostatin, and perlecan-derived endorepellin, to name a few (66), all of which interfere with endothelial cell motility, angiogenesis, and viability. Endostatin cooperates with endorepellin, as well as several other proteoglycans, to modulate autophagy, facilitate degradation of hyaluronan synthase-2 (thus affecting the glycocalyx coat), and elicit angiostasis (67, 68).
The ablation of the microvasculature is believed to involve the following steps: damaged endothelial cells perturb normal blood flow and dampen the flow-dependent shear stress-induced activation of eNOS, leading to further cell damage and apoptosis. The resulting stasis of blood flow in the involved vessel is a prerequisite for its impending obliteration. Though the intimate mechanisms of vessel pruning remain debatable—from the withdrawal of survival factors (e.g., pericyte-synthesized VEGF) to, dislodgement from the basement membrane, to active vasoconstriction (even with pharmacological agents)—the stagnation of blood flow and a decline in shear stress appear to be obligatory for vessel regression (69). Local platelet adhesion and recruitment of leukocytes, eventually disposing of cell debris complete vessel obliteration and regression. At the molecular level, the process is governed, among others, by Wnt and Notch pathways (70) and angiopoietins/Tie signaling (angiopoietin 1/Tie2 signaling promoting PI3K/Akt survival signaling, which is disrupted by angiopoietin 2) (71, 72). In addition, endorepellin, a C-terminal domain V of an ubiquitous matrix proteoglycan, perlecan, achieves its antiangiogenic and proapoptotic effect in endothelial cells by downregulating VEGF receptor 2 (reviewed in Ref. 67). Desquamated apoptotic endothelial cells leave behind a decellularized basement membrane, referred to as a string vessel or empty basement membrane tube (73). Even at this stage, the rarefaction process could be reversed. The remaining scaffold of the basement membrane being rich in endothelial and pericyte growth factors, such as VEGF, basic fibroblast growth factor (FGF), and PDGF, could guide repopulation of string vessels with EC, potentially restoring blood flow to the area (74).
Endothelial-to-Mesenchymal Transition
Endo-MT in embryogenesis represents a physiological process occurring during formation of heart valves and septa. In adulthood, Endo-MT is implicated in pulmonary hypertension, vein graft failure, atherosclerosis, and metastatic spread of malignant cells, or cerebral cavernous malformation, to mention a few (75–81). Endo-MT is believed to be a major contributor to vascular dropout and development of fibrosis. According to various sources, ∼30%–50% of interstitial fibroblasts/myofibroblasts originate from endothelium. However, the recent study by Kuppe et al. (4), while profiling the single-cell transcriptomes from healthy and fibrotic human kidneys, has arrived at the identification of distinct subpopulations of pericytes and fibroblasts as the main cellular sources of scar-forming myofibroblasts (identified as periostin- and PDGF receptor-expressing cells) during human kidney fibrosis, thus questioning the major contribution of endothelial and epithelial cell types to the formation of myofibroblasts. Yet, the states of a partial epithelial- and/or endothelial-mesenchymal transition cannot be ruled out at this time. Furthermore, though Endo-MT does not directly contribute to fibrosis, it does it indirectly via microvascular rarefaction. Hence, these findings relegate to Endo-MT the important role in the demise of phenotypical endothelial cells through the loss of their anticoagulatory, vasoregulatory, and anti-inflammatory functions to instead become conduits for microvascular obliteration.
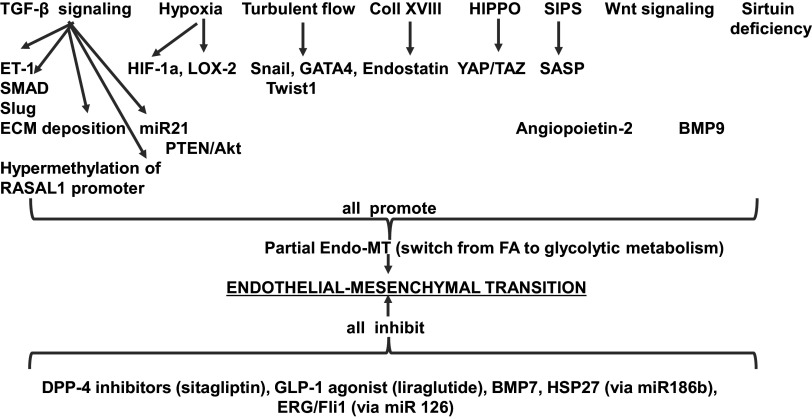
Major pathways triggering Endo-MT encompass TGF-β liberation and signaling, turbulent blood flow, hypoxia, stress-induced premature senescence (SIPS), activation of the HIPPO-YAP/TAZ pathway, endostatin signaling, and reduction of activity and expression of sirtuins, as recently summarized by Sun et al. (82) (schematically depicted in Fig. 3). Partial Endo-MT refers to the potentially reversible reprogramming toward mesenchymal cell phenotype, whereas complete Endo-MT presumes its irreversibility. This subdivision, though apparently helpful in the past, ignores the recent successes of cell reprogramming, which leads to the reversal of Endo-MT, as outlined below.
Figure 3.
Schematic depiction of forces promoting and impeding development of endothelial-to-mesenchymal transition (see text for details). BMP7, bone morphogenetic protein 7; SASP senescence-associated secretory product; SIPS, stress-induced premature senescence.
