Abstract
Background & Aims:
Food insecurity (FI) is a risk factor for nonalcoholic fatty liver disease (NAFLD) and advanced fibrosis in the general population, but its impact on liver disease in people with HIV (PWH) is unknown.
Methods:
We examined the association of FI with prevalence of NAFLD and fibrosis in a diverse cohort of PWH. PWH aged ≥18 years on antiretroviral therapy, HIV RNA<200 copies/mL, and without other known liver diseases were screened for NAFLD (CAP≥263 decibels/meter) and advanced fibrosis (LSM≥11 kilopascals) by vibration controlled transient elastography at eight US centers. Participants were categorized as food insecure using the Six-Item Short Form Household Food Security Survey. We used multivariable logistic regression to estimate odds ratios (OR) and 95% confidence intervals (CI) of NAFLD and advanced fibrosis by FI status.
Results:
Among 654 PWH, NAFLD was present in 348 (53%) and advanced fibrosis in 41 (6%). FI was present in 203 (31%) of participants, including 97/348 (28%) with NAFLD and 18/41 (44%) with advanced fibrosis. In multivariable analysis, FI was associated with lower odds of NAFLD (OR=0.57,95%CI:0.37–0.88) and a greater, but nonsignificant, odds of advanced fibrosis (OR=1.38,95%CI:0.65–2.90). We identified a significant interaction between FI and diabetes (p=0.02) on fibrosis risk, with a greater odds of fibrosis among food insecure PWH and diabetes (OR=3.83,95%CI:1.15–12.73) but not among food insecure nondiabetics (OR=1.12,95%CI:0.47–2.98).
Conclusions:
FI is highly prevalent among PWH and associated with lower odds of NAFLD, and among PWH with diabetes, a greater odds of advanced fibrosis. FI may contribute to hepatic fibrosis through mechanisms other than steatosis in PWH.
Keywords: social determinants of health, liver disease, fatty liver, liver health outcomes, AIDS, HIV, food security, MASLD
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a major cause of liver-related morbidity and mortality among people with HIV (PWH).1 NAFLD encompasses a spectrum of diseases ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), characterized by hepatocyte ballooning and inflammation, and can progress to cirrhosis and hepatocellular carcinoma.2 It is a hepatic manifestation of the metabolic syndrome, and the recent recommendation to change the name from NAFLD to metabolic dysfunction-associated steatotic liver disease (MASLD) reflects this well-recognized association.3 Over 30% of PWH have NAFLD, a prevalence rate that has more than doubled over the past decade.4 Additionally, high rates of fibrosis progression among PWH with NAFLD have been observed.4 With improvements in treatments for hepatitis C virus (HCV), NAFLD is poised to become the leading indication for liver transplantation among PWH.5
There are several risk factors for NAFLD among PWH, including rising rates of the metabolic syndrome, lipodystrophy due to HIV itself and antiretroviral therapy (ART), HIV-associated immune activation, and gut microbial translocation.6–8 In recent years, data has emerged showing the influence of commercial and social determinants of health (SDOH) on the natural history of NAFLD in the general population.9 Uninsured or publicly insured people with NAFLD have higher rates of hospital admissions and in-hospital mortality.10 LatinX persons, who carry a high disease burden of NAFLD, remain underrepresented in NASH clinical trials.11 A key SDOH, food insecurity, or the limited availability of nutritionally adequate food due to lack of financial resources, has also been linked to poorer NAFLD outcomes.12 Food insecurity is an independent risk factor for NAFLD and liver fibrosis, and associated with greater all-cause mortality and health care utilization in this population.13,14 This relationship is, in part, mediated by poor diet quality.14 Adherence to a healthy diet is the cornerstone of NAFLD management, however people who experience socioeconomic disadvantage often face financial barriers to accessing healthy foods and are reliant on cheaper, ultraprocessed foods. Whether the relationship between food insecurity and NAFLD is similar among PWH, however, is unknown. A better understanding of the relationship between food insecurity and NAFLD in PWH is essential to designing targeted food assistance programs that benefit PWH. We aimed to examine the association of food insecurity with the risk of NAFLD and advanced fibrosis in a diverse cohort of PWH.
Methods
Study population
Consecutive adults aged≥18 years with HIV were prospectively enrolled from July 2021 to July 2023 at eight centers across seven states in the U.S., including Duke University, Indiana University, Johns Hopkins University, University of Alabama Birmingham, University of California San Diego, University of California San Francisco, University of Texas Houston, and Virginia Commonwealth University (R01DK121378, HIV NASH Clinical Research Network, ClinicalTrials.gov, NCT04795219, Supplementary Appendix).
