Keywords: biomarkers, extracellular matrix, matrix metalloproteinases, pathology, tissue inhibitors of metalloproteinases
Abstract
The tissue inhibitors of matrix metalloproteinases (TIMPs) are a family of four matrisome proteins classically defined by their roles as the primary endogenous inhibitors of metalloproteinases (MPs). Their functions however are not limited to MP inhibition, with each family member harboring numerous MP-independent biological functions that play key roles in processes such as inflammation and apoptosis. Because of these multifaceted functions, TIMPs have been cited in diverse pathophysiological contexts. Herein, we provide a comprehensive overview of the MP-dependent and -independent roles of TIMPs across a range of pathological conditions. The potential therapeutic and biomarker applications of TIMPs in these disease contexts are also considered, highlighting the biomedical promise of this complex and often misunderstood protein family.
INTRODUCTION
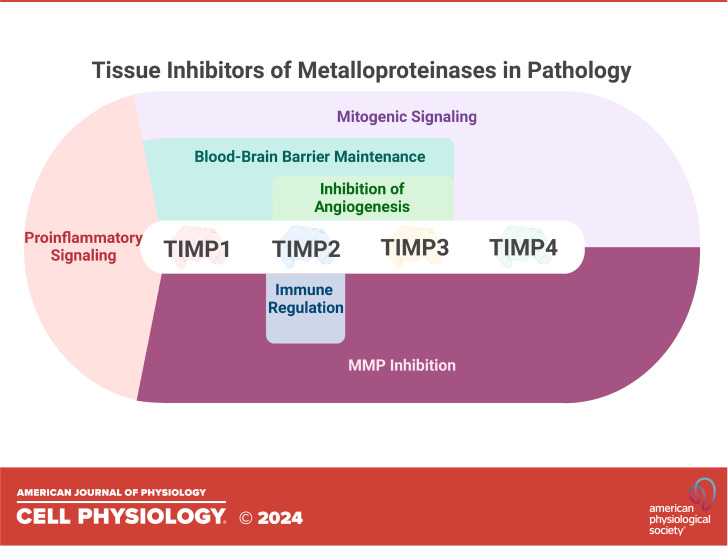
The extracellular matrix (ECM) is a dynamic network of secreted macromolecules that underpin all biological phenotypes in multicellular organisms. The influence of ECM structure and composition on cell phenotype, and vice versa, is referred to as dynamic reciprocity, characterized by reciprocal cross talk between the cellular and extracellular compartments (1). This primordial attribute relies on the continuous remodeling and reshaping of the ECM, a process that is driven by extracellular proteinases. The Matrixin and Adamalysin protease families are the major mediators of ECM degradation, which are members of the Metzincin superfamily of zinc-dependent proteases (2). The Matrixins, also known as matrix metalloproteinases (MMPs), are a family of ∼23 mammalian proteinases that have wide-ranging functions within the ECM and beyond. Similar to the MMPs, the Adamalysins are a large family of important proteinases that are highly active within the ECM, in particular the two subclasses of ADAMs (A Disintegrin and Metalloproteinase) and ADAMTS (ADAM with Thrombospondin Type I repeats). In line with all biological processes, homeostatic mechanisms exist to regulate metalloproteinase activity within tissues, including through degradative processes and direct inhibition. The major mediators of metalloproteinase inhibition are the tissue inhibitors of metalloproteinases (TIMPs). Four paralogous genes encoding TIMP 1 through 4 are conserved throughout mammalian genomes (3). Each contains six disulfide bridges forming six loop regions that produce similar tertiary structures, with sequence similarity between the four proteins ranging from 37 to 51% (4). Despite their sequence and structural similarity, each TIMP possesses unique characteristics and attributes. TIMP1 is an N-linked glycoprotein containing two glycosylation sites at N30 and N78 (5). Similarly, TIMP3 is an N-glycosylated (N184) protein that displays a unique affinity with the ECM through attachments with specific glycosaminoglycans, rendering this TIMP family member anchored with the ECM (6). Furthermore, TIMP3 displays the widest range of inhibitory targets, inhibiting all members of the MMP family, in addition to a range of ADAMs and ADAMTS (7). TIMP2 displays the broadest expression profile of the TIMP proteins and is ubiquitously expressed in normal human tissues (8). TIMP4, however, displays a limited expression profile, with expression detected primarily in the brain (cerebellum), heart, and adipose tissue (4, 9).
Although TIMPs have historically been defined by metalloproteinase inhibitory functions, they contain a wide range of MP inhibition-independent functions (8, 10, 11). TIMPs have a unique relationship with the gelatinases (MMP2 and MMP9), whereby TIMP1/3 can bind to proMMP9 in a noninhibitory complex, and TIMP2/3/4 can similarly bind to proMMP2. These relationships are well-characterized and have direct consequences on the activation of MMP2/9, reviewed extensively elsewhere (11). Furthermore, individual TIMP proteins have been shown to possess specific membrane receptors, including CD63 for TIMP1 and integrin α3β1 for TIMP2 (12–14). These interactions mediate observable signaling pathways that are involved in, but not limited to, granulopoiesis, migration, and growth inhibition (8, 15). The idea that TIMPs are not just tissue inhibitors of metalloproteinases is maturing, and it is anticipated that the catalog of MP-independent functions of TIMPs will continue to expand. Recent work investigating novel functions of TIMP2 has revealed a role in modulating myeloid cell populations (16), expanding on earlier work illustrating how TIMP2 could target myeloid-derived suppressor cells in cancer models (17). The mechanistic details underlying these discoveries are supported by further recent findings describing the expanded proximal interactome for TIMP2 (18).
Most, if not all, diseases in multicellular organisms exhibit signs of ECM dysfunction. In inflammatory conditions, this often manifests as degradation of local ECM architecture and subsequent remodeling to a disease-associated ECM that often amplifies disease phenotypes (19). The Matrixin and Adamalysin proteases play critical roles in these pathological processes, and in multiple instances, they are viable targets for therapeutic intervention (20–23). Consequently, imbalances between metalloproteinases and their inhibitors are often associated with disease etiology, indicative of a breakdown in the homeostatic mechanisms that regulate ECM degradation and remodeling (23, 24). This balance may prove critical, as it was recently described how a molar excess of active MMP9 could cleave and regulate TIMP function, an occurrence that could represent a tipping point for local ECM degradation during development and disease (25). Furthermore, other proteases can cleave TIMP proteins, such as human neutrophil elastase (HNE), which cleaves and inactivates at a site within TIMP1 that is conserved in TIMP2 and substituted in TIMP3/4 (valine to leucine, similar aliphatic hydrophobic amino acids) (26, 27). In addition, a recent report also reveals how ADAMTS7 could cleave TIMP1, modulating atherosclerotic plaque formation (28). Further comprehensive investigation is required to fully map the dynamic relationships between TIMPs and metalloproteinases.
CANCER AND THE TUMOR MICROENVIRONMENT
Excess metalloproteinase (MP) expression and activity have been explicitly linked with cancer for decades. Early findings culminated in the development of a series of broad-spectrum MP inhibitors that failed clinical trials due to a lack of efficacy and severe side-effects (29). Despite these trial failures, inhibition of MPs remains a viable therapeutic approach in oncology. The TIMP family has a complex relationship with cancer, which can be both tumor-suppressive and tumor-promoting. MPs can break down virtually every component of the ECM and their activity is often used by cancer cells to facilitate tumor invasion (23). The activity of different MPs within the tumor microenvironment (TME) affects many aspects of tumor progression. For example, MMP2, 9, and 14 can release the latent form of TGFβ from the ECM, later activating it, resulting in a decreased T-lymphocyte reaction, enhanced expression of several other MMPs, induction of epithelial-to-mesenchymal transition (EMT), and tumor cell migration (30). In this regard, the MP-inhibitory functions of TIMPs are often described to be tumor-suppressive (31). However, it is important to note that not all metalloproteinase activity within the tumor microenvironment is tumor-promoting, and there are various examples of tumor-suppressive metalloproteinase functions that may be lost with excess TIMP activity (19, 32). Notably, MMP8, which works to tighten the adhesions between tumor cells and the ECM, directly suppresses metastasis (32).
Of the TIMP family members, TIMP1 has the most consistent findings regarding its role in tumorigenesis. It is traditionally described as a driver of tumor progression through its distinct MP-independent functions (33). The range of functions TIMP1 exhibits is unique to its two distinct domains. The N-terminal domain is responsible for MP-inhibition, in addition to CD74 and CD82 binding (12, 15). The C-terminal domain of TIMP1 mediates binding to proMMP9 and CD63, the latter of which has been cited as a primary driver of TIMP1’s MP-independent functions and classification as a proinflammatory cytokine (15, 34). Indeed, spatial transcriptomics reveals that TIMP1 expression is strongly associated with regions of pulmonary inflammation, adding credence to this notion (9). A positive correlation between TIMP1 overexpression and disease progression/prognosis has been observed in multiple malignancies, in both solid and hematological tumors (35). Furthermore, the consequences of high TIMP1 levels can be influenced by the glycan status of TIMP1, as aberrant glycosylation of TIMP1 has been shown to decrease its ability to bind to and inhibit MPs (11, 33). Independent of MPs, TIMP1 can stimulate cell growth and displays antiapoptotic functions, contributing to tumor proliferation and migration. Cancer-associated fibroblasts (CAFs) have well-established protumorigenic roles in the TME, and TIMP1 has been reported to mediate fibroblast activation and survival in models of fibrosis through CD63 signaling (36–39). High TIMP1 expression within the tumor-associated stroma has been associated with the accumulation of CAFs within the TME in a manner that likely occurs through CD63 (40). Furthermore, multiple studies have shown that knockdown of TIMP1 results in an inhibition of CAF proliferation in addition to an overall decrease in cancer cell proliferation and metastasis (40–42).
TIMP2 has been the subject of conflicting reports regarding its role in tumor progression. Early studies revealed how TIMP2 could stimulate the proliferation of fibrosarcoma and normal fibroblasts in a dose-dependent manner (43). Later studies using the same fibrosarcoma cell line (HT1080) revealed that TIMP2 could promote an invasive phenotype through interaction with the membrane-type MMP, MMP14 (MT1-MMP) (44). In addition, the TIMP2-MMP14 interaction has been described to protect tumor cells from apoptosis through the Akt pathway (45). Despite these limited reports, numerous studies have described TIMP2 as a tumor suppressor. TIMP2 can indirectly inhibit receptor tyrosine kinase signaling through interaction with integrin α3β1, a mechanism that is mediated through the activity of the tyrosine phosphatase SHP1 (13, 46). This pathway leads to inhibition of growth factor-induced angiogenesis and proliferation in an MP-independent manner. Later studies revealed that TIMP2 could inhibit tumor growth, invasion, and metastasis in various solid cancer models (17, 47–50). In one of these studies that used an orthotopic model of triple-negative breast cancer, the authors found that daily treatment with recombinant TIMP2 decreased vascular permeability, suppressed tumor growth by up to 50%, and strongly inhibited pulmonary metastasis (48). Furthermore, TIMP2 has been shown to display immune regulatory functions, mediated through targeting of myeloid-derived suppressor cells and through inhibition of TGFβ-induced formation of decidual-like natural killer cells, which are classically protumorigenic. In addition, IGF1R is a key regulator of cell proliferation and angiogenesis, and therapeutic targeting of IGF1R has demonstrated promise for the treatment of solid tumors (51, 52). It has been shown that the loop 6 region of TIMP2 can bind to IGF1R in an antagonistic manner to inhibit angiogenesis in endothelial cells (53). Although TIMP2 expression is itself generally quite stable across the spectrum of human cancers, many of its molecular targets and interacting partners are not and show changes that likely result in altered biological activities for TIMP2 in the TME (24).
TIMP3 possesses antiproliferative/angiogenic/metastatic and proapoptotic functions that align with antitumor capabilities (11, 54). This is in line with multiple reports that describe a silencing and downregulation of TIMP3 expression across a range of cancer types (11, 24, 55–57). Overexpression of TIMP3 inhibits tumor invasion and proliferation in vitro and in vivo across many cancer models, including prostate, thyroid, ocular, brain, and colorectal cancers (58–64). There are multiple mechanisms by which TIMP3 imparts these beneficial effects that are independent of MP inhibition. Its activity has been shown to stabilize important death receptor proteins thereby increasing the sensitivity of cancer cells to apoptosis, inhibit angiogenesis through competitive inhibition of VEGF at VEGFR2, and protect the ECM through the inhibition of a range of MMPs, ADAMs, and ADAMTSs (6, 65, 66). TIMP3 can inhibit the widest range of MPs, an attribute that is supported by its C-domain glycosylation site. Mutations at this glycosylation site result in a significant reduction of TIMP3’s MP-binding affinities, suggesting that TIMP3’s unique glycosylation properties regulate its target-binding affinity (11, 67). Interestingly, recent reports describe how active MMP9 can cleave TIMP3 at its C-terminal tail, removing this glycosylation site and potentially producing a truncated TIMP3 with reduced metalloproteinase inhibitory capabilities (25). The effects exerted by TIMP3 on tumor progression may vary between early- and late-stage cancers. In a rare report describing a potential protumor role for TIMP3, it was found that the loss of TIMP3 resulted in delayed tumor initiation and tumor suppression during early-stage mammary tumorigenesis. This was postulated to involve an increase in the shedding of TNF receptor 1, directly as a result of loss of MP inhibition around the mammary epithelium. The same study also highlighted how tumor growth was accelerated in late-stage mammary tumors of TIMP3-deficient mice, highlighting the complex intricacies within the outcomes of TIMP expression at different stages of tumor progression (68).
The mechanisms by which TIMP4 is involved in cancer progression are not well studied, likely due to its highly restricted expression. Within the limited reports available, it has been described that TIMP4 regulates cancer by stabilizing the tumor progenitor cell population. Consequently, upregulation of TIMP4 was found to correspond with rapid tumor growth in nude mouse models (69). Another study found that primary breast tumors with increased levels of TIMP4 were associated with a decreased likelihood of long-term disease-free survival, and an average survival of less than 3 years indicating that TIMP4 may serve as a useful biomarker for aggressive breast carcinomas. The study also showed that patients with early-stage infiltrating ductal carcinoma expressing TIMP4 had on average shorter cancer-free periods, compared with their TIMP4-negative counterparts (70). Like TIMP1, TIMP4 can bind to CD63, although the effects of this interaction remain unstudied (71). The roles of each of the TIMP proteins in cancer are outlined in Fig. 1.
Figure 1.
Summary of the MMP-independent functions of TIMPs 1–3 in the tumor microenvironment. Created with BioRender.com. MMP, matrix metalloproteinase; TIMPs, tissue inhibitors of matrix metalloproteinases.