TGF-β1, a key fibrogenic cytokine, is a major mediator of Endo-MT, whereas another member of the same family, bone morphogenetic protein 7 (BMP7), is a factor counteracting Endo-MT, as demonstrated using an endothelial cell fate-tracing technique. Actions of TGF-β are mediated via alternative stimulation of activin receptor-like kinases 1 or 5 (Alk1 and Alk5). Activation of Alk1, selectively expressed on EC, is proangiogenic (cell migration and proliferation), whereas stimulation of Alk5 induces the transcription of SM22a, fibronectin, and plasminogen activator inhibitor-1 (PAI-1), all mediating the differentiation along the smooth muscle/mesenchymal phenotype of endothelial cells (Endo-MT) and leading to the formation of myofibroblasts. The molecular switch between activation of these two Alk pathways is regulated by endoglin, another specific endothelial TGF-β coreceptor. Prolonged activation with TGF-β1 results in the escape of Alk1 signaling and predominant signaling via Alk 5, thus promoting Endo-MT (83). Profibrogenic effects of TGF-β are achieved via canonical signaling pathway (Alk5 and phosphorylation of Smad transcription factors, P-Smad 2/3); noncanonical signaling (activation of MAPK eventually leading to phosphorylated ERK ½); epigenetic pathways Snail1 or Smad; and microRNAs (fibro-miR-494, miR-21, miR-374) (reviewed in Ref. 84). A recent study of Endo-MT showed that energy-supplying mitochondrial β-oxidation of long-chain fatty acids (FAO) in EC is inhibited by TGF-β signaling (85). Other physiological inhibitors of TGF-β signaling include basic FGF and BMP2, both capable of downregulating TGF-β receptor 1 (84).
Attempts to reverse endothelial-mesenchymal transition were reported. In our studies seeking to reverse Endo-MT, we’ve failed to demonstrate it (86). In contrast, in a model of cardiac fibrosis, Ubil et al. (87) used genetic fate map strategy to show that about one-third of fibroblasts in the postischemic heart gained expression of endothelial-specific markers. This process of mesenchymal-to-endothelial transition in the ischemic heart is governed by p53: its loss decreases, whereas its stimulation increases formation of fibroblast-derived endothelial cells, enhances vascular density in the postinfarcted myocardium, and improves cardiac function. It is noteworthy that p53 is also a senescent cell fate gatekeeper, regulating its apoptosis or persisting survival (reviewed in Ref. 88).
These studies represent earlier examples of senescence and premature senescence affecting organ functions, in particular, renal functions and their reversal by instituting rejuvenating therapies. More recently, this field has been dramatically expanded with the direct demonstration that elimination of p16Ink4a-positive senescent cells delays aging-associated disorders (89). Production of proinflammatory and profibrogenic senescence-associated secretory products (SASPs) appears to be primarily responsible for the propagation of the initial lesions, failed intrinsic regenerative mechanisms, and progression of chronic disease. Several excellent recent reviews detail these processes occurring in renal disease (88, 90–92) and examples of usage of senolytics, like quercetin, in reversing premature kidney senescence and dysfunction. Along with this came the realization that dysfunctional cells are the source of secretory signals, hampering regeneration instead promoting fibrosis (55), somewhat like senescence-associated secretory products; indeed, such cells show an increased frequency of premature stress-induced senescence. The corollary of this line of investigation is represented by attempts to manipulate the secretomes of dysfunctional endothelial or epithelial cells to mitigate fibrogenic programs in adjacent fibroblasts (reviewed in Ref. 55). Such an endothelial and myofibroblast reprogramming through “reconditioning” of cells and their microenvironment represents another emerging trend.
Progression of Fibrosis and Senescence
Both processes engage multicellularly coordinated responses of p53, p16, and p21, unless the stressor is transient. Stress-induced premature senescence (SIPS) is associated with secretion of fibrogenic products, like SASP, promoting irreversibility of fibrogenic processes, their propagation, and organ function demise (Fig. 4). It is remarkable that a host of mechanisms depicted in Fig. 4 (rejuvenation pathway) represents therapeutic targets to concomitantly attenuate SIPS and reduce fibrosis. This fact is suggestive of the importance of SIPS and SASP in the progression of fibrotic diseases.
Figure 4.
Molecular mechanisms leading from noxious stressors to premature cell senescence (SIPS), loss of endothelial functions, cessation of blood flow, and obliteration of the vessel; pathways for their reversibility and persistence. Individual mechanisms are shown in green. Potential consequences of SIPS—irreversibility, propagation of SIPS via senescence-associated secretory products (SASPs), endothelial dysfunction, cell death and senolysis, and cell rejuvenation or fibrosis are shown in yellow boxes. SIPS, stress-induced premature senescence; CR, calorie restriction. For additional mechanistic details, the reader is referred to Refs. 88 and 92.
In the interplay between stressors and vascular endothelium, ECD, Endo-MT, SIPS, and SASP are instruments of developing microvascular rarefaction (Fig. 5) (three probable scenarios are outlined). It is remarkable that angiocrine signals coming from intact vascular endothelium are supportive of parenchymal cells in different organs; dysfunctional endothelial cells not only lack this ability but also aggravate injury to parenchymal cells and fibrogenesis (95, 96). These studies have also demonstrated that normalization of angiocrine signaling attenuates and reverses fibrosis. As there is a distinct overlap between physiological versus pathological angiocrine signaling, on the one hand, and the secretome of intact cells versus SASP, it wouldn’t be a far-fetched conclusion that restoration of microvascular function is capable of alleviating fibrosis.