Participants with a documented history of HIV-1 on suppressive antiretroviral therapy with HIV RNA<200 copies/mL and without other known cause of liver disease were included. Other liver diseases encompassed current or prior chronic hepatitis B virus (HBV; confirmed by a positive hepatitis B surface antigen in serum at any time prior to enrollment), evidence of recent or current HCV (i.e. the presence of anti-HCV antibody with detectable HCV RNA in serum within 3 years prior to enrollment), alpha-1-antitrypsin deficiency, Wilson disease, hemochromatosis, polycystic liver disease, autoimmune hepatitis, or primary biliary cholangitis. For this analysis, patients were also excluded if they were drinking excessively (defined an Alcohol Use Disorder Identification Test [AUDIT] score of ≥8), currently pregnant, had disseminated or advanced malignancy, or unable to complete Fibroscan® assessments. A single institutional review board (sIRB) approved study protocols and consent forms, and all study participants gave written informed consent.
Steatosis and fibrosis assessments
All participants underwent vibration controlled transient elastography by Fibroscan® (VCTE, Echosens, Paris) by a trained study staff. Fibrosis was assessed by liver stiffness measurement (LSM, in kiloPascals [kPA]) and steatosis was assessed by controlled attenuated parameter (CAP, in decibels/meter [dB/m] using either the M or XL probe, as appropriate. Participants were instructed to fast for at least 3 hours prior to VCTE. All examinations had at least 10 reliable measurements. Unreliable LSM was defined as an interquartile range of >30%. Advanced fibrosis (≥F3) was categorized as LSM≥11kPa and hepatic steatosis was defined as CAP≥263 dB/m, cutoffs that have been used in prior studies.15,16 NAFLD was defined as hepatic steatosis with AUDIT<8. We also performed a sensitivity analysis using a higher CAP of ≥285dB/m (a cutoff used in prior studies of PWH) and CAP ≥353dB/m (90% specificity for steatosis).17 CAP has been shown to be an effective alternative to MRI PDFF for noninvasive hepatic steatosis assessment in PWH.17,18
Food insecurity assessment
Food insecurity was assessed using the Six-Item Short Form U.S. Department of Agriculture (USDA) Household Food Security Survey (HFSS).19 The six-item survey is a shortened version of the original 18-item HFSS that identifies food insecurity and hunger among adult household members. It has been validated in the general population and extensively utilized to measure food insecurity among PWH.20,21 Using USDA-validated cut points, adults were categorized as having high, marginal, low, and very low food security based on the number of affirmative responses. We grouped low and very low food security into a combined “food insecure” category and grouped high and marginal food security into the “food secure” category, consistent with prior food insecurity analyses.13,22 We also performed a secondary analysis in which we categorized food security status as high/marginal (i.e. food secure) vs. low vs. very low food security to assess whether there were differences in the association with liver outcomes among those experiencing different degrees of food insecurity.
Covariate assessment
Other covariates included sociodemographic characteristics, including age, sex at birth, race, ethnicity, and educational attainment; anthropometrics, including body mass index (BMI in kg/m2) and waist circumference (in centimeters); metabolic comorbidities (type 2 diabetes mellitus, hyperlipidemia, hypertension); and physical activity (metabolic equivalents[mets] per minute per week. Diabetes was defined as hemoglobin A1c≥6.5%, fasting blood glucose ≥126mg/dL, or use of a medication for type 2 diabetes. Hyperlipidemia was defined as plasma triglycerides ≥150mg/dL, plasma HDL-cholesterol ≤40mg/dL for males and ≤50mg/dL for females, or use of lipid-lowering treatment. Hypertension was defined as blood pressure ≥130/85mmHg or use of antihypertensive drug treatment. factors included self-reported alcohol use (using the AUDIT score) and tobacco use (never, former, current). Laboratory tests included aspartate aminotransferase(AST), alanine aminotransferase(ALT), total bilirubin, albumin, platelet count, fasting lipid panel, hemoglobin A1c, and creatinine. HIV-related factors included HIV RNA, CD4+ cell count, and antiretroviral therapy (ART) class (nucleoside reverse transcriptase inhibitor[NRTI], non-nucleoside reverse transcriptase inhibitor[NNRTI], protease inhibitor[PI], and integrase strand transfer inhibitor[INSTI]).
Statistical analysis
We compared sociodemographic, lifestyle, and clinical characteristics among participants stratified by food security status using the t-test or Mann-Whitney U test for continuous variables, the chi-square or Fisher exact test for categorical variables, and Cochran-Armitage or Jonckheere–Terpstra test trend tests for ordered alternatives. We used multivariable logistic regression to evaluate the association of food insecurity with hepatic steatosis and advanced fibrosis. We adjusted each model for covariates that were chosen a priori, including age, sex, race and ethnicity, educational level, BMI, diabetes, and physical activity. Given the known associations of diabetes with both food insecurity and liver disease, we also performed an interaction analysis to assess whether diabetes modified the relationship between food insecurity and the outcome of steatosis and fibrosis. Statistical tests were two-sided and P<0.05 was considered statistically significant. All analyses were performed using survey procedures in STATA/MP 16.1 (College Station, TX).