DEMENTIA AND OTHER NEUROLOGICAL DISORDERS
Metalloproteinases play a multifaceted role in both the developing and adult brains and consequently are involved in the pathophysiology of ischemia, epilepsy, and dementias (72, 73). Dementia is characterized by cognitive decline that is severe enough to interfere with daily functioning. Alzheimer’s disease (AD) is the most common cause of dementia and occurs due to the accumulation of abnormal proteins within the brain (74, 75). Most research on AD focuses on 1) the accumulation of amyloid beta (Aβ) peptides that represent the predominant components of the β-amyloid plaques, 2) hyperphosphorylation of Tau protein, which in excess results in the formation of neurofibrillary tangles (NFTs), and 3) sustained inflammatory responses within AD brains (76, 77). The roles of TIMPs and MPs in AD progression are poorly defined, despite the established roles that MPs play in the processing of amyloid precursor protein (APP) and Aβ. Nonamyloidogenic (nontoxic) processing of APP is mainly performed by ADAM10 and ADAM17, which are inhibited by TIMP1/3 and TIMP3, respectively (78). Amyloidogenic processing of APP through sequential cleavage by β-secretases and γ-secretase produces Aβ, whose levels are tightly controlled due to their toxicity when in high abundance. MMPs such as MMP2/9 are Aβ-degrading enzymes that help to control the levels of Aβ in healthy tissues (79). As such, dysregulation within the TIMP-MP system may play an important role in the early stages of AD and related dementias. In line with all chronic diseases, inflammation (neuroinflammation) is a major component of the pathogenesis of AD for which MPs (and by association, their inhibitors) play a key role (80, 81).
TIMPs can impact AD progression via both MP-dependent and MP-independent mechanisms. TIMP1 displays enriched expression in the cerebral cortex and the thalamus, and its activity is frequently described as protective in models of AD, often through stimulated cell survival and protection of the blood-brain barrier (BBB), a mechanism that likely relies on interaction with CD63 (82–87). Interestingly, TIMP1 has been described as a ligand for APP in monocytes, suggesting a potential role for TIMP1 in the biology and/or pathogenesis of APP in the brain (88). TIMP2 is ubiquitously expressed throughout the brain and, like TIMP1, it has reported beneficial effects on BBB function that could prove beneficial in AD (85, 89). Several reports have described how TIMP2 can regulate neuronal function, while others have described how circulating TIMP2 can support profound beneficial effects on aged brains, the predominant risk factor for AD (90–94). Specifically in AD, a unique population of AD-limiting glial cells referred to as disease-associated microglia are neuroprotective, and are defined by a series of markers that include TIMP2 (95). Whether TIMP2 plays a role in these microglial-dependent neuroprotective functions remains to be investigated. TIMP3 displays low regional specificity in the brain, with the choroid plexus being the sole region with a moderate expression level (85). Consequently, TIMP3’s functions are poorly defined in AD, despite its inhibitory actions against APP-targeting ADAM10 and ADAM17. The sparse reports describing TIMP3 prevalence in AD are limited to a few inconclusive studies, with an individual report describing how TIMP3 supports AD pathology through inhibition of ADAM10/17 (96). On the contrary, TIMP3 levels in cerebrospinal fluid have been reported to negatively correlate with cognitive decline in AD cases (97). Similar across the TIMPs, TIMP3 has reported beneficial effects supporting BBB function, thus TIMP3 may display conflicting effects upon disease etiology (98, 99). TIMP4 exhibits minimal expression within the central nervous system (CNS), with transcript detection limited to the cerebellum (85). This restricted expression has produced few studies investigating potential roles for TIMP4 AD, with individual reports purporting TIMP4 as a serum biomarker in AD (97, 100–102).
Multiple sclerosis (MS) is an autoimmune disorder caused by the immune cell targeting of the myelin sheath surrounding neurons. In MS, the myelin sheath is degraded leading to the formation of scar tissues called sclerosis, causing nerve damage manifesting as neurological disabilities, decreased motor capabilities, muscle abnormalities, vision problems, and fatigue (103). Studies have described how TIMP1 and TIMP2 display anti-inflammatory properties within the CNS, at least partially through the maintenance of the BBB (87, 89, 104–106). Dysregulation within the BBB and immune cell infiltration is described as a hallmark of MS (107). In addition, TIMP1 has been shown to directly regulate CD4+ T-cell migration across the CNS parenchyma to modulate the neuroinflammatory response (108). TIMPs also exhibit a neuroprotective effect by promoting the survival of both oligodendrocytes and neurons through neurotrophic factor signaling, which is critical since axonal degeneration and demyelination are characteristic features of MS (99, 109, 110). Furthermore, research points toward an influential role for TIMP1 in oligodendrocyte differentiation and remyelination, both of which are crucial for repairing damaged nerves (111). It was described that TIMP1 knockout mice exhibit not only an impaired ability to remyelinate their nerves following injury but also delayed developmental myelination of the CNS (109, 111). These results indicate that TIMP1 overexpression might hold potential as a therapeutic factor for remyelination. Further studies revealed that astrocyte-derived TIMP1 plays a central role in the differentiation of oligodendrocytes in vitro, and mice that were TIMP1-deficient could not spontaneously remyelinate their axons (112). This further bolsters the idea that TIMP1 might have the therapeutic ability to potentiate recovery in demyelinating disorders such as MS. Other neurological sclerosing diseases such as amyotrophic lateral sclerosis have poorly defined associations with TIMP activity, possibly due to poor disease models and a reliance on postmortem tissue. The roles that MPs, and by association TIMPs, play in neurodegenerative diseases are reviewed extensively elsewhere (73). In addition, a recent report highlights important mechanistic details describing TIMP2’s protective effects over BBB function in a model of traumatic brain injury (113). Mediated through α3β1 integrin binding, TIMP2 could limit BBB disruption through inhibition of Src-dependent VE-Cadherin internalization in a manner that is MP-independent, reiterating the therapeutic potential of TIMP2 in neurological disorders. The signaling roles for each of these TIMP proteins in neurological disorders are depicted in Fig. 2.
Figure 2.
TIMP proteins in neurological disease. Diverse TIMP signaling and inhibitory pathways underlying roles in Alzheimer’s disease, neuronal health and inflammation, and blood-brain barrier integrity. Created with BioRender.com. APP, amyloid precursor protein; CNS, central nervous system; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of matrix metalloproteinase.
ARTHRITIC DISEASES
Arthritis is a hypernym describing a variety of inflammatory joint diseases, among them rheumatoid arthritis (RA) and osteoarthritis (OA). While both are common conditions, they differ in their etiology. RA is an autoimmune disease that produces chronic inflammation, often affecting many joints (114, 115). OA is a degenerative joint disease caused by erosion of the joint architecture. Both of these joint disorders have been associated with elevated expression and activity of extracellular proteinases that can degrade ECM components of the afflicted joint (116). Within the synovial tissue of RA-afflicted joints, neutrophils represent the predominant cell type, and their activity has been implicated in the pathogenesis of the disease (117, 118). Neutrophils are a major source of TIMP-free MMP8 and MMP9, collagenolytic MPs whose activity is involved in both RA and OA disease progression (116, 119–121). With regards to the roles that MP activity assumes in disease etiology, it is logical to assume a protective role for TIMP proteins in arthropathies. Despite this assumption, there is little concrete evidence describing the direct role of TIMPs in the pathophysiology or resolution of arthritic conditions. Punctate reports describing changes in TIMP expression in the synovial fluid and serum of individuals with arthritis are conflicting or poorly conclusive (122–126). Despite the lack of definitive studies, there are several reports describing the potential therapeutic effect of TIMP proteins present in arthritis. TIMP2-deficient mice exhibit accelerated OA through a mechanism that involves enhanced angiogenesis, a pathway that likely relies on the MP-independent function of TIMP2. TIMP3 knockout mice also display mild cartilage degradation similar to that observed in patients with OA through enhanced aggrecanase activity, which are MPs of the ADAMTS family. The aggrecanase inhibitory activity of TIMP3, which is unique among the TIMP family members, has led to the description of TIMP3 as a chondroprotective factor that may be clinically used in cases of OA (127–129). In a comparable study, TIMP1 was described as therapeutically ineffective in a murine model of collagen-induced arthritis (130). In line with TIMP4’s highly restricted expression, there have been no reports linking TIMP4 with the pathophysiology of arthritis, and only individual instances describing an increase in the expression of TIMP4 in OA (131, 132). Figure 3 summarizes the functional roles of each of the TIMP proteins in arthritic diseases.
Figure 3.
TIMP proteins in rheumatoid arthritis and osteoarthritis. Created with BioRender.com. ECM, extracellular matrix; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of matrix metalloproteinase.
CARDIOVASCULAR DISEASES
Altered expression patterns of MPs and TIMPs, such as decreased expression of TIMP4, are known to occur following myocardial infarction (MI) (133). These manifest as region-specific imbalances of specific MPs and TIMPs throughout the myocardium (134). Such modifications of protein expression take place in stages, with some occurring as late as 16 wk post-MI (135). In a mouse model, broad-spectrum MP inhibition has been found to reduce post-MI left ventricular dilation, indicating a role of MPs and TIMPs in left ventricular remodeling after infarction (136, 137). In a comparable study in pigs, broad-spectrum inhibition of MPs using PD166793 helped to attenuate infarct expansion and also produced an early-phase increase in TIMP1 levels, with chronic treatment (8 wk post-MI) also increasing the region-specific levels of TIMP4 (137). Unlike the other TIMPs, TIMP4 is largely restricted to structures of the cardiovascular system, where it is upregulated in inflammatory conditions such as atherosclerosis and giant cell arteritis (138). The role of TIMP4 in the heart is not thoroughly understood, although it seems to contribute to cardiac contractility and cardiac cell differentiation, becoming epigenetically silenced in heart failure (139, 140). Spatial transcriptomics in the murine heart reveals that TIMP4 expression is most prevalent in the microvasculature of the cardiac parenchyma, an observation that may impart key context regarding the role of TIMP4 in healthy and infarcted hearts (9). In any event, the impact of TIMP4 on cardiovascular pathology is multifaceted. TIMP4 may play a role in the prevention of atrial fibrosis, which appears to involve an interaction between TIMP4 and TGFβ1 (141, 142). Polymorphisms in TIMP4 are associated with changes in angiographic coronary plaque progression (143). Furthermore, TIMP4 knockout mice show abnormally decreased cardiac function with age, as well as increased mortality following MI. This occurs primarily through left ventricle (LV) rupture, although this effect is reduced by the administration of a broad-spectrum MMP inhibitor or genetic deletion of MMP2 (144). These observations illuminate the importance of TIMP4-mediated MP inhibition in maintaining the structural integrity of the heart. Beyond cardiac protection, TIMP4 may also be important for cardiac recovery, having been found to play an essential role in the recovery of normal cardiac function following myocardial ischemia-reperfusion, in large part due to its ability to inhibit MT1-MMP (MMP14) (145). An important discriminating factor between TIMP2 and TIMP4’s affinity for MMP14 is the fact that the TIMP4-MMP14 interaction is insufficient for the activation of MMP2 (146). On the contrary, trimolecular complex formation between MMP14-TIMP2-MMP2 is a well-established mechanism of proMMP2 activation (147, 148).
Though TIMP4 may be the most prominently studied TIMP in the context of cardiovascular disease, the importance of other TIMPs in this area is not to be left unnoticed. For example, increased TIMP1 levels post-MI appear to be important in the regulation of MP-mediated left ventricular remodeling, as TIMP1 deletion induces adverse remodeling that is rescued by broad-spectrum MP inhibition (149). The important yet complex functions of TIMP1 in cardiac health are seen in other findings showing that TIMP1 knockout mice displayed markedly decreased cardiac fibrosis but increased coronary atherosclerosis (150). Correspondingly, overexpression of TIMP1 reduces atherosclerosis, at least in apolipoprotein E-deficient mice (151). Plasma TIMP1 is associated with risk factors and indices of cardiac disease, although no causal connection has been determined, it likely represents a collateral observation that is linked to TIMP1’s long-standing association with inflammation, a definitive risk factor in cardiomyopathy (152, 153).
The contributions of TIMP2 and TIMP3 to cardiovascular disease are equally intriguing. As with TIMP1 and TIMP4, deficiency of TIMP2 increases post-MI disease severity, largely due to decreased inhibition of MMP14 (154). As mentioned, TIMP1 was previously found to protect against atherosclerosis, but other work comparing TIMP1 and TIMP2 suggests TIMP2 is a more effective cardioprotective factor (155). In accord with all the other TIMPs, TIMP3 knockout mice exhibited worsened pathology and survival following MI, but marked improvement was observed when the knockout mice were treated with a broad-spectrum MP inhibitor quickly after MI (156). TIMP3 may also play a protective role against the development and rupture of atherosclerotic plaques (157, 158). A causal association between elevated serum TIMP3 and lower risks of coronary artery disease and myocardial infarction has been found (159). Interestingly, although both TIMP2 and TIMP3 seem to play protective roles in the context of cardiovascular disease, the pathways underlying these roles may be very different. TIMP2-deficient mice infused with angiotensin II displayed aggravated myocardial hypertrophy and decreased cardiac fibrosis, whereas TIMP3-deficient mice receiving the same infusion displayed severe fibrosis without hypertrophy (160). Although not a focus of the present review, it is also worth noting that associations have been reported between TIMPs and other cardiovascular conditions, such as TIMP3 and hypertension and TIMP2 and atrial fibrillation (161, 162).
Many of the aforementioned roles for TIMPs in cardiovascular diseases lean on the MP-inhibitory functions of TIMP proteins within cardiac tissue, yet the MP-independent functions of TIMPs likely distinguish their beneficial effects. This can be exemplified by an intriguing study that overexpressed individual TIMP family members in cardiac fibroblasts, concluding that each TIMP had heterogenous MP-independent effects on cardiac fibroblast proliferation and apoptosis, despite each instance producing a phenotype reminiscent of myofibroblasts (163). The latter point may be crucial in the early stages of MI, where the time-dependent evolution of activated fibroblast phenotypes is crucial for tissue repair (164). Furthermore, TIMPs have well-established regulatory functions on endothelial cells and cardiomyocytes, reviewed extensively elsewhere (165).