Figure 5.
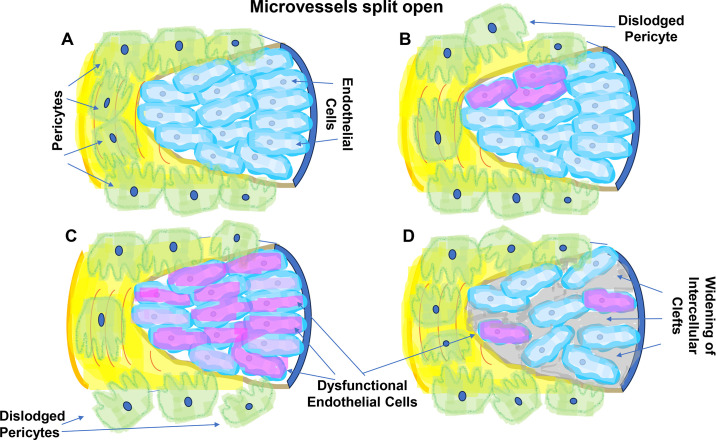
Scenarios depicting possible interplay between fibrosis and microvascular rarefaction. A: a schematic view of an open vessel with visible endothelial cells (light blue), surrounded by pericytes (green). B: diverse stressors lead to detachment of pericytes from the basement membrane resulting in diminution of prosurvival signaling by pericytes and damage to the adjacent endothelial cells, eventuating in the collapse of the capillary bed. C: local or global endothelial dysfunction (shown in shades of violet) characterized by defective NO synthesis leads, among others, to reduced NAD and sirtuins activity, development of stress-induced premature senescence, and generation of fibrogenic and anti-angiogenic secretome; pericytes detach from the basement membrane to further compound vasculopathy; D: stressors activate endothelial cells (shown in shades of violet) followed by breakdown of permeability barrier (gray) to proteins (93) (i.e., fibrin and fibronectin), eventually stiffening the ECM and promoting mobilization of pericytes, formation of myofibroblasts, endothelial dysfunction, fibrogenesis, and eventually vessel dropout. The levels of VEGF may be even elevated, which adds to increased vesicular permeability, but compensatory angiogenesis remains frustrated, as, for example, in hyperglycemic microenvironment characterized by refractoriness to VEGF despite its elevated expression (94). ECM, extracellular matrix.
POTENTIAL PHARMACOLOGICAL MANIPULATION OF MICROVASCULAR RAREFACTION AND FIBROSIS
As discussed earlier, the brunching chain of events initiated by chemical, infectious, metabolic, or other stressors culminating in endothelium-assisted fibrogenesis is potentially helpful in defining therapeutic targets. The course of fibrotic progression, though often indolent, is remarkably unrelenting. Nonetheless, glimpses at potential therapeutic interventions are continuously emerging. Though their detailed description and analyses are beyond the scope of the present essay, it would be amiss not to mention some of those strategies relevant to vascular endothelial involvement and how they could change the slope of progression (here portrayed with the Gompertz curve), as shown in Fig. 6 and Table 1.
Figure 6.
A Gompertz-like curve depicting mediators and inhibitors of progression of fibrosis. A categorical list of groups of factors potentially accelerating or inhibiting fibrosis. For a complete list of participating transcription factors (TFs), epigenetic regulators, and growth factors, the interested reader is referred to Hinz et al. (11, 97) In addition, mesenchymal stromal cells have been advocated for treatment of fibrotic conditions in different organs (98). Sulindac has recently been introduced for prevention of Endo-MT (80) in a model of cerebrovascular malformation associated with Endo-MT. ECD, endothelial cell dysfunction; Endo-MT, endothelial-mesenchymal transition.
Table 1.
Therapeutic strategies based on the knowledge of endothelial contribution to fibrosis (see the text for details)
| Targets | Potential Therapeutic Interventions |
|---|---|
| Increased vascular permeability | Discontinue action of a stressor, anti-inflammatory agents, recombinant kallikrein-related peptidase-10, Hepta-Ang1, follistatin-like 1 protein |
| “Straightjacket” TGF-β and myofibroblast formation | Softening of the ECM, inhibitors of transglutaminase-1, lysyl oxidases, and advanced glycation end-products, PIEZO-1 and Notch 1 |
| Endothelial cell dysfunction | NO donors, activation of sirtuins, NAD, rapalogs |
| SIPS | Sirtuins, rapalogs, calorie restriction, senolytics |
| Prevent/reverse Endo-MT | Sitagliptin, liraglutide, manipulate p53 expression |
| Degraded glycocalyx | Sphingosine-1-phosphate, inhibitors of sheddases (doxycycline, Zn-chelators, heparinase inhibitors), metformin, recombinant syndecan-1 |
ECM, extracellular matrix; Endo-MT, endothelial-mesenchymal transition; NO, nitric oxide; SIPS, stress-induced premature senescence.