Results
Cohort Characteristics
Of 724 recruited participants with HIV, 654 met inclusion criteria (63 excluded due to excessive alcohol intake, 7 excluded due to missing food security data). Among the 654 participants in the study, NAFLD was present in 348(53%) and advanced fibrosis in 41(6%) of participants (Table 1). Food insecurity was present in 203(31%) of the cohort, 97(28%) of those with NAFLD, and 18(44%) of those with advanced liver fibrosis. Participants who were food insecure, compared to food secure, were more likely to be younger (mean 52.8 vs 53.7 years), have BMI≥30 kg/m2 (50% vs 44%), and consume more calories from sugar sweetened beverages (307 vs 236 kilocalories). They were also less likely to have type 2 diabetes (16% vs 21%) or an undetectable HIV-1 RNA (78% vs 85%). Sex at birth, race-ethnicity, BMI, and laboratory parameters were similar between food security groups. Additionally, participants with NAFLD, compared to those without NAFLD, were more likely to be obese (66% vs 24%), have a greater waist circumference (108.5 vs 92.8 cm), be diabetic (26% vs 12%), have hyperlipidemia (43% vs 35%), and have a history of CD4 T-cell count <200 cells/mm3 (all p-values<0.05; Supplementary Table 1),
Table 1.
Baseline characteristics of study participants by food security status
| Total Mean ± SD or n (%) | Food secure Mean ± SD or n (%) | Food insecure Mean ± SD or n (%) | P value | |
|---|---|---|---|---|
| Number of participants | 654 | 451 | 203 | |
| Age, y | 53.4 ± 11.6 | 53.7 ± 11.6 | 52.8 ± 11.7 | 0.41 |
| Sex at birth (male) | 474 (73) | 330 (73) | 144 (71) | 0.65 |
| Transgender (female) | 15 (2) | 8 (2) | 7 (3) | 0.29 |
| Race/ethnicity | 0.23 | |||
| NH White | 167 (25) | 121 (27) | 46 (23) | |
| NH Black | 332 (51) | 224 (50) | 108 (53) | |
| Hispanic | 131 (20) | 86 (19) | 45 (22) | |
| Other/unknown/refused | 24 (4) | 20 (4) | 4 (2) | |
| Educational attainment | 0.36 | |||
| Less than high school | 98 (15) | 62 (14) | 36 (18) | |
| High school or GED | 197 (30) | 140 (31) | 57 (29) | |
| College or above | 355 (55) | 248 (55) | 107 (53) | |
| Body mass index (kg/m2) | 30.5 ± 6.8 | 30.2 ± 6.3 | 31.1 ± 7.7 | 0.12 |
| Body mass index (kg/m2) class | 0.05 | |||
| <25 | 133 (20) | 87 (19) | 46 (23) | |
| 25–29.9 | 221 (34) | 166 (37) | 55 (27) | |
| ≥30 | 300 (46) | 198 (44) | 102 (50) | |
| Waist circumference (cm) | 101.2 ± 16.1 | 100.6 ± 14.9 | 102.4 ± 18.5 | 0.18 |
| Hypertension | 362 (55) | 244 (54) | 118 (58) | 0.34 |
| Type 2 diabetes mellitus | 127 (19) | 95 (21) | 32 (16) | 0.11 |
| Hyperlipidemia | 255 (39) | 175 (39) | 80 (39) | 0.88 |
| Smoking status | <0.01 | |||
| Never | 252 (39) | 169 (38) | 83 (41) | |
| Former | 264 (40) | 199 (44) | 65 (32) | |
| Current | 137 (21) | 82 (18) | 55 (27) | |
| Physical activity (met/min/week) | 6643 ± 6330 | 6729 ± 6297 | 6444 ± 6417 | 0.62 |
| History of cured HCV infection | 87 (13) | 56 (12) | 31 (15) | 0.32 |
| History of past alcohol intake | 413 (63) | 290 (64) | 123 (61) | 0.36 |
| Total kcal from sugar-sweetened beverages | 259 ± 317 | 236 ± 295 | 307 ± 357 | <0.01 |
| Total grams from sugar-sweetened beverages | 688 ± 802 | 63 ± 744 | 800 ± 911 | 0.02 |
| HIV related features | ||||
| History of AIDS | 80 (12) | 56 (12) | 24 (12) | 0.83 |
| History of CD4 T-cell count <200 cells/mm3 | 211 (32) | 144 (32) | 67 (33) | 0.79 |