Therapeutic applications of TIMPs in cardiovascular disease are promising but remain limited to preclinical testing. The approaches often involve targeted inhibition of elevated MPs that contribute to adverse cardiac remodeling. TIMP3’s unique matrix-binding characteristic makes it a predominant interest regarding therapeutic intervention in MI. This can be exemplified by a study that used hydrogel-embedded recombinant TIMP3 implanted post-MI that was successful in reducing inflammation, MI expansion, and LV dilatation (166). Despite the apparent success, implementation of this approach requires an open chest procedure. Intracoronary delivery of recombinant TIMP3 yielded similarly promising results, and the pharmacokinetics of this treatment may be improved through the engineering of TIMP3 with additional glycosylation or fusion with other proteins (167, 168). Other approaches to increase TIMP3 levels post-MI have included adenovirus-based or cell-based (cell transplantation) gene therapy (169–171). Indeed, TIMP3 adenovirus-coated stents were demonstrated in pigs to significantly decrease in-stent restenosis (172). Each of these TIMP3-based cardiovascular therapeutics is reviewed extensively by Fan and Kassiri (6). Applications of TIMP3 to non-MI cardiovascular pathologies are far more limited, although overexpression of TIMP3 by macrophages was found to reduce atherosclerotic plaque size, inflammation, and oxidative stress signaling in mice (173). Along the same lines, adenovirus-based TIMP3 expression following vein grafting in pigs increases neointimal apoptosis, reducing neointima formation considerably, which holds clinical potential in the context of procedures such as coronary artery bypass (174). Exogenous TIMP3 also holds promise for the treatment of abdominal aortic aneurysms, although experiments with live animal models remain to be performed (175). Importantly, a consideration for any exogenous TIMP3 treatment is the potential for TIMP3 to alter the cellular secretome through its interactions with ADAM10 and LRP1 (176). Notably, some therapeutics commonly administered post-MI, such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin II type I receptor blockers, and β-adrenergic receptor blockers, may reduce MP expression and/or activity, although the specific effects vary and more work is needed to understand the relevant pharmacological mechanisms (177). The TIMP functional roles outlined in this section are summarized in Fig. 4.
Figure 4.
Cardioprotective effects of TIMP proteins through MP-dependent and MP-independent mechanisms. Created with BioRender.com. MP, metalloproteinase; TIMP, tissue inhibitors of matrix metalloproteinase.
NONNEOPLASTIC PULMONARY DISEASES
Much of our present understanding of TIMPs in pulmonary disease focuses on the balance between TIMPs and MPs in conditions such as pulmonary fibrosis and emphysema. ECM degradation and remodeling are hallmarks of both pulmonary fibrosis and emphysema, and each harbors phenotypes consistent with dysregulation in MP expression and activity (178–180). Fibrosis has been associated with an imbalance between TIMP and MP expression that favors the expression of TIMPs and suggests a nondegradative environment (181). However, gene-targeted studies reveal that most MPs promote pulmonary fibrosis through diverse mechanisms (179). These conflictions, in addition to the many causal factors that induce pulmonary diseases, create a disjointed understanding of the roles that TIMPs play in pathogenesis and their potential role in disease resolution. TIMP2 and TIMP3 are strongly expressed throughout all compartments of the lung, with TIMP1 displaying a low, but highly inducible, expression profile (9). In line with the inducible nature of TIMP1, increased TIMP1 expression is associated with increased fibrosis in mouse models of silica exposure (182, 183) One study suggests that TIMP1 regulates the fibrotic response by stimulating fibroblast activation and proliferation through a pathway involving the formation of a cell surface TIMP1/CD63/integrin β1 complex that activates ERK signaling (184). Indeed, in its early stages, pulmonary fibrosis may be suppressed using antisense TIMP1 retroviral vectors (185). Somewhat paradoxically, TIMP3 knockout mice displayed both increased total MP activity in the lungs and increased fibrosis following lung injury, and it was determined that this effect was mediated through persistent inflammation and neutrophil recruitment at the injury site, leading to delayed wound resolution (186). This knockout model could be rescued with an MP inhibitor, suggesting that metalloproteinase activity was the causative factor.
In contrast to the high TIMP/MP ratio that may be found in fibrosis, a high MP/TIMP ratio is thought to cause a degradative environment that can destroy normal tissue structure and aggravate injury, which may be the case in acute lung injury and radiation-induced lung injury (187, 188). MP-favored imbalances may occur following acute lung injury that augments disease severity, as has been suggested previously in the case of ventilator-induced lung injury (189). MP and TIMP expression patterns vary significantly between different benign pulmonary diseases, contributing to the incohesive understanding of TIMP biology in the lungs. The MMP9/TIMP1 imbalance is of special interest not only in pulmonary fibrosis but also in other diseases, such as chronic obstructive pulmonary disease (COPD), bronchiectasis, and asthma. However, the magnitude of the MMP9/TIMP1 ratio may vary between these diseases, and the details are complicated by other TIMPs (190, 191). In asthma, overexpression of not only TIMP1 but also TIMP3 has been reported, along with abnormally high or low expression of various ADAM and ADAMTS proteinases (192). MMP9:TIMP1 balance has been found to differ between fibrosis and other pulmonary conditions like COPD, even when inflammatory profiles are similar; for instance, COPD is associated with increased expression of both TIMP1 and MMP9, along with MMP2 (193, 194). Sputum analysis suggests that the increase in MMP9 activity appears to be disproportionately large relative to the increase in TIMP1 expression (195). Furthermore, the increase in TIMP1 may be macrophage-independent, as alveolar macrophages from patients with COPD release less TIMP1 than those from individuals with normal lung function (196). Complicating analysis is the fact that pulmonary patients may possess comorbidities that impact systemic MP-TIMP balance. Osteoporosis, which is common in patients with COPD and is associated with increased MMP9 activity, is often seen in patients with COPD (197). Interestingly, blood from patients with COPD revealed a similar pattern of increased MMP9 expression with no significant difference in TIMP1 or TIMP2 expression (197). This effect was specifically attributed to the patients’ osteoporosis as opposed to their pulmonary disease (197). Other studies of circulating MMP9, TIMP1, and TIMP2, however, have yielded very different results and interpretations, with one set of findings showing increased expression of all three proteins in the plasma of patients with COPD, perhaps suggesting roles for these circulating proteins in COPD-related airway remodeling (198).
Compared with TIMP1, there are relatively few TIMP2-specific findings regarding benign pulmonary disease, but some fundamental questions have been raised. Within the lungs, TIMP2 has been found to colocalize with Ki67 in fibroblast foci (181), an observation in line with previous reports describing proliferative effects of TIMP2 on cardiac fibroblasts and fibrosarcoma cells (43, 163). TIMP2 expressed by myofibroblasts in idiopathic pulmonary fibrosis is thought to contribute to the stable deposition of ECM that is associated with the condition (199). Single nucleotide polymorphisms in TIMP2 have been associated with the development of different lung conditions, including paraseptal emphysema and additional forms of COPD (200–202). Yet, increased TIMP2 expression is associated with improved pulmonary function in COPD and chronic asthma (203). These contradictions may result due to the varying roles of TIMP2 as both an inhibitor of MPs and a signaling molecule in inflammation-related pathways. Interestingly, TIMP2 expression in the lungs seems to increase with age, as does the expression of TGFβ1 and MMP2 (204). The distinct roles that TIMP2 plays in the lungs are specifically reviewed elsewhere (205).
Shifting focus to TIMP3, again the TIMP-MP balance is often described as contributing to pulmonary pathophysiology. TIMP3 knockout mouse studies have revealed critical anti-inflammatory roles for TIMP3 in the lungs including regulation of MP activity, neutrophil influx, and macrophage differentiation (186, 206). MP-TIMP balance is regulated at least in part by TGFβ signaling, which becomes dysregulated in patients with COPD, and the regulation of TIMP3 by TGFβ appears especially important in the pathogenesis of both COPD and idiopathic pulmonary fibrosis (IPF) (207, 208). Indeed, TIMP3 knockout mice are known to exhibit emphysema-like enlargement of the airway with decreased tissue elasticity, and in humans, mutation of TIMP3 may result in the autosomal dominant condition Sorsby fundus dystrophy, which is associated with pulmonary in addition to ocular disease (209, 210). Although TIMP3 expression appears to have a protective effect against pulmonary fibrosis, an excess of TIMP3 may be problematic, with proteomic analysis of human lung tissue indicating increased TIMP3 expression in COPD (211, 212).
Pulmonary hypertension (PH) is another disease of interest regarding TIMP biology. PH induced by hypoxia or monocrotaline is associated with increased abundance of MPs and TIMPs (213, 214). TIMP4 levels may contribute to PH, including in cases of systemic sclerosis (215). This is notable considering the relatively limited expression patterns of TIMP4 in the lungs (9). In a surgical mouse model of pulmonary hypertension, folic acid supplementation was found to raise right ventricular TIMP4 abundance that had been decreased after the surgery and to decrease TIMP1 levels that had been increased after the surgery, improving MP/TIMP balance (216). TIMP4 may also play roles in other pulmonary conditions, notably COPD. TIMP4, along with TIMP1, is increased in COPD following exposure to cigarette smoke (217, 218). Similarly, patients with COPD with mild-to-moderate acute exacerbation displayed increased pulmonary levels of TIMP4 compared with healthy subjects (219).
Several TIMP-related therapeutic approaches have been studied for the treatment of pulmonary disease, though clinical trials with such biologics remain to be completed. By and large, these approaches involve a restoration of normal pulmonary MP/TIMP balance to prevent and possibly reverse pathological airway remodeling. For example, doxycycline administration to inhibit MP activity and thus restore the MMP9/TIMP1 ratio represents a promising treatment modality for COPD (220). Similarly, treating emphysematous mice using glycyl-l-histidyl-l-lysine peptide was found to attenuate the emphysema and partially restore normal MMP9/TIMP1 balance in the lungs (221). This action appears to involve multiple mechanisms, including NF-κB and oxidative stress pathways (221). Mice exposed to cigarette smoke and then treated with rosiglitazone, a drug prescribed for type II diabetes mellitus, also showed reduced MP activity, possibly due to MAPK- or NF-κB-dependent signaling pathways, which helped restore protease/inhibitor balance and ameliorated the development of emphysema-related pathology (222). That work also uncovered a potential role for the peroxisome proliferator-activated receptor γ (PPARγ) in emphysema, with the rosiglitazone treatment raising PPARγ expression levels (222). A subsequent study supports a protective role for PPARγ in maintaining proper MP/TIMP balance, particularly MMP9/TIMP1 balance, in COPD, putting PPARγ forward as a potential therapeutic target in COPD intervention (223). Other promising therapeutics that appear to similarly quell MP activity and reduce MP/TIMP imbalance include the antibiotic erythromycin, thegallic acid (a naturally occurring phenol), and recombinant human keratinocyte growth factor (rHuKGF), also known as palifermin (224–226). An additional interesting player in this therapeutic arena is SIRT1, a protein deacetylase. Reduced SIRT1 is associated with MMP9/TIMP1 imbalance in the lungs of smokers and patients with COPD, which appears to relate to increased TIMP1 acetylation at specific lysine residues and reduced interaction with MMP9 (227, 228). In this regard, it has been suggested that SIRT1 is involved in the expression and deacetylation of TIMP1, facilitating TIMP1-mediated MP inhibition in emphysema and COPD. In any case, redressing the deficiencies in SIRT1 activity in smokers through pharmacological activation of SIRT1 has the potential to restore a normal TIMP1 expression and acetylation patterns, and in turn, reestablish the homeostatic protease/inhibitor balance. The complex roles of TIMP proteins in pulmonary disease are summarized in Fig. 5.
Figure 5.
Complex roles of TIMP proteins in pulmonary disease. TIMP family members are frequently characterized by conflicting functional and associative roles in pulmonary diseases. Created with BioRender.com. MP, metalloproteinase; TIMP, tissue inhibitors of matrix metalloproteinase.
TIMPs AS DISEASE BIOMARKERS
From a clinical standpoint, TIMPs represent an impractical therapeutic due to their short blood half-life of less than 4 h (229), although efforts are described to address this (168, 230). In fact, in considering TIMP biomarker applications, we need not limit the discussion to the diseases discussed above. For example, the NephroCheck test score, which considers urinary TIMP2 and IGFBP7 abundance, is used in the early detection of acute kidney injury (231, 232). Furthermore, TIMPs have shown promise as diagnostic and prognostic biomarkers in diverse cancers, including nonsmall cell lung cancer, prostate cancer, pancreatic cancer, breast cancer, and ovarian cancer (233–237). Serum TIMP1 was found to have upper moderate sensitivity and specificity as a potential diagnostic value for colorectal cancer (238). Though more research is needed, decreases in plasma and cerebrospinal fluid TIMP3 levels may be indicative of Alzheimer’s disease progression (97, 238), and it has been proposed that the measurement of TIMPs and MPs in the cerebrospinal fluid of patients with multiple sclerosis may hold promise for diagnosis and treatment decisions (239).
The relevance of the matrisome to cardiovascular disease is well appreciated. Higher circulating MP levels, especially MMP2 and MMP9, are known to correlate with disease states including both acute coronary disease and post-MI congestive heart failure (240, 241). Also, TIMPs, most notably TIMP1, have been found to possess a significant correlation with vascular events. Associations between increased circulating TIMP1 and MI, heart failure severity, stroke, and cardiovascular death have been reported (242–245). Similarly, in pulmonary hypertension, circulating TIMP1 is elevated, and high TIMP1 appears to reflect more severe disease and predict poorer outcomes (246, 247). In addition to TIMP1, TIMP4 is a very strong biomarker for pulmonary hypertension, with high plasma TIMP4 associated with increased pulmonary arterial pressure, right ventricular hypertrophy severity, and poor survival (248–250). In idiopathic pulmonary hypertension, an increased MMP2/TIMP4 ratio is associated with clinical worsening events and death (251). Measurements of circulating plasma MPs and TIMPs may hold promise for patients with pulmonary hypertension in association with standard hemodynamic indices and various values indicative of pulmonary arterial stiffness (252). Some interest has even been shown in the potential for MPs and TIMPs to be applied as biomarkers for Takayasu’s arteritis, a relatively rare vascular disease (252, 253).
In pulmonary disease, just as the relevant pathways vary within and between conditions, so too do the biomarkers. This translates to a lack of concordance in the utility of TIMPs as biomarkers for pulmonary diseases that often have confounding variables, such as smoking status and pollution exposure. Linked to its emerging role as an inflammatory cytokine, TIMP1 presents with the most consistency as a biomarker in pulmonary diseases such as COPD, as well as respiratory infections involving COVID-19 and respiratory syncytial virus (15, 254, 255). Furthermore, it has been described that plasma MP and TIMP levels have potential use as biomarkers for treatment failure, relapse, and death in patients with pulmonary tuberculosis that may eventually guide clinicians’ judgment regarding treatment regimens (257).