Curtailing increased vascular endothelial permeability obviously would require cessation of the primary insult with or without use of anti-inflammatory medications. Enhancement of endothelial barrier function could be achieved using a recombinant kallikrein-related peptidase-10 (the precise mechanism remains unknown) (47). Another enhancer of the barrier function is angiopoietin-1. Studies by Li et al. (99) have established the role of endothelial-specific angiopoietin1-Tie2 signaling in ameliorating ischemic kidney disease. The investigators used an angiopoietin-mimetic Hepta-ANG1 to demonstrate its renoprotective efficacy, probably involving normalization of paracellular permeability, but also, as discussed earlier, this signaling pathway is essential in providing survival signals to damaged endothelium and, therefore, preventing vascular rarefaction. On the other hand, transcellular permeability could be ameliorated with the use of follistatin-like 1 (100), a protein secreted by intact endothelium subjected to undisturbed flow. Claesson-Welsh et al. (19) advocate the use of drugs that block VEGF-A or VEGF receptor-2 to reduce vascular leakage in general. It is not at all clear how harmful such a therapy could be for kidney disease.
Inhibitors of Endo-MT have been summarized in Fig. 3. Pharmacological application of dipeptidyl peptidase-4 (DPP-4) inhibitors (e.g., sitagliptin) or glucagon-like protein 1 (GLP-1) agonists (e.g., liraglutide) has not been explored in kidney fibrosis.
Attempts to recondition damaged endothelial glycocalyx have uncovered therapeutic role for the sphingosine-1-phosphate (101) that normalized vascular permeability while preserving glycocalyx. Along these lines, endothelial cell reconditioning (102) could be achieved using sheddases inhibitors, like doxycycline, sphingosine-1-phosphate, or Zn-chelators to protect proteoglycans; heparanase inhibitors to protect heparan sulfate; or metformin to prevent degradation of hyaluronan. The first in vivo attempt to restore postmyocardial infarction cardiac endothelial glycocalyx using recombinant syndecan-1 has been reported (103).
The role of p53 in mesenchymal-endothelial transition has been demonstrated (87). These investigators used genetic fate mapping to demonstrate that cardiac fibroblasts acquire an endothelial cell-like phenotype after acute ischemic cardiac injury and that transcription factor p53 regulates this switch. These findings were corroborated in loss-of-function and gain-of-function experiments manipulating the expression of p53, thus establishing that stimulation of the p53 pathway in cardiac fibroblasts results in augmentation of mesenchymal-to-endothelial transition, enhancement of vascular density, and improvement of cardiac function, all occurring in vivo and contributing to neovascularization of the injured heart. Whether a similar strategy could be used in preventing/reversing kidney fibrosis remains to be seen.
Several therapeutic approaches are based on attempts to reduce tissue stiffness. Those include development of inhibitors of cross linkers like transglutaminase-2 (catalyzes γ-glutamyl-lysine bonds resistant to proteolytic degradation), lysyl oxidases (catalyze hydroxy-pyridinium cross links), and inhibitors of advanced glycosylation end-products (form cyclic cross links binding to terminal amino groups) (12). Additional targets for curtailing mechanotransduction include trials to inhibit mechanosensing via PIEZO-1 and Notch 1 inhibitors and integrins inhibitors. Actual ongoing clinical trials have been summarized elsewhere (104).
A modified fragment of C-terminal end of collagen XVIII/endostatin, the recombinant fusion protein, END55, has been shown to exert antifibrotic effects in lung fibrosis models and in humans with idiopathic pulmonary fibrosis or systemic sclerosis by reducing the expression of genes encoding matrix proteins (105). Inhibition of glutaminase-1 has been advocated to reduce matrix deposition and attenuate experimental pulmonary fibrosis (106).
The expanding body of therapeutics trialed to alleviate ECD and premature endothelial cell senescence has been recently reviewed (107–109). Those tend to prevent Endo-MT, rejuvenate endothelial cells, reduce senescence-associated secretory products, and ablate senescent cells. Henderson et al. (6) and Zhao et al. (110) have recently summarized a score of ongoing clinical trials. Interested readers are referred to this comprehensive review.
In conclusion, my premise to follow the chain of pathogenetic events involving endothelial cells, namely increased permeability–stiffening of the matrix–endothelial dysfunction–microvascular rarefaction–tissue fibrosis, has culminated in a rational outline of potential therapeutic interventions. As schematically shown in Fig. 6, any exaggerated fibrotic response (shown as a shift of Gompertz curve to the left) and vascular dropout are precursors of functional decline of an organ. Attempts are on the way to arrest and even partially reverse structural distortion and functional defects by targeting the vascular endothelium, which plays a critical permissive role in fibrosis (all shown as a shift of Gompertz curve to the right). Hopefully, the results of these attempts will not take long to materialize.
GRANTS
Studies in the author’s laboratory are partially supported by the National Institutes of Health grant HL144528 and New York Community Trust funds for research and education.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.S.G. prepared figures; drafted manuscript.
ACKNOWLEDGMENTS
I apologize to many authors whose work has not been cited here due to space constraints.