| Number of participants | 654 | 451 | 203 | |
| Time since HIV diagnosis (years) | 19.1 ± 10.0 | 19.4 ± 9.9 | 18.5 ± 10.3 | 0.27 |
| HIV-RNA undetectable (yes) | 540 (83) | 382 (85) | 158 (78) | 0.03 |
| HIV-RNA copies per-mL | 49.7 ± 32.6 | 49.1 ± 32.7 | 50.5 ± 32.9 | 0.82 |
| CD4+ cell count(cells/mm^3) | 727.1 ± 332.6 | 712.7 ± 324.3 | 758.4 ± 348.6 | 0.11 |
| ART | ||||
| NRTI | 587 (90) | 404 (90) | 183 (90) | 0.82 |
| NNRTI | 122 (19) | 91 (20) | 31 (15) | 0.14 |
| PI/r | 90 (14) | 65 (14) | 25 (12) | 0.47 |
| INSTI | 550 (84) | 371 (82) | 179 (88) | 0.06 |
| Pharm-enhancer (cobicistat) | 120 (18) | 84 (19) | 36 (18) | 0.79 |
| VCTE features | ||||
| CAP dBm | 268 ± 65 | 272 ± 64 | 258 ± 65 | 0.01 |
| ≥263 | 348 (53) | 251 (56) | 97 (48) | 0.06 |
| ≥285 | 267 (41) | 196 (43) | 71 (35) | 0.04 |
| LSM kPa | 6.4 ± 6.0 | 6.3 ± 6.3 | 6.6 ± 5.2 | 0.58 |
| ≥8 | 95 (14) | 64 (14) | 31 (15) | 0.71 |
| ≥11 | 41 (6) | 23 (5) | 18 (9) | 0.07 |
| Laboratory features | ||||
| ALT (U/L) | 29.9 ± 24.6 | 31.2 ± 25.5 | 27.1 ± 22.3 | <0.01 |
| AST (U/L) | 27.6 ± 18.9 | 28.0 ± 18.7 | 26.6 ± 19.6 | <0.01 |
| Albumin (g/dl) | 4.30 ± 0.41 | 4.32 ± 0.37 | 4.25 ± 0.48 | 0.03 |
| Total bilirubin (mg/dl) | 0.58 ± 0.47 | 0.59 ± 0.44 | 0.57 ± 0.54 | 0.73 |
| Glucose (mg/dl) | 104.4 ± 38.6 | 105.1 ± 36.3 | 102.8 ± 43.3 | 0.12 |
| HbA1c (%) | 5.9 ± 1.1 | 5.9 ± 1.1 | 5.9 ± 1.2 | 0.77 |
| Total cholesterol (mg/dl) | 175.1 ± 41.9 | 175.8 ± 43.4 | 173.6 ± 38.4 | 0.55 |
| Triglycerides (mg/dl) | 144.5 ± 135.2 | 149.0 ± 155.0 | 134.3 ± 71.5 | 0.91 |
| HDL (mg/dl) | 48.4 ± 16.5 | 47.9 ± 16.4 | 49.5 ± 16.8 | 0.25 |
| LDL (mg/dl) | 101.6 ± 37.8 | 102.4 ± 39.4 | 99.6 ± 33.7 | 0.40 |
| Creatinine (mg/dl) | 1.15 ± 1.02 | 1.09 ± 0.45 | 1.28 ± 1.69 | 0.72 |
| Platelet x 109/L | 242 ± 72 | 242 ± 74 | 242 ± 65 | 0.96 |
| FIB-4 | 0.35 | |||
| <1.30 | 398 (62) | 268 (60) | 130 (65) | |
| 1.30–2.67 | 216 (33) | 154 (35) | 62 (31) | |
| >2.67 | 31 (5) | 24 (5) | 7 (4) | |
| NFS | 0.64 | |||
| <−1.455 | 313 (49) | 219 (49) | 94 (47) | |
| −1.455 to 0.675 | 279 (43) | 193 (43) | 86 (43) | |
| >0.675 | 52 (8) | 33 (7) | 19 (10) |
Abbreviations: AIDS, Acquired Immunodeficiency Syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAP, controlled attenuated parameter; GED, General Educational Development; HbA1c, hemoglobin A1c; HIV, human immunodeficiency virus; HDL, high density lipoprotein; INSTI, integrase strand transfer inhibitors; LDL, low density lipoprotein; LSM, liver stiffness measurement; NFS, NAFLD Fibrosis Score; NH, nonhispanic, NNRTI, non -nucleoside reverse transcriptase inhibitors; NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; PI, protease inhibitors
Association between food security status and NAFLD
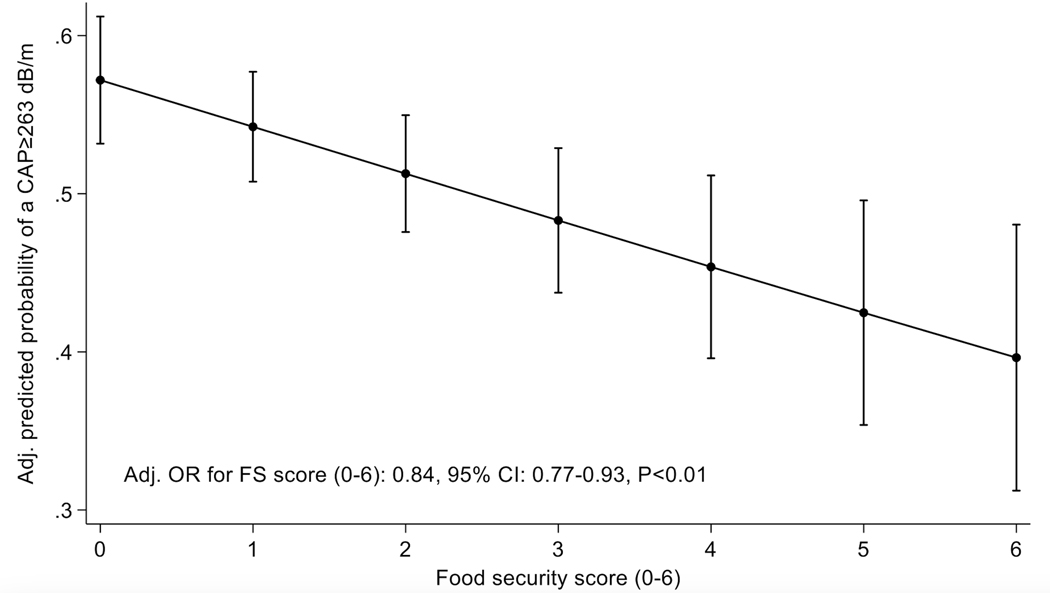
In multivariable logistic regression models, food insecurity was associated with lower odds of NAFLD (OR=0.57,95%CI:0.37–0.88,p=0.01) after controlling for age, sex, race-ethnicity, educational level, presence of type 2 diabetes, BMI, physical activity, and consumption of sugar sweetened beverages (Table 2). We noted an inverse and dose-dependent effect between food security status and odds of steatosis after controlling for relevant covariates (Figure 1). In other words, the more food insecure a participant was, the less likely they