FUTURE PERSPECTIVES
The contribution of TIMPs to the pathophysiology of human disease is becoming increasingly apparent. In large part, these roles are described through their inhibitory action against MPs, whose expression and activity are explicitly intertwined with pathogenesis. However, the everincreasing characterization of MP-independent functions in TIMP proteins across tissues and the spectrum of human disease models adds credibility to their importance in tissue homeostasis and response to pathology. Many investigations probe the ratios between certain MPs and TIMPs, but increasing evidence suggests partial interrogation of an inherently complex system is insufficient, and greater context and understanding will be derived from a holistic approach. This concept is a difficult challenge to engage, considering the complex mechanisms that mediate the levels and activity of MPs and TIMPs. TIMPs and MPs are regulated at multiple levels, through gene expression, microRNA regulation, alternative splicing, zymogen activation, degradation, and posttranslational modifications. The latter point is an important factor, considering that TIMP1 and 3 are glycosylated and TIMP2 can be phosphorylated (3, 258). Furthermore, it is also important to consider the consequences of TIMP binding on the proteinase-independent functions of MPs that have not been fully addressed, whereby TIMP binding can facilitate MP scavenging by the endocytic receptor LRP1 (259, 260). Further work is required to mechanistically map the disease- and tissue-specific functions of each TIMP family member, the dependencies of these functions, and the consequences thereafter. Only then can researchers begin to unlock the therapeutic potential hidden beneath the tissue inhibitors of metalloproteinases appellation.
GRANTS
This research was supported by the Center for Cancer Research, NCI/NIH research grants ZIA BC011204 and ZIA SC 009179 to WGSS (NCI Intramural Research program).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.C-P. and D.P. conceived and designed research; S.C-P., J.R., and D.P. prepared figures; S.C-P., J.R., and D.P. drafted manuscript; S.C-P., J.R., and D.P. edited and revised manuscript; S.C-P., J.R., W.G.S-S., and D.P. approved final version of manuscript.
REFERENCES
- 1. Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol 99: 31–68, 1982. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 2. Huxley-Jones J, Clarke T-K, Beck C, Toubaris G, Robertson DL, Boot-Handford RP. The evolution of the vertebrate metzincins; insights from Ciona intestinalis and Danio rerio. BMC Evol Biol 7: 63, 2007. doi: 10.1186/1471-2148-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803: 55–71, 2010. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol Cancer 7: 85, 2008. doi: 10.1186/1476-4598-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim Y-S, Kim S-H, Kang J-G, Ko J-H. Expression level and glycan dynamics determine the net effects of TIMP-1 on cancer progression. BMB Rep 45: 623–628, 2012. doi: 10.5483/bmbrep.2012.45.11.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fan D, Kassiri Z. Biology of tissue inhibitor of metalloproteinase 3 (TIMP3), and its therapeutic implications in cardiovascular pathology. Front Physiol 11: 661, 2020. doi: 10.3389/fphys.2020.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spano DP, Scilabra SD. Tissue inhibitor of metalloproteases 3 (TIMP-3): in vivo analysis underpins its role as a master regulator of ectodomain shedding. Membranes (Basel) 12: 211, 2022. doi: 10.3390/membranes12020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peeney D, Liu Y, Lazaroff C, Gurung S, Stetler-Stevenson WG. Unravelling the distinct biological functions and potential therapeutic applications of TIMP2 in cancer. Carcinogenesis 43: 405–418, 2022. doi: 10.1093/carcin/bgac037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peeney D, Fan Y, Gurung S, Lazaroff C, Ratnayake S, Warner A, Karim B, Meerzaman D, Stetler-Stevenson WG. Whole organism profiling of the Timp gene family. Matrix Biol Plus 18: 100132, 2023. doi: 10.1016/j.mbplus.2023.100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stetler-Stevenson WG. The continuing saga of tissue inhibitor of metalloproteinase 2: emerging roles in tissue homeostasis and cancer progression. Am J Pathol 193: 1336–1352, 2023. doi: 10.1016/j.ajpath.2023.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson HW, Defamie V, Waterhouse P, Khokha R. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer 17: 38–53, 2017. doi: 10.1038/nrc.2016.115. [DOI] [PubMed] [Google Scholar]
- 12. Grunwald B, Schoeps B, Kruger A. Recognizing the molecular multifunctionality and interactome of TIMP-1. Trends Cell Biol 29: 6–19, 2019. doi: 10.1016/j.tcb.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 13. Seo D-W, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei B-y, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 114: 171–180, 2003. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 14. Seo D-W, Saxinger WC, Guedez L, Cantelmo AR, Albini A, Stetler-Stevenson WG. An integrin-binding N-terminal peptide region of TIMP-2 retains potent angio-inhibitory and anti-tumorigenic activity in vivo. Peptides 32: 1840–1848, 2011. doi: 10.1016/j.peptides.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schoeps B, Fradrich J, Kruger A. Cut loose TIMP-1: an emerging cytokine in inflammation. Trends Cell Biol 33: 413–426, 2023. doi: 10.1016/j.tcb.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 16. Peeney D, Kumar S, Singh TP, Liu Y, Jensen SM, Chowdhury A, Coates-Park S, Rich J, Gurung S, Fan Y, Meerzaman D, Stetler-Stevenson WG. Timp2 loss-of-function mutation and TIMP2 treatment in murine model of NSCLC: modulation of immunosuppression and oncogenic signaling (Preprint). bioRxiv: 2023.12.29.573636, 2023. doi: 10.1101/2023.12.29.573636. [DOI] [PMC free article] [PubMed]
- 17. Guedez L, Jensen-Taubman S, Bourboulia D, Kwityn CJ, Wei B, Caterina J, Stetler-Stevenson WG. TIMP-2 targets tumor-associated myeloid suppressor cells with effects in cancer immune dysfunction and angiogenesis. J Immunother 35: 502–512, 2012. doi: 10.1097/CJI.0b013e3182619c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peeney D, Gurung S, Rich J, Coates-Park S, Liu Y, Toor J, Jones J, Richie C, Jenkins L, Stetler-Stevenson W. Extracellular proximity labeling reveals an expanded interactome for the matrisome protein TIMP2 (Preprint). Res Sq: rs.3.rs-3857263, 2024. doi: 10.21203/rs.3.rs-3857263/v1. [DOI] [Google Scholar]
- 19. Cox TR. The matrix in cancer. Nat Rev Cancer 21: 217–238, 2021. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 20. Fingleton B. MMPs as therapeutic targets—still a viable option? Semin Cell Dev Biol 19: 61–68, 2008. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shay G, Lynch CC, Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol 44-46: 200–206, 2015. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov 13: 904–927, 2014. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- 23. de Almeida LGN, Thode H, Eslambolchi Y, Chopra S, Young D, Gill S, Devel L, Dufour A. Matrix metalloproteinases: from molecular mechanisms to physiology, pathophysiology, and pharmacology. Pharmacol Rev 74: 712–768, 2022. doi: 10.1124/pharmrev.121.000349. [DOI] [PubMed] [Google Scholar]
- 24. Peeney D, Fan Y, Nguyen T, Meerzaman D, Stetler-Stevenson WG. Matrisome-associated gene expression patterns correlating with TIMP2 in cancer. Sci Rep 9: 20142, 2019. doi: 10.1038/s41598-019-56632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coates-Park S, Lazaroff C, Gurung S, Rich J, Colladay A, O'Neill M, Butler GS, Overall CM, Stetler-Stevenson WG, Peeney D. Tissue inhibitors of metalloproteinases are proteolytic targets of matrix metalloproteinase 9. Matrix Biol 123: 59–70, 2023. doi: 10.1016/j.matbio.2023.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okada Y, Watanabe S, Nakanishi I, Kishi J, Hayakawa T, Watorek W, Travis J, Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett 229: 157–160, 1988. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- 27. Nagase H, Suzuki K, Cawston TE, Brew K. Involvement of a region near valine-69 of tissue inhibitor of metalloproteinases (TIMP)-1 in the interaction with matrix metalloproteinase 3 (stromelysin 1). Biochem J 325: 163–167, 1997. doi: 10.1042/bj3250163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharifi MA, Wierer M, Dang TA, Milic J, Moggio A, Sachs N, von Scheidt M, Hinterdobler J, Müller P, Werner J, Stiller B, Aherrahrou Z, Erdmann J, Zaliani A, Graettinger M, Reinshagen J, Gul S, Gribbon P, Maegdefessel L, Bernhagen J, Sager HB, Mann M, Schunkert H, Kessler T. ADAMTS-7 modulates atherosclerotic plaque formation by degradation of TIMP-1. Circ Res 133: 674–686, 2023. doi: 10.1161/CIRCRESAHA.123.322737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown PD. Matrix metalloproteinase inhibitors. Angiogenesis 1: 142–154, 1998. doi: 10.1023/A:1018373520193. [DOI] [PubMed] [Google Scholar]
- 30. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278: 16–27, 2011. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 31. Sun J. Matrix metalloproteinases and tissue inhibitor of metalloproteinases are essential for the inflammatory response in cancer cells. J Signal Transduct 2010: 985132, 2010. doi: 10.1155/2010/985132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141: 52–67, 2010. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bigelow RLH, Williams BJ, Carroll JL, Daves LK, Cardelli JA. TIMP-1 overexpression promotes tumorigenesis of MDA-MB-231 breast cancer cells and alters expression of a subset of cancer promoting genes in vivo distinct from those observed in vitro. Breast Cancer Res Treat 117: 31–44, 2009. doi: 10.1007/s10549-008-0170-7. [DOI] [PubMed] [Google Scholar]
- 34. Ries C. Cytokine functions of TIMP-1. Cell Mol Life Sci 71: 659–672, 2014. doi: 10.1007/s00018-013-1457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forte D, Salvestrini V, Corradi G, Rossi L, Catani L, Lemoli RM, Cavo M, Curti A. The tissue inhibitor of metalloproteinases-1 (TIMP-1) promotes survival and migration of acute myeloid leukemia cells through CD63/PI3K/Akt/p21 signaling. Oncotarget 8: 2261–2274, 2017. doi: 10.18632/oncotarget.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT, Werb Z. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20: 174–186, 2020. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iredale JP, Pellicoro A, Fallowfield JA. Liver fibrosis: understanding the dynamics of bidirectional wound repair to inform the design of markers and therapies. Dig Dis 35: 310–313, 2017. doi: 10.1159/000456581. [DOI] [PubMed] [Google Scholar]
- 38. Takawale A, Zhang P, Patel VB, Wang X, Oudit G, Kassiri Z. Tissue inhibitor of matrix metalloproteinase-1 promotes myocardial fibrosis by mediating CD63-integrin β1 interaction. Hypertension 69: 1092–1103, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09045. [DOI] [PubMed] [Google Scholar]
- 39. Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJP, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem 277: 11069–11076, 2002. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 40. Gong Y, Scott E, Lu R, Xu Y, Oh WK, Yu Q. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS One 8: e77366, 2013. doi: 10.1371/journal.pone.0077366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ando T, Charindra D, Shrestha M, Umehara H, Ogawa I, Miyauchi M, Takata T. Tissue inhibitor of metalloproteinase-1 promotes cell proliferation through YAP/TAZ activation in cancer. Oncogene 37: 263–270, 2018. doi: 10.1038/onc.2017.321. [DOI] [PubMed] [Google Scholar]
- 42. Song G, Xu S, Zhang H, Wang Y, Xiao C, Jiang T, Wu L, Zhang T, Sun X, Zhong L, Zhou C, Wang Z, Peng Z, Chen J, Wang X. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res 35: 148, 2016. doi: 10.1186/s13046-016-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase-2 stimulates fibroblast proliferation via a cAMP-dependent mechanism. J Biol Chem 270: 13453–13459, 1995. doi: 10.1074/jbc.270.22.13453. [DOI] [PubMed] [Google Scholar]
- 44. Sounni NE, Rozanov DV, Remacle AG, Golubkov VS, Noel A, Strongin AY. Timp-2 binding with cellular MT1-MMP stimulates invasion-promoting MEK/ERK signaling in cancer cells. Int J Cancer 126: 1067–1078, 2010. [Erratum in Int J Cancer 126: 2002, 2010]. doi: 10.1002/ijc.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Valacca C, Tassone E, Mignatti P. TIMP-2 interaction with MT1-MMP activates the AKT pathway and protects tumor cells from apoptosis. PLoS One 10: e0136797, 2015. doi: 10.1371/journal.pone.0136797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seo D-W, Kim SH, Eom S-H, Yoon HJ, Cho Y-R, Kim P-H, Kim YK, Han J-W, Diaz T, Wei B-Y, Stetler-Stevenson WG. TIMP-2 disrupts FGF-2-induced downstream signaling pathways. Microvasc Res 76: 145–151, 2008. doi: 10.1016/j.mvr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bourboulia D, Han H, Jensen-Taubman S, Gavil N, Isaac B, Wei B, Neckers L, Stetler-Stevenson WG. TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/beta-catenin complex expression in A549 lung cancer cells. Oncotarget 4: 166–176, 2013. doi: 10.18632/oncotarget.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peeney D, Jensen SM, Castro NP, Kumar S, Noonan S, Handler C, Kuznetsov A, Shih J, Tran AD, Salomon DS, Stetler-Stevenson WG. TIMP-2 suppresses tumor growth and metastasis in murine model of triple-negative breast cancer. Carcinogenesis 41: 313–325, 2020. doi: 10.1093/carcin/bgz172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Escalona RM, Bilandzic M, Western P, Kadife E, Kannourakis G, Findlay JK, Ahmed N. TIMP-2 regulates proliferation, invasion and STAT3-mediated cancer stem cell-dependent chemoresistance in ovarian cancer cells. BMC Cancer 20: 960, 2020. doi: 10.1186/s12885-020-07274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benzing C, Lam H, Tsang CM, Rimmer A, Arroyo-Berdugo Y, Calle Y, Wells CM. TIMP-2 secreted by monocyte-like cells is a potent suppressor of invadopodia formation in pancreatic cancer cells. BMC Cancer 19: 1214, 2019. doi: 10.1186/s12885-019-6429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grimberg A. Mechanisms by which IGF-I may promote cancer. Cancer Biol Ther 2: 630–635, 2003. [PMC free article] [PubMed] [Google Scholar]