REFERENCES
- 1. Kramann R, DiRocco DP, Humphreys BD. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J Pathol 231: 273–289, 2013. doi: 10.1002/path.4253. [DOI] [PubMed] [Google Scholar]
- 2. Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int 87: 297–307, 2015. doi: 10.1038/ki.2014.287. [DOI] [PubMed] [Google Scholar]
- 3. Kuppe C, Kramann R. Role of mesenchymal stem cells in kidney injury and fibrosis. Curr Opin Nephrol Hypertens 25: 372–377, 2016. doi: 10.1097/MNH.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 4. Kuppe C, Ibrahim MM, Kranz J, Zhang X, Ziegler S, Perales-Patón J, Jansen J, Reimer KC, Smith JR, Dobie R, Wilson-Kanamori JR, Halder M, Xu Y, Kabgani N, Kaesler N, Klaus M, Gernhold L, Puelles VG, Huber TB, Boor P, Menzel S, Hoogenboezem RM, Bindels EMJ, Steffens J, Floege J, Schneider RK, Saez-Rodriguez J, Henderson NC, Kramann R. Decoding myofibroblast origins in human kidney fibrosis. Nature 589: 281–286, 2021. doi: 10.1038/s41586-020-2941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014. Jun doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature 587: 555–566, 2020. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorrell JM, Caplan AI. Fibroblasts – a diverse population at the center of it all. Int Rev Cell Mol Biol 276: 161–214, 2009. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 8. Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR Jr, Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol 14: 1251–1260, 2012. doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151, 2014. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asada N, Takase M, Nakamura J, Oguchi A, Asada M, Suzuki N, Yamamura K, Nagoshi N, Shibata S, Rao TN, Fehling HJ, Fukatsu A, Minegishi N, Kita T, Kimura T, Okano H, Yamamoto M, Yanagita M. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest 121: 3981–3990, 2011. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech 43: 146–155, 2010. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 12. Long Y, Niu Y, Liang K, Du Y. Mechanical communication in fibrosis progression. Trends Cell Biol 32: 70–90, 2022. doi: 10.1016/j.tcb.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 13. Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med 2: a006544, 2012. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-β structure and activation. Nature 474: 343–349, 2011. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol 21: 2046–2054, 2011. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 16. Soares CLR, Wilairatana P, Silva LR, Moreira PS, Vilar Barbosa NMM, da Silva PR, Coutinho HDM, de Menezes IRA, Felipe CFB. Biochemical aspects of the inflammatory process: a narrative review. Biomed Pharmacother 168: 115764, 2023. doi: 10.1016/j.biopha.2023.115764. [DOI] [PubMed] [Google Scholar]
- 17. Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46: 927–942, 2017. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510: 92–101, 2014. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Claesson-Welsh L, Dejana E, McDonald DM. Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol Med 27: 314–331, 2021. doi: 10.1016/j.molmed.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 10: 923–934, 2008. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 21. Broermann A, Winderlich M, Block H, Frye M, Rossaint J, Zarbock A, Cagna G, Linnepe R, Schulte D, Nottebaum AF, Vestweber D. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med 208: 2393–2401, 2011. doi: 10.1084/jem.20110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang G, Tiemeier GL, van den Berg BM, Rabelink TJ. Endothelial glycocalyx hyaluronan: regulation and role in prevention of diabetic complications. Am J Pathol 190: 781–790, 2020. doi: 10.1016/j.ajpath.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 23. Goligorsky MS. The cell “coat of many colors”. Am J Pathol 190: 728–731, 2020. doi: 10.1016/j.ajpath.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 24. Butler MJ, Down CJ, Foster RR, Satchell SC. The pathological relevance of increased endothelial glycocalyx permeability. Am J Pathol 190: 742–751, 2020. doi: 10.1016/j.ajpath.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dogné S, Rath G, Jouret F, Caron N, Dessy C, Flamion B. Hyaluronidase 1 deficiency preserves endothelial function and glycocalyx integrity in early streptozotocin-induced diabetes. Diabetes 65: 2742–2753, 2016. doi: 10.2337/db15-1662. [DOI] [PubMed] [Google Scholar]
- 26. Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol 80: 389–402, 2015. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 108 (Pt 6): 2369–2379, 1995. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 28. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039, 1995. [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J, Braet F, Brodsky S, Weinstein T, Romanov V, Noiri E, Goligorsky MS. VEGF-induced mobilization of caveolae and increase in permeability of endothelial cells. Am J Physiol Cell Physiol. 282: C1053–C1063, 2002. doi: 10.1152/ajpcell.00292.2001. [DOI] [PubMed] [Google Scholar]