were to have NAFLD. These results were confirmed in a secondary analysis in which we categorized food security status as high/marginal vs low vs very low (Supplementary Table 2). We found similar results using a CAP≥285 dB/m (N=267) cutoff as with a CAP≥263 dB/m cutoff (Table 3). Using a cutoff of ≥90% specificity (CAP≥353 dB/m, N=72), food insecurity remained associated with a lower odds of NAFLD, though this did not reach statistical significance (adjusted OR= 0.60, 95%CI:0.30–1.17,P-value=0.13), likely due to being underpowered (Supplementary Table 3). Lastly, we found a significant interaction between food insecurity and diabetes on the odds of NAFLD with CAP cutoffs of ≥263 and ≥285 dB/m (P-values<0.01 for all; Supplementary Tables 4 and 5) but not with CAP cutoff of ≥353 dB/m (P=0.16; Supplementary Table 6).
Table 2.
The association of food security and odds of NAFLD (CAP≥263 dB/m).
| Crude OR for NAFLD | 95%CI | P-value | |
|---|---|---|---|
| Food insecure (vs food secure), unadjusted | 0.73 | 0.52–1.01 | 0.06 |
| Adjusted OR for NAFLD | 95%CI | P-value | |
| Food insecure (vs food secure), adjusted for age, sex, race-ethnicity, educational level, type 2 diabetes, BMI, physical activity, and total grams from sugar-sweetened beverages | 0.57 | 0.37–0.88 | 0.01 |
Figure 1.
Odds of NAFLD (≥263 dB/m)* by Household Food Security Survey score**
*Covariate-adjusted logistic regression includes age, sex at birth, race-ethnicity, educational levels, type 2 diabetes, BMI, physical activity
**Score ranges from 0 to 6 (0 represents high food security and 6 represents very low food security)
Table 3.
The association of food security and odds of NAFLD (CAP≥285 dB/m). Results based on univariable and multivariable logistic regression analysis.
| Crude OR for NAFLD | 95% CI | P-value | |
|---|---|---|---|
| Food insecure (vs food secure), unadjusted | 0.69 | 0.49–0.98 | 0.04 |
| Adjusted OR for NAFLD | 95% CI | P-value | |
| Food insecure (vs food secure), adjusted for age, sex, race-ethnicity, educational level, type 2 diabetes, BMI, and physical activity | 0.51 | 0.32–0.80 | <0.01 |
Association between food security status and advanced liver fibrosis
In univariate analysis, there was a trend towards higher odds of advanced fibrosis with food insecurity (OR=1.81;95%CI:0.95–3.43), but this was attenuated and not significant in multivariable logistic regression (OR 1.38;95%CI:0.65–2.90) (Table 4). We similarly did not see an association between low or very low food security and advanced fibrosis (Supplementary Table 7). However, there was a significant interaction between the presence of type 2 diabetes and food insecurity (low+very low) on advanced fibrosis in multivariable analysis (p=0.02). When we further examined the relationship between food insecurity and advanced fibrosis stratified by diabetes status, food insecurity was associated with a significantly greater odds of advanced fibrosis among people with diabetes (OR=3.83,95%CI:1.15–12.73), but not among nondiabetics (OR=1.12,95%CI:0.47–2.98) after controlling for age, sex, race-ethnicity, educational levels, BMI, and physical activity (Table 5). Our findings suggest that food insecurity interacts with type 2 diabetes in promoting advanced liver fibrosis among PWH, though we also note small sample sizes (Supplementary Table 8).