- 52. Alfaro-Arnedo E, López IP, Piñeiro-Hermida S, Canalejo M, Gotera C, Sola JJ, Roncero A, Peces-Barba G, Ruíz-Martínez C, Pichel JG. IGF1R acts as a cancer-promoting factor in the tumor microenvironment facilitating lung metastasis implantation and progression. Oncogene 41: 3625–3639, 2022. doi: 10.1038/s41388-022-02376-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beppu T, Sasaki M, Kudo K, Kurose A, Takeda M, Kashimura H, Ogawa A, Ogasawara K. Prediction of malignancy grading using computed tomography perfusion imaging in nonenhancing supratentorial gliomas. J Neurooncol 103: 619–627, 2011. doi: 10.1007/s11060-010-0433-0. [DOI] [PubMed] [Google Scholar]
- 54. Bond M, Murphy G, Bennett MR, Newby AC, Baker AH. Tissue inhibitor of metalloproteinase-3 induces a Fas-associated death domain-dependent type II apoptotic pathway. J Biol Chem 277: 13787–13795, 2002. doi: 10.1074/jbc.M111507200. [DOI] [PubMed] [Google Scholar]
- 55. Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, Baylin SB, Graff JR. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res 59: 798–802, 1999. [PubMed] [Google Scholar]
- 56. Zochbauer-Muller S, Fong KM, Virmani AK, Geradts J, Gazdar AF, Minna JD. Aberrant promoter methylation of multiple genes in non-small cell lung cancers. Cancer Res 61: 249–255, 2001. [PubMed] [Google Scholar]
- 57. Barski D, Wolter M, Reifenberger G, Riemenschneider MJ. Hypermethylation and transcriptional downregulation of the TIMP3 gene is associated with allelic loss on 22q12.3 and malignancy in meningiomas. Brain Pathol 20: 623–631, 2010. doi: 10.1111/j.1750-3639.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anand-Apte B, Bao L, Smith R, Iwata K, Olsen BR, Zetter B, Apte SS. A review of tissue inhibitor of metalloproteinases-3 (TIMP-3) and experimental analysis of its effect on primary tumor growth. Biochem Cell Biol 74: 853–862, 1996. doi: 10.1139/o96-090. [DOI] [PubMed] [Google Scholar]
- 59. Mahller YY, Vaikunth SS, Ripberger MC, Baird WH, Saeki Y, Cancelas JA, Crombleholme TM, Cripe TP. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res 68: 1170–1179, 2008. doi: 10.1158/0008-5472.CAN-07-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang L, Zhao L, Zhao D, Lin G, Guo B, Li Y, Liang Z, Zhao XJ, Fang X. Inhibition of tumor growth and induction of apoptosis in prostate cancer cell lines by overexpression of tissue inhibitor of matrix metalloproteinase-3. Cancer Gene Ther 17: 171–179, 2010. doi: 10.1038/cgt.2009.59. [DOI] [PubMed] [Google Scholar]
- 61. Smith MR, Kung H, Durum SK, Colburn NH, Sun Y. TIMP-3 induces cell death by stabilizing TNF-alpha receptors on the surface of human colon carcinoma cells. Cytokine 9: 770–780, 1997. doi: 10.1006/cyto.1997.0233. [DOI] [PubMed] [Google Scholar]
- 62. Bian J, Wang Y, Smith MR, Kim H, Jacobs C, Jackman J, Kung HF, Colburn NH, Sun Y. Suppression of in vivo tumor growth and induction of suspension cell death by tissue inhibitor of metalloproteinases (TIMP)-3. Carcinogenesis 17: 1805–1811, 1996. doi: 10.1093/carcin/17.9.1805. [DOI] [PubMed] [Google Scholar]
- 63. Anania MC, Sensi M, Radaelli E, Miranda C, Vizioli MG, Pagliardini S, Favini E, Cleris L, Supino R, Formelli F, Borrello MG, Pierotti MA, Greco A. TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene 30: 3011–3023, 2011. doi: 10.1038/onc.2011.18. [DOI] [PubMed] [Google Scholar]
- 64. Lin H, Zhang Y, Wang H, Xu D, Meng X, Shao Y, Lin C, Ye Y, Qian H, Wang S. Tissue inhibitor of metalloproteinases-3 transfer suppresses malignant behaviors of colorectal cancer cells. Cancer Gene Ther 19: 845–851, 2012. doi: 10.1038/cgt.2012.70. [DOI] [PubMed] [Google Scholar]
- 65. Su C-W, Lin C-W, Yang W-E, Yang S-F. TIMP-3 as a therapeutic target for cancer. Ther Adv Med Oncol 11: 1758835919864247, 2019. doi: 10.1177/1758835919864247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 9: 407–415, 2003. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 67. Qi JH, Anand-Apte B. Deglycosylation increases the aggregation and angiogenic properties of mutant tissue inhibitor of metalloproteinase 3 protein: implications for Sorsby Fundus Dystrophy. Int J Mol Sci 23: 14231, 2022. doi: 10.3390/ijms232214231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jackson HW, Hojilla CV, Weiss A, Sanchez OH, Wood GA, Khokha R. Timp3 deficient mice show resistance to developing breast cancer. PLoS One 10: e0120107, 2015. doi: 10.1371/journal.pone.0120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lizarraga F, Espinosa M, Ceballos-Cancino G, Vazquez-Santillan K, Bahena-Ocampo I, Schwarz-Cruz Y Celis A, Vega-Gordillo M, Garcia Lopez P, Maldonado V, Melendez-Zajgla J. Tissue inhibitor of metalloproteinases-4 (TIMP-4) regulates stemness in cervical cancer cells. Mol Carcinog 55: 1952–1961, 2016. doi: 10.1002/mc.22442. [DOI] [PubMed] [Google Scholar]
- 70. Liss M, Sreedhar N, Keshgegian A, Sauter G, Chernick MR, Prendergast GC, Wallon UM. Tissue inhibitor of metalloproteinase-4 is elevated in early-stage breast cancers with accelerated progression and poor clinical course. Am J Pathol 175: 940–946, 2009. doi: 10.2353/ajpath.2009.081094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rorive S, Lopez XM, Maris C, Trepant A-L, Sauvage S, Sadeghi N, Roland I, Decaestecker C, Salmon I. TIMP-4 and CD63: new prognostic biomarkers in human astrocytomas. Mod Pathol 23: 1418–1428, 2010. doi: 10.1038/modpathol.2010.136. [DOI] [PubMed] [Google Scholar]
- 72. De Stefano ME, Herrero MT. The multifaceted role of metalloproteinases in physiological and pathological conditions in embryonic and adult brains. Prog Neurobiol 155: 36–56, 2017. doi: 10.1016/j.pneurobio.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 73. Brkic M, Balusu S, Libert C, Vandenbroucke RE. Friends or foes: matrix metalloproteinases and their multifaceted roles in neurodegenerative diseases. Mediators Inflamm: 620581, 2015. doi: 10.1155/2015/620581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis 3: 75–80, 2001. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 75. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 263–269, 2011. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rivera S, Garcia-Gonzalez L, Khrestchatisky M, Baranger K. Metalloproteinases and their tissue inhibitors in Alzheimer's disease and other neurodegenerative disorders. Cell Mol Life Sci 76: 3167–3191, 2019. doi: 10.1007/s00018-019-03178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 4: 575–590, 2018. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rapti M, Atkinson SJ, Lee M-H, Trim A, Moss M, Murphy G. The isolated N-terminal domains of TIMP-1 and TIMP-3 are insufficient for ADAM10 inhibition. Biochem J 411: 433–439, 2008. doi: 10.1042/BJ20071430. [DOI] [PubMed] [Google Scholar]
- 79. Zipfel P, Rochais C, Baranger K, Rivera S, Dallemagne P. Matrix metalloproteinases as new targets in Alzheimer’s disease: opportunities and challenges. J Med Chem 63: 10705–10725, 2020. doi: 10.1021/acs.jmedchem.0c00352. [DOI] [PubMed] [Google Scholar]
- 80. Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14: 388–405, 2015. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia 39: 279–291, 2002. [Erratum in Glia 40: 130, 2002]. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 82. Saha P, Sarkar S, Paidi RK, Biswas SC. TIMP-1: A key cytokine released from activated astrocytes protects neurons and ameliorates cognitive behaviours in a rodent model of Alzheimer’s disease. Brain Behav Immun 87: 804–819, 2020. doi: 10.1016/j.bbi.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 83. Ashutosh, Chao C, Borgmann K, Brew K, Ghorpade A. Tissue inhibitor of metalloproteinases-1 protects human neurons from staurosporine and HIV-1-induced apoptosis: mechanisms and relevance to HIV-1-associated dementia. Cell Death Dis 3: e332, 2012. doi: 10.1038/cddis.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sarkar S, Paidi RK, Biswas SC. Astrocyte‐secreted TIMP‐1 binds to CD63 and differentially phosphorylates Akt in protecting neurons and promoting cognitive recovery in 5xFAD mice. Alzheimer's & Dementia 17: e052606, 2021. doi: 10.1002/alz.052606. [DOI] [Google Scholar]
- 85. Sjöstedt E, Zhong W, Fagerberg L, Karlsson M, Mitsios N, Adori C, Oksvold P, Edfors F, Limiszewska A, Hikmet F, Huang J, Du Y, Lin L, Dong Z, Yang L, Liu X, Jiang H, Xu X, Wang J, Yang H, Bolund L, Mardinoglu A, Zhang C, von Feilitzen K, Lindskog C, Pontén F, Luo Y, Hökfelt T, Uhlén M, Mulder J. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 367: eaay5947, 2020. doi: 10.1126/science.aay5947. [DOI] [PubMed] [Google Scholar]
- 86. Moore CS, Milner R, Nishiyama A, Frausto RF, Serwanski DR, Pagarigan RR, Whitton JL, Miller RH, Crocker SJ. Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J Neurosci 31: 6247–6254, 2011. doi: 10.1523/JNEUROSCI.5474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tang J, Kang Y, Huang L, Wu L, Peng Y. TIMP1 preserves the blood-brain barrier through interacting with CD63/integrin β 1 complex and regulating downstream FAK/RhoA signaling. Acta Pharm Sin B 10: 987–1003, 2020. doi: 10.1016/j.apsb.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Eckfeld C, Schoeps B, Häußler D, Frädrich J, Bayerl F, Böttcher JP, Knolle P, Heisz S, Prokopchuk O, Hauner H, Munkhbaatar E, Demir IE, Hermann CD, Krüger A. TIMP-1 is a novel ligand of amyloid precursor protein and triggers a proinflammatory phenotype in human monocytes. J Cell Biol 222: e202206095, 2023. doi: 10.1083/jcb.202206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xu L, Nirwane A, Xu T, Kang M, Devasani K, Yao Y. Fibroblasts repair blood-brain barrier damage and hemorrhagic brain injury via TIMP2. Cell Rep 41: 111709, 2022. doi: 10.1016/j.celrep.2022.111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jaworski DM, Soloway P, Caterina J, Falls WA. Tissue inhibitor of metalloproteinase-2(TIMP-2)-deficient mice display motor deficits. J Neurobiol 66: 82–94, 2006. doi: 10.1002/neu.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Perez-Martinez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci 25: 4917–4929, 2005. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Castellano JM, Mosher KI, Abbey RJ, McBride AA, James ML, Berdnik D, Shen JC, Zou B, Xie XS, Tingle M, Hinkson IV, Angst MS, Wyss-Coray T. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544: 488–492, 2017. doi: 10.1038/nature22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ferreira AC, Hemmer BM, Philippi SM, Grau-Perales AB, Rosenstadt JL, Liu H, Zhu JD, Kareva T, Ahfeldt T, Varghese M, Hof PR, Castellano JM. Neuronal TIMP2 regulates hippocampus-dependent plasticity and extracellular matrix complexity. Mol Psychiatry, 28: 3943–3954, 2023. doi: 10.1038/s41380-023-02296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Britton R, Wasley T, Harish R, Holz C, Hall J, Yee DC, Melton Witt J, Booth EA, Braithwaite S, Czirr E, Kerrisk Campbell M. Noncanonical activity of tissue inhibitor of metalloproteinases 2 (TIMP2) improves cognition and synapse density in aging. eNeuro 10: ENEURO.0031-23.2023, 2023. doi: 10.1523/ENEURO.0031-23.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169: 1276–1290 e17, 2017. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 96. Hoe H-S, Cooper MJ, Burns MP, Lewis PA, van der Brug M, Chakraborty G, Cartagena CM, Pak DTS, Cookson MR, Rebeck GW. The metalloprotease inhibitor TIMP-3 regulates amyloid precursor protein and apolipoprotein E receptor proteolysis. J Neurosci 27: 10895–10905, 2007. doi: 10.1523/JNEUROSCI.3135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Park JH, Cho SJ, Jo C, Park MH, Han C, Kim EJ, Huh GY, Koh YH. Altered TIMP-3 levels in the cerebrospinal fluid and plasma of patients with Alzheimer’s disease. J Pers Med 12: 827, 2022. doi: 10.3390/jpm12050827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, Letourneau P, Redell J, Shen L, Wang J, Peng Z, Xue H, Kozar R, Cox CS, Khakoo AY, Holcomb JB, Dash PK, Pati S. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med 4: 161ra150, 2012. doi: 10.1126/scitranslmed.3004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gibb SL, Zhao Y, Potter D, Hylin MJ, Bruhn R, Baimukanova G, Zhao J, Xue H, Abdel-Mohsen M, Pillai SK, Moore AN, Johnson EM, Cox CS, Dash PK, Pati S. TIMP3 attenuates the loss of neural stem cells, mature neurons and neurocognitive dysfunction in traumatic brain injury. Stem Cells 33: 3530–3544, 2015. doi: 10.1002/stem.2189. [DOI] [PubMed] [Google Scholar]
- 100. Wang XX, Tan MS, Yu JT, Tan L. Matrix metalloproteinases and their multiple roles in Alzheimer’s disease. Biomed Res Int 2014: 908636, 2014. doi: 10.1155/2014/908636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vervuurt M, de Kort AM, Jäkel L, Kersten I, Abdo WF, Schreuder FHBM, Rasing I, Terwindt GM, Wermer MJH, Greenberg SM, Klijn CJM, Kuiperij HB, Verbeek MM. Decreased ratios of matrix metalloproteinases to tissue-type inhibitors in cerebrospinal fluid in sporadic and hereditary cerebral amyloid angiopathy. Alzheimers Res Ther 15: 26, 2023. doi: 10.1186/s13195-023-01171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Qin W, Jia X, Wang F, Zuo X, Wu L, Zhou A, Li D, Min B, Wei C, Tang Y, Xing Y, Dong X, Wang Q, Gao Y, Li Y, Jia J. Elevated plasma angiogenesis factors in Alzheimer’s disease. J Alzheimers Dis 45: 245–252, 2015. doi: 10.3233/JAD-142409. [DOI] [PubMed] [Google Scholar]