- 30. Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science 357: 2579, 2017. doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 31. Marcu R, Choi YJ, Xue J, Fortin CL, Wang Y, Nagao RJ, Xu J, MacDonald JW, Bammler TK, Murry CE, Muczynski K, Stevens KR, Himmelfarb J, Schwartz SM, Zheng Y. Human organ-specific endothelial cell heterogeneity. iScience 4: 20–35, 2018. doi: 10.1016/j.isci.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dumas SJ, Meta E, Borri M, Goveia J, Rohlenova K, Conchinha NV, Falkenberg K, Teuwen LA, de Rooij L, Kalucka J, Chen R, Khan S, Taverna F, Lu W, Parys M, De Legher C, Vinckier S, Karakach TK, Schoonjans L, Lin L, Bolund L, Dewerchin M, Eelen G, Rabelink TJ, Li X, Luo Y, Carmeliet P. Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J Am Soc Nephrol 31: 118–138, 2020. doi: 10.1681/ASN.2019080832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dumas SJ, Meta E, Borri M, Luo Y, Li X, Rabelink TJ, Carmeliet P. Phenotypic diversity and metabolic specialization of renal endothelial cells. Nat Rev Nephrol 17: 441–464, 2021. doi: 10.1038/s41581-021-00411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fließer E, Lins T, Berg JL, Kolb M, Kwapiszewska G. The endothelium in lung fibrosis: a core signaling hub in disease pathogenesis? Am J Physiol Cell Physiol 325: C2–C16, 2023. doi: 10.1152/ajpcell.00097.2023. [DOI] [PubMed] [Google Scholar]
- 35. Majno G, Shea SM, Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol 42: 647–672, 1969. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goligorsky MS. Endothelial cell dysfunction: can’t live with it, how to live without it. Am J Physiol Renal Physiol 288: F871–F880, 2005. May doi: 10.1152/ajprenal.00333.2004. [DOI] [PubMed] [Google Scholar]
- 37. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 18: 684–696, 2019. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 38. Vinters HV, Zarow C, Borys E, Whitman JD, Tung S, Ellis WG, Zheng L, Chui HC. Review: vascular dementia: clinicopathologic and genetic considerations. Neuropathol Appl Neurobiol 44: 247–266, 2018. doi: 10.1111/nan.12472. [DOI] [PubMed] [Google Scholar]
- 39. Rajani RM, Quick S, Ruigrok SR, Graham D, Harris SE, Verhaaren BFJ, Fornage M, Seshadri S, Atanur SS, Dominiczak AF, Smith C, Wardlaw JM, Williams A. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci Transl Med 10: eaam9507, 2018. doi: 10.1126/scitranslmed.aam9507. [DOI] [PubMed] [Google Scholar]
- 40. Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA 107: 10268–10273, 2010. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA 108: 10355–10360, 2011. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol 300: H1762–H1769, 2011. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol 32: 979–987, 2012. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mowbray AL, Kang DH, Rhee SG, Kang SW, Jo H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem 283: 1622–1627, 2008. doi: 10.1074/jbc.M707985200. [DOI] [PubMed] [Google Scholar]
- 45. Mahmoud MM, Kim HR, Xing R, Hsiao S, Mammoto A, Chen J, Serbanovic-Canic J, Feng S, Bowden NP, Maguire R, Ariaans M, Francis SE, Weinberg PD, van der Heiden K, Jones EA, Chico TJ, Ridger V, Evans PC. TWIST1 integrates endothelial responses to flow in vascular dysfunction and atherosclerosis. Circ Res 119: 450–462, 2016. doi: 10.1161/CIRCRESAHA.116.308870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, Dharbandi F, Khalsa B, Bressler S, Barr ML, Li R, Hsiai TK. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal 15: 1379–1388, 2011. doi: 10.1089/ars.2010.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McQueen A, Warboys CM. Mechanosignalling pathways that regulate endothelial barrier function. Curr Opin Cell Biol 84: 102213, 2023. doi: 10.1016/j.ceb.2023.102213. [DOI] [PubMed] [Google Scholar]
- 48. Davis MJ, Earley S, Li YS, Chien S. Vascular mechanotransduction. Physiol Rev 103: 1247–1421, 2023. doi: 10.1152/physrev.00053.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Riordan E, Mendelev N, Patschan S, Patschan D, Eskander J, Cohen-Gould L, Chander P, Goligorsky MS. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol Heart Circ Physiol 292: H285–H294, 2007. doi: 10.1152/ajpheart.00560.2006. [DOI] [PubMed] [Google Scholar]
- 50. Lipphardt M, Song JW, Matsumoto K, Dadafarin S, Dihazi H, Müller G, Goligorsky MS. The third path of tubulointerstitial fibrosis: aberrant endothelial secretome. Kidney Int 92: 558–568, 2017. doi: 10.1016/j.kint.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vasko R, Xavier S, Chen J, Lin CH, Ratliff B, Rabadi M, Maizel J, Tanokuchi R, Zhang F, Cao J, Goligorsky MS. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: relevance to fibrosis of vascular senescence. J Am Soc Nephrol 25: 276–291, 2014. doi: 10.1681/ASN.2013010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21: 2644–2658, 2007. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang XZ, Wen D, Zhang M, Xie Q, Ma L, Guan Y, Ren Y, Chen J, Hao CM. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J Cell Biochem 115: 996–1005, 2014. doi: 10.1002/jcb.24748. [DOI] [PubMed] [Google Scholar]
- 54. Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, Chander PN, Goligorsky MS. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol 26: 817–829, 2015. doi: 10.1681/ASN.2013101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lipphardt M, Dihazi H, Müller GA, Goligorsky MS. Fibrogenic secretome of sirtuin 1-deficient endothelial cells: Wnt, Notch and Glycocalyx rheostat. Front Physiol 9: 1325, 2018. doi: 10.3389/fphys.2018.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lipphardt M, Song JW, Ratliff BB, Dihazi H, Müller GA, Goligorsky MS. Endothelial dysfunction is a superinducer of syndecan-4: fibrogenic role of its ectodomain. Am J Physiol Heart Circ Physiol 314: H484–H496, 2018. doi: 10.1152/ajpheart.00548.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin CH, Chen J, Zhang Z, Johnson GV, Cooper AJ, Feola J, Bank A, Shein J, Ruotsalainen HJ, Pihlajaniemi TA, Goligorsky MS. Endostatin and transglutaminase 2 are involved in fibrosis of the aging kidney. Kidney Int 89: 1281–1292, 2016. doi: 10.1016/j.kint.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Folkow B. The fourth Volhard lecture: cardiovascular structural adaptation; its role in the initiation and maintenance of primary hypertension. Clin Sci Mol Med Suppl 4: S3–S22, 1978. doi: 10.1042/cs055003s. [DOI] [PubMed] [Google Scholar]