Table 4.
Independent association of advanced fibrosis (LSM≥11 kPa) with food security.
| Crude OR for advanced fibrosis | 95% CI | P-value | |
|---|---|---|---|
| Food insecure (vs food secure), unadjusted | 1.81 | 0.95–3.43 | 0.07 |
| Adjusted OR for advanced fibrosis | 95% CI | P-value | |
| Food insecure (vs food secure), adjusted for age, sex, race-ethnicity, educational level, type 2 diabetes, BMI, physical activity, and total grams from sugar-sweetened beverages | 1.33 | 0.64–2.79 | 0.44 |
Abbreviations: LSM, liver stiffness measurement
Table 5.
Effect of type 2 diabetes on the odds of advanced fibrosis (LSM≥11kPa) in adults stratified by food security*
| Food security status | ||
|---|---|---|
|
|
||
| Type 2 diabetes | Food secure (high/marginal) | Food insecure (low/very low) |
|
|
||
| OR (95%CI) | OR (95%CI) | |
|
|
||
| No | Ref. | 1.12 (0.47–2.98) |
| Yes | 2.05 (0.79–5.32) | 3.83 (1.15–12.73) |
Model includes age, sex, education, race-ethnicity, physical activity, BMI. P-value=0.04 for difference in the odds of advanced fibrosis between diabetes + high/marginal food security versus diabetes + very low food security groups.
Discussion
In this large, diverse, prospective multicenter cohort study of adults with HIV infection, food insecurity is highly prevalent and associated with lower odds of NAFLD. By contrast, among diabetic PWH, food insecurity is associated with greater odds of advanced fibrosis. To our knowledge, this is one of the first studies investigating the relationship between food insecurity and liver disease among PWH and highlights that food insecurity may influence liver disease differently in HIV infection.
Surprisingly, our findings show that food insecurity is associated with lower odds of steatosis. These findings were unexpected and contrary to data in the non-HIV population. In a study from the National Health and Nutrition Examination Survey (NHANES), food insecurity was associated with a greater risk of fatty liver even after controlling for demographic, metabolic, and lifestyle factors.13 One prior study evaluating food insecurity and steatosis in PWH, the Miami Adult Studies on HIV, found that food insecurity was associated with an increased odds of steatosis at higher BMIs but reduced odds of steatosis at lower BMIs.23 We did not find a similar interaction between food insecurity and BMI on the outcome of steatosis in our cohort. However, the Miami HIV cohort was predominantly comprised of adults without HIV infection and nearly 25% of participants had active HCV, which may impact the risk of hepatic steatosis.
There are several potential explanations for the observed association between food insecurity and lower NAFLD risk in PWH in our cohort. One possible reason is that food insecurity leads to greater immune activation, T-cell dysregulation, and poorer virologic suppression in PWH.24,25 In our study, food insecure participants were less likely to have undetectable HIV RNA than food secure participants and may represent an overall “sicker” population that experiences lower hepatic steatosis. There are also differences in body composition in PWH, who have lower visceral and subcutaneous adipose tissue deposition compared to people without HIV infection,26 which may be exacerbated among those experiencing hunger, leading to less fat deposition in the liver. Additional studies are needed to better understand the inverse relationship between food insecurity and steatosis.
Interestingly, while we did not see a significant relationship between food insecurity and liver fibrosis in the total cohort, we found a significant interaction between food insecurity and diabetes on the outcome of liver fibrosis. Participants with diabetes who were food insecure had greater fibrosis than those with diabetes who were food secure, suggesting that food insecurity may have a more detrimental effect on liver disease among PWH with underlying metabolic comorbidities. Given the small sample sizes of these groups, larger scale studies are needed to confirm these results. Unlike in the general population in whom food insecurity is associated with steatosis and clinically significant fibrosis,13,14 our findings suggest that there may be factors other than steatosis that mediate the association between food insecurity and liver fibrosis in PWH.
The results of our study have significant clinical and public health implications. First, the prevalence of food insecurity in our diverse multicenter cohort was high, affecting nearly one-third of all participants and 44% of those with advanced fibrosis. These rates are significantly higher than those reported in the general population27 and highlights that lack of access to food is a major public health issue in PWH. This association is consistent with prior literature, as HIV has been demonstrated to manifest through the debilitation of productive household members, decreased household economic capacity, and increased caregiver burden, all of which can exacerbate household food insecurity.25 Second, as the cohort of PWH ages and rates of the metabolic syndrome continue to rise, identifying determinants of fibrosis in this population becomes critical to combating liver disease, which has become a leading cause of non-AIDS related mortality. Our study shows that food insecurity may be a key social determinant and modifiable risk factor for liver fibrosis. In clinical practice, clinicians should consider screening for and referring PWH with food insecurity to case managers who can provide linkage to community food assistance programs. Further research efforts and funding are also needed to (1) explore pathways mediating the association of food insecurity with liver fibrosis, and (2) develop evidence-based dietary interventions that can be tested for feasibility and efficacy among PWH.