- 103. Goldenberg MM. Multiple sclerosis review. P T 37: 175–184, 2012. [PMC free article] [PubMed] [Google Scholar]
- 104. Rosenberg GA, Kornfeld M, Estrada E, Kelley RO, Liotta LA, Stetler-Stevenson WG. TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res 576: 203–207, 1992. doi: 10.1016/0006-8993(92)90681-x. [DOI] [PubMed] [Google Scholar]
- 105. Lee EJ, Kim HS. The anti-inflammatory role of tissue inhibitor of metalloproteinase-2 in lipopolysaccharide-stimulated microglia. J Neuroinflammation 11: 116, 2014. doi: 10.1186/1742-2094-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen F, Radisky ES, Das P, Batra J, Hata T, Hori T, Baine A-MT, Gardner L, Yue MY, Bu G, del Zoppo G, Patel TC, Nguyen JH. TIMP-1 attenuates blood-brain barrier permeability in mice with acute liver failure. J Cereb Blood Flow Metab 33: 1041–1049, 2013. doi: 10.1038/jcbfm.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nishihara H, Perriot S, Gastfriend BD, Steinfort M, Cibien C, Soldati S, Matsuo K, Guimbal S, Mathias A, Palecek SP, Shusta EV, Pasquier RD, Engelhardt B. Intrinsic blood-brain barrier dysfunction contributes to multiple sclerosis pathogenesis. Brain 145: 4334–4348, 2022. doi: 10.1093/brain/awac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Savarin C, Bergmann CC, Hinton DR, Stohlman SA. MMP-independent role of TIMP-1 at the blood brain barrier during viral encephalomyelitis. ASN Neuro 5: e00127, 2013. doi: 10.1042/AN20130033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nicaise AM, Johnson KM, Willis CM, Guzzo RM, Crocker SJ. TIMP-1 promotes oligodendrocyte differentiation through receptor-mediated signaling. Mol Neurobiol 56: 3380–3392, 2019. doi: 10.1007/s12035-018-1310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 50: 329–339, 2005. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 111. Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol 180: 12–16, 2012. doi: 10.1016/j.ajpath.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 112. Houben E, Janssens K, Hermans D, Vandooren J, Van den Haute C, Schepers M, Vanmierlo T, Lambrichts I, van Horssen J, Baekelandt V, Opdenakker G, Baron W, Broux B, Slaets H, Hellings N. Oncostatin M-induced astrocytic tissue inhibitor of metalloproteinases-1 drives remyelination. Proc Natl Acad Sci USA 117: 5028–5038, 2020. doi: 10.1073/pnas.1912910117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tang J, Kang Y, Zhou Y, Shang N, Li X, Wang H, Lan J, Wang S, Wu L, Peng Y. TIMP2 ameliorates blood-brain barrier disruption in traumatic brain injury by inhibiting Src-dependent VE-cadherin internalization. J Clin Invest 134, 2023. doi: 10.1172/JCI164199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Scherer HU, Haupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun 110: 102400, 2020. doi: 10.1016/j.jaut.2019.102400. [DOI] [PubMed] [Google Scholar]
- 115. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 365: 2205–2219, 2011. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 116. Grillet B, Pereira RVS, Van Damme J, Abu El-Asrar A, Proost P, Opdenakker G. Matrix metalloproteinases in arthritis: towards precision medicine. Nat Rev Rheumatol 19: 363–377, 2023. doi: 10.1038/s41584-023-00966-w. [DOI] [PubMed] [Google Scholar]
- 117. Cascao R, Rosario HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev 9: 531–535, 2010. doi: 10.1016/j.autrev.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 118. O'Neil LJ, Kaplan MJ. Neutrophils in rheumatoid arthritis: breaking immune tolerance and fueling disease. Trends Mol Med 25: 215–227, 2019. doi: 10.1016/j.molmed.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 119. Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA 104: 20262–20267, 2007. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol 169: 2643–2647, 2002. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- 121. Luo S, Li W, Wu W, Shi Q. Elevated expression of MMP8 and MMP9 contributes to diabetic osteoarthritis progression in a rat model. J Orthop Surg Res 16: 64, 2021. doi: 10.1186/s13018-021-02208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Li X, Fu X, Gao Y, Li H, Wang W, Shen Y. Expression of tissue inhibitor of metalloproteinases-1 and B-cell lymphoma-2 in the synovial membrane in patients with knee osteoarthritis. Exp Ther Med 15: 885–889, 2018. doi: 10.3892/etm.2017.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Myers A, Lakey R, Cawston TE, Kay LJ, Walker DJ. Serum MMP-1 and TIMP-1 levels are increased in patients with psoriatic arthritis and their siblings. Rheumatology (Oxford) 43: 272–276, 2004. doi: 10.1093/rheumatology/keh032. [DOI] [PubMed] [Google Scholar]
- 124. Davidson RK, Waters JG, Kevorkian L, Darrah C, Cooper A, Donell ST, Clark IM. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther 8: R124, 2006. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hembry RM, Bagga MR, Reynolds JJ, Hamblen DL. Immunolocalisation studies on six matrix metalloproteinases and their inhibitors, TIMP-1 and TIMP-2, in synovia from patients with osteo- and rheumatoid arthritis. Ann Rheum Dis 54: 25–32, 1995. doi: 10.1136/ard.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol 22: 335–338, 2004. [PubMed] [Google Scholar]
- 127. Troeberg L. Engineering TIMP-3 for OA therapy. Osteoarthritis and Cartilage 25: S2, 2017. doi: 10.1016/j.joca.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem 276: 12501–12504, 2001. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 129. Nakamura H, Vo P, Kanakis I, Liu K, Bou-Gharios G. Aggrecanase-selective tissue inhibitor of metalloproteinase-3 (TIMP3) protects articular cartilage in a surgical mouse model of osteoarthritis. Sci Rep 10: 9288, 2020. doi: 10.1038/s41598-020-66233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Apparailly F, Noël DL, Millet V, Baker AH, Lisignoli G, Jacquet C, Kaiser M-J, Sany J, Jorgensen C. Paradoxical effects of tissue inhibitor of metalloproteinases 1 gene transfer in collagen-induced arthritis. Arthritis Rheum 44: 1444–1454, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 131. Huang W, Mabrouk ME, Sylvester J, Dehnade F, Zafarullah M. Enhanced expression of tissue inhibitor of metalloproteinases-4 gene in human osteoarthritic synovial membranes and its differential regulation by cytokines in chondrocytes. Open Rheumatol J 5: 81–87, 2011. doi: 10.2174/1874312901105010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Huang W, Li WQ, Dehnade F, Zafarullah M. Tissue inhibitor of metalloproteinases-4 (TIMP-4) gene expression is increased in human osteoarthritic femoral head cartilage. J Cell Biochem 85: 295–303, 2002. doi: 10.1002/jcb.10138. [DOI] [PubMed] [Google Scholar]
- 133. Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation 114: 1020–1027, 2006. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 134. Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, Edmunds LH, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation 107: 2857–2863, 2003. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 135. Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res 46: 307–315, 2000. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 136. Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation 99: 3063–3070, 1999. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 137. Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation 107: 618–625, 2003. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 138. Koskivirta I, Rahkonen O, Mäyränpää M, Pakkanen S, Husheem M, Sainio A, Hakovirta H, Laine J, Jokinen E, Vuorio E, Kovanen P, Järveläinen H. Tissue inhibitor of metalloproteinases 4 (TIMP4) is involved in inflammatory processes of human cardiovascular pathology. Histochem Cell Biol 126: 335–342, 2006. doi: 10.1007/s00418-006-0163-8. [DOI] [PubMed] [Google Scholar]
- 139. Chaturvedi P, Kalani A, Familtseva A, Kamat PK, Metreveli N, Tyagi SC. Cardiac tissue inhibitor of matrix metalloprotease 4 dictates cardiomyocyte contractility and differentiation of embryonic stem cells into cardiomyocytes: road to therapy. Int J Cardiol 184: 350–363, 2015. doi: 10.1016/j.ijcard.2015.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chaturvedi P, Tyagi SC. Epigenetic silencing of TIMP4 in heart failure. J Cell Mol Med 20: 2089–2101, 2016. doi: 10.1111/jcmm.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ye Q, Liu Q, Ma X, Bai S, Chen P, Zhao Y, Bai C, Liu Y, Liu K, Xin M, Zeng C, Zhao C, Yao Y, Ma Y, Wang J. MicroRNA-146b-5p promotes atrial fibrosis in atrial fibrillation by repressing TIMP4. J Cell Mol Med 25: 10543–10553, 2021. doi: 10.1111/jcmm.16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sun Y, Huang Z-Y, Wang Z-H, Li C-P, Meng X-L, Zhang Y-J, Su F, Ma N. TGF-β1 and TIMP-4 regulate atrial fibrosis in atrial fibrillation secondary to rheumatic heart disease. Mol Cell Biochem 406: 131–138, 2015. doi: 10.1007/s11010-015-2431-1. [DOI] [PubMed] [Google Scholar]
- 143. Chen QJ, Lu L, Peng WH, Hu J, Yan XX, Wang LJ, Zhang Q, Zhang RY, Shen WF. Polymorphisms of MMP-3 and TIMP-4 genes affect angiographic coronary plaque progression in non-diabetic and type 2 diabetic patients. Clin Chim Acta 405: 97–103, 2009. doi: 10.1016/j.cca.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 144. Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kytö V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem 285: 24487–24493, 2010. doi: 10.1074/jbc.M110.136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Takawale A, Fan D, Basu R, Shen M, Parajuli N, Wang W, Wang X, Oudit GY, Kassiri Z. Myocardial recovery from ischemia-reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circ Heart Fail 7: 652–662, 2014. doi: 10.1161/CIRCHEARTFAILURE.114.001113. [DOI] [PubMed] [Google Scholar]
- 146. Hernandez-Barrantes S, Shimura Y, Soloway PD, Sang QA, Fridman R. Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem Biophys Res Commun 281: 126–130, 2001. doi: 10.1006/bbrc.2001.4323. [DOI] [PubMed] [Google Scholar]
- 147. Nagase H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res 8: 179–186, 1998. doi: 10.1038/cr.1998.18. [DOI] [PubMed] [Google Scholar]
- 148. Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem 275: 26411–26415, 2000. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol 288: H149–H158, 2005. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 150. Kremastiotis G, Handa I, Jackson C, George S, Johnson J. Disparate effects of MMP and TIMP modulation on coronary atherosclerosis and associated myocardial fibrosis. Sci Rep 11: 23081, 2021. doi: 10.1038/s41598-021-02508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rouis M, Adamy C, Duverger N, Lesnik P, Horellou P, Moreau M, Emmanuel F, Caillaud JM, Laplaud PM, Dachet C, Chapman MJ. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein E-deficient mice. Circulation 100: 533–540, 1999. doi: 10.1161/01.CIR.100.5.533. [DOI] [PubMed] [Google Scholar]
- 152. Sundström J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Wilson PW, Vasan RS. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J 25: 1509–1516, 2004. doi: 10.1016/j.ehj.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 153. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 18: 169–193, 2021. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kandalam V, Basu R, Abraham T, Wang X, Soloway PD, Jaworski DM, Oudit GY, Kassiri Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ Res 106: 796–808, 2010. doi: 10.1161/CIRCRESAHA.109.209189. [DOI] [PubMed] [Google Scholar]
- 155. Di Gregoli K, George SJ, Jackson CL, Newby AC, Johnson JL. Differential effects of tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 on atherosclerosis and monocyte/macrophage invasion. Cardiovasc Res 109: 318–330, 2016. [Erratum in Cardiovasc Res 112: 616, 2016]. doi: 10.1093/cvr/cvv268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol 299: H1012–H1023, 2010. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F, Federici M. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes 58: 2396–2401, 2009. doi: 10.2337/db09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Johnson JL, Jenkins NP, Huang W-C, Di Gregoli K, Sala-Newby GB, Scholtes VPW, Moll FL, Pasterkamp G, Newby AC. Relationship of MMP-14 and TIMP-3 expression with macrophage activation and human atherosclerotic plaque vulnerability. Mediators Inflamm 2014: 276457, 2014. doi: 10.1155/2014/276457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chen H, Chen S, Ye H, Guo X. Protective effects of circulating TIMP3 on coronary artery disease and myocardial infarction: a mendelian randomization study. J Cardiovasc Dev Dis 9: 277, 2022. doi: 10.3390/jcdd9080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Fan D, Takawale A, Basu R, Patel V, Lee J, Kandalam V, Wang X, Oudit GY, Kassiri Z. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res 103: 268–280, 2014. doi: 10.1093/cvr/cvu072. [DOI] [PubMed] [Google Scholar]
- 161. Basu R, Lee J, Morton JS, Takawale A, Fan D, Kandalam V, Wang X, Davidge ST, Kassiri Z. TIMP3 is the primary TIMP to regulate agonist-induced vascular remodelling and hypertension. Cardiovasc Res 98: 360–371, 2013. doi: 10.1093/cvr/cvt067. [DOI] [PubMed] [Google Scholar]