- 59. Sládek T, Gerová M, Znojil V, Devát L. Morphometric characteristics of cardiac hypertrophy induced by long-term inhibition of NO synthase. Physiol Res 45: 335–338, 1996. [PubMed] [Google Scholar]
- 60. Okruhlicova L, Tribulova N, Weismann P, Sotnikova R. Ultrastructure and histochemistry of rat myocardial capillary endothelial cells in response to diabetes and hypertension. Cell Res 15: 532–538, 2005. doi: 10.1038/sj.cr.7290322. [DOI] [PubMed] [Google Scholar]
- 61. Keshet E. Preventing pathological regression of blood vessels. J Clin Invest 112: 27–29, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goligorsky MS. Microvascular rarefaction: the decline and fall of blood vessels. Organogenesis 6: 1–10, 2010. doi: 10.4161/org.6.1.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Montagna W, Carlisle K. Structural changes in aging human skin. J Invest Dermatol 73: 47–53, 1979. doi: 10.1111/1523-1747.ep12532761. [DOI] [PubMed] [Google Scholar]
- 64. Paavonsalo S, Hariharan S, Lackman MH, Karaman S. Capillary rarefaction in obesity and metabolic diseases – organ-specificity and possible mechanisms. Cells 9: 2683, 2020. doi: 10.3390/cells9122683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285, 1997. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 66. Folkman J. Antiangiogenesis in cancer therapy—endostatin and its mechanisms of action. Exp Cell Res 312: 594–607, 2006. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 67. Chen CG, Iozzo RV. Angiostatic cues from the matrix: endothelial cell autophagy meets hyaluronan biology. J Biol Chem 295: 16797–16812, 2020. doi: 10.1074/jbc.REV120.014391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Poluzzi C, Iozzo RV, Schaefer L. Endostatin and endorepellin: a common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev 97: 156–173, 2016. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Korn C, Augustin HG. Mechanisms of vessel pruning and regression. Dev Cell 34: 5–17, 2015. doi: 10.1016/j.devcel.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 70. Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development 141: 1757–1766, 2014. doi: 10.1242/dev.104422. [DOI] [PubMed] [Google Scholar]
- 71. Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121: 2278–2289, 2011. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Daly C, Eichten A, Castanaro C, Pasnikowski E, Adler A, Lalani AS, Papadopoulos N, Kyle AH, Minchinton AI, Yancopoulos GD, Thurston G. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res 73: 108–118, 2013. doi: 10.1158/0008-5472.CAN-12-2064. [DOI] [PubMed] [Google Scholar]
- 73. McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med 9: 713–725, 2003. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 74. Brown WR. A review of string vessels or collapsed, empty basement membrane tubes. J Alzheimers Dis 21: 725–739, 2010. doi: 10.3233/JAD-2010-100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 76. Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol 175: 1380–1388, 2009. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kato T, Mizuno S, Ito A. A decrease in glomerular endothelial cells and endothelial-mesenchymal transition during glomerulosclerosis in the tensin2-deficient mice (ICGN strain). Acta Histochem Cytochem 47: 265–271, 2014. doi: 10.1267/ahc.14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053, 2013. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498: 492–496, 2013. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 80. Bravi L, Rudini N, Cuttano R, Giampietro C, Maddaluno L, Ferrarini L, Adams RH, Corada M, Boulday G, Tournier-Lasserve E, Dejana E, Lampugnani MG. Sulindac metabolites decrease cerebrovascular malformations in CCM3-knockout mice. Proc Natl Acad Sci USA 112: 8421–8426, 2015. doi: 10.1073/pnas.1501352112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xu X, Friehs I, Zhong Hu T, Melnychenko I, Tampe B, Alnour F, Iascone M, Kalluri R, Zeisberg M, Del Nido PJ, Zeisberg EM. Endocardial fibroelastosis is caused by aberrant endothelial to mesenchymal transition. Circ Res 116: 857–866, 2015. doi: 10.1161/CIRCRESAHA.116.305629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sun X, Nkennor B, Mastikhina O, Soon K, Nunes SS. Endothelium-mediated contributions to fibrosis. Semin Cell Dev Biol 101: 78–86, 2020. doi: 10.1016/j.semcdb.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 83. Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29: 630–638, 2009. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 84. Alvandi Z, Bischoff J. Endothelial-mesenchymal transition in cardiovascular disease. Arterioscler Thromb Vasc Biol 41: 2357–2369, 2021. doi: 10.1161/ATVBAHA.121.313788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xiong J, Kawagishi H, Yan Y, Liu J, Wells QS, Edmunds LR, Fergusson MM, Yu ZX, Rovira II, Brittain EL, Wolfgang MJ, Jurczak MJ, Fessel JP, Finkel T. A metabolic basis for endothelial-to-mesenchymal transition. Mol Cell 69: 689–698.e7, 2018. doi: 10.1016/j.molcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Matsumoto K, Xavier S, Chen J, Kida Y, Lipphardt M, Ikeda R, Gevertz A, Caviris M, Hatzopoulos AK, Kalajzic I, Dutton J, Ratliff BB, Zhao H, Darzynkiewicz Z, Rose-John S, Goligorsky MS. Instructive role of the microenvironment in preventing renal fibrosis. Stem Cells Transl Med 6: 992–1005, 2017. doi: 10.5966/sctm.2016-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 514: 585–590, 2014. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Goligorsky MS. Chronic kidney disease: a vicarious relation to premature cell senescence. Am J Pathol 190: 1164–1171, 2020. doi: 10.1016/j.ajpath.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2011. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol 13: 77–89, 2017. doi: 10.1038/nrneph.2016.183. [DOI] [PubMed] [Google Scholar]
- 91. Goligorsky MS. Chapter 19: stress-induced senescence as a forme fruste of chronic kidney disease—a case for failed regeneration. In: Regenerative Nephrology (2nd ed.)., edited by Goligorsky MS. London: Academic Press, 2022. [Google Scholar]
- 92. Docherty MH, O'Sullivan ED, Bonventre JV, Ferenbach DA. Cellular senescence in the kidney. J Am Soc Nephrol 30: 726–736, 2019. doi: 10.1681/ASN.2018121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens 20: 297–305, 2011. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 94. Kim BS, Chen J, Weinstein T, Noiri E, Goligorsky MS. VEGF expression in hypoxia and hyperglycemia: reciprocal effect on branching angiogenesis in epithelial-endothelial co-cultures. J Am Soc Nephrol 13: 2027–36, 2002. doi: 10.1097/01.asn.0000024436.00520.d8. [DOI] [PubMed] [Google Scholar]
- 95. Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505: 97–102, 2014. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, Fong GH, Sakmar TP, Rafii S, Ding BS. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med 22: 154–162, 2016. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180: 1340–1355, 2012. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis: the mesenchymal stromal cells breakthrough. Stem Cells Int, 2014: 340257, 2014. doi: 10.1155/2014/340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li Y, Liu P, Zhou Y, Maekawa H, Silva JB, Ansari MJ, Boubes K, Alia Y, Deb DK, Thomson BR, Jin J, Quaggin SE. Activation of angiopoietin-Tie2 signaling protects the kidney from ischemic injury by modulation of endothelial-specific pathways. J Am Soc Nephrol 34: 969–987, 2023. doi: 10.1681/ASN.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ghim M, Pang KT, Burnap SA, Baig F, Yin X, Arshad M, Mayr M, Weinberg PD. Endothelial cells exposed to atheroprotective flow secrete follistatin-like 1 protein which reduces transcytosis and inflammation. Atheroscler 333: 56–66, 2021. doi: 10.1016/j.atherosclerosis.2021.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang L, Zeng M, Fan J, Tarbell JM, Curry FR, Fu BM. Sphingosine-1-phosphate maintains normal vascular permeability by preserving endothelial surface glycocalyx in intact microvessels. Microcirculation 23: 301–310, 2016. doi: 10.1111/micc.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ishiko S, Goligorsky MS. Ways and means of cellular reconditioning for kidney regeneration. Am J Nephrol 53: 96–107, 2022. doi: 10.1159/000522050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Vahldieck C, Cianflone E, Fels B, Löning S, Depelmann P, Sabatino J, Salerno N, Karsten CM, Torella D, Weil J, Sun D, Goligorsky MS, Kusche-Vihrog K. Endothelial glycocalyx and cardiomyocyte damage is prevented by recombinant syndecan-1 in acute myocardial infarction. Am J Pathol 193: 474–492, 2023. doi: 10.1016/j.ajpath.2022.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci Transl Med 10: eaa0475, 2018. doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- 105. Mlakar L, Garrett SM, Watanabe T, Sanderson M, Nishimoto T, Heywood J, Helke KL, Pilewski JM, Herzog EL, Feghali-Bostwick C. Ameliorating fibrosis in murine and human tissues with END55, an endostatin-derived fusion protein made in plants. Biomedicines 10: 2861, 2022. doi: 10.3390/biomedicines10112861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cui H, Xie N, Jiang D, Banerjee S, Ge J, Sanders YY, Liu G. Inhibition of glutaminase 1 attenuates experimental pulmonary fibrosis. Am J Respir Cell Mol Biol 61: 492–500, 2019. doi: 10.1165/rcmb.2019-0051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wilson RB, Lee JJ, Pickering GJ, Borradaile NM. Natural products in regeneration. In: Regenerative Nephrology (2nd ed.)., edited by Goligorsky MS. London: Academic Press, 2022, p. 419–437. [Google Scholar]
- 108. Zhu XY, Lerman LO. Senomorphic, senolytic, and rejuvenation therapies. In: Regenerative Nephrology (2nd ed.)., edited by Goligorsky MS. London: Academic Press, 2021, p. 405–417. [Google Scholar]
- 109. Ebert T, Stenvinkel P. Premature vascular aging and senescence in chronic kidney disease. In: Regenerative Nephrology (2nd ed.)., edited by Goligorsky MS. London: Academic Press, 2021, p. 263–279. [Google Scholar]
- 110. Zhao M, Wang L, Wang M, Zhou S, Lu Y, Cui H, Racanelli AC, Zhang L, Ye T, Ding B, Zhang B, Yang J, Yao Y. Targeting fibrosis, mechanisms and clinical trials. Signal Transduct Target Ther 7: 206, 2022. doi: 10.1038/s41392-022-01070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]