We acknowledge several limitations. As with all observational studies, we could not account for confounding factors that were not adequately measured. However, the HIV NASH CRN contains extensive data on sociodemographic and behavioral characteristics for which we adjusted in multivariable analyses. We also measured steatosis and fibrosis using VCTE, which is less reliable than liver biopsy. However, VCTE has been well-validated as a noninvasive measure of steatosis and fibrosis in PWH.17,18 Lastly, we note the high prevalence of steatosis in this cohort; while the HIV NASH CRN recruits with an “all-comers” policy (i.e. does not select for people with risk factors for NAFLD), people are recruited from clinics, which may enrich our population with NAFLD. However, the prevalence rate is overall similar to the rates of steatosis in the US general population and in prior studies of PWH.4,28 There were many strengths. This is one of the first and largest studies examining the impacts of food insecurity on liver disease outcomes in PWH. Additionally, the HIV NASH CRN is a diverse cohort that includes adults with HIV from eight different centers throughout the US, reflecting real-world data that are generalizable to PWH across the country.
In conclusion, food insecurity is highly prevalent and associated with a lower odds of NAFLD in PWH but a greater odds of liver fibrosis in diabetic PWH independent of other SDOH, metabolic, and lifestyle factors. Exploration of the mechanistic underpinnings underlying these associations is needed to inform preventive food insecurity interventions that can be effectively integrated into HIV, diabetes, and liver disease care.
Supplementary Material
What you need to know:
Background:
Food insecurity is a risk factor for NAFLD and liver fibrosis in the general population, but little is known about its influence on liver disease in people with HIV (PWH).
Findings:
Food insecurity is highly prevalent in PWH but associated with lower odds of NAFLD. In PWH and diabetes, it is linked to greater fibrosis. Food insecurity may contribute to hepatic fibrosis through mechanisms other than hepatic steatosis in HIV.
Implications for patient care:
PWH at risk for liver disease should be screened for food insecurity and referred to the appropriate resources. Further studies are needed to better understand these mechanisms and their implications.
Financial support:
This study was funded by R01DK121378; USC CTSI KL2 Award (Kardashian; KL2TR001854). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Abbreviations:
- AIDS
acquired immunodeficiency syndrome
- ALT
alanine aminotransferase
- ART
antiretroviral therapy
- AST
aspartate aminotransferase
- AUDIT
Alcohol Use Disorder Identification Test
- CAP
controlled attenuated parameter
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HFSS
Household Food Security Survey
- HIV
human immunodeficiency virus
- INSTI
integrase strand transfer inhibitor
- LSM
liver stiffness measurement
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- PWH
people with HIV
- SDOH
social determinants of health
- USDA
US Department of Agriculture
- VCTE
vibration controlled transient elastography
Footnotes
Conflicts of Interest:
Dr. Kardashian: None for this paper. For full disclosure, consulting: Gilead.
Dr. Audrey Lloyd: None for this paper. For full disclosure, her institution has received research grant support from Gilead Sciences and AbbVie.
Dr. Susanna Naggie: declares no conflicts of interest.
Dr. Mark S. Sulkowski: declares no conflicts of interest.
Dr. Tinsay Woreta: declares no conflicts of interest.
Dr. Jordan E. Lake: declares no conflicts of interest.
Dr. Sonya Heath: declares no conflicts of interest.
Dr. Jennifer C. Price: None for this paper. She has received research grant support from Gilead Sciences, AbbVie, VIR, Genentech, and Zydus.
Laura Wilson, Dr. Eduardo Vilar Gomez and Holly Crandall declare no conflicts of interest.
Dr. Chalasani: None for this paper. For full disclosure, he has ongoing consulting agreements with Madrigal, Zydus, Ventyx, Pfizer, GSK, Merck, Altimmune, and Foresite. He has research support from DSM and Exact Sciences. He has equity ownership in Avant Sante Therapeutics, a contract research organization.