- 162. Gai X, Zhang Z, Liang Y, Chen Z, Yang X, Hou J, Lan X, Zheng W, Hou J, Huang M. MMP-2 and TIMP-2 gene polymorphisms and susceptibility to atrial fibrillation in Chinese Han patients with hypertensive heart disease. Clin Chim Acta 411: 719–724, 2010. doi: 10.1016/j.cca.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 163. Lovelock JD, Baker AH, Gao F, Dong J-F, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol 288: H461–H468, 2005. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 164. Daseke MJ 2nd, Tenkorang MAA, Chalise U, Konfrst SR, Lindsey ML. Matrix Biol 91-92: 109–116, 2020. Cardiac fibroblast activation during myocardial infarction wound healing: fibroblast polarization after MI. doi: 10.1016/j.matbio.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: 'Embracing the MMP-independent-side of the family'. J Mol Cell Cardiol 48: 445–453, 2010. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 166. Eckhouse SR, Purcell BP, McGarvey JR, Lobb D, Logdon CB, Doviak H, O'Neill JW, Shuman JA, Novack CP, Zellars KN, Pettaway S, Black RA, Khakoo A, Lee T, Mukherjee R, Gorman JH, Gorman RC, Burdick JA, Spinale FG. Local hydrogel release of recombinant TIMP-3 attenuates adverse left ventricular remodeling after experimental myocardial infarction. Sci Transl Med 6: 223ra21, 2014. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Barlow SC, Doviak H, Jacobs J, Freeburg LA, Perreault PE, Zellars KN, Moreau K, Villacreses CF, Smith S, Khakoo AY, Lee T, Spinale FG. Intracoronary delivery of recombinant TIMP-3 after myocardial infarction: effects on myocardial remodeling and function. Am J Physiol Heart Circ Physiol 313: H690–H699, 2017. doi: 10.1152/ajpheart.00114.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Chintalgattu V, Greenberg J, Singh S, Chiueh V, Gilbert A, O'Neill JW, Smith S, Jackson S, Khakoo AY, Lee T. Utility of Glycosylated TIMP3 molecules: Inhibition of MMPs and TACE to improve cardiac function in rat myocardial infarct model. Pharmacol Res Perspect 6: e00442, 2018. doi: 10.1002/prp2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. George SJ, Wan S, Hu J, MacDonald R, Johnson JL, Baker AH. Sustained reduction of vein graft neointima formation by ex vivo TIMP-3 gene therapy. Circulation 124: S135–S142, 2011. doi: 10.1161/CIRCULATIONAHA.110.012732. [DOI] [PubMed] [Google Scholar]
- 170. Takawale A, Zhang P, Azad A, Wang W, Wang X, Murray AG, Kassiri Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 313: H224–H236, 2017. doi: 10.1152/ajpheart.00108.2017. [DOI] [PubMed] [Google Scholar]
- 171. Jia Z-B, Tian H, Kang K, Miao H-Z, Liu K-Y, Jiang S-L, Wang L-P. Expression of the tissue inhibitor of metalloproteinase-3 by transplanted VSMCs modifies heart structure and function after myocardial infarction. Transpl Immunol 30: 149–158, 2014. doi: 10.1016/j.trim.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 172. Johnson TW, Wu YX, Herdeg C, Baumbach A, Newby AC, Karsch KR, Oberhoff M. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler Thromb Vasc Biol 25: 754–759, 2005. doi: 10.1161/01.ATV.0000157582.33180.a9. [DOI] [PubMed] [Google Scholar]
- 173. Casagrande V, Menghini R, Menini S, Marino A, Marchetti V, Cavalera M, Fabrizi M, Hribal ML, Pugliese G, Gentileschi P, Schillaci O, Porzio O, Lauro D, Sbraccia P, Lauro R, Federici M. Overexpression of tissue inhibitor of metalloproteinase 3 in macrophages reduces atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 32: 74–81, 2012. doi: 10.1161/ATVBAHA.111.238402. [DOI] [PubMed] [Google Scholar]
- 174. George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH. Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-3. Circulation 101: 296–304, 2000. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]
- 175. Zhai H, Qi X, Li Z, Zhang W, Li C, Ji L, Xu K, Zhong H. TIMP-3 suppresses the proliferation and migration of SMCs from the aortic neck of atherosclerotic AAA in rabbits, via decreased MMP-2 and MMP-9 activity, and reduced TNF-α expression. Mol Med Rep 18: 2061–2067, 2018. doi: 10.3892/mmr.2018.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Scilabra SD, Pigoni M, Pravatá V, Schätzl T, Müller SA, Troeberg L, Lichtenthaler SF. Increased TIMP-3 expression alters the cellular secretome through dual inhibition of the metalloprotease ADAM10 and ligand-binding of the LRP-1 receptor. Sci Rep 8: 14697, 2018. doi: 10.1038/s41598-018-32910-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther 30: 31–41, 2012. doi: 10.1111/j.1755-5922.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 61: 259–266, 2006. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 53: 585–600, 2015. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix Biol 44-46: 167–174, 2015. doi: 10.1016/j.matbio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 181. Selman M, Ruiz V, Cabrera S, Segura L, Ramírez R, Barrios R, Pardo A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol 279: L562–L574, 2000. doi: 10.1152/ajplung.2000.279.3.L562. [DOI] [PubMed] [Google Scholar]
- 182. Zelko IN, Zhu J, Roman J. Role of SOD3 in silica-related lung fibrosis and pulmonary vascular remodeling. Respir Res 19: 221, 2018. doi: 10.1186/s12931-018-0933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 349: 209–220, 2014. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 184. Dong J, Ma Q. TIMP1 promotes multi-walled carbon nanotube-induced lung fibrosis by stimulating fibroblast activation and proliferation. Nanotoxicology 11: 41–51, 2017. doi: 10.1080/17435390.2016.1262919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Tang H, Mao J, Gao L, Liu J, Wu T. Effect of antisense TIMP-1 cDNA on the expression of TIMP-1 and MMP-2 in lung tissue with pulmonary fibrosis induced by bleomycin. Mol Med Rep 7: 149–153, 2013. doi: 10.3892/mmr.2012.1140. [DOI] [PubMed] [Google Scholar]
- 186. Gill SE, Huizar I, Bench EM, Sussman SW, Wang Y, Khokha R, Parks WC. Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury. Am J Pathol 176: 64–73, 2010. doi: 10.2353/ajpath.2010.090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chen G, Ge D, Zhu B, Shi H, Ma Q. Upregulation of matrix metalloproteinase 9 (MMP9)/tissue inhibitor of metalloproteinase 1 (TIMP1) and MMP2/TIMP2 ratios may be involved in lipopolysaccharide-induced acute lung injury. J Int Med Res 48: 300060520919592, 2020. doi: 10.1177/0300060520919592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Li X, Ma D, Zha X, Quan D, Pan D, Sun M, Hu B, Zhao B. Ilomastat, a synthetic inhibitor of MMPs, prevents lung injury induced by γ-ray irradiation in mice. Oncotarget 8: 60789–60808, 2017. doi: 10.18632/oncotarget.18487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Tao Z, Jie Y, Mingru Z, Changping G, Fan Y, Haifeng W, Yuelan W. The Elk1/MMP-9 axis regulates E-cadherin and occludin in ventilator-induced lung injury. Respir Res 22: 233, 2021. doi: 10.1186/s12931-021-01829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Vignola AM, Paganin F, Capieu L, Scichilone N, Bellia M, Maakel L, Bellia V, Godard P, Bousquet J, Chanez P. Airway remodelling assessed by sputum and high-resolution computed tomography in asthma and COPD. Eur Respir J 24: 910–917, 2004. doi: 10.1183/09031936.04.00032603. [DOI] [PubMed] [Google Scholar]
- 191. Guan W-J, Gao Y-H, Xu G, Lin Z-Y, Tang Y, Gu Y-Y, Liu G-H, Li H-M, Chen R-C, Zhong N-S. Sputum matrix metalloproteinase-8 and -9 and tissue inhibitor of metalloproteinase-1 in bronchiectasis: clinical correlates and prognostic implications. Respirology 20: 1073–1081, 2015. doi: 10.1111/resp.12582. [DOI] [PubMed] [Google Scholar]
- 192. Paulissen G, Rocks N, Quesada-Calvo F, Gosset P, Foidart J-M, Noel A, Louis R, Cataldo DD. Expression of ADAMs and their inhibitors in sputum from patients with asthma. Mol Med 12: 171–179, 2006. doi: 10.2119/2006-00028.Paulissen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Beeh KM, Beier J, Kornmann O, Buhl R. Sputum matrix metalloproteinase-9, tissue inhibitor of metalloprotinease-1, and their molar ratio in patients with chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis and healthy subjects. Respir Med 97: 634–639, 2003. doi: 10.1053/rmed.2003.1493. [DOI] [PubMed] [Google Scholar]
- 194. Zhang Y, Li Y, Ye Z, Ma H. Expression of matrix metalloproteinase-2, matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, and changes in alveolar septa in patients with chronic obstructive pulmonary disease. Med Sci Monit 26: e925278, 2020. doi: 10.12659/MSM.925278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Culpitt SV, Rogers DF, Traves SL, Barnes PJ, Donnelly LE. Sputum matrix metalloproteases: comparison between chronic obstructive pulmonary disease and asthma. Respir Med 99: 703–710, 2005. doi: 10.1016/j.rmed.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 196. Pons AR, Sauleda J, Noguera A, Pons J, Barceló B, Fuster A, Agustí AGN. Decreased macrophage release of TGF-beta and TIMP-1 in chronic obstructive pulmonary disease. Eur Respir J 26: 60–66, 2005. doi: 10.1183/09031936.05.00045504. [DOI] [PubMed] [Google Scholar]
- 197. Bolton CE, Stone MD, Edwards PH, Duckers JM, Evans WD, Shale DJ. Circulating matrix metalloproteinase-9 and osteoporosis in patients with chronic obstructive pulmonary disease. Chron Respir Dis 6: 81–87, 2009. doi: 10.1177/1479972309103131. [DOI] [PubMed] [Google Scholar]
- 198. Uysal P, Uzun H. Relationship between circulating serpina3g, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 and -2 with chronic obstructive pulmonary disease severity. Biomolecules 9: 62, 2019. doi: 10.3390/biom9020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Fukuda Y, Ishizaki M, Kudoh S, Kitaichi M, Yamanaka N. Localization of matrix metalloproteinases-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest 78: 687–698, 1998. [PubMed] [Google Scholar]
- 200. Hirano K, Sakamoto T, Uchida Y, Morishima Y, Masuyama K, Ishii Y, Nomura A, Ohtsuka M, Sekizawa K. Tissue inhibitor of metalloproteinases-2 gene polymorphisms in chronic obstructive pulmonary disease. Eur Respir J 18: 748–752, 2001. doi: 10.1183/09031936.01.00102101. [DOI] [PubMed] [Google Scholar]
- 201. Hegab AE, Sakamoto T, Uchida Y, Nomura A, Ishii Y, Morishima Y, Mochizuki M, Kimura T, Saitoh W, Kiwamoto T, Iizuka T, Massoud HH, Massoud HM, Hassanein KM, Sekizawa K. Association analysis of tissue inhibitor of metalloproteinase2 gene polymorphisms with COPD in Egyptians. Respir Med 99: 107–110, 2005. doi: 10.1016/j.rmed.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 202. Kukkonen MK, Tiili E, Vehmas T, Oksa P, Piirilä P, Hirvonen A. Association of genes of protease-antiprotease balance pathway to lung function and emphysema subtypes. BMC Pulm Med 13: 36, 2013. doi: 10.1186/1471-2466-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ghanei M, Ghalejooghi NA, Nourani MR, Harandi AA, Fooladi AA. Effect of TGFß1 and TIMP2 on disease activity in asthma and COPD. Iran J Allergy Asthma Immunol 9: 79–86, 2010. [PubMed] [Google Scholar]
- 204. Vitenberga Z, Pilmane M. Age-related lung tissue remodeling due to the local distribution of MMP-2, TIMP-2, TGF-β and Hsp70. Biotech Histochem 93: 239–248, 2018. doi: 10.1080/10520295.2017.1421322. [DOI] [PubMed] [Google Scholar]
- 205. Costanzo L, Soto B, Meier R, Geraghty P. The biology and function of tissue inhibitor of metalloproteinase 2 in the lungs. Pulm Med 2022: 3632764, 2022. doi: 10.1155/2022/3632764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Gill SE, Gharib SA, Bench EM, Sussman SW, Wang RT, Rims C, Birkland TP, Wang Y, Manicone AM, McGuire JK, Parks WC. Tissue inhibitor of metalloproteinases-3 moderates the proinflammatory status of macrophages. Am J Respir Cell Mol Biol 49: 768–777, 2013. doi: 10.1165/rcmb.2012-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Su B-H, Tseng Y-L, Shieh G-S, Chen Y-C, Wu P, Shiau A-L, Wu C-L. Over-expression of prothymosin-α antagonizes TGFβ signalling to promote the development of emphysema. J Pathol 238: 412–422, 2016. doi: 10.1002/path.4664. [DOI] [PubMed] [Google Scholar]
- 208. García-Alvarez J, Ramirez R, Checa M, Nuttall RK, Sampieri CL, Edwards DR, Selman M, Pardo A. Tissue inhibitor of metalloproteinase-3 is up-regulated by transforming growth factor-beta1 in vitro and expressed in fibroblastic foci in vivo in idiopathic pulmonary fibrosis. Exp Lung Res 32: 201–214, 2006. doi: 10.1080/01902140600817481. [DOI] [PubMed] [Google Scholar]
- 209. Martin EL, Truscott EA, Bailey TC, Leco KJ, McCaig LA, Lewis JF, Veldhuizen RAW. Lung mechanics in the TIMP3 null mouse and its response to mechanical ventilation. Exp Lung Res 33: 99–113, 2007. doi: 10.1080/01902140701198625. [DOI] [PubMed] [Google Scholar]
- 210. Meunier I, Bocquet B, Labesse G, Zeitz C, Defoort-Dhellemmes S, Lacroux A, Mauget-Faysse M, Drumare I, Gamez A-S, Mathieu C, Marquette V, Sagot L, Dhaenens C-M, Arndt C, Carroll P, Remy-Jardin M, Cohen SY, Sahel J-A, Puech B, Audo I, Mrejen S, Hamel CP. A new autosomal dominant eye and lung syndrome linked to mutations in TIMP3 gene. Sci Rep 6: 32544, 2016. doi: 10.1038/srep32544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Hu R-P, Lu Y-Y, Zhang X-J. MiR-34b-5p knockdown attenuates bleomycin-induced pulmonary fibrosis by targeting tissue inhibitor of metalloproteinase 3 (TIMP3). Eur Rev Med Pharmacol Sci 23: 2273–2279, 2019. doi: 10.26355/eurrev_201903_17276. [DOI] [PubMed] [Google Scholar]
- 212. Åhrman E, Hallgren O, Malmström L, Hedström U, Malmström A, Bjermer L, Zhou X-H, Westergren-Thorsson G, Malmström J. Quantitative proteomic characterization of the lung extracellular matrix in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. J Proteomics 189: 23–33, 2018. doi: 10.1016/j.jprot.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 213. Karoor V, Strassheim D, Sullivan T, Verin A, Umapathy NS, Dempsey EC, Frank DN, Stenmark KR, Gerasimovskaya E. The short-chain fatty acid butyrate attenuates pulmonary vascular remodeling and inflammation in hypoxia-induced pulmonary hypertension. Int J Mol Sci 22: 9916, 2021. doi: 10.3390/ijms22189916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Bai Y, Wang H-M, Liu M, Wang Y, Lian G-C, Zhang X-H, Kang J, Wang H-L. 4-Chloro-DL-phenylalanine protects against monocrotaline-induced pulmonary vascular remodeling and lung inflammation. Int J Mol Med 33: 373–382, 2014. doi: 10.3892/ijmm.2013.1591. [DOI] [PubMed] [Google Scholar]