Dr. Gawrieh consulting: TransMedics, Pfizer. Research grant support: Viking and Zydus,
Dr. Sterling declares none for this paper. For full disclosure, he has research support form Gilead, AbbVie, Abbott, and Roche, has served on a DSMB for Pfizer, AskBio
Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. RL has stock options in 89bio and Sagimet Biosciences. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Michel M, Labenz C, Wahl A, et al. Prevalence and risk factors of nonalcoholic steatohepatitis with significant fibrosis in people with HIV. AIDS. 2022;36(12):1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelotti GA, Machado MV, & Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013;10:656–665. [DOI] [PubMed] [Google Scholar]
- 3.Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023;79:1542–1556. [DOI] [PubMed] [Google Scholar]
- 4.Kalligeros M, Vassilopoulos A, Shehadeh F, et al. Prevalence and Characteristics of Nonalcoholic Fatty Liver Disease and Fibrosis in People Living With HIV Monoinfection: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2023;21:1708–1722. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Varela I, Dodge JL, Terrault NA, Brandman D, & Price JC. Nonviral liver disease is the leading indication for liver transplant in the United States in persons living with human immunodeficiency virus. Am J Transplant. 2021:doi: 10.1111/ajt.16569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology 2008;135:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin. Infect. Dis. 2008;47:250–257. [DOI] [PubMed] [Google Scholar]
- 8.Kardashian A, Peters MG, Tien PC, & Price JC. The Pathogenesis of Liver Disease in People Living With Human Immunodeficiency Virus: The Emerging Role of the Microbiome. Clin Liver Dis 2020;15:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivancovsky-Wajcman D, Brennan PN, Kopka CJ, et al. Integrating social nutrition principles into the treatment of steatotic liver disease. Commun Med (Lond) 2023;3:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adejumo AC, Samuel GO, Adegbala OM, et al. Prevalence, trends, outcomes, and disparities in hospitalizations for nonalcoholic fatty liver disease in the United States. Ann Gastroenterol 2019;32:504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel P, Muller C, & Paul S. Racial disparities in nonalcoholic fatty liver disease clinical trial enrollment: A systematic review and meta-analysis. World J Hepatol 2020;12: 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman-Jensen A, Rabbitt MP, Gregory CA, & Singh A. Household Food Security in the United States in 2020. 2021;ERR-298. [Google Scholar]
- 13.Golovaty I, Tien PC, Price JC, Sheira L, Seligman H, Weiser SD. Food Insecurity May Be an Independent Risk Factor Associated with Nonalcoholic Fatty Liver Disease among Low-Income Adults in the United States. J. Nutr. 2020;150: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardashian A, Dodge JL, & Terrault NA. Food Insecurity is Associated With Mortality Among U.S. Adults With Nonalcoholic Fatty Liver Disease and Advanced Fibrosis. Clin Gastroenterol Hepatol 2022;20(12):2790–2799. [DOI] [PubMed] [Google Scholar]
- 15.Gawrieh S, Lake JE, Debroy P, et al. Burden of fatty liver and hepatic fibrosis in persons with HIV: A diverse cross-sectional US multicenter study. Hepatology 2023;78:578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2019;17:156–163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajmera VH, Cachay ER, Ramers CB, et al. Optimal Threshold of Controlled Attenuation Parameter for Detection of HIV-Associated NAFLD With Magnetic Resonance Imaging as the Reference Standard. Clin Infect Dis 2021;72:2124–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duarte M, Tien P, Ma Y, et al. Controlled attenuation parameter accurately detects liver steatosis in people with HIV. AIDS 2022;36:2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bickel G, Nord M, Price C, Hamilton W, & Cook J. Guide to measuring household food security, revised 2000. Food and Nutrition Service, U.S. Department of Agriculture, 2000. [Google Scholar]
- 20.Blumberg SJ, Bialostosky K, Hamilton WL. & Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health 1999;89: 1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatsu I, Hade E, & Campa A. Food Security Status is Related to Mental Health Quality of Life Among Persons Living with HIV. AIDS Behav 2017;21:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seligman HK, Laraia BA, & Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J. Nutr. 2010;140: 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamargo JA, Sherman KE, Campa A, et al. Food insecurity is associated with magnetic resonance-determined nonalcoholic fatty liver and liver fibrosis in low-income, middle-aged adults with and without HIV. Am J Clin Nutr 2021;113: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamargo JA, Hernandez-Boyer J, Teeman C, et al. Immune Activation: A Link Between Food Insecurity and Chronic Disease in People Living With Human Immunodeficiency Virus. J Infect Dis 2021;224: 2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters BA, Sheira L, Hanna DB, et al. Food Insecurity and T-cell Dysregulation in Women Living With Human Immunodeficiency Virus on Antiretroviral Therapy. Clin Infect Dis 2021;72(5): e112–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kardashian A, Ma Y, Sherzer R, et al. Sex differences in the association of HIV infection with hepatic steatosis. AIDS 2017;31:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.USDA Economic Research Service. Food Security and Nutrition Assistance. https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials/food-security-and-nutrition-assistance/#:~:text=In%202021%2C%2089.8%20percent%20of,had%20very%20low%20food%20security. 2023.
- 28.Kalligeros M, Vassilopoulos A, Vassilopoulos S, et al. Prevalence of Steatotic Liver Disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017–2020. Clin Gastroenterol Hepatol 2023;(23)00914-X:S1542–3565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.