- 215. Elias GJ, Ioannis M, Theodora P, Dimitrios PP, Despoina P, Kostantinos V, Charalampos K, Vassilios V, Petros SP. Circulating tissue inhibitor of matrix metalloproteinase-4 (TIMP-4) in systemic sclerosis patients with elevated pulmonary arterial pressure. Mediators Inflamm 2008: 164134, 2008. doi: 10.1155/2008/164134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Qipshidze N, Tyagi N, Metreveli N, Lominadze D, Tyagi SC. Autophagy mechanism of right ventricular remodeling in murine model of pulmonary artery constriction. Am J Physiol Heart Circ Physiol 302: H688–H696, 2012. doi: 10.1152/ajpheart.00777.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Sun J, Bao J, Shi Y, Zhang B, Yuan L, Li J, Zhang L, Sun M, Zhang L, Sun W. Effect of simvastatin on MMPs and TIMPs in cigarette smoke-induced rat COPD model. Int J Chron Obstruct Pulmon Dis 12: 717–724, 2017. doi: 10.2147/COPD.S110520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Hao W, Li M, Zhang C, Zhang Y, Wang P. Inflammatory mediators in exhaled breath condensate and peripheral blood of healthy donors and stable COPD patients. Immunopharmacol Immunotoxicol 41: 224–230, 2019. doi: 10.1080/08923973.2019.1609496. [DOI] [PubMed] [Google Scholar]
- 219. Hao W, Li M, Zhang Y, Zhang C, Wang P. Comparative study of cytokine levels in different respiratory samples in mild-to-moderate AECOPD patients. Lung 197: 565–572, 2019. doi: 10.1007/s00408-019-00263-y. [DOI] [PubMed] [Google Scholar]
- 220. Singh B, Ghosh N, Saha D, Sarkar S, Bhattacharyya P, Chaudhury K. Effect of doxycyline in chronic obstructive pulmonary disease - an exploratory study. Pulm Pharmacol Ther 58: 101831, 2019. doi: 10.1016/j.pupt.2019.101831. [DOI] [PubMed] [Google Scholar]
- 221. Zhang Q, Yan L, Lu J, Zhou X. Glycyl-L-histidyl-L-lysine-Cu(2+) attenuates cigarette smoke-induced pulmonary emphysema and inflammation by reducing oxidative stress pathway. Front Mol Biosci 9: 925700, 2022. doi: 10.3389/fmolb.2022.925700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Hou G, Yin Y, Han D, Wang Q-Y, Kang J. Rosiglitazone attenuates the metalloprotease/anti-metalloprotease imbalance in emphysema induced by cigarette smoke: involvement of extracellular signal-regulated kinase and NFκB signaling. Int J Chron Obstruct Pulmon Dis 10: 715–724, 2015. doi: 10.2147/COPD.S77514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Zhou X-M, Hou G, Gu D-X, Wang Q-Y, Zhao L. Peroxisome proliferator-activated receptor-γ in induced sputum is correlated with MMP-9/TIMP-1 imbalance and formation of emphysema in COPD patients. J Thorac Dis 9: 3703–3710, 2017. doi: 10.21037/jtd.2017.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Zhou Y, Tan X, Kuang W, Liu L, Wan L. Erythromycin ameliorates cigarette-smoke-induced emphysema and inflammation in rats. Transl Res 159: 464–472, 2012. doi: 10.1016/j.trsl.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 225. Singla E, Dharwal V, Naura AS. Gallic acid protects against the COPD-linked lung inflammation and emphysema in mice. Inflamm Res 69: 423–434, 2020. doi: 10.1007/s00011-020-01333-1. [DOI] [PubMed] [Google Scholar]
- 226. Kotnala S, Tyagi A, Muyal JP. rHuKGF ameliorates protease/anti-protease imbalance in emphysematous mice. Pulm Pharmacol Ther 45: 124–135, 2017. doi: 10.1016/j.pupt.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 227. Yao H, Hwang J-W, Sundar IK, Friedman AE, McBurney MW, Guarente L, Gu W, Kinnula VL, Rahman I. SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD. Am J Physiol Lung Cell Mol Physiol 305: L615–L624, 2013. doi: 10.1152/ajplung.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis JE, Elliott P, Barnes PJ, Ito K. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J 23: 2810–2819, 2009. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- 229. Black RA, Durie FH, Otten-Evans C, Miller R, Slack JL, Lynch DH, Castner B, Mohler KM, Gerhart M, Johnson RS, Itoh Y, Okada Y, Nagase H. Relaxed specificity of matrix metalloproteinases (MMPS) and TIMP insensitivity of tumor necrosis factor-alpha (TNF-alpha) production suggest the major TNF-alpha converting enzyme is not an MMP. Biochem Biophys Res Commun 225: 400–405, 1996. doi: 10.1006/bbrc.1996.1186. [DOI] [PubMed] [Google Scholar]
- 230. Batra J, Robinson J, Mehner C, Hockla A, Miller E, Radisky DC, Radisky ES. PEGylation extends circulation half-life while preserving in vitro and in vivo activity of tissue inhibitor of metalloproteinases-1 (TIMP-1). PLoS One 7: e50028, 2012. doi: 10.1371/journal.pone.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Daubin D, Cristol JP, Dupuy AM, Kuster N, Besnard N, Platon L, Buzançais A, Brunot V, Garnier F, Jonquet O, Klouche K. Urinary biomarkers IGFBP7 and TIMP-2 for the diagnostic assessment of transient and persistent acute kidney injury in critically ill patients. PLoS One 12: e0169674, 2017. doi: 10.1371/journal.pone.0169674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Maizel J, Daubin D, Vong LV, Titeca-Beauport D, Wetzstein M, Kontar L, Slama M, Klouche K, Vinsonneau C. Urinary TIMP2 and IGFBP7 identifies high risk patients of short-term progression from mild and moderate to severe acute kidney injury during septic shock: a prospective cohort study. Dis Markers 2019: 3471215, 2019. doi: 10.1155/2019/3471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Zhu L, Yu H, Liu S-Y, Xiao X-S, Dong W-H, Chen Y-N, Xu W, Zhu T. Prognostic value of tissue inhibitor of metalloproteinase-2 expression in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 10: e0124230, 2015. doi: 10.1371/journal.pone.0124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Lee S, Desai KK, Iczkowski KA, Newcomer RG, Wu KJ, Zhao Y-G, Tan WW, Roycik MD, Sang Q-XA. Coordinated peak expression of MMP-26 and TIMP-4 in preinvasive human prostate tumor. Cell Res 16: 750–758, 2006. doi: 10.1038/sj.cr.7310089. [DOI] [PubMed] [Google Scholar]
- 235. Zhou W, Sokoll LJ, Bruzek DJ, Zhang L, Velculescu VE, Goldin SB, Hruban RH, Kern SE, Hamilton SR, Chan DW, Vogelstein B, Kinzler KW. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev 7: 109–112, 1998. [PubMed] [Google Scholar]
- 236. Zhu D, Zha X, Hu M, Tao A, Zhou H, Zhou X, Sun Y. High expression of TIMP-1 in human breast cancer tissues is a predictive of resistance to paclitaxel-based chemotherapy. Med Oncol 29: 3207–3215, 2012. doi: 10.1007/s12032-012-0239-3. [DOI] [PubMed] [Google Scholar]
- 237. Zhang Y, Xu B, Liu Y, Yao H, Lu N, Li B, Gao J, Guo S, Han N, Qi J, Zhang K, Cheng S, Wang H, Zhang X, Xiao T, Wu L, Gao Y. The ovarian cancer-derived secretory/releasing proteome: A repertoire of tumor markers. Proteomics 12: 1883–1891, 2012. doi: 10.1002/pmic.201100654. [DOI] [PubMed] [Google Scholar]
- 238. Meng C, Yin X, Liu J, Tang K, Tang H, Liao J. TIMP-1 is a novel serum biomarker for the diagnosis of colorectal cancer: a meta-analysis. PLoS One 13: e0207039, 2018. doi: 10.1371/journal.pone.0207039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Rosenberg GA. Matrix metalloproteinases biomarkers in multiple sclerosis. Lancet 365: 1291–1293, 2005. doi: 10.1016/S0140-6736(05)61008-2. [DOI] [PubMed] [Google Scholar]
- 240. Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol 32: 368–372, 1998. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 241. Wagner DR, Delagardelle C, Ernens I, Rouy D, Vaillant M, Beissel J. Matrix metalloproteinase-9 is a marker of heart failure after acute myocardial infarction. J Card Fail 12: 66–72, 2006. doi: 10.1016/j.cardfail.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 242. Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J 151: 1101.e1–1101.e8, 2006. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 243. Goldbergova MP, Parenica J, Jarkovsky J, Kala P, Poloczek M, Manousek J, Kluz K, Kubkova L, Littnerova S, Tesak M, Toman O, Pavek N, Cermakova Z, Tomandl J, Vasku A, Spinar J. The association between levels of tissue inhibitor of metalloproteinase-1 with acute heart failure and left ventricular dysfunction in patients with ST elevation myocardial infarction treated by primary percutaneous coronary intervention. Genet Test Mol Biomarkers 16: 1172–1178, 2012. doi: 10.1089/gtmb.2012.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Hansson J, Vasan RS, Ärnlöv J, Ingelsson E, Lind L, Larsson A, Michaëlsson K, Sundström J. Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: cohort study. PLoS One 6: e16185, 2011. doi: 10.1371/journal.pone.0016185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. West MJ, Nestel PJ, Kirby AC, Schnabel R, Sullivan D, Simes RJ, Pollicino C, Lubos E, Münzel TF, White HD, Tonkin AM, Bickel C, Tiret L, Blankenberg S; LIPID Study Investigators. The value of N-terminal fragment of brain natriuretic peptide and tissue inhibitor of metalloproteinase-1 levels as predictors of cardiovascular outcome in the LIPID study. Eur Heart J 29: 923–931, 2008. doi: 10.1093/eurheartj/ehn007. [DOI] [PubMed] [Google Scholar]
- 246. Tiede SL, Wassenberg M, Christ K, Schermuly RT, Seeger W, Grimminger F, Ghofrani HA, Gall H. Biomarkers of tissue remodeling predict survival in patients with pulmonary hypertension. Int J Cardiol 223: 821–826, 2016. doi: 10.1016/j.ijcard.2016.08.240. [DOI] [PubMed] [Google Scholar]
- 247. Safdar Z, Tamez E, Chan W, Arya B, Ge Y, Deswal A, Bozkurt B, Frost A, Entman M. Circulating collagen biomarkers as indicators of disease severity in pulmonary arterial hypertension. JACC Heart Fail 2: 412–421, 2014. [Erratum in JACC Heart Fail 3: 345, 2015]. doi: 10.1016/j.jchf.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Marc A, Pop C, Sitar-Taut A-V, Budisan L, Berindan-Neagoe I, Pop D. The role of matrix metalloproteinases in patients with pulmonary hypertension: data from a prospective study. BMC Cardiovasc Disord 21: 607, 2021. doi: 10.1186/s12872-021-02424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Arvidsson M, Ahmed A, Säleby J, Hesselstrand R, Rådegran G. Plasma matrix metalloproteinase 2 is associated with severity and mortality in pulmonary arterial hypertension. Pulm Circ 12: e12041, 2022. doi: 10.1002/pul2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Schumann C, Lepper PM, Frank H, Schneiderbauer R, Wibmer T, Kropf C, Stoiber KM, Rüdiger S, Kruska L, Krahn T, Kramer F. Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 15: 523–532, 2010. doi: 10.3109/1354750X.2010.492431. [DOI] [PubMed] [Google Scholar]
- 251. Wetzl V, Tiede SL, Faerber L, Weissmann N, Schermuly RT, Ghofrani HA, Gall H. Plasma MMP2/TIMP4 ratio at follow-up assessment predicts disease progression of idiopathic pulmonary arterial hypertension. Lung 195: 489–496, 2017. doi: 10.1007/s00408-017-0014-5. [DOI] [PubMed] [Google Scholar]
- 252. Schäfer M, Ivy DD, Nguyen K, Boncella K, Frank BS, Morgan GJ, Miller-Reed K, Truong U, Colvin K, Yeager ME. Metalloproteinases and their inhibitors are associated with pulmonary arterial stiffness and ventricular function in pediatric pulmonary hypertension. Am J Physiol Heart Circ Physiol 321: H242–H252, 2021. doi: 10.1152/ajpheart.00750.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Wu G, Mahajan N, Dhawan V. Acknowledged signatures of matrix metalloproteinases in Takayasu's arteritis. Biomed Res Int 2014: 827105, 2014. doi: 10.1155/2014/827105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Schuurhof A, Bont L, Hodemaekers HM, de Klerk A, de Groot H, Hofland RW, van de Pol AC, Kimpen JLL, Janssen R. Proteins involved in extracellular matrix dynamics are associated with respiratory syncytial virus disease severity. Eur Respir J 39: 1475–1481, 2012. doi: 10.1183/09031936.00012311. [DOI] [PubMed] [Google Scholar]
- 255. Zingaropoli MA, Latronico T, Pasculli P, Masci GM, Merz R, Ciccone F, Dominelli F, Del Borgo C, Lichtner M, Iafrate F, Galardo G, Pugliese F, Panebianco V, Ricci P, Catalano C, Ciardi MR, Liuzzi GM, Mastroianni CM. Tissue Inhibitor of Matrix Metalloproteinases-1 (TIMP-1) and Pulmonary Involvement in COVID-19 Pneumonia. Biomolecules 13, 2023. doi: 10.3390/biom13071040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Sivakumar S, Hissar S, Nair D, Banurekha VV, Kornfeld H, Babu S. Association of plasma matrix metalloproteinase and tissue inhibitors of matrix metalloproteinase levels with adverse treatment outcomes among patients with pulmonary tuberculosis. JAMA Netw Open 3: e2027754, 2020. doi: 10.1001/jamanetworkopen.2020.27754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Sánchez-Pozo J, Baker-Williams AJ, Woodford MR, Bullard R, Wei B, Mollapour M, Stetler-Stevenson WG, Bratslavsky G, Bourboulia D. Extracellular phosphorylation of TIMP-2 by secreted c-Src tyrosine kinase controls MMP-2 activity. iScience 1: 87–96, 2018. doi: 10.1016/j.isci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Carreca AP, Pravatà VM, Markham M, Bonelli S, Murphy G, Nagase H, Troeberg L, Scilabra SD. TIMP-3 facilitates binding of target metalloproteinases to the endocytic receptor LRP-1 and promotes scavenging of MMP-1. Sci Rep 10: 12067, 2020. doi: 10.1038/s41598-020-69008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Arai AL, Migliorini M, Au DT, Hahn-Dantona E, Peeney D, Stetler-Stevenson WG, Muratoglu SC, Strickland DK. High-affinity binding of LDL receptor-related protein 1 to matrix metalloprotease 1 requires protease:inhibitor complex formation. Biochemistry 59: 2922–2933, 2020. doi: 10.1021/acs.biochem.0c00442. [DOI] [PMC free article] [PubMed] [Google Scholar]